Abstract
The Arenaviridae family currently comprises 22 viral species, each of them associated with a rodent species. This viral family is important both as tractable experimental model systems to study acute and persistent infections and as clinically important human pathogens. Arenaviruses are enveloped viruses with a bi-segmented negative-strand RNA genome. The interaction with the cellular receptor and subsequent entry into the host cell differs between Old World and New World arenavirus that use α-dystoglycan or human transferring receptor 1, respectively, as main receptors. The recent development of reverse genetic systems for several arenaviruses has facilitated progress in understanding the molecular biology and cell biology of this viral family, as well as opening new approaches for the development of novel strategies to combat human pathogenic arenaviruses. On the other hand, increased availability of genetic data has allowed more detailed studies on the phylogeny and evolution of arenaviruses. As with other riboviruses, arenaviruses exist as viral quasispecies, which allow virus adaptation to rapidly changing environments. The large number of different arenavirus host reservoirs and great genetic diversity among virus species provide the bases for the emergence of new arenaviruses potentially pathogenic for humans.
Keyword: Arenavirus diversity and evolution
1. Introduction
Arenaviruses are a group of enveloped, bi-segmented RNA-containing viruses with unique ambisense genomic organization. Nearly all of the arenaviruses members so far described cause acute or persistent infections of rodents. Specific members of the order Rodentia (Musser and Carleton, 2005) are the principal hosts of the arenaviruses for which natural host relationship has been well characterized (Childs and Peters, 1993). It is assumed that humans usually become infected with arenaviruses by inhalation of virus in aerosolized droplets of secretions or excretions from infected rodents. The family Arenaviridae is divided into Old World and New World complexes (Buchmeier et al., 2007, Gonzalez et al., 2007). Lassa and lymphocytic choriomeningitis virus (LCMV) viruses are considered the most important of the Old World arenaviruses due to their association with severe disease in humans. The New World complex is divided into 3 major clades (A, B and C) with clade B containing all the hemorrhagic fever (HF) associated viruses (Archer and Rico-Hesse, 2002, Bowen et al., 1997, Charrel et al., 2003). Arenaviruses have often been viewed as relatively stable genetically with amino acid sequence identities of 90–95% among different strains of LCMV and of 44–63% for homologous proteins of different arenaviruses (Southern and Bishop, 1987). However, considerable variation in biological properties among LCMV strains has become apparent, with dramatic phenotypic differences among closely related LCMV isolates (reviewed in Sevilla and de la Torre, 2006, Sevilla et al., 2002). These data provide strong evidence of viral quasispecies involvement in arenavirus adaptability and pathogenesis. Here, we will review several aspects of the molecular biology of arenaviruses, phylogeny and evolution and quasispecies dynamics of arenavirus populations for a better understanding of arenavirus pathogenesis.
2. Arenaviruses as model systems to study virus–host interactions, and as human pathogens
Arenaviruses merit significant interest both as tractable experimental model systems to study acute and persistent viral infections (Oldstone, 2002, Zinkernagel, 2002), and as clinically important human pathogens. In addition, compelling evidence indicates that the prototypic arenavirus LCMV is a neglected human pathogen of clinical significance in congenital infections (Barton et al., 2002, Jahrling and Peters, 1992, Mets et al., 2000). Moreover, LCMV poses special threat to immuno-compromised individuals, as tragically illustrated by recent cases of transplant-associated infections by LCMV with a fatal outcome in the USA (Fischer et al., 2006, Peters, 2006) and Australia (Palacios et al., 2008). Besides a public health risk, arenaviruses pose a biodefense threat (Borio et al., 2002) and six of them, including Lassa virus (LASV) and LCMV, are category A agents.
2.1. LCMV infection of the mouse: the Rosetta stone of virus–host interaction
Studies using LCMV have led to major advances in virology and immunology that apply universally to other microbial infections and viral infections of humans, including virus-induced immunopathological disease, MHC restriction, and T cell-mediated killing (Oldstone, 2002, Zinkernagel, 2002). The outcome of LCMV infection of its natural host, the mouse, varies dramatically depending on the strain, age, immunocompetence and genetic background of the host, as well as the route of infection, and the strain and dose of infecting virus (Oldstone, 2002, Zinkernagel, 2002). This provides investigators with a unique model system with which to investigate parameters that critically influence many aspects of virus–host interaction including the heterogeneity of phenotypic manifestations often associated with infection by the same virus.
2.2. Arenaviruses and their impact in human health
Arenaviruses cause chronic infections of rodents with a worldwide distribution (Buchmeier et al., 2007). Infected rodents move freely in their natural habitat and may invade human dwellings. Humans are infected through mucosal exposure to aerosols, or by direct contact of abraded skin with infectious materials. The Old World (OW) Lassa virus (LASV) and several New World (NW) arenaviruses cause HF disease in humans, posing a serious public health problem (Buchmeier et al., 2007, McCormick and Fisher-Hoch, 2002, Peters, 2002). Thus, LASV is estimated to infect several hundred thousand individuals yearly in its endemic regions of West Africa, resulting in a high number of Lassa fever (LF) cases associated with significant mortality and high morbidity. Notably, increased traveling to and from endemic regions has led to the importation of LF into non-endemic metropolitan areas around the globe (Freedman and Woodall, 1999, Holmes et al., 1990, Isaacson, 2001, Macher and Wolfe, 2006). Likewise, the NW arenavirus Junin virus (JUNV) causes Argentine HF, a severe illness with hemorrhagic and neurological manifestations and a case fatality of 15–30% (Harrison et al., 1999, Peters, 2002, Weissenbacher et al., 1987), whereas the NW Machupo (MACV) and Guaranito (GTOV) arenaviruses emerged as causative agents of HF in Bolivia and Venezuela, respectively (Peters, 2002). These concerns are aggravated by the lack of licensed vaccines, and current anti-arenavirus therapies being limited to the use of the nucleoside analogue ribavirin (Rbv), which is only partially effective and associated with significant side effects.
3. Molecular and cell biology of arenaviruses
Arenaviruses are enveloped viruses with a bi-segmented negative strand RNA genome and a life cycle restricted to the cell cytoplasm Eliminate Buchmeier 2001 (Buchmeier et al., 2007, Meyer et al., 2002, Southern, 1996). Each genomic RNA segment, L (ca. 7.3 kb) and S (ca. 3.5 kb), uses an ambisense coding strategy to direct the synthesis of two polypeptides in opposite orientation, separated by a non-coding intergenic region (IGR) with a predicted folding of a stable hairpin structure (Fig. 1 ). The S RNA encodes the viral glycoprotein precursor (GPC) and the nucleoprotein, NP. The L RNA encodes the viral RNA dependent RNA polymerase (RdRp, or L polymerase), and the small (ca. 11 kDa) RING finger protein Z. Viral mRNAs are non-polyadenylated and have extra non-templated nucleotides and appear to have a cap structure at their 5′-ends, but the origin of both the 5′-non-templated nucleotides and cap structure remains to be determined. NP and L mRNAs are transcribed into a genomic complementary mRNA, whereas the GPC and Z are not translated from genomic RNA species, bur rather from genomic sense mRNAs that are transcribed using as templates the corresponding antigenome RNA species, which also function as replicative intermediates. Transcription termination of viral mRNAs was mapped to multiple sites within the distal side of the IGR (Meyer and Southern, 1994, Tortorici et al., 2001). This observation led to the proposal that IGR served as a bona fide transcripton termination signal, which has been confirmed by more recent studies using reverse genetics technology (Lopez and Franze-Fernandez, 2007).
Fig. 1.
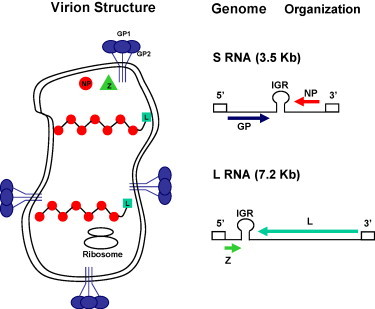
Arenavirus virion structure and genome organization. Virions are spherical to pleomorphic, with a lipid membrane envelope, measuring 50–300 nm (average 110–130) in diameter. The virion contains two filamentous nucleocapsids per envelope, each with “strings of beads” appearance and helical symmetry (indicated in the figure with red circles). The viral glycoprotein (GP) forms trimers GP1/GP2 to form the spikes on the virus surface (blue in the figure). Each virion has two segments of linear ssRNA: L (large) (ca. 7.3 kb) and S (small) (ca. 3.5 kb). Both use an ambisense coding strategy to direct the synthesis of two polypeptides in opposite orientation, separated by a non-coding intergenic region (IGR) with a predicted folding of a stable hairpin structure. The S RNA encodes the viral glycoprotein precursor (GPC) and the nucleoprotein, NP. The L RNA encodes the viral RNA dependent RNA polymerase (RdRp, or L polymerase), and the small (ca. 11 kDa) RING finger protein Z. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Post-translational cleavage of GPC generates the three components that form the GP complex (GPc): the stable signal peptide (SSP, 58aa), GP1 (Mr 40–46 kD), and GP2 (Mr 35 kD) (Buchmeier et al., 2007). The arenavirus SSP is unique in that it remains stably associated with the GP complex following cleavage by signal peptidase, and plays crucial roles in the trafficking of GP through the secretory pathway (Eichler et al., 2003, Eichler et al., 2004, York et al., 2004). Generation of GP1 and GP2 of the OW arenaviruses LCMV and LASV was found to be mediated by the SKI-1/S1P cellular protease (Beyer et al., 2003, Kunz et al., 2003, Pinschewer et al., 2003a, Pinschewer et al., 2003b). This finding has been now extended also to NW HF arenaviruses Guanarito, Machupo and Junin (Rojek et al., 2008). Notably, the protease recognition site present in Guanarito GPC deviates from the originally reported S1P consensus sequence (Rojek et al., 2008), indicating that S1P specificity is broader than previously thought.
Trimers of GP1/GP2 associate via ionic interactions to form the spikes that decorate the virus surface. GP1 is located at the top of the spike and mediates virus interaction with host cell surface receptors. The cellular receptor for LCMV and LASV is α-dystroglycan (α-DG) (Cao et al., 1998, Spiropoulou et al., 2002). Upon initial attachment to the target cell, LCMV virions are taken up in smooth-walled vesicles, which are not associated with clathrin (Borrow and Oldstone, 1994). In contrast, the NW arenaviruses GTOV, JUNV, MACV, and Sabia can use human transferrin receptor 1 as a cellular receptor (Radoshitzky et al., 2007) and clathrin-dependent endocytosis has been reported for JUNV (Martinez et al., 2007). Fusion between the viral and cell membranes is triggered by the acidic environment of the late endosome, which is thought to evoke a conformational changes in the arenavirus GP exposing a fusogoneic peptide (Castilla et al., 1994, Di Simone and Buchmeier, 1995, Di Simone et al., 1994, York and Nunberg, 2006), that mediates fusion of the virion and host cell membranes (Eschli et al., 2006, Gallaher et al., 2001).
Once the virus RNP is delivered into the cytoplasm of the infected cell, the polymerase associated with the virus RNP directs the biosynthetic processes involved in RNA replication and gene transcription (Fig. 2 ). Primary transcription initiated at the genome promoter located at the genome 3′-end results in synthesis of NP and L mRNA from the S and L segments, respectively. Subsequently the virus polymerase can adopt a replicase mode and moves across of the IGR to generate a copy of the full-length antigenome RNA (agRNA). This agRNA will serve as template for the synthesis of the GP (agS) and Z (agL) mRNAs. The agRNA species serve also as templates for the amplification of the corresponding genome RNA species.
Fig. 2.
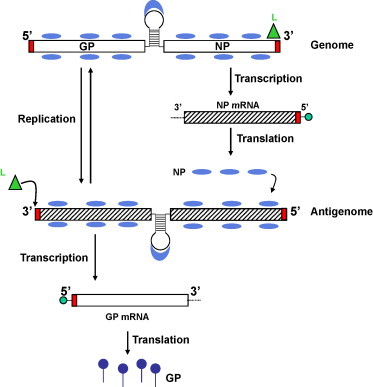
Arenavirus replication and transcription. The virus enters the cell via endosomal route. The viral uncoating in cytoplasm releases the viral genome. NP and L mRNAs (in the figure is shown the replication and transcription of S segment) are transcribed into genomic complementary mRNA, whereas the GP and Z are not translated from genomic species, but rather from genomic sense mRNAs that are transcribed using the corresponding antigenome RNA species, which also function as replicative intermediates. The primary transcription initiated at the genome promoter located at the genome 3′-end results in synthesis of NP mRNA from the S. Viral mRNA has a cap structure at the 5′-ends (green circle). Subsequently the virus polymerase can adopt a replicase mode and moves across of the IGR to generate a copy of the full-length antigenome RNA. This antigenome RNA will serve as template for the synthesis of the GP mRNA. The antigenome species serve also as templates for the amplification of the corresponding genome RNA species or replication. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
As with many other enveloped negative strand (NS) RNA viruses, formation and release (budding) of arenavirus infectious progeny from infected cells requires that assembled viral RNPs associate at the cell surface with membranes that are enriched in viral GPs. For many enveloped NS RNA viruses this association and subsequent viral budding, are mediated by a matrix (M) protein that acts as a bridge between the RNP and GP. We (Perez and de la Torre, 2003) and others (Strecker et al., 2003, Urata et al., 2006) have shown that Z is the main driving force of arenavirus budding and that this process is mediated by the Z proline-rich late (L) domain motifs (PTAP and PPPY) known to control budding of several other viruses via interaction with specific host cell proteins (Freed, 2002). The view of Z as the arenavirus counterpart of the M protein found in many other NS RNA viruses is consistent with recent ultrastructural data on arenavirus virions determined by cryo-electron microscopy (Neuman et al., 2005), as well as genetic and biochemical evidence of a GP-Z association (Capul et al., 2007).
3.1. Arenavirus reverse genetics
The inability to genetically manipulate the arenavirus genome has hampered studies aimed at understanding its molecular and cell biology, as well as pathogenesis. We have developed a reverse genetics system for the prototypic arenavirus LCMV that is allowing us to investigate the cis-acting sequences and trans-acting factors that control arenavirus replication and gene expression, as well as assembly and budding (Lee et al., 2002, Perez and de la Torre, 2003, Pinschewer et al., 2003a, Pinschewer et al., 2003b). Moreover, we can now rescue infectious LCMV entirely from cloned cDNAs (Flatz et al., 2006, Sanchez and de la Torre, 2006), which allows us to examine the phenotypes of rLCMV with predetermined mutations in the context of the virus natural infection of the mouse. This development provides us with unique opportunities to investigate arenavirus–host interactions that influence a variable infection outcome, ranging from virus control and clearance by the host defenses to severe acute disease and long-term chronic infection associated with subclinical disease. Likewise, these advances in arenavirus genetics have opened new approaches for the development of screening strategies to identify and evaluate novel anti-arenaviral drugs targeting specific steps of the virus life cycle.
4. Arenavirus phylogeny and evolution
4.1. Arenaviridae taxonomy
4.1.1. Arenaviridae family
LCMV, the first known arenavirus, was discovered almost simultaneously by three different groups in North America in the 1930s. Armstrong and Lillie coined the name LCMV in 1934 based on the pathological picture this agent caused upon intracerebral inoculation of monkeys and mice. The same year, Traub (1934) isolated LCMV from apparently healthy mice, demonstrating the ability of LCMV to persistently infect its host. Subsequently, Rivers and Scott (1935) demonstrated that LCMV was a human pathogen. The next arenavirus discovered was Junin virus during a hemorrhagic fever outbreak in Argentina (Parodi et al., 1958). In the 1960s, Tacaribe (Downs et al., 1963), Machupo (Johnson et al., 1965) and Amapari (Pinheiro et al., 1966) viruses were identified. In the early-1970s, 10 different arenaviruses were known including Lassa virus (LASV), discovered in Nigeria in 1969 (Frame et al., 1970, Rowe et al., 1970).
The name arenavirus, derived from the latin arenosus, relates to sandy-looking particles observed by EM of ultrathin sections of virion particles. Currently, there are 22 recognized species into the Arenavirus genus, the unique member of the Arenaviridae family, and 2 tentative species: Pampa virus (Lozano et al., 1997) and Rio Cacarana virus. Moreover, 2 new viruses were recently isolated from transplanted patients in Australia, Dandenong virus (Palacios et al., 2008), and from a human case of hemorrhagic fever in Bolivia, Chapare virus (Delgado et al., 2008). There are several evidences that Dandenong virus might be a strain of LCMV based on region of origin, clinical spectrum and genetic data. Field studies allowed the discovery of five arenaviruses: Kodoko virus in Guinea (Lecompte et al., 2007), Morogoro virus in Tanzania (Charrel et al., 2008), Pinhal virus in Brazil (Charrel et al., 2008) and Catarina and Skinner Tank viruses from United States of America (Cajimat et al., 2008, Cajimat et al., 2007). World Health Organization also reported a potential new arenavirus isolate from 4 lethal cases of hemorrhagic fever in Zambia/South Africa. Arenaviruses are organized in two distinct groups: (1) the New World (LCMV and LASV) and the New World on one hand LCMV and LASV, and (2) on the other hand, viruses from the Tacaribe complex, including all the other known arenaviruses.
4.1.2. Species demarcation criteria
The originally established species demarcation criteria (described online on the ICTV website) are: (1) association with a specific host species or group of species, (2) presence in a defined geographical area, (3) etiological agent (or not) of disease in humans, (4) significant differences in antigenic cross-reactivity, including lack of cross-neutralization activity where applicable, and (5) significant sequence difference from other species in the genus. Since the definition of a virus species is polythetic (Van Regenmortel, 1989), a new isolate will be placed into an existing species if it shares with its members 4 or 5 common characters, whereas a tentative species will be proposed if it presents at least two differences with any other arenavirus. The analysis of the information used to make a taxonomic decision could pose significant difficulties. Some isolates may have been isolated only from human patients, precluding the identification of the virus host like for Sabia virus and, more recently, Chapare and Dandenong viruses. Moreover, the antigenic cross-reactivity of a new isolate can be difficult to determine, since it requires the proper containment facility and the ability to manipulate different arenaviruses for comparative purposes. These difficulties have led more recently to favor genetic criteria to define arenavirus species. The genetic criterium relies on a statistical sequence difference between the new isolate and other members of an existing arenavirus species. This was not exempt of some controversies: (1) lack of agreement on which portion of the arenavirus genome should be used and (2) degree of genetic diversity required to consider that two viruses belong to different species. (Bowen et al., 2000) proposed to use as a cutoff value an uncorrected amino acid p distance of 12% in the NP. The rationale for this was: (1) it was the highest intra-species difference reported, and (2) the lowest inter-species distance was of 13% between Junin and Machupo viruses. However, when this criterium, originally designed for full-length NP sequences, was also extended to partial NP sequences it raised significant problems. Thus, several LASV isolates showed differences up to 14.8% in uncorrected amino acid p distances using a small region of the NP gene (Bowen et al., 2000). Likewise, Pirital and Whitewater Arroyo virus isolates presented intra-species distances up to 16 and 15.1%, respectively, using partial NP sequences (Fulhorst et al., 2001, Weaver et al., 2000). Even the recent availability of complete arenavirus genomes has not solved the problem of how to correctly assign a cutoff value for species demarcation partly because the sequence availability is biased: there are many less Old World arenaviruses than New World arenaviruses (5 versus 17, respectively), but the first ones include the majority of complete intra-species sequences (Charrel et al., 2008). So the intra-species diversity is likely underestimated among the New World arenaviruses whereas the inter-species diversity could be also underestimated for Old World arenaviruses. Indeed, when a large number of isolates were compared using the same genomic area, the maximum diversity found in an Old World arenavirus (LASV) was similar to the one found in two New World arenaviruses (Whitewater Arroyo and Pirital viruses) (Bowen et al., 2000, Fulhorst et al., 2001, Weaver et al., 2000). These findings suggest that the best way to use the genetic criterion is to follow the arenavirus species definition and to compare a new isolate with the larger available set of sequences.
4.2. Phylogenetic relationships among arenaviruses
The first analysis of large number of arenaviral NP sequences (Fig. 3 ) (Bowen et al., 1996, Bowen et al., 1997) led to the following conclusions: (1) a genetic separation between Old World and New World arenaviruses, confirming the geographic and serologic data, (2) the New World complex is composed by three different clades (A, B and C), and (3) all the New World arenaviruses responsible of human hemorrhagic fevers are grouped together into clade B.
Fig. 3.
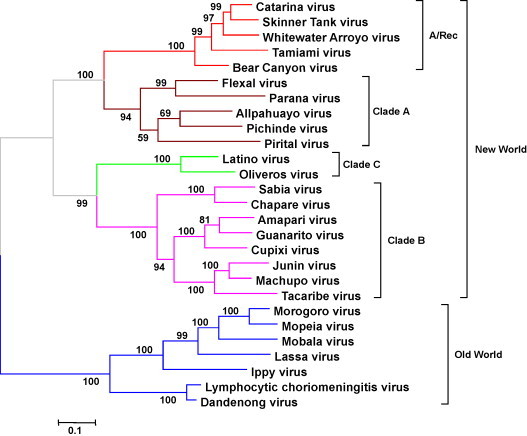
Phylogeny of the arenaviruses based on the analysis of the complete amino acid sequence of the nucleoprotein. Phylograms were obtained by using the tree reconstruction analysis of Treepuzzle 5.2 (Schmidt et al., 2002) with quartet puzzling algorithm (Strimmer and von Haeseler, 1996) and an accurate parameters estimation (quartet sampling for substitution process and NJ tree for rate variation) for both the model of substitution and the mixed model of heterogeneity (1 invariable + 8 gamma rates). The model of substitution, estimated from data set was the VT model (Muller and Vingron, 2000). The number of puzzling steps was set to 10,000 and the outgroup was LCMV Armstrong. The scale indicates the maximum likelihood branch length. Numbers represent support for the internal branches of the quartet puzzling tree topology in percent.
The complete sequence of the S segment of a Whitewater Arroyo virus (WWAV) isolate uncovered a discrepancy between the phylogenetic trees derived from the NP or GPC genes. Based on NP data, WWAV was placed into the clade A, whereas GPC data placed this virus within clade B (Charrel et al., 2001). Bootscanning analysis indicated that the phylogenetic position of WWAV shifted from clade B to clade A at the very end of the GPC gene. WWAV shared more domains (identical sequences of four or more amino acid residues) with clade A viruses than with clade B viruses in the NP sequence, but the opposite using the GPC gene, including a gap of 17 aa unique to WWAV and clade B viruses. This peculiarity of WWAV was soon extended to Tamiami and Bear Canyon viruses, two other North America arenaviruses (Archer and Rico-Hesse, 2002, Charrel et al., 2002). The two North America arenaviruses recently isolated, Catarina virus and Skinner Tank virus, cluster into the North America clade and show the same phylogenetic incongruence using the GPC or NP sequences (Cajimat et al., 2007, Cajimat et al., 2008, Charrel et al., 2008). Based on their peculiar phylogenetic features, the North America arenaviruses were referred as the A/Rec lineage (Charrel et al., 2003), which could be considered as a fourth lineage exists among New World arenaviruses. Phylogentic studies based on partial polymerase (L gene) sequences showed no differences compared with results obtained for the NP gene (Charrel et al., 2003). This finding suggested that highly unlikely an exchange of an entire segment (reassortment) did occur in the past for any currently known arenavirus species. This finding was first demonstrated for Old World arenaviruses (Emonet et al., 2006) and subsequently extended to the New World arenaviruses (Cajimat et al., 2007, Charrel et al., 2008).
The arenavirus family is composed of two groups: New World and the Old World arenaviruses, which are considered to be two clades sharing a common ancestor. This hypothesis, however, is difficult to confirm as both groups appear to be monophyletic, and consequently the out-group used to root the tree, required to confirm the monophyletic nature of the OW and NW groups, must be from another virus family, which raises the problem of selecting the appropriate viral family, genus, and specific gene as the out-group. Using the sequence of an isolate of the tospovirus Impatiens necrotic spot virus as an outgroup (Albarino et al., 1998) found that the Old World group was monophyletic, whereas the New World group was either monophyletic using the NP gene, or polyphyletic using the GPC gene. Using the secondary structure-assisted alignment of polymerase sequences, Vieth et al. (2004) reported that both the New World and the Old World arenavirus groups were monophyletic with respect to a large set of ssRNA negative-strand viruses. The Old World group is composed of five species, and of the newly discovered Kodoko, Morogoro and Dandenong viruses (Charrel et al., 2008, Lecompte et al., 2007, Palacios et al., 2008). If Kodoko virus is finally recognized as a distinct species, then the Old World arenaviruses would be split into two lineages: (1) a group composed of arenaviruses infecting Praomys rodents: LASV, MOBV, IPPYV, MOPV and Morogoro virus and (2) a second group composed of LCMV and Kodoko virus, infecting Mus rodents. The New World group is actually composed of four lineages: clades A, B, C and A/Rec. The clade A is composed of five viruses, FLEV, PARV, PICV, PIRV and ALLV. The internal relationships among those viruses are not established, since they vary from one gene to the other and the internal nodes are poorly supported by boostrap analyses (Charrel et al., 2008). Clade B contains seven arenavirus species plus Chapare virus, and this clade appears to form three different lineages: (1) Chapare virus and SABV, (2) CPXV, GTOV and AMAV, and (3) JUNV, MACV and TCRV. Those three groups are well supported and were previously reported (Archer and Rico-Hesse, 2002, Bowen et al., 1996, Bowen et al., 1997, Cajimat et al., 2007, Charrel et al., 2002, Charrel et al., 2003). The clade C is only composed of two arenaviruses, OLVV and LATV, and a potential third member, Pinhal virus, for which little information is available (Charrel et al., 2008). The fourth clade is the A/Rec clade, composed by all North America arenaviruses: WWAV, TAMV, BCNV and the newly discovered Skinner Tank and Catarina viruses. The intra-clade A/Rec relationships are poorly resolved, due to the incongruence of phylogenetic data from one gene to the other and also due to the fact that only the GPC and NP sequences are available for Catarina and Skinner Tank viruses.
4.3. Genome reorganization
4.3.1. Recombination (intra-segmental)
The hypothesis of a recombination event has been proposed as the cause of the discrepancy between NP- and GPC-based phylogenetic trees (Charrel et al., 2001). Moreover, this recombination event was supposed to be at the origin of the A/Rec lineage (Archer and Rico-Hesse, 2002, Charrel et al., 2002, Charrel et al., 2003). Several findings appeared to support this hypothesis: (1) the number of amino acid domains shared between WWAV and arenaviruses from clades A and B, (2) the 17 amino acid gap common to WWAV and clade B viruses, (3) the bootscanning analysis (Charrel et al., 2001), and (4) the phylogenetic shift seen between GPC- and NP-based phylogenetic trees for all North America arenaviruses (Charrel et al., 2002, Charrel et al., 2003), a feature also reported for the two newly discovered North America arenaviruses, Catarina and Skinner Tank viruses (Cajimat et al., 2008, Charrel et al., 2008). The consistency of that character among close viruses clearly supports a heritable trait, and thereby the idea that a past recombination event was at the origin of that lineage. However, an alternative explanation could be provided based on the peculiarity of the GP1 region within the GPC gene. GP1 is highly variable in clades B and A/Rec, whereas it is much more conserved among clades A and C (Table 1 ). Due to this high variability in clades B and A/rec (the GP1 of TRCV and SABV, both from clade B, share only 19% of common amino acids), the alignment of GP1 is not significant in term of phylogenetic information when the whole arenavirus family is compared and could represent a confounding factor for the phylogenetic analyses based on the GPC gene. Nevertheless, when only the conserved regions of GPC are used (the signal peptide and the GP2 region), the inconsistency of relationships between North America arenaviruses and clades A and B arenaviruses is still present, which strengthens the recombination hypothesis (Charrel et al., 2008).
Table 1.
Genetic diversity among New World species from the same clades for the GP1 protein (p-distances values in percent). Since 2 species are found in clade C, the minimal (Min), maximal (Max) and average values are the same.
| Clade A | Clade B | Clade C | Clade A/Rec | |
|---|---|---|---|---|
| Min | 12.6 | 41.1 | / | 47.3 |
| Max | 24.4 | 80.6 | / | 63.2 |
| Average | 20.6 | 66.7 | 28.4 | 53.9 |
The generation of recombinant arenaviruses requires cells to be simultaneously infected by two different arenaviruses. The possibility to co-infect cultured cells has been demonstrated (Lukashevich, 1992, Lukashevich et al., 1991, Lukashevich et al., 2005, Riviere et al., 1985, Riviere and Oldstone, 1986), but a co-infection event may be significantly more difficult to occur in nature. For this to happen two different arenaviruses must have the opportunity to co-infect the same rodent. From a geographic point of view, some arenaviruses share a common geographic localization, like Guanarito and Pirital viruses in Venezuela (Fulhorst et al., 1999, Fulhorst et al., 2008, Weaver et al., 2000), Junin and Oliveros viruses in Argentina (Mills et al., 1996), Machupo, Latino and Chapare viruses in Bolivia (Delgado et al., 2008, Johnson et al., 1965, Murphy et al., 1969). From a host point of view, different arenaviruses can some times infect the same rodent species, like Guanarito and Pirital viruses, which were isolated both in cotton rats Sigmodon alstoni and in cane mice Zygodontomys brevicauda (Weaver et al., 2000). However, despite an extensive genetic characterization, the existence of a GTOV-PIRV recombinant virus has never been demonstrated (Cajimat and Fulhorst, 2004, Fulhorst et al., 2008). The absence of a GTOV/PIRV recombinant, despite the fact that all the required factors might be present, could be explained by superinfection exclusion, a well-known phenomenon among arenaviruses (Damonte et al., 1983, Ellenberg et al., 2004, Ellenberg et al., 2007). Cells infected with a given arenavirus become highly resistant to superinfection by another genetically closely related arenavirus via mechanisms that remain poorly understood.
Since no A/Rec arenavirus has been isolated in South America and neither clade A nor clade B arenavirus has been isolated in North America, it is difficult to explain how the recombination occurred. Some possibilities to consider are: (1) the two parental arenaviruses were in North America, but disappeared, either by natural extinction or by being outcompeted by the recombinant arenavirus, (2) the recombination occurred in South America, and the recombinant arenavirus migrated up to North America, maybe following its host, and finally (3) the recombination occurred in North America, and all the clades A and B arenaviruses migrated south. Based on the actual and limited knowledge of past and actual arenavirus geographic repartition and host association, it is difficult to state about any of those hypotheses. Among these possibilities, the second one appears to be highly unlikely because South America arenaviruses are supposed to descend from an ancestral arenavirus located in North America (Cajimat et al., 2007). Moreover, North America arenaviruses are primarily associated with rodents from Neotominae subfamily, which are almost exclusively found in North America, and this association was supposed to be a long-term co-evolutionary relationship (Cajimat et al., 2007).
The origin of the North America arenaviruses via a recombination event remains hypothetical. The only finding still supporting this hypothesis is the incongruence between GPC- and NP-based phylogenetic reconstructions. The other originally cited genetic arguments are no longer in favor of this hypothesis. The 17 amino acid gap is located within GP1, whose phylogenetic information is very doubtful due to a poor alignment, and the North America arenaviruses share now the same number (N = 3) of specific conserved domains of 4 or more amino acids with either the clade A or the clade B arenaviruses. Moreover, the superinfection exclusion phenomenon and the absence of any lab-created or characterized PIRV/GTOV recombinant weaken the recombination hypothesis. Finally, the clade C arenaviruses also demonstrated a shift between GPC- and NP-based trees (Charrel et al., 2008), and it seems very doubtful such a rare event would be the origin of two different lineages. It seems more plausible that the phylogenetic information derived from arenavirus GPC is influenced by specific selection pressures (Cajimat et al., 2007). The reasons why GPC would be under a selection pressure different from the other arenavirus genes need to be determined. Likewise, the statistical significance of the recombination event found in North America and clade C arenaviruses using recombination detection softwares should be validated using an up-to-date arenavirus genetic database.
More research in cell culture with marked variants is needed to substantiate the occurrence of true recombination in the arenaviruses, and to estimate its frequency under controlled laboratory conditions.
4.3.2. Reassortment
Due to the bi-segmented nature of their genome, arenaviruses have the potential for reassortment of their segments during co-infection of the same cell. The generation of reassortant viruses between two parental arenaviruses has been well documented in cultured cells (Lukashevich et al., 1991, Lukashevich et al., 2005, Riviere et al., 1985, Riviere and Oldstone, 1986). The reassortment approach has been used to define the function of viral gene products in arenavirus pathogenesis (Riviere et al., 1985, Riviere and Oldstone, 1986), arenavirus induced immunosuppression (Matloubian et al., 1990, Matloubian et al., 1993), and to create live-attenuated vaccines (Lukashevich et al., 2005). The generation of reassortant arenaviruses is subjected to the same constrains as those described for the generation of recombinant arenaviruses. Moreover, currently we have only very limited knowledge about the mechanisms governing packaging of arenavirus genomes, which may pose additional difficulties for the generation of certain reassortant arenaviruses. In addition, to be successful reassortant, arenaviruses need to have a higher or comparable fitness than both of its parents to be maintained and spread. The existing genetic data do not support a role of reassortment in the formation of new arenavirus species (Cajimat and Fulhorst, 2004, Charrel et al., 2003, Charrel et al., 2008, Emonet et al., 2006). Nonetheless, the finding by (Weaver et al., 2000) of a rodent infected by two different genotypes of PIRV indicates that the superinfection exclusion is not absolute and therefore reassortment events may occur often among members of quasispecies replicating in a persistently infected host. However, it has not been determined whether the two genotypes of PIRV isolated from the same rodent were replicating within the same cells, a requirement for the generation of reassortant viruses.
4.4. Co-evolution of arenaviruses and their natural rodent reservoirs
All known arenaviruses, with exception of TCRV, have as natural reservoirs one or two rodent species from the family Muridae (Salazar-Bravo et al., 2002). This association does not appear to be random, and groups of genetically closely related arenaviruses infect closely related rodent species. Thus, the Old World arenaviruses are found in rodents from the subfamily Murinae, whereas rodents from the subfamilies Sigmodontinae and Neotominae are reservoirs for the New World arenaviruses. Furthermore, inside the Old World group, the LASV complex (LASV, MOBV, MOPV and IPPYV) is associated with Praomys rodents, while LCMV and Kodoko virus, which constitute a potential second Old World group, are found in rodents from the genus Mus (Lecompte et al., 2007). In the New World lineage, North America arenaviruses, except TAMV, are primarily associated with rodents from the subfamily Neotominae (Cajimat et al., 2007, Cajimat et al., 2008), whereas the reservoirs of the South America arenaviruses belong to the subfamily Sigmotondinae.
This almost perfect repartition of arenavirus lineages into rodent clades, as well as their ability to persistently infect their rodent hosts led to the proposal of a co-evolution mechanism that could explain both the actual arenavirus diversity and geographic distribution, as well as the virus/rodent adaptation (Bowen et al., 1996, Bowen et al., 1997, Childs and Peters, 1993). This feature was also reported for other groups of viruses, including hantaviruses which are very similar to arenaviruses both from an ecological and overall genomic organization point of view (Jackson and Charleston, 2004, Ramsden et al., 2008, Ramsden et al., 2009). The co-evolution hypothesis requires that both the primary host–virus phylogenies match, exceptions apart like host-switch events. The inherent problem of such a process is the accumulation of hypotheses: the two phylogenetic hypotheses (host and virus phylogenetic trees) are compared and since a perfect match usually does not occur, further hypotheses are proposed to explain the present virus/reservoir association. Therefore, it can be challenging to discuss the final result, since arbitrary choices must be make, like the phylogenetic trees that will be used in the comparison, as well as assumptions in the method used to reconcile the virus and host phylogenies. For arenaviruses, and as explained above, a major caveat is the lack of phylogeny conclusiveness for some clades, like North America arenaviruses or clade A arenaviruses, for which the information is not robust and depends on the gene or the phylogenetic algorithm used to infer it.
Two studies tried to test the co-evolution hypothesis, either using Old World arenaviruses (Hugot et al., 2001), or New World arenaviruses (Jackson and Charleston, 2004). In the first one, Hugot et al. (2001) compared the Old World phylogeny to their murine hosts using a method able to discriminate the virus reservoir (primary host) from secondary hosts. This analysis resulted in 10 different scenarios, but authors focused on the 2 scenarios, which presented the lowest number of extra-hypotheses. Based on those data, authors claimed that the co-evolutionary pathway is an acceptable explanation for the present day Old World arenaviruses/Murinae associations. The second study was based on a phylogenetic reconciliation analysis which first built all the potentially optimal solutions able to reconcile any incongruence between host/parasite phylogenies and then tested the statistical significance of such solutions (Jackson and Charleston, 2004). The New World arenaviruses and their sigmodontine hosts were used, and the result showed an absence of statistical significance in the impact of co-evolution onto the present day virus/host association.
Since the two studies gave opposite results, but used different methods and samples, the impact of co-evolution onto the virus and host evolutionary processes remains hypothetical and to be demonstrated. Such a demonstration will require a larger set of viruses/hosts, to take into account arenavirus phylogenetic issues and most of all, a better determination of the arenavirus reservoirs whose identity is not sure for many arenaviruses (Salazar-Bravo et al., 2002).
5. Recent advances in quasispecies dynamics: collectivities as the unit of selection
Over the last years, important developments on the relevance of quasispecies complexity as a determinant of the phenotype and of the survival capacity of RNA viruses in vivo have taken place. Major progress has resulted from the isolation of a mutant of poliovirus (PV) resistant to the mutagenic nucleoside analogue ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) (R). This R-resistant mutant included amino acid replacement G64S in the viral RNA-dependent RNA polymerase (RdRp). Interestingly, despite this replacement affecting a residue distant from the catalytic site of the RdRp, the mutant enzyme displayed increased template-copying fidelity (Arnold et al., 2005, Castro et al., 2005, Pfeiffer and Kirkegaard, 2005). The PV harboring G64S in its RdRp gave rise to mutant spectra that included about 6-fold lower number of mutations than wild type PV (Vignuzzi et al., 2006), and a 3-fold lower frequency of mutants resistant to guanidine hydrochloride, an inhibitor of picornaviral replication (Pfeiffer and Kirkegaard, 2003). The decreased amplitude of the mutant spectrum produced by PV with G645 in the RdRp compromised the adaptability of the virus to a complex environment, and resulted in decreased pathogenicity for mice (Pfeiffer and Kirkegaard, 2005, Vignuzzi et al., 2006). Most significantly, pathogenicity for mice was regained when the complexity of the mutant spectrum was restored to wild type levels by replicating the virus in the presence of mutagenic nucleoside analogues. These studies are relevant for at least two reasons: they point to mutant spectrum complexity as a significant element in viral pathogenesis, and they document the behavior of the entire quasispecies as a unit of selection in vivo, one of the main features that render quasispecies theory irreducible to classical (Wright–Fisher) formulations.
R-resistant mutants have been isolated for hepatitis C virus (Young et al., 2003) and foot-and-mouth disease virus (FMDV) (Sierra et al., 2007). Despite the RdRps of FMDV and PV being closely related, the R-resistance of FMDV was associated with RdRp replacement M296I, distant from the site of the PV mutation. In contrast to substitution G64S in PV, M296I, is located at a loop close to the active site of the polymerase, and did not give rise to an increase of template-copying fidelity (Arias et al., 2008, Sierra et al., 2007). Comparison of the R-resistant FMDV and PV RdRps indicates that different polymerase sites can be directly or indirectly involved in nucleotide discrimination during RNA synthesis, and that a restriction in the incorporation of R-triphophate (RTP) need not entail a general increase of template copying fidelity. The ongoing research on picornavirus and retrovirus copying fidelity constitutes (once again!) evidence of the subtle molecular mechanisms that a virus can exploit to overcome selective constraints directed to the very core of its biological needs: catalysis of RNA polymerization. In addition, the isolation viral mutants resistant to mutagenic analogues may help in the understanding of the molecular basis of polymerase fidelity (Ferrer-Orta et al., 2007, Friedberg et al., 2006), a key issue for general biology.
Other studies have also reinforced the relevance of mutant collectivities in virus behavior. In one of such studies with reconstructed FMDV quasispecies, complex swarms of escape-mutants were selected by antibodies, and minority components of the selected repertoire were endowed with the potential to recognize an alternative receptor (Martin and Domingo, 2008, Perales et al., 2005). Minority genomes in FMDV quasispecies contribute also to a molecular memory by which genomes that are dominant at an earlier evolutionary phase of the virus are represented at frequencies higher than expected from mere mutational pressure (Ruiz-Jarabo et al., 2000, Ruiz-Jarabo et al., 2002, Ruiz-Jarabo et al., 2003b). Memory genomes can be present during replication of HIV-1 in vivo, and they may influence the course of HIV-1 evolution (Briones et al., 2003, Briones et al., 2006). Therefore, clinically relevant evolutionary events underwent by HIV-1 in vivo could not be explained on the basis of consensus nucleotide sequences, if the composition of mutant spectra was ignored (Briones and Domingo, 2008). The observations on internal interactions among components of a mutant spectrum provide an interpretation of several early observations made with RNA virus populations. Notably, complementation among components of a mutant spectrum explain that individual biological clones isolated from a viral population display decreased fitness relative to the uncloned populations (Domingo et al., 1978, Duarte et al., 1994). Likewise, interfering interactions among components of mutant spectrum explain suppression by a mutant spectrum of a mutant with superior fitness (de la Torre and Holland, 1990) (see also next section).
Experimental and theoretical studies have further supported the concept of an error threshold for maintenance of genetic information, and that high genetic complexity (approximated to the genome size) requires a correspondingly larger accuracy of the replication machinery (Nowak, 2006, Takeuchi and Hogeweg, 2007). Theoretical formulations are in agreement with the presence in replicative DNA polymerases of differentiated organisms of a domain corresponding to 3′ to 5′ exonucleolytic proofreading activity (Friedberg et al., 2006). Because of their limited genetic complexity, RNA viruses can tolerate (and exploit) high error rates, and they generally lack a functional proofreading activity (Domingo et al., 2008). The only animal RNA viruses for which the presence of genomic sequences corresponding to a 3′ to 5′ proofreading-repair activity has been documented are the coronaviruses (Eckerle et al., 2007, Minskaia et al., 2006). One of the nonstructural CoV proteins is nsp14 or ExoN exoribonuclease. The SARS-CoV nsp14 expressed in bacteria showed 3′ to 5′ exonuclease activity in vitro, and mutations at the active site residues resulted in impaired viral RNA synthesis in animal cells (Minskaia et al., 2006). Replacement of the active site residues of the nsp14 ExoN of the coronavirus murine hepatitis virus (MHV) resulted in viable viruses that yielded mutant spectra with 15-fold higher mutation frequencies than wild type, without detectable changes in viral fitness (Eckerle et al., 2007). The authors cautiously recognize that their study did not constitute a direct proof that the mutant virus increased its mutant spectrum complexity through inactivation of the proofreading activity. However, the evidence strongly suggests that some correction mechanism that limits the mutation frequency in standard MHV is not operating (or is operating less efficiently) in MHV harboring substitutions in nsp14 (ExoN). Comparative studies with cellular DNA polymerases and their mutated versions suggest that a typical proofreading activity may contribute up to 100-fold to the accuracy of template copying (Friedberg et al., 2006).
Given the absence of proofreading and post-replicative repair mechanisms, RNA viruses in general produce error copies essentially during each round of replication. It is counterintuitive to some virologists and geneticists that when the consensus genomic sequence of an RNA virus remains invariant during replication, the population is nevertheless highly dynamic, with mutants arising at all times (Domingo et al., 1978). An invariant consensus genome is in a reality a collection of mutating genomes that yield the same consensus. The virus is “mutating towards itself”. Important biological events depend on changes in the mutant spectrum and not in the consensus sequence, as documented in the following sections.
5.1. Contribution of LCMV quasispecies to pathogenesis
Viral quasispecies contain multitudes of variants that can manifest alternative phenotypes in response to environmental demands. The contribution of a quasispecies genetic structure to the biology of RNA viruses is illustrated by the rapid selection of variants present within replicating viral populations in vivo in response to specific selection pressures. LCMV is an excellent experimental model for studying several aspects of viral pathogenesis and their connection with virus variation. The virus in vivo in its natural host, the mouse, is non-cytolytic, and can cause either acute or persistent infections. When immunocompetent adult mice are injected with LCMV, they generate a robust immune response that results in virus clearance, which is mainly mediated by major histocompatibility complex (MHC) class-I restricted CD8+ antiviral cytotoxic T lymphocytes (CTL) (Byrne and Oldstone, 1984, Fung-Leung et al., 1991). By contrast, mice infected neonatally or in uterus with LCMV become persistently infected for life. This persistent infection is due to the infection of thymic cells and the specific removal (negative selection) of lymphocytes with potential responsiveness to LCMV (King et al., 1992, Pircher et al., 1989). In the course of such persistent infections, distinct viral variants can be isolated from the brain and lymphoid tissue (Ahmed et al., 1984). These viral variants have different biological properties that correlate with the type of tissue from which they are isolated. One type of variant predominates in the CNS (Evans et al., 1994, Tishon et al., 1993), whereas the other type predominates in lymphocytes and macrophages of the immune system (Ahmed et al., 1984, Borrow and Oldstone, 1992, Parekh and Buchmeier, 1986, Tishon et al., 1993). Most of the CNS isolates are similar to the parental Armstrong (Arm) strain used to infect the mice and induce potent virus-specific CTL responses in adult mice, in which the infection is cleared within 2 weeks. In contrast, the majority of the isolates derived from the lymphoid tissue (the prototypic virus is called Cl13) cause chronic infections in adult mice associated with a restricted CTL response to LCMV and other viruses, as well as limited antibody responses to foreign antigens (Ahmed and Oldstone, 1988).
The emergence of cell-specific viral variants can be explained by the fact that when a viral infection occurs in the whole animal, the various organs and cell types present in the body provide a rich milieu for the selection of viral variants. During long-term persistence in carrier mice with continuous virus replication, and given the high mutation rate of RNA viruses LCMV variants that have a growth advantage in certain cell types are likely to be selected. The selection of a large number of LCMV variants in vivo has been documented (Sevilla et al., 2000). Sequence analysis of these variants showed consistently an amino acid substitution (F260L or F260I) in GP1 of the immunosuppressive viruses, which correlates with a 200–500-fold enhanced binding to α-DG and a high dependence on α-DG for the infection of cells by the Cl13-like viruses (Kunz et al., 2001, Kunz et al., 2003). The analysis of the anatomic distribution of immunosuppressive variants in the spleen of adult immunocompetent mice 3 days after infection showed a different tropism for both group of variants (Borrow et al., 1995, Sevilla et al., 2000, Smelt et al., 2001), in which Cl13-like viruses localized exclusively in cells of the marginal zone and white pulp of spleen, whereas Arm-like viruses localized primarily in cells within the red pulp. This distinct distribution in spleen indicated that the immunosuppressive and the non-immunosuppressive viruses infected different subsets of cells. The identification of cells in the spleen infected by LCMV revealed two subsets mainly infected by immunosuppressive viruses, CD11c- and DEC-205 positive dendritic cells that are the major cells population expressing α-DG in spleen (Sevilla et al., 2004).
Another evidence of the contribution of LCMV variants to pathogenesis can be found in the neonatal infection of C3H/St mice with LCMV that leads to a persistent infection associated with a growth hormone (GH) deficiency syndrome (GHDS) (Oldstone et al., 1982). This disorder is manifested as marked growth retardation and the development of hypoglycemia, which frequently leads to the death of infected animals (Oldstone et al., 1982, Oldstone et al., 1984). By using reassortants between a LCMV strain that causes GH disease (Arm) and one that does not (WE), the viral genes causing GH disease were mapped to the S RNA (Riviere et al., 1985). Variants with the ability to cause marked growth retardation and rapid death, the hallmarks of GHDS, were present at a proportion of approximately 1 in 20 (5%) within the GHDS-nil WE population. Moreover, WE clones that did not cause GHDS, like the prototypic WEc54, replicated poorly in the GH-producing cells in the pituitaries of infected mice. By contrast, a high viral load was detected in pituitary glands isolated from mice infected with WE clones that caused GHDS, like the prototypic WEc2.5 (Buesa-Gomez et al., 1996). Several experiments have shown that variants like WEc2.2 were preferentially selected in the livers of C3H/St mice infected with the WE parental population, meaning that viral replication in the liver may favor a preferential accumulation of variants like WEc2.2 (Buesa-Gomez et al., 1996). However, this selection did not lead to GHDS, a finding consistent with the requirement of virus replication in GH-producing cells of the anterior pituitary for the development of GHDS (Oldstone et al., 1982, Oldstone et al., 1984). The basis of the differences between WEc5.4 and WEc2.2 has been mapped to one amino acid change from S (153) to F in the viral GP-1 (Teng et al., 1996). Taken together all these findings one may see an illustration of how differences in the sequence distribution between two quasispecies with very similar, or identical, consensus sequences can significantly influence the virus impact on the infected host (Table 2 ).
Table 2.
Biological relevance of single amino acid replacements in Arenavirus biologya.
| Amino acid substitution | Protein/virus | Biological effect |
|---|---|---|
| F260 → L/I | GP1/LCMV ARM → Cl13 | Confer immunosuppressive phenotype (CTL−P+) |
| Specific targeting of dendritic cells | ||
| Increased affinity for α-DG receptor | ||
| S153 → F | GP1/LCMV (Wec54, Wec2.5) | Growth hormone deficiency syndrome (GHDS) |
| Mediates LCMV WE GHDS− → GHDS+ | ||
| Abrogates targeting of dendritic cells | ||
Summarized from Sevilla et al. (2002) and Sevilla and de la Torre (2006).
5.2. LCMV population dynamics and lethal mutagenesis
The evidence of genetic heterogeneity and variation of LCMV in cell culture and in vivo has reinforced the concept that this virus behaves as a typical ribovirus regarding error-prone replication, and quasispecies dynamics (Sevilla and de la Torre, 2006). Indeed, replication of a biological clone of LCMV in cell culture gave rise to a complex distribution of single and multiple mutants (Grande-Perez et al., 2002, Grande-Perez et al., 2005a, Grande-Perez et al., 2005b). Mutation frequencies within mutant spectra of LCMV replicated in BHK-21 cells were in the range of 1.0 × 10−4 to 2.7 × 10−4 substitutions per nucleotide (Grande-Perez et al., 2002). These values of population heterogeneity are comparable to those quantitated for other RNA viruses (Domingo, 2007).
One of the predictions of quasispecies theory is the existence of an error threshold for maintenance of genetic information (Eigen and Biebricher, 1988, Eigen and Schuster, 1979). That an increase of the error rate during virus replication results in loss of infectivity was first documented experimentally by John Holland and colleagues with poliovirus (PV) and vesicular stomatitis virus (VSV), using several mutagenic agents (Holland, 1990, Lee et al., 1997). Subsequent work by several teams confirmed the extinction of several RNA viruses upon replication in the presence of base or nucleoside analogues (reviews in Anderson et al., 2004, Domingo, in press, Graci and Cameron, 2002). This is now known as lethal mutagenesis of viruses, and LCMV has played an essential role in elucidating its underlying molecular mechanisms.
Replication of LCMV in cell culture in the presence of the mutagenic base analogue 5-fluorouracil (FU) resulted in an increase of mutant spectrum complexity and virus extinction (Grande-Perez et al., 2002, Grande-Perez et al., 2005b, Ruiz-Jarabo et al., 2003a). Administration of FU to mice prevented the establishment of a persistent LCMV infection in vivo. This experiment constitutes a proof of principle of the feasibility of a lethal mutagenesis approach in vivo (Ruiz-Jarabo et al., 2003a).
In the course of a persistent LCMV infection of BHK-21 cells, it was observed that FU treatment evoked larger reductions of infectivity than of total viral RNA levels (Grande-Perez et al., 2005a). The results suggested that defective, replication-competent genomes, that were termed defectors, contributed to LCMV extinction. In silico simulations of LCMV replication in the absence or presence of such defector genomes, together with additional experiments, strongly support the participation of defector genomes in LCMV extinction associated with enhanced mutagenesis (Martín et al., 2005, manuscript in preparation). These observations led to the proposal of the lethal defection model of virus extinction (Grande-Perez et al., 2005a). This model has modified current views of the transition of viruses to error catastrophe in a substantial manner, as follows. At low mutagenesis intensities the viral population is enriched with defective genomes that contribute to the replicative collapse of the system. At high mutagenic activity the number of lethal mutations increases and extinction results from the combined action of highly defective genomes and replication-incompetent genomes containing lethal mutations.
This multiple-step, lethal defection mechanism of virus entry into error catastrophe is also supported by studies carried out in parallel with FMDV. First, preextinction FMDV populations interfere with replication of standard, infectious FMDV RNA upon co-electroporation into cultured cells (Gonzalez-Lopez et al., 2004). Second, specific capsid and polymerase mutants of FMDV can exert complementation (at early times of infection) and then interference (at late times of infection) when co-electroporated into cells with standard, infections FMDV RNA (Perales et al., 2007). Evidence of effect of specific mutants on viral replication has been also described for poliovirus (Crowder and Kirkegaard, 2005). Nevertheless, it should be noted that it is not possible to predict, particularly in the context of an enhanced mutagenesis scenario, how many negative mutants must be generated, and what their interfering activity must be, to disrupt replication and expression of standard viral genome RNA species. However, evidence points to a collective interfering activity consistent with previous findings, based on fitness differences between entire viral populations and their component biological clones (Domingo et al., 1978, Duarte et al., 1994), of the collective behavior of mutant spectra. Such behavior renders classical models of population genetics insufficient to account for quasispecies behavior (see previous section). Therefore, the lethal defection model proposed to explain LCMV extinction by enhanced mutagenesis is in very much in line with other results that emphasize the profound biological consequences of mutant spectra behaving as an integrated ensemble (Stich et al., 2007).
It was suggested that in the course of mutagenesis, viral populations could acquire increased robustness to the deleterious effects of mutations, and that such a robustness could jeopardize viral extinction by lethal mutagenesis (Sanjuan et al., 2007). We have examined this possibility with LCMV populations subjected to a history of FU mutagenesis. Neither the uncloned populations, nor biological clones isolated from them, differ form populations or clones treated in parallel in the absence of FU in their capacity to be extinguished by lethal mutagenesis (Martin and Domingo, 2008). Therefore, there is no evidence that LCMV populations acquired mutational robustness in the course of mutagenesis by FU.
6. Perspectives
New arenaviruses have being recently detected, suggesting that many others will be uncovered in the future. It is likely that some of them will be added to the existing, remarkable list of arenaviruses as human pathogens. These new detections are a clear demonstration of the emergent nature of this important group of human pathogens. Arenaviruses naturally inhabit one of the most diverse and dynamic group of hosts: the rodents. Rodent population numbers fluctuate in response to environmental changes such as climatic variations or agricultural practices, among other influences that underlie the emergence of new viral pathogens. The combination of a dynamic RNA virus and a dynamic host species promotes encounters between humans and potential new pathogens, with unpredictable impact for human health. There will be a continuing need for the surveillance of rodent species to detect the presence of new arenaviruses, and characterize them to further understand their taxonomy and origins, and to evaluate their potential to become human pathogens.
Arenaviruses have historically provided excellent model systems for studies on basic immunology and viral pathogenesis. They are likely to continue providing extremely useful systems to understand virus variation in vivo, and its relation to viral pathogenesis. The availability of a reverse genetic system for arenaviruses represents a critical advance for elucidating the molecular basis of pathogenic processes. This can now be achieved through a deeper understanding of the function of arenavirus proteins, and functional variations that result from specific mutations. The disparate response of strains of mice to variant versions of LCMV constitutes an example of a fundamental issue that is amenable to analysis with the new tool.
From a practical side, human arenavirus infections require urgently the development of vaccines and new antiviral agents. Progress in this area is rapid, and should soon be able to produce results with prospects of a clinical application. The research on lethal mutagenesis of viruses is still in its infancy, but its development will require tests with adequate animal models that can sustain either acute or persistent infections. Again, arenaviruses are ideally suited to provide such models, and some research along these lines is now in progress. Either from theoretical or practical points of view, arenaviruses constitute fascinating objects for biological research.
References
- Ahmed R., Oldstone M.B.A. Organ specific selection of viral variants during chronic infection. J. Exp. Med. 1988;167:1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L.D., Chiller J.M., Oldstone M.B.A. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarino C.G., Posik D.M., Ghiringhelli P.D., Lozano M.E., Romanowski V. Arenavirus phylogeny: a new insight. Virus Genes. 1998;16(1):39–46. doi: 10.1023/a:1007993525052. [DOI] [PubMed] [Google Scholar]
- Anderson J.P., Daifuku R., Loeb L.A. Viral error catastrophe by mutagenic nucleosides. Annu. Rev. Microbiol. 2004;58:183–205. doi: 10.1146/annurev.micro.58.030603.123649. [DOI] [PubMed] [Google Scholar]
- Archer A.M., Rico-Hesse R. High genetic divergence and recombination in Arenaviruses from the Americas. Virology. 2002;304(2):274–281. doi: 10.1006/viro.2002.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A., Arnold J.J., Sierra M., Smidansky E.D., Domingo E., Cameron C.E. Determinants of RNA-dependent RNA polymerase (in)fidelity revealed by kinetic analysis of the polymerase encoded by a foot-and-mouth disease virus mutant with reduced sensitivity to ribavirin. J. Virol. 2008;82(24):12346–12355. doi: 10.1128/JVI.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J.J., Vignuzzi M., Stone J.K., Andino R., Cameron C.E. Remote site control of an active site fidelity checkpoint in a viral RNA-dependent RNA polymerase. J. Biol. Chem. 2005;280(27):25706–25716. doi: 10.1074/jbc.M503444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton L.L., Mets M.B., Beauchamp C.L. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 2002;187(6):1715–1716. doi: 10.1067/mob.2002.126297. [DOI] [PubMed] [Google Scholar]
- Beyer W.R., Popplau D., Garten W., von Laer D., Lenz O. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 2003;77(5):2866–2872. doi: 10.1128/JVI.77.5.2866-2872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borio L., Inglesby T., Peters C.J., Schmaljohn A.L., Hughes J.M., Jahrling P.B., Ksiazek T., Johnson K.M., Meyerhoff A., O’Toole T., Ascher M.S., Bartlett J., Breman J.G., Eitzen E.M., Jr., Hamburg M., Hauer J., Henderson D.A., Johnson R.T., Kwik G., Layton M., Lillibridge S., Nabel G.J., Osterholm M.T., Perl T.M., Russell P., Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287(18):2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- Borrow P., Oldstone M.B.A. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J. Virol. 1992;66:7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Oldstone M.B. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198(1):1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- Borrow P., Evans C.F., Oldstone M.B.A. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immunosuppression. J. Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M.D., Peters C.J., Mills J.N., Nichol S.T. Oliveros virus: a novel arenavirus from Argentina. Virology. 1996;217(1):362–366. doi: 10.1006/viro.1996.0124. [DOI] [PubMed] [Google Scholar]
- Bowen M.D., Peters C.J., Nichol S.T. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol. Phylogenet. Evol. 1997;8(3):301–316. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- Bowen M.D., Rollin P.E., Ksiazek T.G., Hustad H.L., Bausch D.G., Demby A.H., Bajani M.D., Peters C.J., Nichol S.T. Genetic diversity among Lassa virus strains. J. Virol. 2000;74(15):6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones C., Domingo E. Minority report: hidden memory genomes in HIV-1 quasispecies and possible clinical implications. AIDS Rev. 2008;10(2):93–109. [PubMed] [Google Scholar]
- Briones C., Domingo E., Molina-Paris C. Memory in retroviral quasispecies: experimental evidence and theoretical model for human immunodeficiency virus. J. Mol. Biol. 2003;331(1):213–229. doi: 10.1016/S0022-2836(03)00661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones C., de Vicente A., Molina-Paris C., Domingo E. Minority memory genomes can influence the evolution of HIV-1 quasispecies in vivo. Gene. 2006;384:129–138. doi: 10.1016/j.gene.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Buchmeier M.J., de la Torre J.C., Peters C.J. Arenaviridae. In: PM K.D., editor. Fields Virology. 5th ed. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 1791–1828. [Google Scholar]
- Buesa-Gomez J., Teng M.N., Oldstone C.E., Oldstone M.B., de la Torre J.C. Variants able to cause growth hormone deficiency syndrome are present within the disease-nil WE strain of lymphocytic choriomeningitis virus. J. Virol. 1996;70(12):8988–8992. doi: 10.1128/jvi.70.12.8988-8992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J.A., Oldstone M.B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J. Virol. 1984;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajimat M.N., Fulhorst C.F. Phylogeny of the Venezuelan arenaviruses. Virus Res. 2004;102(2):199–206. doi: 10.1016/j.virusres.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Cajimat M.N., Milazzo M.L., Bradley R.D., Fulhorst C.F. Catarina virus, an arenaviral species principally associated with Neotoma micropus (southern plains woodrat) in Texas. Am. J. Trop. Med. Hyg. 2007;77(4):732–736. [PubMed] [Google Scholar]
- Cajimat M.N., Milazzo M.L., Borchert J.N., Abbott K.D., Bradley R.D., Fulhorst C.F. Diversity among Tacaribe serocomplex viruses (family Arenaviridae) naturally associated with the Mexican woodrat (Neotoma mexicana) Virus Res. 2008;133(2):211–217. doi: 10.1016/j.virusres.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Henry M.D., Borrow P., Yamada H., Elder J.H., Ravkov E.V., Nichol S.T., Compans R.W., Campbell K.P., Oldstone M.B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus [see comments] Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Capul A.A., Perez M., Burke E., Kunz S., Buchmeier M.J., de la Torre J.C. Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains. J. Virol. 2007;81(17):9451–9460. doi: 10.1128/JVI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla V., Mersich S.E., Candurra N.A., Damonte E.B. The entry of Junin virus into Vero cells. Arch. Virol. 1994;136(3–4):363–374. doi: 10.1007/BF01321064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro C., Arnold J.J., Cameron C.E. Incorporation fidelity of the viral RNA-dependent RNA polymerase: a kinetic, thermodynamic and structural perspective. Virus Res. 2005;107(2):141–149. doi: 10.1016/j.virusres.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel R.N., de Lamballerie X., Fulhorst C.F. The Whitewater Arroyo virus: natural evidence for genetic recombination among Tacaribe serocomplex viruses (family Arenaviridae) Virology. 2001;283(2):161–166. doi: 10.1006/viro.2001.0874. [DOI] [PubMed] [Google Scholar]
- Charrel R.N., Feldmann H., Fulhorst C.F., Khelifa R., de Chesse R., de Lamballerie X. Phylogeny of New World arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem. Biophys. Res. Commun. 2002;296(5):1118–1124. doi: 10.1016/s0006-291x(02)02053-3. [DOI] [PubMed] [Google Scholar]
- Charrel R.N., Lemasson J.J., Garbutt M., Khelifa R., De Micco P., Feldmann H., de Lamballerie X. New insights into the evolutionary relationships between arenaviruses provided by comparative analysis of small and large segment sequences. Virology. 2003;317(2):191–196. doi: 10.1016/j.virol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Charrel R.N., de Lamballerie X., Emonet S. Phylogeny of the genus Arenavirus. Curr. Opin. Microbiol. 2008;11(4):362–368. doi: 10.1016/j.mib.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Childs J.E., Peters C.J. Ecology and epidemiology of arenaviruses and their hosts. In: MS S., editor. The Arenaviridae. Plenum Press; New York: 1993. pp. 331–384. [Google Scholar]
- Crowder S., Kirkegaard K. Trans-dominant inhibition of RNA viral replication can slow growth of drug-resistant viruses. Nat. Genet. 2005;37(7):701–709. doi: 10.1038/ng1583. [DOI] [PubMed] [Google Scholar]
- Damonte E.B., Mersich S.E., Coto C.E. Response of cells persistently infected with arenaviruses to superinfection with homotypic and heterotypic viruses. Virology. 1983;129(2):474–478. doi: 10.1016/0042-6822(83)90185-x. [DOI] [PubMed] [Google Scholar]
- de la Torre J.C., Holland J.J. RNA virus quasispecies populations can suppress vastly superior mutant progeny. J. Virol. 1990;64(12):6278–6281. doi: 10.1128/jvi.64.12.6278-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado S., Erickson B.R., Agudo R., Blair P.J., Vallejo E., Albarino C.G., Vargas J., Comer J.A., Rollin P.E., Ksiazek T.G., Olson J.G., Nichol S.T. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 2008;4(4):e1000047. doi: 10.1371/journal.ppat.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone C., Buchmeier M.J. Kinetics and pH dependence of acid-induced structural changes in the lymphocytic choriomeningitis virus glycoprotein complex. Virology. 1995;209(1):3–9. doi: 10.1006/viro.1995.1225. [DOI] [PubMed] [Google Scholar]
- Di Simone C., Zandonatti M.A., Buchmeier M.J. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology. 1994;198(2):455–465. doi: 10.1006/viro.1994.1057. [DOI] [PubMed] [Google Scholar]
- Domingo E. Virus evolution. In: PM K.D., editor. Fields Virology. 5th ed. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 389–421. [Google Scholar]
- Domingo, E., in press. Microbial evolution and emerging diseases. In: Power, C., Johnson, R.T. (Eds.). Emerging Neurological Infections. Mercel Dekker, Inc., New York.
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Domingo E., Escarmis C., Menendez-Arias A., Perales C., Herrera M., Novella I., Holland J.J. Virla quasispecies: dynamics, interactions and pathogenesis. In: Domingo E., Parrish C., Holland J.J., editors. Origin and Evolution of Viruses. Elsevier; Oxford: 2008. [Google Scholar]
- Downs W., Anderson C.R., Spence L., Aitken T., Greenhal A.H. Tacaribe virus, a new agent isolated from Artibeus bats and mosquitos in Trinidad. Am. J. Trop. Med. Hyg. 1963;12:640–646. doi: 10.4269/ajtmh.1963.12.640. [DOI] [PubMed] [Google Scholar]
- Duarte E.A., Novella I.S., Ledesma S., Clarke D.K., Moya A., Elena S.F., Domingo E., Holland J.J. Subclonal components of consensus fitness in an RNA virus clone. J. Virol. 1994;68(7):4295–4301. doi: 10.1128/jvi.68.7.4295-4301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerle L.D., Lu X., Sperry S.M., Choi L., Denison M.R. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J. Virol. 2007;81(22):12135–12144. doi: 10.1128/JVI.01296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler R., Lenz O., Strecker T., Eickmann M., Klenk H.D., Garten W. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 2003;4(11):1084–1088. doi: 10.1038/sj.embor.7400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler R., Lenz O., Strecker T., Eickmann M., Klenk H.D., Garten W. Lassa virus glycoprotein signal peptide displays a novel topology with an extended endoplasmic reticulum luminal region. J. Biol. Chem. 2004;279(13):12293–12299. doi: 10.1074/jbc.M312975200. [DOI] [PubMed] [Google Scholar]
- Eigen M., Biebricher C.K. Sequence space and quasispecies distribution. In: Domingo E., Ahlquist P., Holland J.J., editors. RNA Genetics. CRC Press; Boca Raton, FL: 1988. pp. 211–245. [Google Scholar]
- Eigen M., Schuster P. Springer; Berlin: 1979. The hypercycle. A Principle of Natural Self-Organization. [DOI] [PubMed] [Google Scholar]
- Ellenberg P., Edreira M., Scolaro L. Resistance to superinfection of Vero cells persistently infected with Junin virus. Arch. Virol. 2004;149(3):507–522. doi: 10.1007/s00705-003-0227-1. [DOI] [PubMed] [Google Scholar]
- Ellenberg P., Linero F.N., Scolaro L.A. Superinfection exclusion in BHK-21 cells persistently infected with Junin virus. J. Gen. Virol. 2007;88(Pt 10):2730–2739. doi: 10.1099/vir.0.83041-0. [DOI] [PubMed] [Google Scholar]
- Emonet S., Lemasson J.J., Gonzalez J.P., de Lamballerie X., Charrel R.N. Phylogeny and evolution of old world arenaviruses. Virology. 2006;350(2):251–257. doi: 10.1016/j.virol.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Eschli B., Quirin K., Wepf A., Weber J., Zinkernagel R., Hengartner H. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J. Virol. 2006;80(12):5897–5907. doi: 10.1128/JVI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.F., Borrow P., de la Torre J.C., Oldstone M.B. Virus-induced immunosuppression: kinetic analysis of the selection of a mutation associated with viral persistence. J. Virol. 1994;68(11):7367–7373. doi: 10.1128/jvi.68.11.7367-7373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Orta C., Arias A., Perez-Luque R., Escarmis C., Domingo E., Verdaguer N. Sequential structures provide insights into the fidelity of RNA replication. Proc. Natl. Acad. Sci. U.S.A. 2007;104(22):9463–9468. doi: 10.1073/pnas.0700518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S.A., Graham M.B., Kuehnert M.J., Kotton C.N., Srinivasan A., Marty F.M., Comer J.A., Guarner J., Paddock C.D., DeMeo D.L., Shieh W.J., Erickson B.R., Bandy U., DeMaria A., Jr., Davis J.P., Delmonico F.L., Pavlin B., Likos A., Vincent M.J., Sealy T.K., Goldsmith C.S., Jernigan D.B., Rollin P.E., Packard M.M., Patel M., Rowland C., Helfand R.F., Nichol S.T., Fishman J.A., Ksiazek T., Zaki S.R. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006;354(21):2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- Flatz L., Bergthaler A., de la Torre J.C., Pinschewer D.D. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. U.S.A. 2006;103(12):4663–4668. doi: 10.1073/pnas.0600652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame J.D., Baldwin J.M., Jr., Gocke D.J., Troup J.M. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am. J. Trop. Med. Hyg. 1970;19(4):670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- Freed E.O. Virology. Rafting with Ebola. Science. 2002;296(5566):279. doi: 10.1126/science.1070868. [DOI] [PubMed] [Google Scholar]
- Freedman D.O., Woodall J. Emerging infectious diseases and risk to the traveler. Med. Clin. North Am. 1999;83(4):865–883. v. [PubMed] [Google Scholar]
- Friedberg E.C., Walker G.C., Siede W., Wood R.D., Schultz R.A., Ellenberger T. American Society for Microbiology; Washington, DC: 2006. DNA Repair and Mutagenesis. [Google Scholar]
- Fulhorst C.F., Bowen M.D., Salas R.A., Duno G., Utrera A., Ksiazek T.G., De Manzione N.M., De Miller E., Vasquez C., Peters C.J., Tesh R.B. Natural rodent host associations of Guanarito and pirital viruses (Family Arenaviridae) in central Venezuela. Am. J. Trop. Med. Hyg. 1999;61(2):325–330. doi: 10.4269/ajtmh.1999.61.325. [DOI] [PubMed] [Google Scholar]
- Fulhorst C.F., Charrel R.N., Weaver S.C., Ksiazek T.G., Bradley R.D., Milazzo M.L., Tesh R.B., Bowen M.D. Geographic distribution and genetic diversity of Whitewater Arroyo virus in the southwestern United States. Emerg. Infect. Dis. 2001;7(3):403–407. doi: 10.3201/eid0703.010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulhorst C.F., Cajimat M.N., Milazzo M.L., Paredes H., de Manzione N.M., Salas R.A., Rollin P.E., Ksiazek T.G. Genetic diversity between and within the arenavirus species indigenous to western Venezuela. Virology. 2008;378(2):205–213. doi: 10.1016/j.virol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W.P., Kundig T.M., Zinkernagel R.M., Mak T.W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J. Exp. Med. 1991;174(6):1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W.R., DiSimone C., Buchmeier M.J. The viral transmembrane superfamily: possible divergence of Arenavirus and Filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 2001;1:1. doi: 10.1186/1471-2180-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.P., Emonet S., de Lamballerie X., Charrel R. Arenaviruses. Curr. Top. Microbiol. Immunol. 2007;315:253–288. doi: 10.1007/978-3-540-70962-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lopez C., Arias A., Pariente N., Gomez-Mariano G., Domingo E. Preextinction viral RNA can interfere with infectivity. J. Virol. 2004;78(7):3319–3324. doi: 10.1128/JVI.78.7.3319-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graci J.D., Cameron C.E. Quasispecies, error catastrophe, and the antiviral activity of ribavirin. Virology. 2002;298(2):175–180. doi: 10.1006/viro.2002.1487. [DOI] [PubMed] [Google Scholar]
- Grande-Perez A., Sierra S., Castro M.G., Domingo E., Lowenstein P.R. Molecular indetermination in the transition to error catastrophe: systematic elimination of lymphocytic choriomeningitis virus through mutagenesis does not correlate linearly with large increases in mutant spectrum complexity. Proc. Natl. Acad. Sci. U.S.A. 2002;99(20):12938–12943. doi: 10.1073/pnas.182426999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande-Perez A., Gomez-Mariano G., Lowenstein P.R., Domingo E. Mutagenesis-induced, large fitness variations with an invariant arenavirus consensus genomic nucleotide sequence. J. Virol. 2005;79(16):10451–10459. doi: 10.1128/JVI.79.16.10451-10459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande-Perez A., Lazaro E., Lowenstein P., Domingo E., Manrubia S.C. Suppression of viral infectivity through lethal defection. Proc. Natl. Acad. Sci. U.S.A. 2005;102(12):4448–4452. doi: 10.1073/pnas.0408871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L.H., Halsey N.A., McKee K.T., Jr., Peters C.J., Barrera Oro J.G., Briggiler A.M., Feuillade M.R., Maiztegui J.I. Clinical case definitions for Argentine hemorrhagic fever. Clin. Infect. Dis. 1999;28(5):1091–1094. doi: 10.1086/514749. [DOI] [PubMed] [Google Scholar]
- Holland J.J. Defective viral genomes. In: Fields B., Knipe D.M., editors. Virology. Raven Press; New York: 1990. pp. 151–165. [Google Scholar]
- Holmes G.P., McCormick J.B., Trock S.C., Chase R.A., Lewis S.M., Mason C.A., Hall P.A., Brammer L.S., Perez-Oronoz G.I., McDonnell M.K. Lassa fever in the United States. Investigation of a case and new guidelines for management. N. Engl. J. Med. 1990;323(16):1120–1123. doi: 10.1056/NEJM199010183231607. [DOI] [PubMed] [Google Scholar]
- Hugot J.P., Gonzalez J.P., Denys C. Evolution of the Old World Arenaviridae and their rodent hosts: generalized host-transfer or association by descent? Infect. Genet. Evol. 2001;1(1):13–20. doi: 10.1016/s1567-1348(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Isaacson M. Viral hemorrhagic fever hazards for travelers in Africa. Clin. Infect. Dis. 2001;33(10):1707–1712. doi: 10.1086/322620. [DOI] [PubMed] [Google Scholar]
- Jackson A.P., Charleston M.A. A cophylogenetic perspective of RNA-virus evolution. Mol. Biol. Evol. 2004;21(1):45–57. doi: 10.1093/molbev/msg232. [DOI] [PubMed] [Google Scholar]
- Jahrling P.B., Peters C.J. Lymphocytic choriomeningitis virus. A neglected pathogen of man. Arch. Pathol. Lab. Med. 1992;116(5):486–488. [PubMed] [Google Scholar]
- Johnson K.M., Mackenzie R.B., Webb P.A., Kuns M.L. Chronic infection of rodents by Machupo virus. Science. 1965;150(703):1618–1619. doi: 10.1126/science.150.3703.1618. [DOI] [PubMed] [Google Scholar]
- King C.C., Jamieson B.D., Reddy K., Bali N., Concepcion R.J., Ahmed R. Viral infection of the thymus. J. Virol. 1992;66(5):3155–3160. doi: 10.1128/jvi.66.5.3155-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S., Sevilla N., McGavern D.B., Campbell K.P., Oldstone M.B. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J. Cell. Biol. 2001;155(2):301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S., Edelmann K.H., de la Torre J.C., Gorney R., Oldstone M.B. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology. 2003;314(1):168–178. doi: 10.1016/s0042-6822(03)00421-5. [DOI] [PubMed] [Google Scholar]
- Lecompte E., ter Meulen J., Emonet S., Daffis S., Charrel R.N. Genetic identification of Kodoko virus, a novel arenavirus of the African pigmy mouse (Mus Nannomys minutoides) in West Africa. Virology. 2007;364(1):178–183. doi: 10.1016/j.virol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Gilbertson D.L., Novella I.S., Huerta R., Domingo E., Holland J.J. Negative effects of chemical mutagenesis on the adaptive behavior of vesicular stomatitis virus. J. Virol. 1997;71(5):3636–3640. doi: 10.1128/jvi.71.5.3636-3640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.J., Perez M., Pinschewer D.D., de la Torre J.C. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 2002;76(12):6393–6397. doi: 10.1128/JVI.76.12.6393-6397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez N., Franze-Fernandez M.T. A single stem-loop structure in Tacaribe arenavirus intergenic region is essential for transcription termination but is not required for a correct initiation of transcription and replication. Virus Res. 2007;124(1–2):237–244. doi: 10.1016/j.virusres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lozano M.E., Posik D.M., Albarino C.G., Schujman G., Ghiringhelli P.D., Calderon G., Sabattini M., Romanowski V. Characterization of arenaviruses using a family-specific primer set for RT-PCR amplification and RFLP analysis. Its potential use for detection of uncharacterized arenaviruses. Virus Res. 1997;49(1):79–89. doi: 10.1016/s0168-1702(97)01458-5. [DOI] [PubMed] [Google Scholar]
- Lukashevich I.S. Generation of reassortants between African arenaviruses. Virology. 1992;188(2):600–605. doi: 10.1016/0042-6822(92)90514-p. [DOI] [PubMed] [Google Scholar]
- Lukashevich I.S., Vasiuchkov A.D., Stel’makh T.A., Scheslenok E.P., Shabanov A.G. The isolation and characteristics of reassortants between the Lassa and Mopeia arenaviruses. Vop. Virusol. 1991;36(2):146–150. [PubMed] [Google Scholar]
- Lukashevich I.S., Patterson J., Carrion R., Moshkoff D., Ticer A., Zapata J., Brasky K., Geiger R., Hubbard G.B., Bryant J., Salvato M.S. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J. Virol. 2005;79(22):13934–13942. doi: 10.1128/JVI.79.22.13934-13942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher A.M., Wolfe M.S. Historical Lassa fever reports and 30-year clinical update. Emerg. Infect. Dis. 2006;12(5):835–837. doi: 10.3201/eid1205.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V., Domingo E. Influence of the mutant spectrum in viral evolution: focused selection of antigenic variants in a reconstructed viral quasispecies. Mol. Biol. Evol. 2008;25(8):1544–1554. doi: 10.1093/molbev/msn099. [DOI] [PubMed] [Google Scholar]
- Martinez M.G., Cordo S.M., Candurra N.A. Characterization of Junin arenavirus cell entry. J. Gen. Virol. 2007;88(Pt. 6):1776–1784. doi: 10.1099/vir.0.82808-0. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Somasundaram T., Kolhekar S.R., Selvakumar R., Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J. Exp. Med. 1990;172(4):1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Kolhekar S.R., Somasundarum T., Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J.B., Fisher-Hoch S.P. Lassa fever. Curr. Top. Microbiol. Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- Mets M.B., Barton L.L., Khan A.S., Ksiazek T.G. Lymphocytic choriomeningitis virus: an underdiagnosed cause of congenital chorioretinitis. Am. J. Ophthalmol. 2000;130(2):209–215. doi: 10.1016/s0002-9394(00)00570-5. [DOI] [PubMed] [Google Scholar]
- Meyer B.J., Southern P.J. Sequence heterogeneity in the termini of lymphocytic choriomeningitis virus genomic and antigenomic RNAs. J. Virol. 1994;68(11):7659–7664. doi: 10.1128/jvi.68.11.7659-7664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B.J., de la Torre J.C., Southern P.J. Arenaviruses: genomic RNAs, transcription, and replication. Curr. Top. Microbiol. Immunol. 2002;262:139–157. doi: 10.1007/978-3-642-56029-3_6. [DOI] [PubMed] [Google Scholar]
- Mills J.N., Barrera Oro J.G., Bressler D.S., Childs J.E., Tesh R.B., Smith J.F., Enria D.A., Geisbert T.W., McKee K.T., Jr., Bowen M.D., Peters C.J., Jahrling P.B. Characterization of Oliveros virus, a new member of the Tacaribe complex (Arenaviridae: Arenavirus) Am. J. Trop. Med. Hyg. 1996;54(4):399–404. doi: 10.4269/ajtmh.1996.54.399. [DOI] [PubMed] [Google Scholar]
- Minskaia E., Hertzig T., Gorbalenya A.E., Campanacci V., Cambillau C., Canard B., Ziebuhr J. Discovery of an RNA virus 3′ → 5′ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103(13):5108–5113. doi: 10.1073/pnas.0508200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Vingron M. Modeling amino acid replacement. J. Comput. Biol. 2000;7(6):761–776. doi: 10.1089/10665270050514918. [DOI] [PubMed] [Google Scholar]
- Murphy F.A., Webb P.A., Johnson K.M., Whitfield S.G. Morphological comparison of Machupo with lymphocytic choriomeningitis virus: basis for a new taxonomic group. J. Virol. 1969;4(4):535–541. doi: 10.1128/jvi.4.4.535-541.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser G.G., Carleton M.D. Superfamily Muroidea. In: Wilson D., Reeder D.M., editors. Mammal Species of the World. A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press; Baltimore: 2005. pp. 894–1522. [Google Scholar]
- Neuman B.W., Adair B.D., Burns J.W., Milligan R.A., Buchmeier M.J., Yeager M. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J. Virol. 2005;79(6):3822–3830. doi: 10.1128/JVI.79.6.3822-3830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M.A. The Belknap Press of Harvard University Press; Cambridge, Massachusetts and London: 2006. Evolutionary Dynamics. [Google Scholar]
- Oldstone M.B. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- Oldstone M.B., Sinha Y.N., Blount P., Tishon A., Rodriguez M., von Wedel R., Lampert P.W. Virus-induced alterations in homeostasis: alteration in differentiated functions of infected cells in vivo. Science. 1982;218(4577):1125–1127. doi: 10.1126/science.7146898. [DOI] [PubMed] [Google Scholar]
- Oldstone M.B., Rodriguez M., Daughaday W.H., Lampert P.W. Viral perturbation of endocrine function: disordered cell function leads to disturbed homeostasis and disease. Nature. 1984;307(5948):278–281. doi: 10.1038/307278a0. [DOI] [PubMed] [Google Scholar]
- Palacios G., Druce J., Du L., Tran T., Birch C., Briese T., Conlan S., Quan P.L., Hui J., Marshall J., Simons J.F., Egholm M., Paddock C.D., Shieh W.J., Goldsmith C.S., Zaki S.R., Catton M., Lipkin W.I. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008;358(10):991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- Parekh B.S., Buchmeier M.J. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986;153(2):168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Parodi A., Greenway D., Rugiero H., Frigerio M., Metler N., Garzón F., Boxaca M., Guerrero L., Nota L. Sobre la etiología del brote epidémico de Junín. Día Médico. 1958;30:2300. [PubMed] [Google Scholar]
- Perales C., Martin V., Ruiz-Jarabo C.M., Domingo E. Monitoring sequence space as a test for the target of selection in viruses. J. Mol. Biol. 2005;345(3):451–459. doi: 10.1016/j.jmb.2004.10.066. [DOI] [PubMed] [Google Scholar]
- Perales C., Mateo R., Mateu M.G., Domingo E. Insights into RNA virus mutant spectrum and lethal mutagenesis events: replicative interference and complementation by multiple point mutants. J. Mol. Biol. 2007;369(4):985–1000. doi: 10.1016/j.jmb.2007.03.074. [DOI] [PubMed] [Google Scholar]
- Perez M., de la Torre J.C. Characterization of the genomic promoter of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2003;77(2):1184–1194. doi: 10.1128/JVI.77.2.1184-1194.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C.J. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 2002;262:65–74. doi: 10.1007/978-3-642-56029-3_3. [DOI] [PubMed] [Google Scholar]
- Peters C.J. Lymphocytic choriomeningitis virus—an old enemy up to new tricks. N. Engl. J. Med. 2006;354(21):2208–2211. doi: 10.1056/NEJMp068021. [DOI] [PubMed] [Google Scholar]
- Pfeiffer J.K., Kirkegaard K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. U.S.A. 2003;100(12):7289–7294. doi: 10.1073/pnas.1232294100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer J.K., Kirkegaard K. Increased fidelity reduces poliovirus fitness and virulence under selective pressure in mice. PLoS Pathog. 2005;1(2):e11. doi: 10.1371/journal.ppat.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro F., Shope R.E., de Andrade A.H.P., Bensabath G., Cacío G.V., Casals J. Amapari, a new virus of teh Tacaribe group from rodents and mites of Amapa territory, Brazil. Proc. Soc. Exp. Biol. Med. 1966;122:531–535. [Google Scholar]
- Pinschewer D.D., Perez M., de la Torre J.C. Role of the virus nucleoprotein in the regulation of lymphocytic choriomeningitis virus transcription and RNA replication. J. Virol. 2003;77(6):3882–3887. doi: 10.1128/JVI.77.6.3882-3887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinschewer D.D., Perez M., Sanchez A.B., de la Torre J.C. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 2003;100(13):7895–7900. doi: 10.1073/pnas.1332709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Burki K., Lang R., Hengartner H., Zinkernagel R.M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Radoshitzky S.R., Abraham J., Spiropoulou C.F., Kuhn J.H., Nguyen D., Li W., Nagel J., Schmidt P.J., Nunberg J.H., Andrews N.C., Farzan M., Choe H. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446(7131):92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden C., Melo F.L., Figueiredo L.M., Holmes E.C., Zanotto P.M. High rates of molecular evolution in hantaviruses. Mol. Biol. Evol. 2008;25(7):1488–1492. doi: 10.1093/molbev/msn093. [DOI] [PubMed] [Google Scholar]
- Ramsden C., Holmes E.C., Charleston M.A. Hantavirus evolution in relation to its rodent and insectivore hosts: no evidence for codivergence. Mol. Biol. Evol. 2009;26(1):143–153. doi: 10.1093/molbev/msn234. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Oldstone M.B. Genetic reassortants of lymphocytic choriomeningitis virus: unexpected disease and mechanism of pathogenesis. J. Virol. 1986;59(2):363–368. doi: 10.1128/jvi.59.2.363-368.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P., Oldstone M.B. Perturbation of differentiated functions during viral infection in vivo. II. Viral reassortants map growth hormone defect to the S RNA of the lymphocytic choriomeningitis virus genome. Virology. 1985;142(1):175–182. doi: 10.1016/0042-6822(85)90431-3. [DOI] [PubMed] [Google Scholar]
- Rojek J.M., Sanchez A.B., Nguyen N.T., de la Torre J.C., Kunz S. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J. Virol. 2008;82(15):7677–7687. doi: 10.1128/JVI.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W.P., Murphy F.A., Bergold G.H., Casals J., Hotchin J., Johnson K.M., Lehmann-Grube F., Mims C.A., Traub E., Webb P.A. Arenoviruses: proposed name for a newly defined virus group. J. Virol. 1970;5(5):651–652. doi: 10.1128/jvi.5.5.651-652.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Jarabo C.M., Arias A., Baranowski E., Escarmis C., Domingo E. Memory in viral quasispecies. J. Virol. 2000;74(8):3543–3547. doi: 10.1128/jvi.74.8.3543-3547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Jarabo C.M., Arias A., Molina-Paris C., Briones C., Baranowski E., Escarmis C., Domingo E. Duration and fitness dependence of quasispecies memory. J. Mol. Biol. 2002;315(3):285–296. doi: 10.1006/jmbi.2001.5232. [DOI] [PubMed] [Google Scholar]
- Ruiz-Jarabo C.M., Ly C., Domingo E., de la Torre J.C. Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) Virology. 2003;308(1):37–47. doi: 10.1016/s0042-6822(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Jarabo C.M., Miller E., Gomez-Mariano G., Domingo E. Synchronous loss of quasispecies memory in parallel viral lineages: a deterministic feature of viral quasispecies. J. Mol. Biol. 2003;333(3):553–563. doi: 10.1016/j.jmb.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Salazar-Bravo J., Ruedas L.A., Yates T.L. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 2002;262:25–63. doi: 10.1007/978-3-642-56029-3_2. [DOI] [PubMed] [Google Scholar]
- Sanchez A.B., de la Torre J.C. Rescue of the prototypic Arenavirus LCMV entirely from plasmid. Virology. 2006;350(2):370–380. doi: 10.1016/j.virol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sanjuan R., Cuevas J.M., Furio V., Holmes E.C., Moya A. Selection for robustness in mutagenized RNA viruses. PLoS Genet. 2007;3(6):e93. doi: 10.1371/journal.pgen.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.A., Strimmer K., Vingron M., von Haeseler A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18(3):502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Sevilla N., de la Torre J.C. Arenavirus diversity and evolution: quasispecies in vivo. Curr. Top. Microbiol. Immunol. 2006;299:315–335. doi: 10.1007/3-540-26397-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N., Kunz S., Holz A., Lewicki H., Homann D., Yamada H., Campbell K.P., de La Torre J.C., Oldstone M.B. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 2000;192(9):1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N., Domingo E., de la Torre J.C. Contribution of LCMV towards deciphering biology of quasispecies in vivo. Curr. Top. Microbiol. Immunol. 2002;263:197–220. doi: 10.1007/978-3-642-56055-2_10. [DOI] [PubMed] [Google Scholar]
- Sevilla N., McGavern D.B., Teng C., Kunz S., Oldstone M.B. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 2004;113(5):737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M., Airaksinen A., Gonzalez-Lopez C., Agudo R., Arias A., Domingo E. Foot-and-mouth disease virus mutant with decreased sensitivity to ribavirin: implications for error catastrophe. J. Virol. 2007;81(4):2012–2024. doi: 10.1128/JVI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt S.C., Borrow P., Kunz S., Cao W., Tishon A., Lewicki H., Campbell K.P., Oldstone M.B. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 2001;75(1):448–457. doi: 10.1128/JVI.75.1.448-457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P.J. Arenaviridae: the viruses and their replication. In: Knipe F.B.N.D.M., Howley P.M., editors. Fields Virology. 3rd ed. Lippincott–Raven Publishers; Philadelphia: 1996. pp. 1505–1519. [Google Scholar]
- Southern P.J., Bishop D.H. Sequence comparison among arenaviruses. Curr. Top. Microbiol. Immunol. 1987;133:19–39. doi: 10.1007/978-3-642-71683-6_3. [DOI] [PubMed] [Google Scholar]
- Spiropoulou C.F., Kunz S., Rollin P.E., Campbell K.P., Oldstone M.B. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 2002;76(10):5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich M., Briones C., Manrubia S.C. Collective properties of evolving molecular quasispecies. BMC Evol. Biol. 2007;7:110. doi: 10.1186/1471-2148-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker T., Eichler R., Meulen J., Weissenhorn W., Dieter Klenk H., Garten W., Lenz O. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected] J. Virol. 2003;77(19):10700–10705. doi: 10.1128/JVI.77.19.10700-10705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimmer K., von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 1996;13:964–969. [Google Scholar]
- Takeuchi N., Hogeweg P. Error-threshold exists in fitness landscapes with lethal mutants. BMC Evol. Biol. 2007;7:15. doi: 10.1186/1471-2148-7-15. (Author reply 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M.N., Borrow P., Oldstone M.B., de la Torre J.C. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J. Virol. 1996;70(12):8438–8443. doi: 10.1128/jvi.70.12.8438-8443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishon A., Borrow P., Evans C., Oldstone M.B. Virus-induced immunosuppression. 1. Age at infection relates to a selective or generalized defect. Virology. 1993;195(2):397–405. doi: 10.1006/viro.1993.1389. [DOI] [PubMed] [Google Scholar]
- Tortorici M.A., Albarino C.G., Posik D.M., Ghiringhelli P.D., Lozano M.E., Rivera Pomar R., Romanowski V. Arenavirus nucleocapsid protein displays a transcriptional antitermination activity in vivo. Virus Res. 2001;73(1):41–55. doi: 10.1016/s0168-1702(00)00222-7. [DOI] [PubMed] [Google Scholar]
- Urata S., Noda T., Kawaoka Y., Yokosawa H., Yasuda J. Cellular factors required for Lassa virus budding. J. Virol. 2006;80(8):4191–4195. doi: 10.1128/JVI.80.8.4191-4195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel M.H. Applying the species concept to plant viruses. Arch. Virol. 1989;104(1–2):1–17. doi: 10.1007/BF01313804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth S., Torda A.E., Asper M., Schmitz H., Gunther S. Sequence analysis of L RNA of Lassa virus. Virology. 2004;318(1):153–168. doi: 10.1016/j.virol.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Vignuzzi M., Stone J.K., Arnold J.J., Cameron C.E., Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439(7074):344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Salas R.A., de Manzione N., Fulhorst C.F., Duno G., Utrera A., Mills J.N., Ksiazek T.G., Tovar D., Tesh R.B. Guanarito virus (Arenaviridae) isolates from endemic and outlying localities in Venezuela: sequence comparisons among and within strains isolated from Venezuelan hemorrhagic fever patients and rodents. Virology. 2000;266(1):189–195. doi: 10.1006/viro.1999.0067. [DOI] [PubMed] [Google Scholar]
- Weissenbacher M.C., Laguens R.P., Coto C.E. Argentine hemorrhagic fever. Curr. Top. Microbiol. Immunol. 1987;134:79–116. doi: 10.1007/978-3-642-71726-0_4. [DOI] [PubMed] [Google Scholar]
- York J., Nunberg J.H. Role of the stable signal peptide of Junin arenavirus envelope glycoprotein in pH-dependent membrane fusion. J. Virol. 2006;80(15):7775–7780. doi: 10.1128/JVI.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J., Romanowski V., Lu M., Nunberg J.H. The signal peptide of the Junin arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J. Virol. 2004;78(19):10783–10792. doi: 10.1128/JVI.78.19.10783-10792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.C., Lindsay K.L., Lee K.J., Liu W.C., He J.W., Milstein S.L., Lai M.M. Identification of a ribavirin-resistant NS5B mutation of hepatitis C virus during ribavirin monotherapy. Hepatology. 2003;38(4):869–878. doi: 10.1053/jhep.2003.50445. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R.M. Lymphocytic choriomeningitis virus and immunology. Curr. Top. Microbiol. Immunol. 2002;263:1–5. doi: 10.1007/978-3-642-56055-2_1. [DOI] [PubMed] [Google Scholar]


