Abstract
In the present study, we performed to selectively deplete glycyrrhizin from Si-Ni-San, a traditional Chinese prescription that consists of 4 Chinese herbs including Radix Glycyrrhizae Uralensis, and examined its influence on the suppressing activity of Si-Ni-San against contact sensitivity in mice. An immunoaffinity column was made by covalently coupling the polyclonal antibody, obtained by the immunization with glycyrrhizin–BSA conjugate, to CNBr-activated Sepharose 4B. By using this column, glycyrrhizin in Si-Ni-San was selectively and almost completely depleted from the whole extract, which was confirmed by high-performance liquid chromatography (HPLC). Both 200 mg/kg of Si-Ni-San and 10 mg/kg of glycyrrhizin, the dose corresponding to its proportion contained in Si-Ni-San, significantly reduced the ear swelling of picryl chloride (PCl)-induced ear contact sensitivity in mice and the inhibition by Si-Ni-San was stronger than that by glycyrrhizin. The adhesion activity to type IV collagen of the isolated spleen cells from PCl-sensitized mice was significantly decreased by both Si-Ni-San and glycyrrhizin. However, the glycyrrhizin-depleted sample of Si-Ni-San (Si-Ni-SanGL−) only showed a slight inhibition on the cell adhesion. Furthermore, the spleen cells from PCl-sensitized mice produced more matrix metalloproteinase (MMP)-2 and -9 than naive spleen cells did, and both Si-Ni-San and glycyrrhizin remarkably reduced MMP-2 and MMP-9 production. In contrast, Si-Ni-SanGL− only showed a slight inhibition. These results suggest that glycyrrhizin may act as one of the active constituents of Si-Ni-San in inhibiting delayed-type hypersensitivity reaction via down-regulating the MMP production and the cell adhesion to extracellular matrix. The present study also provides a new approach to recognize and validate an active constituent in traditional prescription through a selective depletion.
Keywords: Si-Ni-San, Glycyrrhizin, Immunoaffinity column, Contact sensitivity, Matrix metalloproteinase, Adhesion
1. Introduction
Contact sensitivity has served as the classical model for delayed-type hypersensitivity (DTH) reactions in skin [1]. The cellular and molecular mechanisms of DTH reactions have been intensively studied. During the lymphocyte localization and infiltration to inflammatory locus, lymphocytes should interact with endothelial cells and the underlying basement membrane and extracellular matrix [2], [3]. In the process of these immune responses, matrix metalloproteinases (MMPs) are known to degrade basement membrane and extracellular matrix and to enhance the inflammatory infiltration of T lymphocytes into target tissues [4]. Furthermore, there are increasing evidences that some kinds of monoclonal antibodies and synthetic peptides could ameliorate inflammation via inhibiting lymphocyte adhesion to extracellular matrix [3], [5], [6]. These findings demonstrated that inhibition on T cell adhesion and interference with the activities of the proteinases including MMPs may represent a useful approach to the treatment of T cell-mediated immune diseases.
To find such an approach, in addition to the immunosuppressants and anti-inflammatory agents, research has also been focused on the effect of various traditional Chinese medicines. Many extracts from Chinese herbs and their principles have been evidenced to improve T cell-mediated diseases, such as hepatitis and arthritis, via inhibiting the T cell function [7], [8], [9], [10]. Our group has also found the strong effect of a traditional Chinese prescription, Si-Ni-San, in treating immunological liver injury and contact sensitivity [11], [12]. These effects of the prescription have also been recognized to be related to the inhibition on the T cell functions. Si-Ni-San written in the Treatise on Febrile diseases, a medical classic by Zhongjing Zhang in East Han Dynasty, comprises an equal ratio of four Chinese medicines: Chaihu (Radix Bupleuri Chinensis), Shaoyao (Radix Paeoniae Alba), Zhishi (Fructus Citri Aurantii) and Gancao (Radix Glycyrrhizae Uralensis). This ancient prescription has been believed to be effective in curing some inflammatory diseases and widely used as a mediation recipe to treat hepatitis, gastritis, neuralgia, appendagitis during the long history of China [13], [14], [15]. Among the herbal drugs composed in the prescription, Glycyrrhiza Uralensis Fisch (licorice) is a common medicinal herb in traditional Chinese medicine. In clinics, it is used to treat hepatitis, diabetes, and peptic ulcer [16]. As a major component of licorice root, glycyrrhizin, consists of glycyrrhetic acid and two molecules of glucuronic acid (Fig. 1 ). So far, glycyrrhizin exhibits a number of pharmacological effects, including anti-inflammation, anti-ulcer, anti-allergy, anti-carcinogenesis, and immunomodulation [17], [18], [19], [20]. The compound is also used as a potential therapeutic agent for several virus diseases, including chronic hepatitis, acquired immunodeficiency syndrome, and herpes infection [21], [22], [23], [24]. Recently glycyrrhizin was also proved to be effective against severe acute respiratory syndrome (SARS)-associated coronavirus [25]. Taking these reports into consideration, we presumed that glycyrrhizin might make an important contribution to the pharmacological efficacy of Si-Ni-San. Although Si-Ni-San has been widely used in clinics and its action mechanisms have been partially elucidated [11], [12], the active constituents and the pharmacological properties of their potency are not sufficiently clarified yet. For exploring the active principles of a Chinese herb and its blended prescription, a common method is to isolate the compounds contained by a chemical purification or the activity-guided purification [26]. Such assay is usually based on the activity of purified compounds but difficult to reflect the actual contribution of the compound in total prescription.
Fig. 1.
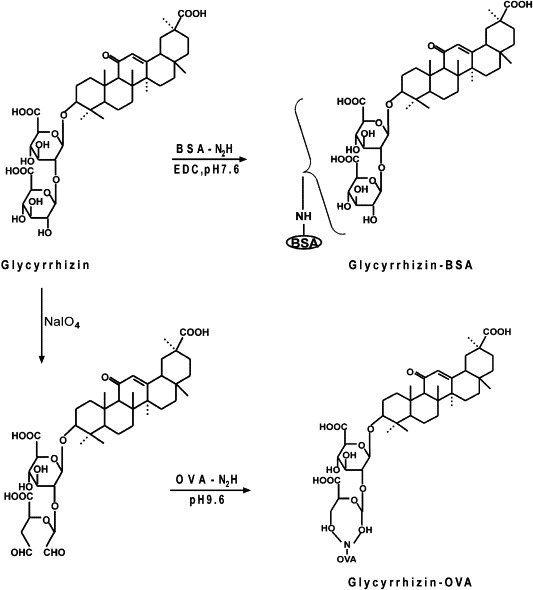
Synthesis of glycyrrhizin–BSA and glycyrrhizin–OVA conjugates.
On the other hand, immunoaffinity chromatography is one of the most powerful techniques to selectively isolate or concentrate minor components of interest from a complex mixture [27]. Its selectivity is derived from the use of an immobilized specific biomolecule, such as antibodies, receptors and specific proteins. In the aspect of traditional Chinese medicines, antibodies to the chemical principles contained in the crude materials have been made and applied to the quantitative determination of some active compounds in various traditional Chinese prescriptions or herbs by using ELISA [28]. In order to obtain antibodies against the components, the compounds are usually bound with a protein, such as bovine serum albumin (BSA), and the complex is then used as an antigen. According to these findings, in the present study, we designed to make the antibody vs glycyrrhizin, and then we tried to selectively deplete glycyrrhizin from Si-Ni-San to define the role of the compound in the whole Si-Ni-San in inhibiting ear contact sensitivity, a typical delayed-type hypersensitivity reaction, with regard to lymphocyte adhesion and MMP production.
2. Materials and methods
2.1. Animals
Female ICR strains of mice (conventional), 6–8-week-old and 20 ± 2 g were obtained from the Experimental Animal House of China Pharmaceutical University (Nanjing, China). They were maintained with free access to pellet food (Jiangsu Cooperation Medical and Pharmaceutical Company, Nanjing, China) and water in plastic cages at 21 ± 2 °C and kept on a 12-h light / dark cycle. Male New Zealand white strain rabbit, 8-month-old, weighing 2–3 kg were purchased from Jiangsu Academy of Agricultural Sciences (Nanjing, China) and maintained on the premises under standard animal house conditions. Animal welfare and experimental procedures were carried out strictly in accordance with the guide for the care and use of laboratory animals (National Research Council of USA, 1996) and the related ethical regulations of our university. All efforts were made to minimize animal's suffering and to reduce the number of animals used.
2.2. Drugs and reagents
The crude drugs used in this study were purchased from Nanjing Medicinal Material Co. (Nangjing, China) and identified as Bupleurum Chinese DC. (Radix Chinensis, Chaihu), Paeonia albiflora Pall. (Radix Paeoniae Alba, Shaoyao), Citrus aurantium L. (Fructus Citri Aurantii, Zhshi) and Glycyrrhiza uralensis Fisch. (Radix Glycyrrhizae Uralensis, Gancao) by Dr Boyang Yu (Department of Chinese Medicinal Prescription, China Pharmaceutical University). They were mixed in an equal ratio (25 g of each drug; in total 100 g) to make up Si-Ni-San, a mixed powder of material crude drugs. These materials were used for making 70% ethanol extracts as reported with small modification. Briefly, the materials (100 g) were extracted twice with 5-fold volumes of 70% ethanol (500 mL) at 70 °C for 1 h each time. Then the supernatant, after centrifuging at 2000 ×g, was pooled and lyophilized to make a powder with 23.2% yields for Si-Ni-San. The dosages of these extracts were indicated as the powders. The contents of main components in the 70% ethanol extracts of Si-Ni-San were determined by high-performance liquid chromatography (HPLC) as 0.25% of saikosaponin a, 1.0% of paeoniflorin, 2.7% of naringin and 4.6% of glycyrrhizin. The powders were dissolved in water for in vivo assay by gavage oral administration to mice and in RPMI 1640 medium for in vitro assay. Other drugs and reagents used in this study were as follows: glycyrrhizin (Wako, Japan), bovine serum albumin (BSA, sigma), ovalbumin (OVA, sigma), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), N,N-dimethylformamide (DMF), Freund's complete and incomplete adjuvants (Pierce), peroxidase-labeled goat anti-rabbit IgG (Wuhan Boster Biotechnology Co., Ltd, China), o-phenylenediamine (OPD), Sephadex G-25 (Beijing Baidier Biotechnology Co., Ltd, China), cellulose DE-32 (Beijing Dingguo Biotechnology Co., Ltd, China), CNBr-activated Sepharose 4B (pharmacia Biotech), injection dexamethasone sodium phosphate (Dex, Nanjing 3rd pharmaceutical factory, Nanjing, China), picryl chloride (PCl, Nacalai tesque Inc, Kyoto, Japan), rat tail type IV collagen (Sigma), phorbol myristate acetate (PMA, sigma), acrylamide and bis-acrylamide (Shanghai Sangon Biotechnical Co., Ltd, Shanghai, China), gelatin and Coomassie brilliant blue R-250 (Sigma), crystal violet (Shanghai Yuanhang reagent factory, Shanghai, China), 96-well culture plates (Nunclon). All other chemicals were standard commercial products of analytical grade.
2.3. Analysis for glycyrrhizin contained in Si-Ni-San by HPLC
Analytical HPLC was performed on a pump (Waters 600) equipped with a UV detector (254 nm, waters 2487). ODS-C18 column, 150 × 4.6 mm, i.d., 5 μm (YMC, Japan) was used. Methanol : water : acetic acid (70 : 25 : 5, (v / v)) was used as mobile phase (flow rate 1.0 mL/min).
2.4. Synthesis of glycyrrhizin–BSA conjugate (glycyrrhizin-BSA) [29]
To 10 mL PBS (pH 7.6) solution containing 25 mg BSA and 20 mg EDC, 21 mg glycyrrhizin in 1.0 mL DMF solution were added dropwise. Five mg EDC were added to the above reaction mixture again 30 min later and then stirred at room temperature for 12 h. The reaction mixture was dialyzed against H2O for 3 days, and then lyophilized to give glycyrrhizin–BSA conjugate (30 mg).
2.5. Synthesis of glycyrrhizin–OVA conjugate (glycyrrhizin–OVA) [30]
MeOH solution 4.0 mL containing 60 mg glycyrrhizin was added to the H2O solution (4.0 mL) of NaIO4 dropwise, and stirred for 60 min. To the above reaction mixture, a carbonate buffer solution (pH 9.6, 14 mL) containing OVA (50 mg) was added. The reaction mixture was adjusted to pH 10.0 with 1 M Na2CO3 solution and stirred at room temperature for 6 h, and so dialyzed for 3 days against H2O, and then lyophilized to give glycyrrhizin–OVA conjugate (80 mg).
2.6. Immunization of rabbits
Rabbits were bled through the marginal ear vein before immunization and the blood was collected from individual rabbits into separate tubes. After clotting for 60 min at 37 °C and overnight at 4 °C, the separated pre-immune serum was taken from the clot and centrifuged at 10,000 ×g to remove any remaining insoluble material for 10 min at 4 °C. These sera were stored in aliquots at − 20 °C and used as blanks for enzyme-linked immunosorbent assay (ELISA). To make polyclonal antiserum against glycyrrhizin, two rabbits were immunized with the glycyrrhizin–BSA conjugates. Namely, 2 mg of glycyrrhizin–BSA was dissolved in 500 μL sterile PBS (pH 7.2) and mixed with Freund's complete adjuvant in a ratio of 1 : 1. Then, these emulsions were injected subcutaneously into 10–20 sites on the back of the respective rabbits. The rabbit was boosted intramuscularly at multiple sites on Day 14 and Day 21 with 3 and 5 mg of the glycyrrhizin–BSA in 500 μL of sterile PBS (pH 7.2) mixed with 500 μL of Freund's incomplete adjuvant, respectively. On Day 28, each rabbit was boosted again with 4 mg glycyrrhizin–BSA in 500 μL of sterile PBS (pH 7.2) by ear vein injection. Blood was sampled via the ear vein and antibody titres were checked by direct ELISA. Once the desired antibody titre had been confirmed, the rabbits were sacrificed and bled out. The immune sera against glycyrrhizin were stored at − 20 °C.
2.7. ELISA for antiserum titre
A 100 μL portion of glycyrrhizin–OVA (500 μg/mL in 50 mM carbonate buffer) was allowed to adsorb onto the wells of a 96-well immunoplate (Nunclon) at 4 °C overnight. The plate was treated with 200 μL of PBS containing 5% newborn calf serum at 37 °C for 1 h to reduce nonspecific adsorption, washed three times with PBS containing 0.05% Tween-20 (PBST), and reacted with 100 μL different dilutions of antiserum at 37 °C for 1 h. Appropriately diluted non-immune serum was used as the control. The plate was washed three times with PBST, and then the antibody was combined with 100 μL of 1 : 1000 dilution of peroxidase-labeled goat anti-rabbit IgG for 1 h at 37 °C. After washing the plate three times with PBST, 100 μL of substrate solution, 0.1 M citrate buffer (pH 4.0) containing 0.15% H2O2 and 0.4 mg/mL of OPD diammonium salt, were added and incubated for 20 min in darkness. Then, 50 μL of 2 M H2SO4 were added to stop the reaction. The absorbance was measured using an ELISA reader (Sunrise Remote/Touch Screen, TECAN, Austria) at 492 nm.
2.8. Polyclonal antibody purification and characterization
2.8.1. Ammonium sulfate fractionation
Antibodies in the sera were precipitated by three-step procedure. The first step consisted in the addition of ammonium sulfate up to 50% saturation and the suspension was stored overnight at 4 °C followed by centrifugation at 10,000 rpm for 20 min. The supernatant was discarded. The precipitate protein was dissolved in ammonium sulfate to the final concentration of 33% saturation and the suspension was kept at 4 °C for 2 h under gentle stirring. The precipitate was collected by centrifugation at 10,000 rpm for 20 min. The protein was precipitated again with ammonium sulfate to the final concentration of 33% saturation.
2.8.2. Sephadex G-25 fractionation
A glass column (2.5 × 45.0 cm) was packed with Sephadex G-25 and equilibrated with 400 mL of 0.0175 M phosphate buffer at pH 6.4. A flow rate of 60 mL/h was maintained. The precipitate resulting from ammonium sulfate fractionation 33% saturation was dissolved in 3 mL of 0.0175 M phosphate buffer and applied to the Sephadex G-25 gel bed and protein was eluted with 0.0175 M phosphate buffer at pH 6.4. Absorbance at 280 nm was monitored for each protein fraction with a UV spectrophotometer (21C-A, Shanghai Kanghua Bio-instrument factory, China). Fractions that showed highest protein content were collected.
2.8.3. Ion-exchange chromatography cellulose DE-32
A glass column (2.5 × 45.0 cm) was packed with slurry of cellulose DE-32 and equilibrated with 200 mL of 0.0175 M phosphate buffer at pH 6.4. The fractions of highest absorbance at 280 nm from gel filtration on Sephadex G-25 column pooled and applied to the cellulose DE-32 column. Elution was performed with 0.0175 M phosphate buffer at pH 6.4, at a flow rate of 60 mL/h. Fractions (5 mL) were collected, protein content for each fraction were monitored. The fractions possessing highest protein content were collected and lyophilized.
2.8.4. Characterization of purified IgG
IgG purity characterization was carried out on a Bio-Rad electrophoresis system. Sodium dodecyl sulfate-polyacrylamide gel eletrophoresis (SDS-PAGE) was performed according to the method of Laemmli, UK [31]. Electrophoresis was performed in 7% polyacrylamide gels with a current of 25 mA for approximately 2 h. Standard molecular weight marker containing 10 proteins ranging in size from 10 to 200 kDa (Bio-Rad, USA) was used as reference in the lane. The gels were stained with Coomassie brilliant blue R-250 for 2 h with constant shaking on a rocker table.
2.9. Preparation of immunoaffinity column using polyclonal antibody and breakthrough column
Purified IgG (10 mg) in 0.01mol/L PBS was added to a slurry of CNBr-activated Sepharose 4B (500 mg) in coupling buffer (0.1 M NaHCO3 containing 0.5 M NaCl, pH 8.0). The slurry was rotated end-over-end for 2 h at room temperature. After washing away the excess ligand with 5 bed volumes of coupling buffer, the slurry was treated with 0.1 M Tris–HCl, pH 8.0, for a further 2 h at room temperature to block remaining activate groups. Then the slurry was washed three times with alternate cycles of 0.1 M acetate buffer, pH 4.0 containing 0.5 M NaCl, and 0.1 M Tris–HCl, pH 8.0 containing 0.5 M NaCl. Finally, the affinity gel was centrifuged and the supernatant removed. The washing solution and the supernatant were combined, and unbound protein measured at 280 nm for determining coupling efficiency, which was found to be 87% of the IgG added. The immunoaffinity gel was washed with PBS and packed in glass mini-columns in volumes of 2.5 mL. Before breakthrough column, the immunoaffinity column was conditioned with 0.01 mol/L PBS at 4 °C. Then 1 mL of the solution of filtered Si-Ni-San in 0.01 mol/L PBS was applied to the column and the effluent was collected and measured by HPLC until glycyrrhizin appeared in the effluent. Thereafter, the fraction without glycyrrhizin was collected and evaporated to dryness on a 50 °C water bath.
2.10. Picryl chloride-induced ear contact sensitivity
Mice were sensitized by painting 0.1 mL of 1% PCl in ethanol on the shaved skin of their abdomens. Five days later, they were challenged by painting 30 μL of 1% PCl in olive oil on right ear lobe [12]. Eighteen hours later, the mice were sacrificed under anesthesia and then the ear thickness of right against left was measured with a digimatic micrometer (0.001 mm, Mitutoyo Co., Tokyo, Japan). The control animals were run parallel with other groups except for gavage p.o. the same volume of water.
2.11. Cell adhesion to collagen IV
Adhesion assay was performed according to the report [32] with some modifications. Briefly, a flat-bottom 96-well microplate was coated with 50 μL solution containing type IV collagen (50 μg/mL) and left at 4 °C overnight. Nonspecific binding sites were blocked with 0.2% BSA for 2 h at room temperature followed by washing three times with phosphate buffer solution. The cells were suspended in RPMI 1640 and spleen cells (5 × 105) were added to each well. The cells were incubated at 37 °C for 1 h with or without PMA (100 nM) and the non-adherent cells were removed by washing three times with RPMI 1640. Then cells were fixed with methanol / acetone (1 : 1), and stained with 0.5% crystal violet in 20% methanol. Unbound dye was removed in tap water and the plate was dried in air. Bound dye was extracted with 1% SDS. The absorbance of the samples was measured at 592 nm. The wells, which were fixed and stained without previous washing, were regarded as the absorbance of total cells. The results were expressed as the mean percentage of total cells from triplicate wells and the experiments were repeated three times. Spleen cells from control animals were subjected to the same assay procedures in parallel. Specificity of cell adhesion assays was corroborated using BSA as substratum.
2.12. Gelatin zymography assay
Analysis by zymography on gelatin gel allows detection of enzymatic activity of the secreted collagenases MMP-2 and MMP-9 [33]. Briefly, spleen cells isolated from various treated mice were suspended in serum-free RPMI 1640 medium at a density of 5 × 105/well and incubated at 37 °C in 5% CO2 for 24 h. Spleen cells from control animals were subjected to the same assay procedures in parallel. Twenty microliters of the supernatants were mixed with 10 μL sample buffer (62.5 mM Tris–HCl containing 10% glycerol, 0.00125% bromophenol blue and 12% sodium dodecyl sulfate (SDS)) without reducing agent, and they were subjected to SDS-PAGE in 5% polyacrylamide gels that were copolymerized with 2 mg/mL of gelatin at 4 °C for 1 h. After electrophoresis, the gels were washed twice in the rinsing buffer (50 mM Tris–HCl containing 2.5% Triton X-100, 5 mM CaCl2, 1 μM ZnCl2, 0.05% NaN3) for 1 h at room temperature to remove SDS. Then, they were incubated for 36 h at 37 °C in the incubation buffer (50 mM Tris–HCl containing 5 mM CaCl2, 1 μM ZnCl2, 0.05% NaN3). The gels were stained with 0.1% Coomassie brilliant blue R250 for 30 min, and destained for 8 h in a solution of 10% acetic acid and 10% isopropanol. The proteolytic activity was evidenced as clear bands (zones of gelatin degradation) against the blue background of stained gelatin.
2.13. Statistical analysis
Results were expressed as mean ± SD of three independent experiments and each experiment includes triplicate sets in vitro and of eight animals of each group in vivo. Statistically evaluated by Student's t test when only two value sets were compared, and one-way ANOVA followed by Dunnett's test when the data involved three or more groups. P < 0.05 was considered to be significant.
3. Results
3.1. Effects of Si-Ni-San, glycyrrhizin, and dexamethasone on PCl-induced ear contact sensitivity in mice
As shown in Table 1 , when orally administered for 6 days from the sensitization, Si-Ni-San at 200 mg/kg significantly inhibited the ear swelling. Glycyrrhizin at the dose of 10 mg/kg, which was corresponding to its proportion contained in Si-Ni-San, also showed a significant reduction in the ear swelling. Such result was confirmed in the repeated experiment where Si-Ni-San and glycyrrhizin at the dose of 100 and 5 mg/kg, respectively showed a tendency of inhibition and those at 200 and 10 mg/kg significantly inhibited the contact sensitivity. Dexamethasone as a positive drug also showed a strong inhibition.
Table 1.
Effects of Si-Ni-San, glycyrrhizin, and dexamethasone on PCl-induced ear contact sensitivity in mice
| Group | No. of mice | Dose (mg/kg− 1) | Ear swelling (10− 3 mm) | Inhibition (%) |
|---|---|---|---|---|
| Experiment 1 | ||||
| Control | 8 | 0 | 110.9 ± 18.5 | 0 |
| Si-Ni-San | 8 | 200 | 53.3 ± 25.3⁎⁎ | 52 |
| Glycyrrhizin | 8 | 10 | 73.4 ± 28.1⁎⁎ | 34 |
| Dexamethasone | 8 | 10 | 21.0 ± 5.3⁎⁎ | 81 |
| Experiment 2 | ||||
| Control | 8 | 0 | 99.8 ± 12.4 | 0 |
| Si-Ni-San | 8 | 100 | 80.9 ± 22.2 | 18.9 |
| 8 | 200 | 58.3 ± 18.4⁎⁎ | 41.6 | |
| Glycyrrhizin | 8 | 5 | 88.1 ± 9.4 | 11.7 |
| 8 | 10 | 72.1 ± 27.0⁎ | 27.7 | |
| Dexamethasone | 8 | 10 | 23.6 ± 10.7⁎⁎ | 76.3 |
Ear contact sensitivity was induced to picryl chloride in mice. Eighteen hours after the challenge, the thickness of right and left ears were measured and the swelling was evaluated by the increase in ear thickness. Si-Ni-San and glycyrrhizin were given orally and dexamethasone was given intramuscularly for 6 days after the sensitization. Each figure indicates the mean ± SD of 8 mice.
P < 0.05.
P < 0.01 vs control; (Dunnett's test).
3.2. Production and characteristics of the polyclonal antibodies against glycyrrhizin
As described in Fig. 1, two conjugate products of glycyrrhizin were obtained with carrier protein. Glycyrrhizin was coupled to BSA to prepare the complete antigen, which was used to immunize rabbits. Glycyrrhizin was also coupled to OVA as coating antigens. The reactivity of obtained antiserum was tested by varying antibody concentration and by performing a dilution curve with direct ELISA method as indicated in Fig. 2 . The crude antiserum was partially purified by fractional precipitation with ammonium sulfate and it was pooled and further purified by Sephadex G-25 and cellulose DE-32 columns. The purity of IgG was confirmed by SDS-PAGE. As shown in Fig. 3 , the purified IgG separated into two subunits with molecular masses of 25 and 50 kDa.
Fig. 2.
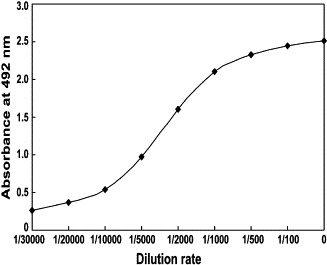
Characterization of anti-glycyrrhizin antiserum with ELISA. To examine the reactivity of the antiserum, gradiently diluted antiserum were added to each well of a 96-well immunoplate coated with 500 μg/mL glycyrrhizin–OVA.
Fig. 3.
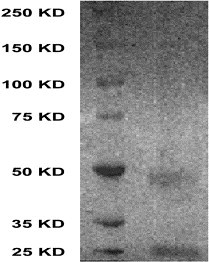
SDS-PAGE of purified IgG from antiserum. The purified IgG was separated by 7% SDS–polyacrylammide. The protein bands of the gel were stained with Commassie brilliant blue R-250. Left lane: molecular weight marker; right lane: purified IgG.
3.3. Depletion of glycyrrhizin from Si-Ni-San by immunoaffinity column chromatography
The purified polyclonal antibody was coupled to CNBr–Sepharose 4B to generate the immunoaffinity column. Si-Ni-San was loaded on the immunoaffinity column and the effluent was collected and measured by HPLC. As shown in Fig. 4B, the content of glycyrrhizin in Si-Ni-San was 4.6% according to the standard (Fig. 4A). After passed through the column, the compound was almost undetectable and its content decreased about 95.5% (Fig. 4C).
Fig. 4.
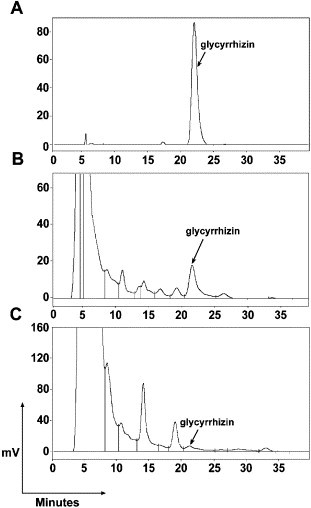
HPLC chromatogram of glycyrrhizin before and after Si-Ni-San was loaded onto the immunoaffinity column. A, HPLC chromatogram of standard glycyrrhizin. B, HPLC chromatogram of Si-Ni-San before it was loaded onto the column. C, HPLC chromatogram of Si-Ni-San after it was passed through the column. Before loaded onto the column, 0.5 mg Si-Ni-San in 2 mL pH 6.4 PBS solution was filtered through a 0.22 mm polysulfone filter. Then it was eluted from the column at 0.5 M NaCl. The fractions were collected and subjected to HPLC analysis. HPLC conditions: YMC ODS-C18 column (150 × 4.6 mm, i.d., 5 μm); column temperature, 30 °C; mobile phase, methanol–water–acetic acid (70 : 25 : 5 (v / v)); flow rate, 1.0 mL/min; UV detection at 254 nm; injection volume, 20 μL.
3.4. Effect of Si-Ni-San, glycyrrhizin and Si-Ni-SanGL− on the adhesion ability to type IV collagen of splenocytes from PCl-sensitized mice
Mice were sensitized by PCl as described in Materials and Methods. Five days after the sensitization, the spleen cells were isolated for adhesion assay. As shown in Fig. 5 , in the presence of PMA, the splenocytes from PCl-sensitized mice showed a significantly increased adhesion to collagen as compared with the cells from naive mice. Against this, Si-Ni-San and glycyrrhizin significantly inhibited the adhesion. In comparison with the original Si-Ni-San, the glycyrrhizin-depleted sample of Si-Ni-San (Si-Ni-SanGL−) only showed a slight inhibition on the cell adhesion.
Fig. 5.
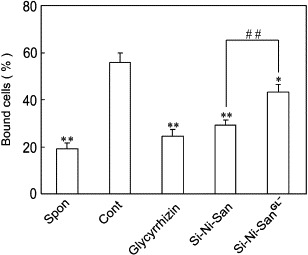
Effect of Si-Ni-San, glycyrrhizin and the glycyrrhizin-depleted sample of Si-Ni-San (Si-Ni-SanGL−) on the adhesion of splenocytes from PCl-sensitized mice to type IV collagen. Mice were sensitized by PCl as described in Materials and methods. Five days after the sensitization, the spleen cells were isolated and incubated with Si-Ni-San, glycyrrhizin and Si-Ni-SanGL− all at 10− 4 g/mL at 37 °C in 5% CO2 for 2 h. Then the cells were washed and used for adhesion assay. Each column represents the mean ± SD of three independent experiments and each experiment includes triplicate sets. Spon: cell alone; Cont: cells + PMA; *P < 0.05, **P < 0.01 vs Cont (Dunnett's test); ##P < 0.01 vs Si-Ni-San (Student's two-tailed t test).
3.5. Effect of Si-Ni-San, glycyrrhizin and Si-Ni-SanGL− on the production of MMP-2 and MMP-9 in splenocytes isolated from PCl-sensitized mice
As shown in Fig. 6A and B, the splenocytes isolated from PCl-sensitized mice secreted a higher level of MMP-2 than those from naive mice. Against the increased secretion, both Si-Ni-San and glycyrrhizin, which had treated the cells for 24 h, significantly decreased the cell adhesion. However, Si-Ni-SanGL− only showed slight redution. In the case of MMP-9, there were the similar results (Fig. 6C and D).
Fig. 6.
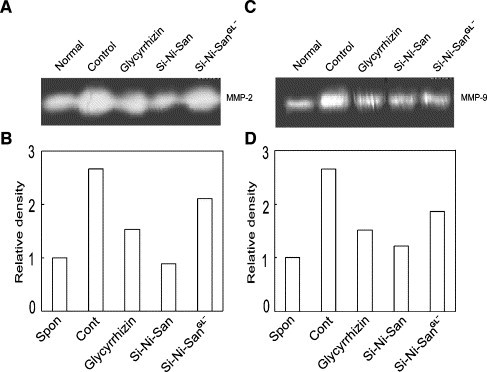
Effects of Si-Ni-San, glycyrrhizin, and the glycyrrhizin-depleted sample of Si-Ni-San (Si-Ni-SanGL−) on the productions of MMP-2 and MMP-9 in splenocytes isolated from PCl-sensitized mice. A and B: MMP-2. C and D: MMP-9. Five days after PCl sensitization, the isolated spleen cells were incubated with Si-Ni-San, glycyrrhizin and Si-Ni-SanGL− all at 10− 4 g/mL at 37 °C for 24 h. After the incubation, the supernatants were aspirated and used for zymography assay. The figure shown here is the representative of three different experiments.
4. Discussion
In the present study, we first confirmed the anti-DTH activity of Si-Ni-San, a traditional Chinese prescription, by using a mouse model of PCl-induced ear contact sensitivity, a typical DTH reaction. Since Si-Ni-San contains high content of glycyrrhizin, which possesses a wide range of pharmacological properties including strong anti-inflammatory activity, the activity of Si-Ni-San at 200 mg/kg was compared with this major component according to its amount (10 mg/kg) contained in 200 mg/kg Si-Ni-San. As the result, both Si-Ni-San and glycyrrhizin showed a significant inhibition on the ear swelling though the inhibition intensity by glycyrrhizin was less than that by Si-Ni-San at the used doses (Table 1). This finding suggests that glycyrrhizin, at least, is one of the main components contained in Si-Ni-San to interfere with the activation of T lymphocytes involved in the ear inflammation.
Next, we attempted to prepare an immunoaffinity column against glycyrrhizin to selectively deplete this compound from Si-Ni-San. For this purpose, we first made an antibody to the compound. As shown in Fig. 1, we conjugated glycyrrhizin with BSA to prepare the complete antigen and used it to immunize rabbits. At the same time, glycyrrhizin–OVA conjugate was also synthesized as coating antigens with different method to avoid cross-reaction. Serum from immunized rabbits was seen to give strong titres when test by ELISA against glycyrrhizin–OVA (Fig. 2). Then the polyconal antibody against glycyrrhizin was purified from the antiserum by ammonium sulfate fractionation, and consecutive column chromatography using Sephadex G-25 and DEAE-cellulose. After these purification steps, two subunits with molecular mass of 25 and 50 kDa were detected in the purified polyconal antibody, which in good agreement with that of the light and heavy chains of IgG being determined under denatured conditions on SDS-PAGE (Fig. 3). These results indicated that we have successfully obtained the purified polyconal antibody against glycyrrhizin.
Today separations based upon imunoaffinity techniques are more and more common in a large field of applications. Due to the specificity of the antigen–antibody interactions, methods based on immunorecognition are very selective. Immunorecognition methods carried out in chromatographic columns can be used for both isolation and analysis [34]. It has been reported that some monoclonal or polyconal antibodies against various bioactive natural products have been made to develop new ELISA methods for the quantification of principles in crude drugs or prescription samples [28]. However, using these antibodies to deplete specific component in traditional Chinese prescription has not been reported yet. In the present study, the antibody raised against glycyrrhizin was covalently bound to the chromatographic packing. The coupling efficiency defined as the percentage of immobilized antibody accounted for the original amount, was observed to be 87%. Then Si-Ni-San extract was passed through this imunoaffinity column, where glycyrrhizin was captured and concentrated by the glycyrrhizin antibody while the rest of components of the samples were eluted. In Fig. 4, we observed the content of glycyrrhizin in Si-Ni-San decreased greatly after passed through the column, accounting for about 95.5% of the original amount. This result indicated that glycyrrhizin has been selectively depleted from Si-Ni-San by this approach.
Considering the crucial role of the adhesion ability to type IV collagen of lymphocytes to extracellular matrix in the progress of DTH reaction [3], we next examined the influence of Si-Ni-San, which had been depleted of glycyrrhizin on the adhesion of spleen cells from PCl-sensitized mice to type IV collagen in vitro. The adhesion activity of the spleen cells was significantly enhanced when the cells were stimulated by protein kinase C (PKC) activator, PMA in vitro. Against the increase, the pretreatment with Si-Ni-San and glycyrrhizin significantly inhibited the adhesion almost to the normal level. However, the glycyrrhizin-depleted sample of Si-Ni-San only showed a slight inhibition. In other words, the depletion of glycyrrhizin significantly blocked the inhibition of Si-Ni-San on the lymphocyte adhesion (Fig. 5). These findings indicated that glycyrrhizin might make an actual contribution to the inhibition of Si-Ni-San on the cell adhesion.
Lymphocyte localization to the inflammatory sites requires cooperation between adhesion molecules and MMPs. As many reports indicated, MMPs that can degrade extracellular matrix is indispensable for the adhesion of lymphocytes to extracellular matrix. Excretion of MMP-2 and MMP-9 conspicuously increases when the integrins on T cell surface bind to their ligands in extracellular matrix [35]. In Fig. 6, we observed the activities of MMP-2 and MMP-9 produced by spleen lymphocytes from antigen-sensitized mice. Such production of MMP-2 and MMP-9 was remarkably inhibited by Si-Ni-San and glycyrrhizin. However, once glycyrrhizin was depleted from Si-Ni-San, the extract only showed a slight inhibition. These results suggested that glycyrrhizin also plays an important role in the inhibition efficacy of Si-Ni-San on MMPs production.
In addition to glycyrrhizin, there are many other active components contained in Si-Ni-San. For instance, saikosaponins from Radix Bupleuri were well known to have excellent anti-inflammatory activity [36]; paeoniflorin from Radix Paeoniae showed a potent analgesic action [37]; and naringin from Fructus Citri Aurantii had antioxidant effects in cholesterol-fed rabbits [38]. All these findings as well as those on the biological activities of glycyrrhizin so far, were obtained by pharmacological assays using the purified compounds. However, in these investigations, it is usually difficult to know whether the compounds actually act in the total prescription. On the other hand, our previous study focused on the effect of Si-Ni-San and its effective constituents on T cell-mediated disease models. Our data demonstrated that the activities of Si-Ni-San were better than its single herbs on the animal inflammation. By analyzing the composition of Si-Ni-San, we found that the effect of this prescription was mainly contributed by the combination of Chaihu and Shaoyao, as well as Shaoyao and Gancao [12]. We also found that saikosaponin mainly inhibited T lymphocyte activation and proliferation (unpublished data), while glycyrrhizin showed strong inhibition on T lymphocyte adhesion to extracellular matrix and MMPs secretion (Fig. 5, Fig. 6). These data indicated a fact that Si-Ni-San showed an integrated effect from many components contained and there still remain a lot of questions. From this view, the attempt to specifically deplete one or more components from a traditional Chinese prescription as used in the present study may be helpful for the elucidation of the activity of traditional Chinese medicines. Overall, glycyrrhizin may act as an active constituent of Si-Ni-San in inhibiting DTH reaction involving down-regulation of MMPs production and the cell adhesion to extracellular matrix. A further study of the detailed mechanisms is now in progress.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No.90209040) and Natural Science Foundation of Jiangsu Province (No. BK2003206). We should thank Mr. Dongfeng Liu for his technical assistance on the antibody preparation.
References
- 1.Baumer W., Tschernig T., Sulzle B., Seegers U., Luhrmann A., Kietzmann M. Effects of cilomilast on dendritic cell function in contact sensitivity and dendritic cell migration through skin. Eur J Pharmacol. 2003;481(2–3):271–279. doi: 10.1016/j.ejphar.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Au B., Seabrook T., Andrade W., McCulloch C.A., Hay J.B. Tissue specificity of lymphocyte migration into sheep gingival tissue. Arch Oral Biol. 2001;46(9):835–845. doi: 10.1016/s0003-9969(01)00038-3. [DOI] [PubMed] [Google Scholar]
- 3.de Fougerolles A.R., Sprague A.G., Nickerson-Nutter C.L., Chi-Rosso G., Rennert P.D., Gardner H. Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105(6):721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetzl E.J., Banda M.J., Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996;156(1):1–4. [PubMed] [Google Scholar]
- 5.Haworth D., Rees A., Alcock P.J., Wood L.J., Dutta A.S., Gormley J.J. Anti-inflammatory activity of c(ILDV-NH(CH2)5CO), a novel, selective, cyclic peptide inhibitor of VLA-4-mediated cell adhesion. Br J Pharmacol. 1999;126:1751–1760. doi: 10.1038/sj.bjp.0702511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattei M., Carnieri E., Politi V., D'Alessio S., Sella A., Cassol M. Inhibition of contact hypersensitivity reaction to picryl chloride: effect of small molecular weight peptidomimetic compounds possessing inhibitory activity against metalloproteinases. Int Immunopharmacol. 2002;2(5):699–710. doi: 10.1016/s1567-5769(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 7.Wu M.J., Weng C.Y., Ding H.Y., Wu P.J. Anti-inflammatory and antiviral effects of Glossogyne tenuifolia. Life Sci. 2005;76(10):1135–1146. doi: 10.1016/j.lfs.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Asano K., Matsuishi J., Yu Y., Kasahara T., Hisamitsu T. Suppressive effects of Tripterygium wilfordii Hook f., a traditional Chinese medicine, on collagen arthritis in mice. Immunopharmacology. 1998;39(2):117–126. doi: 10.1016/s0162-3109(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Zhao Y., Xu Q. Astilbin prevents concanavalin A-induced liver injury by reducing TNF-alpha production and T lymphocytes adhesion. J Pharm Pharmacol. 2004;56(4):495–502. doi: 10.1211/0022357023033. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y., Chen T., Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res. 2003;52(8):334–340. doi: 10.1007/s00011-003-1179-3. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J., Zhou C., Xu Q. Alleviating effects of Si-Ni-San, a traditional Chinese prescription, on experimental liver injury and its mechanisms. Biol Pharm Bull. 2003;26(8):1089–1094. doi: 10.1248/bpb.26.1089. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y., Chen T., Xu Q. Si-Ni-San, a traditional Chinese prescription, and its drug-pairs suppress contact sensitivity in mice via inhibiting the activity of metalloproteinases and adhesion of T lymphocytes. J Pharm Pharmacol. 2003;55:839–846. doi: 10.1211/002235703765951465. [DOI] [PubMed] [Google Scholar]
- 13.Guo X.P., Li D.L., Li J.M., Liu Z.G., Wang Y.M. Clinical pathological study of Si-Ni-San on treating chronic hepatitis and hepatic fibrosis. Chin J Inform Tradi Chin Med. 1999;6:71–72. [in Chinese] [Google Scholar]
- 14.Cai S.R., Liu H.T. Treatment of chronic gastritis by Si-Ni-San, one hundred and eighty-six cases. J Sichuan Tradi Chin Med. 2004;22:47–48. [in Chinese] [Google Scholar]
- 15.Zhang F.W., Zhang Y. Treatment of chronic atrophic gastritis by Si-Ni-San, fifty-eight cases. Chin J Tradi Chin Med & Pharm. 2000;15:79–80. [in Chinese] [Google Scholar]
- 16.Arase Y., Ikeda K., Murashima N., Chayama K., Tsubota A., Koida I. The long-term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Matsui S., Matsumoto H., Sonoda Y., Ando K., Aizu-Yokota E., Sato T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;4(13):1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J.X., Akao T., Nishino T., Tani T. The influence of commonly prescribed synthetic drugs for peptic ulcer on the pharmacokinetic fate of glycyrrhizin from Shaoyao-Gancao-tang. Biol Pharm Bull. 2001;24(12):1395–1399. doi: 10.1248/bpb.24.1395. [DOI] [PubMed] [Google Scholar]
- 19.Park H.Y., Park S.H., Yoon H.K., Han M.J., Kim D.H. Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-d-glucuronide. Arch Pharm Res. 2004;27(1):57–60. doi: 10.1007/BF02980047. [DOI] [PubMed] [Google Scholar]
- 20.Chayama K. Management of chronic hepatitis C and prevention of hepatocellular carcinoma. J Gastroenterol. 2002;37((Suppl.): 13):69–73. doi: 10.1007/BF02990103. [DOI] [PubMed] [Google Scholar]
- 21.van Rossum T.G., Vulto A.G., Hop W.C., Schalm S.W. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am J Gastroenterol. 2001;96(8):2432–2437. doi: 10.1111/j.1572-0241.2001.04049.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyaji C., Miyakawa R., Watanabe H., Kawamura H., Abo T. Mechanisms underlying the activation of cytotoxic function mediated by hepatic lymphocytes following the administration of glycyrrhizin. Int Immunopharmacol. 2002;2(8):1079–1086. doi: 10.1016/s1567-5769(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology. 2002–2003;70(4):229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- 24.Sekizawa T., Yanagi K., Itoyama Y. Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virol. 2001;45(1):51–54. [PubMed] [Google Scholar]
- 25.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kase Y., Saitoh K., Makino B., Hashimoto K., Ishige A., Komatsu Y. Relationship between the antidiarrhoeal effects of Hange-Shashin-To and its active components. Phytother Res. 1999;13(6):468–473. doi: 10.1002/(sici)1099-1573(199909)13:6<468::aid-ptr504>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Afyan N.B., Gordon N.F., Regnier F.E. Automated real-time immunoassay of biomolecules. Nature. 1992;358:603–604. doi: 10.1038/358603a0. [DOI] [PubMed] [Google Scholar]
- 28.Lu Z., Morinaga O., Tanaka H., Shoyama Y. A quantitative ELISA using monoclonal antibody to survey paeoniflorin and albiflorin in crude drugs and traditional Chinese herbal medicines. Biol Pharm Bull. 2003;26(6):862–866. doi: 10.1248/bpb.26.862. [DOI] [PubMed] [Google Scholar]
- 29.Goodfriend T.L., Levine L., Fasman G.D. Antibodies to bradykinin and angiotensin: a use of carbodiimides in immunology. Science. 1964;144:1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka H., Shoyama Y. Formation of a monoclonal antibody against glycyrrhizin and development of an ELISA. Biol Pharm Bull. 1998;21:1391–1393. doi: 10.1248/bpb.21.1391. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U.K. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Franitza S., Hershkoviz R., Kam N., Lichtenstein N., Vaday G.G. TNF-alpha associated with extracellular matrix fibronectin provides a stop signal for chemotactically migrating T cells. J Immunol. 2000;165(5):2738–2747. doi: 10.4049/jimmunol.165.5.2738. [DOI] [PubMed] [Google Scholar]
- 33.Torimura T., Ueno T., Kin M., Harada R., Nakamura T., Sakamoto M. Laminin deposition to type IV collagen enhances heptotaxis, chemokinesis, and adhesion of hepatoma cells through β1-integrins. J Hepatol. 2001;35(2):245–253. doi: 10.1016/s0168-8278(01)00127-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M., Liu Y., Li Y., Zhang X., Chang W. Development and characterization of an immunoaffinity column for the selective extraction of bisphenol A from serum samples. J Chromatogr, B, Analyt Technol Biomed Life Sci. 2003;783:401–410. doi: 10.1016/s1570-0232(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 35.Yakubenko V.P., Lobb R.R., Plow E.F., Ugarova T.P. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha4beta1-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp Cell Res. 2000;260(1):73–84. doi: 10.1006/excr.2000.5002. [DOI] [PubMed] [Google Scholar]
- 36.Bermejo B.P., Abad M.M., Silvan S.A., Sanz G.A., Fernadez M.L. In vivo and in vitro anti-inflammatory activity of saikosaponins. Life Sci. 1998;63(13):1147–1156. doi: 10.1016/s0024-3205(98)00376-2. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi M., Ueda C., Aoki S., Taiima K., Tanaka N., Yamahara J. Anticholinergic action of Paeony root and its active constituents. Yakugaku Zasshi. 1990;110(12):964–968. doi: 10.1248/yakushi1947.110.12_964. [DOI] [PubMed] [Google Scholar]
- 38.Jeon S.M., Bok S.H., Jang M.K., Kim Y.H., Namc K.T., Jeong T.S. Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta. 2002;317:181–190. doi: 10.1016/s0009-8981(01)00778-1. [DOI] [PubMed] [Google Scholar]


