Abstract
Mammalian reoviruses (MRVs) are associated with pulmonary infections and have been isolated from humans and various animals experiencing respiratory illness. We report here the first case of an MRV detected in the masked palm civet, which showed the highest similarity to the serotype 3 MRV. Reovirus particles were identified by electron microscopic examination of both negative-stain and thin-section. Genomic pattern analysis on SDS-PAGE showed that MPC/04 had 10-segmented double-strand RNA genome. Intranasal infection of four-week-old female BALB/c mice resulted in fatal respiratory distress but not other routes. Infections caused tissue damage and inflammation. MPC/04 grew to higher titers in the lungs than in other tissues. This research strongly suggests a need for additional experimentation to understand the pathogenic mechanisms of mammalian orthoreoviruses in infected animals and humans.
Keywords: Mammalian orthoreovirus, Genome sequencing, Pathogenesis
Highlights
-
•
An MRV strain was isolated from masked palm civet cats in China.
-
•
Phylogenetic analysis indicates that MPC/04 is the product of reassortment strains.
-
•
Pathogenesis studies suggest a propensity for infection via the respiratory route.
1. Introduction
Mammalian reoviruses (MRVs), members of the genus Orthoreovirus, are non-enveloped double-stranded RNA viruses with a genome composed of 10 segments (Song et al., 2008). They were found in hosts with or without clinical manifestations (Dermody et al., 2013). MRVs are known to cause mild enteric and respiratory infections in humans. They are widespread and infect a broad spectrum of mammals. To date, MRVs have been reported in various mammalian hosts, including humans and animal species (Steyer et al., 2013, Decaro et al., 2005). In the last few years, they have often been described as the sole pathogen in various hosts presenting severe clinical manifestations, such as hemorrhagic enteritis, acute respiratory infections, central nerve system implications, and others. Recently, several groups reported infections of bat species with MRVs which resulted in visible pathologies in the organs of infected bats (Kohl et al., 2012, Lelli et al., 2013). They speculated on the possibility of bat-to-human interspecies transmission, but no evidence to support this hypothesis was provided. A MRV isolation and molecular characterization of a MRV-3 strain from a dog with diarrhea are reported. The reovirus isolate showed an atypical hemagglutination pattern and a retarded electrophoretic mobility of the S1 segment, which is characteristic of MRV type 3 (MRV-3). The authors proposed that these viruses might be important zoonotic pathogens (Decaro et al., 2005).
The masked palm civet is increasingly recognized as a reservoir of various viruses. During the epidemic, SARS-CoV was isolated from most marketplace civets (Guan et al., 2003). It persisted in civets for weeks. Biochemical and structural studies of virus–receptor interactions reveal close evolutionary relationships among the civet and human viral strains and thus support the critical roles of civets in transmitting SARS-CoV to humans. The zoonotic potential of reoviruses has been described and discussed elsewhere (Chua et al., 2008, Chua et al., 2011, Kohl et al., 2012). The transmission of reoviruses from one host to another is not limited to close contact but can be achieved through indirect transmission.
Although reovirus infection of humans usually induces mild symptoms, the infection of newborn mice can lead to severe pathologic conditions such as lethal encephalitis depending on the inoculation route and strain (Tyler et al., 1986). In this study, we report the isolation of a novel MRV strain from organ tissue of the masked palm civet. To evaluate the genetic properties of the MPC/04 strain, its genome was sequenced and analyzed. The characterized genome of MPC/04 provides clues to aid the identification of characteristics of Chinese field strains. To the best of our knowledge, the MPC/04 strain can readily establish infection by the respiratory route, and it demonstrated fatal respiratory infection in mice.
2. Material and methods
2.1. Ethics statement
Animal experiments were approved by the Animal Ethics Committee of Harbin Veterinary Research Institute (HVRI) of the Chinese Academy of Agricultural Sciences (CAAS) and performed in accordance with animal ethics guidelines and approved protocols. The Animal Ethics Committee approval number was SYXK (Hei) 2011-022.
2.2. Animals, cells and virus
Four-week-old female BALB/c mice were obtained from the experimental animal center of HVRI and housed in individually ventilated cages. All animals were maintained in the animal facility at HVRI under standard conditions prescribed by the Institutional Guidelines. The study protocol was approved by the Institutional Animal Care and Use Committee. Vero E6 cells (African green monkey kidney cell line) were obtained from the ATCC (ATCC® CRL-1586™) and grown at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM glutamine, 5% fetal calf serum and antibiotics. The type 3 strain Dearing (T3D) was obtained from ATCC (ATCC® VR-824™) and propagated in Vero E6 cells on cell culture flask (Corning®, 75 cm2, Canted Neck).
2.3. Viral isolation, purification and production
Samples were homogenized in phosphate-buffered saline (PBS) and centrifuged at 3000 g for 15 min. The supernatant was filtered through a 0.22-μm-pore-size filter and inoculated in confluent monolayers of Vero E6 at 37 °C with 5% CO2. After incubation for 1 h, the inoculum was removed, and the cells were supplemented with the original growth medium and observed daily for 7 days to monitor development of the cytopathic effect (CPE). To harvest virus particles, cells were homogenized by three freeze–thaw cycles and the resulting suspension was purified from cell debris by low-speed centrifugation (1000 g 10 min). Aliquots were stored at − 80 °C. One aliquot was titrated on Vero E6 cells to estimate a titer by plaque assay (Berard and Coombs, 2009). If CPE was negative after 4 passages, the virus isolation result was considered negative.
In addition to CPE, the indirect immunofluorescence assay (IFA) was done to detected MRV proteins in infected cell cultures. Briefly, after washing with PBS, the infected Vero E6 cells were fixed with 4% paraformaldehyde for 15 min and then incubated with 1% BSA for 1 h. After washing, the cells were incubated with a mouse anti-MRV (T3D) antibody (1:50) at 37 °C for 1 h. The cells were washed again with PBS and incubated gout anti-mouse IgG-FITC secondary antibody (1:100) (Santa Cruz, USA) for 1 h at 37 °C in the dark. After washing, fluorescence was observed under an AMG EVOS F1 inverted microscope (AMG, USA). Normal mouse sera, diluted 1:50, was used as the negative control.
Samples were prepared for negative-stain and thin-section examination by electron microscopy (EM) following previously described procedures with modifications. The viral suspension was subjected to centrifugation at 3000 g for 15 min to generate a suspension, followed by 20,000 g for 15 min to pellet the virus particles, which were then negatively stained with 2% phosphotungstic acid and examined on a HITACH H-7650 electron microscope. In addition, infected cells were prepared for thin sectioning. Briefly, the specimen was fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M PBS for 2 h, washed 3 times with PBS, and postfixed with 1% (vol/vol) OsO4 for 2 h. After being washed, the specimen was dehydrated in a graded ethanol series and embedded in Epon812 resin, following the standard protocol. Ultrathin sections were collected on carbon-coated 100 mesh copper grids and stained with 1% uranyl acetate and 1% lead citrate. In addition, clarified stool suspension was used for direct EM examination of the sample after negative staining with 2% phosphotungstic acid. EM grids were screened at 80 kV on a Hitachi H-7650 electron microscope.
2.4. Electropherotype
Viral dsRNA (MRV/04 and T3D) was extracted from purified virus using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. dsRNA segments were separated by electrophoresis in 8% (w/v) polyacrylamide slab gels, 1.5 mm thick with a 7.3 cm path length. About 30 μl of each sample was loaded onto the gels and electrophoresis was carried out at 120 V for 4 h at room temperature.
2.5. RT-PCR and nucleic acid sequencing
A total of 22 primer pairs were designed to amplify the complete genome of MPC/04. All information regarding the primers is provided in Table 1 . RT-PCR for the amplification of each viral genome segment was carried out using the One Step RT-PCR kit (Qiagen). Amplified products were analyzed by electrophoresis on 1% agarose gel and purified using the High Pure PCR Product Purification Kit (Roche). The PCR products were purified and cloned into the pMD18-T vector (TaKaRa). Sequence of positive colonies was performed by Shanghai Invitrogen Biotechnology Co. Ltd. using the traditional Sanger method. At least three positive colonies for each amplification product were sequenced to guarantee the fidelity of results.
Table 1.
Primers used in this study for amplification of the full-length genome of MPC/04 strain.
| Gene | Primer | Start | Sequence (5′–3′) |
|---|---|---|---|
| L1 | L1a | 9 | TTCCACGACAATGTCATCCA |
| L1b | 1019 | AGTTCGCGCGCTTTCTTATC | |
| L1c | 951 | GGGAGTCATGCCATTGTCCA | |
| L1d | 1964 | TGAATCATGTTCTGCATTCC | |
| L1e | 1886 | CTGCATCCATTGTAAATGACGAGTC | |
| L1f | 2339 | GCTATGTCATATTTCCATCCGAATTC | |
| L1g | 1930 | GCTAGGCCGATATCGGGAATGCAG | |
| L1h | 2278 | CTTGAGATTAGCTCTAGCATCTTCTG | |
| L1i | 2212 | CCAAGGTGACGACGGACTGA | |
| L1j | 2901 | CGCTCGTCCAGATTTCGTAG | |
| L1k | 2806 | AACGCAGATTATCGCAGGTG | |
| L1l | 3839 | CACGACCCATGGTAGACTCA | |
| L2 | L2a | 13 | ATGGCGAACGTY(T/C)TGGGGR(A/G)GTGAG |
| L2b | 817 | GGACGTTGGCTGAGAATTGCTCTA | |
| L2c | 803 | CTTCGCGGCTAACGTGAGA | |
| L2d | 1573 | GCTGATCCGCTCCCGAATG | |
| L2e | 1362 | ATCCGAGCTGCCGCAGTTAC | |
| L2f | 2395 | CGCGCCATTAATGTTATCAAA | |
| L2g | 2207 | CGAATGTGGGAAATGCACGTA | |
| L2h | 3492 | GGGTTTGCTATCTGCCATTGAC | |
| L2i | 3301 | GGAATCTAY(C/T)ACM(A/C)ATGCAGGC | |
| L2j | 3895 | GAGGGACRR(A/G)TGAGTTACAGAGG | |
| L3 | L3a | 13 | GATGAAGCGGATTCCAAGGA |
| L3b | 3882 | GATGAATCGGCCCAACTAGC | |
| M1 | M1a | 1 | GCTATTCGCGGTCATGGC |
| M1b | 1466 | CCTGTCATCATGCGGAATGAG | |
| M1c | 1382 | GAGCAK(T/G)GCGGTTATGGAR(G/A)AT | |
| M1d | 1764 | TGCGCR(G/A)CTAGTR(A/G)GCATACAT | |
| M1e | 1590 | CATTCGCTCATGCCGATAGTG | |
| M1f | 22,304 | GATGAAGCGCGTACGTAGTCTTAG | |
| M2 | M2a | 2 | GCTAATCTGCTGACCGTCACTC |
| M2b | 2199 | TGTGCCTGCATCCCTTAACC | |
| M3 | M3a | 1 | GCGGTCGGTCGACGCTAAAGTGACCGTGGTCATGGCTTCATTCAAGGG |
| M3b | 2241 | GCAGGGGATCCGATGAATGGGGGTCGGGAAGGCTTAAGGG | |
| S1 | S1a | 1 | ATGGATCCTCGCTTACGTGA |
| S1b | 510 | GACCGCTGTACGCTCTAAT | |
| S1c | 340 | ACCACGAGTTGACAGTCTGGAT | |
| S1d | 1436 | CGCGCTAGATTCACCTCACATT | |
| S2 | S2a | 1 | GCTATTCGCTGGTCAGTTATGGC |
| S2b | 1331 | GATGAATGTGTGGTCAGTCGTGAG | |
| S3 | S3a | 1 | GCTAAAGTCACACCTGTCGTCGTC |
| S3b | 1198 | GATGATTAGGCGTCACCCACCAC | |
| S4 | S4a | 1 | GCGAATTCGCTATTTTTGCCTCTTCCCAGA |
| S4b | 1215 | CAS4TGCCTGCAGATGAATGAAGCCTGTCCCACGTC |
2.6. Genome analyses
Sequences were assembled using the Seqman program (DNASTAR, Madison, WI) and manually edited. The complete nucleotide sequence of MPC/04 has been submitted to GenBank (GQ468266–GQ468275GQ468266GQ468267GQ468268GQ468269GQ468270GQ468271GQ468272GQ468273GQ468274GQ468275). Several local and web-based bio-information tools, including DNASTAR, Emboss, and software from NCBI were employed at various stages of sequence analysis. Homology searches were conducted using the NCBI BLAST programs. Multiple alignments of nucleotide sequences were generated using Bioedit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Phylogenetic analyses were performed using Neighbor-Joining (NJ) methods with Kimura 2-parameter model in MEGA 5. The support for the tree nodes was calculated with 1000 bootstrap replicates.
2.7. Mouse experiments
Four-week-old female BALB/c mice were inoculated intranasally (i.n.), intracranially (i.c.), intraperitoneally (i.p.) or intragastrically (i.g.) with purified reovirus MPC/04 diluted in PBS. The doses of viruses were 105, 106, and 107 PFU. PBS alone was used as a control. All mice were weighed daily and observed twice a day for clinical signs of disease. Three mice in each group were euthanized 7 days post inoculation (p.i.), and 14 p.i., respectively. Organs (lung, liver, intestine, spleen, and brain) were collected and prepared for virus titration and histological studies. Viral titers in organ homogenates were determined by plaque assay using Vero E6. A histopathological scoring system was developed, and the severity of the lesions scored from 0 (no lesion) to 3 (severe lesion). Data from both scoring systems were analyzed using the Kruskal–Wallis non-parametric mean comparison test (Elliott and Hynan, 2011) and differences were considered significant at p < 0.05. The remaining mice were monitored for 30 days for weight loss and mortality.
3. Results
3.1. Virus isolation and visualization
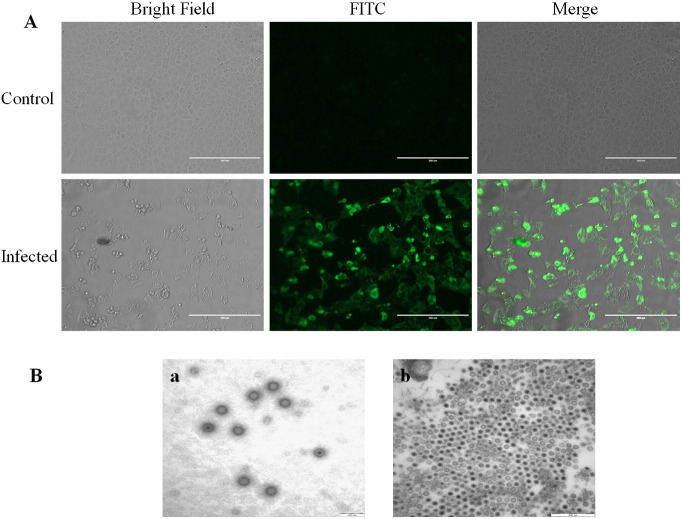
During 2003 and 2004, 192 oropharyngeal and cloacal swabs were collected from masked palm civets in Guangdong Province in southern China. After inoculation of Vero E6 cells with homogenized samples and four serial passages, one MRV isolate designated that MPC/04 was successfully obtained from the oropharyngeal and cloacal swabs of a masked palm civet in Guangdong Province in southern China and CPE was observed at 4–6 days post-inoculation, included granulating, shrinking, rounding, seining, and falling off (Fig. 1A). Indirect immunofluorescence assay results showed that serum from mice immunized with T3D recognized viral antigens (Fig. 1A). Virus particles were visualized by negative staining electron microscopy, revealing typical inner and outer icosahedral, non-enveloped capsids of approximately 70 nm in diameter, which is characteristic of reoviruses (Fig. 1B–a). Ultra-thin sections of infected Vero E6 cells displayed typical electron-dense virus particles organized in a paracrystalline pattern within the cytoplasm (Fig. 1B–b). Genomic pattern analysis on SDS-PAGE showed that MPC/04 had 10 dsRNA segments that migrated in a pattern highly consistent with those of members of the MRV species, dividing MPC/04 RNAs into three size classes: three large (L1, L2 and L3), three medium (M1, M2 and M3) and four small (S1, S2, S3 and S4) segments in the gel (Fig. 2 ).
Fig. 1.
(A) Indirect immunofluorescence detection of MRV in Vero E6 cells infected with strain MPC/04. The cells were fixed at 4% paraformaldehyde, blocked with 1% BSA, washed, and incubated with mouse anti-MRV (T3D) antibody. The cells were then incubated with FITC-labeled goat anti-mouse IgG secondary antibody. (B) Electron micrographs. a: negative staining of cell-culture supernatant. Non-enveloped reoviral particles with double-layered capsid structure were observed (diameter = approximately 70 nm). b: ultra-thin sections of infected Vero E6 cells displayed typical contrast-rich virus particles, organized as paracrystalline structures within the cytosol.
Fig. 2.
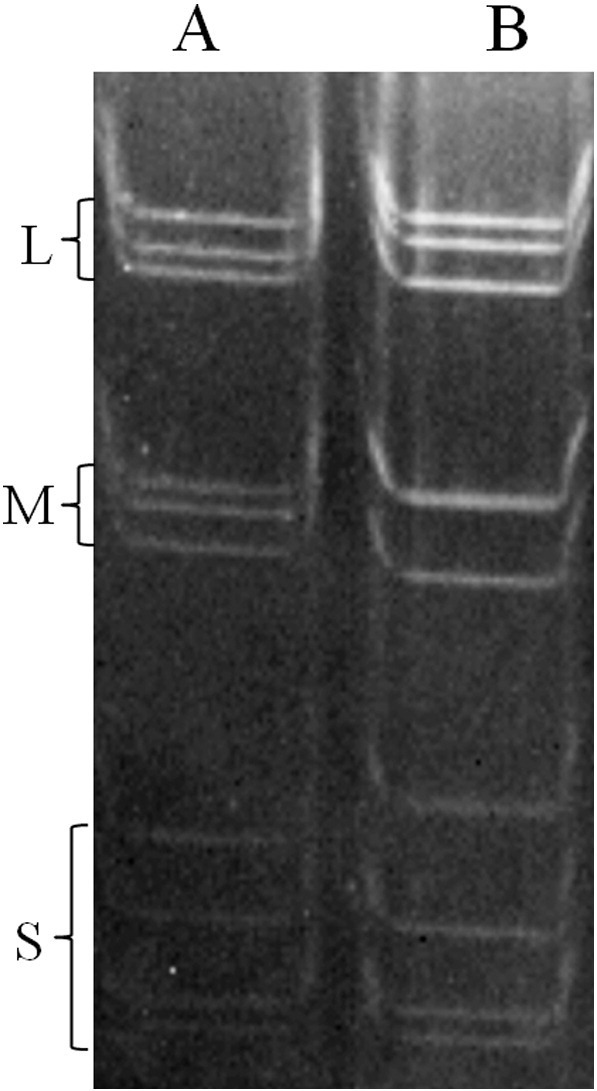
SDS-PAGE demonstrating electrophoresis pattern. L, M and S represent large, medium and small segments, respectively. dsRNA segments were separated by electrophoresis in 8% (w/v) polyacrylamide slab gels. Lane A: MPC/04 (cell culture supernatant after virus propagation), lane B: T3D (cell culture supernatant).
3.2. Full-length nucleotide sequence and phylogenetic analysis
The complete genome sequence of MPC/04 was generated and comparative analysis with other reovirus strains was performed. The full RNA genome of MPC/04 was amplified using the 22 pairs of primers listed in Table 1. The complete sequences of 10 segments were submitted to a GenBank database under accession numbers GQ468266–GQ468275GQ468266GQ468267GQ468268GQ468269GQ468270GQ468271GQ468272GQ468273GQ468274GQ468275. The complete genome of MPC/04 was 22,830 bp in length and consisted of segments L1 to L3, M1 to M3, and S1 to S4 (3804 bp, 3870 bp and 3828 bp; 2211 bp, 2127 bp and 2166 bp; and 1368 bp, 1257 bp, 1101 bp and 1098 bp, respectively) encoding proteins λ1 (1275 aa), λ2 (1289 aa), λ3 (1267 aa), μNS (721 aa), μ1 (708 aa), μ2 (736 aa), σ1 (455 aa), σ2 (418 aa), σNS (366 aa) and σ3 (365 aa).
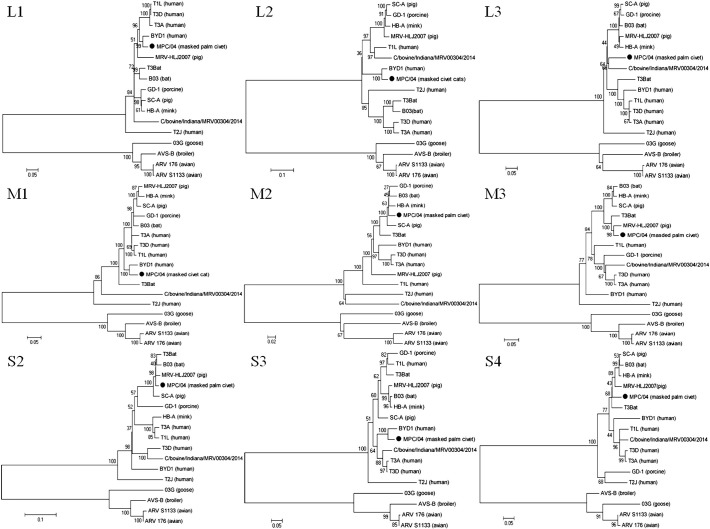
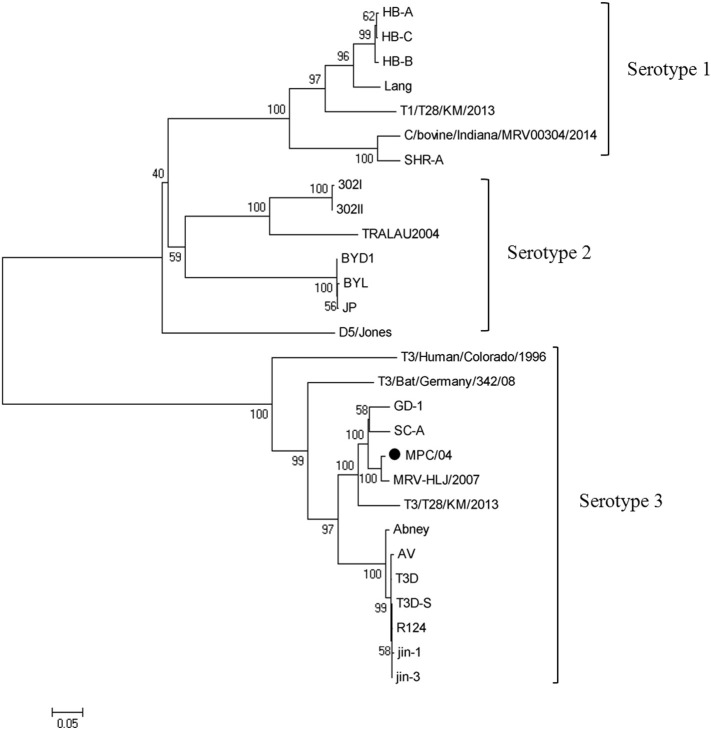
Nucleotide and deduced amino acid sequences were obtained and further analyzed. Following BLAST of the nucleotide sequence, the L1, L2, M1 and S3 segments of MPC/04 showed the highest nucleotide sequence identity (78.2–93.7%) with reovirus strain BYD1 (isolated from human), the M3, S1, S2 segments showed highest identities (95.2–97.9%) to MRV-HLJ/2007 (isolated from swine) (Fig. 3 ). The MPC/04 S1 segment was clustered in the mammalian reovirus serotype 3 group and shared the highest identity with the porcine strains MRV-HLJ/2007, SC-A and GD-1 (nucleotide identities were 97.9%, 93.4% and 94.5%) (Fig. 4 ). Phylogenetic analysis of MPC/04 showing that each segment was derived from an independent evolutionary path indicates that reassortment occurred during evolution.
Fig. 3.
Phylogenetic analysis of the L1, L2, L3, M1, M2, M3, S2, S3 and S4 genome segments for the MPC/04 strain and most related whole-genome strains from GenBank. Neighbor-joining was used for the construction of phylogenetic tree with bootstrap values of 1000 replicates shown at the branches. The scale bar represents the p-distance.
Fig. 4.
Phylogenetic trees based on S1 gene of MPC/04 from this study and 27 previously published reovirus sequences from GenBank. Neighbor-joining was used for the construction of phylogenetic tree with bootstrap values of 1000 replicates shown at the branches. The scale bar represents the p-distance.
3.3. Pathogenicity experiments
To determine the route of infection, we inoculated BALB/c mice with purified reovirus MPC/04 in three ways. Mice inoculated i.n. exhibited noticeable respiratory distress and body weight loss following inoculation with different doses of MPC/04, but the time to death and clinical symptoms of infection varied with dose (Fig. 5 ). The highest dose of MPC/04 (107 PFU) induced signs of disease and resulted in the death of mice commencing 7 days post infection that was associated with piloerection, hunching and respiratory distress that was consistent with pneumonia. Mice exposed to the same doses by either i.c. i.p. or i.g. inoculation did not exhibit any signs of respiratory distress or changes in body weight during the course of the experiment, and no histological changes were observed in their organs at any time point. Between 41.7 and 66.7% of mice inoculated i.n with the higher doses of MPC/04 (106 and 107 PFU) died within 17 days of exposure. In contrast, the overall survival rate of mice inoculated i.c., i.p., i.g. and control mice were 100% (data not shown).
Fig. 5.
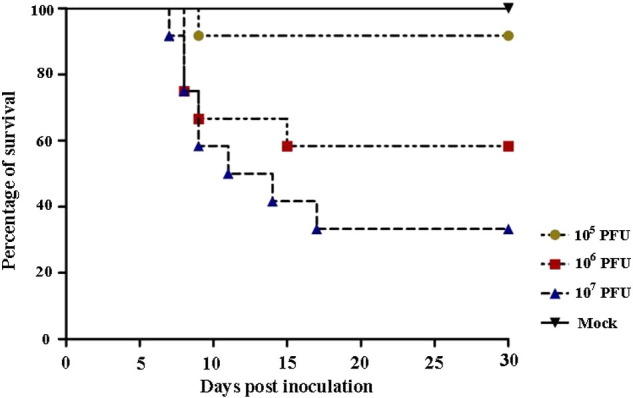
Survival of BALB/c mice infected with different doses of MPC/04 (105, 106 or 107 PFU) via the intranasal route. Survival of infected mice was monitored daily for a total of 30 days. The time of BALB/c mice to death and clinical symptoms of infection varied with dose. Other routes of infection did not induce fatality (not show).
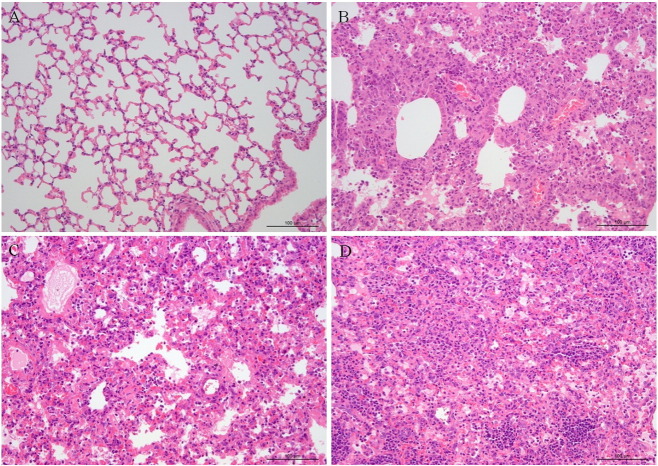
To assess the gross pathological consequences of infection, organs were paraffin-embedded, sectioned and stained with hematoxylin and eosin (H–E) at 7 dpi and 14 dpi. The mice that were infected i.n. displayed greater signs of pathology; mice inoculated via other routes were not significantly different from control mice. There were obvious lesions in the lungs due to tissue damage and inflammation associated with alveolar thickening and lymphocytic infiltration, as well as accumulation of cellular debris and distended bronchioles and alveoli (Fig. 6 ). A composite analysis using the histopathological scoring system was performed, which yielded a mean histopathological score (means ± S.D.) of 0 in panel A, 1.75 ± 0.25 in panel B, 2.8 in panel C, and 2.75 ± 0.28 in panel D. The liver exhibited minor foci of necrosis and inflammatory cells. The brain, intestines, kidneys and spleen appeared relatively normal. In summary, significant and severe pathology was restricted to the lungs in mice inoculated i.n. with MPC/04. These results show that the route of infection is intranasal. This route is used for inoculation in subsequent experiments.
Fig. 6.
Pathology of lung of virus infected BALB/c mice. Four-week-old female BALB/c mice were infected intranasally with 107 PFU MPC/04 before sectioning formalin fixed, paraffin embedded sections for histopathological assessment by H and E. staining. Panel A shows uninfected control images for BALB/c mice lung. Panel B and D show the MPC/04 infected lung at 7 dpi and 14 dpi. Panel C shows mice infected with MPC/04 developed to severe respiratory distress and death on day 8.
3.4. Viral titers in different organs of mice
To further characterize MRV MPC/04 strain replication in different tissues, viral titers were detected in the lung, liver, intestine, spleen, and brain at 3, 5, 7, 14 and 21 dpi. Animals inoculated with 107 and 106 PFU MPC/04 strain showed higher viral titers (up to 106 PFU/g) than animals inoculated with 105 PFU. Higher levels of viral RNA were detected in the lungs than in the brain, liver, intestine and spleen with 107 PFU (Fig. 7 ). However, the tissue of inoculated i.c., i.p. and i.g. with 107 PFU was shown 3 to 5 orders of magnitude fewer titers in each organ (data not shown). No viral titer was detected in the two uninfected control animals.
Fig. 7.
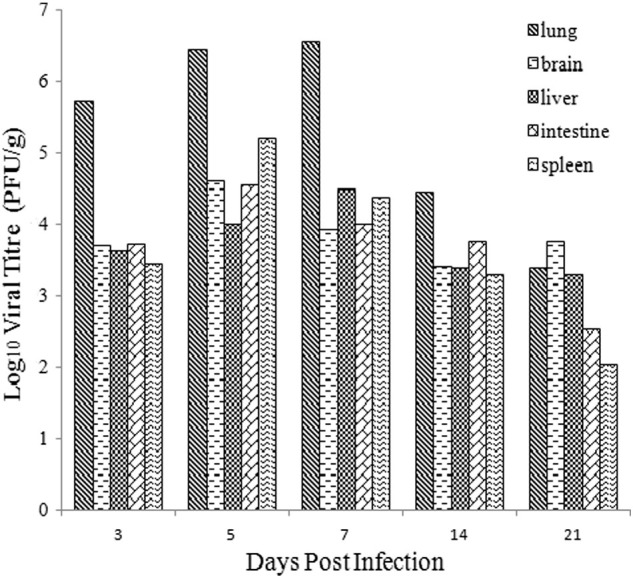
Viral titers in lung, brain, liver, intestine and spleen of BALB/c mice (i.n. inoculation) with 107 PFU at 3, 5, 7, 14, 21 dpi.
4. Discussion
MRV can infect many mammalian species, including cattle, sheep, horses, pigs, dogs, cats, mice and humans. However they are rarely associated with disease and mortality risk (Dermody et al., 2013, Attoui et al., 2011). The masked palm civets (Paguma larvata) are usually found in the tropical jungles and rainforests of south-east Asia, India and China. The masked palm civet has been confirmed to be a host of mammalian orthoreoviruses that cause mild respiratory and enteric infections in humans and many domestic animals. This study is the first description of an MRV strain found in masked palm civet, and one of the 192 samples (0.52%) was classed as positive by PCR and electron microscopy. Although the sample quality could have been a contributing factor to the low success rate, the isolation procedures also need to be further improved to increase the success rate of MRV. Because of the apparent lack of species barriers, MRV may potentially switch from animal to human.
MRV particle contains 10 genome segments, on the basis of electrophoretic mobility it can be divided into three size classes (Attoui et al., 2011, Day, 2009). In this study, the genotypes of MPC/04 were analyzed and verified by electrophoresis of viral genomic RNA. Electropherotype of MPC/04 shows 3-3-4 migration pattern that is typical of the mammalian reovirus. The whole-genome sequence comparison of our strain to MRV genomes available in GenBank clearly shows that MPC/04 isolation is most closely related to the BYD1 and MRV-HLJ/2007. The MRV BYD1 strain analyzed was isolated from the throat swabs of one SARS patient of China, which can cause clinical symptoms similar to those of severe acute respiratory syndrome in guinea pigs and macaques (Song et al., 2008). Comparative analyses of the BYD1 genome demonstrated it is a novel reassortant virus: its S1 gene segment derives from a previously unidentified serotype 2 isolate and its other nine segments derive from ancestors of homologous type 1 strain Lang (T1L) and type 3 strain Dearing (T3D) segments. MRV-HLJ/2007 was isolated from swine in Heilongjiang Province, China (Zhang et al., 2011). Phylogenetic analysis of MPC/04 showed that the isolation from palm civets was the reassortment of gene segments during mixed infections with more than one MRV isolates in nature. The results indicate that the isolated virus MPC/04 may be a novel and reassortant virus. Moreover, the potential function of these genes may be important for understanding the pathogenic mechanisms and should be studied further.
MRVs were traditionally believed to be causative agents of mild respiratory and enteric infections without significant clinical impact (Steyer et al., 2013). However, several recent studies have suggested that MRV can cause serious illnesses, even death in humans and other mammals, including upper respiratory tract infections, diarrhea and encephalitis (Tyler et al., 2004, Ouattara et al., 2011, Steyer et al., 2013). Indeed, the pathogenesis of reovirus infections has been most extensively studied using both suckling and adult animals, and infections lead to systemic viral replication, morbidity, and mortality (Dermody et al., 2013, Doyle et al., 2015, Organ and Rubin, 1998). Natural infection with MRV may involve either the nasal and/or oral routes of transmission. Therefore, we firstly tested the route of infection in four ways: intranasally, intracranially, intraperitoneally and intragastrically with different doses of the MPC/04 strain. We found that mice were easily infected via intranasal inoculation with MPC/04, exhibited noticeable respiratory distress and body weight loss. To measure the potential pathogenicity of the strain, experimental infection using animal models is necessary. Furthermore, mice inoculated i.n. result in extensive lung infection. We observed approximately 70% mortality in mice inoculated i.n with 107 PFU of MPC/04 strain and 0.93% mortality in other routes. Previous study of reovirus infection to respiratory tract showed that both T1L and T3D reoviruses can infect the adult and suckling mice via i.n. inoculation (Gauvin et al., 2013). The highest dose of T1L (1 × 107 PFU) induced signs of disease and resulted in the death of 4 of 6 mice commencing 7 days post infection. However T3D does not efficiently infect mice by oral route, because the T3D σ1 protein is hypersensitive to cleavage by digestive enzymes (Chappell et al., 1998, Nibert et al., 1995). Our results confirm that MPC/04 strain is pathogenic to four-week-old female BALB/c mice. Additional studies are needed to understand that pathogenicity of MPC/04 to other animals.
Mammalian reovirus strains display serotype-specific in cell tropism, tissue tropism and mechanism of viral dissemination (Dichter and Weiner, 1984, Tardieu and Weiner, 1982, Tyler et al., 1986). To determine the kinetics of replication, we analyzed viral titers in lung, brain, liver, intestine and spleen after intranasal infection with MPC/04. We found that the viral titers in the lung were higher at 7 days for mice in comparison with other tissue. Moreover, mouse in 107 PFU inoculation group displayed severe acute respiratory symptoms and died from day 7 dpi. Recent studies have indicated that adult mice were infected intranasally with 107 PFU of both T1L and T3D for histopathological assessment of brain, lungs, heart, liver, spleen, kidney and intestine (Gauvin et al., 2013). The histological observations were consistent with the virological findings in that organs with higher viral replication revealed greater signs of pathology, namely tissue damage and inflammation in the lungs. Our results indicate that MPC/04 replicated to higher levels in the lung, in agreement with its greater ability to cause acute respiratory distress in the mouse. We also provide evidence that these novel MRV strains are pathogenic to mice, leading to lethal respiratory system disease.
In conclusion, the MPC/04 strain can readily establish infection via the respiratory route, causing a potentially fatal respiratory infection of the host mouse. The discovery and characterization of MPC/04 demonstrate the increasing risk posed by unknown animal viruses which are capable of infecting and causing disease in humans. The reovirus provides a rare opportunity to gain insights into possible transmission events and to trace the evolution of masked palm civet viruses. This further highlights the urgent need to systematically survey wild animal borne viruses in the international community so as to enable us to conduct more effective risk assessment, to provide forecast for potential future outbreaks and to devise better prevention and control strategies. Further large-scale studies are necessary to understand the roles of MPC/04 in animal-to-human infection.
Acknowledgments
This work was supported by funds from the National Natural Science Foundation of China (31402204), the State Key Laboratory of Veterinary Biotechnology (SKLVBP201421), and the Basic Scientific Research Operation Cost of State-leveled Public Welfare Scientific Research Courtyard (0302014011).
Contributor Information
Shengwang Liu, Email: SWLIU@hvri.ac.cn.
Liandong Qu, Email: qld@hvri.ac.cn.
References
- Attoui H., Mertens P.P.C., Becnel J., Belaganahalli S., Bergoin M., Brussaard C.P. Virus Taxonomy. Classification and Nomenclature of Viruses: Ninth Report of the International Committee on the Taxonomy of Viruses. Elsevier Academic Press; London: 2011. Orthoreovirus, Reoviridae; pp. 546–554. ( http://www.elsevier.com/books/virus-taxonomy/king/978-0-12-384684-6) [Google Scholar]
- Berard A., Coombs K.M. Mammalian reoviruses: propagation, quantification, and storage. Curr. Protoc. Microbiol. 2009 doi: 10.1002/9780471729259.mc15c01s14. (Chapter 15: Unit15C.1) [DOI] [PubMed] [Google Scholar]
- Chappell J.D., Barton E.S., Smith T.H., Baer G.S., Duong D.T., Nibert M.L., Dermody T.S. Cleavage susceptibility of reovirus attachment protein sigma1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the sigma1 neck. J. Virol. 1998;72:8205–8213. doi: 10.1128/jvi.72.10.8205-8213.1998. ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC110170/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B., Voon K., Crameri G., Tan H.S., Rosli J., McEachern J.A., Suluraju S., Yu M., Wang L.F. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One. 2008;3:e3803. doi: 10.1371/journal.pone.0003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B., Voon K., Yu M., Keniscope C., Abdul Rasid K., Wang L.F. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS One. 2011;6:e25434. doi: 10.1371/journal.pone.0025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.M. The diversity of the orthoreoviruses: molecular taxonomy and phylogenetic divides. Infect. Genet. Evol. 2009;9:390–400. doi: 10.1016/j.meegid.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Desario C., Ricci D., Camero M., Lorusso E., Elia G., Lavazza A., Martella V., Buonavoglia C. Virological and molecular characterization of a mammalian orthoreovirus type 3 strain isolated from a dog in Italy. Vet. Microbiol. 2005;109:19–27. doi: 10.1016/j.vetmic.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody T.S., Parker J.S., Sherry B. Orthoreoviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1304–1346. [Google Scholar]
- Dichter M.A., Weiner H.L. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann. Neurol. 1984;16:603–610. doi: 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- Doyle J.D., Stencel-Baerenwald J.E., Copeland C.A., Rhoads J.P., Brown J.J., Boyd K.L., Atkinson J.B., Dermody T.S. Diminished reovirus capsid stability alters disease pathogenesis and littermate transmission. PLoS Pathog. 2015;11:e1004693. doi: 10.1371/journal.ppat.1004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A.C., Hynan L.S. A: SAS(®) macro implementation of a multiple comparison post hoc test for a Kruskal–Wallis analysis. Comput. Methods Prog. Biomed. 2011;102:75–80. doi: 10.1016/j.cmpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Gauvin L., Bennett S., Liu H., Hakimi M., Schlossmacher M., Majithia J., Brown E.G. Respiratory infection of mice with mammalian reoviruses causes systemic infection with age and strain dependent pneumonia and encephalitis. Virol. J. 2013;10:67. doi: 10.1186/1743-422X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Kohl C., Lesnik R., Brinkmann A., Ebinger A., Radonic A., Nitsche A., Muhldorfer K., Wibbelt G., Kurth A. Isolation and characterization of three mammalian orthoreoviruses from European bats. PLoS One. 2012;7:e43106. doi: 10.1371/journal.pone.0043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli D., Moreno A., Lavazza A., Bresaola M., Canelli E., Boniotti M.B., Cordioli P. Identification of mammalian orthoreovirus type 3 in Italian bats. Zoonoses Public Health. 2013;60:84–92. doi: 10.1111/zph.12001. [DOI] [PubMed] [Google Scholar]
- Nibert M.L., Chappell J.D., Dermody T.S. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved sigma 1 protein. J. Virol. 1995;69:5057–5067. doi: 10.1128/jvi.69.8.5057-5067.1995. ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC189323/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organ E.L., Rubin D.H. Pathogenesis of reovirus gastrointestinal and hepatobiliary disease. Curr. Top. Microbiol. Immunol. 1998;233:67–83. doi: 10.1007/978-3-642-72095-6_4. [DOI] [PubMed] [Google Scholar]
- Ouattara L.A., Barin F., Barthez M.A., Bonnaud B., Roingeard P., Goudeau A., Castelnau P., Vernet G., Paranhos-Baccala G., Komurian-Pradel F. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg. Infect. Dis. 2011;17:1436–1444. doi: 10.3201/eid1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Zhou Y., He J., Zhu H., Huang R., Mao P., Duan Q. Comparative sequence analyses of a new mammalian reovirus genome and the mammalian reovirus S1 genes from six new serotype 2 human isolates. Virus Genes. 2008;37:392–399. doi: 10.1007/s11262-008-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer A., Gutiérrez-Aguire I., Kolenc M., Koren S., Kutnjak D., Pokorn M., Poljšak-Prijatelj M., Racki N., Ravnikar M., Sagadin M., Fratnik Steyer A., Toplak N. High similarity of novel orthoreovirus detected in a child hospitalized with acute gastroenteritis to mammalian orthoreoviruses found in bats in Europe. J. Clin. Microbiol. 2013;51:3818–3825. doi: 10.1128/JCM.01531-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Weiner H.L. Viral receptors on isolated murine and human ependymal cells. Science. 1982;215:419–421. doi: 10.1126/science.6276976. [DOI] [PubMed] [Google Scholar]
- Tyler K.L., McPhee D.A., Fields B.N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986;233:770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Tyler K.L., Barton E.S., Ibach M.L., Robinson C., Campbell J.A., O'Donnell S.M., Valyi-Nagy T., Clarke P., Wetzel J.D., Dermody T.S. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J. Infect. Dis. 2004;189:1664–1675. doi: 10.1086/383129. [DOI] [PubMed] [Google Scholar]
- Zhang C., Liu L., Wang P., Liu S., Lin W., Hu F., Wu W., Chen W., Cui S. A potentially novel reovirus isolated from swine in northeastern China in 2007. Virus Genes. 2011;43:342–349. doi: 10.1007/s11262-011-0642-4. [DOI] [PubMed] [Google Scholar]