Abstract
Introduction
There are suggestions that virus co-infections may influence the clinical outcome of respiratory virus illness. We performed a systematic review of the literature to summarise the evidence.
Methods
MEDLINE, EMBASE, Ovid and WEB of Science databases, major organisation websites and reference lists of published studies were searched. The quality of studies was assessed using the STROBE tool (von Elm et al., 1) Individual study data was analyzed using odds ratios and 95% confidence intervals as a measure of association between exposure (co-infection), patient outcome and results summarised using forest plots and tables
Results
Nineteen (19) studies from all over the world were identified and included in the review. Most of the studies 73.7% (14/19) recruited children ≤6 years old. Evidence on the role of co-infection in increasing disease severity was inconclusive. In five out of eight studies, co-infection significantly increased risk of admission to general ward (OR: 2.4, 95% CI: 1.3 - 4.4, p = 0.005; OR: 2.4, 95% CI: 1.1 - 7.7, P = 0.04; OR: 3.1, 95% CI: 2.0 - 5.1, p = <0.001; OR: 2.4, 95% CI: 1.7-3.4, p = <0.0001 and OR: 2.3, 95% CI: 1.1 - 5.1, p = 0.34), one found it did not (OR: 0.59, 95% CI: 0.4 - 0.9, p = 0.02) and the other 2 had insignificant results. Similarly on risk of admission to ICU, some studies found that co-infection significantly increased risk of admission to ICU (OR: 2.9, 95% CI: 1.4 - 5.9, p = 0.004 and OR: 3.0, 95% CI: 1.7 - 5.6, p = <0.0001), whereas others did not (OR: 0.18, 95% CI: 0.05 - 0.75, p = 0.02 and OR: 0.3, 95% CI: 0.2 - 0.6, p = <0.0001). There was no evidence for or against respiratory virus co-infections and risk of bronchiolitis or pneumonia.
Conclusion
The influence of co-infections on severe viral respiratory disease is still unclear. The observed conflict in outcomes could be because they were conducted in different seasons and covered different years and periods. It could also be due to bias towards the null, especially in studies where only crude analysis was conducted. Future studies should employ stratified analysis.
Keywords: repisratory virus infections, co-infections, dual or multiple infections, admission to a general ward, admission to ICU, disease severity
Introduction
Respiratory viruses including; influenza virus types A and B (Flu A/B), respiratory syncytial virus (RSV), rhinovirus (RV), adenovirus (AdV), human metapneumovirus (hMPV), human coronavirus (hCoV), human bocavirus (hBoV) and human parainfluenza viruses type 1, 2 and 3 (hPIV1-3), have been singly or jointly detected from patients suffering from respiratory diseases [2], [3], [4], [5]. Incidence studies have indicated that 15-38% of respiratory infections develop into acute lower respiratory infections (ARIs) with severe signs and symptoms including wheezing, bronchiolitis, croup, high fever and pneumonia with subsequent increases in hospitalization to a general ward (GW), admission to intensive care unit (ICU), or mortality [6], [7], [8], [9], [10], [11]. A number of factors have been attributed to the severity of respiratory viral disease including; underlying chronic diseases such as chronic respiratory diseases, diabetes, chronic liver disease, chronic heart disease, chronic renal disease; and other factors such as immunodeficiency, old age, young age, pregnancy, viral genome mutations [11], [12], [13], [14]. There are suggestions that the presence of more than one type of virus in the respiratory specimen may also affect the clinical presentation of respiratory tract infection [15], [16], [17], [18]. However, the relationship between co-infection and severity of illness remains unclear. This review investigates the relation between co-infection in general and co-infection between influenza and other respiratory viruses and clinical outcome.
Methodology
We searched the electronic databases; MEDLINE, EMBASE and WEB of Science for primary epidemiological studies on the role of co-infections in causing severe clinical disease; i.e. risk of hospitalization to the GW, admission to ICU or death, and risk of developing bronchiolitis and pneumonia. We also searched websites of health organisations e.g. the World Health Organisation (WHO), United Kingdom's Health Protection Agency (HPA), United States of Americas Centre for Disease Control (CDC), World Influenza Network Centre for bibliography or any published reports on respiratory viruses’ co-infections and patient outcome. The MEDLINE and EMBASE system have studies published from May, 1946 to date, whereas the Web of Science has studies published from 1945 to date. The search was refined to include studies related to medicine in general or to specific branches i.e. infectious diseases, virology, internal or respiratory system, pathology and critical care. Reference lists of good quality studies, were also manually searched to identify studies addressing the question under review.
For the electronic databases, the search technique involved combining a number of subject headings and keywords and the scoping of text words; words used included: Viruses, virus, virus diseases, virus infection, respiratory tract infections, respirovirus, respirovirus infections, lower respiratory tract infection(s), upper respiratory tract infection(s), orthomyxoviridae, orthomyxoviridae infections, orthomyxovirus, influenza human, influenza A virus, influenza A virus H1N1 subtype, 2009 H1N1 influenza, influenza A(H1N1)pdm09, influenza A virus H3N2 subtype, rhinovirus, human rhinovirus, rhinovirus infection, adenovirus, adenovirus infection(s), respiratory syncytial virus(es), respiratory syncytial virus infection(s), metapneumovirus, metapneumovirus human, parainfluenza virus 1 human, parainfluenza virus 2 human, parainfluenza virus 3 human, bocavirus, bocavirus infection, coronavirus, coronavirus infection, co-infection(s), mixed infection, dual infection(s), multiple infection(s), virulence, virus virulence, prognosis, pathogenicity, virus pneumonia, bronchiolitis, viral bronchiolitis, hospital, hospitalisation, hospitalization, hospital care, hospital admission, patient admission, length of stay, intensive care, critical care, intensive care unit, ICU admission, fatality, mortality, death.
Study quality assessment and selection criteria
The “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)” tool for critical appraisal of epidemiological studies (von Elm et al., [1]), was used to assess the studies identified in the search. Only studies which measured co-infection as a risk factor for disease outcome and included the outcome measures; hospitalization to general ward, admission to ICU, bronchiolitis or pneumonia were included. Studies that investigated exposures other than those investigated in this review i.e. did not include influenza and ≥4 of the other respiratory viruses considered as exposures of interest in this study, did not give risk outcome in co-infections vs. single infections, did not report risk of hospitalization to ICU, or general ward, bronchiolitis and pneumonia, did not use PCR or RT-PCR as a diagnostic method, were conducted among patients with underlying chronic diseases or impaired immunostatus, were duplicates of other included studies or had data incompatible with odds ratios calculation, (i.e. with some cells having a zero) were excluded.
Statistical analysis
The exposure of interest was co-infection among eleven respiratory viruses i.e. Flu A/B, RSV, RV, AdV, hMPV, hCoV, hBoV and hPIV1-3. Association between co-infection and severe disease (admission to general ward or ICU, bronchiolitis or pneumonia) was assessed using odds ratios and 95% confidence intervals calculated using single infection(s) as the baseline, or single influenza A or B infection as the baseline, in the analysis of influenza co-infections and severity of respiratory disease. Results from individual studies were summarised using tables and all analyses were done using the Comprehensive Meta-Analysis software – version 2 (BIOSTAT, Englewood, NJ 07631 USA).
Results
Characteristics of the studies included in this review
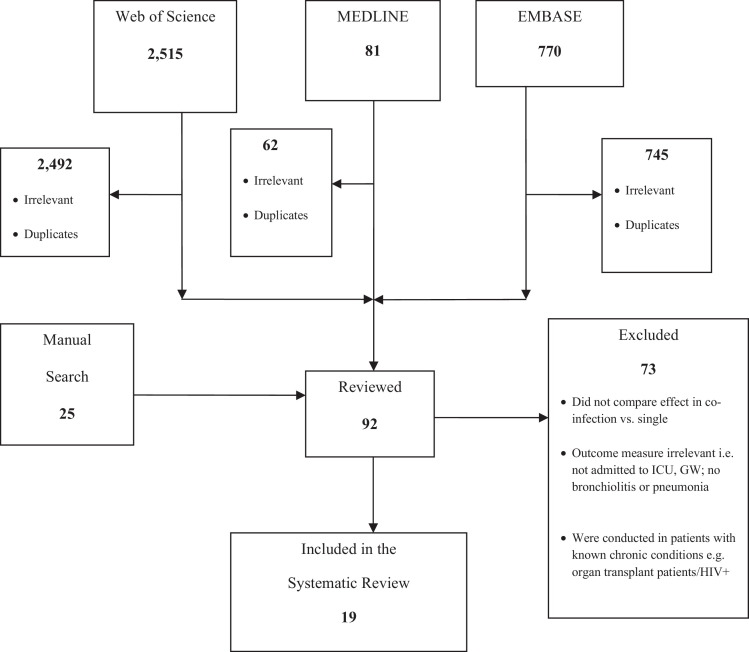
A summary of the number of studies that were retrieved from each database and the studies that were selected and included in this systematic review is provided in Figure 1 . Out of the 3,391 papers identified through electronic and manual search, ninety two (92) papers were reviewed of which 19 were included.
Figure 1.
Number of studies that were identified, included and excluded.
Notes: ICU – intensive care unit, GW - general ward.
Studies included in this review were from all over the world, i.e. 6 of the included studies were from Europe, 5 from North America, 3 from South America, 3 from Asia and 2 from Africa. The details of the included studies are provided in Table 1 . A large number of the studies, 11/19 (57.9%), involved patients hospitalized to a general ward or the intensive care unit with acute respiratory disease, some (6/19; 31.6%) recruited in and outpatients and 2/19 (10.5%) were case-control studies recruiting hospitalized patients and healthy controls. The highest proportion of studies 52.6% (10/19) recruited children <6 years old, 6 (31.6%) studies included children ≤18 years old, 3 (15.9%) included both adults and children. Most of the studies 14/19 (73.7%) applied a prospective design covering periods ranging from 3 months to 4 years, and 5/19 (26.3%) analysed patients data retrospectively. Together all the studies recruited 12,320 people with 48 as the smallest sample size and 4,336 as the largest sample size, the majority recruiting between 200 and 900 patients.
Table 1.
Characteristics of studies included in this review
| No | Study name (Ref No.) Country |
Study design | Age group | Sample size & +ve rate | No & co-infe rate | Protocol & Viruses analysed | Outcome measure of interest |
|---|---|---|---|---|---|---|---|
| 1 | Richard et al., [30] France |
hospitalised GW or ICU with severe bronchiolitis, 2 yrs prospective |
< 1 yr | 180 (96.1) | 44 (25.4) | RT-PCR, PCR & tissue culte. All RVI's’except hBoV | admission to ICU |
| 2 | Cilla et al., [21] Spain |
attended at paediatric emergency dpt, 2 yrs prospective |
<3 yrs | 315 (66.9) | 61 (27.0) | PCR & Direct IF, tissue culture. All RVIs | admission to ICU admission to GW |
| 3 | Huguenin et al., [19] France |
hospitalised to GW or icu with acute bronchiolitis, 1 yr prospective |
< 1 yr | 138 (91.0) | 85 (62.0) | RT-PCR & direct IF assay. All RVI's |
admission to ICU, |
| 4 | Franz et al., [22] Germany |
admitted with LRTI, 2 yrs prospective |
<16 yrs | 404 (78.0) | 127 (34.0) | RT-PCR, All RVI's |
pneumonia |
| 5 | Singleton et al., [28] Alaska USA |
hospitalised & community controls, 2 years prospective |
<3 yrs | 865 (71.2) | 35 (5.7) | RT-PCR, All RVI's except hBoV |
admission to GW bronchiolitis, pneumonia |
| 6 | Drews et al., [25] USA |
outpatients and hospitalised patients, 4 yrs retrospective |
children & adults | 4,336 (30.9) | 67 (5.0) | PCR, ELISA, tissue culture. All RVI's except hBoV | admission to GW |
| 7 | Martin et al., [20] USA |
outpatients and hospitalised 1 yr retrospective |
<4 yrs | 893 (63.0) | 103 (18.0) | RT-PCR All RVI's except hBoV & RV |
admission to ICU, admission to GW |
| 8 | Boivin et al., [23] Canada |
admitted to paediatrics dpt with ARTIs 6 months prospective |
<3 yrs | 259 (61.9) | 23 (14.0) | RT-PCR, All RVI's except hBoV & hCoV |
bronchiolitis pneumonia |
| 9 | Camargo et al., [35] Brazil |
hospitalised to GW or ICU, 3 months prospective |
Children & adults |
159 (65.4) | 15 (14.4) | RT-PCR, All RVIs | admission to ICU |
| 10 | Do et al., [31] Vietnman |
hospitalised to GW & ICU with ARI, 3 yr prospective |
<13 yrs | 309 (72.0) | 62 (20.0) | RT-PCR, All RVI's |
Admission to ICU bronchiolitis pneumonia |
| 11 | Venter et al., [29] South Africa |
outpatients, hospitalized patients and healthy controls, 2 years retrospective |
< 5 yrs | 610 (83.6) | 279(54.7) | RT-PCR and IFA assays All RVI's |
admission to ICU, admission to GW, pneumonia |
| 12 | Sung et al., [42] Taiwan |
admitted with ALRTI, 8 months prospective |
<3 yrs | 48 (70.83) | 8 (23.5) | RT-PCR & direct IF, All RVI's |
pneumonia |
| 13 | O’Çallaghani-Gordo et al., [26] Mozambique |
outpatients, 1 year prospective |
<1 yr | 333 (55.6) | 38 (20.5) | PCR. All RVI's except hBoV & hCoV |
admission to GW |
| 14 | Rhedin et al., [43] Sweden |
admitted to paediatric ward 6 months prospective |
< 17 yrs | 502 (61.6) | 45 (14.6) | RT-PCR All RVIs | admission to ICU |
| 15 | Marcone et al., [27] Argentina |
in and outpatients 2 years prospective |
<6 yrs | 620 (76.8) | 61 (12.8) | RT-PCR & IF All RVI's except hBoV & hCoV |
admission to a GW |
| 16 | Kouni et al., [24] Greece |
in and out patients at emergency dpt 1 year prospective |
<14 yrs | 611 (65.0) | 169 (45.6) | RT-PCR All RVIs |
admission to a GW |
| 17 | Echenique et al., [32] USA |
hospitalised to a GW or ICU 2 months retrospective |
Children & aduts | 1,192 (55.2) | 49 (7.4) | RT-PCR All RVIs |
admission to ICU |
| 18 | Libster et al., [38] Argentina |
hospitalised to a GW or ICU. 3 months prospective |
<18 yrs | 391 (64.2) | 47 (18.7) | RT-PCR FluA, RSV, AdV & PIV1-3 |
admission to ICU |
| 19 | Bicer et al., [44] Turkey |
Hospitalised to GC or ICU, 1 year retrospective |
<9 yrs | 155 (66.5) | 21 (13.5) | RT-PCR All RVIs |
pneumonia |
Notes: RT-PCR – real time polymerase chain reaction, IF – immunofluorescence assay, ICU intensive care unit, GW – general ward, RVIs - respiratory virus infections, hBoV –human bocavirus, CoV – human coronavirus, RSV respiratory syncytial virus, AdV – adenovirus, hPIV1-3 – human parainfluenza virus types 1 to 3.
Factors associated with positivity and co-infection rates
Positivity rates ranged from 30.9% to 96.1% (mean 68.2%) whereas co-infection ranged from 5.0% to 62.0% (mean 23.0%). Respiratory syncytial virus was the most predominant co-infecting virus with most of the studies reporting RSV being the most common among all the viruses involved in the co-infections (Supplementary Table S1). RSV was reported as the most frequent co-infecting with adenovirus by Huguenin et al., [19] and Martin et al., [20] co-infecting with bocavirus by Cilla et al., [21] and Franz et al., [22] and co-infecting with influenza A virus by Boivin et al., [23] and Kouni et al., [24] There was a weak negative correlation between age and high positivity/co-infection rate, such that studies that recruited young children were more likely to report high rates of infection and co-infection (correlation coefficients - 0.56 and 0.35 for infection and co-infection respectively). In studies that recruited both adults and children, the rates of co-infection were 5.0% 7.2% and 14.4%, compared to co-infection rates of 5.7% to 62.0% in studies that recruited children < 6 years old (Table 1).
Co-infection and risk of hospitalisation to a general ward
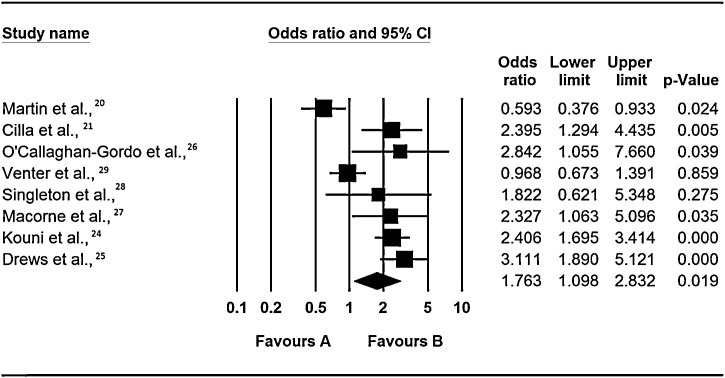
Evidence from the review of the role of co-infections on risk of admission to a general ward is inconclusive as 5 of the 8 included studies (Drews et al., [25], Cilla et al., [21], O’Callaghani-Gordo et al., [26], Marcone et al., [27] and Kouni et al., [24]) found a significant positive association (OR: 3.11, 95% CI: 2.0 – 5.12, p = < 0.0001, OR: 2.40, 95% CI: 1.29 – 4.44, p = 0.005, OR: 2.84, 95% CI: 2.84, p = 0.04, OR: 2.33, 95% CI: 1.10 – 5.10, p = 0.04 and OR: 2.41, 95% CI: 1.70 – 3.41, p = <0.0001), one study (Singleton et al., [28]) found insignificant increase in risk, and 2 studies; Martin et al., [20] and Venter et al., [29] did not (i.e. Martin et al., [20] found co-infection was associated with a significant reduction in risk of hospitalization to a general ward OR: 0.59, 95% CI: 0.38 – 0.93, p = 0.02, whereas Venter et al., [29] also found a reduction in risk, but this was not statistically significant) – Figure 2 .
Figure 2.
Respiratory virus co-infections and risk of admission to a general ward.
Notes: Odds ratios are for occurrence of event (hospitisation to a general ward) in multiple infections vs. single infections as the baseline. The squares represent the estimated odds ratios, the diamond represent their summary, the horizontal lines give their 95% confidence intervals and the size of the squares represent the weight of the study.
Despite Cilla et al., [21] and Venter et al., [29] studying the same viruses, under five years old children, they reported conflicting results (Cilla et al., [21] reporting increase in risk and Venter et al., [29] reporting insignificant slight lowering of risk). The differences in the findings of these two studies could be due to the difference in the study design. Venter et al., [29] studied only a fraction (627 out of 1,702) of patients presenting with respiratory illness had their samples screened by RT-PCR, whereas Cilla et al., [21] screened all patients. Probably, Venter et al., [29] would have arrived at a different result if all patient samples were screened for respiratory viruses.
Similarly, Martin et al., [20] and Drews et al., [25] reported conflicting results despite the two having recruited both in and out patients, studying the same respiratory viruses. However the differences in Drews and Martin's studies could be due to the differences in the size and duration of the studies. Drews et al., [25] summarised findings of epidemiological reports conducted over a 4 year period whereas Martin et al., [20] covered a period of only 1 year or it could be because of the age difference of the study groups.
Lastly, the differences between Singleton et al., [28] and O’Callaghan-Gordo et al., [26] could be due to bias towards the null as there was some difference in the number of viruses studied by the 2 studies O’Callaghan-Gordo [26] did not test for hCoV. Some viral co-infections of low severity influence the estimates of co-infection patterns, this resulted in bias of severe illness towards the null, when crude analysis is applied. Infact it could also be possible that the variations in Cilla et al., [21] and Venter et al., [29] and also between Martin et al., [20] and Drews et al., [25] were also partly due to bias towards the null. The above complexity emphasizes the importance of identifying individual viral agents in influencing the outcome of disease, with and without co-infection.
Co-infection and risk of admission to intensive care unit (ICU)
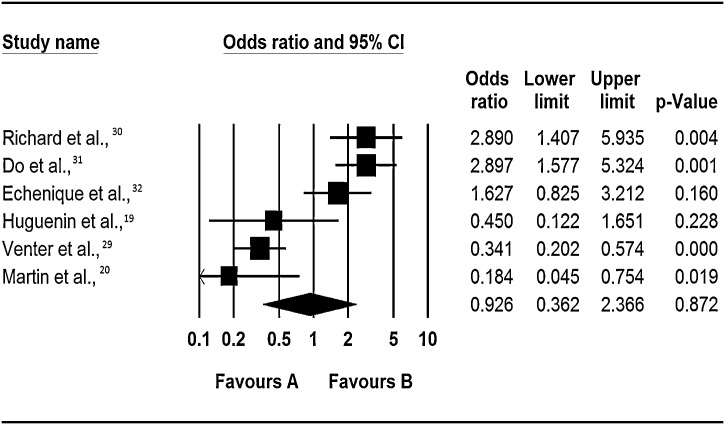
Just as in above section, the evidence from this review on the role of co-infection on risk of admission to the ICU is inconclusive. Two of the six studies that carried out a crude analysis on the effect of co-infection on the risk of admission to the ICU (Richard et al., [30] and Do et al., [31]), found that co-infection significantly increased the risk of admission to the ICU (OR: 2.9 95% CI: 1.4 – 5.9, p = 0.004 and OR: 3.0, 95% CI: 1.6 – 5.6, p = <0.0001), whereas two studies (Martin et al., [20]; Venter et al., [29]) found it significantly reduced this risk (OR: 0.18, 95% CI: 0.05 – 0.75, p = 0.02 and OR: 0.34, 95% CI: 0.20 – 0.57, p = < 0.0001), and two studies; Echenique et al., [32] and Huguenin et al., [33] found insignificant increase and reduction in risk respectively (Figure 3 ). As all the six studies included in this part of the review used RT-PCR for virus identification and studied the same viruses, the differences in their findings could be attributed to the differences in study designs as Martin et al., [20] and Venter et al., [29] recruited both out-patients and hospitalized individuals whereas Richard et al., [30] and Do et al., [31] recruited patients hospitalized with acute respiratory infections. This may have skewed the outcomes in Richard et al., [30] and Do et al., [31] studies towards a more severe outcome.
Figure 3.
Respiratory virus co-infections and risk of admission to the intensive care unit.
Notes: Odds ratios are for occurrence of event (hospitisation to a general ward) in multiple infections vs. single infections as the baseline. The squares represent the estimated odds ratios, the diamond represent their summary, the horizontal lines give their 95% confidence intervals and the size of the squares represent the weight of the study.
Co-infections and risk of developing bronchiolitis or pneumonia
Co-infection and risk of bronchiolitis
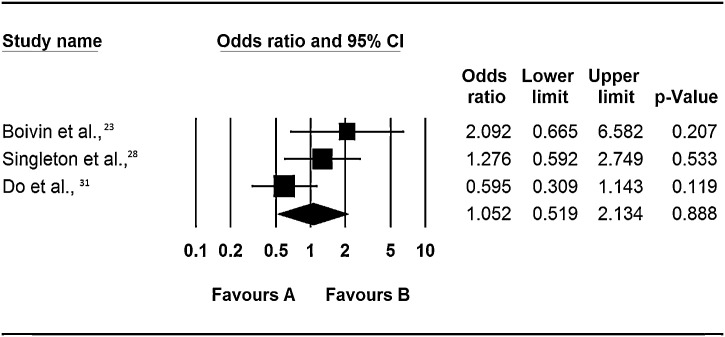
Again the evidence from the systematic review was inconclusive as none of the studies had found a statistically significant association for or against the role of co-infection in increasing the risk of bronchiolitis (Figure 4 ). Two of the three studies included in this analysis recruited hospitalised patients and community based controls whereas one recruited patients admitted to the general ward or ICU. However all the 3 studies used RT-PCR for identification of viruses. The difference in their findings could therefore be either because of the variability in the number and types of viruses they investigated, or due to the age differences of recruited patients. Boivin et al., [23] recruited patients infected with Flu A/B, RSV A/B, AdV, hMPV and PIV1-4, to which Singleton et al., [28] and Do et al., [31] also included hCoV. This observation suggests that in children <3 years, coronaviruses cause disease of different severity than in teens. However our interpretation of this interaction is hampered by the lack of a statistically significant finding in the studies.
Figure 4.
Respiratory virus co-infections and risk of bronchiolitis.
Notes: Odds ratios are for occurrence of event (hospitisation to a general ward) in multiple infections vs. single infections as the baseline. The squares represent the estimated odds ratios, the diamond represent their summary, the horizontal lines give their 95% confidence intervals and the size of the squares represent the weight of the study.
Co-infection and risk of pneumonia
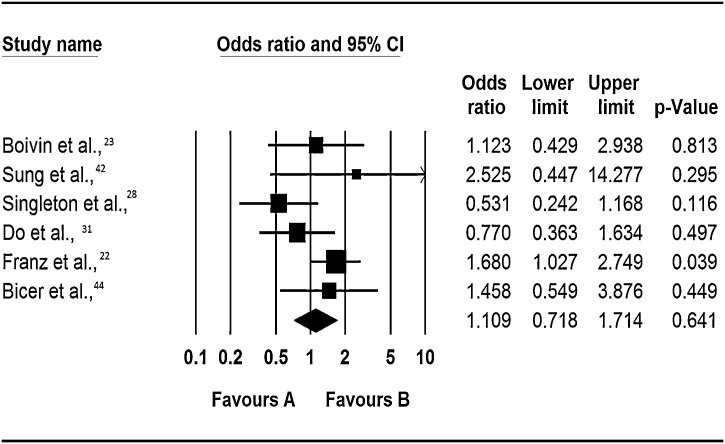
Respiratory viruses have previously been identified as significant causes of community acquired viral pneumonia (Ruuskanen et al., [34]); however the role of co-infection among respiratory viral infections has not been previously explored. In this review no significant association was found between co-infection and the risk of developing pneumonia (Figure 5 ). Specifically, 4 of the 6 included studies found that co-infection increased risk of pneumonia by between 12% to 2.5-fold, but only Franz et al., [22] reported a statistically significant association (OR: 1.68, 95% CI: 1.03 – 2.75, p = 0.04). In the 4 other studies, co-infection was protective, but again the odds ratios were not statistically significant. Similar issues as indicated in the preceding sections i.e. types of viruses studied should be born in mind in interpreting our findings on this subject.
Figure 5.
Respiratory virus co-infections and risk of pneumonia
Notes: Odds ratios are for occurrence of event (hospitisation to a general ward) in multiple infections vs. single infections as the baseline. The squares represent the estimated odds ratios, the diamond represent their summary, the horizontal lines give their 95% confidence intervals and the size of the squares represent the weight of the study.
Influenza virus single and multiple infections and disease severity
Eight studies were included in a review investigating the relationship between single and multiple influenza virus infection and disease outcome (supplementary Table 2). Two of the 7 studies, Venter et al., [29] and Singleton et al., [28] reported on the risk of admission to a general ward; 5 reported on risk of admission to ICU, development of bronchiolitis and pneumonia and 2 each reported on risk of developing bronciolitis and pneumonia. There is insufficient evidence in support of an association or lack of association between influenza A virus and other respiratory viruses’ co-infection and severity of disease outcome. Thus whilst Boivin et al., [23] reported a statistically significant association between co-infection and increased risk of bronchiolitis (OR: 4.69, 95% CI: 1.38 – 15.95, p = 0.01), Singleton et al., [28] found it was protective (OR: 0.43, 95% CI: 0.18 – 0.99, p = 0.05) despite the two studies having recruited children < 3 years old and used RT-PCR for virus identification. On the other hand, Camargo et al., [35] and Singleton et al., [28] found that co-infection was actually protective against admission to ICU, however this was also not statistically significant. There is therefore a need for a larger well designed study investigating the impact of co-infection on the outcome of influenza disease.
Discussion and Conclusion
In conclusion, this review found inconclusive results on the role of co-infections among respiratory viruses on risk of admission to a general ward or the ICU; some studies found co-infection increased the risk yet others did not. We did not find any studies that reported a significant association between co-infections and bronchiolitis and only one study reported a statistically significant association between co-infections and pneumonia.
Some of the studies included in this review were highly heterogeneous and because of this, our interpretation of the results leaned on findings from individual studies. Despite these challenges, it is important to investigate whether co-infection could increase disease severity across the age spectrum or it would only be a burden in children <5 years or the elderly >65 years old (holding other factors constant). The results here are unable to answer this question because, for example, while one of the studies that recruited both adults and children Drews et al., [25] found increased risk of admission to a general ward, the other study that recruited patients of the same age profile Echeniqu et al., [32], did not find a significant risk of admission to the ICU.
Several factors contribute to heterogeneity in the findings of the studies on respiratory virus infections: the types and number of viruses tested and the year and season the study was conducted; the type of confounding factors controlled for e.g. co-morbidities, patients age, gender, immune status,; and the differences in study designs (i.e. whether study recruited both out-patients and hospitalized individuals and the size and duration of the studies); difference in the diagnostic tests that were used.
In this review, severity varied with the type of viruses involved in the co-infection. For example, in studies where RSV/hBoV and Flu A/hCoV co-infections were predominant, a significantly increased risk of admission to a general ward was reported - Cilla et al., [21] and Drews et al., [25], whereas in studies where RSV/AdV and RSV/RV co-infections were predominant, a reduced risk of admission to a general ward was reported - Martin et al., [20], and Venter et al., [29] (Figure 2; Supplementary table S1). The crude analysis adopted by many authors could have introduced bias towards the null i.e. some viral co-infections of low severity influenced the estimates of co-infection patterns towards the null when crude analysis was applied. Future studies should employ stratified analysis on the effect of co-infections on disease outcome where effects of specific pairs of viruses e.g. Flu A/RSV, RSV/hMPV or RSV/AdV are investigated so as to elucidate the type of virus pairs which increase or decrease disease severity. The variations could also be because of the differences in the co-infection patterns because of the differences in types of viruses that circulated in different seasons and years the different studies were conducted; Influenza A viruses, RSV, hMPV and AdV follow seasonal patterns, with higher virus activity in winters and minimal activity in summers, RV circulate all year round whereas hPIV1-4 are predominantly in summer, and the studies included here spanned over different time periods.
Co-infection was negatively associated with age; studies that recruited young children <5 years were likely to report higher co-infection rates than those that recruited teenagers (13 to 18 years old) and young adults. We only included crude odds ratios and this could influence the outcome of our review. The estimated odds ratios might be different if controlled for confounding factors and this should be born in mind when interpreting the results of this review. Indeed there could be other additional factors contributing to a great variation in the frequencies of co-infections reported by different studies included in this review e.g., the differences in the season the studies were conducted, differences in the diagnostic assays (primers used in PCR experiments), and again probably due to differences in study design.
Evidence from other studies indicate that the rate of co-infection is higher when studies recruit hospitalised patients and is lower when they recruit both hospitalised and outpatients or when only outpatients are recruited [16;18;38;39;39-41]. Specifically, the studies that recruited hospitalised patients; Calvo et al., [16], Libster et al., [38], and Aberle et al., [18] reported higher co-infection rates (17%, 19% and 20% respectively) and were more likely to find an association between co-infection and severe outcome. The studies that recruited both hospitalised and outpatients; Laguna-Torres et al., [41], Nisii et al., [40], and Esper et al., [39] reported comparatively lower co-infection rates (3.9%, 6%, and 13.1% respectively) and were more likely to find no association between co-infection and severe disease. Martin et al., [20] and Venter et al., [29] recruited both outpatients and hospitalised individuals whereas Richard et al., [30] and Do et al., [31] recruited patients hospitalised with acute respiratory infections. This may have skewed the outcomes in Richard et al., [30] and Do et al., [31] studies towards a more severe outcome.
As for diagnostic method, the role of polymerase chain reaction (PCR) in giving better sensitivity and specificity than other diagnostic methods was previously discussed by Henrickson [36] and Lee et al., [37]. In this review, only studies that used RT-PCR, PCR were included. If there is any yet unknown systematic error due to application of RT-PCR or PCR, then the effect would be carried over into the results of our study. However, at the present time, PCR remains the gold standard for diagnosis, as some of the respiratory viruses cannot be cultured in laboratories; hence we believe that the results summarized here closely resemble the epidemiological situation.
Literature has suggested that virus-virus interactions may influence host immune response in driving other respiratory viruses’ virulence or a virulence. Respiratory virus proteins are detected by host cell tall like receptors; TL2, TLR4 and TLR6; TLR3 TLR7, TLR8 and TLR9 and by the protein kinase RNA - activated (PKR), the melanoma differentiation associated gene 5 (MAD-5), the retinoic inducible gene I (RIG-I) and the 2’,5’-Oligoadenylate synthetase (2’,5’-OAS1&2) which in turn triggers host production of cytokines including; tumour necrosis factor (TNF), type 1 proinflammatory cytokines; interferon-alpha (IFN-α), and interferon-beta (IFN-β), interleukin-6 (IL-6), interleukin-18 (IL-18) [11], [45], [46], which counteract virus infection. Depending on the type of virus, infection may lead to cytokine storm resulting into severe disease characterised by organ failure. Casalegno et al., [46] and other researcher [47], [48] suggested that rhinoviruses interfered with circulation of other viruses, and some studies [15], [18] indicated that co-infections with rhinoviruses resulted in low risk. However the precise mechanisms in co-infections that may affect virulence are not well understood and more research is needed to understand the biomedical processes in respiratory virus co-infections and the co-infection patterns that may increase or decrease virulence.
The fact that only one study found a significant association between co-infection and risk of pneumonia and no study found significant association between co-infection and bronchiolitis, yet to be admitted patients must have some form of acute lower or upper respiratory disease, merits discussion. It is possible that patients could have presented with different signs and symptoms. The use of proxies for measuring disease severity e.g. hospitalisation or death, other than signs and symptoms, could avoid these problems. It is possible that some of the co-infections indicated by different studies were nosocomial infections, however, in all the included studies, ascertainment of disease status was performed during the time of hospitalisation or during the first consultation, ruling out the possibility of nosocomial infections. Also the possibilities of publication and study selection bias should be born in mind. Conversely, we employed a standard search strategy, making sure that we are able to capture all the possible studies covering the subject under study. The search was performed on MEDLINE, EMBASE and WEB of Science, databases which summarise publication in a wide variety of medical journals. We also manually searched studies of good quality to include in the review and in this way hope to have eliminated any study selection or publication bias.
In conclusion, this review found inconclusive results on the role of co-infections on severity of respiratory disease. Many of the problems in interpretation of the evidence were because the authors adopted crude analysis. Future studies should employ stratified analysis on the effect of co-infections on disease outcome where the effects of specific pairs of viruses e.g. Flu A/RSV, RSV/hMPV or RSV/AdV are studied so as to elucidate the type of virus pairs which increase or decrease disease severity.
Educational Aims
-
•
To inform scientists on the role of co-infection in acute respiratory tract infection (ARI) leading to hospitalization to a general ward or the ICU, bronchiolitis or pneumonia.
-
•
To highlight the problems of confounding and bias when crude analysis is applied and the importance or need of conducting stratified analysis in research on respiratory virus co-infections.
-
•
To present evidence for multiple testing of respiratory virus infections in patients presenting with influenza like illness.
Funding
This work had no funding.
Conflict of interest
All authors, no conflict of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prrv.2013.11.001.
Appendix A. Supplementary data
References
- 1.von E.E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 2.Allander T. Human bocavirus. J Clin Virol. 2008;41(1):29–33. doi: 10.1016/j.jcv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Chanock R., Chambon L., Chang W., Goncalves F.F., Gharpure P., Grant L. WHO respiratory disease survey in children: a serological study. Bull World Health Organ. 1967;37(3):363–369. [PMC free article] [PubMed] [Google Scholar]
- 4.Pyrc K., Berkhout B., van der Hoek L. Identification of new human coronaviruses. Expert Rev Anti Infect Ther. 2007;5(2):245–253. doi: 10.1586/14787210.5.2.245. [DOI] [PubMed] [Google Scholar]
- 5.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de G.R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monto A.S., Sullivan K.M. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110(1):145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hak E., Rovers M.M., Kuyvenhoven M.M., Schellevis F.G., Verheij T.J. Incidence of GP-diagnosed respiratory tract infections according to age, gender and high-risk co-morbidity: the Second Dutch National Survey of General Practice. Fam Pract. 2006;23(3):291–294. doi: 10.1093/fampra/cmi121. [DOI] [PubMed] [Google Scholar]
- 8.Fleming D.M., Zambon M., Bartelds A.I. Population estimates of persons presenting to general practitioners with influenza-like illness, 1987-96: a study of the demography of influenza-like illness in sentinel practice networks in England and Wales, and in The Netherlands. Epidemiol Infect. 2000;124(2):245–253. doi: 10.1017/s0950268899003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glezen W.P., Decker M., Joseph S.W., Mercready R.G., Jr. Acute respiratory disease associated with influenza epidemics in Houston, 1981-1983. J Infect Dis. 1987;155(6):1119–1126. doi: 10.1093/infdis/155.6.1119. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y., Zhang R.F., Xie Z.P., Yan K.L., Gao H.C., Song J.R. Newly identified respiratory viruses associated with acute lower respiratory tract infections in children in Lanzou, China, from 2006 to 2009. Clin Microbiol Infect. 2012;18(1):74–80. doi: 10.1111/j.1469-0691.2011.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham N.M. The epidemiology of acute respiratory infections in children and adults: a global perspective. Epidemiol Rev. 1990;12:149–178. doi: 10.1093/oxfordjournals.epirev.a036050. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation. Acute Respiratory Infections (Update September 2009). Available at http://www.who.int/vaccine_research/diseases/ari/en/index1.html. Accessed on 12 October 2011.
- 14.Health Protection Agencu, UK. Surveillance of influenza and other respiratory viruses in the UK, 2010-2011 report. Available at http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1296687414154. Accessed on 1 August 2011.
- 15.Esper F.P., Spahlinger T., Zhou L. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. Journal of Infection. 2011;63(4):260–266. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo C., Garcia-Garcia M.L., Blanco C., Vazquez M.C., Frias M.E., Perez-Brena P. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42(3):268–272. doi: 10.1016/j.jcv.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. Journal of Infectious Diseases. 2005;191(3):382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberle J.H., Aberle S.W., Pracher E., Hutter H.P., Kundi M., Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatric Infectious Disease Journal. 2005;24(7):605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 19.Huguenin A., Moutte L., Renois F., Leveque N., Talmud D., Abely M. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol. 2012;84(6):979–985. doi: 10.1002/jmv.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin E.T., Kuypers J., Wald A., Englund J.A. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza and Other Respiratory Viruses. 2011;6(1):71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cilla G., Onate E., Perez-Yarza E.G., Montes M., Vicente D., Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J Med Virol. 2008;80(10):1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franz A., Adams O., Willems R., Bonzel L., Neuhausen N., Schweizer-Krantz S. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48(4):239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boivin G., De S.G., Cote S., Gilca R., Abed Y., Rochette L. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9(6):634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouni S., Karakitsos P., Chranioti A., Theodoridou M., Chrousos G., Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013;19(8):772–777. doi: 10.1111/1469-0691.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drews A.L., Atmar R.L., Glezen W.P., Baxter B.D., Piedra P.A., Greenberg S.B. Dual respiratory virus infections. Clinical Infectious Diseases. 1997;25(6):1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Callaghan-Gordo C., Diez-Padrisa N., Abacassamo F., Perez-Brena P., Casas I., Alonso P.L. Viral acute respiratory infections among infants visited in a rural hospital of southern Mozambique. Trop Med Int Health. 2011 Sep;16(9):1054–1060. doi: 10.1111/j.1365-3156.2011.02811.x. [DOI] [PubMed] [Google Scholar]
- 27.Marcone D.N., Ellis A., Videla C., Ekstrom J., Ricarte C., Carballal G. Viral etiology of acute respiratory infections in hospitalized and outpatient children in Buenos Aires, Argentina. Pediatr Infect Dis J. 2013 Mar;32(3):e105–e110. doi: 10.1097/INF.0b013e31827cd06f. [DOI] [PubMed] [Google Scholar]
- 28.Singleton R.J., Bulkow L.R., Miernyk K., DeByle C., Pruitt L., Hummel K.B. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82(7):1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venter M., Lassauniere R., Kresfelder T.L., Westerberg Y., Visser A. Contribution of common and recently described respiratory viruses to annual hospitalizations in children in South Africa. J Med Virol. 2011;83(8):1458–1468. doi: 10.1002/jmv.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard N., Komurian-Pradel F., Javouhey E., Perret M., Rajoharison A., Bagnaud A. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatric Infectious Disease Journal. 2008;27(3):213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 31.Do A.H.L., van Doorn H.R., Nghiem M.N., Bryant J.E., Hoang THt, Do Q.H. Viral Etiologies of Acute Respiratory Infections among Hospitalized Vietnamese Children in Ho Chi Minh City, 2004GÇô2008. PLoS ONE. 2011;6(3):e18176. doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echenique I.A., Chan P.A., Chapin K.C., Andrea S.B., Fava J.L., Mermel L.A. Clinical characteristics and outcomes in hospitalized patients with respiratory viral co-infection during the 2009 H1N1 influenza pandemic. PLoS ONE. 2013;8(4):e60845. doi: 10.1371/journal.pone.0060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huguenin A., Moutte L., Renois F., Leveque N., Talmud D., Abely M. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. Journal of Medical Virology. 2012;84(6):979–985. doi: 10.1002/jmv.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camargo C., Guatura S.B., Bellei N. Respiratory viral coinfection among hospitalized patients with H1N1 2009 during the first pandemic wave in Brazil. Braz J Infect Dis. 2012;16(2):180–183. doi: 10.1016/S1413-8670(12)70302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henrickson K.J. Advances in the laboratory diagnosis of viral respiratory disease. Pediatric Infectious Disease Journal. 2004;23(1 Suppl) doi: 10.1097/01.inf.0000108187.63151.ea. Suppl-10. [DOI] [PubMed] [Google Scholar]
- 37.Lee M.S., Walker R.E., Mendelman P.M. Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials. Hum Vaccin. 2005;1(1):6–11. doi: 10.4161/hv.1.1.1424. [DOI] [PubMed] [Google Scholar]
- 38.Libster R., Bugna J., Coviello S., Hijano D.R., Dunaiewsky M., Reynoso N. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362(1):45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 39.Esper F.P., Spahlinger T., Zhou L. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. J Infect. 2011;63(4):260–266. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nisii C., Meschi S., Selleri M., Bordi L., Castilletti C., Valli M.B. Frequency of Detection of Upper Respiratory Tract Viruses in Patients Tested for Pandemic H1N1/09 Viral Infection. Journal of Clinical Microbiology. 2010;48(9):3383–3385. doi: 10.1128/JCM.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laguna-Torres V.A., Sanchez-Largaespada J.F., Lorenzana I., Forshey B., Aguilar P., Jimenez M. Influenza and other respiratory viruses in three Central American countries. Influenza Other Respi Viruses. 2011;5(2):123–134. doi: 10.1111/j.1750-2659.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung C.C., Chi H., Chiu N.C., Huang D.T., Weng L.C., Wang N.Y. Viral etiology of acute lower respiratory tract infections in hospitalized young children in Northern Taiwan. J Microbiol Immunol Infect. 2011;44(3):184–190. doi: 10.1016/j.jmii.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhedin S., Hamrin J., Naucler P., Bennet R., Rotzen-Ostlund M., Farnert A. Respiratory viruses in hospitalized children with influenza-like illness during the H1n1 2009 pandemic in Sweden. PLoS ONE. 2012;7(12):e51491. doi: 10.1371/journal.pone.0051491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bicer S., Giray T., Col D., Erdag G.C., Vitrinel A., Gurol Y. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013;39:22. doi: 10.1186/1824-7288-39-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biron C.A., Sen G.C. Innate Immune Responses to Viral Infections. In: Knipe D.M., Griffin D.E., Lamb R.A., Straus S.E., Howley P.M., Martin M.A., editors. Fields Virology. 5 ed. Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 249–278. [Google Scholar]
- 46.Casalegno J.S., Ottmann M., Duchamp M.B., Escuret V., Billaud G., Frobert E. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clinical Microbiology & Infection. 2010 Apr;16(4):326–329. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 47.Greer R.M., McErlean P., Arden K.E., Faux C.E., Nitsche A., Lambert S.B. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45(1):10–15. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linde A, Rotzen-Ostlund M, Zweygberg-Wirgart B, Rubinova S, Brytting M. Does viral interference affect spread of influenza? Euro Surveillance: Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin 2009; 14(40). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.