1. Introduction
The modern electrophysiology (EP) laboratory is a complex environment providing an array of interventions for the diagnosis and treatment of heart rhythm disorders and is a result of many transformations over the last three decades. The EP field has witnessed rapid expansion in the number of therapeutic procedures treating a wide range of arrhythmias and in the new technologies available to perform these procedures. Because of the increasing complexity of equipment and procedures and an ever-expanding knowledge base, it was concluded that the field would benefit from a consensus document that would define the critical components and processes of a modern EP laboratory. To this end, the Heart Rhythm Society (HRS) convened a multidisciplinary team to review EP laboratory design, ergonomics, personnel, equipment, occupational hazards, and patient safety, as well as clinical and ethical issues related to diagnostic and therapeutic EP procedures. The goal is to provide physicians, administrators, and regulatory personnel with the recommended requirements for building, staffing, and running a modern EP laboratory to optimize patient outcomes, minimize patient risk, and provide a safe and positive environment for physicians and staff.
The writing committee was formed by the Scientific and Clinical Documents Committee of the HRS, with approval by the President of the HRS and the HRS Executive Committee. The composition of the committee was meant to represent the range of stakeholders in the EP laboratory. The choice of the writing committee members was in accordance with the HRS Relationships With Industry policy.1 All members of the writing committee were required to fully disclose all potential conflicts of interest (see Appendix 1).
Relatively little published literature addresses the EP laboratory environment, staffing, and processes. Therefore, many of the statements in this document are the product of expert consensus by the writing committee and reviewers. For cases in which there were divergent opinions on a statement, a vote among writing committee members was taken, and if a two-third majority supported the statement, it was adopted in the document. The sections pertaining to pediatric and adult congenital heart disease were reviewed and approved by the Pediatric and Congenital Electrophysiology Society (PACES), a nonprofit organization dedicated to the treatment of arrhythmia disorders in children and individuals with congenital heart disease (CHD). The final document was approved by the Board of Trustees of the HRS. This document is directed to all health care professionals who design, manage, and/or work in the EP laboratory environment.
2. Evolution of the EP Laboratory
The field of clinical cardiac electrophysiology (CCEP) has grown from its origin as a field of clinical research for arrhythmogenesis to its present-day incarnation as an important specialty offering advanced therapies for a wide variety of disorders. Clinical EP laboratories emerged in the late 1960s, and by the early 1970s, formal fellowships had been established and EP laboratories were taking shape. First-generation EP laboratories often shared space with cardiac catheterization laboratories and were typically subordinate to coronary angiographic and hemodynamic procedures. When a space was dedicated for electrophysiological testing, it was often small, and fluoroscopy was delivered with portable C-arm units. These laboratories were sufficient for diagnostic EP studies and electropharmacological testing. Second-generation EP laboratories developed in the 1980s with the introduction of catheter ablation and cardiac implantable electronic devices (CIEDs) to the electrophysiologist’s armamentarium. Pacemaker implantation was shifting from the domain of surgery to that of cardiac EP. With increasingly complex procedures being performed in EP laboratories, more space was allocated to new dedicated laboratories and fluoroscopy equipment began to be upgraded to systems commensurate with those used in cardiac catheterization laboratories.
The third generation of interventional cardiac EP has been driven by the success of catheter ablation and advanced device therapy. The precise anatomy and physiology of a wide variety of arrhythmias has been elucidated through the development of advanced mapping systems and improvements in ablation catheter technologies. Modern device therapy incorporates multimodal multisite pacing, sophisticated therapies for tachyarrhythmias, and advanced diagnostics. With the increasing complexity of EP procedures and equipment has come increasing sophistication of laboratory processes and greater demands on laboratory personnel. The cost and complexity of the modern EP laboratory now demands that standards are developed to ensure a high level of care.
3. Laboratory Environment
Laboratory Environment Recommendations
|
3.1. Procedure Room Options
There are multiple options and practice settings for performing EP and implantable device procedures. Medical centers may adopt one or more of the following laboratory operations for their practice. The choice among the following options involves a trade-off between increasing capability for procedure complexity and increasing construction and operating costs.
3.1.1. Dedicated EP Laboratory
In a dedicated EP laboratory, the staff space and procedure room space are separate from the cardiac catheterization laboratory and/or radiology laboratory, although the staff space and procedure room space often exist within a common area. The preparatory and recovery rooms are often shared with other subspecialties. Procedures that can be performed in this laboratory setting include diagnostic EP studies, ablation procedures, use of cardiac implantable devices, implantable device extractions, use of temporary pacemakers, three-dimensional (3D) mapping, intracardiac echocardiography (ICE), and use of robotics. The advantages of using a dedicated EP laboratory include greater availability of more highly trained allied personnel, room equipment dedicated to only EP procedures, and decreased overall equipment costs per room.
3.1.2. Shared EP and Catheterization Laboratory
A shared procedural laboratory program is usually in association with a cardiac catheterization laboratory program, but can also be shared with an interventional radiology program. A shared room allows for two or more practices to share common equipment that includes fluoroscopic equipment, recording systems, emergency equipment, and anesthesia equipment, as well as the space. This is helpful in circumstances of low overall volumes when sharing a room allows for flexibility in patient care while controlling costs and space requirements.
3.1.3. Device-Only Laboratory
These types of procedure rooms have been created at large-volume institutions that can support a procedure room dedicated only to CIED surgery. The procedures performed in this type of room include the use of pacemakers and defibrillators that are single chamber, dual chamber, or biventricular in operation. Other procedures can include the use of temporary pacemakers, the use of implantable loop recorders, and lead and device extractions. Device and lead extractions may also be performed in a surgical operating room (OR) on the basis of the patient’s condition or on the standard agreed on by the institution. Advanced mapping and EP recording systems are not required, and the costs of equipping this type of laboratory are lower, which is the key advantage of this type of room. Device-only laboratories are appropriate for high-volume centers that already have one or more fully outfitted EP laboratories.
3.1.4. Advanced Mapping, Ablation, and Combined Hybrid Laboratories
These procedure rooms are designed to the rigorous standards of ORs (positive airflow, medical gas availability, surgical lighting, and substerile scrub area) but have high-quality fixed fluoroscopy and a full complement of EP and/or cardiac catheterization equipment. These rooms are ideal for procedures that may be combined with open or minimally invasive cardiac surgery and for lead extraction procedures. When not being used for hybrid surgical procedures, these laboratories can function either as fully functional ORs or as fully functional EP/catheterization suites. Procedures that can be performed include complex ablation procedures that involve EP and surgical components, left atrial appendage occlusion or clipping, epicardial lead placement, and minimally invasive valve replacement.
3.1.5. Special Procedure Rooms
Some organizations incorporate special noninvasive rooms into their practice to accommodate patient care that does not require fluoroscopy or other specialty equipment. These rooms are often used to perform minor procedures such as cardioversions, tilt table studies, and noninvasive programmed stimulation defibrillation threshold testing. Autonomic testing with head-up tilt table testing requires a procedure table that has the capability for 70º head-up tilt, an electrocardiogram (ECG) monitor, noninvasive blood pressure monitor, supplemental oxygen, and basic supplies. Equipping these rooms is much less expensive than equipping a full procedural laboratory and can help improve patient flow and volume through a busy EP department.
3.1.6. Pediatric EP Laboratory
The room and equipment standards for pediatric EP procedures are similar to those for adult EP procedures, except for the availability of pediatric resuscitation equipment and drug doses as well as a wider inventory of smaller catheters. Pediatric and congenital EP patients can require a combined procedure of EP and the need for cardiac catheterization, including angiography and possible intervention. Thus, it is optimal (although not a necessity) for a pediatric/congenital EP laboratory to meet all the standards of a pediatric catheterization laboratory. Pediatric EP procedures in young children should be performed in pediatric hospitals or hospitals that have a pediatric cardiology and EP service.
3.2. Freestanding Cardiac EP Laboratory
An EP laboratory that is not physically attached to a hospital is considered a freestanding laboratory. Freestanding EP laboratories can be privately owned, and when owned by physicians, there may be concerns about conflicts of interest (as discussed in Section 12). This arrangement presents challenges that stem from the separation of the laboratory from vital hospital services. In the event of a life-threatening complication, such as pericardial tamponade2 or endovascular tear during lead extractions,3 an emergency response from certain hospital-based services such as cardiothoracic surgery can become necessary, and even possibly lifesaving. Performing EP procedures in freestanding EP laboratories on patients with clinical conditions that confer increased risk are relatively contraindicated. These include preexisting advanced heart failure and severe left ventricular dysfunction4; recent myocardial infarction, recent stroke, chronic kidney disease, severe chronic obstructive pulmonary disease, pulmonary hypertension, and severe/morbid obesity5; and severe valvular dysfunction or prosthetic heart valve, CHD (including atrial septal defect repair), active oral anticoagulation, advanced age, and pediatric age. Procedures that necessitate lesion creation close to coronary arteries, such as aortic cusp ablation6 and epicardial ablation,7 carry a higher risk of intraprocedural myocardial infarction and should not be performed outside a hospital. As part of the consent process, patients should be informed that the procedure is being performed without on-site surgical backup. In order to ensure the safety of a patient undergoing a procedure in a freestanding EP laboratory, a functional and tested system must be in place to quickly transfer patients to a hospital with immediate surgical support in case of an unanticipated complication. The receiving program should be familiar with complications unique to the EP laboratory. There must be a standing agreement between the laboratory and the receiving hospital so that there is no unnecessary delay in the transfer process.
3.3. Hospital and EP Laboratory
The hospital environment plays an important role in shaping the structure and function of the EP laboratory. A “closed EP laboratory” is commonly present in academic institutions and limits physician practice to faculty members of the particular institution or university. In contrast, “open EP laboratories” allow credentialing and the participation of multiple physician groups, including those who do not hold faculty level appointments. Such laboratory structuring is common in community and private institutions and is also present in some academic settings. Whether an EP laboratory is open or closed is determined by the institution’s leadership on the basis of economic, historical, political, and geographical factors that are often beyond physician control. An inherent difficulty in the open EP laboratory format lies in procedure scheduling for multiple physicians; a centralized scheduling structure that can arrange scheduling while organizing and prioritizing procedures on the basis of urgency and acuity is important to avoid conflicts and optimize patient care.
The complexity and degree of invasiveness of EP procedures is dependent on the level of support provided by the hospital or other health care organization in terms of personnel, facilities, and equipment. Anesthesia support is desirable for the safe performance of potentially lengthy and complex procedures. The role of anesthesia services in the EP laboratory is detailed in Section 6. Surgery backup must be immediately present for lead extraction procedures in which a lead to be removed is older than 1 year (or require tools other than a standard stylet to be removed if younger than 1 year from implantation)8, and mapping/ablation procedures require pericardial access. Complex ablation procedures, such as atrial fibrillation and ventricular tachycardia (VT) ablation, should be performed only in hospitals equipped and prepared to manage these types of emergencies, with access to emergency surgical support when required. Finally, high-risk procedures in critically ill patients, such as ablation of VT in patients requiring hemodynamic support with extracorporeal membrane oxygenation, can only be safely performed in institutions offering comprehensive programs with active engagement from electrophysiologists, surgeons, and anesthesiologists. Although such collaborations were limited to advanced tertiary care institutions in the past, the increasing availability of institutional resources and support has expanded the range of facilities in which complex procedures are performed to include private institutions.
3.4. Regulatory Standards Related to EP Laboratories
Federal guidelines for the construction and retrofitting of health care facilities have been influenced by recent catastrophic events, such as the Northridge earthquake of 1994, Hurricane Katrina in 2005, and the F5 tornado that made a direct hit on a hospital in Joplin, MO, in 2011. In the mid-1990s, three formerly competing code writing agencies united to form the International Code Council. Their mission was to develop a national construction code that, among other entities, would regulate the construction of health care facilities to mitigate the risk of damage due to seismic, wind, and flood dangers. Known as the International Building Code, one of its versions has been adopted by every state. In addition, the Federal Emergency Management Agency, a branch of the Department of Homeland Security, published revised guidelines for improving hospital safety in earthquakes, floods, and high winds.
The primary legislative avenues for controlling the dissemination of expensive health care services are Certificate of Need (CON) laws. As of 2009, 39 states still have a CON process, law, or set of requirements. In most cases, the approval of CON is based on the actual or projected volume of services provided in the procedural laboratories. As procedural volumes for percutaneous coronary arterial interventions have diminished at most tertiary referral hospitals, many hospitals have shifted some coronary interventional laboratory CONs to EP laboratories. Once an EP laboratory is established, the primary government body overseeing its operations, policies, and procedures is the Joint Commission (TJC).
4. Laboratory Design
Laboratory Design Recommendations
|
The American Institute of Architects and the Facility Guidelines Institute regularly publish the Guidelines for Design and Construction of Hospitals and Health Care Facilities.9 This document is recognized by federal and state authorities, and recently this document has included EP laboratories. It provides defined standards in terms of the space and functionality of EP laboratories with a goal to specifically improve work flow in the EP environment, acknowledging that the EP laboratory requires more space than an angiographic/interventional laboratory for supporting equipment and supplies. Traditionally, however, the construction of an EP laboratory had no specific guidelines because of its special applications. The typical layout is generally derived from a cardiac catheterization laboratory,9 which is not ideal for the performance of the full range of EP procedures. The limitations of direct adaptation of an angiography suite design to the practice of cardiac EP include space constraints relative to the special equipment used in EP procedures, the necessity to work on either side of the patient table, and the requirement to access the patient’s upper chest for device implantation. EP laboratory plans should take into account not only the available space within the procedure room but also its location relative to pertinent services such as the patient prep area, recovery area, OR, intensive care unit, the ward, and specialized resources such as an adjacent magnetic resonance imaging (MRI) suite that might permit real-time MRI imaging during procedures in the future. The rationale is to consider the proximity of all needed services in the overall design during the planning stage so that enhanced patient flow can be achieved. The aim of the planning committee should be to build a consensus on a minimum set of specifications that will meet the needs of the clinicians and support staff, and enable them to provide optimal patient care, while maintaining occupational safety for the staff.
4.1. Space Requirements
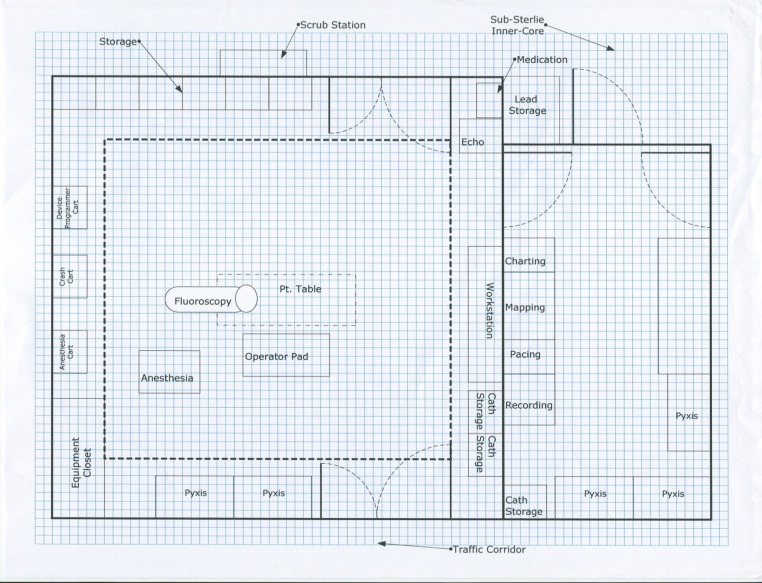
The EP laboratory needs as much space as is practical to ensure the freedom of movement of the operator and staff, to accommodate all equipment used, and to facilitate movement of staff in emergency situations. The recommended procedural area of a complete EP laboratory (not including control room space) is 500 sq ft or greater of clear floor area, although 350 sq ft is the absolute minimum requirement. There should be a minimum of 8 ft of clear space between the wall and the edges of each side of the patient table when it is positioned at the isocenter. Enough clearance at the head of the bed should be allocated for anesthesia equipment on either side and sterile access to jugular vein entry sites, if employed, while allowing for free range of movement of the fluoroscopy C-arm. The ceiling height is dependent on the requirements of the X-ray/fluoroscopic equipment9 ( Figure 1). Preexisting laboratories that are being renovated where it is impossible to expand the gross area because of building and location constraints should follow federal and state code requirements, but due caution should be taken to meet suggested recommendations.
Figure 1.
Space requirements. The sample layout of EP laboratory with adjacent control room area. Note the availability of enough free space at the head of bed area allowing freedom of movement of fluoroscopy arm and anesthesia equipment. EP = electrophysiology.
4.2. Room Layout
The fluoroscopic equipment plays a major role in determining the amount of ideal space in the procedural area and could serve as the reference point. Equipment can be either mounted on the floor or suspended from the ceiling. The latter configuration allows for the floor to be optimally cleaned; however, because of the amount of equipment that would need to be suspended from the ceiling (monitors, surgical lights, X-ray barriers, equipment racks, and anesthesia gas supply), a floor-mounted configuration may be more practical in some laboratories. It is best if X-ray generators and tanks are located in a space separate from the procedure and control rooms. The size and portability of the fluoroscopy unit is important in planning room size, especially when cabinetry and other fixtures are planned for installation on the walls within the procedural area. Installation of cabinetry at the head of the bed is discouraged because it further limits space to allow free movement of the X-ray arm, anesthesia supply cart, and life support equipment. Cabinetry for supplies frequently used during cases should be positioned on the side walls for easy access. The room should be wide enough to accommodate the cabinet and open door swing without impinging on the sterile field and traffic flow through the laboratory.
Most peripheral equipment such as recording systems, stimulators, and radiofrequency (RF) generators are made from multiple components, some of which need to be in a control room and others in the laboratory itself. It is strongly recommended that none of the modules sit on the floor. This can reduce sterility and cleanliness as well as put the equipment at risk of being damaged by fluids. A ceiling-mounted boom removes all equipment from the floor and reduces damage to cables by allowing them to remain connected at all times. By placing the recording system amplifier, the RF generator, the mapping system amplifier, the stimulator amplifier and router, and other peripheral equipment together on a ceiling-mounted equipment boom, all cabling will be permanently placed and connected, reducing cable wear. The removal of rolling equipment carts from the room improves staff access to the patient. Removing cables and equipment from the floor reduces the tripping hazard to the staff and risk of equipment damage. Because additional portable EP equipment is often employed during a procedure, it is necessary to have ample power outlets installed to accommodate such needs.
Anesthesia gases are best supplied via a ceiling-mounted anesthesia boom, which should include two oxygen lines, one nitrous oxide line, one medical air line, two vacuum lines, and one waste anesthetic gas disposal line.10 It should be equipped with at least one slide clamp for vacuum canister placement, which should allow the canisters to be located within 4 in. of the floor for ease of removal when full. The anesthesia boom should have a minimum of six electrical outlets, at least some of which should be on emergency (red plug) circuits in case of general power outage during a procedure. A mounted light controlled independently from the room lighting for charting in a dark room is a useful option. Video can be routed from the anesthesia boom to display data from an anesthesia cart to monitors placed around the room.
4.3. Hybrid Laboratory
The hybrid laboratory has all the requirements of a full EP laboratory but has added features that allow it to serve as a fully functional operating suite. These laboratories are often larger and have the fluoroscopy equipment on a track so that it can be entirely removed from the surgical field. It is typically located within or contiguous to the other ORs and has a full substerile scrub and supply area. The use of a hybrid laboratory for EP procedures is evolving. Hybrid laboratories in which EP procedures are performed need to be outfitted with the appropriate EP-specific equipment, including EP recording systems, mapping systems, and programmed stimulators. Procedures that might benefit from performance in this setting would include those where surgical intervention or extracorporeal hemodynamic support might be required, such as lead extractions, VT ablation procedures in patients with structural heart disease, and hybrid atrial fibrillation ablation procedures.
4.4. Control Room
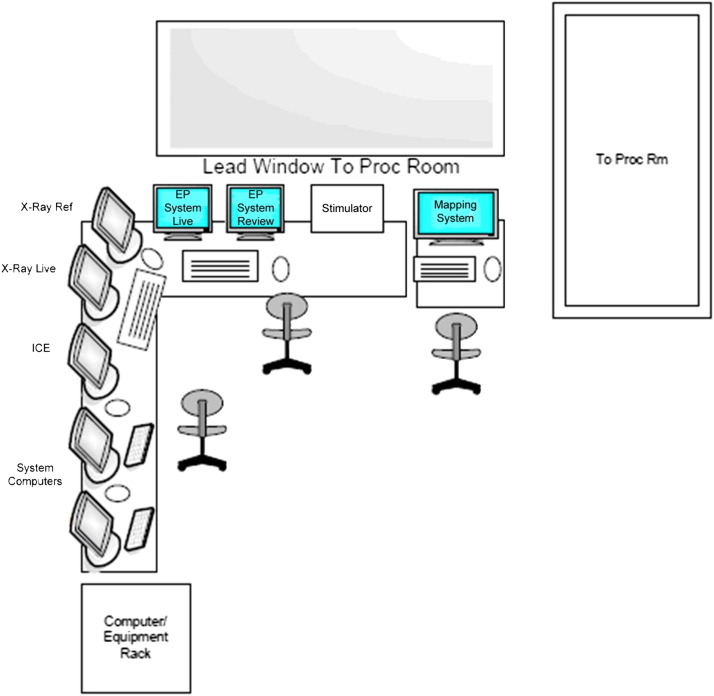
Although some EP laboratories house all the monitoring and stimulating equipment in the procedure room, it may be preferable to have a contiguous control room with an interposed leaded wall and large viewing window so that members of the team (apart from the primary operator, the circulating nurse, and the anesthesia professional) can work without exposure to ionizing radiation. The control rooms can be shared among two or more laboratories. A separate control room demands a full duplex intercom system so that there is no barrier to communication. The space required for a control room is not inclusive of the procedural area measurements. Adequate ventilation should be supplied to account for excess heat production from the electronics. The counters should be at least 30 in. deep so that the monitors can be 20 in. away from the user. At least 160 in. of desk space is suggested for a laboratory with a single-plane fluoroscopy system and 180 in. of desk space for a biplane fluoroscopy system to allow for fluoroscopy monitors, a mapping system, a recording system, and a stimulator. An additional 45 in. of desk space is suggested for a two-monitor reading station or a single-monitor workstation ( Figure 2). The participation of an ergonomics expert in the planning should be considered as a measure to comply with Occupational Safety and Health Administration standards.
Figure 2.
Control room. Example of a simplified layout of the control room and EP equipment. Recommended counter measurements should be applied as mentioned in the text. EP = electrophysiology; ICE = intracardiac echocardiography.
4.5. Traffic Flow
The ideal design for an EP suite should be similar to that of an OR, including a substerile entrance with scrub sinks (dedicated or common). Patient transport from the prep area to the EP laboratory and vice versa should be limited to a common egress that connects to hallways leading to the hospital wards and other areas. If the EP laboratories are placed in existing space that does not allow for OR-quality substerile entrances and hallways, every effort should be made to prevent through traffic flow past the entrance to the EP laboratories where sterile procedures are being performed.
4.6. Conduits and Cabling
EP suites require special consideration from electrical design engineers because there are multiple high load and electrically sensitive pieces of equipment in this wet environment. Conduits used as wireways should follow the specifications of Articles 376, 378, and 392 of the NEC Handbook.11 The EP laboratory setup primarily involves the data and power cabling layout that connects equipment between the control room and the procedural area, and the following requirements should suffice, considering the few cables that need to be run in these enclosures. For rooms that are not equipped with ceiling-mounted equipment booms, the conduits should be at least two runs of 4-in.-diameter tubes that connect the procedure room to the control room through the floor, dedicated solely to EP equipment cabling (separate from X-ray equipment and power receptacle requirements). This conduit should be conductive and bonded to equipotential grounding. Floor openings or ports should be concealed by an enclosure that should be fluid tight with protective grommets that will prevent cable insulation damage. The length/reach is dependent on the location of each cable termination linking the equipment, as specified by the EP representative who oversees the room project and design. For rooms equipped with ceiling-mounted equipment booms, cabling runs through ceiling trays connecting the control room to the procedure room boom. The trays can be used in conjunction with other equipment that terminates at the equipment boom as long as there is enough separation between power lines and data transmission lines to prevent electromagnetic interference (EMI) induced by adjacent power lines running in parallel. Open trays are preferable for ease of access above the ceiling and should be conductive and bonded to equipotential grounding. The length/reach is dependent on the location of each cable termination linking the equipment, as specified by the EP representative who oversees the room project and design. Backup temporary cabling should be available in case of failure of conduit cabling during a case.
4.7. Electrical System/Noise Immunity
Current regulations for health care facilities should follow Article 517 of the NEC Handbook. Because the EP procedure room is classified as a “wet procedure location,” the installation of an isolated power system with line isolation monitoring is required, which provides a layer of protection from the hazards of electric shock with the added benefit of line noise isolation because of its design.9 In addition, all computer equipment directly related to the ongoing monitoring and treatment of a patient must have an uninterruptible power supply (UPS). The UPS may be integrated into the power for the entire suite, or individual UPS may be placed in line for each central processing unit. The main purpose of the UPS is to prevent the EP system, mapping system, or other critical imaging or monitoring system from going through a hard shutdown and full reboot procedure in case of a transient power outage or surge. Other important electrical components of the laboratory, such as the imaging train, should be connected to emergency backup power so that cases can be completed even if line power is lost. Power lines and data lines should be run separately and isolated from each other in different conduits to prevent EMI from power line wiring induced through data line wiring that could affect optimal performance of the EP equipment. If open cable trays are used above the ceiling, careful consideration should be given to the placement of power lines and other fixtures that can be sources of EMI. Although power lines used on these runs do not necessarily involve enough energy to induce heating, it is still a good rule to follow the specifications of Article 300.20 of the NEC Handbook as a reference.11 Adequate spacing of EP laboratory equipment in the procedural area should be followed. Interface cables between the patient and the equipment (e.g., ECG cables and intracardiac catheter cables) should not dangle by the X-ray tube and should be kept neatly arranged by the side of the patient to provide easy access for troubleshooting purposes during the procedure.
4.8. Air Flow/Heating, Ventilation, and Air Conditioning
Air flow should be of OR quality. The design should comply with the Guidelines for Environmental Infection Control in Health-Care Facilities Recommendations from the Centers for Disease Control and Prevention and 5, 6 of the Healthcare Infection Control Practices Advisory Committee document.10 Emphasis should be placed on the use of in-line filters or mechanical smoke evacuation systems to prevent airborne infective and toxic particles from the plume produced by electrocautery and similar equipment. The temperature control should support effective configuration for temperatures as low as 60ºF. This allows comfort for practitioners who are wearing sterile gowns, hats, and masks on top of lead aprons during long procedures. Patient comfort should also be addressed, particularly as they are fully draped and may be only lightly sedated.
4.9. Lighting
The patient table should be flanked by large lighting squares or the equivalent to flood the main procedure area with light. Appropriate grounding is required to prevent EMI from these lights. The lighting squares should be tied to an X-ray pedal switch that can be turned on and off at will by the X-ray operator. Additional spotlights that are dimmable from a distant wall switch are also recommended for procedures that require a darker environment to optimize glare reduction and visualization of display systems in front of the operator. There should be at least one overhead OR light of surgical quality mounted on an articulating arm, strategically placed to be accessible for use on the left shoulder, right shoulder, or abdomen at either side of the patient. There should be sufficient range of motion to be able to focus light intensity at a steeper angle toward and into the implant pocket. Two lights are optimal for reducing shadows. The preferred OR light is mounted on a boom that extends from the ceiling and has free range of movement to focus the beam at the angles and distance optimal to adequately light the surgical field and device implant pockets. Anesthesia and/or nursing should have a light over their workspace that is independent of the room lighting on either side of the patient table, which should be oblique at a distance from the X-ray C-arm.9
4.10. Sound Systems/Communications Equipment
For laboratory designs that employ a separate control room, there may be difficulties with the use of communication systems that link the operator in the procedure room to the control room staff. Because critical processes such as timing of ablation onset and offset require close coordination between the bedside and the control room, the importance of good two-way communication for patient safety and quality of care cannot be overstated. The ideal equipment is capable of a always-on, two-way system because of the constant and instantaneous need to communicate. The ideal system is an always-on, full-duplex, two-way intercom system, with a toggle to silence unnecessary chatter from the control room. This requires electronic noise cancellation to prevent acoustical feedback and has variable effectiveness depending on room acoustics. A simpler solution is a one-way push-to-talk intercom, but this does not allow spontaneous back-and-forth communication. The use of wireless headsets is a favorable solution, which broadcasts spoken words directly to the headphone users, with simultaneous talk paths open as needed. Whatever system is selected should be high fidelity, spectrum friendly, and encrypted to prevent eavesdropping and potential HIPAA violations, making it a more expensive solution.
4.11. Data Network
Procedural charting and operative reports should be part of the institution’s electronic medical record. The network cabling and hardware should have a minimum capability of support for gigabit Ethernet speed.9 The data demands of imaging systems, including 3D electroanatomic mapping systems, are great and require larger storage repositories in comparison with the compressed images of major imaging equipment such as ultrasound and X-ray radiograph systems. There is an increased use of imaging created by computed tomography (CT) and MRI, which are 3D in nature, necessitating high transfer speeds between the picture archiving and communication system (PACS) and the EP laboratory environment. Collaboration with the information technology (IT) department and its infrastructure within the institution is necessary in this venture. EP systems gather information in digitized format for patient records and review at a later time. It will be important for industry to develop a better and unified standard for storing and retrieving cardiac electrogram information. Waveform information in EP is constructively different from image information and needs to be handled in a different manner. The complexity involved in translating the files without losing the ability to utilize the tool sets needed during review, and to scroll through the whole EP study, is a challenge. The Digital Imaging and Communications in Medicine standard is a more robust model to follow and should be the preferred method, when feasible.12
For current equipment standards and needs, the recommendation is to involve the IT department in the safekeeping of digital records of patient information. Storing information in an enterprise-wide network repository managed by the health care IT staff within the institution is recommended, as they are adequately equipped to comply with policies governing hospital data. Data storage must be HIPAA compliant13 and must be maintained according to the laws of each individual state—typically 5–7 years for adults and 5–7 years past the age of maturity for pediatric patients. Practically, the duration of data storage should be longer than the minimum requirement, because old invasive study data are often important in the management of patients decades later. Electronic storage of all EP laboratory information could require 5–10 terabytes of space annually; therefore, the IT department must anticipate commitment of these resources for this process. Regardless of the equipment’s capability to store to the network, the IT department should be involved as long as they comply with the EP equipment manufactureres’ recommendations.
5. Laboratory Equipment
Laboratory Equipment Recommendations
|
5.1. Procedure Table
Patient safety and comfort are the most important considerations for the modern EP laboratory table. The ability to support a heavy patient is one of the most important features of the modern EP procedure table, with tables capable of supporting more than 200 kg being commercially available. The length and width of the table are also important considerations. Although standard table lengths are usually sufficient to accommodate most patients, there is growing need for the increased width provided by bariatric surgical tables. Motorized tables with adjustable height and a tilting capacity of up to 20º have become standard. Tilting into the Trendelenburg position may be helpful in cases of difficult subclavian venous access or internal jugular venous access in ablation and device procedures. Reverse Trendelenburg positioning can be helpful for patients unable to lie flat because of musculoskeletal or respiratory difficulties. Table rotation up to 180º facilitates patient transport but more importantly provides better access to the table head in cases of emergency. This feature, as well as the ability to tilt sideways, may also be helpful for maximizing surgical exposure in hybrid OR laboratories. Given the need to perform both right- and left-sided procedures, having rails on both sides of the table is particularly useful for mounting equipment and tableside controls. Finally, given the length of some EP procedures, in which patients may lay supine for several hours, a comfortable and supportive EP table pad is important. Foam material is commonly used in EP table pads, but other materials are also available.
5.2. Radiographic Equipment
Although fluoroscopy remains the mainstay of EP procedures, it is imperative to reduce ionizing radiation exposure to patients, operators, and staff as best possible. Specific issues related to radiation and limiting exposure are detailed in Section 11. The complexity of procedures performed in the laboratory is the primary determinant of the specific fluoroscopy features needed. Both single- and biplane fluoroscopic systems are suitable for the modern EP laboratory, and the choice of the system is dictated by the specific needs of the laboratory. In basic EP laboratories designed primarily for device implantation, a single-plane system is usually sufficient. Biplane systems are often preferred in more advanced laboratories where ablation is performed, as these biplane systems can be converted to single-plane units for device insertion; however, the advent of 3D mapping technology has diminished operator reliance on biplane fluoroscopy.
The introduction of digital imaging has been the most important recent change in fluoroscopic imaging. Digital flat panel detectors permit reduction in radiation and provide excellent image quality with a physically smaller and thinner detector. These systems allow greater temporal resolution and contrast ratio with less image distortion and veiling glare and allow the acquisition of high-quality still images. The latter feature is particularly useful for procedures depending on the imaging of vascular structures such as coronary arteries, the coronary sinus, and its branches. Floor- and ceiling-mounted units are available depending on the exact specifications and setup of the laboratory space. Some digital fluoroscopic systems offer advanced imaging capabilities, which may be useful in EP procedures including rotational angiography, rotational CT imaging, and multimodality integration of 3D magnetic resonance and CT images. These features are generally more suited for advanced laboratories performing complex ablation procedures. Three-dimensional reconstructed images from CT, MRI, and rotational fluoroscopy can guide ablation planning, catheter navigation, and catheter ablation.14 The pattern of myocardial scarring defined by delayed enhancement MRI scanning can influence the method of access (endocardial vs. epicardial), catheter type, and type of mapping technology.15 In the setting of atrial fibrillation ablation, a preprocedural 3D image can be helpful in cases of unusual atrial or pulmonary vein anatomy. Creation of a 3D map during the procedure using a mapping system can obviate the need for a preprocedural 3D image.
5.3. EP Systems
An EP system refers to the hardware and software programs that allow clinicians to record, display, store, and review data acquired during EP procedures. The monitoring system includes a computer workstation with both local and bedside high-resolution color display monitors, a recorder, amplifiers and filters for signal acquisition and processing, a printer, and device interface cables. The workstation contains an integrated computer that uses data processing software with amplifiers and adjustable filters to process and display electrogram signals and waveforms. At a minimum, the system should contain 12-lead surface ECG and 24 intracardiac electrogram channels, which is sufficient for the basic EP laboratory. Advanced laboratories performing complex ablation procedures require EP systems with 64–128-channel capabilities to simultaneously record signals from different multipolar catheters and display hemodynamic data from arterial and/or left atrial pressure transducers. Useful features for EP systems include a triggered sweep, template matching, and capability to save fluoroscopic images. These data are displayed on color monitors that include both real-time and review screens for visualization and analysis of electrogram signals during mapping and ablation. The number of available channels displayed on color monitors is configurable and differs among the various EP systems. Storage capabilities are often included in EP systems with various hard disk capacities and digital media for archival purposes and retrieval of data. Ideally, data should be stored in a central repository and be available to any workstation over the network. Integration and interfacing with RF-generating devices, fluoroscopy, mapping, and ablation systems are also important components of the system. Finally, the systems should be capable of communicating with institutional information systems and electronic medical records.
5.4. Resuscitation Equipment
Resuscitation equipment is mandatory, given the potential for induction of malignant arrhythmias. A biphasic external defibrillator is required in each EP laboratory, with a backup defibrillator immediately accessible. Routine preventative maintenance of external defibrillators should be performed, according to U.S. Food and Drug Administration (FDA) guidelines and manufacturer recommendations.16 A crash cart containing standard advanced cardiac life support (ACLS) medications must be available to assist with the management of tachy- and bradyarrhythmias. Standard ACLS medications should be available, including, but not limited to, epinephrine, atropine, dopamine, vasopressin, adenosine, amiodarone, and lidocaine, in addition to magnesium sulfate, calcium chloride, potassium chloride, and sodium bicarbonate. Sedative reversal agents should also be available, including flumazenil and naloxone. It is essential that the laboratory be stocked with appropriate long needles, guide wires, and catheters for emergency pericardiocentesis and that all operators and staff are familiar with the use of this equipment.
Given the increasing complexity of EP procedures and the potential need for general anesthesia, an anesthesia cart that contains endotracheal intubation equipment as well as sedative, paralytic, and anesthetic agents is highly recommended. This includes a resuscitator bag and mask, a non-rebreather mask, suction equipment, and arterial blood gas kits. Such a cart should also contain a separate monitoring system for ECG and hemodynamics, including a pressure transducer and end-tidal carbon dioxide monitor, and should be available even in cases not staffed by an anesthesiologist. Finally, all modern EP laboratories should possess high-flow oxygen and vacuum for suctioning as detailed in Section 9.
5.5. Stimulators
Programmable electrical stimulators are the mainstay of EP studies and must provide reliable, accurate, and effective electrical stimulation. Modern programmable electrical stimulators have multiple output channels, usually ranging from two to four channels. It is important for these channels to be independent and isolated and to accurately provide stimuli of adjustable amplitude and pulse duration. Burst pacing and delivery of one or more premature extrastimuli are standard features of all stimulators. In addition, some modern stimulators are fully automated and have the capacity of delivering several types of preprogrammed stimulation protocols to assess physiological parameters such as thresholds, sinus node recovery times, refractory periods, and Wenckebach periods.
5.6. Ablation Systems
In order to perform catheter ablation of cardiac arrhythmias, an ablation system is required in the EP laboratory. Ablation systems generally consist of a generator, cables, and catheters for the delivery of energy and may or may not include a ground patch, depending on the energy source. The ablation systems should interface with EP monitoring and electroanatomic mapping systems. Energy sources can be in the form of RF ablation, cryoablation, ultrasound ablation, microwave ablation, and laser ablation. RF and cryotherapy sources are the most widely clinically utilized, and a discussion of the other sources is beyond the scope of this document.
RF ablation as a therapeutic modality is the most commonly used and has been proven to be highly effective and safe for the treatment of a wide array of arrhythmias.17 Irrigated RF energy ablation systems require an irrigation pump to infuse saline in either a closed- or an open-irrigated tip catheter. Cryoablation systems consist of a cryocatheter, a refrigeration console with nitrous oxide, a coaxial tube for the delivery of nitrous oxide, and an electrical cable. During cryoablation, heat is removed from the tissue by using a refrigerant (nitrous oxide) in a closed-irrigated tip catheter. Cryoablation can be delivered at a single site (catheter based) or over a larger tissue area (balloon device). The selection of ablation modality depends on operator preference, patient size,18 and ablation target. RF energy remains the most established modality for ablation. Cooled RF technologies are generally employed where deep and/or transmural lesions are required, such as with VT ablation. Either irrigated RF energy or the cyrothermic balloon ablation system is commonly used for atrial fibrillation ablation procedures, depending on operator preference.
It is desirable for an EP laboratory to have more than one type of ablation system, but the selection of an ablation system and energy type is entirely discretionary. Different catheters have different handling characteristics, and different ablation systems have different strengths and weaknesses. It is recommended that all EP laboratory personnel using the ablation systems are able to demonstrate familiarity and proficiency with the setup, operation, and characteristics of all ablation system(s) employed at their site.
5.7. Mapping Systems
Three-dimensional electroanatomic mapping systems are commonly used in the EP laboratory for the acquisition of accurate and reproducible electrical and anatomic information and display in 3D. Reconstruction of complex cardiac geometry with direct nonfluoroscopic catheter visualization is combined with endocardial electrogram data to create a 3D map of the cardiac chamber. Advanced signal processing can present acquired electrophysiological data in a variety of formats to direct the operator to optimal ablation targets. In addition, standard fluoroscopy, CT, MRI, and intracardiac ultrasound images can be integrated with electroanatomic mapping systems to link electrogram information with anatomical structures. This allows nonfluoroscopic catheter localization, reducing radiation exposure during catheter ablation procedures.19 Mapping systems consist of a workstation computer, local and bedside monitors, fiber-optic media converter with a fiber-optic cable, an amplifier, diagnostic and ablative catheters, and a patient interface unit that provides the central connection of the computer system to catheters, cables, and the amplifier. The system can interface with recording systems and integrate with ultrasound, fluoroscopy, and CT/MRI systems. The system consists of a workstation computer, local and bedside monitors, an amplifier, fiber-optic media converter with a fiber-optic cable, and a multielectrode array catheter.
5.8. ICE Systems
ICE is often useful as an adjunctive imaging modality during complex procedures. It has the potential to improve both the safety and the efficacy of a procedure. Dynamic visualization of intracardiac structures, catheters, and other procedural devices is possible using ICE. The ability to use this modality in real time is an advantage that improves the work flow of the procedure compared with using other pre- or postprocedural augmentative imaging modalities. Using ICE to directly visualize and confirm the proper position of the transseptal needle on the atrial septum can minimize procedural complications, such as cardiac perforation. Pulmonary vein stenosis can be avoided by using ICE to confirm an ostial position of the lasso catheter during pulmonary vein isolation.20 Early detection of complications, such as pericardial effusion or intracardiac thrombus formation, can lead to earlier and more effective interventions.21 Fluoroscopic exposure and its associated risks can be minimized when navigation of catheters and procedural devices are guided by using ICE.22 The success of a procedure can depend on the recognition and successful navigation of challenging anatomy that can be detectable through ICE, such as a prominent Eustachian ridge during atrial flutter ablation, a crista terminalis ectopic tachycardia focus, or a ventricular arrhythmia involving the papillary muscles or aortic cusps.23 Contact of the ablation catheter with tissue can be verified before the delivery of ablative energy, and ablative effects on the tissue can be monitored by assessing morphological changes, including tissue swelling and increased tissue echogenicity. Presently, two different types of ICE systems are available: systems using a linear phased array transducer that produces a 90º image longitudinal to the catheter and systems that use a rotational transducer to display a 360º image perpendicular to the catheter. Each system has relative advantages and disadvantages, and their selection is based on operator preference. Some ultrasound catheters can work with 3D electroanatomic mapping systems and can import 2D ultrasound images to augment 3D electroanatomic mapping.24
Despite the potential value of ICE, reviewed in detail above, it is important to recognize that clinical trials are not available to demonstrate that the use of ICE improves the outcomes or safety of ablation procedures. Although some operators and centers depend heavily on ICE, many others use it only in selective situations. ICE substantially increases procedure costs, requires an additional site for vascular access, and requires extensive training in order to accurately interpret the images.25
5.9. Robotic Navigation Systems
Catheter movement can be performed using robotic navigation systems, allowing for reproducible complex catheter manipulation, improved tissue contact and stability, and the potential for more efficient and efficacious lesion formation. Because of the automated nature of catheter navigation using 3D anatomic mapping systems, fluoroscopic exposure may be reduced, especially for the primary operator, who typically performs the ablation procedure seated in the control room. This may also translate into less orthopedic strain from the use of lead aprons.
Two distinctly different types of robotic navigation systems are currently available. Robotic arm systems use steerable sheaths to direct catheter movement. These systems can use a full array of conventional catheters, including irrigated ablation catheters. The rigidity of the sheath and the lack of tactile feedback increase the risk of cardiac perforation and pericardial tamponade.26 Pressure sensor technology is used to assess appropriate tissue contact and to avoid perforation, but can be confounded by indirect forces and tortuous catheter positions. A simpler robotic approach to control the catheter movement involves the use of a robotic arm to remotely manipulate a steerable ablation catheter exactly as an operator would manipulate the catheter directly.27 Although the operator sacrifices the tactile feel of catheter manipulation with this system, it allows the operator to move to a radiation-free space and to perform the ablation from a seated position.
Magnetic systems use two large banks of external magnets to manipulate a magnetized catheter. These magnets can be either solid magnets that are physically moved or electromagnets using electromagnetic field manipulation. Specialized ablation catheters for these systems are available, including open-irrigated tip catheters. Because the body of the catheter has no rigidity and the catheters are directed solely by a limited low-intensity magnetic field, the risk of cardiac perforation is virtually eliminated.28 The constant magnetic force holds the catheter in contact with tissue, even during cardiac and respiratory motion, translating to potentially more precise and efficacious lesions.29 The use of robotic navigation systems takes the primary operator away from the patient’s side during the procedure; thus, subtle changes in clinical status that are usually noticed in close proximity to the patient or the tactile sensation of a steam pop may no longer be detectable. Hence, close monitoring by an anesthesiologist and the nursing staff is of paramount importance when robotic navigation is being used.
5.10. Integrated Data Display Systems
As the breadth of technologies in the modern EP laboratory has grown, so too has the challenge of displaying information in a meaningful and useful way. The model using a fixed number of separate monitors, each displaying a single signal, is not well suited for laboratories using multiple systems and performing complex procedures. Modern advanced laboratories have increasingly taken advantage of integrated data display systems (IDDSs). These IDDSs replace the multiple fixed monitors with a single large screen that displays multiple signals, thereby allowing the physician and laboratory staff to display as many images as required in whatever layout they choose. Not only do IDDSs enhance flexibility, they also diminish the physical requirements for monitoring, thereby liberating space within the EP laboratory. The drawback of IDDSs is the addition of another layer between the operator and the source systems that may be susceptible to image distortion or complete failure that would affect all signals. Thus, it is necessary to have separate backup monitors for critical functions in case of failure. Lastly, IDDSs should have a simple, intuitive user interface; otherwise, any benefit they provide would be outweighed by issues relating to the complexity of use.
5.11. Telemedicine Applications
Telemedicine has grown in many areas of medicine over the past decade, and EP is no exception. In fact, EP is better suited than most specialties to leverage this growing trend, thanks in part to the integration of many laboratory systems into a single interface and to advances in remote catheter navigation systems. Remote diagnostics are already a reality because of the growth of several networks that link various laboratories and facilities together. Physicians from a number of institutions can broadcast live and prerecorded procedures and perform real-time consultations with other participating facilities. Remote surgery has been demonstrated using the current generation of remote catheter navigation technologies and has been further bolstered by the addition of newer laboratory integration systems. While the requirements for remote surgery are similar to those of remote diagnostics, there should be much less tolerance for latency and system responsiveness as well as enhanced fail-safe measures and the ability for local override. Significant gaps in state, federal, and international regulations will need to be addressed before telemedicine can reach its full potential in this field.
6. Laboratory Staffing
Laboratory Staffing Recommendations
|
6.1. Physicians
6.1.1. Qualifications
Staff physicians must have prerequisite training and appropriate credentialing reflecting expertise in the management and treatment of cardiac arrhythmias. Training requirements and guidelines for pacemaker/ICD selection, implantation, and follow-up as well as catheter ablation procedures have been addressed by the American Heart Association (AHA), American College of Cardiology (ACC), and HRS30, 31, 32, 33, 34 and are addressed in Section 7.
Physicians performing procedures in the EP laboratory often supervise the administration of intravenous sedatives given by the nursing personnel in the laboratory. Therefore, all physicians in the laboratory should demonstrate proficiency in sedation pharmacology, patient monitoring, and airway management. There should be a credentialing process in the institution that establishes a standard for conscious sedation management.
6.1.2. EP Laboratory Medical Director
The EP laboratory medical director must be an expert in CCEP and satisfy the above requirements, in addition to carrying out important administrative duties that include physician leadership, patient care clinical leadership, quality of care, and education. As a physician leader, the medical director is responsible for providing overall medical direction and supervision within the EP laboratory. The roles and responsibilities of the other EP staff physicians must be specifically outlined by the director so that there are clear measures by which the EP staff physicians are evaluated. Ensuring staff members are appropriately credentialed and that they are maintaining cognitive and procedural competency is important for maintaining up-to-date health care provider standards. The laboratory director should work with the institution’s leadership to establish specific training- and volume-based credentialing and recredentialing criteria based on published clinical care guidelines (when available). Those criteria should be understood and adhered to by all.
The medical director must develop and implement quality measures that result in fewer complications, reduced cost, and successful patient outcomes. Working closely with administrative staff to develop policies, procedures, and practice guidelines impacts accountability measures used by accreditation authorities, including TJC and the National Committee for Quality Assurance. Additional responsibilities may include planning or coordinating ongoing educational opportunities for all EP personnel, championing the EP service line, identifying budgetary savings and efficiencies, participating in or initiating purchasing of capital items that keep the service line current, and assisting as requested with the development and review of EP-related policies and procedures. Policies should be compatible with other areas with which the EP service interacts, such as the prep and recovery areas, anesthesia, surgery, and the cardiac catheterization laboratory.
6.1.3. Faculty/Teaching Attending Physician
Faculty physicians typically work in a teaching hospital or affiliate institution. They must satisfy the same qualifications as above, in addition to those set forth by the Accreditation Council for Graduate Medical Education (ACGME). These requirements are quite rigorous, and failure to adhere to requirements may result in the program being placed on probation or loss of accreditation.
6.1.4. EP Laboratory Attending Physician
Although certain components of the procedure can be delegated to a trainee or other secondary operator, the laboratory’s attending physician of record is ultimately responsible for all activities within the laboratory and for patient welfare. It is important for the staff physician to recognize that patient safety and successful outcomes depend greatly on effective communication in the EP laboratory. This communication should include preoperative discussions with all members of the team before the case is underway regarding specific patient needs. The physician should review the diagnosis, indications for the procedure, anticipated equipment needed, and potential findings of the procedure. The patient should have a clear understanding of what to expect postprocedure in order to minimize anxiety. After the procedure, clear communication of the procedure findings, postprocedure orders, and recommendations should be exchanged with the treatment team, including physicians, APNs, PAs, and nurses.
6.1.5. Secondary Operators
Secondary operators are those physicians assisting with a procedure who might or might not participate in certain aspects of EP procedures and who might bill separately for an area of expertise not provided by the primary physician in the laboratory ( Table 1). Their role is planned and limited to nonemergency procedures. The patient should be informed before the procedure of any secondary operators expected to be assisting with the case.
Table 1.
Secondary Operators in the Cardiac EP Laboratory
| Secondary operator | Role/duties |
|---|---|
| Cardiac electrophysiologist |
|
| Interventional cardiologist |
|
| Interventional radiologist or interventional cardiologist |
|
| Noninterventional cardiologist |
|
| Cardiothoracic surgeon |
|
| Anesthesiologist |
|
EP = electrophysiology, LVOT = left ventricular outflow tract; VT = ventricular tachycardia.
6.1.6. Cardiovascular Trainee (Fellow)
The role of the fellow can be variable and dependent on the attending physician present in the laboratory. There are specific requirements that each fellow in training must satisfy in order to successfully complete his or her training and be eligible for the American Board of Internal Medicine (ABIM) certification examination (or American Board of Osteopathic Medicine for those individuals following the osteopathic route). The fellow should begin under the direct supervision of a key clinical faculty member from the training program. With ongoing evaluation and feedback, the fellow is given graduating responsibility. Varying levels of supervision are appropriate depending on skill level and level of training. It is appropriate for fellows to perform components of the procedure without direct supervision (such as vascular access, catheter placement, device pocket incisions, and pocket closures), but the attending physician must be available to intervene promptly if any issues arise.
6.2. Anesthesiology
It is desirable that anesthesia services be an integral part of clinical practice in the EP laboratory. An anesthesia group composed of anesthesiologists and certified registered nurse anesthetists (CRNAs) can provide a high level of perioperative/periprocedural care to patients undergoing EP procedures. Having anesthesia services readily available for the EP service is advantageous. The anesthesia service can provide important educational assistance to nonanesthesia staff administering conscious sedation, such as training on the use of various sedation agents, and the use of special monitoring techniques such as capnography. Patients undergoing EP procedures present special challenges related to sedation. It is imperative that sedation/anesthesia personnel function collaboratively with the electrophysiologist in the management of these patients during procedures. Procedural issues relating to anesthesia management are discussed further in Section 8.
6.3. Allied Professional Personnel
To ensure optimal safety and efficacy of interventional EP, it is important to emphasize the necessity of a multidisciplinary team approach. In this respect, the term allied professionals has been employed. Allied professionals are defined as all nonphysician members of the health care team involved with the care of the patient in the EP laboratory. This includes, but is not limited to, registered nurses (RNs), EP technologists, radiological technologists, certified nurse practitioners (NPs), PAs, CRNAs, patient prep and recovery staff, and OR staff. Other key personnel that are important for the safe and efficient function of the laboratory include quality assurance (QA) staff; information technologists; biomedical engineers; scheduling coordinators; purchasing, inventory, and supply personnel; and housekeeping. Based on evidence-based practice and best practice patterns, it is important to acknowledge that there is limited published research regarding the roles and responsibilities inherent in EP. Recommendations as to how these positions may be filled by any one of the several categories of personnel are discussed below.
6.3.1. Advanced Practice Nurses and Physician Assistants
APNs and PAs can play major roles and serve many functions in the EP laboratory, as determined by the director of the laboratory. They should be placed in those areas where they will have maximum impact on patient care and assume roles and responsibilities unique to their training and certification. APNs are often placed in clinic settings where they may evaluate and treat arrhythmia or device-related issues. They can make rounds on inpatients, make assessments, develop plans for care, write histories and physical exams, and admit and discharge patients. They can perform pre- and postprocedural evaluations and follow-up. Particularly in nonacademic institutions or practices, an APN or PA may function as the most experienced or skilled nonphysician practitioner in the laboratory setting and thus function as a first assistant for many technical aspects of the procedure. Each institution should have established policies defining the role of the APN and/or PA in the care of hospital patients.
6.3.2. Registered Nurses
An RN should be present for every invasive procedure in the EP laboratory. The nurse must be familiar with the overall function of the laboratory as well as coordinate with the physician operator and the other team members. The nurse (either RN or CRNA) is the primary individual responsible for the direct observation, sedation, and nursing care of the patient during the EP procedure and must be prepared to respond to any emergency. The number and type of nursing personnel required in the EP laboratory will vary depending on the type of procedure, equipment used, and additional support staff assigned to the procedure.35 EP procedures are complex by their nature, and it is essential that the nursing staff participating in such procedures provide safe, evidence-based care.
In institutions where nurses are responsible for the administration of intraprocedural sedation, they are to follow institutional training and guidelines for the care of the patient.
When a nurse is administering deep sedation, his or her focus should be only on monitoring patient status, vital signs, oxygenation, and level of sedation. However, during moderate or light sedation, this individual may assist with minor, interruptible tasks once the patient’s level of sedation/analgesia and vital signs have stabilized, provided that adequate monitoring of the patient’s level of sedation is maintained.36 The nurse can also manage point-of-care testing for activated clotting times (ACTs), oxygen saturation, and blood gas measurements. In most states, only RNs may administer medications and blood products. The nurse optimizes patient safety by adhering to policies, protocols, and procedures, such as completing the “active time-out” preprocedure and ensuring that the proper airway assessments are completed before the administration of sedation. Keeping a record or charting during the procedure is generally the responsibility of nurses. In addition, training on the use of stimulators, infusers, and ablation generators is recommended so that nurses are able to function in multiple roles. Overall, nurses are coordinators of all patient care in the laboratory and they oversee the care other allied professional-EP personnel are providing.
6.3.3. Technologists
Because of the extremely complex and technical aspects of many EP procedures, there should be at least one additional person involved in the more complex procedures, in addition to the nurse who provides direct patient care. Depending on the complexity of the procedure, there may need to be more than one additional person. In this arena, specific training, experience, and certifications may determine which team member occupies each role. For example, a nurse and a technologist may be equally capable of performing a certain duty or responsibility but economics, staffing availability, and the simultaneous performance of multiple duties can dictate who does each job in the laboratory. Because of the multiplicity of roles, it is useful for members of the EP team to be cross-trained and be able to function in multiple roles and situations. There is a wide array of additional equipment that requires training to operate. This includes, but is not limited to, lasers, energy source generators, electroanatomic mapping systems, robotic and magnetic catheter navigation systems, echocardiography (transesophageal and intracardiac), and CT and MRI imaging.
In many laboratories, it is the technologist or nurse who monitors and operates the recording system. This activity requires a thorough understanding and knowledge of the electrophysiological properties of the heart as well as pacing protocols and ablation. The operator must be able to troubleshoot pacing problems and remain calm and functional in emergency situations. All technologists must have basic cardiac life support certification, and ACLS certification is preferred. In the pediatric laboratory, pediatric cardiac life support certification is required. As with the nurse, the technologist should have the ability to review, understand, and synthesize into practice new knowledge and practices.
EP technologists perform as essential team members. They may be a first assistant, which requires in-depth knowledge of percutaneous procedures, catheters, sterile technique, energy generators, and integrated noninvasive imaging. They should be trained in the use, maintenance, and troubleshooting of all the equipment. EP technologists should be skilled in sterile technique, passing sterile supplies, and obtaining and performing point-of-care testing on blood samples. They are often the person who assists on device implant cases and lead extractions—roles that require fastidious adherence to sterile technique and an in-depth understanding of the implant process along with its risks and goals. A technologist or nurse may serve as the first assistant for an invasive case. The circulator is typically a nurse, but this role can be filled by a technologist, depending on the staffing mix in the laboratory, the scope of practice in this job description, and the institutional requirements.
At least one department member should be a certified radiological technologist or equivalent technologist with expertise in the operation of fluoroscopic equipment as well as expertise in radiographic and angiographic imaging principles and techniques. Requirements for the participation of a radiological technologist in fluoroscopic procedures vary from state to state. In conjunction with a qualified medical physicist, the radiological technologist should monitor radiation safety techniques for patients and laboratory personnel. In many states, the Nuclear Regulatory Commission has specific regulations for who may operate ionizing radiation equipment and under what circumstances. It is imperative that these regulations are understood and followed in the laboratory for the protection of patients and staff.
6.3.4. Industry Employed Allied Professionals
Device programmers, mapping and recording systems, and some ablation systems may sometimes be operated by industry representatives. Industry representatives must function according to clear policies under the direction of the laboratory manager, staff, or physician. They are often required to provide the institution with evidence of appropriate immunizations, competency documentation, and endorsement from their company before being allowed in the laboratory. They are generally allowed to have patient contact only under direct staff supervision.25
During device implants or other device-related procedures, a clinical industry representative may be present under the direct supervision of the attending physician. They may bring device equipment to the laboratory, provide intraprocedural programming and testing, and may even be asked to participate in data collection related to device registries. A member of the EP laboratory staff, however, should be assigned the ultimate responsibility for the accurate and complete submission of data to national device registries. These industry representatives are often excellent sources of information and education for the regular EP laboratory staff. They may assist in the provision of formal training and education on device-related issues.25
6.3.5. Staffing Patterns
To ensure optimal safety and efficacy of interventional EP, it is important to emphasize the necessity of a multidisciplinary team approach. EP procedures are complex and include diagnostic, interventional, and therapeutic measures and should be performed by experienced personnel. Because of the complexity of the EP procedures, patient safety and positive outcomes are highly dependent on the skill levels of the staff ( Table 2). Therefore, personnel dedicated to EP laboratory procedures are recommended. Additional personnel are needed as the complexity of the case increases, and more equipment is required. The staffing mix may be influenced by regulations, regional practice patterns, type of institution (academic vs. nonacademic), credentialing bodies, and economics. Cross-training of staff within the EP department maximizes staffing flexibility and is strongly recommended.
Table 2.
Staffing Recommendations for Electrophysiology Procedures
| Type of procedure | Recommended personnel (Alternatives/additional ad hoc personnel) | Pediatric laboratory staffing personnel (Alternatives/additional ad hoc personnel) |
|---|---|---|
| Basic EP study | 1 EP credentialed MD performing the procedure | 1 EP credentialed MD performing the procedure |
| (Fellow, NP, PA, and technician performing under the supervision of an MD responsible for the procedure [as approved by the institution]) | 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation | |
| 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation⁎ | 1 Nurse | |
| 1 cardiovascular technologist/radiology technologist | ||
| (1 nurse or 1 cardiovascular technologist/radiology technologist⁎) | ||
| Tilt table tests | 1 nurse, NP, or PA | 1 nurse, NP, or PA |
| MD must be on the premises, readily available, and aware that testing is occurring | Pediatric cardiologist must be on the premises, readily available, and aware that testing is occurring | |
| (1 tilt table technician) | (1 tilt table technician) | |
| Cardioversions | 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation | 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation |
| Noninvasive programmed stimulation | ||
| Defibrillation threshold testing | 1 MD performing/supervising the procedure | 1 MD performing/supervising the procedure |
| (1 nurse or technologist circulating and documenting) | 1 nurse monitoring and recovering the patient | |
| Ablation procedures | 1 EP credentialed MD performing the procedure | 1 specialist in pediatric EP performing the procedure |
| (Secondary MD operators may be desirable to perform certain parts of the procedure) | (Many laboratories have a working standard of a secondary MD operator for all cases) | |
| (Fellow, NP, PA, and technician performing under the supervision of an MD responsible for the procedure [as approved by the institution]) | (Fellows or other students assisting or observing) | |
| 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation | 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation | |
| (1 nurse giving medications, and patient care during the procedure may do charting) | 1 nurse giving medications, and patient care during the procedure, may do charting | |
| (Physician extenders such as an NP or PA) | (Physician extenders such as an NP or PA) | |
| 1 technologist or nurse running the recording system, stimulator, and ablation system; may be a radiation technologist | 1 technologist or nurse running the recording system, stimulator, and ablation system; may be a radiation technologist | |
| (Vendor representative running the mapping system) | (Vendor representative running the mapping system) | |
| (Vendor representative or hospital technologist assisting with echocardiography) | (Vendor representative or hospital technologist assisting with echocardiography) | |
| (Vendor representatives assisting with the operation of other specialized equipment, such as lasers, cryoablation generators, and intracardiac echo machines) | (Vendor representatives assisting with the operation of other specialized equipment, such as lasers, cryoablation generators, and intracardiac echo machines) | |
| Device implant procedure | 1 EP or device-credentialed MD performing the procedure | 1 specialist in pediatric EP performing the procedure |
| (Fellow, NP, PA, and technician performing under the supervision of an MD responsible for the procedure [as approved by the institution]) | (Secondary operator, including physician extenders) | |
| (Fellows or other students assisting or observing) | ||
| 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation⁎ | 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation | |
| (1 nurse or technologist circulating⁎) | 1 nurse circulating | |
| (1 technologist or nurse may be a surgical assistant) | 1 technologist or nurse may be a surgical assistant | |
| (Vendor representative from the device manufacturer) | (Vendor representative from the device manufacturer) | |
| (Vendor representative assisting with the operation of other specialized equipment, such as lasers and other extraction equipment) | (Vendor representative assisting with the operation of other specialized equipment, such as lasers and other extraction equipment) | |
| Lead extraction procedure | 1 EP or device-credentialed MD performing the procedure | 1 MD specialist in pediatric EP performing the procedure ) |
| (Secondary operator including physician extenders) | (Secondary operator, including physician extenders | |
| (Fellow, NP, PA, and technician performing under the supervision of an MD responsible for the procedure [as approved by the institution]) | (Fellows or other students assisting or observing) | |
| 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist, or 1 nurse trained and credentialed in procedural sedation | 1 CRNA administering anesthesia under the supervision of an MD anesthesiologist | |
| 1 CV surgeon to be immediately available (may be required to be in the room for the critical part of the procedure) | 1 congenital CV surgeon in the operating room at the time of extraction and immediately available periprocedure | |
| 1 CV surgery fellow on call for surgical assistance | ||
| 1 nurse circulating | 1 perfusionist | |
| (1 technologist or nurse scrubbing) | 1 nurse | |
| 1 cardiovascular technologist/radiology technologist | ||
| (May require a second scrub person for the surgical procedure) | 1 CV OR scrub nurse | |
| 1 CV OR circulating nurse | ||
| (An operator to monitor a TEE that may be in place. This function is sometimes performed by the anesthesiologist) | (An operator to monitor a TEE that may be in place. This function is sometimes performed by the anesthesiologist) | |
| (Vendor representative from the device manufacturer) | (Vendor representative from the device manufacturer) | |
| (Vendor representative assisting with the operation of other specialized equipment, such as lasers and other extraction equipment) | (Vendor representative assisting with the operation of other specialized equipment, such as lasers and other extraction equipment) |
CRNA = certified registered nurse anesthetist; CV = cardiovascular; EP = electrophysiology; MD = physician; NP = nurse practitioner; PA = physician assistant; TEE = transesophageal echocardiography.
In procedures performed with deep sedation/analgesia, the CRNA or nurse administering sedation/anesthesia should have no responsibilities other than monitoring the patient. A second nurse or technologist must be available to circulate and document. However, in procedures performed with moderate or light sedation, this individual may assist with minor, interruptible tasks once the patient’s level of sedation/analgesia and vital signs have stabilized, provided that adequate monitoring for the patient’s level of sedation is maintained.52
6.4. Administrator/Manager
The role of the EP department administrator is typically held by someone with broad knowledge of the field of EP. Depending on the size and volume of the laboratory, the administrator may have no clinical obligations or may serve as the head nurse of the laboratory. The responsibilities can include, but are not limited to, the following: strategic planning in association with the medical director, managing operational issues, capital planning, budgeting, hiring, planning orientations and training programs for allied professionals, and other general administration duties. A nurse or a cardiovascular technologist, preferably with some business training or experience, is best suited for this role. In shared or combined cardiac catheterization laboratories and EP laboratories, there may be a common administrator overseeing both areas.
In many departments, the manager is a nurse. The responsibilities of a nurse manager include an overall understanding of the day-to-day operations of the laboratory, management of pre- and postprocedural care areas, and direct participation in the observation and care of patients undergoing EP procedures. Additional areas of responsibility include application of institutional guidelines for patient monitoring, medication administration, procedural sedation, and patient safety. Staff competencies and proficiency in performing tasks required before, during, and after the procedure must be developed, updated, and reviewed on a regular basis. The nurse manager will collaborate with anesthesia, pharmacy, biomedical engineering, purchasing, equipment vendors, and housekeeping to coordinate the operation of the EP laboratory.
7. Laboratory Personnel Credentialing
Laboratory Personnel Credentialing Recommendations
|
7.1. Attending Physicians
7.1.1. Credentialing
A range of procedures is performed in cardiac EP suites. Procedures that fall within the domain of physicians trained and ABIM certified in cardiovascular diseases include performance of electrical cardioversions and placement of temporary pacemaker wires. Invasive cardiac EP procedures in adults, including diagnostic electrophysiological testing and catheter ablation, should be restricted to physicians who are ABIM certified in CCEP.31 All CCEP board–certified physicians have completed at least 1 year of comprehensive subspecialty training in CCEP at an ACGME-approved training program30 or had substantial experience and a career focus in CCEP if they trained in an era before the development of formal CCEP training programs. The majority of programs encourage a second year non-ACGME advanced fellowship. The 2013 guidelines for advanced training in pediatric and congenital EP represent procedural requirements for those completing training.37 The clinical competence statement on invasive electrophysiology studies, catheter ablation, and cardioversion,34 supplemented by expert consensus statements on transvenous lead extraction,8 catheter and surgical ablation of atrial fibrillation,38 and catheter ablation of ventricular arrhythmias,39 provides guidelines for appropriate training in CCEP. This document is scheduled to be updated in the near future. As training standards evolve, these minimum requirements will be updated regularly. A successful passage of the ABIM CCEP board examination is required to receive the Certificate of Added Qualification in CCEP. A similar but alternate pathway is available for doctors of osteopathy through the American Board of Osteopathic Medicine. Physicians for whom these pathways are unavailable because of international training or pathway choices and who are actively involved in the clinical management of EP patients may choose to certify through the IBHRE with the Certification Examination for Competency in Cardiac Electrophysiology. Physicians performing complex catheter ablation procedures, such as atrial fibrillation/complex atrial tachycardia ablation and VT ablation, should treat at least 25 cases of each with an experienced mentor before becoming independent. Alternatively, they should perform these procedures during their CCEP training program, as recommended by the 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation38 and the 2009 EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias.39
A number of training pathways can lead to the practice of implanting CIEDs. Although physicians who are board certified in CCEP have met minimum training standards for CIED implantation, some cardiovascular diseases (CVD) board–certified physicians and some American Board of Surgery–certified cardiac, thoracic, and general surgeons also devote a substantial portion of their practice to the prescription, implantation, and follow-up of CIEDs. The criteria established by the ABIM program requirements28 and the Recommendations for Training in Adult Cardiovascular Medicine Core Cardiology Training document33 strongly recommend that any non–CCEP-certified physician who wants privileges for ICD implantation in adults completes formal training in this field. Achieving CCDS from the IBHRE for CIED prescription, implantation, and follow-up is strongly recommended.
Credentialing is ultimately the responsibility of the credentialing committee of the individual hospital, who should be familiar with the training and credentialing standards for specialists in cardiac arrhythmias. Although most major centers already follow the guidelines above, some centers allow less qualified practitioners to perform these EP laboratory procedures. Unfortunately, hospital credentialing committees have substantial conflicts of interest that could lead to granting privileges to physicians without appropriate board certification and experience, as refusal of these credentials usually results in a loss of patients and revenues to competing institutions. Ultimately, the patient pays the price when inappropriate credentials are allowed.
7.1.2. Evaluation and Recredentialing
All EP laboratory physicians should be subject to periodic peer review and recredentialing. Important components in the recredentialing process should include a review of ABIM board certification and IBHRE CCDS status, case volume, patient outcomes, peer evaluation, and continuing medical education (CME). The specific criteria for recredentialing are determined by each individual hospital, but should generally parallel the following recommendations: ABIM CCEP board certification and IBHRE certification are limited to 10 years; to stay current for CCEP, the physician must complete a series of CME and/or practice improvement activities9; recertification examination for CCEP and CCDS are each required at 10-year intervals; to ensure that cognitive and technical skills are maintained, the physician’s clinical competence must be evaluated and documented on a regular basis; it is the responsibility of the medical staff credentialing committee to ensure that physicians perform the necessary number of evaluations and procedures needed to maintain their expertise31 and also that they participate in regular CME activities. The EP laboratory should have a robust QA process (see Section 9), and physician outcomes should be compared with national benchmarks derived from the literature or databases such as the National Cardiovascular Data Registry (NCDR) on a regular basis; regular 360º evaluations, including evaluations from physician coworkers, fellows, nursing staff, technical staff, and patients, should be considered as part of the recredentialing review process.
For the benefit of patients, it is paramount that physicians be held to a high performance standard and that remediation, withholding recredentialing, or revocation of privileges occurs if criteria are not met. The physician leaders must be committed to working aggressively to maintain the highest standards of patient care in their laboratories.40
7.1.3. Pediatric Training and Credentialing
PACES, working in conjunction with HRS, has developed guidelines for advanced training in EP focused on pediatric patients and patients of any age with CHD. The original guidelines, endorsed by the HRS and published in 2005, required at least 12 months of specialized training, and were followed by a 2008 document focused on the implantation of pacemakers and defibrillators in these populations.41, 42 There are currently no third-tier board examinations for diplomates of the pediatric cardiology subboard of the American Board of Pediatrics. However, pediatric electrophysiologists are eligible to take the IBHRE examination for physicians with special competency in EP, as that examination includes a pediatric module. PACES has recently written a competency statement for training in this field.43 That competency statement strongly recommended that all graduating fellows and active pediatric EP clinicians take the IBHRE EP examination.
7.1.4. Adult Congenital Heart Disease Training and Credentialing
The field of adult congenital heart disease (ACHD) is an important and expanding clinical domain that is typically staffed by clinicians who are competent in both pediatrics and internal medicine. Cardiologists providing invasive EP care for this unique patient population can enter the field from either specialty, but a portion of their formal training must be focused on the complex anatomy and unique EP of ACHD. Further recommendations on the expertise necessary to care for this patient group can be found in a recent consensus statement supported by AHA/ACC/HRS.44 A board certification process for ACHD is being developed and is scheduled for implementation by 2015.
7.2. Nurses
7.2.1. Training and Credentialing
Nursing licensure, credentialing, recredentialing, continuing education, and laboratory training are affected by the requirements of multiple agencies, including federal and state governments, the health care organization, and Occupational Safety and Health Administration.
The standards of professional practice for nurses employed in the EP laboratory environment have been defined.33 All EP nurses should have a critical care or a strong cardiology background, ACLS certification, and, in the pediatric EP laboratory, pediatric ACLS. An extensive knowledge of cardiac anatomy and physiology, electrocardiography, pharmacology, and training in sterile technique is also required. Nurses need to have a thorough understanding of catheter-based interventions and surgical procedures, cardioversion, arrhythmia discrimination, and emergency treatment of life-threatening arrhythmias and complications/emergencies. Familiarity with fluoroscopic, electroanatomic, and echocardiographic imaging is a required skill set. Annual or biannual competencies required for nurses working in heart rhythm service operations should include basic life support, ACLS, infection control, emergency and TJC preparedness, training in conscious sedation, charting, and patient safety. Demonstration of competency in radiation safety, sterile technique, external defibrillator operation, unit-specific nursing protocols, ACT operation, and temporary pacemaker operation should also be mandatory. Depending on the roles and responsibilities assumed by RNs, competencies may be needed in the EP recording system and programmed stimulator operation, ablation, generator operation, mobile laboratory operations, sheath insertion/removal, operation of vascular ultrasound, 3D mapping operation, and ICE operation.
Certification offered by the (IBHRE is an integral part of heart rhythm education, training expectations, and requirements. In addition to the standard certification requirements for being an RN, the IBHRE offers two certification examinations for allied professionals designed to demonstrate a mastery of knowledge in cardiac rhythm management.
7.2.2. Evaluation and Recredentialing
Continuing education requirements are highly variable by state and nurse specialty. Nurses need to review requirements for the state in which they are practicing to ensure proper compliance and maintenance of certification, as requirements can range from no required continuing education to as many as 30 hours every 2 years.45 The Certification Examination for Competency in Cardiac Rhythm Device Therapy and the Certification Examination for Competency in Cardiac Electrophysiology are required at 10-year intervals.
7.3. Advanced Practice Nurses
7.3.1. Training and Credentialing
The APN (usually a NP) is educated through a certified graduate level NP program, meets the requirements of the state credentialing bodies, and practices according to the American Nurses Association consensus model.46 An NP is trained to perform preprocedural evaluation, order and interpret diagnostic tests, and conduct postprocedural follow-up. After on-the-job training, NPs may assist in diagnostic EP studies, catheter ablation procedures, and device implants but cannot serve as a primary operator. NPs working in heart rhythm services should be certified in basic and advanced cardiac life support and have knowledge of radiation safety, sterile technique, external defibrillator operation, ICE operation, and temporary pacemaker operation. Among laboratories represented by writing committee members, NPs (or PAs) are involved in pre- and postprocedural care in more than 75% of the centers but only 19% of the centers employ these individuals for assistance in the procedural laboratory.
7.3.2. Evaluation and Recredentialing
There are no uniform recredentialing criteria for NPs in the EP laboratory. NPs are expected to maintain certification and licensure as per certification and state guidelines. The institution should establish volume criteria for the maintenance of procedural competencies.
7.4. Technologists
7.4.1. Training and Credentialing
Individuals with a variety of backgrounds and qualifications can work in an EP laboratory as a cardiovascular technologist or technician. There is no professional regulatory body for cardiac technologists, although efforts are underway in some regions to achieve this goal. Most technologists have postsecondary education (university degree or college diploma) with extensive on-the-job training. Industry-sponsored courses often provide supplemental education specific to technologies used in the EP laboratory environment. Some cardiovascular technologist programs offer EP as a component within a cardiovascular technology program; there are also certificate programs available at some accredited colleges.47 Cardiovascular technologists can be credentialed as a registered cardiovascular invasive specialist, registered cardiology technologist, registered cardiopulmonary technologist, or registered cardiac electrophysiology specialist through an accredited association such as Cardiovascular Credentialing International.46 Advanced EP specialty certification is achieved through the IBHRE.
7.4.2. Evaluation and Recredentialing
Satisfactory institutional performance appraisals (e.g., through 360º assessments of skills, competency, professional development, decision making, and leadership) are recommended. The individual should treat a sufficient volume of cases to maintain competency. If the technologist is IBHRE certified, then the maintenance of certification through continuing education or certification examination is required.
7.5. Physician Assistants
7.5.1. Training and Credentialing
The PA is an advanced practice professional who is trained through a graduate level university program to perform many tasks, including preprocedural evaluation (history and physical examination and diagnostic tests) and postprocedural follow-up under the direct supervision of the physician.48, 49 After on-the-job training, PAs may assist in diagnostic EP studies, 3D mapping, catheter ablation procedures, and device implants, but cannot serve as a primary operator. Institutional internal certification, minimum volume of annual cases to maintain competency in invasive EP, performance appraisal, and maintenance of continuing education should be a requirement for a PA practicing in the clinical EP laboratory.
7.5.2. Evaluation and Recredentialing
There are no uniform recredentialing criteria for PAs. They should perform a minimum number of procedures as determined by the EP laboratory director and EP laboratory manager and demonstrate current competence on the basis of the results of ongoing professional practice evaluation and outcomes.
7.6. Industry Employed Allied Professionals
Industry Employed Allied Professionals (IEAPs) are hired employees of a medical device company who may serve as assistants in the EP laboratory. The HRS published a statement on the clinical role of IEAPs in 2008. IEAPs should provide technical assistance only on the manufacturer-specific products they represent, and they must work under the direct supervision of the responsible physician.25 Although IEAPs may contribute substantially to patient care in some settings, overreliance on their service may lead to a lack of continuity of care, suboptimal patient education and counseling, and issues with liability and accountability. Among laboratories represented by writing committee members, approximately 90% of the laboratories use IEAP support in most or all device implant cases, and two thirds of the laboratories use IEAP support in most or all 3D mapping cases.
8. Procedural Issues
Procedural Recommendations
|
8.1. Patient Preparation
8.1.1. History, Physical Examination, and Laboratory Examination
Preparation for EP procedures requires a careful preprocedural history and physical examination by a physician, NP, or PA to confirm the reason for the procedure that day and identify all comorbidities that could adversely impact procedure outcome. A thorough medication history, including allergies, must be gathered. The patient needs to be evaluated for factors that will impact anesthesia management (adequacy of airway, history of anesthesia experiences, obstructive sleep apnea, and physical indicators for difficult intubation). All adult patients should have recent (usually within 2 weeks) laboratory work, including electrolytes, blood urea nitrogen, creatinine, complete blood count, and, if taking anticoagulants, prothrombin time. All women of childbearing potential, including girls older than 12 years, should have serum or urine pregnancy testing within 2 weeks before the procedure. The need for a preprocedure laboratory exam in healthy children undergoing elective electrophysiological testing is not clear and is not common practice.
8.1.2. Patients Receiving Oral Anticoagulants or Antiplatelet Medications
As many management strategies for arrhythmias require chronic and/or periprocedural anticoagulation, careful evaluation, assessment, and planning are needed. Among the considerations are the agent used, thromboembolic risk, bleeding risk, comorbidities, laboratory values, and availability of reversal agents or blood products such as fresh frozen plasma. Consideration should be given to performing additional preprocedural transesophageal echocardiograms or the use of intracardiac ultrasound to reduce the risk of complications. In patients undergoing pacemaker or defibrillator lead extraction or who require pericardial access for epicardial ablation or left atrial ablation ligation, additional preparations may be required, including typing and crossmatching of blood products, availability of thoracic surgical backup and/or OR, and, in some cases, intraprocedural transesophageal echocardiogram.
8.1.3. Patients Receiving Antiarrhythmic Drugs
Many patients are taking one or more medications to control the heart rate and/or rhythm at the time of an EP procedure. In most cases, rhythm active drugs (including β-blockers and calcium-channel blockers) are discontinued five half-lives before the procedure to allow the target arrhythmia to be induced, mapped, and ablated. In patients undergoing anatomically based ablation, withholding these drugs may not be necessary.
8.1.4. Patient Education and Consent
For most patients, the EP laboratory is an unfamiliar and intimidating environment, one in which an equally unfamiliar procedure is about to be performed. A complete description of the anticipated events is best delivered in the outpatient setting before the procedure day. Education as to the planned agenda, the other participants (nurses, technologists, EP doctors, and anesthetists/anesthesiologists), and the nature of some of the equipment in the laboratory is important to ease the patient’s anxiety and aid in their cooperation during the procedure. Many of the technical terms used to describe the procedure are foreign to the patient; the staff must take care to use lay language in their descriptions, to evaluate the patient’s ability to learn and preference how to learn, and to assess the patient’s comprehension. This role is usually filled by an RN familiar with the procedure. The requisite process for informed consent is detailed in Section 12. Critical components include ensuring patient understanding, full disclosure of the risks and alternatives to the planned procedure, and the opportunity for the patients to ask questions and fully discuss their concerns. The patient education and consent process must be completed before the administration of any sedative or anxiolytic agents. In the case of pediatric patients or adult patients with cognitive impairment, education must be given and consent requested of the patient and the legal guardian.
8.1.5. Time-Out
A time-out must be performed immediately before the initiation of the procedure when all key personnel are present. All members of the team are to cease their activities while one member recites two patient identifiers (i.e., name, date of birth, and medical record number), the type and laterality of the procedure, the name of the operator, and any known allergies. All members of the team must agree on all points before the procedure can commence.50
8.2. Procedural Issues—EP Catheter Procedures
8.2.1. Sedative agents, Relaxants, and Anesthesia
The goal of analgesia and anesthesia in the EP laboratory should be to provide a safe, nontraumatic experience for the patient. The administration of anesthesia varies among case types and among institutions, from anxiolysis or moderate procedural sedation by an Advanced Cardiac Life Support (ACLS)/Pediatric Advanced Life Support (PALS)-certified nurse under the supervision of the cardiac electrophysiologist51, 52 to monitored anesthesia care or general anesthesia administered by an anesthesiologist or CRNA under the supervision of the anesthesiologist. The health care institution must require those administering or supervising moderate sedation who are not anesthesiologists to meet the requirements of the American Society of Anesthesiologists to obtain privileges.53 Credentialing for this privilege must be periodically renewed. If intravenous procedural sedation will be used, the physician must establish an American Society of Anesthesiologists classification and Mallampati score for the patient before the procedure.36 It is necessary to have these assessments done before the procedure so that, if necessary, alternate plans for sedation may be arranged to optimize patient safety and minimize procedural delays. Medications typically employed include etomidate, propofol, ketamine, fentanyl, midazolam, methohexital, and inhalational agents. Individual states regulate what medications can be administered by nonanesthesiologists. As the provision of sedation is a continuum and the depth of sedation may vary, best practice is that all patients receiving moderate or deep sedation be evaluated by continual observation of qualitative clinical signs, pulse oximetry, noninvasive blood pressure monitoring, heart rate and rhythm, and monitoring for the presence of exhaled carbon dioxide to ensure the adequacy of ventilation unless precluded or invalidated by the nature of the patient, procedure, or equipment.51 Monitoring equipment should be in working order and have appropriate audible alarms. In pediatric cases, factors to be considered for the choice of sedation or general anesthesia include young age, preexisting medical conditions, presence of CHD, airway issues, physician or family choice, and the length and complexity of the procedure. The 2002 NASPE Position Statement on Pediatric Ablation delineated the types of anesthesia (conscious sedation, moderate sedation, and general anesthesia), and these remain applicable.54
Regardless of whether the administration of sedative agents is under the control of the electrophysiologist or another caregiver, the electrophysiologist must have a working knowledge of the effects of the agents used and how they might impact the electrical aspects of the procedure (such as arrhythmia inducibility and effects on blood pressure) or interact with other medications that may be given. Deep sedation or general anesthesia can minimize patient discomfort, can benefit the EP procedure by preventing patient movement, and is necessary during defibrillation threshold testing. An immobile patient facilitates accurate and precise 3D mapping and reduces risk during transseptal puncture, pericardial access, or ablation in close proximity to critical structures. Note that in cases where the assessment of phrenic nerve function is important for a favorable case outcome (such as placement of a coronary vein branch pacing lead or ablation within the right superior pulmonary vein), paralytic dosing should be reduced or eliminated. Improved safety, efficacy, and procedure times have been shown with the use of general anesthesia with certain procedures such as atrial fibrillation ablation.55 Using high-frequency ventilation can further minimize respiration-related cardiac movement during ablation.56
Although adequate sedation should be administered to ensure patient comfort because certain arrhythmias such as atrial tachycardias and outflow tract VTs can be dependent on adrenergic tone, excessive sedation can result in the inability to induce the clinical arrhythmia. In cases in which an adrenergic-dependent arrhythmia is suspected, sedation must be minimized until the clinical arrhythmia is induced and mapped. Deep sedation is usually administered while performing ablation to prevent patient movement. When assessing the end point in these cases, care must be used to differentiate the effect of sedation from the actual elimination of the arrhythmia.
8.2.2. Sterile Preparation of the Access Site and Vascular Access
Although the risk of infection is extremely low with EP catheter procedures,57 appropriate sterile techniques should be maintained. This includes sterile preparation of all access sites, such as the groin and neck. If there is the potential for pericardial access, the subxiphoid region, and possibly the parasternal and apical regions, should also be prepped and draped. In cases with a higher risk of cardiac tamponade, sterile preparation of the subxiphoid region can be considered at the onset of the procedure.
8.2.3. Diagnostic Catheter Selection
Catheters with a smaller French size and fewer electrodes are more flexible, exert lower axial force, and may carry a lower risk of perforation,58 but should perhaps be avoided because of difficulty maintaining stable catheter position. Catheters with more electrodes can facilitate rapid recognition of arrhythmia activation patterns and are particularly beneficial at sites such as the coronary sinus. Smaller electrodes and narrower interelectrode spacing detect a more local activation signal and can provide more precise activation mapping, but may be less maneuverable. Activation confined to a small structure or circuit, such as the His bundle or an accessory pathway, however, may be difficult to localize with narrow electrode spacing. The field of signal detection can be increased either by changing to a catheter with wider electrode spacing or by reconfiguring the electrode pairing. With this wider field of view, anatomical localization is decreased. Some diagnostic catheters are designed with electrodes in a specialized spatial configuration, such as circular/ring, basket, or star-shaped catheters. These catheters can enable rapid deciphering of an activation pattern even with a paucity of arrhythmia. The number of catheters and recording sites should be adequate to achieve the desired end points of the procedure, but not so many that vascular damage or obstruction or intracardiac entanglement could occur.
8.2.4. Anticoagulation
Anticoagulation is necessary for all left heart procedures with heparin or bivalirudin in patients allergic to heparin.38, 39 Even in patients with therapeutic international normalized ratio on warfarin, heparin must be administered for left heart procedures, though typically in lower doses than in patients not taking warfarin. For right heart procedures, there is no evidence favoring routine use of anticoagulation; in cases where there is concern that the patient is at an increased risk for thromboembolic complications (prolonged procedure, known or discovered patent foramen ovale), some centers administer heparin for anticoagulation.
8.2.5. Selection of Ablation Catheters
Selection of the mode and catheter for catheter ablation is operator dependent. Catheter factors such as torque delivery, axial stiffness, steerability, and introducer diameter affect device selection. Ablation modes, including RF, cooled RF, laser, and cryothermy all have their strengths and weaknesses. The goal is to achieve a therapeutic ablation by identifying the ablation target, maneuvering to that site, and then destroying enough tissue to prevent arrhythmia initiation/propagation while minimizing the risk of collateral injury. The use of open-irrigated tip catheter ablation may result in the infusion of 2–3 L of volume during the case, which can precipitate heart failure in susceptible patients.59 Multielectrode or “single-shot” ablation systems are being developed for the treatment of complex substrates such as atrial fibrillation. Experience with many of these technologies is limited. A preferred device for these applications may emerge in the future as our experience increases. Selecting the appropriate ablation catheter is a process that involves a correct interpretation of the arrhythmia mechanism, a firm understanding of the advantages and disadvantages of the different catheters and energy sources, and the need for the responsible use of resources.60
8.2.6. Optimizing Signal Recording
Bipolar intracardiac recordings are standard in most laboratories because they theoretically detect only near-field signals, unlike unipolar recordings that incorporate both near and far-field components.61, 62 Far-field signals can still be detected in bipolar recordings but are typically a lower amplitude and frequency. Unipolar recordings can be helpful in mapping sites of focal activation, such as ventricular insertion sites of accessory pathways in preexcitation syndromes and idiopathic outflow tract ventricular arrhythmias, in which deep sharp QS configurations signify a site from which activation emanates (i.e., site of the earliest ventricular activation). The unipolar signal can help clarify the content of the bipolar electrogram (near vs. far-field), the timing of actual local depolarization (intrinsicoid deflection of the unipolar signal), and the relative proximity of the tip vs. the ring electrode to the ablation target.
The accurate interpretation of potential ablation target sites involves correctly differentiating local from distant activation as well as from electrical noise. Noise troubleshooting is a complex issue and involves many variables. It is necessary to be familiar with the basics of signal acquisition provided in Appendix 1 to correct noise issues. An inadequate signal-to-noise ratio will result in physiological signals being obscured by ambient noise with loss of critical information. At the onset of the procedure, steps should be taken to ensure that the signal quality is optimized for successful mapping. This should include the following: (1) choosing the appropriate electrode spacing, (2) setting the high-pass filter high enough to exclude low-frequency artifacts, such as respiratory drift, (3) setting the low-pass filter low enough to exclude high-frequency noise artifacts, (4) turning on the notch filter that excludes the 50–60-Hz bandwidth typical of electrical interference, and (5) gaining the signal appropriately to visualize low-amplitude signals of interest while minimizing the magnification of noise artifacts. It should be noted that using the notch filter on bipolar intracardiac signals can introduce ringing to sharp simple signals, making them appear fractionated. This is a particular concern when targeting fractionated potentials in cases of VT and atrial fibrillation. If the laboratory and equipment are properly grounded and the electricity in the laboratory is conditioned, there should be no 50–60 Hz noise on the intracardiac signals. Lower gain recording should be employed if the electrogram signals exceed the recording range of the amplifier. Efforts should be made, working with the facility’s biomedical engineering personnel, to achieve the lowest noise signals possible. Steps toward this goal include appropriate equipment grounding and shielding of cables, scheduled maintenance of connecting cables with replacement if contact plugs lose continuity, maintaining the shortest distance traveled by all electrical cables, ensuring that cables are off the floor and removed from potential hazards such as wheeled carts and cleaning solutions, and maintaining separation between high-voltage lines, such as power cables, and low-voltage lines used for transmitting the patient’s electrical signals.
8.3. Acute EP Catheter Procedural Complications
Dealing with complications in the EP laboratory has several components: avoidance, recognition, and response. To the extent possible, complications should be avoided by adhering to standard techniques and practices. When a complication occurs, the outcome for the patient hinges on how quickly a problem is recognized and appropriately evaluated as well as how quickly and appropriately the response to the incident rectifies the situation. Even the most careful and skilled operator will have occasional unavoidable complications (Table 3)63, 64, 65, 66, 67, 68.
Table 3.
Procedural Complications
| Complication | Prevention | Diagnosis | Treatment |
|---|---|---|---|
| EP catheter procedural complications | |||
| Pericardial effusion/tamponade | Avoid excess catheter force | Fluoroscopy of cardiac border,63 2D echocardiography | Reversal of anticoagulation, urgent pericardiocentesis |
| AV nodal block | Monitor for accelerated junctional rhythm, VA block, AV block during overdrive atrial pacing | ECG | Pacemaker |
| Phrenic nerve palsy | Phrenic nerve mapping, phrenic nerve pacing during RSPV, SVC, and LAA ablation64, 65, 66 | Fluoroscopy, chest radiography | Conservative therapy |
| Stroke | Anticoagulation (ACT >300 or 350 s in the LA), avoid char formation | Neurological exam, MRI scan (DWI and FLAIR imaging) | Conservative therapy, Merci thrombectomy |
| Coronary artery injury | Avoid excess power delivery in the CS, coronary angiography before epicardial ablation, coronary ostia visualization by angiography and ICE before ablation in the aortic root | ECG | Percutaneous intervention |
| Access site complications (hematoma, AV fistula, pseudoaneurysm) | Site selection, excellent technique, vascular ultrasound to guide puncture,67 micropuncture | Physical exam, ultrasound | Manual pressure, bed rest |
| Radiation burn | Minimize radiation exposure | Physical exam (presentation typically 2–8 wk postprocedure, but can be >40 wk) | Avoid repeat exposure |
| CIED implant procedural complications | |||
| Pneumothorax | Extrathoracic vascular access (axillary or cephalic vein) | Chest radiography | 100% oxygen rebreather, chest tube |
| Lead dislodgement | Test lead for acute fixation (“tug test”) | ECG, device interrogation, chest radiography | Reoperation and repositioning |
| Pericardial effusion/tamponade | Avoid excess forward pressure during lead placement | Fluoroscopy of the cardiac border,63 2D echocardiography | Urgent pericardiocentesis |
| Pocket hematoma | Avoid heparin and clopidogrel | Exam | Conservative therapy, pressure wrap, reoperation for pocket evacuation |
| Infection | Preoperative antibiotics, prevent hematoma,67 score or excise chronic pocket fibrosis | Exam, wound culture, blood culture | Antibiotics, extraction of the entire system (unless superficial) |
| Air embolism | Use introducer sheaths with hemostatic valves | Fluoroscopy | 100% oxygen rebreather |
| Postprocedural complications | |||
| Hematoma | Avoid heparin and clopidogrel | Exam | Conservative therapy |
| Infection | Good wound care | Exam, wound culture, blood culture | Antibiotics, extraction of entire system (unless superficial) |
| Late pericardial effusion/tamponade | None | 2D echocardiography | Pericardiocentesis |
| Phrenic nerve palsy | Phrenic nerve mapping, phrenic nerve pacing during RSPV, SVC, and LAA ablation64, 65, 66 | Loss of diaphragmatic motion on fluoroscopy, elevated hemidiaphragm | Conservative therapy |
| Stroke | Postprocedure anticoagulation68 | Neurological exam, MRI scan (DWI and FLAIR imaging) | Conservative therapy, consider Merci thrombectomy |
| Myocardial infarction | Appropriate anticoagulation | ECG, biomarkers | Medical therapy, percutaneous intervention |
| Atrial-esophageal fistula | Limit power, time, temperature, pressure during posterior wall ablation, monitor esophageal temperature | Fever, malaise, leukocytosis, systemic embolism, CT or MRI findings | Surgery |
| New arrhythmias | Avoid creation of gaps in linear ablation | ECG, ambulatory monitor | Antiarrhythmic drugs, repeat catheter ablation |
| Radiation burn | Minimize radiation exposure | Physical exam (presentation typically 2–8 wk postprocedure, but can be >40 wk) | Avoid repeat exposure |
2D = two-dimensional; ACT = activated clotting time; AV = atrioventricular; CS = coronary sinus; CT = computed tomography; DWI = diffusion weighted imaging; ECG = electrocardiogram; EP = electrophysiology; FLAIR = fluid-attenuated inversion recovery; ICE = intracardiac echocardiography; LA = left atrium; LAA = left atrial appendage; MRI = magnetic resonance imaging; RSPV = right superior pulmonary vein; SVC = superior vena cava; VA = ventricular atrial.
8.4. Procedural Issues—CIED Implantation
8.4.1. The OR Environment
One of the most important risks in device implantation procedures is infection. The device implantation laboratory should be regarded as an OR, with the same attention to sterile technique. Hats, masks, and shoe covers should be worn in the procedure room when the sterile field is exposed. Efforts should be made to restrict traffic in and out of the procedure rooms and minimize the number of personnel in the room. Studies have demonstrated that microbial counts increase significantly in unoccupied ORs when the door is left open to the hallway.69 After explanation of a device from an infected pocket, the room should undergo cleaning according to standard procedures employed in the OR for contaminated cases.
8.4.2. Antibiotic Prophylaxis
The use of preoperative antibiotics has been conclusively proven to reduce CIED infection.70 Administration of an antibiotic, usually a first-generation cephalosporin, 1 hour before implantation is required. In light of the prevalence of the colonization with methicillin-resistant strains of Staphylococcus, some operators choose vancomycin in patients at higher risk of infection, although data are lacking to support this practice. Vancomycin is a suitable choice for patients with penicillin allergies. If vancomycin is selected, it should be administered within 2 hours of the procedure.71
8.4.3. Sterile Technique
The instruments and components used in the device implant laboratory must be opened in a clean air environment. By doing so, bacterial contamination of these instruments and components will be kept to a minimum. All personnel in the room must wear a cap and mask at all times, and all who are in contact with the sterile field must perform a complete surgical scrub and must be gowned and gloved.
8.4.4. Sterile Preparation of the Surgical Site
The operative site(s) should be prepared with an antiseptic agent. Although these agents eliminate the immediate bacterial count on the skin surface at the operative site, hair follicles may prevent complete sterilization of the skin. Hair clipping close to the skin in the prep room, rather than shaving, is recommended because bacteria on the skin surface begin to recolonize within 30 minutes in the presence of hair follicles despite complete sterilization.72 Some centers instruct patients to use preprocedural home cleansing kits. Site preparation with alcohol-based solutions should be allowed to dry completely before draping. The combination of evaporating alcohol, supplemental oxygen, and electrocautery poses a significant fire risk in the surgical field.
8.4.5. Concomitant Groin Access During Implant Procedures
In some cases, additional femoral venous access for the placement of a temporary pacing catheter may be warranted (e.g., lead extraction and pacemaker-dependent patients with an inadequate escape rhythm who are undergoing generator change).8 Large-bore venous sheaths are useful for rapid volume resuscitation in the event of vascular tears during lead extraction, and arterial access facilitates beat-to-beat monitoring of blood pressure.
8.5. Acute CIED Implant Procedural Complications (Table 4)
Table 4.
Definitions and Terminology Used in Clinical Fluoroscopy
| Roentgen (R) is the unit of radiation exposure in air. The total charge produced in air per unit mass by ionizing radiation is easily measurable with survey instruments. |
|---|
| Gray (Gy) is the International System of Units (SI) of absorbed radiation dose of ionizing radiation, which has replaced the term radiation absorbed dose (in rad). One gray is the absorption of 1 J of ionizing radiation by 1 kg of matter (equivalent to 100 rad), and it assesses the potential biological risk to that tissue. The U.S. unit for absorbed dose is rad. 1 Gy = 100 rad. |
| Equivalent dose Sievert (Sv) is a measure of equivalent dose, and is also an SI unit. It takes into account the different probability of effects that occur when the same amount of absorbed dose is delivered by different types of radiation (protons vs. X-rays). It is equal to the absorbed dose in Gy multiplied by the radiation weighting factor, WR, and other modifying factors. The WR is 1 for X-rays. The equivalent dose can be used to assess radiation risk if the person’s whole body is uniformly irradiated. For partial body exposure, such as cardiac electrophysiology, additional correction is necessary to assess the radiation risk. |
| Effective dose is used to assess the risk when only a part of the body absorbs energy from radiation. Since some organs in the body are more sensitive to radiation effects than others, the equivalent dose is multiplied by the appropriate tissue weighting factors. This terminology is used to assess the risk of radiation-induced cancer and hereditary effects. For example, the effective dose for a typical chest X-ray radiograph is 0.0001 Sv (0.1 mSv). The U.S. unit for sievert (Sv) is rem. 1 Sv = 100 rem. |
| As low as reasonably achievable (ALARA) standard is a system for limiting the amount of radiation a person receives. Radiation exposure should be justified on the basis of the assumption that there is no threshold below which ionizing radiation is free from harmful biological effects and that shielding from radiation exposure is needed, no matter how low the dose. |
| Kerma is an acronym for kinetic energy released in the material. Kerma is measured in Gy. |
| Kerma-area product (PKA) is the integral of air kerma (absorbed dose to air) across the entire X-ray beam emitted from the X-ray tube. PKA is a surrogate measurement for the entire amount of energy delivered to the patient by the beam. PKA is measured in Gy·cm2. Another term for this is the dose-area product (in Gy·cm2). |
| Reference air kerma (Ka,r) is the kerma-area product at a specific point in space relative to the fluoroscopic gantry (the interventional reference point) during a procedure. It is measured in Gy. |
| Peak skin dose is the highest dose of radiation exposure on any portion of a patient’s skin during a procedure. |
Complications associated with acute CIED implantation are often technique related. Attention to detail will minimize the risk of difficulties related to the implant procedure.
8.6. Postprocedural Issues
8.6.1. Vascular Hemostasis
Venous sheaths may be removed at the end of the procedure if no anticoagulant has been administered, with pressure held for 10–20 minutes. If heparin has been administered, waiting until the ACT is more than 175 seconds (<250 seconds if the patient is receiving therapeutic warfarin) before sheaths are removed decreases the likelihood of bleeding and hematoma formation. Reversal of heparin effect with protamine is employed in many laboratories to more rapidly reverse heparin effect and allow almost immediate sheath removal, although one must be prepared to treat uncommon but sometimes severe protamine reactions.64 Vascular closure devices are uncommonly used in EP,64, 65 but are an appropriate choice for arterial closure. After atrial fibrillation ablation, the reestablishment of therapeutic anticoagulation soon after sheath removal is desirable to lessen periprocedural stroke risk but there is no consensus on the optimal regimen or timing.
8.6.2. Postanesthesia Recovery
When mild anesthesia is used, vital signs and oxygen saturation should be monitored continuously until the patient is conscious and communicative. Access sites, cardiac rhythm, and neurological state should be assessed every 15 minutes during the first hour and then periodically thereafter. Late complications, such as access site hematomas and hemodynamically significant pericardial effusion, can develop after the patient leaves the postprocedural recovery area. If a patient received midazolam during the procedure and a dose of its antidote (Romazicon/flumazenil) was administered, the patient must be monitored for a rebound effect of midazolam. If general anesthesia was used, patients usually recover in a postanesthesia care unit.
8.6.3. Postprocedural Complications
Procedural complications that can arise after the patient leaves the laboratory area (or even after hospital discharge) are listed in Table 4. A process for tracking postprocedural complications should be in place as part of the laboratory’s QA process (see Section 9).
8.6.4. Medication
Postprocedure anticoagulation is recommended in patients who are at high risk of stroke on the basis of evaluation tools such as CHADS2 or CHA2DS2-VASc scores. In many laboratories, chronic warfarin therapy is not interrupted during either device implantation or ablation procedures in patients who are taking it for stroke prevention in the setting of atrial fibrillation or mechanical heart valves; the procedure is safe, with the international normalized ratio ranging from 2.0 to 3.5.35, 65
In patients with insulin-dependent diabetes, the morning insulin dose is typically halved on the day of the procedure and glucose is periodically monitored during the procedure and the patient is treated accordingly.
8.7. Hospital Discharge
The setting for EP procedures may be outpatient, 23-hour observation, or inpatient. The decision for discharge takes into account procedural detail, patient age, and health status, the potential for complications (such as blood loss), and the ability of the patient (or caregivers) to evaluate signs of concern.67 This is a medical decision and should be determined irrespective of reimbursement issues.
8.8. Reporting Procedural Results
The procedure report should include, at a minimum, the following: the primary and secondary operators; the indication for the procedure; names and doses of any medications administered; intake, output, and estimated blood loss; catheter/pacing/ICD lead model numbers, serial numbers, insertion sites, and intracardiac destinations; findings and procedure performed; complications encountered; and fluoroscopic exposure (minutes; mGy; dose-area product). Patients who receive excessive radiation exposure during a procedure (typically >3000 mGy, but requirements vary by state) must be notified and followed up for evidence of skin damage. Ideally, this information is stored in a database for QA purposes. Recordings made during the procedure (electrograms and fluoroscopic and mapping system images) should be archived on digital media (ideally on a network, or alternatively on a CD or DVD) for future reference, if needed.64
9. Pediatric and Adult Congenital Heart Disease
Pediatric and Adult Congenital Heart Disease Recommendations
|
9.1. Patient Factors Different From Adults
Issues that pertain to EP laboratory standards and practice for pediatric EP and patients with pediatric and adult CHD with rhythm abnormalities differ from those pertaining to adults and are not confined to issues of patient and cardiac size compared with the adult patient. The decision-making process for interventions has implications for patient quality of life and development. Success, failure, and procedural factors related to intervention therefore span many decades. A significant factor in treating cases involving young patients in the EP laboratory is the need for age-appropriate supportive care.
9.1.1. Arrhythmia Substrate, Patient Size, and Future Patient Growth
Arrhythmia mechanisms in young patients vary by age72 and influence decision making. Patient size can dictate the use of and expertise with smaller ablation catheter sizes or the use of esophageal pacing if vascular access is limited, such as is the case in neonates. Knowledge of ablation lesion formation and potential expansion in an immature myocardium is critical to the care of pediatric patients.73 Children and young adults will experience decades of device-related issues compared with the typical adult patient. These issues should affect decision making in terms of timing and location of cardiac EP devices, accounting for growth, potential need for multiple extractions and replacements, and issues of venous occlusion. For patients with CHD, these factors are complex and affect the decision to intervene. Surgical interventions for all forms of CHD have resulted in improved survival rates,72 and the details of these surgical interventions are critical in the analysis of rhythm substrates in the EP laboratory. More complex pediatric CHD survivors comprise an increasing percentage of the adult CHD population.72 It is recommended that procedures in pediatrics and patients with CHD be performed by (1) pediatric cardiologists, (2) a collaboration of adult and pediatric cardiologists, or (3) an adult cardiologist with established interest and expertise in adult CHD.
9.2. Indications for EP Procedures in Pediatric Patients and Patients With CHD
The indications for catheter ablation in the pediatric population derived from an understanding of the natural history of arrhythmias in young patients, likely rates of procedural success and complications, and the risk of recurrence and have been reviewed in prior publications.54, 74 The guidelines for the assessment of the asymptomatic young patient with Wolff-Parkinson-White syndrome are published as a joint PACES/HRS statement.75 In the pediatric population, the presence of CHD affects the expected results of ablation and recommendations for intervention. The guidelines for the implantation of cardiac rhythm devices in young patients and patients with CHD were last updated in 2008.76 Epicardial pacing is used for those in whom transvenous pacing is contraindicated, such as prosthetic tricuspid valves, right-to-left intracardiac shunts, and small patient size, or for those undergoing concomitant heart surgery. The majority of ICDs are implanted via the transvenous route, but this may not be possible in some individuals because of anatomical constraints. Because ICD leads are larger and prone to fibrosis, patient size limitations for transvenous systems are considerable. Although established in adults, the indications for cardiac resynchronization therapy in children are less certain and are based on retrospective reviews, rather than randomized trials.77, 78
9.3. Patient Safety Concerns
Because younger patients may have a longer life span after EP procedures than do adults, the lifetime risks of malignancy and birth defects (stochastic risks) are higher. Adult patients with CHD incur increased radiation exposure resulting from the electroanatomic complexity of cases and the need for multiple procedures. Strategies for radiation dose reduction as detailed in Section 11 should be aggressively implemented. The most significant complications in small children include pericardial effusion, pneumothorax, atrioventricular block, and death.79, 80 Animal and clinical studies have shown the potential expansion of scar tissue with maturation70 and the risk of late coronary artery injury79 potentially related to ablation location. The risk of atrioventricular block is increased by small patient size and septal pathways, presumably because of the smaller anatomical dimensions of structures. Smaller tip catheters, lowered RF energy, shorter lesion duration, and the use of apnea and pacing techniques may diminish risk.81 Cryoablation is perceived as being safer, but may have a higher recurrence rate compared with RF lesions.82 It is recommended that EP laboratories conducting procedures on pediatric patients have cryoablation capability.
9.4. Procedural Issues
9.4.1. Inpatient vs. Outpatient Setting
The procedure setting for invasive EP studies and ablation for pediatric and congenital EP patients may be outpatient or inpatient. The decision for discharge takes into account procedural detail, patient size, potential for complications, and the ability of the parents to evaluate signs of concern. The protocol for EP device placement or revision is mostly less than 1 day of observation, but generator changes in older patients may not require more than 6 hours. Young patients with new devices are monitored overnight to administer periprocedural antibiotics, evaluate for pneumothorax or hemothorax, evaluate the device parameters, check lead location, and manage pain.
9.4.2. Sedation, Anesthesia, and Medications
The goal of sedation in the pediatric and congenital EP laboratory should be to provide a safe, nontraumatic experience for the patient by considering young age, preexisting conditions, presence of CHD, airway issues, family choice, and complexity of the procedure. The 2002 NASPE Position Statement on Pediatric Ablation delineated the types of anesthesia (e.g., conscious sedation, moderate sedation, and general anesthesia), and these remain applicable.54 Personnel responsible for sedation, anesthesia, and administration of medications must be experienced with pediatric and congenital EP patients, and PALS and ACLS certification should be maintained. All physicians performing EP procedures should be knowledgeable regarding sedation, monitoring, and airway management. Allied health professionals (e.g., nurses, APNs, and PAs) can be involved with sedation of a patient, if directly supervised by a physician.
9.4.3. Facilities
The room and equipment standards for pediatric EP procedures are similar to those for adult EP procedures (see 4, 5) but must have a cardiac defibrillator specifically for use with children, a code cart meeting pediatric needs, and age-appropriate anesthesia equipment. Pediatric and congenital EP patients may require a combined procedure of EP and hemodynamic catheterization, including angiography and possible intervention. Thus, it is desirable that a pediatric/CHD EP laboratory meet the same standards as a pediatric catheterization laboratory.
9.5. Lab Staffing
General recommendations for EP laboratory staffing are detailed in Section 6 and Table 2. Pediatric and congenital EP guidelines recommend that the facility and laboratory staff should be appropriate for the patient population and pediatric and CHD interventional and surgical experts be urgently available during laboratory procedures.42 All members of the team should be trained in PALS (when treating a child/infant) and ACLS (if treating an adult).
9.5.1. Physicians
Training and board certification pathways employed by physicians who perform EP procedures on pediatric and congenital EP patients include the pediatrics and internal medicine pathways (see 6, 7).
9.5.2. EP Laboratory Personnel
Recommended staffing for pediatric cases is detailed in Table 3. While laboratory staff roles are similar to those outlined in Section 6 for pediatric cases, these staff should have expertise in performing cardiac procedures in pediatric and congenital EP patients.
9.6. Emergency Supportive Care and Surgical/Intensive Care Unit Backup
Pediatric EP procedures should be performed (earlier than the teenage years) in centers where there is pediatric cardiovascular surgical availability. For procedures performed on patients in their teenage years or procedures on adult patients with CHD that are performed in adult or mixed pediatric and adult institutions, a CV surgical plan should be formalized to accommodate on-site emergencies (notably, cardiac perforation) and a rapid transfer made to a pediatric cardiovascular center should the anatomy of the patient require this expertise.
9.7. Postprocedural Care
The postprocedural care can be performed in a separate periprocedural area, the general cardiology floor, or the intensive care unit. Continuous telemetry should be available for the evaluation of heart rhythm. The environment for postprocedural care should be appropriate for patient age and development. The nursing and physician staff should be experienced in the care of pediatric and congenital EP patients.
10. Quality
Quality Recommendations
|
10.1. The QA/QI Process
High-quality, consistent care delivery in a busy cardiac EP laboratory requires standard protocols for procedures, communication channels, and documentation. An essential component of a successful EP laboratory is to have an internal QA/QI process in place, in addition to public reporting requirements. This requires a commitment from facility administrators to provide adequate staffing, including a committee chair, staff coordinator, and funding for collecting and managing data. The goal of the program should be to motivate and encourage physicians and staff to participate and to take initiative in the QA/QI process and overall success of the laboratory. It is acknowledged that there is little published data on the QA/QI process in the EP laboratory and that expert consensus is the primary basis for our recommendations. Further research and development of quality metrics specific to the practice of cardiac EP is ongoing.
To begin the QA/QI process, its components should be identified. These components include national requirements for tracking (e.g., device implants), minimum acceptable complication rates (e.g., infections), and compliance with national registries, including the NCDR ICD Registry. A QA/QI program must ensure that key data are collected prospectively and systematically. When the QA/QI team is identifying potential quality metrics, either strong scientific evidence or expert consensus must support the metric. The metric must measure areas important to patient care, and it is best if it covers an aspect of practice where there is a gap in patient care. The data must be available in a usable format for future analysis. These data allow the laboratory to benchmark its performance and provides a reference by which appropriate changes can be made.
Provider qualifications are typically well established by national guidelines and certification bodies. It is the responsibility of each institution to ensure staff credentialing, maintenance of certification, and necessary CME requirements are met (see Section 7). Practitioners should be expected to adhere to published practice guidelines unless the reason for deviation is documented. Clinical situations not directly addressed by the guidelines inevitably arise and require judgment and skill to address. Periodic peer review of cases that fall outside the guidelines is recommended to ensure appropriate delivery of care. The guidelines evolve and, by definition, require a consensus of data before they can be written and revised. The writing group recognizes and encourages the development of new clinical pathways and tools. This development is best achieved through research protocols that adequately capture patient demographic characteristics, procedure characteristics, and outcomes. The research consent process ensures that patients are aware that the treatment being offered is beyond the current recommendations of the practice guidelines.
Procedure outcomes, including success rates and complications (and ideally including 30-day outcomes), should be documented and recorded. The writing group recognizes that success for some procedures requires clinical follow-up (particularly atrial fibrillation and VT ablation procedures). In these instances, an acute end point for the procedure should be specified (e.g., pulmonary vein isolation, noninducibility for VT, or creation of a planned RF ablation lesion set based on the results of an endocardial voltage scar map) and documentation should indicate if the end point was achieved. An assessment of freedom from arrhythmia recurrence after 1 year of follow-up should be performed. Risk-adjusted models are not well developed by which the relative frequency of complications and successful outcomes among different patient populations can be interpreted and physician results compared. Therefore, the interpretation of success rates and complication rates requires judgment by peers. Physicians should participate in regularly scheduled QI and/or peer review meetings to maintain privileges and evaluate procedural appropriateness. A quarterly EP laboratory morbidity and mortality conference (stand-alone or as a component of another conference) should be mandatory, with attendance documented.
The modern cardiac EP laboratory depends on many complex hemodynamic and physiological recording systems, advanced imaging systems, advanced mapping systems, and multiple ablation systems. Rigorous processes must be established to ensure that (1) a QA process for equipment is established; (2) equipment is tested and demonstrated to be functioning appropriately, both on a routine basis and immediately before a case in which the specific item will be used; verification of equipment function should be included in the time-out procedure; (3) EP laboratory staff are appropriately trained in the maintenance, setup, and operation of the equipment; and/or (4) representatives from the vendor are available, qualified, and cleared by administration and occupational health to participate in the operation of the equipment before, during, and after the procedures. Competencies in clinical skills, ACLS, PALS (when appropriate), sterile technique, radiation safety, and fire safety should be assessed on a regular basis.
The EP laboratory requires processes be in place to ensure proper communication within the EP laboratory and with other hospital services. Within the laboratory, protocols for emergency situations (such as tamponade or ventricular fibrillation refractory to defibrillation) can make the difference between an organized, streamlined, successful resuscitation effort and a chaotic effort during which leadership is absent, team members duplicate some activities and neglect others, and ultimately a tragic but potentially avoidable outcome occurs. Beyond the boundaries of the EP laboratory, communication between the EP team and other health care professionals is essential, particularly during handoffs from one care team to another and at the time of patient discharge. It is the role of the QA/QI committee to oversee that excellent communication processes are developed, maintained, and adhered to by the staff.
10.2. Clinical Outcomes and Complications
Data acquired from the EP laboratory QA process should be used to benchmark the complication rates and outcomes of both individual practitioners and the overall EP laboratory. For practitioners with complication rates above the benchmark, an objective unbiased peer review of the relevant cases is critical to determining whether a deviation in the standard of care occurred. Because event rates are low and risk-adjusted models are not well developed for the EP laboratory, peer review is particularly important. Practitioners should not be penalized for accepting higher risk and/or more challenging cases. However, if reckless behavior or inadequate skills or knowledge is deemed to be present and a deviation in care occurs, verbal and written communication by the chair of QA is imperative. This communication should include a clear plan for corrective action and documentation of potential future actions if corrective action is not successful.
10.3. Case Volumes
A link between operator case volume, skills, and outcomes has been documented in some but not all areas of cardiac EP, yet controversy and conflicting data remain. Specific case volumes for training are outlined by the ABIM29 (Table 3), and for clinical competency they are available in the HRS clinical competency statements.8, 17, 33, 38, 39, 42 Given the often poorly defined relationship between case volumes and outcomes, a more appropriate measure is to ensure that all major complications (see Table 3) are reviewed by the QA committee and handled as described in the previous section.
10.4. Database
A prospective plan to acquire data in an accessible and functional database is an essential building block for any QA/QI process. Without objective, reliable data to measure outcomes, no meaningful effort at QI can be undertaken. Minimum data that should be recorded in a searchable aggregate form include patient demographic characteristics, relevant history of present illness, medications, CIED product information data, and data on outcome and complications from any invasive procedures. Patients with CIED should be identifiable by the device that has been implanted to permit rapid identification of patients who may have received defective hardware in the event of a recall or other notification from the manufacturer or FDA.
The field of medical informatics is on the verge of exponential growth. Registries such as the ACC/NCDR ICD Registry have identified specific data fields that should be captured for participation in the registry and compliance with payer mandates.83 Beyond these basic requirements, recommendations for more detailed database fields are included in clinical guidance documents for the management of patients with atrial fibrillation and for the management of patients with VT.38, 39 These standard data elements provide an opportunity for EP laboratories invested in research to aggregate and/or compare their data with that of other laboratories working in the same field.
10.5. Pediatric and Adult Congenital Heart Disease
To date, QA efforts in pediatric and congenital EP have centered on the creation of large EP procedural registries.84, 85 In late 2010, a PACES taskforce began to develop and implement a self-sustaining multicenter QI registry known as MAP-IT. Presently, the MAP-IT taskforce is creating a registry of patient-centered late outcome measures of catheter ablation procedures. For the first time, an empirical and data-derived method of risk/complexity adjustment for pediatric and CHD EP procedures, known as the COMPASS score, has been developed. The future of QA efforts for procedure-based subspecialties will require the benchmarking and reporting of risk-adjusted “patient-centered” outcome measures. Widespread implementation of the MAP-IT initiative within PACES should satisfy this need.
11. Occupational Health Concerns
Occupational Health Concern Recommendations
|
11.1. Radiation Safety
The field of cardiac EP is greatly dependent on fluoroscopic imaging for the placement of catheters and device leads into the heart. This results in significant radiation exposure to the patient, the operator, and the laboratory staff. While this exposure cannot be eliminated in most cases, attention to fluoroscopic technique can minimize radiation dose. Competencies in radiation safety should be completed yearly by the EP laboratory staff. Some states mandate fluoroscopy licenses be obtained by all personnel using fluoroscopy and renewed periodically, including physicians and radiation technologists.
11.1.1. Terms for Understanding Radiation Exposure in the Cardiac EP Laboratory
Nonionizing radiation, such as microwave or infrared radiation, can cause heating but not molecular damage to cells. Ionizing radiation, such as β, γ, and X radiation, strips electrons from atoms and causes molecular injury to DNA. Ionizing radiation has great potential for damage to tissue, including burns and malignancy. Understanding the basic definitions and terminology used to describe ionizing radiation is helpful when trying to understand the potential effects on the human body (see Table 4).86
11.1.2. Biological Risks From Radiation Exposure
Radiation effects are described by their deterministic and stochastic effects.87 Deterministic effects are harmful tissue reactions that are determined by an absorbed threshold dose. Radiation-induced skin burns are an example of deterministic effects.88 Stochastic effects include malignancy and heritable effects and are not determined directly by the dose, but a higher dose increases the probability of an adverse outcome. Human tissue radiosensitivity varies directly with the rate of cellular proliferation and number of future divisions and indirectly with the degree of morphological and functional differentiation. The most sensitive tissues include the bone marrow, spermatocytes, and intestinal crypt cells.87 Local skin injury is the most commonly encountered deterministic effect in cardiovascular medicine, with changes noted at doses above 2 Gy. Findings often do not appear until weeks after exposure.
Radiation exposure increases lifetime risk of fatal malignancy. The as low as reasonably achievable standard was derived from the Biological Effects of Ionizing Radiation VII Report.89 This report makes the assumption that cancer risk increases proportionally with radiation exposure and that there is no radiation dose that is without risk. All personnel working in the laboratory must be aware of the as low as reasonably achievable standard. A skin threshold dose of 2 Sv should not be exceeded. Because the prevalence of fatal malignancies continues to increase over time after radiation exposure, children and young adults are more susceptible to these complications in their lifetimes. Adult patients with CHD incur increased radiation exposure resulting from the electroanatomic complexity of cases and the need for multiple procedures.
11.1.3. Measuring Radiation Exposure
A normalized X-ray dose at a specific kilovolt peak 1 m from the source, total fluoroscopic time, backscatter correction factor, and source-to-skin distance can be used to estimate radiation exposure.90 A direct measurement of radiation doses at multiple sites can be performed with lithium fluoride thermoluminescent dosimeter sensors and optically stimulated luminescence. The highest exposure to patients has been observed with a median skin entrance dose of 7.26 rem (range 0.31-135.7 rem) at the ninth vertebral body. This dose is predicted to be associated with a lifetime excess risk of malignancy to the female breast, active bone marrow, and lung of greater than 700 cases per million undergoing routine catheter ablation. Operator exposure was highest at the left hand, waist, and left maxilla.91 Fluoroscopy equipment should report three parameters: fluoroscopy time; radiation dose (air kerma, in Gy), a measure of deterministic injury potential; and the dose-area product (in cGy·cm2), a measure of stochastic injury potential. Radiation exposure is a superior metric to fluoroscopy time; reliance on fluoroscopy time is discouraged. The United States Nuclear Regulatory Commission annual dose limits for radiation are 0.50 Sv for skin, arms, and legs; 0.15 Sv for eyes; and 0.05 Sv for the whole body. The fluoroscopy dose for each case should be recorded in the medical record and accessible to patients.
11.1.4. Minimizing Radiation Exposure to Patients
The predominant strategy for minimizing radiation exposure to patients is to minimize the radiation dose.86, 92 The most effective approach is to minimize fluoroscopy pedal time. Operators should develop the habit of tapping the fluoroscopy pedal rather than standing on the pedal for a long period of time. If the eyes stray from the fluoroscopy screen, the foot should come off the pedal immediately. Decreasing the fluoroscopy pulse rate will significantly reduce dose at the cost of temporal resolution of the image. Supplementation of fluoroscopic imaging with nonfluoroscopic electroanatomic guidance systems by using stored fluoroscopy loops rather than cine loops and using pulsed fluoroscopy will reduce the total procedural dose. As the X-ray tube gets closer to the patient, X-rays at the skin entry point increase and the risk of deterministic injury also increases; thus, the operator should position the table at a comfortable height with some distance between the tube and the patient. If the image intensifier is not positioned as close to the patient as possible, the image will be magnified but the radiation dose will be much higher. To magnify the image, use the appropriate magnification mode. Both geometric and electronic magnification increases dose to the patient. Doses may be limited effectively by collimation, limiting the field of view with shutters as much as possible. For example, reducing the diameter of the field of view by 29% will reduce the radiation dose by half. Steeply angulated projections should be avoided, and if used, the C-arm should be repositioned somewhat throughout the procedure to avoid delivering radiation to the identical skin entry site. Depending on which procedure is being performed, local shielding of the patient’s thyroid and gonads can be employed.93
Since the stochastic effects of radiation exposure are cumulative, the caregiver should be sensitive to the lifetime exposure of the patient to ionizing radiation. There has been active discussion among regulators on implementing a system for lifetime tracking of radiation exposure to patients, but the tools for this type of system are not available, and therefore it is not presently mandated.94 It is important to emphasize that radiation exposure is dependent on the age and condition of the equipment. Thus, aggressive limitation of fluoroscopy pedal time is desirable, but those efforts may be futile if the dose rate is high because of a high frame rate or employment of an old imaging train. It is now an accepted standard that fluoroscopy dose, not only fluoroscopy time, is entered in the permanent medical record for each fluoroscopic procedure. Ideally, the peak skin dose, the reference air kerma, the kerma-area product, and the fluoroscopy time should be recorded for every case.95 A review of fluoroscopy use by individual operators should be part of every laboratory’s QA process.
11.1.5. Minimizing Occupational Radiation Exposure
The primary approaches used to reduce radiation exposure to the operator and laboratory staff are increasing distance from the source, scatter reduction, and dose limitation. Radiation dissipates in proportion to the square of the distance from the source, and so even a modest effort to move away from the tube will significantly reduce exposure. Radiation scatter occurs as radiation from the generator tube enters the patient and is partially reflected or refracted by body tissues. Scatter from the patient is the main source of radiation exposure to the patient outside the imaging field and to the operator. The operator and laboratory personnel must be protected from exposure to the scatter radiation with shielding. A minimum of 0.5-mm lead-equivalent protective apron, thyroid shield, and eye protection should be used.
Proper table shielding can dramatically reduce the scatter radiation escaping into the environment. Scattered radiation exits the body at all angles but is greatest on the same side of the patient as the X-ray source because only 1%–5% of radiation completely penetrates the patient’s body and exits on the other side.91 Therefore, proper undertable shielding is paramount. Shield extensions above the table rail and a contoured ceiling-mounted shield in contact with the patient’s torso will substantially reduce operator exposure. Since radiation doses decrease with the square of the distance from the source, the operator should perform the procedure as far from the radiation tube as is practical. Barium-impregnated drapes can further reduce radiation scatter in the procedure field.96 All recommendations described above for limiting total fluoroscopy dose will also reduce the operator and laboratory staff radiation exposure.
Radiation exposure to a pregnant EP laboratory worker is a special situation and should be resolved on a case-by-case basis. It is recommended that radiation exposure to the pregnant staff member, as measured by a waist dosimeter (under the lead apron), should not exceed 0.05 rem/mo, or 0.5 rem for the entire pregnancy.35 Additional layers of lead can be worn over the abdomen to further protect the fetus.
11.1.6. Quality Management
The FDA regulates fluoroscopy equipment manufacturing and has dose limits for systems with automatic exposure control.97 States regulate the safe use and operation of radiation-producing machines, such as fluoroscopic imaging systems. TJC’s sentinel event is an unexpected occurrence involving death or serious injury. Prolonged fluoroscopy that exceeds doses of 1500 rad (15 Gy) to the skin is a reportable sentinel event and requires an institutional response. A qualified medical physicist should perform initial acceptance testing and annual testing to ensure optimal image quality and radiation dose. Unfortunately, assuming that fluoroscopic equipment is functioning properly, the most important factor in radiation exposure to patients and staff is operator knowledge and behavior. TJC recommended (but did not mandate) credentialing standards for fluoroscopists,98 and the recommended curriculum was endorsed by HRS, American College of Cardiology Foundation, AHA, and the Society for Cardiovascular Angiography and Interventions (SCAI).86 The training of EP physicians and staff in radiation physics, radiation biology, and technological developments in X-ray imaging systems and X-ray dose management is highly variable, and physician credentialing and recredentialing has no requirement regarding knowledge of radiation safety. It is therefore incumbent on the hospital leaders to establish high local standards and to track fluoroscopy use and behavior.
11.2. Occupational Health Risks of Wearing Lead
11.2.1. Lead Aprons
Lead-equivalent aprons required for radiation protection of staff are heavy and present a substantial physical burden to the interventional cardiologist.99 Historically, lead aprons were made using 0.5-mm lead-equivalent materials, with weight per unit area of these garments being 7 kg/m2.98 An increased risk of cervical spondylosis is a known consequence for cardiologists who wear protective garments while standing for long hours performing procedures.87 Orthopedic problems and user fatigue associated with the continued use of heavy aprons contributed to the development of lower-weight lead-equivalent materials that are commonly used for protective garments today. Modern nonlead 0.5-mm lead-equivalent protective garments have a weight reduction of 30% or more than do traditional lead aprons.98 That modern aprons are lighter might be expected to lessen, but not eliminate, orthopedic discomfort and injury. It is important to emphasize that all lead should be tested at 6-month intervals to check for cracks or leaks.
Along with weight, there are several other physical and ergonomic aspects of radiation protection garments that should be considered. To minimize discomfort, garments must fit correctly and should be tightened around the midsection to shift weight from the shoulders to the hips as much as possible. There are several garment designs that are customized for various uses. Many interventional cardiologists choose a two-piece garment consisting of a skirt and vest (including internal frame), both of which should be snugly tightened around the midsection. Such garments are frequently designed to “wraparound” the wearer and typically provide the fully specified lead-equivalent protection from radiation only in the front where the garment overlaps. A thyroid shield should always be used,100 and leaded glasses should be worn to minimize the risk of developing cataracts.101
11.2.2. Alternatives to Wearing Lead
Tableside alternatives to lead-equivalent garments include a floor-mounted radiation protection cabin and a ceiling- or gantry-mounted suspended radiation protection system.99, 102, 103 Both these systems are designed to remove the weight burden of radiation protection while allowing tableside access in a sterile working environment. Because the weight of the shielding is not borne by the operator, thicker, heavier materials can be used. This results in a 16–78-fold decrease in radiation exposure to the operator.103 The disadvantages of these systems are that they restrict the motion of the operator to some degree, and they increase the equipment that is present in an already crowded procedure laboratory environment.
Robotic manipulation of ablation catheters can be accomplished using external magnetic fields to guide magnetic tipped catheters or robotic armatures that actuate sheath and catheter movement remotely. These systems allow electrophysiologists to perform most of the procedure behind fixed radiation barriers, thereby eliminating exposure to scattered radiation for the operator (but not the patient or in-laboratory staff) and eliminating the need to wear protective lead.26, 104, 105 Although there is a learning curve associated with these technologies, they appear to yield results equivalent to conventional manual catheter procedures. Recent developments in electroanatomic mapping and ICE have been used to perform catheter ablation of atrial fibrillation22 with minimal use of X-ray imaging. Future developments of these and other imaging technologies that do not use ionizing radiation may obviate the need for radiation protection garments in the EP laboratory of the future.
11.3. Laboratory Ergonomics
The physical stresses associated with working in the EP/interventional laboratory have been identified as a high prevalence of orthopedic problems, particularly those related to the spine, hips, knees, and ankles.102, 106 The primary contributor to orthopedic problems is wearing personal radiation protection apparel. Other factors may be those related to ergonomic design, increasing complexity and duration of interventional procedures, falls, and lengthy careers. A Multi-Specialty Occupational Health Group has been formed to evaluate risks and hazards and advocate for efforts to reduce these hazards.107 Table 5 provides a list of measures to be considered in laboratory design and procedural processes to reduce the incidence of ergonomic stresses on the EP laboratory physicians and staff.
11.4. Operator Safety During Cardiac EP in Patients With Communicable Diseases
Preventing the transmission of infectious agents to ensure the safety of the operator and other staff in the EP laboratory as much as possible involves, first and foremost, adherence to standardized uniformly applied universal precautions in every aspect of patient care. Risks of acquisition of infectious diseases by health care workers can be minimized by adherence to current infection control guidelines.108
11.4.1. Individual Personal Precautions
Institutions have policies on required annual or biannual staff inoculations. These may include inoculations such as hepatitis B, influenza, pertussis, and rubeola (measles). Such inoculations can provide immunity from certain highly transmissible diseases, enabling staff to care for these patients without inordinate risk. For example, health care workers not immune to chicken pox should not be required to care for patients with chicken pox.109 Because of the specialization of staff in the EP laboratory, it can be difficult to provide adequate staffing if multiple concessions have to be made for noncompliant staff. The interval for regular testing of staff for tuberculosis is defined by every institution. In special situations, staff may be fitted for N95 filter masks, which are particulate respirators that are used to prevent inhalation of small infectious airborne particles transmitted by patients with active tuberculosis. Institutional policies should be followed closely in these situations.
11.4.2. Standard Precautions
The major features of universal precautions and body substance isolation are incorporated in standard precautions and are used universally to prevent transmission of highly contagious or virulent infectious agents that may be spread by air and/or contact. The basic principle is that all blood, body fluids, secretions, excretions (except sweat), nonintact skin, and mucous membranes may contain transmissible infectious agents. Since it is impossible to identify all sources of all infectious agents, the same precautions are applied to every patient at every encounter. Each institution has a detailed description of how standard precautions are implemented. The following text highlights certain aspects particularly pertinent to EP laboratory processes.
Hand hygiene is the single most important factor in controlling and preventing the transmission of germs. Additional protection is offered by the appropriate use of medical gloves; however, the unnecessary and inappropriate use of gloves results in a waste of resources and may increase the risk of germ transmission.110 Hands are washed each time after gloves are removed. Hand-washing supplies should be readily accessible in the EP laboratory and environments. Sterile gloves are indicated for any surgical procedure, invasive radiologic procedure, and procedures involving vascular access (central lines). Examination gloves are indicated in clinical situations where there is potential for touching blood, body fluids, secretions, excretions, and items visibly soiled by body fluids. Splash protection with masks and eye shields is recommended when working with arterial and venous catheterization. Gloves are not indicated (except for contact precautions) for tasks involving direct patient exposure, including taking blood pressure, pulse, and temperature; administering intramuscular and subcutaneous injections; bathing and dressing the patient; transporting the patient; and any vascular line manipulation in the absence of blood leakage, or with indirect patient exposure, including using the phone, writing in the chart, removing and replacing linen for the patient’s bed, and placing noninvasive ventilation equipment and oxygen cannula.110
Safe injection practices, including the use of needleless injection systems and proper disposal of any sharps and other equipment used for invasive procedures, are paramount. Single-dose vials are preferable to multiple-dose vials, particularly if the vial will be used for multiple patients.
Transmission-based precautions are as described in each institution’s infection control policies. Contact precautions require the use of gowns and gloves when there is any contact with the patient or the surrounding area. Droplet precautions are invoked when infectious respiratory droplets can travel directly from the patient’s respiratory tract to the recipient, generally over short distances. This necessitates facial protection, such as surgical mask, eye protection, and/or face shield. Personal eyeglasses and contact lenses are not considered adequate eye protection.110 Historically, the area of defined risk has been a distance of 3 ft or more around the patient, although depending on the size of the droplets (smallpox and SARS are examples of small droplets), the infective area may be up to 6 ft from the patient. Droplets do not remain infectious over long periods of time and therefore do not require special air handling and ventilation. Airborne infection isolation precautions prevent transmission of infectious agents that remain infectious over long distances when suspended in the air (e.g., rubeola virus [measles], varicella virus [chicken pox], Mycobacterium tuberculosis, and possibly SARS coronavirus). If at all possible, EP procedures should be deferred in these patients unless a negative airflow procedure room is available. If an EP procedure is unavoidable, a regular positive pressure procedure room with airflow similar to an OR and a portable high-efficiency particulate air filtration unit may be used.110, 111 The patient should wear a surgical mask, and the staff should wear N95 filter masks. The procedure should be the last case of the day, and the room left empty until adequate air exchange has taken place to clear the room.
11.4.3. Laboratory Processes
Protocols and regulations as established by the institution for the disposal or processing of infectious material, drapes, and fluids should be followed. Care should be taken to avoid shaking drapes or linens, which can aerosolize contaminants. All sharps that are to be reprocessed should be placed in puncture-resistant containers, leak proof on all sides and bottom, and labeled with a biohazard label. Blood, suction material, or other contaminated liquids can be converted to a solid by the addition of a gel-forming substance to avoid risk of fluid leaking out of containers. “Clean” and “dirty” areas of the laboratory should be maintained during procedures. Personnel touching a contaminated patient/wound/area should try to avoid touching surrounding objects before removing gloves and washing hands. After procedures, all touched surfaces need to be cleaned with a hospital-approved disinfectant, leaving the surfaces wet for the amount of time as directed on the bottle. This cleaning includes monitoring cables, intravenous pumps, transfer equipment, and all nondisposable objects within 3–6 ft of a patient on droplet precautions and within 6 ft if the patient has been coughing.
11.4.4. Catheter Reprocessing
Catheter reprocessing is being adopted by many EP laboratories nationwide, which is aimed at reducing the cost of expensive single-use device and to lessen the environmental impact from discarded supplies. With the passage and enactment of the Medical Device User Fee and Modernization Act of 2002, the reprocessing of single-use devices is now supported in federal law. Physicians and the entire EP team must come to an agreement on whether reprocessing is going to positively impact their clinical operation and patient care from the financial, legal, and/or ethical point of view. Ablation catheters are classified by the FDA as class III devices; therefore, they cannot be reprocessed at this time. There are a few third-party FDA-approved reprocessors used by many EP and catheterization laboratories. In choosing a vendor for reprocessing, each team should consider not only the price of the reprocessed catheters but also the particular vendor’s turnaround time, catheter collection logistics, and reprocessing success rate.
11.4.5. Transportation
When a patient must be transported to other units or departments, the referring area should communicate the patient’s isolation needs to the receiving area before transporting the patient. All precautions should be followed throughout the transport process. Gloves and other appropriate barriers should be worn by the patient and/or health care worker during all transfers. Protective equipment is then to be removed, the hands washed, and the patient is to be transported to or from the laboratory. Patients on droplet precautions must have their mouths and/or tracheostomy covered with a surgical mask. Transporting a patient on airborne precautions should be discouraged, but if absolutely necessary, consult with the infection control officer as to specific transportation methods.
12. Ethical Concerns
Laboratory Environment Recommendations
|
12.1. Informed Consent
Obtaining an informed consent is a process whereby communication between a patient and a physician results in the patient authorizing the medical procedure. This is an integral procedure that safeguards patient autonomy and provides the patient with information about the procedure that allows them to understand and agree to the procedure being recommended. This process is not only a legal requirement but also an ethical obligation.
To ensure appropriate communication, sufficient time should be allocated to this process. A physician, APN, or PA that is part of the EP team should obtain the informed consent, and it should be made clear if trainees, PAs, or APNs will be operators. The process must take place before premedication with sedation so that the patient is able to understand the discussion and communicate a decision. The discussion should take place out of the procedure room and be in a language that the patient adequately understands, with a qualified interpreter when needed. Common risks, even if not considered serious, should be discussed, as should serious risks that are potentially life-threatening, even if exceedingly rare. All aspects of the procedure that can be reasonably anticipated should be included in the discussion.
Informed consent in the majority of pediatric cases will be granted by the parent or guardian acting on the patient’s behalf. Adult patients with CHD are similar to other adults except in cases of cognitive handicaps, as in some genetic syndromes. A minor should be invited to participate in the process, but the consent should be obtained from a legal guardian. Consent/assent forms for young patients older than 12 years are frequently used. Serum or urine pregnancy testing is generally applied for females older than 12 years within 2 weeks before the procedure.
The physician should be aware that they can have a substantial influence on their patient’s decision. As an expert, the patient looks to the physician for guidance. It is an ethical obligation for the physician to present a rational argument for undergoing the procedure. In contrast, any form of coercion with the use of verbal or nonverbal threat or manipulation through the incomplete or nontruthful presentation of information is always unethical. Patients should be given ample opportunity to discuss their concerns about the procedure and to have their questions answered satisfactorily. If the patient is incapacitated, every effort should be made to seek out and obtain consent from an appropriate surrogate.
12.2. Ethics of Teaching in the EP Laboratory Setting
Teaching invasive procedural skills is a necessary part of maintaining the availability of widespread medical expertise to patients. The use of simulators can be a helpful adjunct to teaching invasive procedures but cannot replace the experience gained from actual patient procedures.112 Although teaching is the charge of institutions with postgraduate training programs and affiliations with medical schools, the teaching of invasive procedures can occur in any type of institution.
It is the ethical duty of physicians to communicate to patients when and in what way trainees will be involved in their procedures. When asked before the procedure, the majority of patients consent to allowing trainees to practice their skills.113 Most teaching institutions have patients sign a form during the admission process that acknowledges their implicit consent to allow trainees to perform procedures under the supervision of senior physicians. Although this may be sufficient communication to patients for minor procedures such as venipuncture or acquisition of a surface ECG, in more invasive procedures it is mandatory that a separate discussion takes places during the informed consent process that clearly states the participation and the role of the trainee. Allowing a trainee to practice on a patient under general anesthesia without the patient’s explicit consent does not respect the patient’s autonomy and compromises the trainee’s moral integrity. Trainees should be invited to participate in the procedure only as an integral part of their training, not for mere curiosity. It is the responsibility of the supervising physician to allow the trainee to participate in the capacity that they are capable. The trainee should not be left performing the procedure unsupervised.
12.3. Clinical Research Studies During Clinical Procedures
Without clinical research, promising potentially lifesaving technologies and therapies cannot be safely made available for patients. Our understanding of disease processes progresses because of ongoing research. Clinical research, however, exposes patients to some risk without proven direct benefit. As a researcher, the operator’s primary responsibility is generating knowledge, whereas as a physician, the operator’s primary responsibility is the well-being of the patient. When there is a conflict between these two roles, the role of the physician must override the role of the researcher.
Patients should be considered for a research protocol if they are looking for improved outcomes compared with those of current therapies. Recruitment of patients should be carried out in a manner that does not coerce participation. Similarly, the opportunity for participation in available investigation protocols should be presented to all patients fulfilling inclusion and exclusion criteria even if the attending physician does not believe that the patient is an ideal candidate. A necessary amount of time should be given to the informed consent process in sufficient advance of the procedure so that the patient is not unduly pressured into making a decision. When children or adult patients unable to provide an informed consent are being considered for a research protocol, it may be helpful to have an independent advocate ensure that parents or legally authorized representatives are making a rational decision exposing the subject to risk.
It is not enough to obtain a patient’s consent to participate in a study. It is the ethical duty of an investigator to ensure that the research protocol does not place the participants at unreasonable risk that is disproportionate to the expected benefits of the research study. The protocol must have scientific merit with an adequate likelihood of success as well as an expectation of social benefit. All research protocols performed in the EP laboratory must be reviewed and approved by an independent group, such as an institutional review board, to ensure that the study is ethically acceptable. Results must be reported honestly, not only to honor the risk that research participants took but also to protect future recipients of the technology and therapies made available through research.
12.4. Physician-Industry Relations
Industry can partner with physicians to promote medical knowledge and improve patient care through examples such as supporting CME programs, physician training, and clinical research. Although physicians and industry may share a common goal of advancing medical care, the primary interest of the physician is the well-being of the patient whereas that of the industry is profitability. Any interaction with industry, therefore, has the potential to influence a physician’s decision regarding a company’s product or service and a physician must stay vigilant to avoid being biased by these interactions.1
Any gift or amenity that is offered has the potential to exert influence.114 Institutions and physician groups may define policies regarding the acceptance of industry gifts, but anyone approached by industry must be able to judge whether accepting the gift carries the danger of biasing a medical decision and compromising patient care. It is unethical to accept any gift that is predicated on using a particular product or service. It is imperative that the conflict of interest disclosures to employers, editors, professional organizations, and audiences be accurate and up to date. The conflict of interest policies of each physician’s institution and professional organization must be followed. Physicians who are on product review and new product introduction committees for their hospital or professional organization and who have consulting or other relationships with industry must disclose those relationships and recuse themselves from product decisions in those cases.
Footnotes
Developed in collaboration with and endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), and the Pediatric and Congenital Electrophysiology Society (PACES). Endorsed by European Heart Rhythm Society (EHRA), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE)-Latin American Society of Cardiac Pacing and Electrophysiology. EHRA endorses the recommendations of the expert consensus statement, with the exception of those statements specifically addressing US regulations.
Appendix 1
Table A1.
Writing Group Disclosure Table
| Writing group | Consultant/ advisory board | Speakers’ bureau/ honoraria | Research grant | Fellowship support | Board Mbs/stock options/partner | Others |
|---|---|---|---|---|---|---|
| Joseph G. Akar, MD, PhD, HRS | None | None | None | None | None | None |
| Janice L. Baker, MSN, CCRN, CEPS, FHRS | None | None | None | None | None | None |
| Salwa Beheiry, MSN, RN, BSN, CCRN, HRS | None | None | None | None | None | None |
| Douglas Beinborn, RN, MA, HRS | 1: Mediasphere Medical, Abiomed | None | None | None | None | None |
| John F. Beshai, MD, FHRS | 4: Lifewatch | None | None | None | 1: American College of Cardiology | None |
| Neil Brysiewicz, BSBME, MS, HRS | None | None | None | None | None | None |
| Christine Chiu-Man, MS, CEPS, CCDS, FHRS | None | None | None | None | None | 1: St. Jude Medical, Medtronic |
| Kathryn K. Collins, MD, FHRS | None | None | None | None | None | None |
| Matthew Dare, CEPS, HRS | 1: Stereotaxis, Medtronic, Biosense Webster | None | None | None | None | None |
| Kenneth Fetterly, PhD | None | None | None | None | None | None |
| John D. Fischer, MD, FHRS | 2: Medtronic | 1: Sanofi-Aventis | 1: Medtronic, Boston Scientific, St. Jude Medical, Biotronik, Sanofi-Aventis | 2: Biotronik; 3: Medtronic, Boston Scientific, St. Jude Medical | None | None |
| David E. Haines MD, FHRS | None | None | None | None | None | Equity interest; 0: nContact Surgical |
| Richard Hongo, MD, FHRS | 1: Medtronic, St. Jude Medical, Boston Scientific | 1: Boston Scientific, Medtronic, St. Jude Medical, Sanofi-Aventis | None | None | None | None |
| Samuel Irefin, MD | None | None | None | None | None | None |
| John Lopez, BS, BSN, RN, HRS | None | None | None | None | None | 1: St. Jude Medical, Boston Scientific, Medtronic, Biotronik, Biosense Webster |
| John M. Miller, MD, FHRS | 1: Biotronik, Boston Scientific, Biosense Webster, Medtronic, Stereotaxis, St. Jude Medical, Topera | None | None | 3: Medtronic; 4: Biosense Webster, Biotronik | None | None |
| James C. Perry, MD, FHRS | 1: Medtronic, U.S. Department of Justice | None | 2: Medtronic | None | None | None |
| David J. Slotwiner, MD, HRS | None | None | None | None | None | None |
| Gery F. Tomassoni, MD, FHRS, FACC | 1: St. Jude Medical, Biosense Webster, Boston Scientific, Stereotaxis, Medtronic | 1: Biosense Webster, Boston Scientific, Medtronic, Stereotaxis, Pfizer, Sanofi-Aventis; 2: St. Jude Medical | None | None | None | 1: Stereotaxis |
| Esther Weiss, RN | None | None | None | None | None | None |
0 = $0; 1 = <$10,000; 2 = >$10,000 to <$25,000; 3 = >$25,000 to <$50,000; 4 = >$50,000 to <$100,000; 5 = >$100,000.
Table A2.
Peer Reviewer Disclosure Table
| Peer reviewer | Consultant/ advisory board | Speakers’ bureau/ honoraria | Research grant | Fellowship support | Board Mbs/stock options/partner | Others |
|---|---|---|---|---|---|---|
| Alfred Buxton, MD, HRS | 1: Medtronic, Boston Scientific, St. Jude Medical | None | 0: Biosense Webster, Medtronic | 0: Biosense Webster, Medtronic, Boston Scientific | None | None |
| Anne Dubin, MD , FHRS | None | None | None | 2: Medtronic | None | None |
| Craig Swygman, CEPS, FHRS | 1: St. Jude Medical | None | None | None | None | None |
| David Benditt, MD, FHRS, CCDS | 0: Medtronic; 1: CardioNet; 2: St. Jude Medical; 3: CVRx | None | None | None | None | Equity interests; 0: Medtronic; 1: Advanced Circulatory Systems; 2: CardioNet; 3: St. Jude Medical |
| George Crossley III, MD, FHRS, CCDS | 2: Medtronic, Boston Scientific | 2: Sanofi-Aventis, Boston Scientific, Medtronic | 2: Medtronic | None | None | None |
| James Tcheng, MD | 1: American Board of Internal Medicine, Philips Medical Systems; 3: American College of Cardiology | None | 2: U.S. Food and Drug Administration, National Institutes of Health | None | None | Fiduciary role and salary position funding; 3: American College of Cardiology |
| Kenneth Ellenbogen, MD, FHRS | 1: Boston Scientific Corp, CardioNet, American Heart Association, American College of Cardiology Foundation, Cameron Health, Biotronik; 2: Medtronic, North American Center for Continuing Medical Education; 3: St. Jude Medical | None | 0: National Institutes of Health, Blue Ash Therapeutics; 2: Medtronic; 3: Biosense Webster, Boston Scientific | 3: Biosense Webster, Boston Scientific, Medtronic | None | Royalty; 0: Wiley-Blackwell; 2: Elsevier |
| Marianne Beardsall, NP, FHRS, CCDS | 1: Medtronic, St. Jude Medical | None | None | None | None | None |
| Michael Lloyd, MD, FHRS | 1: St. Jude Medical, Biotectix Corporation | None | 1: Boston Scientific, Medtronic | None | None | None |
| Noel Boyle, MD, PhD, FHRS | None | None | None | 3: Medtronic, St. Jude Medical, Boston Scientific | None | None |
| Paul Wang, MD, FHRS, CCDS | 1: Medtronic, Biosense Webster, Boston Scientific, St. Jude Medical | None | None | 2: Medtronic, Boston Scientific, Bionsense Webster, St. Jude Medical | None | Equity interests; 1: Vytronus |
| Sumeet Chugh, MD, FHRS | None | None | 5: National Institutes of Health | 2: Boston Scientific, Medtronic | None | Salary; 5: National Institutes of Health |
| Susan Etheridge, MD, FHRS, CEPS | None | None | None | None | None | Officer, 0: American College of Cardiology |
| Timm-Michael Dickfeld, MD, PhD, FHRS | 1: Biosense Webster | None | 5: GE Healthcare, Biosense Webster | None | None | None |
| Ulrika Maria Birgersdotter-Green, MD, FHRS | 0: Biotronik, St. Jude Medical, Medtronic | 0: St. Jude Medical, Medtronic | 2: St. Jude Medical, Medtronic | None | None | None |
0 = $0; 1 = <$10,000; 2 = >$10,000 to <$25,000; 3 = >$25,000 to <$50,000; 4 = >$50,000 to <$100,000; 5 = >$100,000.
References
- 1.Lindsay B.D., Asirvatham S.J., Curtis A.B. Guidance for the Heart Rhythm Society pertaining to interactions with industry endorsed by the Heart Rhythm Society on April 26, 2011. Heart Rhythm. 2011;8(7):e19–e23. doi: 10.1016/j.hrthm.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Tokuda M., Kojodjojo P.P., Epstein L.M., Koplan B.A., Michaud G.F., Tedrow U.B., Stevenson W.G., John R.M. Outcomes of cardiac perforation complicating catheter ablation of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2011;4(5):660–666. doi: 10.1161/CIRCEP.111.963413. [DOI] [PubMed] [Google Scholar]
- 3.Rickard J., Wilkoff B.L. Extraction of implantable cardiac electronic devices. Curr Cardiol Rep. 2011;13(5):407–414. doi: 10.1007/s11886-011-0198-x. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson W.G., Wilber D.J., Natale A. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118(25):2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishna G., Sprung J., Ravi B.S., Chandrasekaran K., McGoon M.D. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005;45(10):1691–1699. doi: 10.1016/j.jacc.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 6.Yokokawa M., Good E., Crawford T. Ventricular tachycardia originating from the aortic sinus cusp in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2011;8(3):357–360. doi: 10.1016/j.hrthm.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Sacher F., Roberts-Thomson K., Maury P. Epicardial ventricular tachycardia ablation: a multicenter safety study. J Am Coll Cardiol. 2010;55(21):2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 8.Wilkoff B.L., Love C.J., Byrd C.L., Bongiorni M.G., Carillo R.G., Crossley G.H., Epstein L.M., Friedman R.A., Kennergren C.E., Mitkowski P., Schaerf R.H., Wazni O.M. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm. 2009;6(7):1085–1104. doi: 10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Facilities Guidelines Institute. Guidelines for design and construction of health care facilities. http://www.fgiguidelines.org/. Published January 1, 2014. Accessed December 5, 2012.
- 10.Sehulster L, & Chin R. Guidelines for Environmental Infection Control in Health-Care Facilities. Center for Disease Control and the Healthcare Infection Control Practices Advisory Committee (HICPAC). 2003. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm Published December 5, 2011. Accessed December 5, 2012. [PubMed]
- 11.Earley MW, Sargent JS, Sheehan JV & Buss W. NEC 2008 Handbook. Quiny, MA: National Fire Protection Association; 2008
- 12.Medical Imaging & Technology Alliance. The DICOM Standard: Digital Imaging and Communications in Medicine. http://medical.nema.org/standard.html. Accessed December 5, 2012.
- 13.US Department of Health and Human Services. Health Information Privacy. http://www.hhs.gov/ocr/privacy/hipaa/understanding/special/healthit/index.html Accessed December 5, 2012.
- 14.Gupta S., Desjardins B., Baman T., Ilg K., Good E., Crawford T., Oral H., Pelosi F., Chugh A., Morady F., Bogun F., Delayed-enhanced M.R. scar imaging and intraprocedural registration into an electroanatomical mapping system in post-infarction patients. JACC Cardiovasc Imaging. 2012;5(2):207–210. doi: 10.1016/j.jcmg.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haqqani H.M., Tschabrunn C.M., Tzou W.S. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8(8):1169–1176. doi: 10.1016/j.hrthm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Strategies for Clinical and Biomedical Engineers to Maintain Readiness of External Defibrilators. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CardiovascularDevices/ExternalDefibrillators/ucm233451.htm. 2010. Published March 21, 2013. Accessed December 5, 2012.
- 17.Scheinman M., Calkins H., Gillette P., Klein R., Lerman B.B., Morady F., Saksena S., Waldo A. NASPE policy statement on catheter ablation: personnel, policy, procedures, and therapeutic recommendations. Pacing Clin Electrophysiol. 2003;26(3):789–799. doi: 10.1046/j.1460-9592.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Chanani N.K., Chiesa N.A., Dubin A.M., Avasarala K., Van Hare G.F., Collins K.K. Cryoablation for atrioventricular nodal reentrant tachycardia in young patients: predictors of recurrence. Pacing Clin Electrophysiol. 2008;31(9):1152–1159. doi: 10.1111/j.1540-8159.2008.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoury D.S., Taccardi B., Lux R.L., Ershler P.R., Rudy Y. Reconstruction of endocardial potentials and activation sequences from intracavitary probe measurements. Localization of pacing sites and effects of myocardial structure. Circulation. 1995;91(3):845–863. doi: 10.1161/01.cir.91.3.845. [DOI] [PubMed] [Google Scholar]
- 20.Banchs J.E., Patel P., Naccarelli G.V., Gonzalez M.D. Intracardiac echocardiography in complex cardiac catheter ablation procedures. J Interv Card Electrophysiol. 2010;28(3):167–184. doi: 10.1007/s10840-010-9474-8. [DOI] [PubMed] [Google Scholar]
- 21.Ren J.F., Marchlinski F.E., Callans D.J. Left atrial thrombus associated with ablation for atrial fibrillation: identification with intracardiac echocardiography. J Am Coll Cardiol. 2004;43(10):1861–1867. doi: 10.1016/j.jacc.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson J.D., Helms A., Mangrum J.M., Mahapatra S., Mason P., Bilchick K., McDaniel G., Wiggings D., DiMarco J.P. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2(6):611–619. doi: 10.1161/CIRCEP.109.872093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokokawa M., Good E., Desjardins B., Crawford T., Jongnarangsin K., Chugh A., Pelosi F., Oral H., Morady F., Bogun F. Predictors of successful catheter ablation of ventricular arrhythmias arising from the papillary muscles. Heart Rhythm. 2010;7(11):1654–1659. doi: 10.1016/j.hrthm.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartzman D., Zhong H. On the use of CartoSound for left atrial navigation. J Cardiovasc Electrophysiol. 2010;21(6):656–664. doi: 10.1111/j.1540-8167.2009.01672.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay B.D., Estes N.A., III, Maloney J.D., Reynolds D.W. Heart Rhythm Society Policy Statement Update: Recommendations on the Role of Industry Employed Allied Professionals (IEAPs) Heart Rhythm. 2008;5(11):e8–10. doi: 10.1016/j.hrthm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Bai R., Di Biase L., Valderrabano M. Worldwide experience with the robotic navigation system in catheter ablation of atrial fibrillation: methodology, efficacy and safety. J Cardiovasc Electrophysiol. 2012;23(8):820–826. doi: 10.1111/j.1540-8167.2012.02316.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan E., Frumkin W. A Novel Remote Catheter System, Demonstrates Safety and Efficacy. Heart Rhythm. 2013 (ARTICLE IN PRINT) [Google Scholar]
- 28.Faddis M.N., Chen J., Osborn J., Talcott M., Cain M.E., Lindsay B.D. Magnetic guidance system for cardiac electrophysiology: a prospective trial of safety and efficacy in humans. J Am Coll Cardiol. 2003;42(11):1952–1958. doi: 10.1016/j.jacc.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt J.D., Saliba W.I., Schweikert R.A., Cummings J., Natale A. Remote magnetic navigation to map and ablate left coronary cusp ventricular tachycardia. J Cardiovasc Electrophysiol. 2006;17(10):1142–1144. doi: 10.1111/j.1540-8167.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 30.American Board of Internal Medicine: Accreditation Coucil for Graduate Medical Education. Program Requirements for Graduate Medical Education in Clinical Cardiac Electrophysiology (Internal Medicine). http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/154_clinical_card_electrophys_int_med_07012012_1-YR.pdf. 2012. Published July 1, 2012. Accessed December 5, 2012.
- 31.American Board of Internal Medicine. Policies and Procedures for Certification. http://www.abim.org/pdf/publications/Policies-and-Procedures-Certification-August-2012.pdf. 2012. Published December 1, 2012. Accessed December 5, 2012.
- 32.Baughman K.L., Duffy F.D., Eagle K.A., Faxon D.P., Hills L.D., Lange R.A. Task force 1: training in clinical cardiology. J Am Coll Cardiol. 2008;51(3):339–348. doi: 10.1016/j.jacc.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Naccarelli G.V., Conti J.B., DiMarco J.P., Tracy C.M. Task force 6: training in specialized electrophysiology, cardiac pacing, and arrhythmia management endorsed by the Heart Rhythm Society. J Am Coll Cardiol. 2008;51(3):374–380. doi: 10.1016/j.jacc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Tracy C.M., Akhtar M., DiMarco J.P., Packer D.L., Weitz H.H., Creager M.A., Holmes D.R., Jr, Merli G., Rodgers G.P., Tracy C.M., Weitz H.H. American College of Cardiology/American Heart Association 2006 update of the clinical competence statement on invasive electrophysiology studies, catheter ablation, and cardioversion: a report of the American College of Cardiology/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training developed in collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2006;48:1503–1517. doi: 10.1016/j.jacc.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Bashore T.M., Balter S., Barac A. 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update. A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents. J Am Coll Cardiol. 2012;59(24):2221–2305. doi: 10.1016/j.jacc.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 36.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologist Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Walsh E.P., Bar-Cohen Y., Batra A.S., Dick M., II, Erickson C., Fish F., Hamilton R.M., Kanter R.J., Reed J.H., Van Hare G.F., Vetter V.L., Webster G. Recommendations for advanced fellowship training in clinical pediatric and congenital electrophysiology: a report from the training and credentialing committee of the pediatric and congenital electrophysiology society. Heart Rhythm. 2013;10:775–781. doi: 10.1016/j.hrthm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 38.Calkins H., Kuck K.H., Cappato R. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9(4):632–696 e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Aliot E.M., Stevenson W.G., Almendral-Garrote J.M. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6(6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Accreditation Council for Graduate Medical Education. Frequently Asked Questions: Clinical Cardiac Electrophysiology: Review Committee for Internal Medicine. http://www.acgme.org/acgmeweb/Portals/0/PDFs/FAQ/154_ClinicalCardiacElectrophysiology_FAQ.pdf. 2012. Published July 1, 2012. Accessed December 5, 2012.
- 41.Vetter V.L., Silka M.J., Van Hare G.F., Walsh E.P. ACCF/AHA/AAP recommendations for training in pediatric cardiology. Task force 4: recommendations for training guidelines in pediatric cardiac electrophysiology endorsed by the Heart Rhythm Society. J Am Coll Cardiol. 2005;46(7):1391–1395. doi: 10.1016/j.jacc.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Saul J.P., Epstein A.E., Silka M.J., Berul C.I., Dick M., Dimarco J.P., Friedman R.A., Rosentahl E., Stephenson E.A., Vetter V.L. Heart Rhythm Society/Pediatric and Congenital Electrophysiology Society Clinical Competency Statement: training pathways for implantation of cardioverter-defibrillators and cardiac resynchronization therapy devices in pediatric and congenital heart patients. Heart Rhythm. 2008;5(6):926–933. doi: 10.1016/j.hrthm.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Walsh E.P., Bar-Cohen Y., Batra A.S. Recommendations for Advanced Fellowship Training in Clinical Pediatric and Congenital Electrophysiology: A Report from the Training and Credentialing Committee of the Pediatric and Congenital Electrophysiology Society. Heart Rhythm. 2013;10:775–781. doi: 10.1016/j.hrthm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 44.Warnes C.A., Williams R.G., Bashore T.M. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(23):e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Nurse.com. Nursing Continuing Education Requirements by State. http://ce.nurse.com/RStateReqmnt.aspx. Published December 1, 2012. Accessed December 13, 2012.
- 46.National Council of State Boards of Nursing. Model for APRN Regulation: Licensure, Accreditation, Certification and Education. https://www.ncsbn.org/Consensus_Model_for_APRN_Regulation_July_2008.pdf. 2008. Published July 7, 2008. Accessed January 30, 2013.
- 47.Commission on Accreditation of Allied Health Education Programs. Profession Description & Certficaition Information. www.caahep.org/. 2012. Published December 1, 2012. Accessed December 7, 2012.
- 48.Mittman D.E., Cawley J.F., Fenn W.H. Physician assistants in the United States. BMJ. 2002;325(7362):485–487. doi: 10.1136/bmj.325.7362.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikhael N., Ozon P., Rhule C. Defining the Physician Assistant Role in Ontario: Ontario Physician Assistant Scope of Practice Statement and Ontario Physician Assistant Competency Profile. HealthForce Ontario. 2007 [Google Scholar]
- 50.The Joint Commission. National Patient Safety Goals: Hospital Accreditation Program. http://www.jointcommission.org/assets/1/6/NPSG_Chapter_Jan2012_HAP.pdf. 2012. Accessed December 5, 2012.
- 51.American Society of Anesthesiologists. Standards for Basic Anesthetic Monitoring. http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx. 2011.Pubblished July 1, 2011. Accessed December 5, 2012.
- 52.Sayfo S., Vakil K.P., Alqaqa׳a A., Flippin H., Bhakta D., Yadav A.V., Miller J.M., Groh W.J. A retrospective analysis of proceduralist-directed, nurse-administered propofol sedation for implantable cardioverter-defibrillator procedures. Heart Rhythm. 2012;9(3):342–346. doi: 10.1016/j.hrthm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 53.American Society of Anesthesiologists. Statement on Granting Privileges for Administration of Moderate Sedation to Practitioners Who Are Not Anesthesia Professionals. http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx. 2011. Published October 18, 2006. Accessed December 5, 2012.
- 54.Friedman R.A., Walsh E.P., Silka M.J., Calkins H., Stevenson W.G., Rhodes L.A., Deal B.J., Wolff G.S., Demaso D.R., Hanisch D., Van Hare G.F. NASPE Expert Consensus Conference: Radiofrequency catheter ablation in children with and without congenital heart disease. Report of the writing committee. North American Society of Pacing and Electrophysiology. Pacing Clin Electrophysiol. 2002;25(6):1000–1017. doi: 10.1046/j.1460-9592.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 55.Di Biase L., Conti S., Mohanty P. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm. 2011;8(3):368–372. doi: 10.1016/j.hrthm.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 56.Elkassabany N., Garcia F., Tschabrunn C., Raiten J., Gao W., Chaichana K., Dixit S., Speck R.M., Zado E., Marchlinski F., Mandel J. Anesthetic management of patients undergoing pulmonary vein isolation for treatment of atrial fibrillation using high-frequency jet ventilation. J Cardiothorac Vasc Anesth. 2012;26(3):433–438. doi: 10.1053/j.jvca.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Doppalapudi H., Yamada T., Kay G.N. Complications during catheter ablation of atrial fibrillation: identification and prevention. Heart Rhythm. 2009;6(12 Suppl):S18–S25. doi: 10.1016/j.hrthm.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 58.Perna F., Heist E.K., Danik S.B., Barrett C.D., Ruskin J.N., Mansour M. Assessment of catheter tip contact force resulting in cardiac perforation in swine atria using force sensing technology. Circ Arrhythm Electrophysiol. 2011 Apr;4(2):218–224. doi: 10.1161/CIRCEP.110.959429. [DOI] [PubMed] [Google Scholar]
- 59.Latchamsetty R., Oral H. Ablation of atrial fibrillation using an irrigated-tip catheter: open or closed? Pacing Clin Electrophysiol. 2012;35(5):503–505. doi: 10.1111/j.1540-8159.2012.03333.x. [DOI] [PubMed] [Google Scholar]
- 60.Andrade J.G., Khairy P., Guerra P.G., Deyell M.W., Rivard L., Macle L., Thbault B., Talajic M., Roy D., Dubuc M. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8(9):1444–1451. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 61.Venkatachalam K.L., Herbrandson J.E., Asirvatham S.J. Signals and signal processing for the electrophysiologist: part I: electrogram acquisition. Circ Arrhythm Electrophysiol. 2011;4(6):965–973. doi: 10.1161/CIRCEP.111.964304. [DOI] [PubMed] [Google Scholar]
- 62.Venkatachalam K.L., Herbrandson J.E., Asirvatham S.J. Signals and signal processing for the electrophysiologist: part II: signal processing and artifact. Circ Arrhythm Electrophysiol. 2011;4(6):974–981. doi: 10.1161/CIRCEP.111.964973. [DOI] [PubMed] [Google Scholar]
- 63.Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250–278. doi: 10.1086/501620. (quiz 279-280) [DOI] [PubMed] [Google Scholar]
- 64.Bashore T.M., Bates E.R., Berger P.B. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(8):2170–2214. doi: 10.1016/s0735-1097(01)01346-8. [DOI] [PubMed] [Google Scholar]
- 65.Malloy P.C., Grassi C.J., Kundu S. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20(7 Suppl):S240–S249. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 66.Cano O., Munoz B., Tejada D., Osca J., Sancho-Tello M.J., Olague J., Castro J.E., Salvador A. Evaluation of a new standardized protocol for the perioperative management of chronically anticoagulated patients receiving implantable cardiac arrhythmia devices. Heart Rhythm. 2012;9(3):361–367. doi: 10.1016/j.hrthm.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Haines D.E., Wang Y., Curtis J. Implantable cardioverter-defibrillator registry risk score models for acute procedural complications or death after implantable cardioverter-defibrillator implantation. Circulation. 2011;123(19):2069–2076. doi: 10.1161/CIRCULATIONAHA.110.959676. [DOI] [PubMed] [Google Scholar]
- 68.Haeusler K.G., Kirchhof P., Endres M. Left atrial catheter ablation and ischemic stroke. Stroke. 2012;43(1):265–270. doi: 10.1161/STROKEAHA.111.627067. [DOI] [PubMed] [Google Scholar]
- 69.Scaltriti S., Cencetti S., Rovesti S., Marchesi I., Bargellini A., Borella P. Risk factors for particulate and microbial contamination of air in operating theatres. J Hosp Infect. 2007;66(4):320–326. doi: 10.1016/j.jhin.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Baddour L.M., Epstein A.E., Erickson C.C. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 71.Movahed M.R., Kasravi B., Bryan C.S. Prophylactic use of vancomycin in adult cardiology and cardiac surgery. J Cardiovasc Pharmacol Ther. 2004;9:13–20. doi: 10.1177/107424840400900i103. [DOI] [PubMed] [Google Scholar]
- 72.Perry J.C. Sudden cardiac death and malignant arrhythmias: the scope of the problem in adult congenital heart patients. Pediatr Cardiol. 2012;33(3):484–490. doi: 10.1007/s00246-012-0171-5. [DOI] [PubMed] [Google Scholar]
- 73.Saul J.P., Hulse J.E., Papagiannis J., Van Praagh R., Walsh E.P. Late enlargement of radiofrequency lesions in infant lambs. Implications for ablation procedures in small children. Circulation. 1994;90(1):492–499. doi: 10.1161/01.cir.90.1.492. [DOI] [PubMed] [Google Scholar]
- 74.Dubin A.M., Van Hare G.F. Radiofrequency catheter ablation: indications and complications. Pediatr Cardiol. 2000;21(6):551–556. doi: 10.1007/s002460010133. [DOI] [PubMed] [Google Scholar]
- 75.Cohen M.I., Triedman J.K., Cannon B.C. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS) Heart Rhythm. 2012;9(6):1006–1024. doi: 10.1016/j.hrthm.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 76.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 77.Janousek J., Gebauer R.A. Cardiac resynchronization therapy in pediatric and congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S21–S23. doi: 10.1111/j.1540-8159.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 78.Cecchin F., Frangini P.A., Brown D.W., Fynn-Thompson F., Alexander M.E., Triedman J.K., Gauvreau K., Walsh E.P., Berul C.I. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: five years experience in a single institution. J Cardiovasc Electrophysiol. 2009;20(1):58–65. doi: 10.1111/j.1540-8167.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 79.Kugler J.D., Danford D.A., Houston K.A., Felix G. Pediatric radiofrequency catheter ablation registry success, fluoroscopy time, and complication rate for supraventricular tachycardia: comparison of early and recent eras. J Cardiovasc Electrophysiol. 2002;13:336–341. doi: 10.1046/j.1540-8167.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 80.Blaufox A.D., Felix G., Saul J.P. Radiofrequency catheter ablation in infants < 18 months old: when is it done and how do they fare? Short-term data from the Pediatric Ablation Registry. Circulation. 2001;104:2803–2808. doi: 10.1161/hc4801.100028. [DOI] [PubMed] [Google Scholar]
- 81.Pecht B., Maginot K.R., Boramanand N.K., Perry J.C. Techniques to avoid atrioventricular block during radiofrequency catheter ablation of septal tachycardia substrates in young patients. J Interv Card Electrophysiol. 2002 Aug;7(1):83–88. doi: 10.1023/a:1020828401929. [DOI] [PubMed] [Google Scholar]
- 82.Chanani N.K., Chiesa N.A., Dubin A.M., Avasarala K., Van Hare G.F., Collins K.K. Cryoablation for atrioventricular nodal reentrant tachycardia in young patients: predictors of recurrence. Pacing Clin Electrophysiol. 2008;31:1152–1159. doi: 10.1111/j.1540-8159.2008.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buxton A.E., Calkins H., Callans D.J. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology) J Am Coll Cardiol. 2006;48(11):2360–2396. doi: 10.1016/j.jacc.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Collins K.K., Schaffer M.S. Use of cryoablation for treatment of tachyarrhythmias in 2010: survey of current practices of pediatric electrophysiologists. Pacing Clin Electrophysiol. 2011;34(3):304–308. doi: 10.1111/j.1540-8159.2010.02953.x. [DOI] [PubMed] [Google Scholar]
- 85.Van Hare G.F., Colan S.D., Javitz H., Carmelli D., Knilans T., Schaffer M., Kugler J., Byrum C.J., Saul J.P. Prospective assessment after pediatric cardiac ablation: fate of intracardiac structure and function, as assessed by serial echocardiography. Am Heart J. 2007;153(5):815–820. doi: 10.1016/j.ahj.2007.02.009. (820 e1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirshfeld J.W., Jr, Balter S., Brinker J.A. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures. A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2004;44(11):2259–2282. doi: 10.1016/j.jacc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 87.Vano E., Rosenstein M., Liniecki J., Rehani M.M., Martin C.J., Vetter R.J. ICRP Publication 113. Education and training in radiological protection for diagnostic and interventional procedures. Ann ICRP. 2009;39(5):7–68. doi: 10.1016/j.icrp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Glazier J.J., Dixon S.R. Skin injury following prolonged fluoroscopy: early and late appearances. QJM. 2012;105(6):571–573. doi: 10.1093/qjmed/hcr089. [DOI] [PubMed] [Google Scholar]
- 89.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2 2006 [PubMed]
- 90.Rosenthal L.S., Mahesh M., Beck T.J. Predictors of fluoroscopy time and estimated radiation exposure during radiofrequency catheter ablation procedures. Am J Cardiol. 1998;82(4):451–458. doi: 10.1016/s0002-9149(98)00356-7. [DOI] [PubMed] [Google Scholar]
- 91.Calkins H., Niklason L., Sousa J., el-Atassi R., Langberg J., Morady F. Radiation exposure during radiofrequency catheter ablation of accessory atrioventricular connections. Circulation. 1991;84(6):2376–2382. doi: 10.1161/01.cir.84.6.2376. [DOI] [PubMed] [Google Scholar]
- 92.Einstein A.J., Moser K.W., Thompson R.C. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116(11):1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 93.United States Nuclear Regulatory Commission. NRC: Annual Dose Limits for Radiation Workers. http://www.nrc.gov/reading-rm/doc-collections/nuregs/staff/sr1556/v6/fig012.html. 2012. Published June 28, 2013. Accessed December 5, 2012.
- 94.Gerber T.C., Carr J.J., Arai A.E. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119(7):1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 95.Miller D.L., Balter S., Dixon R.G., Nikoli B., Bartal G., Cardell J.F., Dauer L.T., Stecker M.S. Quality improvement guidelines for recording patient radiation dose in the medical record for fluoroscopically guided procedures. J Vasc Interv Radiol. 2012;23(1):11–18. doi: 10.1016/j.jvir.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Germano J.J., Day G., Gregorious D., Natarajan V., Cohen T. A novel radiation protection drape reduces radiation exposure during fluoroscopy-guided electrophysiology procedures. J Invasive Cardiol. 2005;17:469–472. [PubMed] [Google Scholar]
- 97.U.S. Food and Drug Administration. CFR-Code of Federal Regualtions Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1020.32. 2012. Published April 1, 2013. Accessed December 14, 2012.
- 98.Joint Commission on Accreditation of Healthcare Organizations. Accreditation Manual for Hospitals Update 3. Oakbrook Terrace, IL; 2002.
- 99.Birnie D., Healey J.S., Krahn A.D. Prevalence and risk factors for cervical and lumbar spondylosis in interventional electrophysiologists. J Cardiovasc Electrophysiol. 2011;22(9):957–960. doi: 10.1111/j.1540-8167.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 100.von Boetticher H., Lachmund J., Hoffmann W. Cardiac catheterization: impact of face and neck shielding on new estimates of effective dose. Health Phys. 2009;97(6):622–627. doi: 10.1097/01.HP.0000363843.01041.99. [DOI] [PubMed] [Google Scholar]
- 101.Jacob S., Boveda S., Bar O., Brezin A., Maccia C., Laurier D., Bernier M.O. Interventional cardiologists and risk of radiation-induced cataract: results of a French multicenter observational study. Int J Cardiol. 2013;167(5):1843–1847. doi: 10.1016/j.ijcard.2012.04.124. [DOI] [PubMed] [Google Scholar]
- 102.Ross A.M., Segal J., Borenstein D., Jenkins E., Cho S. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol. 1997;79(1):68–70. doi: 10.1016/s0002-9149(96)00678-9. [DOI] [PubMed] [Google Scholar]
- 103.Dragusin O., Weerasooriya R., Jais P., Hocini M., Ector J., Takahashi Y., Haissaguerre M., Bosmans H., Heidbuchel H. Evaluation of a radiation protection cabin for invasive electrophysiological procedures. Eur Heart J. 2007;28(2):183–189. doi: 10.1093/eurheartj/ehl420. [DOI] [PubMed] [Google Scholar]
- 104.Di Biase L., Wang Y., Horton R. Ablation of atrial fibrillation utilizing robotic catheter navigation in comparison to manual navigation and ablation: single-center experience. J Cardiovasc Electrophysiol. 2009;20(12):1328–1335. doi: 10.1111/j.1540-8167.2009.01570.x. [DOI] [PubMed] [Google Scholar]
- 105.Pappone C., Vicedomini G., Manguso F. Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol. 2006;47(7):1390–1400. doi: 10.1016/j.jacc.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein J.A., Balter S., Cowley M., Hodgson J., Klein L.W. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63(4):407–411. doi: 10.1002/ccd.20201. [DOI] [PubMed] [Google Scholar]
- 107.Klein L.W., Miller D.L., Balter S., Laskey W., Haines D., Norbash A., Mauro M.A., Goldstein J.A. Occupational health hazards in the interventional laboratory: time for a safer environment. Heart Rhythm. 2009;6(3):439–444. doi: 10.1016/j.hrthm.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 108.Weber D.J., Rutala W.A., Schaffner W. Lessons learned: protection of healthcare workers from infectious disease risks. Crit Care Med. 2010;38(8 Suppl):S306–S314. doi: 10.1097/CCM.0b013e3181e69ebd. [DOI] [PubMed] [Google Scholar]
- 109.Siegel J.D., Rhinehart E., Jackson M. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35(10 Suppl 2) doi: 10.1016/j.ajic.2007.10.007. (S65-164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.World Health Organization. Glove Use Information Leaflet. http://www.who.int/gpsc/5may/Glove_Use_Information_Leaflet.pdf. 2009. Published August 1, 2009. Accessed December 5, 2012.
- 111.Ninomura P., Bartley J. New Ventilation Guidelines for Health-Care Facilities. American Society of Heating, Refrigerating and Air Conditioning Engineers Journal. 2001:29–33. [Google Scholar]
- 112.Engum S.A., Jeffries P., Fisher L. Intravenous catheter training system: computer-based education versus traditional learning methods. Am J Surg. 2003;186(1):67–74. doi: 10.1016/s0002-9610(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 113.McNamara R.M., Monti S., Kelly J.J. Requesting consent for an invasive procedure in newly deceased adults. JAMA. 1995;273(4):310–312. [PubMed] [Google Scholar]
- 114.Chren M.M., Landefeld C.S. Physicians’ behavior and their interactions with drug companies. A controlled study of physicians who requested additions to a hospital drug formulary. JAMA. 1994;271(9):684–689. [PubMed] [Google Scholar]