Abstract
From 2005 to 2013, 17 ferrets were diagnosed with mycobacteriosis at the authors’ practices. Clinical findings included ocular, respiratory, and digestive abnormalities. Diagnosis was based on histopathology, specific histochemical stains, and/or on polymerase chain reaction. All bacteria identified belonged to the nontuberculous Mycobacterium complex. Several treatment protocols were attempted, frequently based on the use of enrofloxacin. In all, 3 ferrets were considered cured. Mycobacteriosis in ferrets is a polymorphous disease with diverse clinical presentations. It is also likely an underdiagnosed disease in pet ferrets, which appear to be particularly susceptible to environmental sources. Mycobacteriosis should be included in the differential diagnosis for ocular, respiratory, and gastrointestinal diseases; in particular, it should be differentiated from systemic coronavirus infection.
Key words: Ferret, Mustela putorius furo, mycobacteriosis, Mycobacterium, pyogranulomatous
Mycobacteria are Gram-positive, aerobic, acid-fast, nonsporulating, facultative intracellular, rod-shaped bacilli that were first described in the 1880s. The genus Mycobacterium comprises more than 70 species.1 Several nomenclatures exist for mycobacteria. They can be classified into 2 groups: the Mycobacterium tuberculosis complex and the group of nontuberculous Mycobacteria (NTM). The nontuberculous Mycobacterium spp. includes Mycobacterium avium, Mycobacterium marinum, Mycobacterium ulcerans, Mycobacterium lepraemurium, and atypical mycobacteria. Nontuberculous Mycobacterium spp. are found in many places, including soil and water, and can infect humans and animals from the environment. Another nomenclature separates mycobacteria into a rapidly growing group (e.g., Mycobacterium fortuitum); a slow-growing group separated into tuberculous mycobacteria, mycobacteria of the M. avium complex, and atypical mycobacteria; and a third group consisting of bacteria with complex, difficult growth requirements, such as Mycobacterium leprae.2, 3, 4
Mycobacteriosis in ferrets was reported as early as 1953.5 However, before 2000, almost all reports of mycobacterial infection in ferrets originated from New Zealand where feral ferrets were infected with Mycobacterium bovis.6, 7, 8 Other descriptions of mycobacteriosis in ferrets have consisted of sporadic case reports of 1 or 2 cases in Europe, Australia, and the United States ( Table 1).9, 10, 11, 12, 13, 14 Infections reported in ferrets include M. bovis and M. avium (mostly in ferrets in New Zealand)15 and Mycobacterium microti, Mycobacterium triplex, M. fortuitum, Mycobacterium florentinum, Mycobacterium interjectum, Mycobacterium septicum, Mycobacterium chelonae, Mycobacterium celatum, and Mycobacterium intracellulare.9, 15, 16
TABLE 1.
Details of cases of mycobacteriosis previously diagnosed in ferrets
| Country | Age, y | Sex | Complaints/Findings | Species | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Italy9 | 5 | NM | Splenitis | M. celatum | Enrofloxacin, rifampicin, and azithromycin | Improvement for 3 mo, died after treatment discontinued, cause of death was uncertain |
| Australia11 | 5 | NM | Eyelid lesion and peripheral lymph node enlargement | M. genavense | Rifampicin, clofazimine, clarithromycin, and chloramphenicol ointment locally | Death after 10 mo, cause was uncertain |
| Australia11 | 4 | F | Eyelid lesion and subcutaneous nasal lesions | M. genavense | Rifampicin | Improvement, treated for 2 mo, died 2 mo later, possible ovarian neoplasia |
| Australia10 | 2 | NF | Weight loss, cough | M. abscessus | Clarithromycin | Treated for 3 mo, discontinued, then treated for 6 mo; alive at time of publication |
| Australia10 | 1½ | NF | Weight loss, cough | M. abscessus | Clarithromycin | Treated for 6 mo then discontinued; alive at time of publication |
| Norway12 | 4 | M | Weight loss and coughing | M. celatum | No treatment | Euthanized |
| USA13 | 6 | NM | Anorexia, vomiting and diarrhea | M. avium | No treatment | Death due to mycobacterial infection after 10 mo |
| USA14 | 6 | NM | Weight loss | M. avium | No treatment | Euthanized |
F, female; M, male; NF, neutered female; NM, neutered male.
Human infection with NTM is often linked with other diseases or an immunocompromised state.9, 17, 18 Pathogenicity of NTM in many animals appears to be related to species susceptibility, immune system, the number and virulence of the bacteria, and the means of contamination. Moreover, NTM have also been found in clinically normal animals.
Although person-to-person infection with NTM has not been described in humans, animal-to-animal contamination has been described, specifically in fish.19, 20, 21 Ferret-to-ferret contamination appears unlikely other than through fighting or cannibalism (rare in domestic ferrets).6, 7 In a case report, 2 infected ferrets lived in the same household but a common source of contagion from the environment was considered more probable than ferret-to-ferret transmission.10 Contamination from food sources might be possible in ferrets that are fed whole prey.
Several methods are used to diagnose mycobacteriosis and include culture, polymerase chain reaction (PCR), and histopathology. Culture on solid media has traditionally been used to isolate and identify mycobacteria, but most species are difficult and slow growing.11, 22 Some mycobacteria species may also be challenging to identify because of difficulties in differentiating these bacteria from closely related mycobacterial species or from species belonging to genera Corynebacterium, Nocardia, and Rhodococcus.23 PCR testing is now commonly used to diagnose mycobacterial infection and appears to be a highly reliable diagnostic tool.23 Mycobacteria are acid-fast because of a complex lipid-rich cell wall and a thick mycolic acid layer. In most cases, histopathology demonstrates readily identified, acid-fast intracellular organisms with Ziehl-Neelsen or Fite-Faraco (modified Ziehl-Neelsen) staining.
Treatment of mycobacteriosis is challenging because antibiotics must be adapted to the intracellular particularities of mycobacteria. Several treatments have been described in humans and in ferrets. Question of treatment raises the important issues of zoonotic potential of the mycobacteria identified and the ethics of using antituberculous drugs intended for humans.24, 25, 26
Few cases of mycobacteriosis have been described in domestic ferrets, with published works primarily being case reports involving 1 or 2 animals (Table 1). This article is the first retrospective study in which a substantial number (17 cases) of ferret mycobacteriosis cases have been assessed.
Materials and Methods
All ferret cases reviewed in this article were presented to the private practices of both authors between 2005 and 2013. Cases were selected based on either consistent clinical findings (e.g., eyelid edema and respiratory signs) and positive PCR or consistent histopathology with identification of acid-fast bacilli on microscopic examination.
PCR was performed on biopsy samples, conjunctival swabs, feces and rectal swabs, or blood. The PCR technique used was based on DNA extraction (digestion bands Bste II and Hae III) followed by qualitative PCR and the restriction fragment length polymorphism technique. The results were interpreted with the aid of the http://app.chuv.ch/pls/pranet/consultation_pkg.resultat_recherche website.
Histopathology was performed on biopsy samples and systematic staining (Ziehl-Neelsen or Fite-Faraco) was used on the collected tissue samples. In suspected cases with consistent histopathology, but without demonstration of acid-fast bacilli, immunohistochemistry was used to exclude cases of systemic coronavirus, which can produce similar lesions. Several cases were excluded from this study by immunohistochemistry.
Results
Results are tabulated in Table 2.
TABLE 2.
Summary of the details of 17 cases of mycobacteriosis in ferrets
| Case | Sex | Age at Time of Diagnosis | Presentation/Exam Findings | Gross Lesions | Body Condition | Pathology | Staining | PCR | Treatment | Cause of Death | Time Between Diagnosis and Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NF | 4 y | Eyelid edema and coughing | Bilateral eyelid edema | Ok | Pyogranulomatous conjunctivitis | ZN+ | Eyelid swab was positive (moderate) | Amoxicillin/clavulanic acid PO and steroid and antibiotic ophthalmic ointment | NR | 5 mo |
| 2 | NF | 2½ y | Eyelid abscess | Unilateral eyelid abscess | Ok | NP | Abscess swab was positive (strongly); M. chelonae | Enrofloxacin PO and chloramphenicol ophthalmic ointment | NR, considered asymptomatic after 3 mo, treatment stopped after 2 y | 3½ y | |
| 3 | NM | 5½ y | Eyelid edema | Bilateral third eyelid edema | Ok | Granulomatous conjunctivitis | ZN+ | Eyelid swab was negative | Enrofloxacin PO | Probably NR | 3 mo |
| 4 | NM | 11 mo | Chronic weight loss palpable abdominal masses | Enlarged mesenteric and hepatic lymph nodes | Thin | NP | Lymph node biopsy was positive (moderate); M. vanbaalenii | Enrofloxacin PO | Mycobacteriosis | 10 d | |
| 5 | NM | 4½ y | Dyspnea and thickened stomach | Thickened stomach and pleural effusion pulmonary lesions | Thin | NP | Blood analysis was positive (moderate); M. canariense | Enrofloxacin PO and monolaurin PO | Mycobacteriosis | 3 mo | |
| 6 | NF | 5 y 3 mo | Eyelid edema and upper respiratory noise | Bilateral third eyelids edema | Ok | NP | Eyelid swab was positive (strongly) | Enrofloxacin PO and monolaurin PO | Probably NR | 2 mo | |
| 7 | NF | 5 y | Discovered during insulinoma laparotomy | Intestinal nodules | Ok | Granulomatous infiltration of the Peyer patches | ZN+ | Feces and rectal swab were positive (moderate); M. shimoidei | Enrofloxacin PO | NR | 1 y 1 mo |
| 8 | NF | 4 y | Thickened stomach, unilateral lower eyelid edema, and mandibular lymph node enlargement | Eyelid edema and mandibular lymph node hypertrophy | Ok | Granulomatous gastritis and lymphadenitis | ZN+ on both | Feces and rectal swab were positive (moderate); M. aurum | Enrofloxacin PO | Mycobacteriosis, enrofloxacin stopped after 1 y because of vomiting, death 9 mo later | 1½ y |
| 9 | NM | 1½ y | Severe upper respiratory noise | None | Ok | NP | Blood analysis was positive (strongly); M. vaccae | Enrofloxacin PO and monolaurin PO | NR, asymptomatic after 1 mo, treatment stopped after 1½ y | 3½ y | |
| 10 | M | 2 y 10 mo | Unilateral upper eyelid edema | Unilateral upper eyelid edema | Thin | Severe granulomatous conjunctivitis highly consistent with mycobacteriosis | ZN− | Eyelid swab was positive (weakly); not identifiable | Enrofloxacin PO and monolaurin PO | Alive, asymptomatic after 1 mo, treatment stopped after 1 y | |
| 11 | NM | 1 y 8 mo | Weight loss, vomiting, and palpable abdominal masses | Enlarged abdominal lymph nodes | Very thin | NP | Lymph node biopsy was positive (strongly) | Enrofloxacin PO | Mycobacteriosis | 1 mo | |
| 12 | NF | 3½ y | Chronic severe upper respiratory noise and sneezing, weight loss, and head deformation | Pulmonary lesions and sinusal mass | Very thin | Granulomatous pneumonia, liver and lymph nodes | FF+ | NP | Enrofloxacin PO and monolaurin PO | Mycobacteriosis | 4 mo |
| 13 | NM | 4 y 10 mo | Bilateral eyelid edema | Bilateral eyelid edema | Ok | NP | Eyelid swab was positive (moderate); M. cosmeticum | Amoxicillin/clavulanic acid PO | Probably NR | 2 wk | |
| 14 | NF | 4½ y | Bilateral eyelid edema | Bilateral eyelid edema | Very thin | NP | Eyelid swab was positive (moderate); M. gordonae | Enrofloxacin PO | Mycobacteriosis | 6 mo | |
| 15 | NM | 6½ y | Dyspnea and severe upper respiratory noise | Pulmonary lesions | Very thin | NP | Blood was positive (moderate); M. gordonae | Enrofloxacin PO and monolaurin PO | Probably NR | 4 mo | |
| 16 | FN | 5½ y | Diarrhea and lethargy and stomach thickening | Endoscopy: stomach mass | Thin | Severe granulomatous gastritis and lymphadenitis, ZN+ spleen | ZN+ | Stomach biopsy was positive (strongly); M. montefiorense | None | Mycobacteriosis | 2 wk |
| 17 | MN | 5½ y | Gingival mass | Gingival mass | Thin | Severe granulomatous gingivitis | ZN+ | Positive (weakly); not identifiable | Amoxicillin/clavulanic acid and microsolone PO then enrofloxacin PO after diagnosis | Alive | 3½ mo |
F, female; M, male; FF+, Fite-Faraco positive; NF, neutered female; NM, neutered male; NP, not performed; NR, not related to mycobacteriosis; PO, per orally; ZN+, Ziehl-Neelsen positive.
The mean age of the ferrets was approximately 4 years but ranged from 11 months to 6.5 years. Only 1 ferret was younger than 1 year (5.88%), 8 ferrets were aged between 1 and 4.5 years (47.06%), and 8 ferrets were older than 4.5 years (46.06%). Age was reported at the time of actual diagnosis and not time of onset of clinical disease signs. Of the 17 ferrets, 8 were female and 9 male. Only 1 animal was intact (male) and all the others had been neutered, surgically or with implantation of deslorelin acetate (Suprelorin; Virbac France, Carros, France). Almost all ferrets had been purchased from pet shops (94.1%). One (5%) was obtained from a private breeder. Each ferret had a different owner.
The following 3 different groups of lesions were identified: eyelid, respiratory (upper or lower), and digestive. Eyelid lesions included unilateral or bilateral swelling or nictitating membrane, without conjunctival or corneal lesions ( Fig. 1). Patient history included topical ophthalmic treatment failure, with both antibiotic and steroid therapy. In the group with respiratory signs, both upper and lower clinical disease signs were observed. Upper respiratory disease signs included sneezing and increased upper respiratory sounds. Pulmonary signs included dyspnea and coughing. Thoracic radiographic images revealed pleural effusion and patchy pulmonary alveolar densities. In 1 case, lesions were found both in the lungs and in the nasal cavity ( FIGURE 2, FIGURE 3, FIGURE 4). Digestive tract lesions were identified in the oral cavity, stomach ( Fig. 5), intestine ( Fig. 6), liver ( Fig. 7), spleen, a mandibular lymph node, and the mesenteric and hepatic lymph nodes ( Fig. 8). Of the 17 ferrets, 10 (58.82%) presented with lesions identified at only 1 site and 7 (41.18%) presented with lesions in multiple sites. Of the ferrets with lesions identified at only 1 anatomic location, 5 presented with eyelid edema. Complete blood count results were available for 7 animals, but no significant abnormalities typically associated with mycobacteriosis in other animals (e.g., leukocytosis, neutrophilia, and monocytosis) were identified. Similarly, no significant abnormalities were noted in the biochemical profile of the animals tested (5 animals).
FIGURE 1.

Case 1: Edema of eyelids and third eyelid in a ferret with mycobacteriosis. In this case, edema was severe.
FIGURE 2.

Case 12: Macroscopic aspect of the lung from a ferret with mycobacteriosis at necropsy. The multiple granulomatous areas (arrows) should be noted.
FIGURE 3.

Case 12: Aspect of the head of a ferret with mycobacteriosis. Significant deformation of the sinuses should be noted. Necropsy showed complete granulomatous filling of the sinuses with partial destruction of the bone.
FIGURE 4.

Case 12: Right lateral thoracic radiograph of a ferret with mycobacteriosis. Areas of increased lobular density in the caudal lung field and mediastinal enlargement cranial to the heart should be noted.
FIGURE 5.

Case 16: Endoscopic view of the mucosa of the stomach in a ferret with mycobacteriosis. Localized thickening and edema of the mucosa (arrows) should be noted. Biopsies allowed diagnosis of mycobacteriosis through histopathology.
FIGURE 6.

Case 7: Thickened and nodular portion of the small intestine (arrow) discovered during surgery for insulinoma in a ferret. Histopathological examination revealed granulomatous infiltration of the Peyer patches.
FIGURE 7.
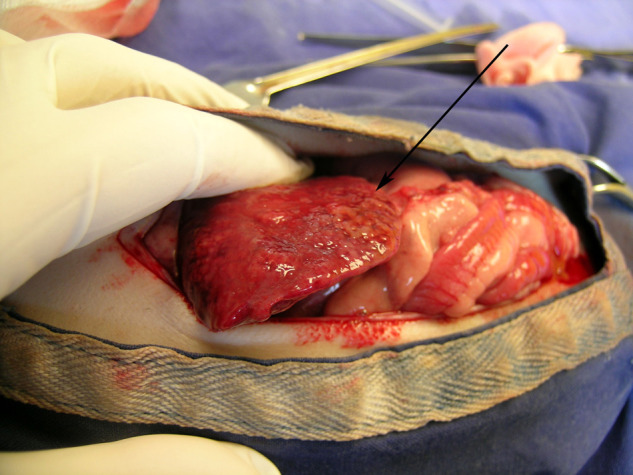
Case 12: Gross appearance of the liver of a ferret with mycobacteriosis at necropsy. The liver is irregular and granulomatous (arrow).
FIGURE 8.

Case 4: Enlarged mesenteric lymph node (arrow) in a ferret with mycobacteriosis. The “cauliflower” feature of this lymph node is typical of pyogranulomatous lesions but is nonspecific for either mycobacteriosis or systemic coronavirus.
PCR was performed on 16 of the 17 ferrets. In the single case where PCR was not performed, diagnosis was made postmortem via histopathology. Of the 16 PCR tests, 15 had positive results, ranging from weakly to strongly positive. The bacterium was further identified in 11 cases. In 2 cases, the owners declined speciation owing to cost. Information for 1 case was unavailable at the time of publication. In 1 case, identification was not possible because of an insufficient amount of DNA retrieved. All the bacteria identified were atypical mycobacteria, and included M. chelonae, Mycobacterium vanbaalenii, Mycobacterium canariense, Mycobacterium shimoidei, Mycobacterium aurum, Mycobacterium vaccae, Mycobacterium gordonae, Mycobacterium cosmeticum, and Mycobacterium montefiorense ( Table 3). M. gordonae and M. cosmeticum were identified twice.
TABLE 3.
Identification of mycobacterial organisms identified in 17 cases in pet ferrets
| Case 1 | Data lost at time of writing |
| Case 2 | M. chelonae |
| Case 3 | Negative |
| Case 4 | M. vanbaalenii |
| Case 5 | M. canariense |
| Case 6 | Testing refused by owner |
| Case 7 | M. shimoidei |
| Case 8 | M. aurum |
| Case 9 | M. vaccae |
| Case 10 | Negative, not enough DNA |
| Case 11 | Testing refused by owner |
| Case 12 | Not performed (postmortem diagnosis) |
| Case 13 | M. cosmeticum |
| Case 14 | M. gordonae |
| Case 15 | M. gordonae |
| Case 16 | M. montefiorense |
| Case 17 | M. cosmeticum |
Histopathology was performed on samples from 8 cases. Lesions were granulomatous or pyogranulomatous in all cases in which histopathology was used. In all, 7 cases were tested for acid-fast bacilli via Ziehl-Neelsen staining (6 were positive and 1 negative) and 1 case was tested via Fite-Faraco staining. Histopathology was not performed in 9 cases. In 4 of the cases where submitted samples were stained, eyelid lesions highly consistent with mycobacteriosis were present, and the owners also elected to have PCR testing performed. In 2 cases, there was no observable disease lesion that could be collected for histopathological examination. In 1 case, the owner declined surgery. In 2 cases, the excision of a lesion was performed, but owners refused histopathological examination owing to the cost of testing.
After identification of the bacteria, all owners were informed of the minimal, but potential zoonotic risk, given that all the mycobacteria identified were atypical. None of the owners opted for euthanasia. Regarding treatment, 2 ferrets were treated with an amoxicillin/clavulanic acid combination at a dose of 12.5 mg/kg, orally every 12 hours (Synulox gouttes; Zoetis France, Paris, France), whereas all the others were treated with enrofloxacin at a dose of 10 mg/kg, orally every 12 hours (Baytril injectable 5%; Bayer Santé Division Animale, Puteaux, France).
Of the 17 ferrets, 3 (17.65%) were considered cured (more than 1 year posttreatment without reoccurrence of clinical disease signs). Of these 3 ferrets, 1 presented with conjunctival edema, 1 with a conjunctival abscess, and 1 with upper respiratory tract signs. There was 1 ferret that was still undergoing treatment at the time of the submission of this article. Of the ferrets included in this study, 14 died (82.35%). Of the 14 ferret deaths, 7 were attributed to mycobacteriosis as no other cause of death was determined, and 7 ferrets (50%) died of disease conditions thought to be unrelated to mycobacteriosis, including 1 that died of insulinoma more than 1 year after diagnosis. Survival of the ferrets in this retrospective clinical review ranged from 10 days to 3.5 years after diagnosis. Mean survival time after diagnosis was 9.4 months.
Source of exposure for all ferrets is unknown, but the authors have speculated as to the risk factors involved. Some of the ferrets were taken outdoors by their owners, but most lived exclusively indoors. Food sources varied. Some ferrets ate chicks and some ate home-prepared meals, whereas most were fed dry kibble only (ferret, cat, or kitten). For ferrets living in groups, no companion ferrets were diagnosed with mycobacteriosis and no owners reported human infection.
Discussion
Few cases of mycobacteriosis have been described in domestic ferrets (Table 1). This article is the first retrospective study of mycobacteriosis in ferrets that involves a substantial number of cases (17). The results of this retrospective clinical study suggest no age or sex predisposition. The low number of affected younger ferrets is likely because of the chronic nature of mycobacterial infections, as mycobacteriosis is a slow progressing disease (several months to several years).10 In the cases described in this report, the time between diagnosis and death was often less than 6 months, but most of the animals had a history of disease signs of months to years before the initial clinic presentation.
There were 3 different groups of lesions identified: those confined to the eyelids, the upper or lower respiratory tract, and/or the gastrointestinal tract. A total of 9 ferrets (56.25%) presented with only 1 lesion site and 7 ferrets (43.75%) presented with multiple lesions sites. The 5 ferrets with a single lesion site were presented for eyelid edema. Complete necropsies were not performed on all ferrets; therefore, the exact extent of lesions in these animals is unknown.
Although immunosuppression is linked with expression of disease in humans and other animals, in our cases and other reported cases in ferrets, no severe concomitant disease or immunosuppressive condition was identified.9, 11 This supports speculation that ferrets may have a natural susceptibility to mycobacterial infection. Source of infection for the ferrets in this report is unknown. Complete history was not available for every animal. In this case series, 5 ferrets were known to live with other ferrets. None of the contact ferrets displayed clinical disease signs that were potentially associated with mycobacteriosis, and some were followed up for many years after diagnosis. Ferret-to-ferret contamination therefore seems unlikely. Furthermore, it is interesting that none of the owners reported mycobacterial infection. In our cases, contamination probably occurred through environmental sources.
In publications on feral ferrets diagnosed with mycobacteriosis, the primary route of infection appears to be oral. This was the likely route of infection in our cases with gastrointestinal lesions. For the ferrets in this report observed with respiratory and eyelid lesions, the principle route of infection was likely by inhalation or contact with aerosolized particles.
In cases in which PCR was attempted, 14 of 15 showed positive results. A negative result was obtained for 1 case, but the histopathological results were conclusive for mycobacteriosis. A possible explanation for this negative result was that the sample was obtained by swabbing the conjunctiva and likely contained too few bacteria. In case 4, the initial PCR on a collected conjunctival swab showed negative results, but a second PCR on biopsy samples of the conjunctiva showed positive results and led to the identification of the mycobacteria. The 9 bacteria identified were atypical mycobacteria, and had not been previously described in ferrets. Similar to findings in other published case reports of ferrets diagnosed with mycobacteriosis, no significant changes were found in hematologic or biochemical analysis.9 When performed on granulomatous or pyogranulomatous lesions, histopathology was consistently effective in disease diagnosis. All but 1 case contained demonstrable acid-fast bacilli. In the case where Ziehl-Neelsen staining showed negative results, the pathologist found the lesions highly consistent with mycobacteriosis, and the PCR result was positive, allowing the diagnosis to be confirmed.
It should be noted that systemic coronavirus can also produce granulomatous or pyogranulomatous lesions.27, 28 Immunohistochemistry for coronavirus antigen can help confirm the diagnosis of ferret systemic coronavirus and exclude mycobacteriosis from the disease differential list of the patient. In our study, several cases were excluded based on positive coronavirus immunohistochemistry.
Based on our cases, diagnosis of mycobacteriosis in ferrets should therefore be made based on both the presence of compatible clinical signs (e.g., third eyelid edema and positive PCR, consistent pathology with positive staining). Presentation of mycobacteriosis in ferrets is polymorphous, and variations in clinical disease conditions make it difficult to characterize a single “mycobacterial disease.” When collecting biopsy samples of lesions, additional samples should be saved and frozen for potential specific pathogen identification, as repeat biopsy is typically not desirable.
The question of the zoonotic potential of mycobacterial infection is a key element of the information provided to the owner and of the decision to treat or euthanize. Many mycobacteria can cause diseases in man. The zoonotic risk is clear for bacteria of the M. tuberculosis group. However, for bacteria of the M. avium group or for rapid growing bacteria such as M. fortuitum or M. chelonae, the actual zoonotic risk appears to be minimal for humans with normal immune systems. Most human infections with atypical organisms originate from the environment, and not from other humans or from animals.11, 29 Nevertheless, precise diagnosis is essential before discussing treatment with an owner.2 In the cases described in this article, owners were encouraged to pursue mycobacterial identification. In 11 cases where identification was achieved, all identified mycobacteria were atypical mycobacteria. The decision to treat should be made carefully, and in conjunction with the owner’s physician. Several treatment protocols have been described in ferrets (Table 1). The ferrets in this report were primarily treated with fluoroquinolones, in particular, enrofloxacin. Enrofloxacin treatment has the advantage of being relatively inexpensive, easily accessible for veterinarians, and easy to administer by owners. In France, fluoroquinolones are considered critical antibiotics, and their use for infection prevention or for minor infections is discouraged; however their use for treatment of mycobacterial infections is likely justified. The use of human antituberculous drugs such as rifampicin, isoniazid, pyrazinamide, rifabutin, or ethambutol could be considered, but there may be serious ethical concerns regarding the use of these drugs, as antibiotic resistance is of critical concern.25, 26 Another antibiotic to consider is azithromycin, which is well tolerated in ferrets and can be used in association with fluoroquinolones. Existing information regarding the typical susceptibility of each bacterium in humans can also help with antimicrobial selection. The use of interferon should also be investigated. It has been suggested that interferon-gamma may play an important role in host resistance to mycobacteria.30, 31 Human interferon alpha-A, which is inexpensive and easy to use, has proved useful in containing or treating other ferret diseases such as systemic coronavirus infection (authors’ personal observations) and should be investigated as a concurrent treatment for mycobacteriosis. The slow growth of mycobacterial organisms necessitates long-term treatment, in some cases, many months. Gauging treatment success is difficult. In the cases assessed in this case series, treatment ended with the death of the animal or after more than 1 year without return of clinical disease signs.
Conclusion
This article is the first retrospective description of multiple cases of mycobacteriosis in domestic ferrets. Mycobacteriosis may infect ferrets more commonly than once previously thought; it is also likely being underdiagnosed. The aim of the article is to illustrate the polymorphous aspect of the disease and the number of cases diagnosed at the veterinary clinics involved. Veterinarians should include mycobacteriosis in their differential disease diagnoses of ferret patients that present with eyelid swelling, upper and lower respiratory disease, and digestive diseases. For cases of abdominal pyogranulomatous lesions, mycobacteriosis should also be considered along with systemic coronavirus infection.
Acknowledgments
The authors would like to thank Dr. Claire Dally, pathologist at LAPVSO Laboratory; Dr. Alexandra Nicolier, pathologist at Vetdiagnostics Laboratory; and Dr. Denis Fritz, from the CAL Laboratory, for their help and proofreading.
References
- 1.Goodfellow M., Magee J.G. Taxonomy of mycobacteria. In: Gangadharam P.R., Jenkins P.A., editors. vol. 1. International Thomson Publishing; London, UK: 1997. pp. 1–71. (Mycobacteria: Basic Aspects). Chapman and Hall Medical Microbiology Series. [Google Scholar]
- 2.Solatges C: Les dermatoses provoquées par les mycobactéries chez les carnivores domestiques. Thèse véto Toulouse, pp 3-4093, 2008
- 3.Rastogi N., Legrand E., Sola C. The mycobacteria: an introduction to nomenclature and pathogenesis. Rev Sci Tech. 2001;20:21–54. doi: 10.20506/rst.20.1.1265. [DOI] [PubMed] [Google Scholar]
- 4.Reavill D.R., Schmidt R.E. Mycobacterial lesions in fish, amphibians, reptiles, rodents, lagomorphs, and ferrets with reference to animal models. Vet Clin N Am Exot Anim Pract. 2012;15:25–40. doi: 10.1016/j.cvex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Symmers W.C., Thomson A.P.D., Iland C.N. Observations on tuberculosis in the ferret (Mustela furo L.) J Comp Pathol. 1953;63:20–31. doi: 10.1016/s0368-1742(53)80004-4. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi T., Labes R.E., Lambeth M. Transmission of mycobacterium bovis from experimentally infected ferrets to non-infected ferrets (Mustelo furo) N Z Vet J. 2000;48:99–104. doi: 10.1080/00480169.2000.36173. [DOI] [PubMed] [Google Scholar]
- 7.Lugton I.W., Wobeser G., Morris R.S. Epidemiology of Mycobacterium bovis infection in feral ferrets (Mustela furo) in New Zealand: I. Pathology and diagnosis. N Z Vet J. 1997;45:140–150. doi: 10.1080/00480169.1997.36014. [DOI] [PubMed] [Google Scholar]
- 8.Ragg J.R., Waldrup K.A., Moller H. The distribution of gross lesions of tuberculosis caused by Mycobacterium bovis in feral ferrets (Mustela furo) from Otago, New Zealand. N Z Vet J. 1995;43:338–341. doi: 10.1080/00480169./1995.35916. [DOI] [PubMed] [Google Scholar]
- 9.Piseddu E., Trotta P., Tortoli E. Detection and molecular characterization of Mycobacterium celatum as a cause of splenitis in a domestic ferret (Mustela putorius furo) J Comp Pathol. 2011;144:214–218. doi: 10.1016/j.jcpa.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Lunn J.A., Martin P., Zaki S. Pneumonia due to Mycobacterium abscessus in two domestic ferrets (Mustelo putorius furo) Aust Vet J. 2005;83(9):542–546. doi: 10.1111/j.1751-0813.2005.tb13325.x. [DOI] [PubMed] [Google Scholar]
- 11.Lucas J., Lucas A., Furber H. Mycobacterium genavense infection in two aged ferrets with conjunctival lesions. Aust Vet J. 2000;78(10):685–689. doi: 10.1111/j.1751-0813.2000.tb10406.x. [DOI] [PubMed] [Google Scholar]
- 12.Valheim M., Djonne B., Heiene R. Disseminated Mycobacterium celatum (Type 3) infection in a domestic ferret (Mustela putorius furo) Vet Pathol. 2001;38:460–463. doi: 10.1354/vp.38-4-460. [DOI] [PubMed] [Google Scholar]
- 13.Schultheiss P.C., Dolginow S.Z. Granulomatous enteritis caused by Mycobacterium avium in a ferret. J Am Vet Med Assoc. 1994;204(8):1217–1218. [PubMed] [Google Scholar]
- 14.Saunders G.K., Thomsen B.V. Lymphoma and Mycobacterium avium infection in a ferret (Mustela putorius furo) J Vet Diagn Invest. 2006;18:513–515. doi: 10.1177/104063870601800521. [DOI] [PubMed] [Google Scholar]
- 15.De Lisles G.W., Kawakami R.P., Yates G.E. Isolation of Mycobacterium bovis and other mycobacterial species from ferrets and stoats. Vet Microbiol. 2008;132:402–407. doi: 10.1016/j.vetmic.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Xavier E.F., Seagar A.L., Doig C. Human and animal infections with Mycobacterium microti. Scotland. Emerg Infect Dis. 2007;13:1924–1927. doi: 10.3201/eid1312.061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner D., Young L.S. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32:257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- 18.Rell L.A., Woods L., Cromie R.L. Mycobacteriosis in birds. Rev Sci Tech. 2001;20(1):180–203. doi: 10.20506/rst.20.1.1273. [DOI] [PubMed] [Google Scholar]
- 19.Landsell W.R., Dixon N., Benjamin L. Isolation of several Mycobacterium species from fish. J Aquat Anim Health. 1993;5:73–76. [Google Scholar]
- 20.Conroy D.E. Observaciones sobre cassos espontaneos de la tuberculosis ictica. Microbiol Esp. 1966;9:93–113. [PubMed] [Google Scholar]
- 21.Frerichs G.N. Mycobacteriosis: nocardiosis. In: Inglis V., Roberts R.J., Bromage N.R., editors. Bacterial Diseases of Fish. Halsted Press; New York, NY: 1993. pp. 219–234. [Google Scholar]
- 22.Eisenach K.D. Molecular diagnosis. In: Rutledge C., Dale J., editors. Mycobacterium: Molecular Biology and Virulence. Blackwell Science; Oxford, UK: 1998. pp. 161–179. [Google Scholar]
- 23.Skuce R.A., Hughes M.S., Taylor M.J. Detection of pathogenic mycobacteria of veterinary importance. Meth Mol Biol. 2003;216:201–221. doi: 10.1385/1-59259-344-5:201. [DOI] [PubMed] [Google Scholar]
- 24.Iyengar K.P., Nadkarni J.B., Gupta R. Mycobacterium chelonae hand infection following ferret bite. Infection. 2013;41:237–241. doi: 10.1007/s15010-012-0309-7. [DOI] [PubMed] [Google Scholar]
- 25.Almeida Da Silva P.E., Palomino J.C. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother. 2011;66(7):1417–1430. doi: 10.1093/jac/dkr173. [DOI] [PubMed] [Google Scholar]
- 26.Zymla A., Abubakar I., Raviglione M. Drug-resistant tuberculosis—current dilemmas, unanswered questions, challenges and priority needs. J Infect Dis. 2012;205(2):228–240. doi: 10.1093/infdis/jir858. [DOI] [PubMed] [Google Scholar]
- 27.Garner M.M., Ramsell K., Morera N. Clinicopathologic features of a systemic coronavirus-associated disease resembling feline infectious peritonitis in the domestic ferret (Mustela putorius) Vet Pathol. 2008;45:236–246. doi: 10.1354/vp.45-2-236. [DOI] [PubMed] [Google Scholar]
- 28.Garner MM: “osis,” “itis,” and virus: differentiating mycobacteriosis, disseminated idiopathic myofasciitis, and systemic coronavirus in the domestic ferret. Proceedings of NAVC Conference, Orlando, FL pp 1705-1706, 2011
- 29.Hoop R.K. Public health implications of exotic pet mycobacteriosis. Semin Avian Exot Pet Med. 1997;6(1):3–8. [Google Scholar]
- 30.Chai N: Mycobacteries atypiques chez les amphibiens. Thèse pour obtenir le grade de docteur du museum d’histoire naturelle, 2008
- 31.Holland S.M. Nontuberculous mycobacteria. Am J Med Sci. 2001;321:49–55. doi: 10.1097/00000441-200101000-00008. [DOI] [PubMed] [Google Scholar]


