Abstract
Emergence of new virus and their heterogeneity are growing at an alarming rate. Sudden outburst of Nipah virus (NiV) has raised serious question about their instant management using conventional medication and diagnostic measures. A coherent strategy with versatility and comprehensive perspective to confront the rising distress could perhaps be effectuated by implementation of nanotechnology. But in concurrent to resourceful and precise execution of nano-based medication, there is an ultimate need of concrete understanding of the NIV pathogenesis. Moreover, to amplify the effectiveness of nano-based approach in a conquest against NiV, a list of developed nanosystem with antiviral activity is also a prerequisite. Therefore the present review provides a meticulous cognizance of cellular and molecular pathogenesis of NiV. Conventional as well several nano-based diagnosis experimentations against viruses have been discussed. Lastly, potential efficacy of different forms of nano-based systems as convenient means to shield mankind against NiV has also been introduced.
Key words: Nanomedicine, Diagnostics, Nipah virus, Antiviral, Virucide
Graphical Abstract
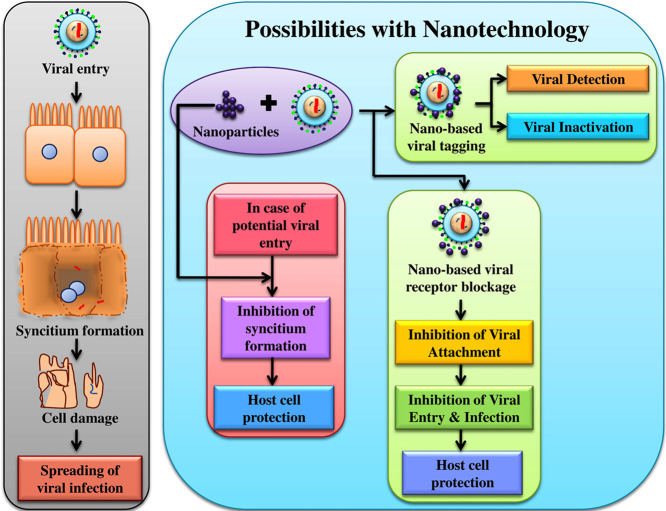
Nipah virus (NiV) infects the host cell via cell surface receptor leading to the formation of syncytium, gradual degradation of which leads to the release of the virus in to blood streams, and transmission of infection to different organs. With nanotechnology it could be possible to detect and inactivate NiV infection specifically through targeted tagging or by blocking viral surface proteins. As these viral surface proteins are also expressed in NiV-infected cell which contributes towards the formation of syncytium, therefore designing a targeted nano-system against these expressed proteins, could also be helpful in prohibiting the spread of viral infection.
In view of the adaptable nanotechnological achievements in the past decade, it is certain that, nanoscience is consistently intensifying its horizon across the globe in present and will be in centuries to come. Despite the expendability and plasticity in nanotechnological application, there is a wide gap in intrigue experimental biomedical application and their substantial commercialization. Moreover, their application in biomedical science is still lurking, and should be prioritized in order to bridge the gap. Additionally, the efficacy of conventional therapies is gradually fading away specifically in case of viral infections due to the development of resistance,1 which could be certainly due to accelerated adaptation in peripheral protein sequence resulting in newfangled viral strain. Van Woensel et al for instance have stated that viruses are the ubiquitous causes of lower respiratory tract disease in babies and youngsters. Later a variation of coronavirus associated with severe acute respiratory syndrome and human metapneumovirus, lately recognized as a new respiratory pathogen over the entire age range has emerged.2
And presently Nipah virus (NiV) another respiratory tract infecting virus with an estimated case fatality rate ranging from 40% to 75% is causing serious issue through the world.3 As indicated by WHO fact-sheet report, lower respiratory infection is ranked 1st and 6th leading cause of mortalities in low income countries and high income countries, respectively, and 4th leading cause of mortalities worldwide.4 The BSL-4 virus with this high rate of morbidity and mortality, up to some extent has strangled the virologist since past 10 years or so for the exigency for a novel therapeutics.5 Moreover, the conventional unidirectional serological diagnosis and therapeutics orchestrate numeral shortcomings such as cost deficit, sophisticated preparation procedures, efficient only on post-exposure prophylaxis and reduced amount of the product in consistent with human population and livestock to be vaccinated.5, 6 Majority of the therapeutics developed basically includes monoclonal antibodies targeting viral surface proteins such as F, G or M proteins.7, 8, 9 Further a number of broad spectrum antiviral agents such as nucleoside analogues namely rebavirin, GS-441524, GS-5734 (remdesivir), R1479 (balapiravir) and most recent drug favipiravir (T-705; 6-flouro-3-hydroxy-2-pyrazinecarboxamine) which is an inhibitor of viral RNA-dependent RNA polymerase have shown certain degree of efficiency against this virus.6 But lack of target specific action and probability of host cell toxicity are the major limitations of these synthetic drugs.10, 11 Taking into consideration, the severity of the infection and imperative necessity for a novel multidirectional, target specific and, non-toxic nature; the tunable nanotechnology based approaches seems to be a promising alternative. This technology harbors certain fascinating properties such as superparamagnetism, high surface plasmon resonance, luminescence, photon upconversion, bioavailability, biocompatibility, immunocompatibility / tolerability and biodegradability. The capacity to channelize through translucently breachable blood–brain barrier (BBB) and blood–air barrier (BAB), tunability and targeted control discharge are chief exclusive points which qualify them to be novel candidate for their utilization in biomedical therapy (antiviral).1, 12 The focal point of present review paper includes cellular molecular pathogenesis of NiV infection. Heterogeneous forms of nano-based approaches used as a means of therapy or diagnostic against other viruses, and finally, provised the future possibilities for nanotechnology in concern with the NiV detection and inhibition.
Cellular and molecular changes in NiV infection
In humans, severe acute respiratory sickness and/ or encephalitis can be provoked by a bat-borne zoonotic, paramyxoviral pathogen NiV.13, 14 This negative-sense single-stranded RNA virus belongs to the family Paramyxoviridae, genus Henipavirus and is closely related to Hendra viruses.13, 15 Like Hendra virus, NiV may also cause fatal pneumonia or an acute respiratory-distress-like syndrome.13, 16 It has been hypothesized that the respiratory disease transmission and pathogenesis are linked to infection of epithelial cells by the NiV at lower respiratory tract at the primary phase.17 Recently Escaffre et al have described two types of infection in association to NiV, namely NiV-M and NiV-B of human respiratory tract based on differentiated epithelial models of trachea and small airways due to their close anatomical resemblance with epithelial cells of human lower respiratory tract.13 According to the epidemiological reports NiV-M and NiV-B infections vary greatly in multiple aspects.18 For instance the percentage of respiratory disease and case fatality rate of NiV-B infections is much higher than NiV-M infection,19 whereas in NiV-M infection studies show that both human and hamster develop similar pathological changes such as presence of vasculitis, necrosis and inflammation. And certain neuronal changes such as antigen positive neurons, microvascular vasculitis and necrosis in hamster which in case of human progresses to encephalitis.18, 20
An extracellular pleomorphic bilayer envelope spiked with two glycoproteins namely trimeric fusion protein (F) and tetrameric attachment protein (G) at the exterior surface and matrix proteins (M) at the interior surface, encapsulates NiV virion.16, 21, 22 Further, the negative-sense single-stranded RNA, along with three other critically vital proteins namely nucleocapsid proteins (N), phosphoproteins (P) and large polymerase proteins (L) is contained within the virion made up of linear ribonucleoprotein (RNP).21 Binding of viral G to the conserved receptors ephrin-B2 and ephrin-B3 marks the beginning of host cell and viral membrane fusion mediated by prior complex formation of viral G and F protein.22
From an evolutionary point of view it could be assumed that, during initial epidemic period of NiV, it has developed a specific mechanism to evade innate immune action of Pterous bat. The evolutionary conservation of this particular immune mechanism within all mammals has made NiV a broad spectrum virus against them.23, 24 The virus blocks the elicitation of innate and adaptive immune response by inhibiting synthesis of cytokines, interferon type I (IFN α/β).25 The IFN α/β in case of viral infection, activate pathways within the viral infected cell which degrades viral mRNA and inhibits viral protein synthesis, hence hindering the rate of viral replication inside the infected cell and subsequently lowering viral load.26, 27 Song et al describe that IFN α/β can act as both autocrine and paracrine signaling molecule, where IFN α and IFN β are producers.28 Therefore it could be said that inhibition of IFN α/β synthesis may severely affect both autocrine and paracrine activity, which may further lead to impaired immune response (Barth et al 2016).29 The cellular and molecular changes during and post NiV infection starting right from NiV attachment and invasive pathogenesis leading to multiple organ failure have been explained in the Figure 1, Figure 2, Figure 3 . Understanding these changes will significantly improve present knowledge essential for developing and evaluating therapeutics against NiV.
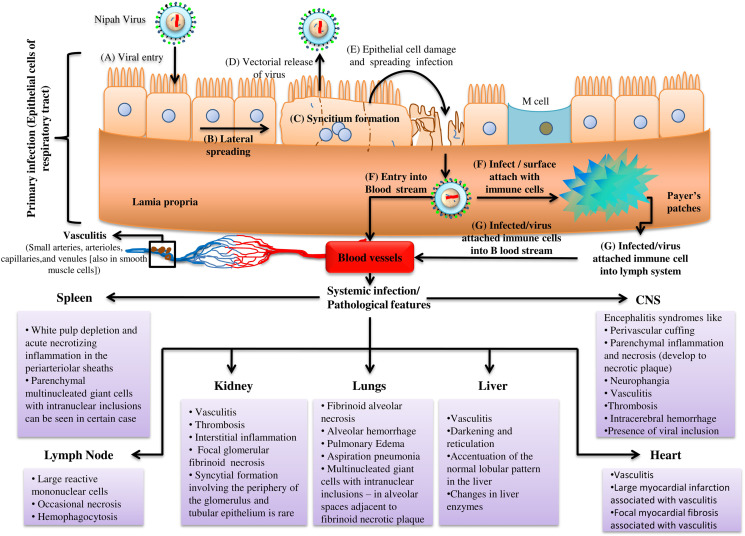
Figure 1.
General representation of Nipah virus infection and pathological features.
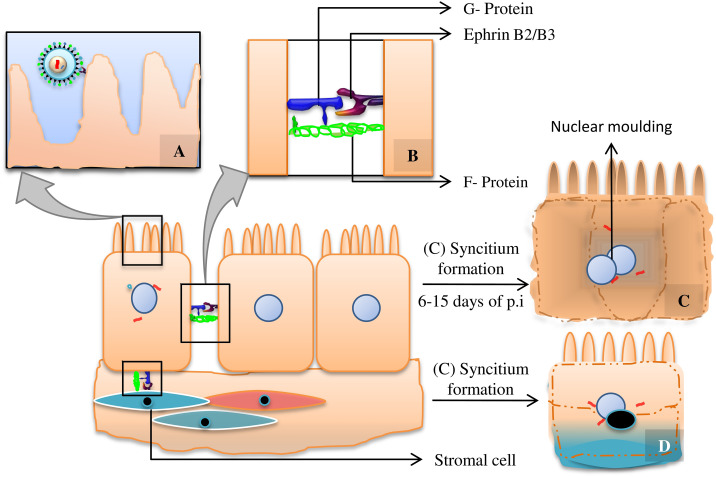
Figure 2.
(A) Viral attachment via interaction between G protein and cell surface receptor Ephrin B2/B3, (B) Interaction between G protein with receptor Ephrin B2/B3 and F protein, (C) Multinucleated giant endothelial cells due to homologous fusion of epithelial cells, (D) Multinucleated giant cell formation due to heterologous fusion of infected epithelial cell and stromal cell. The concept was derived from Wynne et al (2016).35
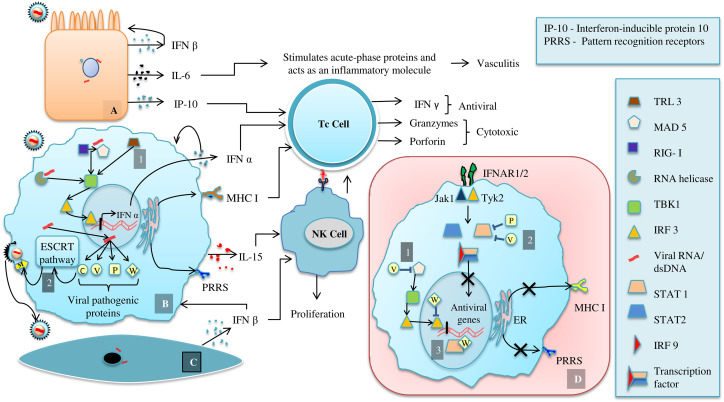
Figure 3.
Immune response to viral infection (A) Infected endothelial cell, (B) Infected Macrophage, (C) Infected stromal cell, (D) Schematic representation of immune suppressing mechanism of NiV proteins. The concept was taken from Prescott et al (2012)193; de Weerd and Nguyen (2012)194; Welsh et al (2012)195; Au-Yeung et al (2013)46; Basler (2012)37; Tanguy et al (2017).47
Cellular changes in detail
NiV infection in the primary stage is restricted within the epithelial cells of host's upper and lower respiratory tract.17 And NiV-F and G necessary for its fusion with the bipolar epithelial cell are at the epical side during initial infection period.30 Here, G protein induced virus-cell fusion begins with the interaction between tetrameric G-protein head region and Ephrin B2/B3 on the epithelial cell. Simultaneously C-terminus region of G interacts with NiV-F protein, whose two hydrophobic N-terminal alpha helical heptad repeat domains (HR-1, HR-2) form a six-helical bundle structure that transverses through cell membrane of the host initiating pH independent class I type membrane fusion (Figure 2).31, 32, 33 After the viral entrance through apical region, it replicates within the cell, sorting of proteins such as F and G on infected cell surface, is further destined for infection could also be regarded as markers.32, 33 Unlike G, precursor form of F protein, F0 get lodged within the membrane and activated through endocytosis followed by cathepsin L mediated endosomal degradation to form mature F1/2 (Cifuentes-Muñoz et al 2017).34
The local infection starts with the lateral spreading of virus occurred by basolaterally expressed protein (G, F) mediated cell–cell merging that gives rise to a consequential configuration of syncytia which are pathological hallmarks for NiV infection of epithelial cells. This has been seen at 14 h of post infection (p.i) which is the earliest time needed for protein detection in in vivo study.30 The cell–cell fusion can be between epithelial cell and stromal cell, heterologous fusion or between two epithelial cells, homologous fusion (Figure 2).35 After disrupting monolayer epithelial cells the newly formed virus buds out from the apical side of the multinucleated epithelial cells. This vectorial release of virus is confirmed by the preferential localization of viral F, G and necessary for budding, M protein on the apical side (88%, 86%, 95% of total G, F and M respectively), which can be seen after 24 h p.i.30 The released virus can either infect the immune cells of secondary lymphoid organs (Mucosal Associated Lymphoid Tissues [MALT], Gut Associated Lymphoid Tissues [GALT]) which are present just under the layer of mucosal epithelia cells. They might also contribute towards impairing antiviral activity by inhibiting synthesis of IFN α/β and reducing expression of cytokines involved in inflammatory process like CCL4, CCL5 and TNF-α. Again IFN induced antiviral genes such as MxA, ISG54 and repression of viral antigen presenting class-I MHC molecules might be another output (Figure 3, D).36, 37 The unconstrained virus could infiltrate the blood stream attaching to leukocytes such as macrophages, dendritic cells, CD6+CD8+ T cells, NK cells causing systemic infection and multiple organ failure in detailed brain, lungs, liver, heart, kidney and spleen ((Figure 1).38 Histopathological lesions are necrosis and vasculitis in non-CNS organs. In case of CNS organs, perivascular cuffing, parenchymal inflammation and neurophangia are most common clinical signs, but are not exclusive for NiV infection and may also be detected in other acute viral encephalitis (Figure 1).36, 39
Molecular changes in detail
With mortality rate of 40%-75% and an outrageous range of host, this atrocious human pathogen also infects neurons apart from endothelial cells.40 In general, paramyxoviruses evade host immune system either by suppressing its detection through lowering the expression of pattern recognition receptors (PRRS) or by impeding the secretion of antiviral signaling molecules.37 In case of NiV, viral P gene encoded post-transcriptionally modified phosphoproteins antagonize the secretion of IFN α/β and elicit antiviral activity at both cytoplasm and nucleolus.37, 41 The various proteins encoded by P genes are P, V, W and C with 407 amino acid length similarity at their N terminal end.41 V and W mRNAs are frame shift mutates P mRNA at a particular conservation site, with insertion of one and two G nucleotide respectively whereas the C mRNA is nested within each P, V, W mRNA.37, 42
The degree of immune response inhibition by each proteins P, V, W varies with P being less than V and W with highest inhibitory activity.37, 43 The elevated inhibition by W protein might be because of its nuclear localization ability.41, 47 This allows it to suppress expression of IFN α/β and antiviral gene at gene expression level and confirms lower probability of eliciting antiviral activity, than as happens in case of cytoplasmic inhibition by V proteins.37, 44, 45 The cytoplasmic inhibitory activity of V proteins is achieved by its binding with dsRNA responding RLR receptor MAD5 hence down regulating the activation of TBK1 and IKKε which activates IFN α/β gene transcription factor IRF3 and IRF7 (Figure 3, D.1). This suppression of IFN β gene expression can be overcome by another TLR3 mediated alternative pathway by producing more amount of adaptor protein IRF1 which activates TBK1 (Figure 3, B.1).37
Further, attenuation of IFNs responded antiviral activity of an infected cell can be discussed considering the JACK/STAT signaling pathway necessary for transcriptional level activation of antiviral genes.37, 46, 47 Responding to IFN α/β and γ, the interactions between phosphorylated STAT1 and STAT2 form their hetero or homodimer (by STAT1 only) respectively (Figure 3, D.2). These dimmers are precursor molecule of IFN α/β gene transcription factor.48, 49 Hence inhibitions of STAT1 and STAT2 interaction result in the absence of antiviral activity. Protein P, V and W interact with STAT1 with their amino terminal domain while only protein V can interact with STAT2.37 Re-localization of non-phosphorylated STAT1 from its typically cytoplasmic location by protein W also makes its unavailability for STAT dimer formation and hence inhibition of IFN α/β gene transcription factor formation (Figure 3, D.3).37 The viral C protein which is predominantly localized in cytoplasm and partly in nucleus is responsible for enveloped viral budding and release by recruiting host ESCRT pathway.50, 51 The C terminal domain of NiV-C, as it possesses homology in its sequence with host protein Vps28 could interact directly with TSG 101, an adaptor molecule between ESCRT I and ESCRT II. However, the reciprocal action among ESCRT I–NiV C and ESCRT II is not fully understood.50 But it has been speculated that viral C protein interacts with M protein present in the inner leaflet of plasma membrane and thereby direct vectorial release of virus particles (Figure 3, B.2).50, 52
Therapeutics against NiV
Guillaume et al developed a potent Vaccinia virus (VV) mediated VV-NiV-F and VV-NiV-G recombinant vaccines in a hamster model.53 They found immunized organism shows complete immunity against NiV induced fatal encephalitis by producing neutralizing antibodies whereas it occurs within 7 to 10 days after intraperitoneal inoculation of NiV isolates. The low neutralizing ability of the produced antibodies as confirmed by the increasing level of serum antibodies gives an idea of studying effect of passive immunization against NiV. Intraperitoneal injection of polyclonal as well as monoclonal (mAb) anti-F and anti-G, separately shows that anti-F and anti-G mAb are more efficient in neutralizing NiV viral infection as compared to polyclonal Ab, with anti-F mAb having high degree of antiviral activity than anti-G mAb.54 A recent hit on broad-spectrum small molecule antiviral purine analogue previously mentioned Favipiravir (T-705) which inhibits NIV and HeV replication in vitro with a minimal cytotoxic effect of highest concentration tested, having a CC50 value of >1000 μl (as observed in Hamster model) has provided a way to fight against the cytopathic effect of deadly NiV, HeV like paramyxoviruses.6
It is not late when one day due to the consistent change in their genome these pathogens would get resistance to the conventional drugs which are unidirectional and have no specific targeted. These limitations could be ameliorated by finding a novel therapy with multiple pathways and biomarkers for the target oriented action. For example in NiV depletion of NiV-C recruited ESCRT pathway mechanism and deposition of other viral proteins (N, M, F, G and L) results in cytoplasmic budding and impaired release of virus from the infected cell.50, 51 There are numerous intermediate molecules associated with ESCRT pathway; targeting conserved intermediate molecules or their precursor might be a novel strategy to eliminate the virus. Further the ligands or receptors with a binding efficiency with viral intermediate molecules could be further enhanced by their amalgamation with nanotechnology.1
Nano-based antiviral therapy
The versatile mode of viral inhibition primarily depends on the type and form of NPs used. Therefore to understand the antiviral activity of NPs a general overview about different types and forms of NPs/ nano-based systems is a necessity. Nano-based antiviral agents extend from simple inorganic NPs to complex organic and hybrid nanosystems. Metallic NPs (MNPs) and NCs in the form of nanospheres, nanocapsule and nanocage could be categorized under inorganic NPs (INPs). Organic NPs (ONPs) include nanocapsule as in the form of NCs, primarily differentiated based on chemical composition. Hybrid nanosystems are standardized amalgamation of inorganic–organic, inorganic–inorganic (nanocomposites), and organic–organic nanoparticulated systems (lipid–polymer hybrid NPs) engineered based on specific requirement or use at targeted site.55
Inorganic nanoparticles
The other properties such as luminescence, tunable size, shape, composition and large surface-to volume ratio have invigorated the importance of INPs in biomedical field for multifarious application. Among these applications the ability of these INPs to expose their multiple surface binding sites as well as control their in vivo behavioral properties is widely explored.12 This phenomenon of multiple interactions at a specific site with the targeted molecule further contributes towards the utilization of these NPs in active targeted imaging for diagnostics, hyperthermia therapy and medication.56 The most common iNPs are MNPs such as silver (Ag), gold (Au), iron oxide (FeO) and NCs of silica, titanium, carbon etc.
Silver nanoparticles (AgNPs)
Silver nanoparticles (AgNPs) are most keenly researched nano-based approach to treat viral infection or their detection. Numerous evaluations of the NPs to develop a novel strategy to either annihilate or ameliorate the severity of infection had already been carried out. To date the efficiency of AgNPs as an antiviral has been checked in Human immunodeficiency virus (HIV)-1, Herpes simplex virus (HSV)-1, 2, 3, Feline Calicivirus, Poliovirus type-1, Peste des petits ruminants virus (PPRV), Murine norovirus-1, Avian influenza A virus subtype H7N3, Coxsackievirus B3, Infectious bursal disease virus and S. cerevisiae dsRNA viruses (Table 1 ). The AgNPs based mechanism for viral inhibition or virucidal activity varies virus to virus. For example Lara et al elucidated the antiviral efficacy of commercially available AgNPs against HIV-1 and some resistant strain such as IIIB, Eli, Beni, 96USSN20, Bal, BCF01, AZTRV, NNRTIRV, PIRV, 3TCRV, and SaquinavirRV along with NP's mode of action.57 Normally gp120 interaction with host receptor CD4 and associated co-receptor results in further conformational changes in virus, exposing gp41 which concordantly interacts and releases viral core into cytoplasm.58 Their findings suggested that AgNPs impedes CD4-dependent virion binding, merging and pathogenesis by interacting with viral gp120 in both cell-free and cell-associated virus.58 In case of HSV-2 the viral inhibition is portrayed by interacting with sulfhydryl group present on the membrane glycoproteins which prevent viral internalization. In Peste des petits ruminants' virus the NPs act by interacting with virion surface and core protein, impairing viral replication and entry.59, 60 Likewise in dsRNA viruses the AgNPs after interaction with viral genome, the NPs were found to inhibit viral replication.61 The antiviral efficacies of AgNPs by these modes of viral entry or attachment inhibition or viral replication inhibition have also been evaluated in other viruses shown in Table 1.
Table 1.
Inorganic Nano-Based Approach for Viral Diagnosis and Inhibition.
| Nanoparticles | Virus | Model Organism | Mechanism of Antiviral Action | Purpose | References | |
|---|---|---|---|---|---|---|
| Silver (Ag) | ||||||
| AgNPs | Human immunodeficiency virus (HIV)-1 | HeLa-CD4-LTR-β-gal cells, MT-2 cells, human PBMC | Interaction with gp120 in order to prohibits CD4-based virion binding, blending, and pathogenesis | Viral entry inhibition and as virucidal agent | Lara et al (2010)57 | |
| Coxsackie virus B3 Nancy strain | Vero cells | Fusion inhibition between virus and cells | Viral entry inhibition | Salem et al (2012)138 | ||
| Herpes Simplex Virus (HSV)-1, 2, 3 | Vero cells | Binds with viral envelop or its protein | Viral entry inhibition and as virucidal agent | Gaikwad et al (2013)139 | ||
| HSV-2 | Vero cells | Bonding with sulfhydryl groups on membrane glycoprotein of virus | Prevents viral internalization | Hu et al (2014)59 | ||
| Peste des petits ruminants (PPR) virus | Vero cells | Interaction with the virion surface as well with the virion core | Impaired viral replication during viral entry | Khandelwal et al (2014)60 | ||
| Avian influenza A virus, subtype H7N3 | Vero cells | Blocking HA function and anomalous interaction viral replication pathway | Viral entry inhibition and possible deformation of viral replication | Fatima et al (2016)140 | ||
| AgNPs immobilized on textile fabrics | Influenza A and Feline Calicivirus | Interaction with viral envelope | Impaired viral envelope resulting viral inhibition | Seino et al (2016)141 | ||
| AgNPs | Poliovirus type-1 | Human Rhabdomyosarcoma | Interaction with viral protein resulting in impaired interaction with the host cell | Inhibition of viral internalization | Huy et al (2017)142 | |
| Murine norovirus-1 | RAW 264.7 cells | Virucidal agent | Castro-Mayorgaa et al (2017)143 | |||
| Feline calicivirus | CRFK cells | |||||
| Infectious Bursal Disease (IBD) virus | Embryonated chicken eggs | Interaction with viral envelope | Viral inhibition | Pangestika et al (2017)144 | ||
| S. cerevisiae dsRNA viruses | HeLa cells, NIH/3 T3 cells | Interaction with viral genome and inhibition of viral replication | Virucidal agent | Rónavári et al (2017)61 | ||
| Titanium (Ti) | ||||||
| Nanoparticles | Biomolecule Loaded/ Conjugated | Virus | Model Organism | Mechanism of Antiviral action | Purpose | References |
| TiO2 NPs | - | MS2, PRD1, ΦX174, Fr | - | Interaction with viral capsid protein (alanine, glycine, and proline residues) | Photocatalytic inactivation of phages | Gerrity et al (2008)74 |
| TiO2 NPs | - | H3N2 | - | Direct contact with virus | Virucidal agent | Mazurkova et al (2010)75 |
| TiO2 particles | - | MS2, ΦX174, PR772 | - | Interaction with viral capsid/ surface proteins | Photocatalytic inactivation of phages | Misstear and Gill (2012)145 |
| TiO2~DNA nanocomposites | DNA | H1N1, H5N1, and H3N2 | MDCK cells | Targeted binding to conservative regions in the viral genome and inhibition of viral reproduction | Viral inhibition | Levina et al (2015)146 |
| TiO2 NPs | - | MS2 | - | Interaction with viral surface proteins | Inactivation of phages | Syngouna and Chrysikopoulos (2017)76 |
| Gold NPs | ||||||
| Au NPs | - | HIV-1 | HeLa-CD4-LTR-B-gal cell | Binding with viral gp120 and prevent CD4 attachment | Inhibition of viral entry | Vijayakumar and Ganesan (2012)62 |
| Au NPs | - | Foot- and- mouth diseases virus (FMDV) | BHK-21 | Arrests viral replication along with transcription | Virucidal agent | Hafashjani et al (2016)63 |
| Au NPs | - | Influenza virus A (H1N1, H3N2) | Peroxidase-mimic enzymatic reaction | Viral detection | Ahmed et al (2016)64 | |
| Gold NPs (AuNPs) | Synthetic peptide resembling FMDV protein |
FMDV | BALB/c mice | Antibody mediated immunity | Size dependent immunization | Chen et al (2010)147 |
| AuNPs | DNAzyme (DDZ) | Dengue virus (DENV) | Aedes albopictus C6/36 cells | DDZ activation mediated salt-induced aggregation of AuNP | Viral detection | Carter et al (2013)148 |
| AuNPs | Anti-A/Udorn/307/1972 antibody | H3N2 | - | Nanodot deposition on the periphery of viral surface | Viral detection | Gopinath et al (2013)149 |
| AuNPs | Small interfering RNA | DENV | Vero cells | Inhibiting viral replication and infectious virion release | Efficient delivery and viral inhibition | Paul et al (2014)150 |
| AuNPs | Viral matrix 2 protein (M2e) | H1N1 | BALB/c female mice | Antibody mediated CpG (cytosine-guanine rich oligonucleotide) immunity | Influenza vaccine | Tao et al (2014)151 |
| AuNPs | Monoclonal anti-hemagglutinin antibody (mAb) | H3N2 | - | Viral surface deposition of NPs- mAb | Colorimetric immunosensor viral detection | Liu et al (2015)152 |
| AuNPs | Recombinant trimetric A/Aichi/2/68 (H3N2), Hemagglutinin (HA) and TLR5 agonist flagellin (FliC) | H3N2 | Female BALB/c mice, HEK 293 T cells, JAWS II cells | Antigen-specific T cell-mediated immunity | Improved immunization | Wang et al (2017)153 |
| Unmodified AuNPs | Charge-neutral peptide nucleic acids (PNA) | Bovine Viral Diarrhea virus (BVDV) | - | BVDV-RNA based PNA induced aggregation of the AuNPs | Colorimetric based viral detection assay | Askaravi et al (2017)154 |
| AuNPs | Viral consensus matrix 2 peptide (M2e) | H1N1, H3N2, H5N1 | BALB/c mice, | Strong humoral and cellular response | Universal influenza A vaccine | Tao et al (2017)155 |
| AuNPs | Recombinant viral Hemagglutinin (HA) | H3N2 | BALB/c mice | Higher level of viral specific IgA and IgG, promotion of antigen-specific interferon-γ (IFN-γ)-secretion, CD4+ cell proliferation and induced activation of strong effector CD8+ T cell | Enhanced mucosal cellular immunity | Wang et al (2018)156 |
| Toll- like receptor 5 (TLR5) agonist flagellin (FliC) | ||||||
| Iron (Fe) | ||||||
| Fe3O4 NPs | Antibodies against viral HA protein, capped with methoxy-terminated ethylene glycol | H5N2 | - | - | Rapid and specific viral detection | Chou et al (2011)69 |
| Magnetic iron oxide NPs (γ-Fe2O3, α-FeOOH) | - | Bacteriophage MS2 | - | Virus removal | Park et al (2014)157 | |
| Magnetic NPs | Aptamers specific for binding to the E1E2 glycoprotein of HCV | HCV | - | Aptamer targeted viral envelope protein binding |
Efficient removal of viral particles | Delaviz et al (2015)71 |
| Iron Oxide NPs | Anti-zika envelope protein (ZENV) antibody, AXL receptor, HSP70 receptor, TIM-1 receptor | Zika virus | - | AXL has the highest affinity for virus; HSP70, TIM-1, and phosphatidylserine might also play active roles in viral tropism | Timely and sensitive analysis of host pathogen interaction | Shelby et al (2017)66 |
| Silica (Si) based NPs | ||||||
| SiNPs | Biotin derivative dCTP bases | Human Papilloma Virus | - | Nucleic acid hybridization | Viral detection | Enrichi et al (2010)85 |
| Hollow Mesoporous SiNPs |
Porcine circovirus type 2-ORF2 protein | Porcine circovirus | BALB/c mice | Stimulate antibody and cell-mediated immune responses | Immunization against the virus | Guo et al (2012)84 |
| SiNPs | Hepatitis B virus core (HBc) protein | Hepatitis B virus | BALB/c mice | Activates antibody and cell-mediated immune responses | Silica-adjuvant vaccines against the virus | Skrastina et al (2014)158 |
| Mesoporous SiNPs | Glycosaminoglycans | HSV-1, 2 | Vero cells | Inhibition of viral attachment to the host cell | Inhibition of viral entry | Lee et al (2016)159 |
| Mesoporous SiNPs | Alkoxysilane (Hydrophobic, Hydrophilic) | GFP lentiviral vector harboring a vesicular stomatitis virus G glycoprotein, GFP lentivirus harboring an HIV-gp120 derived envelope | HEK293T cells | Attachment with the viral envelope protein | Immobilization of the virus resulting in inhibition of viral entry | de Souza et al (2016)82 |
| Europium doped fluorescent SiNPs | Streptavidin | HIV (HIV-1 p24 antigen) | - | Binding to HIV-1 p24 antigen | Fluorescence based sandwich immunoassay detection of virus | Chunduri et al (2017)83 |
| Carbon (C) based NPs | ||||||
| Fullerenes | ||||||
| Glycofullerenes | Carbohydrate moieties (Mannose) | Pseudotyped viral particles (Ebola virus) | Jurkat cells | Blocking the dendritic cell–specific intercellular adhesion molecule-3–grabbing non-integrin receptor (DC-SIGN) | Inhibition of viral attachment leading to inhibited viral entry | Luczkowiak et al (2013)88 |
| Fullerenes | Maximin H5 peptide | Bacteriophage λ | - | Attachment with viral capsid or envelope and inhibiting viral entry / disruption of viral lipid layer lyses if of virion / inhibition of viral replication | Virucidal agent | Dostalova et al (2016)90 |
| Fullerene derivatives 1, 2, 3 and 4 | HIV-1 (Wild type) | SupT1 cells | Inhibition of Gag processing through a protease-independent mechanism | Blocks viral maturation | Martinez et al (2016)160 | |
| HIV-1 (Resistant virus) | SupT1 cells | Impairing viral polyprotein processing through a protease-independent mechanism | ||||
| Globular multivalentglyco fullerenes (Tridecafullerene) | Carbohydrate moieties (Mannose) | Pseudotyped viral particles (Ebola virus) | Jurkat cells | Blocking of DC-SIGN | Inhibition of viral attachment leading to inhibited viral entry | Muñoz et al (2016)161 |
| Glycodendrofullerenes | Carbohydrate moieties (Mannose, Galactose) | Pseudotyped viral particles (Ebola virus glycoprotein) | Jurkat cells | Blocking of DC-SIGN | Inhibition of viral attachment leading to inhibited viral entry | Muñoz et al (2017)162 |
| Carbon nano-tubes | ||||||
| Acid-functionalized multi-walled carbon nanotubes (MWNTs) |
Protoporphyrin IX (PPIX) | H3N2 | NCIH292 | Photoactivated PPIX mediated interaction with viral envelope and its destabilization | Inactivates the virus | Banerjee et al (2012)87 |
| Functionalized single-walled carbon nanotubes (SWCNTs) | Recombinant VP7 subunit vaccine | Grass carp reovirus (GCRV) | Grass carp | VP7 subunit vaccine mediated immunity against virus | Immunization | Zhu et al (2014)89 |
| Ammonium-functionalized SWCNT | pEGFP-vp5vaccine | GCRV | Grass carp | Activation of pro-inflammatory factors (IL-1β, TNF-α) and DNA vaccine mediated immunity against virus | Immunization | Wang et al (2015)163 |
| SWCNT | Ribavirin | GCRV | Grass carp | Affects viral transcription | Viral inhibition | Zhu et al (2015)135 |
| SWCNT | pcDNA-vp7 | GCRV | Grass carp | Activation of pro-inflammatory factors (IL-1β, TNF-α) and DNA vaccine mediated immunity against virus | Immunization | Zhu et al (2015)165 |
| Carbon nanohorns | ||||||
| Carbon nanohorns | T7 tag antibody | T7 bacteriophage | Attachment to the viral surface and photo-exothermic destruction | Viral elimination | Miyako et al (2008)166 | |
| Graphene | ||||||
| Graphene oxide (GO) | Cationic polymer PDDA, nonionic PVP | Pseudorabies Virus, porcine epidemic diarrhea virus |
Vero cells, PK-15 cells | Negatively charged GO interacts with viruses prior to viral entry resulting in virus damage due to its single-layer structure and sharp edge | Viral inhibition | Ye et al (2015)167 |
| β-cyclodextrin (CD) functionalized-GO | Curcumin | Respiratory syncytial virus (RSV) | HEp-2 cells | The NP mimics heparin sulfate on the host cell surface and inhibits viral attachment | Viral inhibition | Yang et al (2017)91 |
| Quantum dots | ||||||
| Quantum dots | Streptavidin | RSV | HEp-2 cells | Streptavidin interact with G-protein in virus | Viral detection | Bentzen et al (2005)168 |
| Carbon dots | ||||||
| Carbon dots | - | Pseudorabies virus, porcine reproductive and respiratory syndrome virus |
MARC-145 cells, PK-15 cells | Inhibits viral replication by inducing type I interferon production | Viral inhibition | Du et al (2016)169 |
| Carbon dots | - | Human NoV GI.1 virus-like-particles (VLPs), GII.4 VLPs | - | Inhibits viral attachment to histo-blood group antigens (HBGA) receptors of host cell | Viral inhibition | Dong et al (2017)170 |
Gold nanoparticles (AuNPs)
Unlike AgNPs though AuNPs could also be synthesized by green synthesis method but their direct use as an antiviral agent is scant. Except in some findings, that too when, AuNPs stabilized with certain biocompatible polymer could act as an effective antiviral agent against HIV-1, H1N1, H3N2, H5N1, dengue virus, bovine viral diarrhea virus and Foot-and-mouth disease virus (FMDB).62, 63, 64 Vijayakumar and Ganesan et al had speculated that the mode of antiviral activity of polyethylene glycol encapsulated AuNPs against HIV-1, might be by blocking gp120 attachment with CD4, which results in inhibited viral entry.62 Hafashjani et al in their finding later suggested that AuNPs arrested FMDV replication at post-entry stage, associated specifically with transcription within the host cell.63 But the exact virucidal mechanism is unclear. The currently used AuNPs in combination with other nano-based formulation are applied for photothermal nanotherapy, bio-sensing, bio-imaging, catalytic activities.65 The unique property of AuNPs contributing to these applications is different from its bulk form that is, Surface Enhanced Raman Scattering (SERS).66 For example Ahmed et al described the capability of positively charged AuNPs for H1N1 and H3N2 detection by mimicking peroxidase enzymatic reaction assay. Likewise there are other examples where hybridized AuNPs demonstrated effective antiviral activity (Table 1).64
Magnetic nanoparticles (MNPs)
Iron oxide or superparamagnetic iron oxide in the form of either magnetite (Fe3O4) or maghemite (Fe2O3, γ-Fe2O3) NPs is the most imperative component of present day magneto responsive nanoparticulated systems.67 These iron based nanoparticulated systems have created a tremendous impact in biomedical/ clinical diagnostics including magnetic resonance imaging, magnetic particle imaging, magnetic fluid hyperthermia and magnetic labeling and cell separation.68 Therefore the direct application of MNPs as a therapeutic is limited. Presently the direct use of MNP as an antiviral agent has been evaluated against Bacteriophages such as MS2 and other highly infectious viruses like zika virus, HCV, H5N2 (Table 1).69, 70, 71, 72 There have been many other findings of exploiting MNPs but their application in hybridized form is much more appreciated, which is described later under Hybrid nanoparticles (Table 3).
Table 3.
Hybrid Nano-Based Approach for Viral Diagnosis and Inhibition.
| Nanoparticles | Bioactive Compound | Virus | Model Organism | Mechanistic Mode of Action | Purpose | References |
|---|---|---|---|---|---|---|
| Nano-based viral detection | ||||||
| Silica (SiO2) coated magnetic Fe3O4 NPs | - | Hepatitis B virus (HBV), Epstein–Barr virus (EBV) | - | - | Higher sensitivity in PCR-based viral detection | Quy et al (2013)180 |
| Au102 (paramercaptobenzoic acid)44 clusters |
- | Enterovirus, Echovirus 1 and Coxsackie virus B3 | - | Site-specific covalent Conjugation on viral surface protein |
Viral targeting | Marjomäki et al (2014)126 |
| Gold/Copper Sulfide Core/Shell NPs | - | Human Norovirus Virus-Like Particles | - | Capsid protein degradation and capsid damage | Viral detection and inactivation | Broglie et al (2015)124 |
| AuNPs-carbon nanotubes (CNTs) hybrid | Antibody | H3N2 | Peroxidase-like activity of the nanohybrid | Colorimetric viral detection assay | Ahmed et al (2016)127 | |
| Chiral AuNPs-quantum dot nanocomposites | - | Avian influenza A (H4N6) virus, fowl adenovirus and coronavirus | - | Chiral plasmon–exciton systems | Viral detection | Ahmed et al (2017)181 |
| Graphene-AuNPs nanohybrid | Anti-NoV antibody | Norovirus-like particles | - | Intrinsic peroxidase-like activity | Colorimetric immunoassays for viral detection | Ahmed et al (2017)182 |
| Glycan-functionalized AuNPs (gAuNPs) | - | hRSV, vieH5N1, anhH5N1, guaH5N1, shaH5N1, guaH5N6, hebH5N8, wsnH1N1, shaH1N1, mosH3N2, aicH3N2, leeB, yamB, shaH7N9, anhH7N9 | Vero cell | Aggregation of gAuNP probes on the viral surface | Viral detection and differentiation | Zheng et al (2017)183 |
| Poly(DL-lactide-co-glycolide) (PLGA) encapsulated superparamagnetic iron oxide NPs | Clocking with RBC membrane vesicles with surface rich in sialic acids - |
H1N1 | - | Virus targeting and isolation via magnetic extraction | Enhanced viral detection | Chen et al (2017)184 |
| Silica-shelled magnetic (Fe3O4) nanobeads (MagNBs) and AuNPs | - | H1N1, H3N2 | - | MagNB mediated target separation and signal amplification by the enzyme-like activity of AuNZs | Ultra-sensitive colorimetric assay (magnetic nano(e)zyme - linked immunosorbent assay [MagLISA]) for viral detection |
Oh et al (2018)128 |
| Gold (Au)/iron-oxide magnetic NP-decorated Carbon nanotubes (CNTs) (Au/MNP-CNT) | Thiol-group-functionalized probe DNA | H1N1, norovirus | - | DNA hybridization | High sensitivity and selectivity detection of viral DNA | Lee et al (2018)125 |
| Nano-based viral inhibition | ||||||
| Silica doped TiO2(P25) NPs | - | Bacteriophage MS2 | - | Hydroxide radical mediated catalytic inactivation | Viral inactivation | Jafry et al (2011)123 |
| Photocatalytic silver doped titanium dioxide (nAg/TiO2) NPs |
- | Bacteriophage MS2 | Hydroxide radical mediated catalytic inactivation | Viral inactivation | Liga et al (2011)185 | |
| Dextran-coated magnetic iron oxide NPs labeled with Cy5.5 fluorescence dye | DNAzyme, cell-penetrating peptide | Hepatitis C virus (HCV) | BALB/c mice, C57BL/6N mice | Silencing of HCV NS3 gene expression resulting in inhibited viral replication | Efficient delivery and viral inhibition | Ryoo et al (2012)129 |
| Fullerene-liposome complex | - | H1N1 | BALB/c mice | Reactive oxygen species regulation | Viral inhibition | Du et al (2012)186 |
| Tannic acid modified AgNPs | - | HSV-2 | C57BL6 mice | Direct inhibition of virus attachment, penetration and post-infection spread | Viral inhibition | Orlowski et al (2014)130 |
| Aminopropyl-functionalized Fe3O4-SiO2 core-shell magnetic hybrid colloid (MHC) decorated AgNPs | - | Bacteriophage ɸX174, Murine norovirus (MNV), Adenovirus serotype 2 (AdV2) | - | Damages viral coat proteins | Viral inhibition | Park et al (2014)157 |
| Silver nanorods conjugated with sodium 2-mercaptoethane sulfonate (Ag-MES) |
- | HIV, HSV-1 | - | Inhibition of viral replication | Viral inhibition | Etemadzade et al (2016)187 |
| Oseltamivir modified AgNPs (Ag@OTV) | - | H1N1 | MDCK cells | Inhibits the activity of Neuraminidase (NA) and Hemagglutinin (HA) prevents viral attachment, inhibit the accumulation of reactive oxygen species (ROS) by virus, activates AKT and p53 phosphorylation | Viral inhibition | Li et al (2016)188 |
| Titanium dioxide nanoparticles and polylysine (PL)-containing oligonucleotides (TiO2·PL-DNA) nanocomposite | Polylysine (PL)-containing oligonucleotides | H1N1, H5N1, H3N2 | MDCK cells | Targeted binding to conservative regions in the viral genome and inhibition of viral reproduction | Viral inhibition | Levina et al (2016)136 |
| Graphene oxide (GO) sheets | - | Feline Coronavirus (FCoV) | fcwf-4 cells, DF-1 cells | Association with viral lipid tails leading to aggregation and rupture of the envelop | Viral inhibition | Chen et al (2016)131 |
| Bursal Disease Virus (IBDV) | Inefficient in binding | Viral release | ||||
| GO sheets with silver particles (GO-Ag) | - | Feline coronavirus (FCoV) | Association with viral lipid tails leading to aggregation with attachment of AgNPs with –SH group of protein and rupture of the envelop | Viral inhibition | ||
| Infectious Bursal Disease Virus (IBDV) | AgNPs mediated interaction with -SH group of viral protein | Synergistic effect | ||||
| Polyethylenimine (PEI) encapsulated AgNPs | Small interfering RNA (siRNA), | Enterovirus 71 (EV71) | Vero cell | Block EV71 from infecting host cells and prevent DNA fragmentation, chromatin condensation and activation of caspase-3 | Viral inhibition | Li et al (2017)189 |
| OTV decoration of SeNPs (Se@OTV) | H1N1 | MDCK cells | Inhibits the activity of Neuraminidase (NA) and Hemagglutinin (HA) prevents viral attachment, inhibit the accumulation of reactive oxygen species (ROS) by virus, activates AKT and p53 phosphorylation | Viral inhibition | Li et al (2017)134 | |
| Alginate (ALG) and stearic acid- poly ethylene glycol (SA-PEG) hybrid NPs |
Zidovudine (AZT) | HIV | Glioma Neuro2a, HeLa cells | - | Efficient drug delivery | Joshy et al (2017)176 |
| Mannosylated Niosomal system | AuNPs and Efavirenz (EFV) | HIV-1 | HeLa cells | - | Viral inhibition | Malik et al (2017)190 |
| AuNPs and AgNPs | Peptide FluPep | H1N1, H3N2, H5N1 |
MDCK cells | - | Efficient delivery and viral inhibition | Alghrair et al (2018)191 |
| AuNPs Covered with SiO2 and SiO2 carrier conjugated with AuNPs | - | Human Adenovirus | MDCK cells | Interaction with viral surface protein leading to coagulation | Antiviral and virucidal action | Lysenko et al (2018)192 |
| Surface decoration of selenium NPs by Amantadine (AM) (Se@AM) | - | H1N1 | MDCK cells | Induces apoptosis, inhibits generation of ROS, and activates phosphorylation and AKT pathway | Viral inhibition | Li et al (2018)132 |
Titanium nanoparticles (TiNPs)
Due to its brightness and high refractive index, titanium dioxide (TiO2) a white pigmented molecule is widely used for a number of purposes. In various sectors of science and industries its nanoform that is TiO2NPs is more abundantly produced and used specifically because of their high solubility, photocatalytic and anticorrosive properties.73 Although their application in biomedical sector is marvels but still than their there is a large gap between classic and current research. Out of every possible implementation that could bridge this gap, antiviral application of TiO2NPs is one of them. Present antiviral evaluation of TiNPs is only limited to influenza virus (H3N2) and certain bacteriophages such as MS2, PRD1, ɸX174, Fr and many others.74, 75, 76 Moreover, the existing toxicity evaluations of the NPs though cannot completely eliminate health risks therefore, their biomedical application as an antiviral agent is questionable.77 But considering beneficial role of TiNPs in biomedical sector, its antiviral activity however, needs to be perfectly conceptualized in animal models too.
Silica nanocarriers (SiNCs)
NPs based on silica due to their unique characteristics like tunable diameter, pore size, convenience in functionalization, biocompatibilities etc. are well appreciated for research all over the globe.78, 79 Apart from these, there are two other important properties, which display manipulative applicable properties including self- immobilization of ligands onto surface of SiNP and stimuli-responsive gatekeepers.80 The practical application lies in their capability to control or accelerate release of the drug payloads simultaneously excluding premature release. Currently, exploiting these properties for photodynamic therapy by functionalizing SiNPs with photosensitizers, is one of the fastest emerging research.79
There are different forms of SiNPs, categorized as porous, mesoporous and non-porous and are utilized for diverse biomedical application.78, 81 Out of the multiple biomedical applications of SiNPs, the present review focuses on antiviral activity as the research in antiviral therapeutics or diagnostics, exploiting silica based NPs is limited.82, 83 Currently antiviral efficacy of SiNPs against some viruses like Human Papilloma Virus, Porcine circovirus, Hepatitis B virus, HSV-1, 2, HIV and recombinant viruses has been evaluated (Table 1). And the mode of antiviral activity is either mediated by immunization of the host against virus or by inhibiting viral entry.82, 84 Viral protein based fluorescent detection and nucleic acid hybridization are the two major methods of viral detection.83, 85 Despite the progress made in the therapeutics and diagnostics against viral infections, an achievement that cannot be questioned, the growing demand for a novel antiviral therapeutics or diagnostics can only be supplemented by further research and evaluations.
Carbon-based nanocarriers
Carbon-based nanocarriers are among the most frequently and widely evaluated nanoparticulated systems in biomedical application. The carbon-based materials such as fullerenes, carbon nano-tubes, nanohorons, nanodots, grapheme and nanodiamonds etc. that also possess some of the above mentioned inimitable properties such as optical, electronic, mechanical and thermal properties have gained themselves much attention in the past decade. Among various beneficiary roles, biocompatibility, in vivo biodistribution, biodegradation and bio-corona formation etc. are few crucial properties that constantly impress the present scientific community till today.86 Although carbon-based nanomaterials are supported by these spectacular advantageous properties, but still their utilization and evaluation in the field of virology are limited to some viruses (Table 1). Antiviral or virucidal activities of these carbon-based nanomaterials have been evaluated against some viruses such as Ebola virus (pseudotyped viral particles), Bacteriophage λ, HIV-1, H3N2, Grass carp reovirus (GCRV) and Respiratory syncytial virus (RSV).87, 88, 89, 90, 91 Various modes of viral inhibition by carbon-based nanomaterials used as carriers are represented in Table 1. Advancement in the evaluation of carbon-based nanosystems against viral infection, though have developed immensely, but their commercialization is a rate-limiting phase and will be resolved with respect to time.86, 92
Quantum dots
The nano-sized semiconductor crystals commonly known as quantum dots (QDs) with their exceptional electronic and optical properties are highly appreciated.93, 94 Specifically intensive fluorescence, high quantum yield, size-tunable light emission, appreciable chemical and photo-stability have revolutionized the concept of nano-based sensing or diagnostics. General elemental composition of QDs includes cadmium (Cd), lead (Pb), mercury (Hg) and zinc (Zn). But recently developed ternary I-III-VI QDs contain elements in different form, here “I” represents cupper (Cu) or silver (Ag), “III” represents gadolinium (Ga) or indium (In) and “VI” represents sulfur (S) or selenium (Se).94 Owing to their extremely small size ranging from 2 to 10 nm QDs are well appreciated and used in biomedical sector as they could conveniently cross the BBB in conjugation with a therapeutic molecule.94, 95 Mahajan et al showed that fluorescent quantum rods (QRs) a form of QD in conjugation with transferring, a targeting molecule and HAAT (Highly Active Antiretroviral Therapy) drug Sequinavir could freely cross an in vitro model of BBB. They also showed that the nano-formulation showed promising antiviral activity against HIV-1.96 Viral detection by QD based approaches is exceedingly escalating research of virology. Norouzi et al developed a cadmium–tellurium QD in association with a biotin acceptor and NH2-receptor probes with target DNA for recognition of human T-lymphotropic virus-1 (HTLV-1) in vitro.97 Likewise, there are many other evaluations where QDs have been utilized either as therapeutics or for in vitro diagnosis of viral pathogens.
Organic nanoparticles
If the size of therapeutic compound is very large, then INPs might not prove promising in carrying or delivering the desired compound. In those conditions Organic nanoparticles (ONPs) might prove beneficial in order to efficient carry or deliver the drug load.98 Further the use of ONPs over INPs is well appreciated in biomedical sector specifically due to various safety issues.99 Encapsulation of drugs favors avoiding off-target toxicity which results in enhanced augmentation at targeted site. Again, the mode of delivery of therapeutics could be modulated by selecting specific design features and chemical requirements for the synthesis purpose. Since, sustained release kinetics can directly influence therapeutic competence and toxicity of the nanosystem.98 Multiple forms of ONPs are exploited in field of nanomedicine but only certain specific ONPs are presently being evaluated for their antiviral activity. These include polymeric NPs, lipid based NPs, dendrimers, neosomes and nanomicelles (Table 2 ).
Table 2.
Organic Nano-Based Approach for Viral Diagnosis and Inhibition.
| Nanoparticles | Bioactive Compound | Virus | Model Organism | Mechanistic Mode of Action | Purpose | References |
|---|---|---|---|---|---|---|
| Polymeric NPs | ||||||
| Poly (D,L-lactide-co-glycolide) NPs | Hemagglutinin (HA) | H1N1 | - | Unaltered molecular weight and antigenicity | Future viral detection and immunization | Lemoine and Préat (1998)100 |
| p-HA | H1N1 | Female Balb/c mice | Enhanced antigen particulate hydrophobicity | Immunization | Lemoine et al (1999)171 | |
| N-Trimethyl chitosan (TMC) NPs | Monovalent influenza (H3N2) subunit vaccine | H3N2 | Female C57BL/6 (B6) mice | Enhanced systemic and local immune responses | Effective carrier for nasal delivery of influenza antigens | Amidi et al (2007)172 |
| Polystyrene NPs | Mannose-specific lectin concanavalin A | HIV-1 | - | Mannose-specific lectin concanavalin A and viral gp120 antigen binding | Mucosal vaccine | Wang et al (2007)173 |
| Poly (ε- caprolactone) (PCL) NPs coated with poly(ethylene oxide) (PEO) | Dapivirine | HIV-1 | CaSki, Caco-2, VK2/E6E7, TZM-bl cells, pig tissue models of vaginal and rectal mucosa | NPs differently modulated permeability and monolayer/tissue retention kinetics of dapivirine | Vaccine adjuvant | das Neves et al (2013)102 |
| Chitosan-PEG NPs | Rabies whole attenuated viral antigen | Rabies virus | - | Effective and sustained elicitor of immune system with negligible toxicity | Immunization | Nivedh et al (2016)101 |
| Poly (D,L-lactic-co-glycolic acid)-b-poly(ethylene glycol) NPs | ||||||
| Methoxy-poly (ethylene glycol) 3000-poly (lactic acid) 34,000 (MePEG-PLA) NPs |
Nonnucleoside reverse transcriptase inhibitor (NNRTI) DAAN-14f (14f), surface-conjugated with HIV-1 fusion inhibitor T1144 | laboratory adapted HIV-1 strains, primary HIV-1 isolates, NNRTI resistant HIV-1 strains,T1144-resistant HIV-1strains | Sprague–Dawley rats | Viral entry inhibition by inhibition viral attachment, if compromised breach then inhibition of transcriptase | Viral inhibition | Li et al (2016)174 |
| Maleimide-poly (ethylene glycol) 3400-poly (lactic acid) (Mal-PEG–PLA) NPs | ||||||
| Poly(aniline-co-pyrrole) polymerized nanoregulators (PASomes) | - | Influenza A virus (H1N1, H3N2, and H9N2) | MDCK cells | ROS mediated viral replication inhibition and cell death | Antiviral agent | Kim et al (2017)175 |
| Amide functionalised alginate NPs | Zidovudine | HIV | C6 glioma cells, neuro 2a cell lines | Inhibition of viral transcriptase | Effective antiviral delivery | Joshy et al (2017)176 |
| Double-layered protein (Core Matrix protein 2 [M2e] coated with headless Hemagglutinin [HA]) NPs | - | Influenza A virus | BALB/c mice | Viral inhibition by antibody-dependent cell-mediated cytotoxicity or Antibody-dependent cellular phagocytosis and | Universal influenza vaccine with long lasting immunity | Deng et al (2018)104 |
| Double-layered polypeptide (nucleoprotein epitopes at core coated with matrix protein 2 ectodomain epitopes) NPs | - | Influenza A virus | BALB/c strain | CD8+ T cells involved protection against virus | NPs-vaccine | Deng et al (2018)177 |
| Liposome | ||||||
| Nano-liposomes | Acyclovir | - | - | - | Intravenous drug delivery and release | Mukherjee et al (2007)178 |
| Solid lipid NPs | shRNA (Targeting internal ribosome entry site [IRES]) | Hepatitis C virus | Huh-7 cells | Silencing of hepatitis C virus replication | Viral inhibition | Torrecilla et al (2016)137 |
| Solid lipid NPs | Ritonavir | Lentiviral-based pseudo-HIV-1 particles | Human 293T cells | Inhibition of viral protease | Efficient encapsulation, release and antiviral activity | Javan et al (2017)179 |
| Solid lipid NPs modified with gelatin | Zidovudine (AZT) | HIV | MCF-7 and neuro 2a brain cells | Appreciable cellular internalization | Favorable loading, controlled discharge, hemocompatibility and nontoxicity | Joshy et al (2017)103 |
| Polyvinylpyrrolidone (PVP)/stearic acid (SA)-polyethylene glycol (PEG) NPs | AZT | HIV | Murine neuro-2a and HeLa Cells |
Appreciable cellular internalization | Favorable loading, sustained release, hemocompatibility and nontoxicity | Joshy et al (2018)106 |
| Nanostructured nanolipid carriers | Podophyllotoxin (POD) | Human papillomavirus (HPV) | VK2/E6E7 | Cell cycle arrest of virally infected cells at G2/M phase | Sustained release, hemocompatibility, nontoxic viral inhibition | Gao et al (2018)107 |
| Dendrimer | ||||||
| Peptide-derivatized dendrimers | Acyclovir (ACV) | HSV-1,2 | Vero cells, HELFs cells, | Inhibition of viral entry by binding to glycosaminoglycan moiety of cell surface heparan sulfate proteoglycans, in virto viral replication | Antiviral activity | Luganini et al (2011)110 |
| Dendrimer NPs | mRNA replicons (multiple antigen expressing replicons) | H1N1, Ebola virus | Wild-type female C57BL/6 and BALB/c mice | Activation of both CD8+ T-cell and antibody responses | mRNA-vaccine | Chahal et al (2016)111 |
| Modified dendrimer NPs | Venezuelan equine encephalitis virus (VEEV) replicon RNAs | Zika virus | C57BL/6 mice | Activation of both CD8+ T-cell and viral E protein-specific IgG responses | mRNA-vaccine | Chahal et al (2017)113 |
| Carbosilane dendrimer NA | 16-mer oligoribonucleotide (RNA) decoy (genomic RNA of HIV) | HIV | HIV-infected MT4 lymphocytes | RNA decoy | Inhibition of HIV encapsidation | Parboosing et al (2017)114 |
| Nonlinear globular G2 dendrimer | Citric acid and polyethylene glycol 600 (PEG-600) | Rabies virus | J774A.1 cell line and NMRI mice | - | Adjuvanticity efficacy | Asgary et al (2018)112 |
| Niosome | ||||||
| Nano-niosome | Acyclovir | - | - | - | Intravenous drug delivery and release | Mukherjee et al (2007)178 |
| HSV-1 | HeLa cell line | - | Drug delivery and anti-viral activity | Monavari et al (2014)119 | ||
| - | Improved drug delivery, release, and anti-viral activity | Javad et al (2014)118 | ||||
| Nanomicelle | ||||||
| Polymeric micelle | Efavirenz (EFV) | HIV | Male Wistar rats | - | Improved oral bioavailability | Chiappetta et al (2010)121 |
| Nanomicelle | Curcumine | Hepatitis C Virus | APC49 Huh7.5 cells | Regulation of viral attachment and entry | Improved bioavailability and anti-viral activity | Naseri et al (2017)122 |
| Polymeric nanomicelle | Biotinylated lipid prodrug of cyclic cidofovir (B-C12-cCDF) |
- | D407, HCE-T cells | - | Efficient anti-viral drug delivery | Mandal et al (2017)55 |
Polymeric NPs
The exploitation of polymeric NPs for progress in the effectual antiviral treatment has been going on for more than a decade. Various forms of polymers (synthetic and natural) have immensely contributed towards meticulous understanding of definite properties such as biodistribution/ bioavailability, biocompatibility, immunocompatibility etc. in biomedical sector. But their uses either for development or for improvement of an antiviral have been investigated only for some pathogenic viruses like influenza, HIV and rabies virus (Table 2).100, 101, 102 Their efficient self-assembly properties, nanosize, shape, and ability to be functionalized based on requirement to either carry the desired therapeutic agent in its core or conjugate the target molecule on its surface are simply magnificent. Recently, Joshy et al have demonstrated that amine functionalized polymeric gelatin NPs loaded with zidovudine against HIV. Simultaneously, they have also conducted hemolysis and aggregation studies to gain deeper understanding of other compatible properties of NPs. From their research they summarized that drug conjugated NPs showed promising biocompatibility and antiviral activity.103 Deng et al on the other hand took a different approach by developing a universal influenza vaccine with double-layered protein NPs composed of tetramaric Matrix protein 2 (M2e) at its core and headless Hemagglutinin (HA) coating at its surface. The protein vaccine could induce substantial and prolonged immunity with complete protection of BALB/c mice against verity influenza A virus challenges.104 With the contemporary improvement made in nanoscience it is certain that these impeccable polymeric NPs in near future will be soon used for the development of a novel antiviral against divergent array of infectious viruses like NiV.
Lipid based NPs
Currently developed solid-lipid NPs and nanostructured lipid nanocarriers have demonstrated their ability as an exceptional efficient biocompatible vehicle for therapeutics in the field of advanced medicine. Among the multiple possible applications of lipid based NPs, their exploitation to formulate a novel antiviral could be assumed primary concern of ongoing researches. Further their anomalous bio-absorbable and biocompatible properties have granted these nanosystems a unique prospective to be viewed at.105 Despite past limitations, where it seemed difficult for a therapeutic to migrate across BBB, it seems promising now with the introduction of multifunctional lipid based nanocarriers. In a study Joshy et al again had developed zidovudine loaded polyvinylpyrrolidone (PVP)/ stearic acid (SA)-polyethylene glycol (PEG) NPs and have determined the in vitro drug loading, controlled discharge, hemocompatibility and non-toxicity in murine neuro-2a and HeLa cells under HIV challenge.106 Similarly, Gao et al designed a nanostructured nanolipid carrier loaded with podophyllotoxin (POD) evaluated drug release parameters hemocompatibility and non-toxic viral inhibition in VK2/E6E7 cell lines challenged with Human papillomavirus (HPV).107 Lipid based NPs in both of its form that is solid-lipid NPs and nanostructured lipid nanocarriers could serve as an exceptional addition to the group of vehicles needed for effective antiviral delivery. The current investigation of lipid-based NPs based antiviral against some viruses such as Hepatitis C virus, HIV and HPV has been documented (Table 2). But certainly in future this marvelous nanosystem could get even better through hybridization, so that it could be used in efficiency of therapy against NiV.
Dendrimers
Dendrimer an organic compound with well-organized, hyperbranched molecule is receiving much attention lately owing to their improving monodispersity, biocompatibility and biodegradability in the field of nanomedicine.108 This molecule is also an extraordinary vehicle for gene, peptide or drugs (natural/ synthetic) delivery at the targeted site within the biological system.109 They are widely used in biomedical science, but their application and utilization for evaluating an antiviral are limited. To date, dendrimers with antiviral activity in conjugation with therapeutic agents have already been documented against some viruses including influenza virus, ebola virus, zika virus, HSV-1, 2 and HIV (Table 2).111, 112, 113 The antiviral efficiency of nanosystems was a result of viral entry inhibition, activation of CD8+ T cell and/ or antibody mediated response and in some case RNA decoys.111, 113, 114 Investigations regarding the efficacy of dendrimers as a nanoperticulated system for designing an antiviral therapy have been introduced by some of the pioneering researches, but their efficiency against NiV is yet to be evaluated.
Niosomes
The non-ionic surfactant-based vesicle in short niosomes structurally similar to liposome is slowly becoming the center of attraction of current investigators. Its physical and chemical properties such as stability, optical activity, biocompatibility, biodegradability, non-immunogenicity, and convenience in functionalization have greatly influenced the scientific community.115, 116 Additionally, efficiency to load both lipophilic and hydrophilic drug with superior bioavailability and guarded release at the intended site have granted niosome a unique prospective in the field of nanomedicine.117 Despite their immense significance, the use of niosomes in biomedical sector specifically in formulation of antiviral agents is limited. For example till now antiviral activity against only HSV has been documented (Table 2). Javad et al and Monavari et al have evaluated the efficacy of acyclovir loaded nano-niosome against HSV-1. They found that the neosomes loaded with the drug showed promising antiviral activity with improved delivery and release kinetics.118, 119 Further in vivo evaluations are required to display the antiviral efficacy against other broad range of infectious viruses such as NiV.
Nanomicelles
Nanomicelles are supramolecular assembly of a surfactant molecule disseminated in a colloidal liquid giving rise to a globular micelle ranging in nano-size. Efficient encapsulation, biocompatibility, colloidal stability and prolonged circulation time etc. are some of the influential properties of polymeric micelles.120 Moreover, a compound possessing these properties is of huge significance in nanomedicine and drug delivery system (Table 2).121 Specifically to formulate an antiviral agent these polymeric nanomicelles is appreciated. For example Naseri et al formulated a nanomycelle encapsulating a bioactive phytocompound curcumin and verified bioavailability and antiviral activity in vitro. They documented that the nanoformulation displayed better bioavailability and satisfactory antiviral activity.122 Present application of this marvelous compound is somewhat stagnant and needs more contribution from researchers all over the world.
Hybrid nanoparticles
Hybrid NPs are by far the most advanced form of NPs that could be thought of in the present century. INPs such as Ag, Au, magnetic NPs, TiO2, SiO2, CNT, fullerene and graphene oxide and ONPs such as polymeric NPs, lipid NPs and niosomes have been explored in their antiviral activity.123, 124, 125 There are a number of viruses against which these NPs in their hybridized state have been evaluated; these include HIV, influenza virus, hepatitis virus, human adenovirus etc. (Table 3 ).
Hybridized NPs in virology may be categorized according to their utilization that is either diagnostic or therapy. Current investigations, where efforts have been made towards diagnosis or detection of the viruses have been documented by many investigators from different corners of the globe. Starting from the primary application that is detection, Marjomäki et al earlier had reported an accurate and precise enteroviruses labeling procedure and described it at the atomic level. Here they reported water-soluble thiol-stabilized gold cluster or chemically called Au102 (para-mercaptobenzoic acid)44 cluster which by its metal core could covalently bind to cysteine molecule nearer to the surface of the virus. Through the above procedure enteroviruses echovirus 1 and coxsackievirus B3 viruses were precisely detected.126 Ahmed et al have synthesized a multifunctional nanohybrid where AuNPs are first bonded with CNT surface, and then specific antibody (anti-influenza A virus HA H1 antibody) is conjugated to it. The nanohybrid displayed oxidative catalysis of 3, 3′, 5, 5′-tetramethyl-benzidine (TMB) by H2O2 gave rise to a deep blue color, the optical density of which depended on concentration of virus.127 Lately, Oh et al developed an ultrasensitive magnetic nanozyme-linked immunosorbent assay for detection of H1N1 virus. The assay had two major components, the silica-shelled magnetic nanobeads (MagNBs) for binding and preliminary detection of the virus, and a gold nanozyme (AuNZ) an enzyme-like acting molecule for amplification of the preliminary detection signals. Together in combination the nanosystem could even detect virus concentration up to femtogram per milliliter.128 There are a number of examples where hybridized nano-based systems showed promising results in viral detection; some of them have been listed in Table 3.
Viral inhibition is foremost important after diagnosis which needs to be addressed immediately; thereafter the requirement of a novel antiviral to combat the viral infectivity has become the primary concern. Further, development of an antiviral within a stipulated time is actually a race against time. Under such case formulation of an antiviral that is biocompatible, non-immunogenic and non-toxic is of the critical requirement to which hybridized nanoparticulated systems are an appreciable option. It is because of the promising antiviral activity of these systems that has been portrayed by various scientific investigators. Ryoo et al had reported iron-oxide NP-based delivery of DNAzyme as a therapeutic for treating hepatitis C infection. Here, the magnetic NPs were linked to a cell-penetrating peptide (CPP) and functional DNAzyme which could induce knockdown of HVC gene NS3 (non-structural protein 3) encoding viral helicase and protease.129 Orlowski et al developed tannic acid functionalized AgNPs of specific size range that could reduce HSV-2 infectivity by blocking viral attachment, penetration as well as viral spread; provided a prior direct attachment of the nano-system with the virus is a basic requirement.130 Chen et al compared the antiviral activity of both graphene oxide (GO) and GO conjugated AdNPs against both coronavirus (FCoV) and infectious bursal disease virus (IBDV). Their analyses showed GO in its non-conjugated and conjugated form had different effect on two different taken viruses. Overall they concluded that GO in its conjugated form that is GO-AgNPs could inhibit both the virus that is FCoV and IBDV effectively.131 After diagnosis and therapy the next arising huddle is drug resistance that soon must be focused and acted accordingly. With this intention Li et al, after much evaluations and strategic understanding the drawbacks of conventional and nano-based antiviral, designed nanoparticulated system by decorating amantadine (AM) on surface of selenium NPs (SeNPs). Their designed Se-AM nanosystem could reverse the drug resistance induced by H1N1 virus.132 Similarly, there are many other investigations which orchestrate the experimental efficiencies of hybrid nanoparticulated systems as antivirals listed in Table 3. Despite the heterogeneous research and investigations exploiting multiple ways and means to diagnose, inhibit the virus by a nano-based therapeutic agent or reverse the drug resistance, still there is hardly any sign of these applications concerning NiV.
Possible nano-based approach for NiV diagnosis
The elevated cost and in some case toxicity of conventionally available specific antiviral therapeutics have created an apparent requirement for specific and rapid viral diagnosis. Specific viral diagnosis may be helpful in discontinuation of broad spectrum antibiotic therapy and determination of prognosis, and it is also believed that rapid test result greatly influences case management. Prior to therapy, diagnosis therefore may be considered as an important aspect in viral infection treatment. There are multiple methods in the present era for laboratory based diagnosis of viral infection; these include serology, viral culture, antigen and nucleic acid detection.
The conventional technique of viral detection that is viral culture may be considered as the only technique through which viable isolate could be obtained for future characterization. Additionally, detection of a diverse array of viruses seems promising with this approach, which is also an advantage over antigen detection and nucleic acid detection. In order to preserve medically relevant viruses, maintenance of heterogeneous types of cultured cell is required as a support, which is a major limitation of this technique. Viral recovery through cell culture becomes difficult when the viral growth is slow; antigen detection in such cases is much useful. Rapidity and non-requirement of viral viability render immense plasticity in management and shifting of the specimens, which together may be considered as the major advantages of the technique. Fluorescent Antibody (FA) staining, Immunoperoxidase staining and Enzyme Immunoassay (EIA) etc. are crucial techniques involved in these methods. But viruses with antigenic heterogeneity and lack of cross-reacting antigens are tedious to be detected even through this technique. Drastic transformation in diagnosis has emerged with the development of analysis based on PCR. This transformation has allowed the detection of specific viral sequence with uttermost sensitivity. More specifically, viral RNA could be easily detected only by amalgamating reverse transcriptase with conventional PCR analysis. Real-Time PCR assay runs on highly sophisticated automated instruments; exploiting optical excitation systems and dyes labeled with fluorescent molecules has tremendously reduced time and probable contamination. The versatility of nucleic acid detection is growing day-by-day and will be in future with the progression of scientific innovation. Finally, the efficacy serology the traditional method viral diagnosis cannot be disregarded. The technique is exclusively promising for unambiguous antiviral immunity determination, but when speculated on clinical utility, assessment of acute and convalescent antibody titers, could be one of its limitations.133 In case of NiV detection these mentioned detection techniques may be fruitful.
Upon considering the present encroachment in the field of nanoscience and virology, their intermediated research has initiated a new direction in rapid diagnosis of infectious viruses. Like Au/CuS core/shell NPs developed by Broglie et al for human norovirus-like particle detection and inactivation by capsid protein degradation and damage,124 similarly a hybrid nanosystem could also be designed for binding with NiV virion two spiked glycoproteins F and G as well as RNA, and capsid proteins such as N, P, L contained within the virion (Figure 4 ).21 A colorimetric viral detection assay exploiting peroxidase-like activity of AuNPs-CNT nanohybrid could also be developed for detection of NiV detection like Ahmed et al, who synthesized and examined the same nanohybrid for the detection of H3N2.127 Likewise there are a number of versatile experimentation (Table 1, Table 2, Table 3) where diagnoses of a verity of viral infections have been summarized. Contemplating on these possibilities, the probabilities of developing a novel nano-based diagnosis technique for detecting NiV could be achieved.
Figure 4.
Speculated role of nanoparticles at different stages of NiV pathogenesis, (A) Inhibition of initial attachment and membrane fusion during viral entry, (B) Inhibition of infected cell NiV-G protein interaction with Ephrin B2/B3 of a healthy cell and activated NiV-G protein's C-terminus region mediated activation of NiV-F, (C) Activation of AKT, (D) Activation of p53 phosphorylation, (E) Inhibition of viral transcription, translation and replication, (F) Modulation of viral transcription.
Possible nano-based approach for NiV inhibition
If NiV detection could be possible, then elimination of the virus through nano-based system is not far or out of reach from the present investigators. The conventional means of viral inhibition or treatment / therapy have been documented under the topic “Therapeutics against NiV”. The nano-based approaches like the use of acid functionalized multi-walled carbon nanotube (MWCTs) made of photoactivated molecules (Protoporphyrin IX) to inactivate H3N2 virus have been demonstrated by Banerjee et al.87 The possible molecular mechanism involved is ROS-based inactivation through which oxidation of viral protein (neuraminidase [NA] and hemagglutinin [HA]) might have taken place; single stranded break in viral genome and protein-RNA crosslinking might have also been a possibility. Similar nano-based approach including pH or photothermal medium accompanied with ROS-based inactivation of NiV could also be evaluated in vitro. Inhibition of viral binding with the host cell surface receptor could also be achieved by preventing viral binding of gp120 and CD4 attachment. In a study, Vijayakumar and Ganesan have demonstrated this mode of inhibition of HIV-1 virus entry using AuNPs in vitro.62 Similarly, NPs with the capacity to interact with NiV surface glycoproteins like G and F could prove to be promising candidates as viral entry inhibitors. Inhibition of virus mediated accumulation of ROS, activation of AKT, and p53 phosphorylation could also be a strategy for inhibiting the entry of infectious viruses. Li et al demonstrated this strategy of H1N1 viral inhibition using oseltamivir (OTV) decorated SeNPs (Se@OTV).134 This strategy could be effectively evaluated in vitro in case of NiV inhibition. Likewise modulation of viral transcription using MWCTs- ribavirin by Zhu et al,135 targeted binding on conservative regions on viral genome using TiO2NPs and polylysine (PL)-containing oligonucleotides nanocomposite by Levina et al.136 and silencing viral replication with the help of solid lipid NPs by Torrecilla et al137 etc. all point in the same direction (Table 1, Table 2, Table 3). Though the efficiency of NPs as an antiviral agent against NiV is yet to be evaluated; still it could be roughly speculated that these investigations are direct representation of the possibility that NPs holds an enormous potential as an antiviral agent against NiV described in Figure 4.
Conclusion
Nanotechnology holds a tremendous opportunity for both viral disease diagnosis and their therapeutics. It is evidenced that NPs could be used as a measure to revert the antiviral resistance which is a slowly developing problem of conventional therapeutic available. Though it is possible that nano-based approaches are the next convenient strategy to deal with NiV, but still developing NPs is difficult if the NiV pathogenesis is not properly understood. Therefore the present review provides a brief knowledge about NiV pathogenesis at cellular and molecular level, where it would be easy to strategize a novel antiviral target using NPs. A brief introduction of various NPs in inorganic, organic and hybrid forms would be helpful in determining their suitability to be evaluated as diagnostics or therapeutics. Finally the overview of major antiviral approaches that have already been studied is represented in tabular format, which could help to strategize possible novel antiviral approach.
Acknowledgments
Authors are grateful to their respective universities for support.
Footnotes
Conflict of interest: Authors declare no conflict of interest with the manuscript.
Contributor Information
Jayanta Kumar Patra, Email: jkpatra.cet@gmail.com.
Sanatan Majhi, Email: sanatanm@gmail.com.
References
- 1.Singh L., Kruger H.G., Maguire G.E.M., Govender T., Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther Adv Infect Dis. 2017;4(4):105–131. doi: 10.1177/2049936117713593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Woensel J.B.M., van Aalderen W.M.C., Kimpen J.L.L. Viral lower respiratory tract infection in infants and young children. BMJ. 2003;327(7405):36–40. doi: 10.1136/bmj.327.7405.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Case fatality rate. 2018. http://www.who.int/news-room/fact-sheets/detail/nipah-virus
- 4.World Health Organization Listing of the top ten causes of death compiled by the WHO. 2018. http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 5.Broder C.C., Xu K., Nikolov D.B., Zhu Z., Dimitrov D.S., Middleton D. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antivir Res. 2013;100(1):8–13. doi: 10.1016/j.antiviral.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawes B.E., Kalveram B., Ikegami T., Juelich T., Smith J.K., Zhang L. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci Rep. 2018;8:7604. doi: 10.1038/s41598-018-25780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossart K.N., Zhu Z., Middleton D., Klippel J., Crameri G., Bingham J. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2009;5(10) doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisbert T.W., Mire C.E., Geisbert J.B., Chan Y.P., Agans K.N., Feldmann F. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci Transl Med. 2014;6(242) doi: 10.1126/scitranslmed.3008929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satterfield B.A., Dawes B.E., Milligan G.N. Status of vaccine research and development of vaccines for Nipah virus. Vaccine. 2016;34(26):2971–2975. doi: 10.1016/j.vaccine.2015.12.075. [DOI] [PubMed] [Google Scholar]
- 10.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):e00221-18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao M., Zhang P., Meng J., Li Y., Liu C., Luo X. Recent advances of biocompatible inorganic nanoparticles towards biomedical applications. Biomater Sci. 2018;6:726–745. doi: 10.1039/c7bm01020f. [DOI] [PubMed] [Google Scholar]
- 13.Escaffre O., Borisevich V., Vergara L.A., Wen J.W., Long D., Rockx B. Characterization of Nipah virus infection in a model of human airway epithelial cells cultured at an air–liquid interface. J Gen Virol. 2016;97(Pt 5):1077–1086. doi: 10.1099/jgv.0.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan M.Z., Sazzad H.M.S., Luby S.P., Sturm-Ramirez K., Bhuiyan M.U., Rahman M.Z. Nipah virus contamination of hospital surfaces during outbreaks, Bangladesh, 2013–2014. Emerg Infect Dis. 2018;24(1):15–21. doi: 10.3201/eid2401.161758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aljofan M., Saubern S., Meyer A.G., Marsh G., Meers J., Mungall B.A. Characteristics of Nipah virus and Hendra virus replication in different cell-lines and their suitability for anti-viral screening. Virus Res. 2009;142(1–2):92–99. doi: 10.1016/j.virusres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni D.D., Tosh C., Venkatesh G., Senthil Kumar D. Nipah virus infection: current scenario. Indian J Virol. 2013;24(3):398–408. doi: 10.1007/s13337-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valbuena G., Halliday H., Borisevich V., Goez Y., Rockx B. A human lung xenograft mouse model of Nipah virus infection. PLoS Pathog. 2014;10(4) doi: 10.1371/journal.ppat.1004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBuysscher B.L., de Wit E., Munster V.J., Scott D., Feldmann H., Prescott J. Comparison of the pathogenicity of Nipah virus isolates from Bangladesh and Malaysia in the Syrian Hamster. PLoS Negl Trop Dis. 2013;7(1) doi: 10.1371/journal.pntd.0002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo M.K., Rota P.A. The emergence of Nipah virus, a highly pathogenic paramyxovirus. J Clin Virol. 2008;43:396–400. doi: 10.1016/j.jcv.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Stachowiak B., Weingartl H.M. Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yabukarski F., Lawrence P., Tarbouriech N., Bourhis J.M., Delaforge E., Jensen M.R. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat Struct Mol Biol. 2014;21(9):754–759. doi: 10.1038/nsmb.2868. [DOI] [PubMed] [Google Scholar]
- 22.Weis M., Maisner A. Nipah virus fusion protein: importance of the cytoplasmic tail for endosomal trafficking and bioactivity. Eur J Cell Biol. 2015;94(7–9):316–322. doi: 10.1016/j.ejcb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo Presti A., Cella E., Giovanetti M., Lai A., Angeletti S., Zehender G. Origin and evolution of Nipah virus. J Med Virol. 2016;88(3):380–388. doi: 10.1002/jmv.24345. [DOI] [PubMed] [Google Scholar]
- 24.Angeletti S., Lo Presti A., Cella E., Ciccozzi M. Molecular epidemiology and phylogeny of Nipah virus infection: a mini review. Asian Pac J Trop Med. 2016;9(7):630–634. doi: 10.1016/j.apjtm.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virtue E.R., Marsh G.A., Baker M.L., Wang L.-F. Interferon production and signaling pathways are antagonized during Henipavirus infection of fruit bat cell lines. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel C.E. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesfay M.Z., Yin J., Gardner C.L., Khoretonenko M.V., Korneeva N.L., Rhoads R.E. Alpha/Beta interferon inhibits cap-dependent translation of viral but not cellular mRNA by a PKR-independent mechanism. J Virol. 2008;82(6):2620–2630. doi: 10.1128/JVI.01784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J., Guan M., Zhao Z., Zhang J. Type I interferons function as autocrine and paracrine factors to induce autotaxin in response to TLR activation. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barth K., Genco C.A. Microbial degradation of cellular kinases impairs innate immune signaling and paracrine TNFα responses. Sci Rep. 2016;6:34656. doi: 10.1038/srep34656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamp B., Dietzel E., Kolesnikova L., Sauerhering L., Erbar S., Weingartl H. Nipah virus entry and egress from polarized epithelial cells. J Virol. 2013;87(6):3143–3154. doi: 10.1128/JVI.02696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar H.C., Matreyek K.A., Choi D.Y., Filone C.M., Young S., Lee B. Polybasic KKR motif in the cytoplasmic tail of Nipah virus fusion protein modulates membrane fusion by inside-out signaling. J Virol. 2007;81(9):4520–4532. doi: 10.1128/JVI.02205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitman S.D., Smith E.C., Dutch R.E. Differential rates of protein folding and cellular trafficking for the Hendra virus F and G proteins: implications for F-G complex formation. J Virol. 2009;83(17):8998–9001. doi: 10.1128/JVI.00414-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q., Stone J.A., Bradel-Tretheway B., Dabundo J., Montano J.A.B., Santos-Montanez J. Unraveling a three-step spatiotemporal mechanism of triggering of receptor-induced Nipah virus fusion and cell entry. PLoS Pathog. 2013;9(11) doi: 10.1371/journal.ppat.1003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cifuentes-Muñoz N., Sun W., Ray G., Schmitt P.T., Webb S., Gibson K. Mutations in the transmembrane domain and cytoplasmic tail of Hendra virus fusion protein disrupt virus-like-particle assembly. J Virol. 2017;91(14):e00152-17. doi: 10.1128/JVI.00152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wynne J.W., Woon A.P., Dudek N.L., Croft N.P., Ng J.H., Baker M.L. Characterization of the antigen processing machinery and endogenous peptide presentation of a bat MHC class I molecule. J Immunol. 2016;196(11):4468–4476. doi: 10.4049/jimmunol.1502062. [DOI] [PubMed] [Google Scholar]
- 36.Seto J., Qiao L., Guenzel C.A., Xiao S., Shaw M.L., Hayot F. Novel Nipah virus immune-antagonism strategy revealed by experimental and computational study. J Virol. 2010;84(21):10965–10973. doi: 10.1128/JVI.01335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basler C.F. Nipah and hendra virus interactions with the innate immune system. Curr Top Microbiol Immunol. 2012;359:123–152. doi: 10.1007/82_2012_209. [DOI] [PubMed] [Google Scholar]
- 38.Escaffre O., Borisevich V., Rockx B. Pathogenesis of Hendra and Nipah virus infection in humans. J Infect Dev Ctries. 2013;7(4):308–311. doi: 10.3855/jidc.3648. [DOI] [PubMed] [Google Scholar]
- 39.Mire C.E., Satterfield B.A., Geisbert J.B., Agans K.N., Borisevich V., Yan L. Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: implications for antibody therapy. Sci Rep. 2016;6:30916. doi: 10.1038/srep30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talekar A., Pessi A., Porotto M. Infection of primary neurons mediated by nipah virus envelope proteins: role of host target cells in antiviral action. J Virol. 2011;85(16):8422–8426. doi: 10.1128/JVI.00452-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw M.L., García-Sastre A., Palese P., Basler C.F. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J Virol. 2004;78(11):5633–5641. doi: 10.1128/JVI.78.11.5633-5641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo M.K., Peeples M.E., Bellini W.J., Nichol S.T., Rota P.A., Spiropoulou C.F. Distinct and overlapping roles of Nipah virus P gene products in modulating the human endothelial cell antiviral response. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw M.L., Cardenas W.B., Zamarin D., Palese P., Basler C.F. Nuclear localization of the Nipah virus W protein allows for inhibition of both virus- and toll-like receptor 3–triggered signaling pathways. J Virol. 2005;79(10):6078–6088. doi: 10.1128/JVI.79.10.6078-6088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoneda M., Guillaume V., Sato H., Fujita K., Georges-Courbot M.-C., Ikeda F. The nonstructural proteins of Nipah virus play a key role in pathogenicity in experimentally infected animals. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida S., Horie R., Sato H., Kai C., Yoneda M. Possible role of the Nipah virus V protein in the regulation of the interferon beta induction by interacting with UBX domain-containing protein1. Sci Rep. 2018;8:7682. doi: 10.1038/s41598-018-25815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Au-Yeung N., Mandhana R., Horvath C.M. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAKSTAT. 2013;2(3) doi: 10.4161/jkst.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanguy M., Véron L., Stempor P., Ahringer J., Sarkies P., Miska E.A. An Alternative STAT signaling pathway acts in viral immunity in Caenorhabditis elegans. mBio. 2017;8(5):e0092417. doi: 10.1128/mBio.00924-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoggins J.W., Rice C.M. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1(6):519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schoggins J.W. Recent advances in antiviral interferon-stimulated gene biology. F1000Res. 2018;7:309. doi: 10.12688/f1000research.12450.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park A., Yun T., Vigant F., Pernet O., Won S.T., Dawes B.E. Nipah virus C protein recruits Tsg101 to promote the efficient release of virus in an ESCRT-dependent pathway. PLoS Pathog. 2016;12(5) doi: 10.1371/journal.ppat.1005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horie R., Yoneda M., Uchida S., Sato H., Kai C. Region of Nipah virus C protein responsible for shuttling between the cytoplasm and nucleus. Virology. 2016;497:294–304. doi: 10.1016/j.virol.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Marsh G.A., Netter J.H. Henipavirus infection: natural history and the virus-host interplay. Curr Treat Options Infect Dis. 2018;10:197–216. doi: 10.1007/s40506-018-0155-y. [DOI] [Google Scholar]
- 53.Guillaume V., Lefeuvre A., Faure C., Marianneau P., Buckland R., Lam S.K. Specific detection of Nipah virus using real-time RT-PCR (TaqMan) J Virol Methods. 2004;120(2):229–237. doi: 10.1016/j.jviromet.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Guillaume V., Aslan H., Ainouze M., Guerbois M., Wild T.F., Buckland R. Evidence of a potential receptor-binding site on the Nipah virus G protein (NiV-G): identification of globular head residues with a role in fusion promotion and their localization on an NiV-G structural model. J Virol. 2006;80(15):7546–7554. doi: 10.1128/JVI.00190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mandal B., Bhattacharjee H., Mittal N., Sah H., Balabathula P., Thoma L.A. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9(4):474–491. doi: 10.1016/j.nano.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Li L., Lu Y., Jiang C., Zhu Y., Yang X., Hu X. Actively targeted deep tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@mesoporous silica core–shell nanoparticles. Adv Funct Mater. 2018;28(5):1704623. doi: 10.1002/adfm.201704623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilen C.B., Tilton J.C., Doms R.W. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2(8):a006866. doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu R.L., Li S.R., Kong F.J., Hou R.J., Guan X.L., Guo F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet Mol Res. 2014;13(3):7022–7028. doi: 10.4238/2014.March.19.2. [DOI] [PubMed] [Google Scholar]
- 60.Khandelwal N., Kaur G., Chaubey K.K., Singh P., Sharma S., Tiwari A. Silver nanoparticles impair Peste des petits ruminants virus replication. Virus Res. 2014;190:1–7. doi: 10.1016/j.virusres.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Rónavári A., Kovács D., Igaz N., Vágvölgyi C., Boros I.M., Kónya Z. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int J Nanomedicine. 2017;12:871–883. doi: 10.2147/IJN.S122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vijayakumar S., Ganesan S. Gold nanoparticles as an HIV entry inhibitor. Curr HIV Res. 2012;10(8):643–646. doi: 10.2174/157016212803901383. [DOI] [PubMed] [Google Scholar]
- 63.Hafashjani S.R., Rezatofighi S.E., Ardakani M.R., Rastegarzadeh S. Gold nanoparticles impair foot-and-mouth disease virus replication. IEEE Trans Nanobioscience. 2016;15:34. doi: 10.1109/TNB.2015.2508718. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed S.R., Kim J., Suzuki T., Lee J., Park E.Y. Detection of influenza virus using peroxidase-mimic of gold nanoparticles. Biotechnol Bioeng. 2016;113(10):2298–2303. doi: 10.1002/bit.25982. [DOI] [PubMed] [Google Scholar]
- 65.Zharov V.P., Mercer K.E., Galitovskaya E.N., Smeltzer M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys J. 2006;90(2):619–627. doi: 10.1529/biophysj.105.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suchomel P., Kvitek L., Prucek R., Panacek A., Halder A., Vajda S. Simple size-controlled synthesis of Au nanoparticles and their size dependent catalytic activity. Sci Rep. 2018;8:4589. doi: 10.1038/s41598-018-22976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tombácz E., Turcu R., Socoliuc V., Vékás L. Magnetic iron oxide nanoparticles: recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem Biophys Res Commun. 2015;468(3):442–453. doi: 10.1016/j.bbrc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 68.Kratz H., Taupitz M., Ariza de Schellenberger A., Kosch O., Eberbeck D., Wagner S. Novel magnetic multicore nanoparticles designed for MPI and other biomedical applications: from synthesis to first in vivo studies. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou T.-C., Hsu W., Wang C.-H., Chen Y.-J., Fang J.-M. Rapid and specific influenza virus detection by functionalized magnetic nanoparticles and mass spectrometry. J Nanobiotechnol. 2011;9:52. doi: 10.1186/1477-3155-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park J.-A., Kim S.-B., Lee C.-G., Lee S.-H., Choi J.-W. Adsorption of bacteriophage MS2 to magnetic iron oxide nanoparticles in aqueous solutions. J Environ Sci Health A. 2014;49(10):1116–1124. doi: 10.1080/10934529.2014.897147. [DOI] [PubMed] [Google Scholar]
- 71.Delaviz N., Gill P., Ajamia A., Aarabic M. Aptamer-conjugated magnetic nanoparticles for the efficient removal of HCV particles from human plasma samples. RSC Adv. 2015;5:79433. [Google Scholar]
- 72.Shelby T., Banerjee T., Zegar I., Santra S. Highly sensitive, engineered magnetic nanosensors to investigate the ambiguous activity of Zika virus and binding receptors. Sci Rep. 2017;7:7377. doi: 10.1038/s41598-017-07620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerrity D., Ryu H., Crittenden J., Abbaszadegan M. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43(11):1261–1270. doi: 10.1080/10934520802177813. [DOI] [PubMed] [Google Scholar]
- 75.Mazurkova N.A., Spitsyna Y.E., Shikina N.V., Ismagilov Z.R., Zagrebel'nyi S.N., Ryabchikova E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol Russ. 2010;5(5–6):417–420. [Google Scholar]
- 76.Syngouna V.I., Chrysikopoulos C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J Colloid Interface Sci. 2017;497:117–125. doi: 10.1016/j.jcis.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 77.Winkler H.C., Notter T., Meyer U., Naegeli H. Critical review of the safety assessment of titanium dioxide additives in food. J Nanobiotechnol. 2018;16:51. doi: 10.1186/s12951-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J., Liu T., Pan J., Liu S., Lu G.Q.M. Advances in multicompartment mesoporous silica micro/nanoparticles for theranostic applications. Annu Rev Chem Biomol Eng. 2018;9:389–411. doi: 10.1146/annurev-chembioeng-060817-084225. [DOI] [PubMed] [Google Scholar]
- 79.Bayir S., Barras A., Boukherroub R., Szunerits S., Raehm L., Richeter S. Mesoporous silica nanoparticles for recent photodynamic therapy applications. Photochem Photobiol Sci. 2018 doi: 10.1039/C8PP00143J. [DOI] [PubMed] [Google Scholar]
- 80.Sun R., Wang W., Wen Y., Zhang X. Recent advance on mesoporous silica nanoparticles-based controlled release system: intelligent switches open up new horizon. Nanomaterials (Basel) 2015;5(4):2019–2053. doi: 10.3390/nano5042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang L., Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8(3):290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Souza E.S.J.M., Hanchuk T.D., Santos M.I., Kobarg J., Bajgelman M.C., Cardoso M.B. Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl Mater Interfaces. 2016;8(26):16564–16572. doi: 10.1021/acsami.6b03342. [DOI] [PubMed] [Google Scholar]
- 83.Chunduri L.A.A., Kurdekar A., Haleyurgirisetty M.K., Bulagonda E.P., Kamisetti V., Hewlett I.K. Femtogram level sensitivity achieved by surface engineered silica nanoparticles in the early detection of HIV infection. Sci Rep. 2017;7:7149. doi: 10.1038/s41598-017-07299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo H.C., Feng X.M., Sun S.Q., Wei Y.Q., Sun D.H., Liu X.T. Immunization of mice by hollow mesoporous silica nanoparticles as carriers of porcine circovirus type 2 ORF2 protein. Virol J. 2012;9:108. doi: 10.1186/1743-422X-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Enrichi F., Riccò R., Meneghello A., Pierobon R., Canton G., Cretaio E. Enhancing the sensitivity of DNA microarray using dye-doped silica nanoparticles: detection of human papilloma virus. AIP Conf Proc. 2010;1275:154–157. doi: 10.1063/1.3505067. [DOI] [Google Scholar]
- 86.Bhattacharya K., Mukherjee S.P., Gallud A., Burkert S.C., Bistarelli S., Bellucci S. Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomedicine: NBM. 2016;12(2):333–351. doi: 10.1016/j.nano.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banerjee I., Douaisi M.P., Mondal D., Kane R.S. Light-activated nanotube-porphyrin conjugates as effective antiviral agents. Nanotechnology. 2012;23(10):105101. doi: 10.1088/0957-4484/23/10/105101. [DOI] [PubMed] [Google Scholar]
- 88.Luczkowiak J., Muñoz A., Sánchez-Navarro M., Ribeiro-Viana R., Ginieis A., Illescas B.M. Glycofullerenes inhibit viral infection. Biomacromolecules. 2013;14(2):431–437. doi: 10.1021/bm3016658. [DOI] [PubMed] [Google Scholar]
- 89.Zhu B., Liu G.L., Gong Y.X., Ling F., Song L.S., Wang G.X. Single-walled carbon nanotubes as candidate recombinant subunit vaccine carrier for immunization of grass carp against grass carp reovirus. Fish Shellfish Immunol. 2014;41(2):279–293. doi: 10.1016/j.fsi.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 90.Dostalova S., Moulick A., Milosavljevic V., Guran R., Kominkova M., Cihalova K. Antiviral activity of fullerene C60 nanocrystals modifiedwith derivatives of anionic antimicrobial peptide maximin H5. Monatsh Chem. 2016;147:905. [Google Scholar]
- 91.Yang X.X., Li C.M., Li Y.F., Wang J., Huang C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–16092. doi: 10.1039/c7nr06520e. [DOI] [PubMed] [Google Scholar]
- 92.Langer R., Weissleder R. Nanotechnology. JAMA. 2015;313(2):135–136. doi: 10.1001/jama.2014.16315. [DOI] [PubMed] [Google Scholar]
- 93.Li J., Zhu J.J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst. 2013;138(9):2506–2515. doi: 10.1039/c3an36705c. [DOI] [PubMed] [Google Scholar]
- 94.Matea C.T., Mocan T., Tabaran F., Pop T., Mosteanu O., Puia C. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomedicine. 2017;12:5421–5431. doi: 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu G., Mahajan S., Roy I., Yong K.-T. Theranostic quantum dots for crossing blood–brain barrier in vitro and providing therapy of HIV-associated encephalopathy. Front Pharmacol. 2013;4:140. doi: 10.3389/fphar.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahajan S.D., Roy I., Xu G., Yong K.T., Ding H., Aalinkeel R. Enhancing the delivery of anti retroviral drug "Saquinavir" across the blood brain barrier using nanoparticles. Curr HIV Res. 2010;8(5):396–404. doi: 10.2174/157016210791330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norouzi M., Ghobadi M.Z., Golmimi M., Mozhgani S.-H., Ghourchian H., Rezaee S.A. Quantum dot-based biosensor for the detection of human T-lymphotropic virus-1. Anal Lett. 2017;50(15):2402–2411. doi: 10.1080/00032719.2017.1287714. [DOI] [Google Scholar]
- 98.Mitragotri S., Stayton P. Organic nanoparticles for drug delivery and imaging. MRS Bull. 2014;39(3):219–223. doi: 10.1557/mrs.2014.11. [DOI] [Google Scholar]
- 99.McClements D.J., Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. npj Sci Food. 2017;6:1. doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lemoine D., Préat V. Polymeric nanoparticles as delivery system for influenza virus glycoproteins. J Control Release. 1998;54(1):15–27. doi: 10.1016/s0168-3659(97)00241-1. [DOI] [PubMed] [Google Scholar]
- 101.Nivedh K., Namasivayam S.K.R., Nishanth A.N. Effect of functionalization of polymeric nanoparticles incorporated with whole attenuated rabies virus antigen on sustained release and efficacy. Resource-Efficient Technol. 2016;2:S25–S38. [Google Scholar]
- 102.das Neves J., Araújo F., Andrade F., Michiels J., Ariën K.K., Vanham G. In vitro and ex vivo evaluation of polymeric nanoparticles for vaginal and rectal delivery of the anti-HIV drug dapivirine. Mol Pharm. 2013;10(7):2793–2807. doi: 10.1021/mp4002365. [DOI] [PubMed] [Google Scholar]
- 103.Joshy K.S., Snigdha S., Kalarikkal N., Pothen L.A., Thomas S. Gelatin modified lipid nanoparticles for anti-viral drug delivery. Chem Phys Lipids. 2017;207(Pt A):24–37. doi: 10.1016/j.chemphyslip.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Deng L., Mohan T., Chang T.Z., Gonzalez G.X., Wang Y., Kwon Y.-M. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun. 2018;9:359. doi: 10.1038/s41467-017-02725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das S., Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharm Sci Tech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joshy K.S., Snigdha S., Anne G., Nandakumar K., Laly A.P., Sabu T. Poly (vinyl pyrrolidone)-lipid based hybrid nanoparticles for anti viral drug delivery. Chem Phys Lipids. 2018;210:82–89. doi: 10.1016/j.chemphyslip.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Gao Y., Han K., Wang Q., Hu Z., Liu Q., Liu L. Development of podophyllotoxin-loaded nanostructured lipid carriers for the treatment of condyloma acuminatum. Mol Med Rep. 2018;17(5):6506–6514. doi: 10.3892/mmr.2018.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sepúlveda-Crespo D., Ceña-Díez R., Jiménez J.L., Muñoz-Fernández M.A. Mechanistic studies of viral entry: an overview of dendrimer-based microbicides as entry inhibitors against both HIV and HSV-2 overlapped infections. Med Res Rev. 2017;37(1):149–179. doi: 10.1002/med.21405. [DOI] [PubMed] [Google Scholar]
- 109.Xu B., Li A., Hao X., Guo R., Shi X., Cao X. PEGylated dendrimer-entrapped gold nanoparticles with low immunogenicity for targeted gene delivery. RSC Adv. 2018;8:1265. doi: 10.1039/c7ra11901a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luganini A., Nicoletto S.F., Pizzuto L., Pirri G., Giuliani A., Landolfo S. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Antimicrob Agents Chemother. 2011;55(7):3231–3239. doi: 10.1128/AAC.00149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chahal J.S., Khan OF, Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci U S A. 2016;113(29):E4133–E4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Asgary V., Shoari A., Moayad M.A., Ardestani M.S., Bigdeli R., Ghazizadeh L. Evaluation of G2 citric acid-based dendrimer as an adjuvant in veterinary rabies vaccine. Viral Immunol. 2018;31(1):47–54. doi: 10.1089/vim.2017.0024. [DOI] [PubMed] [Google Scholar]
- 113.Chahal J.S., Fang T., Woodham A.W., Khan OF, Ling J., Anderson D.G. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep. 2017;7(1):252. doi: 10.1038/s41598-017-00193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parboosing R., Chonco L., de la Mata F.J., Govender T., Maguire G.E., Kruger H.G. Potential inhibition of HIV-1 encapsidation by oligoribonucleotide-dendrimer nanoparticle complexes. Int J Nanomedicine. 2017;12:317–325. doi: 10.2147/IJN.S114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sagar G.H., Arunagirinathan M.A., Bellare J.R. Self-assembled surfactant nano-structures important in drug delivery: a review. Indian J Exp Biol. 2007;45:133–159. [PubMed] [Google Scholar]
- 116.Kailash V., Vidyasagar G., Pratibha C., Ashwini P., Mangesh K., Jagtap V. Niosomes (non ionic surfactant vesicles) preparation and stability in biological environment. Int J Res Control Release. 2013;1(1):1–9. [Google Scholar]
- 117.Ghalandarlaki N., Alizadeh A.M., Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int. 2014;2014:394264. doi: 10.1155/2014/394264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Javad M.P.M., Reza M.S.H., Simin D., Ahmed E.S., Bahram B., Azadeh H. Preparation and evaluation of the antiviral activity of acyclovir loaded nano-niosomes against herpes simplex virus type 1. Pharmaphore. 2014;5(4):483–493. [Google Scholar]
- 119.Monavari S.H., Parsa M.J.M., Bolouri B., Ebrahimi S.A., Ataei-Pirkooh A. The inhibitory effect of acyclovir loaded nano-niosomes against herpes simplex virus type-1 in cell culture. Med J Islam Repub Iran. 2014;28:99. [PMC free article] [PubMed] [Google Scholar]
- 120.Talelli M., Barz M., Rijcken C.J., Kiessling F., Hennink W.E., Lammers T. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today. 2015;10(1):93–117. doi: 10.1016/j.nantod.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiappetta D.A., Hocht C., Taira C., Sosnik A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability. Nanomedicine. 2010;5(1):11–23. doi: 10.2217/nnm.09.90. [DOI] [PubMed] [Google Scholar]
- 122.Naseri S., Darroudi M., Aryan E., Gholoobi A., Rahimi H.R., Ketabi K. The antiviral effects of curcumin nanomicelles on the attachment and entry of hepatitis C virus. Iran J Virol. 2017;11(2):29–35. [Google Scholar]
- 123.Jafry H.R., Liga M.V., Li Q., Barron A.R. Simple route to enhanced photocatalytic activity of p25 titanium dioxide nanoparticles by silica addition. Environ Sci Technol. 2011;45(4):1563–1568. doi: 10.1021/es102749e. [DOI] [PubMed] [Google Scholar]
- 124.Broglie J.J., Alston B., Yang C., Ma L., Adcock A.F., Chen W. Antiviral activity of gold/ copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee J., Morita M., Takemura K., Park E.Y. A multi-functional gold/iron-oxide nanoparticle-CNT hybrid nanomaterial as virus DNA sensing platform. Biosens Bioelectron. 2018;102:425–431. doi: 10.1016/j.bios.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 126.Marjomäki V., Lahtinen T., Martikainen M., Koivisto J., Malola S., Salorinne K. Site-specific targeting of enterovirus capsid by functionalized monodisperse gold nanoclusters. Proc Natl Acad Sci U S A. 2014;111(4):1277–1281. doi: 10.1073/pnas.1310973111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahmed S.R., Kim J., Suzuki T., Lee J., Park E.Y. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosens Bioelectron. 2016;85:503–508. doi: 10.1016/j.bios.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 128.Oh S., Kim J., Tran V.T., Lee D.K., Ahmed S.R., Hong J.C. Magnetic nanozyme-linked immunosorbent assay for ultrasensitive influenza a virus detection. ACS Appl Mater Interfaces. 2018;10(15):12534–12543. doi: 10.1021/acsami.8b02735. [DOI] [PubMed] [Google Scholar]
- 129.Ryoo S.R., Jang H., Kim K.S., Lee B., Kim K.B., Kim Y.K. Functional delivery of DNAzyme with iron oxide nanoparticles for hepatitis C virus gene knockdown. Biomaterials. 2012;33(9):2754–2761. doi: 10.1016/j.biomaterials.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 130.Orlowski P., Tomaszewska E., Gniadek M., Baska P., Nowakowska J., Sokolowska J. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Y.-N., Hsueh Y.-H., Hsieh C.-T., Tzou D.-Y., Chang P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int J Environ Res Public Health. 2016;13(4):430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y., Lin Z., Guo M., Zhao M., Xia Y., Wang C. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int J Nanomedicine. 2018;13:2005–2016. doi: 10.2147/IJN.S155994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Storch G.A. Diagnostic virology. Clin Infect Dis. 2000;31(3):739–751. doi: 10.1086/314015. [DOI] [PubMed] [Google Scholar]
- 134.Li Y., Lin Z., Guo M., Xia Y., Zhao M., Wang C. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int J Nanomedicine. 2017;12:5733–5743. doi: 10.2147/IJN.S140939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu B., Liu G.L., Ling F., Wang G.X. Carbon nanotube-based nanocarrier loaded with ribavirin against grass carp reovirus. Antivir Res. 2015;118:29–38. doi: 10.1016/j.antiviral.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 136.Levina A.S., Repkova M.N., Bessudnova E.V., Filippova E.I., Mazurkova N.A., Zarytova V.F. High antiviral effect of TiO2·PL–DNA nanocomposites targeted to conservative regions of (−) RNA and (+) RNA of influenza a virus in cell culture. Beilstein J Nanotechnol. 2016;7:1166–1173. doi: 10.3762/bjnano.7.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Torrecilla J., Del Pozo-Rodríguez A., Solinís M.Á., Apaolaza P.S., Berzal-Herranz B., Romero-López C. Silencing of hepatitis C virus replication by a non-viral vector based on solid lipid nanoparticles containing a shRNA targeted to the internal ribosome entry site (IRES) Colloids Surf B Biointerfaces. 2016;146:808–817. doi: 10.1016/j.colsurfb.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 138.Salem A.N.B., Zyed R., Lassoued M.A., Nidhal S., Sfar S., Mahjoub A. Plant-derived nanoparticles enhance antiviral activity against Coxsackievirus B3 by acting on virus particles and Vero cells. Dig J Nanomater Biostruct. 2012;7(2):737–744. [Google Scholar]
- 139.Gaikwad S., Ingle A., Gade A., Rai M., Falanga A., Incoronato N. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine. 2013;8:4303–4314. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fatima M., Zaidi N.U., Amraiz D., Afzal F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza a virus. J Microbiol Biotechnol. 2016;26(1):151–159. doi: 10.4014/jmb.1508.08024. [DOI] [PubMed] [Google Scholar]
- 141.Seino S., Imoto Y., Kosaka T., Nishida T., Nakagawa T., Yamamoto T.A. Antiviral activity of silver nanoparticles immobilized onto textile fabrics synthesized by radiochemical process. Nanomater Synth. 2016;1(11):705–710. [Google Scholar]
- 142.Huy T.Q., Thanh N.T.H., Thuy N.T., Chung P.V., Hung P.N., Le A.T. Cytotoxicity and antiviral activity of electrochemical - synthesized silver nanoparticles against poliovirus. J Virol Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 143.Castro-Mayorgaa J.L., Randazzoab W., Fabraa M.J., Lagarona J.M., Aznarab R., Sánchez G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT - Food Sci Technol. 2017;79:503–510. [Google Scholar]
- 144.Pangestika R., Ernawati R., Suwarno . The Veterinary Medicine International Conference 2017. KnE Life Sciences; 2017. Antiviral activity effect of silver nanoparticles (AgNPs) solution against the growth of infectious bursal disease virus on embryonated chicken eggs with ELISA test; pp. 536–548. [DOI] [Google Scholar]
- 145.Misstear D.B., Gill L.W. The inactivation of phages MS2, ΦX174 and PR772 using UV and solar photocatalysis. J Photochem Photobiol B. 2012;107:1–8. doi: 10.1016/j.jphotobiol.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 146.Levina A.S., Repkova M.N., Zarytova V.F., Mazurkova N.A. Proceedings of the 15th IEEE International Conference on Nanotechnology. 2015. TiO2~DNA nanocomposites as efficient site-specific antiviral agents against influenza A virus in cell culture. Rome, Italy. [Google Scholar]
- 147.Chen Y.S., Hung Y.C., Lin W.H., Huang G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010;21(19):195101. doi: 10.1088/0957-4484/21/19/195101. [DOI] [PubMed] [Google Scholar]
- 148.Carter J.R., Balaraman V., Kucharski C.A., Fraser T.S., Fraser M.J. A novel dengue virus detection method that couples DNAzyme and gold nanoparticle approaches. Virol J. 2013;10:201. doi: 10.1186/1743-422X-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gopinath S.C.B., Awazu K., Fujimaki M., Shimizu K., Shima T. Observations of immuno-gold conjugates on influenza viruses using waveguide-mode sensors. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paul A.M., Shi Y., Acharya D., Douglas J.R., Cooley A., Anderson J.F. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95(Pt8):1712–1722. doi: 10.1099/vir.0.066084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tao W., Ziemer K.S., Gill H.S. Gold nanoparticle–M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond) 2014;9(2):237–251. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y., Zhang L., Wei W., Zhao H., Zhou Z., Zhang Y. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst. 2015;140(12):3989–3995. doi: 10.1039/c5an00407a. [DOI] [PubMed] [Google Scholar]
- 153.Wang C., Zhu W., Wang B.-Z. Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int J Nanomedicine. 2017;12:4747–4762. doi: 10.2147/IJN.S137222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Askaravi M., Rezatofighi S.E., Rastegarzadeh S., Seifi Abad Shapouri M.R. Development of a new method based on unmodified gold nanoparticles and peptide nucleic acids for detecting bovine viral diarrhea virus-RNA. AMB Express. 2017;7:137. doi: 10.1186/s13568-017-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tao W., Hurst B.L., Shakya A.K., Uddin M.J., Ingrole R.S., Hernandez-Sanabria M. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza a viruses. Antivir Res. 2017;141:62–72. doi: 10.1016/j.antiviral.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang C., Zhu W., Luo Y., Wang B.Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomedicine. 2018;14(4):1349–1360. doi: 10.1016/j.nano.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park S., Park H.H., Kim S.Y., Kim S.J., Woo K., Ko G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl Environ Microbiol. 2014;80(8):2343–2350. doi: 10.1128/AEM.03427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Skrastina D., Petrovskis I., Lieknina I., Bogans J., Renhofa R., Ose V. Silica nanoparticles as the adjuvant for the immunisation of mice using hepatitis B Core virus-like particles. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee E.C., Davis-Poynter N., Nguyen C.T., Peters A.A., Monteith G.R., Strounina E. GAG mimetic functionalised solid and mesoporous silica nanoparticles as viral entry inhibitors of herpes simplex type 1 and type 2 viruses. Nanoscale. 2016;8:16192–16196. doi: 10.1039/c6nr03878f. [DOI] [PubMed] [Google Scholar]
- 160.Martinez Z.S., Castro E., Seong C.-S., Cerón M.R., Echegoyen L., Llano M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob Agents Chemother. 2016;60(10):5731–5741. doi: 10.1128/AAC.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Muñoz A., Sigwalt D., Illescas B.M., Luczkowiak J., Rodríguez-Pérez L., Nierengarten I. Synthesis of giant globular multivalentglyco fullerenes as potent inhibitors in a model of Ebola virus infection. Nat Chem. 2016;8(1):50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 162.Muñoz A., Illescas B.M., Luczkowiak J., Lasala F., Ribeiro-Viana R., Rojo J. Antiviral activity of self-assembled glycodendro [60] fullerene mono adducts. J Mater Chem B. 2017;5:6566–6571. doi: 10.1039/c7tb01379e. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y., Liu G.-L., Li D.-L., Ling F., Zhu B., Wang G.-X. The protective immunityagainst grass carp reovirus in grass carp induced by a DNA vaccination using single-walled carbonnanotubes as delivery vehicles. Fish Shellfish Immunol. 2015;47(2):732–742. doi: 10.1016/j.fsi.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 165.Zhu B., Liu G.L., Gong Y.X., Ling F., Wang G.X. Protective immunity of grass carp immunized with DNA vaccine encoding the vp7 gene of grass carp reovirus using carbon nanotubes as a carrier molecule. Fish Shellfish Immunol. 2015;42(2):325–334. doi: 10.1016/j.fsi.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 166.Miyako E., Nagata H., Hirano K., Sakamoto K., Makita Y., Nakayama K. Photoinduced antiviral carbon nanohorns. Nanotechnology. 2008;19(7) doi: 10.1088/0957-4484/19/7/075106. [DOI] [PubMed] [Google Scholar]
- 167.Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015;7(38):21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- 168.Bentzen E.L., House F, Utley T.J., Crowe J.E., Jr., Wright D.W. Progression of respiratory syncytial virus infection monitored by fluorescent quantum dot probes. Nano Lett. 2005;5(4):591–595. doi: 10.1021/nl048073u. [DOI] [PubMed] [Google Scholar]
- 169.Du T., Liang J., Dong N., Liu L., Fang L., Xiao S. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278–285. [Google Scholar]
- 170.Dong X., Moyer M.M., Yang F., Sun Y.-P., Yang L. Carbon Dots' antiviral functions against noroviruses. Sci Rep. 2017;7:519. doi: 10.1038/s41598-017-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lemoine D., Deschuyteneer M., Hogge F., Préat V. Intranasal immunization against influenza virus using polymeric particles. Aust J Biol Sci. 1999;10(8):805–825. doi: 10.1163/156856299X00892. [DOI] [PubMed] [Google Scholar]
- 172.Amidi M., Romeijn S.G., Verhoef J.C., Junginger H.E., Bungener L., Huckriede A. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine. 2007;25(1):144–153. doi: 10.1016/j.vaccine.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 173.Wang X., Akagi T., Akashi M., Baba M. Development of core-corona type polymeric nanoparticles as an anti-HIV- 1 vaccine. Mini Rev Org Chem. 2007;4:51–59. [Google Scholar]
- 174.Li W., Yu F., Wang Q., Qi Q., Su S., Xie L. Co-delivery of HIV-1 entry inhibitor and nonnucleoside reverse transcriptase inhibitor shuttled by nanoparticles: cocktail therapeutic strategy for antiviral therapy. AIDS. 2016;30(6):827–838. doi: 10.1097/QAD.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 175.Kim H.O., Yeom M., Kim J., Kukreja A., Na W., Choi J. Reactive oxygen species-regulating polymersome as an antiviral agent against influenza virus. Small. 2017;13(32):1700818. doi: 10.1002/smll.201700818. [DOI] [PubMed] [Google Scholar]
- 176.Joshy K.S., George A., Jose J., Kalarikkal N., Pothen L.A., Thomas S. Novel dendritic structure of alginate hybrid nanoparticles for effective anti-viral drug delivery. Int J Biol Macromol. 2017;103:1265–1275. doi: 10.1016/j.ijbiomac.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 177.Deng L., Chang T.Z., Wang Y., Li S., Wang S., Matsuyama S. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc Natl Acad Sci U S A. 2018;115(33):E7758–E7767. doi: 10.1073/pnas.1805713115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mukherjee B., Patra B., Layek B., Mukherjee A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: an in vitro study. Int J Nanomedicine. 2007;2(2):213–225. [PMC free article] [PubMed] [Google Scholar]
- 179.Javan F., Vatanara A., Azadmanesh K., Nabi-Meibodi M., Shakouri M. Encapsulation of ritonavir in solid lipid nanoparticles: in-vitro anti-HIV-1 activity using lentiviral particles. J Pharm Pharmacol. 2017;69(8):1002–1009. doi: 10.1111/jphp.12737. [DOI] [PubMed] [Google Scholar]
- 180.Quy D.V., Hieu N.M., Tra P.T., Nam N.H., Hai N.H., Son N.T. Synthesis of silica-coated magnetic nanoparticles and application in the detection of path. J Nanomater. 2013;2013:603940. [Google Scholar]
- 181.Ahmed S.R., Nagy E., Neethirajan S. Self-assembled star-shaped chiroplasmonic gold nanoparticles for ultrasensitive chiro-immunosensor of viruses. RSC Adv. 2017;7:40849–40857. [Google Scholar]
- 182.Ahmed S.R., Takemeura K., Li T.C., Kitamoto N., Tanaka T., Suzuki T. Size-controlled preparation of peroxidase-like graphene-gold nanoparticle hybrids for the visible detection of norovirus-like particles. Biosens Bioelectron. 2017;87:558–565. doi: 10.1016/j.bios.2016.08.101. [DOI] [PubMed] [Google Scholar]
- 183.Zheng L., Wei J., Lv X., Bi Y., Wu P., Zhang Z. Detection and differentiation of influenza viruses with glycan-functionalized gold nanoparticles. Biosens Bioelectron. 2017;91:46–52. doi: 10.1016/j.bios.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 184.Chen W., Fang Z.S., Chen Y.T., Chen Y.I., Yao B.Y., Cheng J.Y. Targeting and enrichment of viral pathogen by cell membrane cloaked magnetic nanoparticles for enhanced detection. ACS Appl Mater Interfaces. 2017;9(46):39953–39961. doi: 10.1021/acsami.7b09931. [DOI] [PubMed] [Google Scholar]
- 185.Liga M.V., Bryant E.L., Colvin V.L., Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011;45(2):535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 186.Du C.-X., Xiong H.-R., Ji H., Liu Q., Xiao H., Yang Z.-Q. The antiviral effect of fullerene-liposome complex against influenza virus (H1N1) in vivo. Sci Res Essays. 2012;7(6):706–711. [Google Scholar]
- 187.Etemadzade M., Ghamarypour A., Zabihollahi R., Shabbak G., Shirazi M., Sahebjamee H. Synthesis and evaluation of antiviral activities of novel sonochemical silver nanorods against HIV and HSV viruses. Asian Pac J Trop Dis. 2016;6(11):854–858. [Google Scholar]
- 188.Li Y., Lin Z., Zhao M., Xu T., Wang C., Hua L. Silver nanoparticle based codelivery of oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS-mediated signaling pathways. ACS Appl Mater Interfaces. 2016;8(37):24385–24393. doi: 10.1021/acsami.6b06613. [DOI] [PubMed] [Google Scholar]
- 189.Li Y., Lin Z., Xu T., Wang C., Zhao M., Xiao M. Delivery of VP1 siRNA to inhibit the EV71 virus using functionalized silver nanoparticles through ROS mediated signaling pathways. RSC Adv. 2017;7:1453. [Google Scholar]
- 190.Malik T., Chauhan G., Rath G., Kesarkar R.N., Chowdhary A.S., Goyal A.K. Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif Cells Nanomed Biotechnol. 2017:1–12. doi: 10.1080/21691401.2017.1414054. [DOI] [PubMed] [Google Scholar]
- 191.Alghrair Z.K., Fernig D.G., Ebrahimi B. Enhanced inhibition of influenza virus infection by peptide-noble metal nanoparticle conjugates. bioRxiv. 2018 doi: 10.1101/324939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lysenko V., Lozovski V., Lokshyn M., Gomeniuk Y.V., Dorovskih A., Rusinchuk N. Nanoparticles as antiviral agents against adenoviruses. Adv Nat Sci Nanosci Nanotechnol. 2018;9 [Google Scholar]
- 193.Prescott J., de Wit E., Feldmann H., Munster V.J. The immune response to Nipah virus infection. Arch Virol. 2012;157(9):1635–1641. doi: 10.1007/s00705-012-1352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.de Weerd N.A., Nguyen T. The interferons and their receptors—distribution and regulation. Immunol Cell Biol. 2012;90(5):483–491. doi: 10.1038/icb.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Welsh R.M., Bahl K., Marshall H.D., Urban S.L. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8(1) doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]