Abstract
Mouse hepatitis virus A59 (MHV A59) induces autoantibodies (autoAb) to fumarylacetoacetate hydrolase (FAH), a soluble cytosolic enzyme present in the liver and kidneys, in various mouse strains. The aim of this work was to amplify and diversify the autoimmune response restricted to FAH through the use of the exogenous adjuvant called PADRE. Accordingly, C57BL/6 mice were chosen, because these animals respond to PADRE better than other mouse strains. Results presented herein indicate that, surprisingly, C57BL/6 mice developed signs of autoimmune hepatitis-like disease (AIH), including transient hypergammaglobulinemia, elevated transaminases, autoAb directed against different liver proteins and hepatic cellular infiltrates, indicating that a new model of experimental AIH could be generated by a viral inoculation.
Furthermore, PADRE administration amplified the MHV effect, extending the duration of hypergammaglobulinemia and increasing the binding of autoAb as well as the degree of hepatic infiltrates. However, the adjuvant did not expand the time of the symptoms. Additionally, since plasmatic uric acid and high-mobility group box protein 1 (HGMB1) concentrations augmented in MHV- and/or PADRE-treated mice, it is suggested that both alarmins were probably involved in the spreading of the immune response induced by the viral infection and the adjuvant administration.
Keywords: AIH, Autoimmune response, Mouse hepatitis virus, PADRE adjuvant
Research highlights
► Mouse hepatitis virus A59 induced autoantibodies to mouse tissues in C57BL/6 mice. ► The exogenous adjuvant PADRE amplified the autoimmune response induced by MHV. ► C57BL/6 mice infected with MHV developed signs of autoimmune hepatitis-like disease.
1. Introduction
Autoimmune hepatitis (AIH) is a progressive and chronic condition in which the immune system attacks the liver, causing inflammation and liver cell death. In humans, AIH is characterized by the presence of interface hepatitis and portal plasma cell infiltration, hypergammaglobulinemia, increased serum transaminases and autoantibodies (autoAb) to liver antigens (Ag). Like most other autoimmune diseases, this disorder results from a complex interaction between triggering factors, such as viral infections, genetic predisposition, and immunoregulatory networks [1], [2], [3], [4].
An animal model of AIH could be useful to provide information on the mechanism by which the liver is protecting itself against an immune attack and how various triggering agents disrupt this immune tolerance [3]. Besides, getting insight into this mechanism could lead to preventive or therapeutic measures [5], [6]. Thus, in the present paper, we report the results obtained with a hepatotropic virus, a mouse hepatitis virus (MHV-A59) in a susceptible mouse strain, C57BL/6 (B6), immunized with a strong T cell stimulator, PADRE for "Pan HLA-DR binding epitope” [7], [8].
MHV-A59 is a coronavirus that causes various mouse pathologies, including hepatitis, thymus involution [9], IgG2a-restricted hypergammaglobulinaemia [10] and transient demyelination [11]. We have reported the presence of autoantibodies (autoAb) to fumarylacetoacetate hydrolase (FAH) in sera from various mouse strains after MHV infection [12]. The autoAb recognized conformational as well as linear antigenic determinants in the enzyme, and the autoimmune response was partly related to molecular mimicry [13], [14], [15]. Furthermore, we have shown that the induction of the anti-FAH autoAb was associated with the MHV-induced release of some danger signals [16], also called DAMPs (damage-associated molecular patterns) or alarmins [17].
In a recent work [18], we inoculated BALB/c mice with carbon tetrachloride (CCl4) 30 days after MHV infection, and autoAb and total Ig were assayed in serum 20 days later. The association of MHV infection with the toxic effects of CCl4 resulted in hypergammaglobulinemia and the production of autoAb to various liver and kidney proteins [18]. Liver histology showed several pathological changes, but without the cellular infiltrate that characterizes AIH [5], [6].
To improve the previous experimental model of AIH-like disease, herein we used the synthetic peptide PADRE [7], [8], which was injected together with the virus. Now, B6 mice were chosen rather than BALB/c because B6 strain is known to better respond to PADRE than other mouse strains [7]. The present results indicate first that MHV-infected B6 mice developed several signs of AIH, i.e., hypergammaglobulinaemia, autoAb to liver antigens (Ag), elevated transaminases and, interestingly, liver infiltrates. In addition, the simultaneous treatment with PADRE amplified some signs of the AIH-like disease.
2. Materials and methods
2.1. Mice
Specific-pathogen-free (SPF) female C57BL/6 mice from the University of La Plata, Argentina, were used at the age of 8–10 weeks. All animals were maintained in isolators, on standard laboratory chow, under SPF conditions until the end of the experiments, and received care in compliance with international legal requirements.
2.2. Preparation of MHV stock
The NCTC 1469 adherent cell line derived from normal mouse liver was purchased from the American Type Culture Collection. Cells growing in T-75 bottles were inoculated with MHV A59 virus at a multiplicity of 1–5 TCID50/cell.
After an adsorption period of 1 h at 37 °C, 15 ml of NCTC 135 medium with 10% fetal calf serum was added to each bottle and incubated at 37 °C. Several cycles of freezing and thawing were used to release the virus 24 h after inoculation. The harvested virus was centrifuged at 400 g for 10 min to removed debris and the supernatant was frozen at − 70 °C for storage.
Virus titration by endpoint method was performed by inoculating serial dilutions of the MHV stock onto cell monolayers in 96-multiwell plates. After 24 h, wells with viral cytopathic effect were counted for each dilution and titer was expressed as 50 % tissue infectious doses (TCID50) [19].
2.3. Viral infection and PADRE inoculation
Twelve C57BL/6 mice were inoculated subcutaneously with 50 μg of PADRE/100 μl of PBS diluted 1/2 in complete Freund's adjuvant from Sigma-Aldrich Inc., Illinois, MO, USA (mice called “PADRE”). From the latter, after 24 h, 6 mice were infected intraperitoneally with 104 TCID50 of MHV-A59 (animals “MHV + PADRE”). As a control, a third group of 6 mice was infected only with the virus (“MHV” mice). The mice were bled 15, 30 and 45 days after infection.
2.4. Immunoglobulin assays
For total IgG determination in mouse serum, ELISA microplates (Corning Incorporated, Corning, NY, USA) were coated with 100 μl of phosphate buffer saline (PBS) containing a 1:500 diluted rabbit antiserum directed against mouse Ig. The plates were blocked 1 h at 37 °C with 0.01 M Tris, 0.13 M NaCl, pH 7.4 (TMS) containing 5% of non-fat milk (TMS-M) and were incubated with serial dilutions of mouse serum in the same medium. After 2 h at 37 °C and washing with PBS containing 0.125 ml of Tween 20 per liter (PBS-Tween), the plates were incubated 1 h at 37 °C with peroxidase-labeled anti-mouse IgG Ab. These peroxidase-labeled goat anti-mouse IgG (Santa Cruz Biotechnology, CA, USA) were used at a 1:10,000 dilution in TMS-M.
2.5. Synthesis and purification of PADRE
The sequence of the peptide PADRE is, in a single letter code, aK (X) VAAWTLKAa, where X is the L-cyclohexylalanine, D-amino acids are designated by lowercase letters, and the C-terminal is an amide [7], [8]. The peptide synthesis was performed by solid phase Fmoc methodology on a 0.25 mmol scale. Peptides were assembled on an automatic peptide synthesizer model 431 A (Applied Biosystems Inc. Foster City, CA, USA). Fmoc amino acids (Peptides International, Louisville, KY, USA) were incorporated onto Rink amide-p-methyl P-benzhydrylamine resin (Peptides International Inc., Louisville, Kentucky, USA) as hydroxybenzotriazole active esters. The Fmoc protecting group was removed with 20% piperidine in N-methylpyrrolidone (NMP).
Peptide cleavage of the resin was achieved with trifluoracetic acid (TFA)/ethanedithiol/water 9.5:0.25:0.25 (v/v) for 2 h at room temperature. The suspension of resin was filtered and the crude material was precipitated by adding 15 ml of cold diethyl ether and washed three times with diethyl ether. The residual ether was removed by evaporation under reduced pressure and the peptide was lyophilized.
The crude peptide material was purified by high performance liquid chromatography (HPLC) on a C18 Vydac semi preparative column, 1 × 25 cm (The Separation Group, Hesperia. CA, USA) eluted with a linear acetonitrile gradient (24–80%, in the presence of 0.1% of TFA) over 35 min at 1.5 ml/min). The main peak was collected, lyophilized and repurified on the same column. Peptide purity was verified by amino acid analysis, sequence determination and mass spectrometry performed at the LANAIS-PRO (National Protein Sequencing Facility, UBA-CONICET, Buenos Aires, Argentina).
2.6. ELISA to test anti-PADRE Ab
Essentially, the method described by Ball et al. [20], was used. ELISA plates (Corning Incorporated, Corning, NY, USA) were coated with 2 μg of poly-l-lysine (45–50 kDa, Sigma-Aldrich Inc., Illinois, MO, USA) contained in 50 μl of 0.05 M sodium bicarbonate buffer, pH 9.6. After 1 h at room temperature and a wash with PBS, 50 μl of 1% (v/v) glutaraldehyde was added to each well and the plates washed after 15 min of incubation. The peptide PADRE was diluted to a concentration of 10 μg/ml in PBS and 50 μl was added to the wells coated with poly-l-lysine and activated with glutaraldehyde. The plates were incubated overnight at room temperature and then washed twice with PBS. Reactive aldehyde sites were blocked by the addition of 1 M glycine, 200 μl/well, followed by incubation for 1 h at room temperature and washed.
The plates were then incubated overnight at room temperature with mouse serum diluted in 0.01 M Tris, 0.13 M NaCl, pH 7.4, containing 5% of non-fat milk (TBS-M), and after washing with PBS containing 0.125 ml of Tween 20 per liter (PBS-Tween), bound Ab were revealed with peroxidase-labeled goat anti-mouse IgG (Santa Cruz Biotechnology, CA, USA) diluted 1:10,000 in TBS-M. As a substrate, ortho-phenylene-diamine-dihydrochloride (OPD, Sigma Chemical Co, St. Louis, MO, U.S.A.) with freshly added H2O2 was used. The reaction was stopped after 10 min by addition of 1 M H2SO4. The absorption was measured by ELISA reader (Metertech Inc., Taipei, Taiwan) at 490 nm. Non-specific values of optical density were obtained in the absence of mouse serum.
2.7. Preparation of liver lysates
Livers from non-infected C57BL/6 mice were removed, soaked in chilled PBS and ground in an Omni Mixer Homogenizer (Omni International Inc, USA) at 4 °C with 20 volumes of PBS containing 1 mM phenylmethyl–sulfonyl fluoride (PMSF) and 1 U/ml of trypsin inhibitor. The homogenates were centrifuged for 10 min at 400 g and the clarified extracts kept at − 20 °C until used. A sample of each suspension was solubilized by heating for 30 min at 100 °C in 1 M NaOH and protein concentration was determined by the method of Lowry et al. [21].
2.8. Purification of rat liver FAH
The enzyme was prepared as previously described [12]. Briefly, livers from 90-day-old Wistar rats were homogenized in 5 vol (v/w) of chilled 0.3 M sucrose, 5 mM Tris/HCl buffer containing 0.5 mM CaCl2, 1 U/ml of trypsin inhibitor and 1 mM PMSF, pH 7.4. After centrifugation at 10,000 g for 20 min and then at 100,000 g for 1 h, ethyl alcohol was added to the supernatant as to obtain a final concentration of 50% ethanol. This mixture was allowed to stand overnight at 4 °C and then centrifuged at 16,300 g for 15 min. The supernatant was mixed with 95% ethyl alcohol to yield a final alcohol concentration of 70%. After incubating overnight at 4 °C the precipitated enzyme was packed by centrifugation at 16,300 g for 15 min. The pellet was resuspended in 25 mM phosphate buffer pH 7.2 and stirred for 30 min. The supernatant fluid was recovered after centrifugation of the suspension at 16,300 g for 10 min and solid ammonium sulfate was added so as to obtain a final salt concentration of 40%. After 1 h at 4 °C and centrifugation at 16,300 g for 10 min the pellet was resuspended in 20 mM Tris/HCl pH 8.0 and dialyzed against the same buffer.
2.9. Western blot analysis
Liver extract (100 μg of protein) or purified FAH protein (50 μg) was submitted to 10% SDS-PAGE and then transferred onto nitrocellulose sheets (Amersham, Buckinghamshire, UK). After reversible staining with Ponceau S to check satisfactory transfer, non-specific Ab-binding sites were blocked by incubating the sheets with 5% non-fat milk in 30 mM Tris, 0.14 M NaCl, 0.1% (v/v) Tween 20, pH 8.0 (TBS-M-T) for 1 h at room temperature with shaking. The strips were then incubated overnight at 4 °C with an Ab dilution in TBS-M-T. After several washings with TBS containing 0.1% Tween 20, bound Ab were revealed with peroxidase-labeled goat anti-mouse IgG (Ig-PO, Santa Cruz Biotechnology, CA, USA) diluted 1:10000 in TBS-M-T and ECL Plus reagents (Amersham, Buckinghamshire, UK). In every experiment, a negative control (pool of sera from the same naïve B6 mice used afterward) and a positive control (pool of sera from MHV-infected B6 mice) were included. The apparent molecular mass (kDa) of the detected bands was determined using a wide range protein standard (BDH Laboratory Supplies Poole BH15 1TD, UK).
2.10. Dot blot assays
The FAH protein was diluted in 0.03 M Tris, 0.14 M NaCl pH 8.0 (TBS), then placed (100 μl containing 10 or 15 μg of protein) on the nitrocellulose paper using the Bio-Dot equipment (BioRad Laboratories, Hercules, CA, USA). The membranes were then blocked by incubating for 1 h at 20 °C with 0.03 M Tris, 0.14 M NaCl pH 8.0 (TBS) containing 5% non-fat milk and 0.1% (v/v) Tween 20 (TBS-M-T). After washing with TBS containing 0.1% (v/v) Tween 20 (TBS-T) to remove excess of protein, membranes were incubated overnight at 4 °C with the indicated serum dilution, washed with TBS-T and incubated 1 h at 20 °C with a dilution of 1:10,000 of Ig-PO in TBS-M-T. After four washes with TBS-T, bound Ab were detected by a chemiluminescent reaction (ECL Plus, Amersham, Buckinghamshire, UK) and the fluorescence was subsequently captured on a radiographic film in a frame for X-rays. After exposure, the film was immersed in developing solution for 2 min (Eastman Kodak Company, New York, USA), rinsed with water and then treated for 5 min in development solution (Eastman Kodak Company, New York, USA). To quantify the intensity of the bands obtained, we used the Gel Pro Analyzer program 4 (Media Cybernetics Inc, Silver Spring, MD, USA).
2.11. High-mobility group box protein 1 (HMGB1) assay
Mouse sera were filtered with Centricon YM-100 (Millipore Corp, USA) to clear the samples from macromolecular complexes and then concentrated 15-fold with Centricon YM-30 and separated on 12% SDS-polyacrilamide gels. Western blot analysis was carried out as described above, and HMGB1 was identified with MAb anti-HMGB1 HAP46.5 (Santa Cruz Biotechnology, CA, USA) diluted 1:1000 and peroxidase-labeled goat anti-mouse IgG. Recombinant human HMGB1 (HMGBiotech SRL, Milano, Italy) was used as positive control.
2.12. Histology
Livers from “MHV”, “MHV + PADRE” or control mice (no treatment) were cut into blocks and fixed by immersion into 10% formalin in 0.1 M phosphate buffer, pH 7.4. After fixation, the tissues were dehydrated in graded alcohols, and then embedded in paraffin. 5-μm sections were cut, stained with Harris’ hematoxylin for 2 min and counterstained with eosin for 2 min. The sections were then washed with distilled water, dehydrated in graded alcohols and xylene, and mounted with Canada Balsam.
2.13. Transaminase determination
Serum aspartate aminotransferase (AST) was determined using the GOT (AST) Unitest (Wiener Lab., Rosario, Argentina).
2.14. Measurement of uric acid concentration in plasma and liver tissue
Uric acid concentration was determined enzymatically using the assay kit Uricostat (Wiener Lab, Rosario, Argentina) in 1:50 diluted mouse sera or liver extracts as indicated by the manufacturer. Liver extracts (1 g/20 ml) were prepared as indicated above, except that distilled water was used instead of PBS and that the suspension was centrifuged twice for 10 min at 400 g.
3. Results
3.1. Total immunoglobulin (Ig)
As expected, since MHV produces hypergammaglobulinemia [10], [19], higher Ig levels were found in sera from “MHV” and “MHV + PADRE” mice than in controls at 15 and 30 days post-infection; Ig concentration decreased to normal values after 45 days (Fig. 1 ). PADRE inoculation also caused a significant increase of Ig over control at 15 and 30 days post-treatment (Fig. 1). Moreover, the values of serum Ig concentration were significantly higher in serum from “MHV + PADRE” than in “MHV” animals after 30 days of treatment (Fig. 1).
Fig. 1.

-
1)“PADRE”, “MHV” and “MHV + PADRE” vs. Control in A and B.
-
2)“PADRE” vs. “MHV + PADRE” in A.
-
3)“MHV” vs. “MHV + PADRE” in B.
3.2. AutoAb in sera from mice infected with MHV and/or treated with PADRE
The positions of the various bands of autoAg detected by Western blot assays (relative mobility, RM) were calculated according to their mobilities in relation to the endogenous IgG heavy and light chains location (arbitrary values of 1 and 0, respectively) (Fig. 2 -I).
Fig. 2.

-
I)Example of the calculation of the Relative Mobility (RM) of the autoAg. The IgG heavy and light chains already present in tissues were used as conventional markers (values of 1 and 0, respectively) to calculate the RM of the different proteins. In the figure, the FAH position corresponded to a RM of 0.7.
-
II)Representative results of Western blot assays. A, B and C: 15, 30 and 45 days after the indicated treatments. Control: sera from untreated mice.
Anti-FAH autoAb were detected in sera from “MHV” and “MHV + PADRE” mice at all tested times, as previously described for BALB/c, 129/Sv and CBA/Ht mice infected with MHV [12] (Fig. 2-II). Unexpectedly, in addition to the anti-FAH autoAb, the B6 mice used in this study produced Ab against other liver proteins of different molecular weight after either MHV infection alone or MHV infection together with PADRE treatment. Even mice that were treated with PADRE alone produced autoAb against various liver proteins (see representative results in Fig. 2-II) whereas sera from mice inoculated with complete Freund's adjuvant alone did not react at all (data not shown).
The whole results, presented as individual values of RM, indicated that autoAb from “PADRE” animals detected a similar number of bands corresponding to liver autoAg at 15 and 30 days after treatment (10 and 12 bands, respectively) and a decrease of Ab reactivity at 45 days (4 bands) (Fig. 3 ). As described above, B6 mice inoculated with MHV developed autoAb against other proteins than FAH (Fig. 3). The number of bands detected, excluding the FAH, was higher at 30 days (9 bands) than at 15 or 45 days (3 and 4 bands, respectively). Similarly, Ab from “MHV + PADRE” animals detected the highest number of autoAg (11 bands) at 30 days, whereas at 15 or 45 days post-treatment seven bands were obtained (Fig. 3).
Fig. 3.

Relative mobility of liver autoAg. Dots indicate the individual values of relative mobility (RM) obtained for each group of six mice submitted to the indicated treatments (see Fig. 2). A, B and C: 15, 30 and 45 days post-infection, respectively. Relative mobility of FAH in A: 0.7; B and C: 0.5.
3.3. Reactivity of anti-FAH autoAb determined by Western blot and dot blot analyses
Since Western blot results showed a higher intensity of autoAb reactivity in sera of “MHV + PADRE” mice than in “PADRE” or “MHV” animals (Fig. 2-II), we measured the reactivity of autoAb specific to FAH. Western blot analyses indicated that the reactivity of anti-FAH autoAb increased about 30% in sera from “MHV + PADRE” compared with values from “MHV” mice at 30 days post-infection (Fig. 4 -A). Identical results were obtained with sera after 15 days of treatment (data not shown).
Fig. 4.
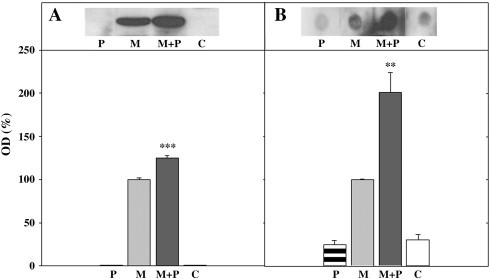
-
A)Reactivity towards FAH (purified from rat liver) of a pool of six sera from mice after 30 days of the indicated treatments. P: PADRE; M: MHV; M + P: MHV + PADRE; C: control, untreated mice. The intensities of the bands were measured by densitometry and results were expressed as % of controls, taken as 100% the reactivity of sera from mice infected with MHV (grey bars). Black and white bars indicated results from M + P and C, respectively, whereas horizontal shows PADRE results. Values are means ± SD of three independent determinations and statistical significance of M + P in comparison with M was calculated with the Student t test. *** P < 0.001.
-
B)Reactivity towards FAH (purified from rat liver) of a pool of six sera from mice after 30 days of the indicated treatments. P: PADRE; M: MHV; M + P: MHV + PADRE; C: control, untreated mice. The intensities of the dots obtained with 15 μg of FAH were analyzed by densitometry. A value of DO = 100% was assigned to the reactivity of sera from mice infected with MHV (grey bars). Black and white bars indicated results from M + P and C, respectively, whereas horizontal shows results obtained with PADRE. Values are means ± SD of three independent determinations and statistical significance of M + P in comparison with M were calculated with the Student t test ** P < 0.005.
Under Western blot conditions the proteins are mostly denatured, so the anti-FAH autoAb would recognize mainly linear and/or cryptic epitopes of the protein. Thus, to determine the autoAb reactivity towards native (or conformational) epitopes of the enzyme, dot blot assays were used. Results indicated that binding reactivity of the anti-FAH Ab was higher in sera from “MHV + PADRE” than in “MHV” mice after 30 days of treatment (Fig. 4-B), whereas no such differences were detected in samples taken 15 days post-treatment (data not shown).
3.4. Ab towards PADRE
Anti-PADRE Ab titers (expressed as the serum dilution to obtain an OD = 0.5) were similar in non-treated and MHV-infected mice, ranging from 200 to 1200 at 15, 30 and 45 days post-treatment (Table 1 -a). On the other hand, Ab titers in “PADRE” animals raised from 1200 to 2800 and 5000 at 15, 30 and 45 days, respectively, whereas those found in “MHV + PADRE” mice were 800, 8000 and 15,000 at the three bleeding times tested (Table 1-a).
Table 1.
Immunological and biochemical parameters determined in this work.
| Treatment | Days post-treatment and/or infection |
||
|---|---|---|---|
| 15 | 30 | 45 | |
| Anti-PADRE Ab (a) 1/dilution (DO 490nm = 0.5) | |||
| Control | 200 | 500 | 300 |
| PADRE | 1200 | 2800 | 5000 |
| MHV | 1200 | 700 | 350 |
| MHV + PADRE | 800 | 8000 | 15,000 |
| AST concentration (b) (expressed as a ratio of sample/normal control) | |||
| PADRE | 0.7 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.2 |
| MHV | 3.6 ± 2.1* | 10.1 ± 4.3* | 0.4 ± 0.1 |
| MHV + PADRE | 3.5 ± 2.5* | 7.2 ± 1.3** | 0.4 ± 0.1 |
| Uric acid (mg/l) (c) | |||
| Control | 10.5 ± 0.3 | 11.3 ± 0.7 | 11.2 ± 0.3 |
| PADRE | 17.3 ± 0.9** | 15.0 ± 0.3* | 17.5 ± 0.8** |
| MHV | 9.1 ± 1.5 | 17.5 ± 1.0** | 19.8 ± 0.8*** |
| MHV + PADRE | 13.5 ± 1.2 | 13.8 ± 1.5 | 14.6 ± 0.0 *** |
| HMGB1 (μg/ml) (d) | |||
| Control | ND | ND | ND |
| PADRE | ND | 79.3 ± 4.8 | 140.1 ± 21.5 |
| MHV | ND | 150.7 ± 0.8 | 168.2 ± 5.7 |
| MHV + PADRE | ND | 120.6 ± 30.1 | 194.3 ± 9.6 |
a) A pool of sera from six mice submitted to each treatment was diluted as indicated in Materials and methods and then added to the ELISA wells. Values are the mean of serum dilution to reach an OD of 0.5.
b) The levels of AST in sera from six animals under each condition were determined. Normal control values were 15–30 U/l, depending on the sample used. Statistical significance in comparison with PADRE treatment was calculated using the Mann–Whitney U-test. * P < 0.05; **P < 0.01.
c) Uric acid was assayed on pooled sera from six mice in triplicate, and statistical significance was calculated with the Student test * P < 0.01, ** P < 0.005, *** P < 0.0001. Control: sera from untreated mice.
d) Pools of sera from six mice submitted to each treatment were used to detect HMGB1 by Western blot assays. The protein concentration was calculated by densitometry in comparison with a positive control (500 μg/ml of recombinant human HMGB1 from HMGBiotech SRL Milano, Italy). ND: not detected.
The autoAb to liver tissue in “PADRE” and “MHV + PADRE” mice could be due to a possible cross-reaction of anti-PADRE Ab with a variety of liver proteins. However, the highest titers of Ab to PADRE were found 45 days post-infection in either “PADRE” or “MHV + PADRE” mice (Table 1-a), whereas autoAb to liver proteins were more abundant at 30 days (Fig. 3), suggesting a lack of correlation between both findings and probably the absence of cross-reaction between anti-PADRE Ab with liver proteins.
3.5. Serum aspartate aminotransferase (AST)
“PADRE” animals did not show elevated levels of the enzyme at any time after treatment (Table 1-b). In contrast, “MHV” and “MHV + PADRE” mice displayed significant higher AST serum levels than controls at 15 and 30 days post-infection, whereas values at 45 days were similar to those found in non-treated animals (Table 1-b).
3.6. Uric acid and HMGB1
As uric acid and HGMB1 are potent alarmins (DAMPs, damage-associated molecular patterns, or endogenous adjuvants) [22], [23], we followed their releases in the four mouse groups. A single inoculation of PADRE significantly increased serum uric acid over control values at 15, 30 and 45 days post-treatment (Table 1-c). Besides, “MHV” mice showed elevated uric acid concentrations in sera obtained after 30 and 45 days of virus inoculation, whereas in serum from “MHV + PADRE” animals the uric acid levels were higher than in controls only at 45 days post-infection. We have also measured the uric acid concentration in hepatocyte extracts from four mice 30 days after treatment, and the following results were obtained: 3.3 ± 0.2 mg/g in controls, 3.5 ± 0.1 mg/g in “PADRE” animals, 6.4 ± 0.5 mg/g (P < 0.005) in “MHV” mice, and 5.9 ± 0.6 mg/g (P < 0.005) in “MHV + PADRE” mice. It should be noted that, at 30 days, the level of uric acid was not significantly increased in serum from “MHV + PADRE” mice, whereas it was clearly elevated in the hepatocyte extract.
The other alarmin, HMGB1, was undetectable in the sera of the four groups at 15 post-infection or post-treatment, but its level strongly increased after 30 and 45 days in "MHV", "MHV + PADRE" and even in "PADRE" groups (Table 1-d).
3.7. Liver pathology
Liver sections of some control animals contained small foci of lymphocytes and neutrophils, although most samples did not show cellular injuries (Fig. 5 ). Livers from “MHV” mice exhibited more severe damages than controls: edema and necrosis of hepatocytes, and significant infiltrates of lymphocytes and neutrophils, mainly 45 days post-infection (Fig. 5). Livers from “MHV + PADRE” mice showed abundant foci of cellular infiltrates at 30 and 45 days post-infection, suggesting that PADRE aggravated in some degree the pathology induced by the virus (Fig. 5). No fibrosis was observed in any group.
Fig. 5.

-
A)“MHV”: hyperemia, hepatocytes with cell swelling and the presence of a small focus of lymphocytic infiltration; “MHV + PADRE”: a small focus of lymphocytic infiltration around the portal space.
-
B)“MHV”: hepatocyte necrosis; “MHV + PADRE”: a major focus of lymphocytic infiltration near the portal space.
-
C)“MHV”: a major focus of lymphocytic infiltration near a blood vessel; “MHV + PADRE”: an important focus of lymphocytic infiltration near a blood vessel and liver cells showing cellular edema.
4. Discussion
In animal models, AIH is induced by various mechanisms including xenoimmunization with human antigens [24], [25], adenovirus vectors [5], [26], transgenesis [27], or polyclonal activation of T cells by inoculation of concanavalin A [28]. In those models, liver damage was observed, and in some conditions elevated transaminases. However, no Ab to liver proteins other than those directed to the immunogen, neither high Ig plasmatic levels were reported. In addition, most models were described as transient, since the mechanisms critical for perpetuation of liver damage are still unknown [for a review see Refs. [3], [5], [6]]. Besides, we have recently found that a simple and common phenomenon, i.e., a viral infection inducing autoAb to an autologous protein followed by a toxic liver injury, could induce some signs of AIH [18]. It was proposed that the release of a greater amount of alarmins [22] could induce the expansion of the autoimmune response and establish a pathology that resembles AIH [18].
The purpose of the present work was to establish a better model of experimental AIH-like disease using MHV-infected mice simultaneously treated with a peptide that is a universal T lymphocyte epitope (PADRE) [7], [8] and so could have amplify and diversify the autoimmune response induced by the virus and restricted to FAH in other mouse strains.
It should be stressed that C57BL/6 (B6) mice, when infected with MHV, were found to behave differently from other mouse strains. Indeed, BALB/c, CBA/Ht and 129/Sv mice, after viral infection, had autoantibodies restricted to FAH [12] whereas MHV-A59 infected B6 mice developed autoantibodies to multiple liver antigens.
Furthermore, sera from B6 mice inoculated with “PADRE” alone also showed autoAb against liver proteins, principally at 30 days post-treatment. In the “MHV + PADRE” group, these autoAb were more abundant and more diversified in their specificities than in “PADRE” animals. In addition, Western blot and dot blot assays proved that autoAb induced in “MHV + PADRE” mice did bind FAH protein stronger than those found in “MHV” animals. Ab from “MHV + PADRE” animals were mainly directed to native epitopes of the enzyme, indicating that PADRE adjuvant should influence B-cell responses.
Because the induction of autoAb detected in both groups, “PADRE” and “MHV + PADRE”, could be related to the presence of anti-PADRE Ab and their possible cross-reactions with liver proteins, anti-PADRE Ab were tested by ELISA. As expected, no anti-PADRE Ab were detected in control or “MHV” animals, although autoAb to liver proteins were found in the latter. Moreover, anti-PADRE Ab peaked at 45 days post-infection or post-treatment in both groups “PADRE” and “MHV + PADRE”, whereas autoAb to liver proteins peaked at 30 days, indicating that anti-PADRE Ab and autoAb had a different kinetics and, probably, were unrelated in their antigenic specificities.
The pathologic features of the liver in mice submitted to the various treatments indicated that some control mice presented small cellular infiltrates, not observed previously in BALB/c mice coming from the same facility [12], [18]. In spite of that, it was observed that MHV and/or PADRE inoculation worsened the condition, since more important infiltrates were observed, mainly in “MHV + PADRE” animals. Injured hepatocytes liberated transaminases, as indicated by the elevated concentrations of the enzymes at 15 and 30 days post-infection although, intriguingly, their plasmatic values were similar to those of controls after 45 days of treatment.
Uric acid, one of the most studied alarmins [23], increased in serum from “PADRE” animals at all times tested, whereas its level in “MHV” augmented after 30 and 45 days of treatment. Furthermore, uric acid concentration was higher than controls only after 45 days of viral infection in “MHV + PADRE” mice. One could speculate that plasmatic uric acid in “MHV + PADRE” mice after 30 days of treatment was similar to controls, and lower than values found in “MHV” animals, due to urate crystals retention within the hepatocytes. In fact, once the uric acid concentration was tested in whole liver tissue 30 days after treatment, results showed identical values in “MHV” and “MHV + PADRE” animals. On the other hand, serum HGMB1 concentration significantly augmented with time in the three animal groups, even in “PADRE” mice.
PADRE is presented by MHC class II molecules and is expected to act only on T helper cells and, indirectly on B cells [7], [8]. Consequently, the effects of PADRE on the release of both alarmins are unexpectedly and intriguing. As it was found that the uric acid levels present in plasma from ”PADRE” animals were elevated, but not in the hepatocyte extracts, the origins of both alarmins are probably not the hepatocytes, but perhaps the stimulated lymphocytes or other non antigen-specific leucocytes. One can also speculate that PADRE is recognized by some PRRs (pattern-recognizing receptors such as the Toll-like receptors and NOD-like receptors), a fact that has, to our knowledge, not been reported so far.
The liver is known to be a classical immunoprivileged site, which is well illustrated by the experiment showing that ectopic expression of myelin basic protein (MBP) in the mouse liver protected against autoimmune neuroinflammation through the involvement of regulatory T cells [29]. In addition, regulatory CD4+CD25+ T cells (Tregs) were found numerically and functionally defective in human AIH [30]. However, this control could be inhibited by non-specific activation of innate immunity through Toll-like receptor 3 [31]. Moreover, it has been reported that uric acid could amplify humoral immune responses [22] and that various Toll-like receptors associated with the inhibition of Tregs may bind alarmins such as heat shock proteins, HMGB1 and chromatin [32], [33]. Thus, it is possible that alarmins released under the effects of MHV and PADRE could bind to some Toll-like receptors and indirectly inhibit Tregs, so inducing some of the AIH signs.
In conclusion, MHV infection alone was capable to cause a hepatitis associated with transient hypergammaglobulinemia, elevated transaminases, and autoAb directed against different liver proteins in B6 mice, signs that are reminiscent of those of AIH. In addition, PADRE administration intensified the effect of viral infection, prolonging the hypergammaglobulinemia and increasing the autoAb reactivity as well as hepatic infiltrations.
As stated by Vergani and Mieli-Vergani [4], “The ideal model for AIH should have a well-defined initiating event followed by chronic inflammation leading to fibrosis”. Therefore, the first part of the objective was reached, since our present results demonstrate that infection of susceptible individuals with a hepatotropic virus triggers some AIH signs. Moreover, since the administration of PADRE adjuvant seemed to expand some MHV effects, one can speculate that a simple vaccination of an infected genetically vulnerable person may eventually initiate the illness. Nevertheless, the chronic phase of the disease, as well as the fibrosis normally found in liver of AIH patients is still lacking in our model. Although more efforts are needed to achieve that goal, the findings indicate that a simple infection with a virus, instead of complex and not natural protocols of immunization, may simulate the onset of the illness and contribute to the study of its pathophysiology.
Acknowledgements
The authors are indebted to Dr. Pierre L. Masson (de Duve Institute, ICP, Brussels, Belgium) for helpful discussions and critical revision of the manuscript. This work was supported by grants from CONICET and Universidad de Buenos Aires, Argentina.
References
- 1.Béland K., Lapierre P., Alvarez F. Influence of genes, sex, age and environment on the onset of autoimmune hepatitis. World J Gastroenterol. 2009;15:1025–1034. doi: 10.3748/wjg.15.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czaja A.J. Understanding the pathogenesis of autoimmune hepatitis. Am J Gastroenterol. 2001;96:1224–1231. doi: 10.1111/j.1572-0241.2001.03707.x. [DOI] [PubMed] [Google Scholar]
- 3.Czaja A.J. Animal models of autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 2010;4:429–443. doi: 10.1586/egh.10.42. [DOI] [PubMed] [Google Scholar]
- 4.Vergani D., Mieli-Vergani G. Aetiopathogenesis of autoimmune hepatitis. World Gastroenterol. 2008;14:3306–3312. doi: 10.3748/wjg.14.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christen U., Holdener M., Hintermann E. Animal models for autoimmune hepatitis. Autoimmun Rev. 2007;6:306–311. doi: 10.1016/j.autrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Hardtke-Wolenski M., Jaeckel E. Mouse models for experimental autoimmune hepatitis: limits and chances. Dig Dis. 2010;28:70–79. doi: 10.1159/000282067. [DOI] [PubMed] [Google Scholar]
- 7.Alexander J., del Guercio M.-F., Frame B., Maewal A., Sette A., Nahm M.H. Development of experimental carbohydrate-conjugate vaccines composed of Streptococcus pneumonia capsular polysaccharides and the universal helper T-lymphocyte epitope (PADRE) Vaccine. 2004;22:2362–2367. doi: 10.1016/j.vaccine.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 8.del Guercio M.-F., Alexander J., Kubo R.T., Arrhenius T., Maewal A., Appella E. Potent immunogenic short linear peptide constructs composed of B cell epitopes and Pan DR T helper epitopes (PADRE) for antibody responses in vivo. Vaccine. 1997;15:441–448. doi: 10.1016/s0264-410x(97)00186-2. [DOI] [PubMed] [Google Scholar]
- 9.Godfraind C., Holmes K.V., Coutelier J.-P. Thymus involution induced by mouse hepatitis virus A59 in BALB/c mice. J Virol. 1995;69:6541–6547. doi: 10.1128/jvi.69.10.6541-6547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutelier J.-P., van der Logt J.T., Heessen F.W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavi E., Gilden D.H., Wroblewska Z., Rorke L.B., Weiss S.R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984;34:597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- 12.Mathieu P.A., Gómez K.A., Coutelier J.-P., Retegui L.A. Identification of two liver proteins recognized by autoantibodies elicited in mice infected with mouse hepatitis virus A59. Eur J Immunol. 2001;31:1447–1455. doi: 10.1002/1521-4141(200105)31:5<1447::AID-IMMU1447>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duhalde-Vega M., Loureiro M.E., Mathieu P.A., Retegui L.A. The peptide specificities of the autoantibodies elicited by mouse hepatitis virus A59. J Autoimmun. 2006;27:203–209. doi: 10.1016/j.jaut.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duhalde-Vega M., Aparicio J.L., Retegui L.A. Fine specificity of autoantibodies induced by mouse hepatitis virus A59. Viral Immunol. 2009;22:287–294. doi: 10.1089/vim.2009.0019. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu P.A., Gómez K.A., Coutelier J.-P., Retegui L.A. Sequence similarity and structural homologies are involved in the autoimmune response elicited by mouse hepatitis virus A59. J Autoimmun. 2004;23:117–126. doi: 10.1016/j.jaut.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhalde-Vega M, Retegui LA. Uric acid and HMGB1 are involved in the induction of autoantibodies elicited in mice infected with mouse hepatitis virus A59. Autoimmunity. in press. doi:10.3109/08916934.2011.579927. [DOI] [PubMed]
- 17.Matzinger P. The Danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 18.Aparicio J.L., Duhalde-Vega M., Loureiro M.E., Retegui L.A. The autoimmune response induced by mouse hepatitis virus A59 is expanded by an hepatotoxic agent. Int Immunopharmacol. 2009;9:627–631. doi: 10.1016/j.intimp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutelier J.-P., Coulie P.G., Wauters P., Heremans H., van der Logt J.T.M. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol. 1990;64:5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball J.M., Henry N.L., Montelaro R.C., Newman M.J. A versatile synthetic peptide-based ELISA for identifying antibody epitopes. J Immunol Methods. 1994;171:37–44. doi: 10.1016/0022-1759(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 21.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Behrens M.D., Wagner W.M., Krco C.J., Erskine C.L., Kalli K.R., Krempski J. The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity. Blood. 2008;111:1472–1479. doi: 10.1182/blood-2007-10-117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapierre P., Djilali-Saiah I., Vitozzi S., Alvarez F. A murine model of type 2 autoimmune hepatitis: xenoimmunization with human antigens. Hepatology. 2004;39:1066–1074. doi: 10.1002/hep.20109. [DOI] [PubMed] [Google Scholar]
- 25.Mori Y., Mori T., Yoshida H., Ueda S., Iesato K., Wakashin Y. Study of cellular immunity in experimental AIH in mice. Clin Exp Immunol. 1984;57:85–92. [PMC free article] [PubMed] [Google Scholar]
- 26.Holdener M., Hintermann E., Bayer M., Rhode A., Rodrigo E., Hintereder G. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voehringer D., Blaser C., Grawitz A.B., Chisari F.V., Buerki K., Pircher H. Break of T cell ignorance to a viral antigen in the liver induces hepatitis. J Immunol. 2000;165:2415–2422. doi: 10.4049/jimmunol.165.5.2415. [DOI] [PubMed] [Google Scholar]
- 28.Hegde V.L., Hegde S., Cravatt B.F., Hofseth L.J., Nagarkatti M., Nagarkatti P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lüth S., Huber S., Schramm C., Buch T., Zander S., Stadelmannet C. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longhi M.S., Hussain M.J., Mitry R.R., Arora F.K., Mieli-Vergani G., Vergani D. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 31.Lang K.S., Georgiev P., Recher M., Navarini A.A., Bergthaler A., Heikenwalder M. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G., Zhao Y. Toll-like receptors and immune regulation: their direct and indirect modulation on regulatory CD4+CD25+ T cells. Immunology. 2007;122:149–156. doi: 10.1111/j.1365-2567.2007.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshak-Rothstein A., Rifkin I.R. Immunologically active autoantigens: the role of Toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]


