Abstract
Objectives
To describe the clinical-radiological-pathological characteristics and treatment outcomes of children with suspected exogenous lipoid pneumonia (ELP).
Design
Systematic review. We searched electronic databases and reference lists published between 1967 and 2018, restricted to non-accidental cases.
Results
Forty-four studies including 489 participants aged 1 day to 17 years from 13 countries were included. Cultural, medical, and behavioural rationale for oil-use was described. The clinical-radiological presentation varied widely. Diagnostic certainty was deemed highest if ELP was confirmed on bronchoalveolar lavage/frozen section lung biopsy with documented extracellular lipid on cytological staining and/or fat analysis. Non-tuberculous mycobacteria infection was identified in six studies: Mycobacterium fortuitum/chelonei, Mycobacterium smegmatis and Mycobacterium abscessus. Treatment comprised supportive therapy, corticosteroids, stopping oil, therapeutic lung-lavage and surgical resection. Outcomes were reported inconsistently.
Conclusion
Paediatric ELP resulting from cultural and medical practices continues to be described globally. Preventive interventions, standardized reporting, and treatment efficacy studies for cases not averted, are lacking.
Protocol registration: PROSPERO CRD42017068313.
Abbreviations: ALD, adrenoleukodystrophy; BAL, bronchoalveolar lavage; CT, computed tomography; ELP, exogenous lipoid pneumonia; GOR, gastro-oesophageal reflux; ILD, interstitial lung disease; MRI, magnetic resonance imaging; NTM, non-tuberculous mycobacteria; ORO, Oil-Red-O; PAS, periodic acid schiff
Keywords: Children’s interstitial lung disease (chILD), Global Health, Oil
Introduction
Exogenous lipoid pneumonia (ELP) is a disorder caused by inhalation or aspiration of mineral, plant-based or animal-based oils and is generally uncommon [1], [2]. ELP related to the use of nonvolatile oils in children has been reported in the literature in several parts of the world. One of the earliest articles published on this condition was based on autopsy findings in Canadian children who developed pneumonia following nasopharyngeal injections of oil in hospital [3]. Subsequently, literature on ELP related to medical use of oil-based products in children as well as the use of various folk remedies involving nasal or oral administration of these oils in children and related cultural practices have also been documented [1], [4], [5].
In addition to a history of oil ingestion or aspiration, children with ELP have been reported to present with non-specific clinical and radiologic findings and variable treatment outcomes [1], [2]. Expert reviews provide a consensus approach of discontinuing oil, treating infections and identifying underlying risk factors [1] however to the best of our knowledge, no formal systematic reviews and meta-analyses have been conducted. Systematically reporting on the global context of non-accidental ELP in children regarding clinical-radiological-pathological characteristics and treatment outcomes may provide a holistic perspective with a robust evidence base for management of these children and identify areas for further research.
Materials and methods
The systematic review methodology was registered on PROSPERO, an international prospective register for systematic reviews as CRD42017068313.
Eligibility criteria
We included studies conducted in any context globally involving children less than 18 years old suspected to have ELP. Exogenous lipoid pneumonia was suspected under the following conditions: (1) history of oil administration related to time of presentation/diagnosis, (2) clinical presentation of persistent or recurrent unexplained pneumonia associated with hypoxia or tachypnea, (3) radiological evidence of persistent diffuse alveolar infiltrates on chest radiograph or computed tomography (CT) chest, and/or (4) cytohistological findings on bronchoalveolar lavage (BAL) or lung biopsy that were consistent with exogenous lipid content. Two levels of case definitions were applied in this systematic review: (a) suspected ELP, for cases in which a cytohistological diagnosis was not available/documented; and (b) confirmed ELP, for cases that included cytohistological findings. Eligible study designs included retrospective or prospective: (a) analytic studies (cross sectional, case-control, cohort or intervention studies), (b) descriptive studies (case reports, case series, cross sectional studies), and (c) qualitative studies. We included studies that had adults and children if the data for adults could be separated and excluded. Letters, editorials, commentaries, conference abstracts, all types of reviews, meta-analyses, non-human studies and non-English studies were excluded.
Information sources, search and study selection
We employed a multi-concept Boolean search strategy based on the Population, Intervention, Control, Outcome, Timing and Setting (PICOTS) framework [6]. This search strategy used keywords related to ELP in children and was restricted to English publications published within the last 50 years. This period was arbitrarily deemed to represent data that were currently relevant. The first author systematically searched Pubmed, EMBASE, Web of Science, SCOPUS and CINAHL Complete to identify studies describing ELP in children published from 1967 to 2018. The Pubmed search strategy used was: (“child”[MeSH Terms] OR Child[tw]) OR (“infant”[MeSH Terms] OR infant[tw]) AND (“pneumonia, lipid”[MeSH Terms] OR (“pneumonia”[All Fields] AND “lipid”[All Fields]) OR “lipid pneumonia”[All Fields] OR (“exogenous”[All Fields] AND “lipoid”[All Fields] AND “pneumonia”[All Fields]) OR “exogenous lipoid pneumonia”[All Fields]) AND (1967:2018[dp]) AND “English”[la]. Thereafter the primary reviewer screened abstracts for eligibility, retrieved full texts to confirm eligibility and extracted data for quantitative synthesis.
Data collection process
Using a standardized tool, the first author independently extracted data from eligible articles and consulted senior reviewers during this process whenever required. Broad characteristics under which data were extracted included: clinical-radiological characteristics and treatment outcomes in children with suspected ELP. Specific information recorded from each included study and details on study quality assessment are provided as Supplementary material (Appendix A).
Synthesis of results and analyses
A narrative summary for the studies eligible for this systematic review is provided classified by diagnostic certainty and restricted to non-accidental etiologies. Given that the predominant designs of the studies included were case series and case reports, summary measures could not be pooled nor could quantitative data be consolidated in a metanalysis.
Results
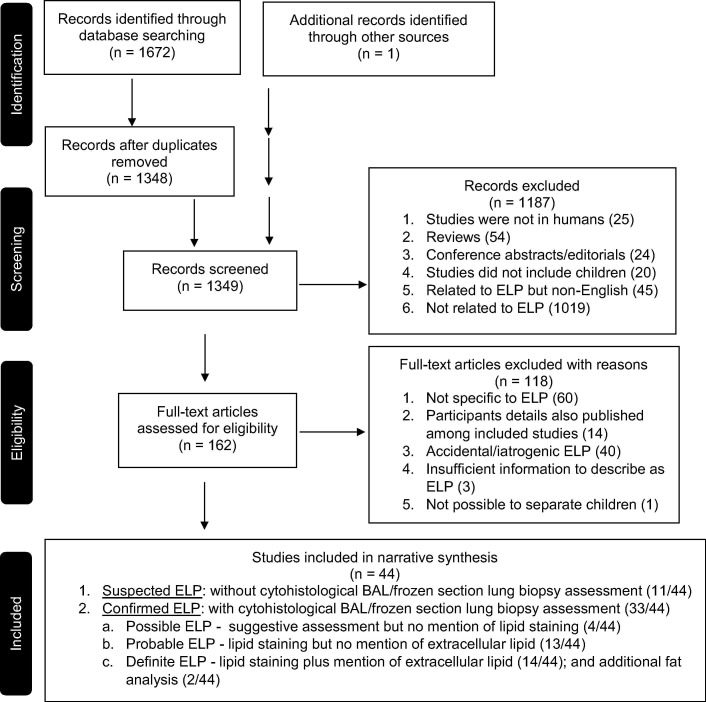
We identified 1672 articles through the electronic database search and one additional record through hand search of references in the current literature. Duplicates were excluded and of the remaining 1349 titles and abstracts, 162 were eligible for full text assessment. Of these, 44 studies were included for qualitative synthesis as depicted in the PRISMA flow chart (Fig. 1 ).
Fig. 1.
PRISMA flow chart.
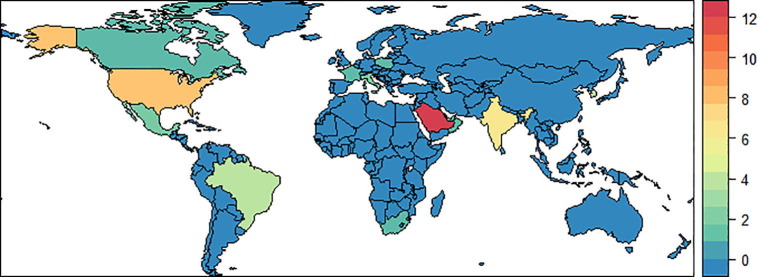
Eligible studies were conducted between 1st January 1967 and 12th August 2018 and included 489 participants from 13 countries: 24 studies from Asia, 15 from the Americas, 4 from Europe and 1 from Africa. Participants’ age at presentation ranged from 1 day to 17 years. Of the 44 studies, 22 were case reports, 18 were case series, three cross-sectional studies and one cohort study. Thirty-three of the 44 included studies documented cytohistological assessment of bronchioalveolar lavage and/or lung biopsy (Fig. 2 and Appendix B).
Fig. 2.
Global map depicting the number of studies published on non-accidental paediatric exogenous lipoid pneumonia in the English literature.
Diagnostic certainty of ELP
We further characterized studies based on their diagnostic certainty of ELP: (1) suspected ELP without BAL/frozen section lung biopsy assessment (11/44); (2) possible ELP with suggestive BAL/frozen section lung biopsy assessment but no mention of lipid staining (4/44); (3) probable ELP with lipid staining on BAL/frozen section lung biopsy cytohistology but no mention of extracellular lipid (13/44); and (4) definite ELP with lipid staining on BAL/frozen section lung biopsy cytohistology plus mention of extracellular lipid (14/44)/ and additional fat analysis (chromatography) of BAL specimen (2/44) (Fig. 1 and Appendix B).
Oil use and rationale
Mineral, animal-based, plant-based and combinations of oils were reported in the studies included in this systematic review. These oils were used for three main reasons: (1) cultural (2) medical or (3) behavioural. Ghee (rendered animal fat) and olive oil were commonly documented in studies from Saudi Arabia and India for which they were used culturally for oral/nasal cleansing purposes in oil baths, as a laxative, for nutritional supplements, to prevent coughs and colds and to generally promote good health [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Shark liver oil was mainly reported in South Korea for cultural reasons [23]. Plant-based cooking oil or mineral oil was given to infants to aid in digestion and prevent constipation for cultural reasons in South Africa [24]. In Brazil, mineral oils were predominantly used for medical reasons as a laxative in the treatment of chronic constipation and partial intestinal obstruction resulting from ascariasis [25], [26], [27], [28]. In North America, mineral oil was predominantly given to children as a laxative in the treatment of chronic constipation [29], [30], [31], [32], [33], with few studies reporting on immigrants from Guatemala and Mexico using olive oil for cultural reasons to help with colic, digestion and nasal congestion [34], [35]. Infrequent rationale for oil use in children at risk of aspiration due to their neurological conditions. included the ketogenic diet for the medical management of intractable seizures [36], [37], and the use of Lorenzo's Oil which is extracted from rapeseed oil and olive oil in the management of adrenoleukodystrophy (ALD) [38]. One study reported oil ingestion in a child with a behavioural disorder who drank large amounts of oil when frustrated [39]. Regarding duration of oil use, majority of the studies reported on prolonged/repeated administration. Few studies reported on acute oil use, and some did not provide this information. The amount and frequency varied widely within and across studies, ranging from 2.5 ml to 20 ml, given once to thrice daily, in documented cases (Appendix B).
Clinical presentation
Children with ELP presented with varied respiratory symptoms and signs including cough, chest pain, tachypnea, reduced breath sounds, crepitations, hypoxia and clubbing. In addition, some studies had well described risk factors or comorbidities, whereas others did not. These risk factors and co-morbidities included gastro-oesophageal reflux, aspiration syndromes; force-feeding, choking, feeding in the recumbent position, infancy; neurological conditions including cerebral palsy, intractable seizures and ALD; gastrointestinal conditions such as chronic constipation, partial intestinal obstruction, Hirschsprung’s disease, colic, and failure to thrive/malnutrition; and nasal congestion (Appendix B).
Microbiology
Of the 44 studies, 25 reported co-infections. Six studies identified non-tuberculous mycobacteria (NTM) co-infection cultured from blood or respiratory tract secretions. These included Mycobacterium fortuitum [10], [40], [41], M. fortuitum/chelonei [42], Mycobacterium smegmatis [31] and Mycobacterium abscessus complex [24]. Other infections described in the selected studies included Branhamella catarrhalis [35]; Pseudomonas, Acinetobacter, Klebsiella [20]; respiratory viruses including Adenovirus, Human Metapneumovirus, Human Bocavirus, Human Rhinovirus, Parainfluenza, Respiratory Syncytial virus A and B, Coronavirus and various bacteria and fungi [24]; Chlamydia on serology [39]; and Enterococcus, Escherichia coli, Klebsiella, coagulase positive Staphylococcus and Clostridium from peritoneal exudate [29] (Appendix C).
Radiological imaging
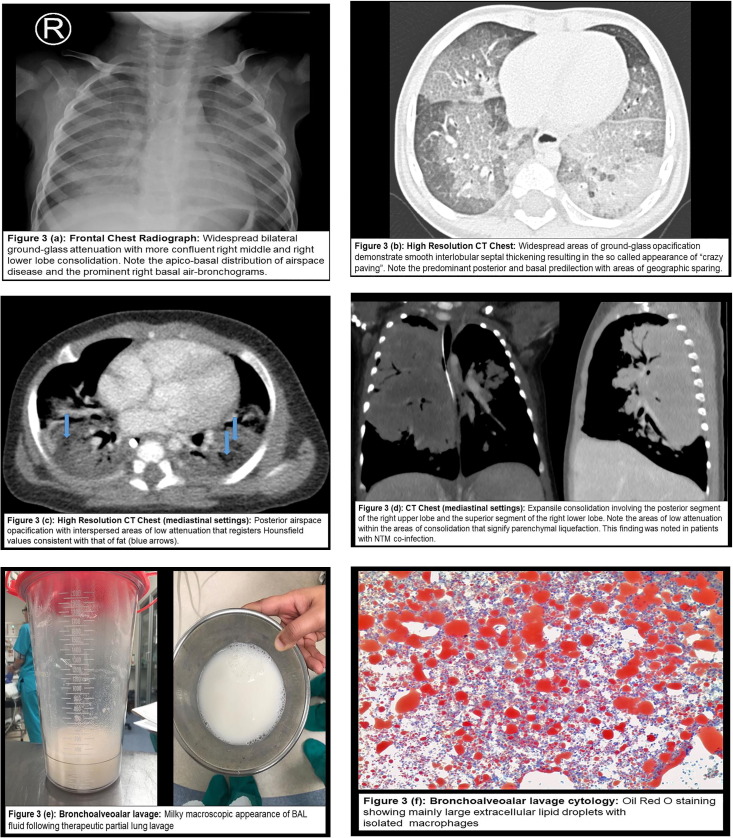
Plain chest radiography was the most common imaging modality described and was reported in 41 of the 44 included studies. The most frequent radiological patterns on plain chest radiography was airspace/ground glass opacification in various lobes. A few studies reported expansile pneumonia in the right upper lobe [7], [17], [19], [24] and features suggestive of bronchiectasis/chronic lung changes [15], [22]. Chest CT was documented in 26 studies. Diffuse airspace/ground glass opacities were noted in most studies with basal/lower lobe predominance. Less frequent findings on CT were fatty attenuation [23], [24], [26], [27], [33], [35], [39], [43], [44], [45], interstitial septal thickening/crazy-paving appearance [23], [24], [26], [27], [35], [38], [46], expansile pneumonia [7], [19], [24] and nodules [26]. Magnetic resonance imaging (MRI) of the chest was performed in one study that utilized the fat saturation technique and showed fat depressed signal in the middle of the lobe not identified on prior chest CTs [19] (Fig. 3 and Appendix C).
Fig. 3.
Examples of radio-pathological characteristics of paediatric exogenous lipoid pneumonia from an African Case Series [24].
Treatment and clinical-radiological outcomes
Treatment reported in most studies comprised supportive therapy which included antibiotics, oxygen and assisted ventilation. Corticosteroid use was reported in 20 studies and administered by different routes; intravenous dexamethasone at 0.3 mg/kg was given for two weeks [45], prednisone was administered at 1–2 mg/kg/day from 2 weeks to 14 months [10], [18], [19], [24], [28], [31], [47], and inhaled beclomethasone was given at 250 mcg 12 hourly for 6 months [47]. Therapeutic partial or whole lung lavage [12], [24], [25], [27], [39], [43], [46] and surgical resection were additional treatment modalities reported in children with ELP [7], [10], [11], [22], [42], [44].
There were no cohort or randomized control studies conducted to determine the efficacy of the various therapeutic modalities used. The descriptive data tended to suggest that corticosteroids and therapeutic partial/whole lung lavage seemed to be associated with shorter time to clinical resolution; whereas surgical lung resection may be associated with more complications including death. In one study the number of activated T lymphocytes and alveolar macrophages was monitored in the BAL to assess treatment outcome, whereby over the 6, 12, and 18 month follow-up period after stopping oil, the macrophage numbers increased and lymphocyte numbers decreased, specifically those in the activated state [46]. Overall, clinical resolution was reported with discontinuation of oil administration and other interventions. Radiological resolution, however, was significantly delayed in comparison to clinical disease. Mortality was reported inconsistently (Appendix C).
Discussion
In the past half-century, paediatric ELP resulting from cultural, medical and behavioural practices has continued to be described in the literature globally, despite being a potentially preventable condition. However, the trend in reporting studies of ELP in children has been decreasing over the last five decades, with the highest peak of published articles noted between 1987 and 2006. Increased awareness of the complications of these oil practices may explain this decline over time. Notably, paediatric ELP from Africa has been described for the first time in the literature only recently [24]. Besides cultural practices, the medical use of Lorenzo’s oil and ketogenic diets in children with severe neurological conditions should be recognized as a possible albeit rare cause of ELP [36], [37], [38]. There is a dearth of studies reporting on interventions geared towards averting paediatric ELP. Designing effective behaviour change prevention strategies that are theoretically sound, contextually relevant and potentially translatable to similar global settings is imperative.
The type, amount, frequency and duration of oil use documented in the selected studies varied widely; and may partly explain the heterogeneous clinical-radiological pattern of paediatric ELP that makes it indistinguishable from other causes of persistent pneumonia or diffuse lung disease in children. NTM disease in patients with ELP is an important comorbidity that is postulated to be favored by the lipophilic environment, and is a finding that is consistent with previous experimental studies [48] and literature in adults [49], [50]. Supportive management particularly antibiotic treatment was the most common treatment offered to children with ELP. Although qualitative findings from this systematic review seem to suggest that steroids and lavage may improve time to clinical resolution, surgery could be associated with many complications including death, and radiologic resolution may be delayed in comparison to clinical resolution, prospective studies to assess the efficacy of treatment interventions in paediatric ELP are needed.
Furthermore, the definitions of paediatric ELP are too heterogenous to make comparisons across studies. We therefore propose a scale of diagnostic certainty comprising four levels: suspected, probable, possible, and definite on cytological assessment/and fat analysis. This scale is envisaged to provide standardized case definitions in reporting new cases and would facilitate research across studies of optimal management strategies and outcomes. Diagnostic certainty levels have been proposed for randomized control trials in other conditions in children such as wheezing, where standardized definitions are lacking [51]. Additionally, standardized reporting could be adapted from international registries such as the international management platform for ChILD [52] or other global registries. Preferably data collection should be prospective to prevent pitfalls of bias and missing data.
The main limitations of this systematic review were the studies included comprised case reports, case series and few cross-sectional studies making it difficult to pool data; exclusion of non-English articles; and a single reviewer. Studies reviewed were highly biased with respect to patient selection due to the nature of their design. Notwithstanding these limitations, we believe that our study provides a current and robust perspective on ELP in children resulting from non-accidental aetiologies.
Conclusion
This systematic review highlights that paediatric ELP is uncommon but may be easily overlooked in societies where oil administration to infants and children is a common cultural practice. Although clinical-radiological patterns vary widely making them non-specific to diagnosing paediatric ELP, health-workers should not forget that ELP is an important cause of ILD in children with persistent pneumonia. Prevention strategies geared at effective behaviour change; and standardized reporting and treatment efficacy studies for cases that are not averted, are needed.
Directions for future research
Behaviour change interventions to avert paediatric ELP are imperative. These preventive strategies should be theoretically sound, contextually-relevant and globally translatable. Additionally, further understanding of the association between NTMs and ELP is needed, and treatment trials are lacking.
Funding
Diana Marangu is a recipient of the African Paediatric Fellowship Program scholarship and the Margaret McNamara Education Grant for Africa 2017.
Educational aims
-
•
To recognize that exogenous lipoid pneumonia (ELP) in children resulting from cultural and medical practices is a rare albeit preventable interstitial lung disease.
-
•
To review the clinical-radiological patterns of paediatric ELP which vary widely making this diagnosis indistinguishable from other causes of persistent pneumonia.
-
•
To describe the association of non-tuberculous mycobacteria (NTM) infections and paediatric ELP resulting in significant comorbidity.
-
•
To rank studies on ELP in children in the existing literature based on diagnostic certainty and propose standardized case definitions and consistent reporting of treatment outcomes and efficacy.
Acknowledgements
We wish to thank Professor Komala Pillay and Dr. Ebrahim Banderker for collecting and reporting on samples and images cited from the African case series.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.prrv.2019.01.001.
Appendix A. Supplementary data
References
- 1.Hadda V., Khilnani G.C. Lipoid pneumonia: an overview. Expert Rev Respir Med. 2010;4(6):799–807. doi: 10.1586/ers.10.74. [DOI] [PubMed] [Google Scholar]
- 2.Betancourt S.L., Martinez-Jimenez S., Rossi S.E., Truong M.T., Carrillo J., Erasmus J.J. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010;194(1):103–109. doi: 10.2214/AJR.09.3040. [DOI] [PubMed] [Google Scholar]
- 3.Laughlen G.F. Studies on pneumonia following naso-pharyngeal injections of oil. Am J Pathol. 1925;1(4) 407–14.1. [PMC free article] [PubMed] [Google Scholar]
- 4.Pinkerton H. Oils and fats: their entrance into and fate in the lungs of infants and children: a clinical and pathologic report. Am J Dis Child. 1927;33(2):259–285. [Google Scholar]
- 5.Ikeda K. Oil aspiration pneumonia (lipoid pneumonia): clinical, pathologic and experimental consideration. Am J Dis Child. 1935;49(4):985–1006. [Google Scholar]
- 6.Samson D., Schoelles K.M. AHRQ methods for effective health care developing the topic and structuring systematic reviews of medical tests: utility of PICOTS, analytic frameworks, decision trees, and other frameworks. In: Chang S.M., Matchar D.B., Smetana G.W., Umscheid C.A., editors. Methods Guide for Medical Test Reviews. Agency for Healthcare Research and Quality (US); Rockville (MD): 2012. [PubMed] [Google Scholar]
- 7.Bakshi S., Bhakoo O.N., Singh S., Bannerjee A.K. Lipoid pneumonia. (A case report) Indian Pediatr. 1971;8(11):793–795. [PubMed] [Google Scholar]
- 8.Balakrishnan S. Lipoid pneumonia in infants and children in South India. Br Med J. 1973;4(5888):329–331. doi: 10.1136/bmj.4.5888.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riff E.J., Moore C., Tufenkeji H., Harfi H. Infantile lipoid pneumonia. Ann Saudi Med. 1990;10(4):378–382. [Google Scholar]
- 10.Annobil S.H., Benjamin B., Kameswaran M., Khan A.R. Lipoid pneumonia in children following aspiration of animal fat (ghee) Ann Trop Paediatr. 1991;11(1):87–94. doi: 10.1080/02724936.1991.11747483. [DOI] [PubMed] [Google Scholar]
- 11.Hugosson C.O., Riff E.J., Moore C.C.M., Akhtar M., Tufenkeji H.T. Lipoid pneumonia in infants: a radiological-pathological study. Pediatr Radiol. 1991;21(3):193–197. doi: 10.1007/BF02011045. [DOI] [PubMed] [Google Scholar]
- 12.Kameswaran M., Annobil S.H., Benjamin B., Salim M. Bronchoscopy in lipoid pneumonia. Arch Dis Child. 1992;67(11):1376–1377. doi: 10.1136/adc.67.11.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta M., Chowdhury M.S.A. A common practice of traditional medication with oil and/or ghee, as folk medicine in children of Southern Saudi Arabia. Saudi Med J. 1992;13(2):106–108. [Google Scholar]
- 14.Annobil S.H., Ogunbiyi A.O., Benjamin B. Chest radiographic findings in childhood lipoid pneumonia following aspiration of animal fat. Eur J Radiol. 1993;16(3):217–220. doi: 10.1016/0720-048x(93)90077-z. [DOI] [PubMed] [Google Scholar]
- 15.Hugosson C., Bahabri S., Rifai A., al-Dalaan A. Hypertrophic osteoarthropathy caused by lipoid pneumonia. Pediatr Radiol. 1995;25(6):482–483. doi: 10.1007/BF02019076. [DOI] [PubMed] [Google Scholar]
- 16.Haddad M.C., Fehaid M.A. Exogenous infantile lipoid pneumonia. Saudi Med J. 1996;17(4):524–527. [Google Scholar]
- 17.Al-Orainy I.A., Ahmed M.F., Patel P.J. The radiological manifestations of childhood lipoid pneumonia in saudi arabia: Literature review and report of two new cases. J Bahrain Med Soc. 1996;8(2):120–123. [Google Scholar]
- 18.Annobil S.H., el Tahir M., Kameswaran M., Morad N. Olive oil aspiration pneumonia (lipoid) in children. Trop Med Int Health: TM & IH. 1997;2(4):383–388. doi: 10.1111/j.1365-3156.1997.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 19.Czechowski J. Childhood lipoid pneumonia due to animal fat aspiration diagnosed by MRI. Saudi Med J. 1997;18(3):318–320. [Google Scholar]
- 20.Kumar M., Biswal N., Bhuvaneswari V., Srinivasan S. Persistent pneumonia: underlying cause and outcome. Indian J Pediatr. 2009;76(12):1223–1226. doi: 10.1007/s12098-009-0272-1. [DOI] [PubMed] [Google Scholar]
- 21.Ramdass K., Soma V., Venkatesh C., Gunasekaran D., Srinivasan S. Lipoid pneumonia in a 10 month old infant. Res J Pharm, Biol Chem Sci. 2015;6(4):1333–1335. [Google Scholar]
- 22.Annobil S.H., Morad N.A., Khurana P., Kameswaran M., Ogunbiyi O., al-Malki T. Reaction of human lungs to aspirated animal fat (ghee): a clinicopathological study. Virchows Archiv. 1995;426(3):301–305. doi: 10.1007/BF00191368. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.Y., Lee K.S., Kim T.S., Yoon H.K., Han B.K., Han J. Squalene-induced extrinsic lipoid pneumonia: serial radiologic findings in nine patients. J Comput Assist Tomogr. 1999;23(5):730–735. doi: 10.1097/00004728-199909000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Marangu D., Pillay K., Banderker E., Gray D., Vanker A., Zampoli M. Exogenous lipoid pneumonia: an important cause of interstitial lung disease in infants. Respirol Case Rep. 2018;6(7) doi: 10.1002/rcr2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira G.A., Del Caro S.R., Bender Lamego C.M., Mercon de Vargas P.R., Vervloet V.E. Radiographic plain film and CT findings in lipoid pneumonia in infants following aspiration of mineral oil used in the treatment of partial small bowel obstruction by Ascaris lumbricoides. Pediatr Radiol. . 1985,;15(3):157–160. doi: 10.1007/BF02388601. [DOI] [PubMed] [Google Scholar]
- 26.Zanetti G., Marchiori E., Gasparetto T.D., Escuissato D.L., Soares Souza A., Jr. Lipoid pneumonia in children following aspiration of mineral oil used in the treatment of constipation: high-resolution CT findings in 17 patients. Pediatr Radiol. 2007;37(11):1135–1139. doi: 10.1007/s00247-007-0603-1. [DOI] [PubMed] [Google Scholar]
- 27.Sias S.M., Daltro P.A., Marchiori E., Ferreira A.S., Caetano R.L., Silva C.S. Clinic and radiological improvement of lipoid pneumonia with multiple bronchoalveolar lavages. Pediatr Pulmonol. 2009;44(4):309–315. doi: 10.1002/ppul.20918. [DOI] [PubMed] [Google Scholar]
- 28.Salgado I.A., Santos C.C., Salgado J.V., Ferraz P.C., Haidar D.M., Pereira H.A. Exogenous lipoid pneumonia in children: a disease to be reminded of. Rev Assoc Med Bras. 1992;58(2):135–137. 2012. [PubMed] [Google Scholar]
- 29.Rabah R., Evans R.W., Yunis E.J. Mineral oil embolization and lipid pneumonia in an infant treated for hirschsprung's disease. Fetal Pediatr Pathol. 1987;7(4):447–455. doi: 10.3109/15513818709161406. [DOI] [PubMed] [Google Scholar]
- 30.Fan L.L., Graham L.M. Radiological cases of the month. Lipoid pneumonia from mineral oil aspiration. Arch Pediatr Adolesc Med. 1994;148(2):205–206. doi: 10.1001/archpedi.1994.02170020091016. [DOI] [PubMed] [Google Scholar]
- 31.Cox E.G., Heil S.A., Kleiman M.B. Lipoid pneumonia and Mycobacterium smegmatis. Pediatr Infect Dis J. 1994;13(5):414–415. [PubMed] [Google Scholar]
- 32.Bandla H.P., Davis S.H., Hopkins N.E. Lipoid pneumonia: a silent complication of mineral oil aspiration. Pediatrics. 1999;103(2):E19. doi: 10.1542/peds.103.2.e19. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein M. First do no harm: the dangers of mineral oil. Paediatr Child Health. 2001;6(3):129–131. doi: 10.1093/pch/6.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Requena-Kassarjian Y., Flores G. An infant with respiratory distress. Clin Pediatr. 2001;40(9):507–509. doi: 10.1177/000992280104000906. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman L.R., Yen E.H., Kanne J.P., Effmann E.L., Gibson R.L., Van Niel C.W. Lipoid pneumonia due to Mexican folk remedies: cultural barriers to diagnosis. Arch Pediatr Adolesc Med. 2005;159(11):1043–1048. doi: 10.1001/archpedi.159.11.1043. [DOI] [PubMed] [Google Scholar]
- 36.Buda P., Wieteska-Klimczak A., Wlasienko A., Mazur A., Ziolkowski J., Jaworska J. Lipoid pneumonia–a case of refractory pneumonia in a child treated with ketogenic diet. Pneumonol Alergol Pol. 2013;81(5):448–452. [PubMed] [Google Scholar]
- 37.Kang H.C., Chung D.E., Kim D.W., Kim H.D. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45(9):1116–1123. doi: 10.1111/j.0013-9580.2004.10004.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheon K.R., Cho H.J., Kim S.S., Woo Y.J. Lipoid pneumonia following aspiration of lorenzo’s oil in a child with x-linked adrenoleukodystrophy. Hong Kong J Paediatr. 2017;22(4):225–228. [Google Scholar]
- 39.Hochart A., Thumerelle C., Petyt L., Mordacq C., Deschildre A. Chronic lipoid pneumonia in a 9-year-old child revealed by recurrent chest pain. Case Rep Pediatr. 2015;2015 doi: 10.1155/2015/402926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verghese S., Ramesh S., Pararmasivan C.N., Kubendiran G., Vijayasekaran D. Mycobacterium fortuitum infection complicating lipoid pneumonia. Indian J Pract Pediatr. 2007;9(4):326–328. [Google Scholar]
- 41.Sharma D., Hilinski J.A. Refractory pneumonia in a Mexican American infant. Clin Pediatr. 2010;49(7):710–712. doi: 10.1177/0009922808325462. [DOI] [PubMed] [Google Scholar]
- 42.Ridaura-Sanz C., Lopez-Corella E., Salazar-Flores M. Exogenous lipoid pneumonia superinfected with acid-fast bacilli in infants: a report of nine cases. Fetal Pediatr Pathol. 2006;25(2):107–117. doi: 10.1080/15513810600788798. [DOI] [PubMed] [Google Scholar]
- 43.Ciravegna B., Sacco O., Moroni C., Silvestri M., Pallecchi A., Loy A. Mineral oil lipoid pneumonia in a child with anoxic encephalopathy: treatment by whole lung lavage. Pediatr Pulmonol. 1997;23(3):233–237. doi: 10.1002/(sici)1099-0496(199703)23:3<233::aid-ppul11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 44.Furuya M.E., Martinez I., Zuniga-Vasquez G., Hernandez-Contreras I. Lipoid pneumonia in children: clinical and imagenological manifestations. Arch Med Res. 2000;31(1):42–47. doi: 10.1016/s0188-4409(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 45.Kim E.S., Kim K.W., Song T.W., Cho S.H., Kim M., Kim K. Squalene-induced exogenous lipoid pneumonia in an infant. Pediatr Int. 2009;51(5):751–753. doi: 10.1111/j.1442-200X.2009.02902.x. [DOI] [PubMed] [Google Scholar]
- 46.Midulla F., Strappini P.M., Ascoli V., Villa M.P., Indinnimeo L., Falasca C. Bronchoalveolar lavage cell analysis in a child with chronic lipid pneumonia. Eur Respir J. 1998;11(1):239–242. doi: 10.1183/09031936.98.11010239. [DOI] [PubMed] [Google Scholar]
- 47.Al-Kindi H., Abdoani R., El-Iraqi M., Praseeda I. Lipoid pneumonia following aspiration of ghee (animal fat) in an Omani infant. Oman Med J. 2008;23(2):108–111. [PMC free article] [PubMed] [Google Scholar]
- 48.Kudoh S. [The virulence of saprophytic acid-fast bacteria coated with oil or fat, with special reference to an observation on the so-called atypical acid-fast bacteria. 1. Experiments with guinea-pigs on intrapulmonal and subcutaneous inoculation of saprophytic bacteria coated with liquid paraffin]. Nihon saikingaku zasshi. Jpn J Bacteriol. 1962;17:154–161. [PubMed] [Google Scholar]
- 49.Hutchins G.M., Boitnott J.K. Atypical mycobacterial infection complicating mineral oil pneumonia. JAMA. 1978;240(6):539–541. [PubMed] [Google Scholar]
- 50.Okamori S., Asakura T., Nishimura T., Tamizu E., Ishii M., Yoshida M. Natural history of Mycobacterium fortuitum pulmonary infection presenting with migratory infiltrates: a case report with microbiological analysis. BMC Infect Dis. 2018;18(1):1. doi: 10.1186/s12879-017-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marangu D., Kovacs S., Walson J., Bonhoeffer J., Ortiz J.R., John-Stewart G. Wheeze as an adverse event in pediatric vaccine and drug randomized controlled trials: a systematic review. Vaccine. 2015;33(41):5333–5341. doi: 10.1016/j.vaccine.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griese M., Seidl E., Hengst M., Reu S., Rock H., Anthony G. International management platform for children's interstitial lung disease (chILD-EU) Thorax. 2018;73(3):231–239. doi: 10.1136/thoraxjnl-2017-210519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.