Abstract
Adenoviruses have transitioned from tools for gene replacement therapy to bona fide vaccine delivery vehicles. They are attractive vaccine vectors as they induce both innate and adaptive immune responses in mammalian hosts. Currently, adenovirus vectors are being tested as subunit vaccine systems for numerous infectious agents ranging from malaria to HIV-1. Additionally, they are being explored as vaccines against a multitude of tumor-associated antigens. In this review we describe the molecular biology of adenoviruses as well as ways the adenovirus vectors can be manipulated to enhance their efficacy as vaccine carriers. We describe methods of evaluating immune responses to transgene products expressed by adenoviral vectors and discuss data on adenoviral vaccines to a selected number of pathogens. Last, we comment on the limitations of using human adenoviral vectors and provide alternatives to circumvent these problems. This field is growing at an exciting and rapid pace, thus we have limited our scope to the use of adenoviral vectors as vaccines against viral pathogens.
Keywords: adenoviruses, vaccines
Introduction
Traditional viral vaccines are based on inactivated or attenuated pathogens. Advances in molecular virology in concert with viral immunology now allow for the genetic engineering of vectors expressing solely those viral antigens that induce immune correlates of protection. Adenoviruses were initially vectored as vehicles for gene therapy. Attempts to replace missing or faulty genes by adenoviral gene transfer were largely unsuccessful in experimental animals and human volunteers alike due to innate and adaptive immune responses induced by the adenoviral antigens. While this reduced their appeal as gene replacement vehicles it invited their use as vaccine carriers. Adenoviral vectors are attractive candidates for transfer of foreign genes for a number of reasons. The adenoviral genome is well characterized and comparatively easy to manipulate. Most adenoviruses cause mild diseases in immunocompetent human adults and by deletion of crucial regions of the viral genome the vectors can be rendered replication-defective, which increases their predictability and reduces unwanted side effects. Adenoviruses have a broad tropism infecting a variety of dividing and nondividing cells. They can be grown to high titers in tissue culture. They can be applied systemically as well as through mucosal surfaces and their relative thermostability facilitates their clinical use.
Thus far most efforts have focused on vectors derived from adenovirus of the human serotype 5 (AdHu5) for eventual use as vaccines for humans, while bovine, porcine, and ovine adenoviruses have been explored for veterinary use.
Adenoviral genes and polypeptides
Adenoviruses are double-stranded DNA viruses with a genome of ∼34–43 kb, a size that is amenable for easy manipulation. The virus compensates for its small genome by encoding polypeptides from both DNA strands and by alternative splicing and the use of different poly(A) sites. Adenoviruses are species-specific and different serotypes, i.e., types of viruses that are not cross-neutralized by antibodies, have been isolated from a variety of mammalian species, such as 51 serotypes from humans and 27 serotypes from simians, including 7 from chimpanzees. Human serotypes, studied most extensively, are divided into six subgroups (A–F; B is subdivided into B1 and B2) based on sequence homology and on their ability to agglutinate red blood cells (Table 1 ).
TABLE 1.
| Human adenovirus serotype A | 12, 18, 31 |
| Human adenovirus serotype B | B1: 3, 7, 16, 21, 50 |
| B2: 11, 14, 34, 35 | |
| Human adenovirus serotype C | 1, 2, 5, 6 |
| Human adenovirus serotype D | 8, 9, 10, 13, 15, 17, 19, 20, 22–30, 32, 33, 36, 37, 38, 39, 42–49, 51 |
| Human adenovirus serotype E | 4 |
| Human adenovirus serotype F | 40, 41 |
| Chimpanzee adenoviruses | C1, C2, C5, C6, C7, C68 |
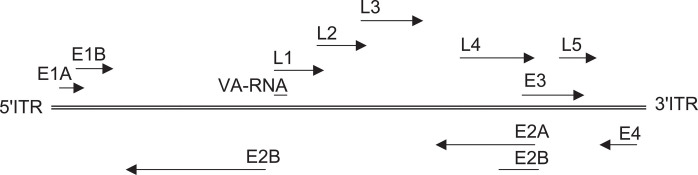
The linear genome flanked by two origins for DNA replication (ITRs) has eight units for RNA polymerase II-mediated transcription (Fig. 1 ). The genome carries five early units (E1A, E1B, E2, E3, E4, and E5, reviewed in [1]), two units that are expressed with a delay after initiation of viral replication (IX and IVa2), and one late unit (L) that is subdivided into L1–L5. Adenoviruses furthermore encode one or two species of RNA called virus-associated (VA) RNA, which are under the control of internal polymerase III promoters.
Fig. 1.
Organization of the adenovirus genome. The different early and late transcription units and the orientation of transcription are shown.
The E1A unit located next to the left terminal ITR is transcribed first and, with the help of cellular factors, activates transcription of the other viral genes. Deletion of E1A renders the virus replication-defective. E1A stimulates viral DNA synthesis, dysregulates cell-cycle control by increasing the stability of p53, and promotes apoptosis through p53-dependent and -independent mechanisms. E1A plays a role in immunoevasion by inhibiting the activity of STAT1, which is needed for activation of interferon-responsive genes. While E1A proteins promote apoptosis, E1B proteins have antiapoptotic functions. E1B polypeptides turn off host cell protein synthesis and help to stabilize, transport, and translate selectively viral RNA. The E1 early gene products can cause transformation of rodent cells; notwithstanding, adenoviruses are not associated with malignancies in humans. The E2 unit encodes DNA-binding proteins and a polymerase and is essential for viral replication. The E3 unit, which is under the control of an NF-κB-inducible promoter, is nonessential for virus replication. E3 proteins allow the virus to escape immunosurveillance by reducing expression of major histocompatibility complex (MHC) class I determinants. E3-derived polypeptides bind directly to the groove of MHC class I molecules and thus prevent binding of peptides and export to the cell surface. Furthermore, E3 proteins associate with TAP and thereby reduce efficient transport of peptides derived from proteolytic cleavage of de novo synthesized viral proteins from the cytoplasm to the endoplasmic reticulum where they can associate with MHC class I molecules. E3 antigens block Fas-mediated apoptosis through binding and internalization of CD95 (Fas receptor) and inactivation of FLICE, an apoptosis-causing caspase. E3 proteins block the TNF signaling pathways and thus prevent TNF-mediated killing of infected cells. At a late stage of viral replication, the E3 unit encodes a polypeptide called the adenovirus death protein that promotes death of the infected cell and hence release of virus particles. The E4 transcription unit encodes seven polypeptides through distinct open reading frames (ORFs), which affect viral transcription and a number of host cell functions including cell proliferation and apoptosis, in part by promoting degradation of p53. E4 is essential for nuclear export of viral RNA. Both ORF3 and ORF6 bind to the 55-kDa unit of E1B. The oncogenic potential of adenoviruses of subgroups A and B relates largely to the activities of E1A and E1B and some of the E4-encoded polypeptides. Products of ORF3 and ORF6, the latter in concert with E1B, inhibit the cellular DNA repair system by redistribution (ORF3) or degradation (ORF6-E1B) of pertinent cellular enzymes and thus prevent formation of concatemers through end-to-end joining of adenoviral genomes [2]. Adenoviruses of the human subgroups B1, C, D, and E and of the chimpanzee serotypes encode two VA RNAs while adenoviruses of the other human subgroups or derived from other simian species encode one VA RNA. VA RNA-encoding genes are under the control of internal promoters, which are transcribed to high levels by polymerase III [3]. The resulting transcripts are short stretches of RNA (∼200 bases), which form double-stranded hairpin–loop structures. VA RNA is essential for translation of adenoviral genes by inhibiting the PKR pathway, which is initiated in virally infected cells and reduces translation by phosphorylation of eIF-2α.
The IX gene product is a transcriptional transactivator and a minor component of the viral capsid that increases virion stability [4]. The IVa2 protein is needed for assembly of adenovirus and packaging of viral DNA [5].
The late transcription units, divided into five subunits (L1–L5) encode as many as 45 different species of RNA, which are differentially spliced during the early and late phase of adenovirus replication (reviewed in [6]). Products of the late transcription unit form the viral capsid (reviewed in [7]). Polypeptide II (hexon), encoded by the L3 unit, is the most abundant of the capsid proteins. The hexon forms thermostable trimers and formation of this complex structure requires the assistance of the 100K protein, a product of the L4 transcriptional unit. The hexon base has three conserved double barrels, while the top has three towers, each tower containing a loop from each subunit that forms most of the capsid. The base of hexon is highly conserved between adenoviral serotypes, while the surface loops, the targets of virus-neutralizing antibodies, are variable. Polypeptide III (encoded by L2), the penton base, is a pentameric structure, which upon binding of polypeptide IV (fiber) trimers (product of L5) assembles into the penton complex. The fiber is composed of a shaft-like region and carries a knob-like domain at the distal end. Minor coat proteins include polypeptides IIIa, VI, VIII, and XI, which stabilize the viral capsid and connect the coat proteins to the viral chromosome.
Adenoviral infections
Adenoviruses cause acute symptomatic and persistent asymptomatic infections. Different serotypes preferentially target distinct organs during the acute infection. Serotypes that commonly infect humans, such as AdHu1, AdHu2, and AdHu5 (subgenus C), cause in general mild upper respiratory infections in children, while subgenera A and F trigger gastrointestinal symptoms. Adenoviruses can lead to serious and even fatal infections such as pneumonia (AdHu4 and 7) or meningioencephalitis (AdHu7, 12, and 32), especially in immunocompromised individuals [8], [9] and children [10]. Symptoms caused by chimpanzee-derived adenoviruses [11] that are currently being explored for vaccine development [12] remain ill defined. Upon acute infections adenoviruses are known to persist in lymphoid cells [13], [14]. Persistence, which does not appear to have clinical sequelae, is linked to the E3 gene products [15], which not only protect infected cells from destruction by the adaptive immune system but also through inhibition of apoptosis confer a survival advantage to persistently infected cells. During persistence the viral genome is retained episomally and virus can be rescued with, albeit low, efficiency from lymphatic tissues years after an acute infection [13], [14].
Some human adenovirus serotypes are ubiquitous and infect most children during early infancy. Infections with AdHu5 and AdHu2 viruses, which have been studied most extensively, are common and depending on the region 45 to 80% of adults carry AdHu5-neutralizing antibodies [12], [16]. AdHu35, which is being developed as a vaccine carrier, is less common and virus-neutralizing antibodies to this virus can be detected depending on the region in ∼5–15% of adults [16]. The chimpanzee viruses tested thus far establish common infections in chimpanzees but are prevalent neither in humans (1–2%) nor in other simian species such as rhesus macaques [12], frequently used for preclinical vaccine testing.
Tropism of adenoviruses
The tropism of adenoviruses is determined by their ability to attach to host cell receptors. This involves an initial binding of the distal knob domain of the fiber to a host cell surface molecule followed by binding of the RGD motif within the penton base with αv integrins (reviewed in [17]). Most human serotype adenoviruses such as AdHu5 and AdHu2 initially bind to the coxsackie adenovirus receptor (CAR) [18], which is expressed on many cell types, including hepatocytes, the basolateral surface of epithelial cells, endothelial cells, myoblasts, and heart muscle cells. Lymphoid cells do not express CAR but nevertheless provide a reservoir for persistent infections with adenoviruses that use CAR. CAR also facilitates entry of the chimpanzee-origin adenovirus AdC68 [19]. Adenoviruses of the subgroup B do not bind CAR. B2 subgroup adenoviruses, including AdHu35, associate with CD46 [20], a complement regulatory protein, expressed on multiple cell types including hematopoietic stem cells and dendritic cells. It should be noted that CD46 is only expressed in mice on spermatocytes. AdHu3 virus, a subgroup B1 virus, attaches to CD80 and CD86 [21], two costimulatory molecules expressed on antigen-presenting cells.
Immune responses to adenoviral antigens
Adenoviruses are highly immunogenic. They activate the innate immune system presumably by expressing so-called pathogen-associated molecular patterns (PAMPs). PAMPs bind to pathogen recognition receptors on host cells, including those of the innate immune system, thus initiating production of proinflammatory cytokines and differentiation of immature dendritic cells into professional antigen-presenting cells [22]. Systemic administration of high doses of AdHu5 vectors into mice [23] or monkeys [24] was shown to trigger rapid release of IL-6, IL-12, and TNF-α and accumulation of transduced macrophages and dendritic cells in lymphatic tissues. Adenovirus-induced maturation of dendritic cells was shown to be NF-κB-dependent [25]. One study described activation of dendritic cells linked to the β-stranded region of the fiber knob [26]. Another study showed activation of dendritic cells linked to an autocrine TNF-α-mediated mechanisms by which TNF-α induced by the RGD sequence within the penton base drove differentiation of immature dendritic cells [27].
Adaptive immune responses are directed to both early and late antigens of adenovirus. Virus-neutralizing antibodies induced by adenoviral infections or upon adenoviral vector delivery are primarily directed against the surface loops of the viral hexon [28] although antibodies to the penton base or the fiber can also neutralize adenovirus [29]. CD4+T cells to adenoviral antigens have been demonstrated in humans and their frequencies were shown to decrease with age. CD4+T cells, which are mainly of the Th1 type, cross-react between different adenoviral serotypes [30]. In experimental animals adenovirus and adenoviral vectors induce CD8+ T cell responses to different structural proteins. Human- as well as mouse-derived CD8+ T cells cross-react between human serotypes as well as between human and simian serotypes [31], as would be expected considering the extensive sequence homology of their target antigens.
Construction of adenoviral vectors
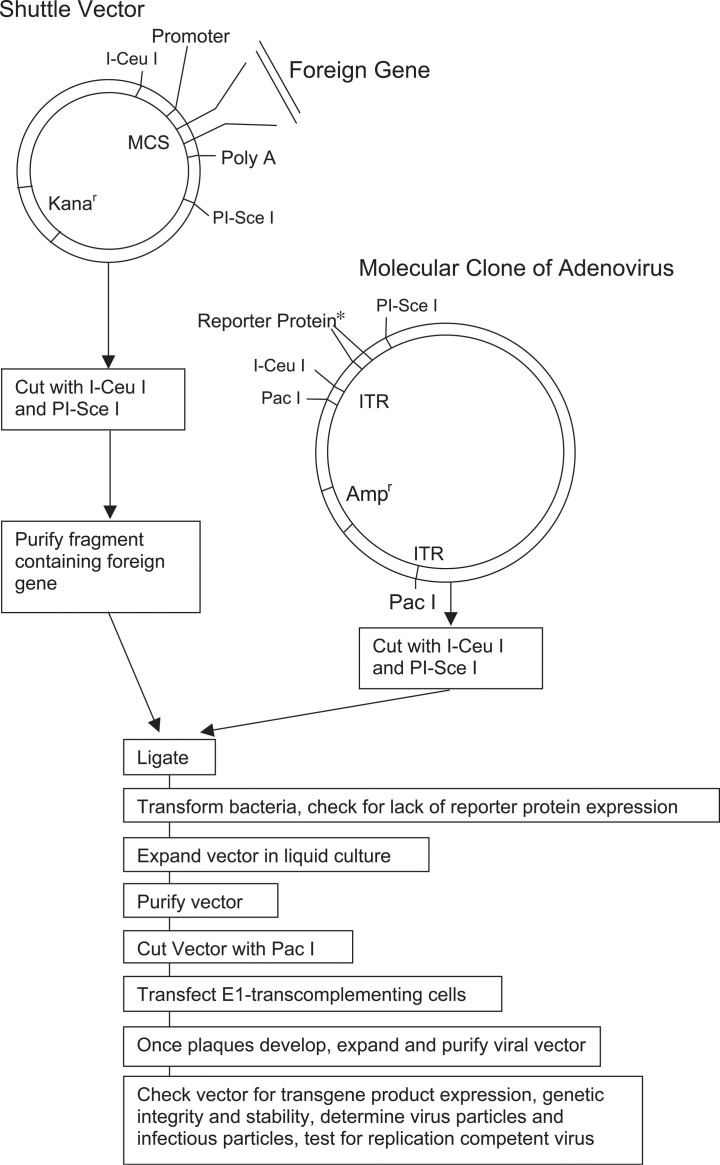
The original E1-deleted adenoviral vectors were constructed by homologous recombination [32]. Cells were cotransfected with purified adenoviral genome deleted of E1 by restriction enzyme digest and a shuttle vector that contained the left-handed ITR, the E1a enhancer, the encapsidation signal, the cytomegalovirus (CMV) IE promoter (or another suitable promoter), a multicloning site for insertion of the gene of interest, and the SV40 poly(A) signal followed by sequences from the adenoviral genome located 3′ of the E1 domain. Now most E1-deleted adenoviral vectors are generated from so-called molecular clones [33] in which the entire adenoviral genome including the ITRs is carried in a bacterial plasmid, allowing for its propagation in Escherichia coli (Fig. 2 ). The sequence of interest can be ligated directly into the molecular clone, which then upon removal of the bacterial sequences is transfected into suitable packaging cell lines. The use of molecular clones not only facilitates the generation of AdHu5 vectors, which can be constructed from commercially available kits, but will also facilitate the eventual clinical use of adenoviral vectors derived from species other than humans. Adenoviruses isolated from chimpanzees are being generated as vaccine carriers [12]. Chimpanzee-derived viruses could potentially be contaminated with yet to be identified pathogens that could propagate unnoticed in parallel with the adenoviral vectors in tissue culture. Considering that animal viruses, especially those from species closely related to humans, such as chimpanzees, have the potential to cross into humans as is well documented for simian immunodeficiency virus [34], the use of chimpanzee-origin adenoviral vectors generated by exclusive propagation in mammalian cell lines would present an unacceptable risk for humans. Generation of vaccine vectors from molecular clones on the other hand eliminates the peril of hidden contaminants from the original isolates.
Fig. 2.
Generation of an E1-deleted adenovirus vector from a molecular viral clone.
Identification of recombinant viral plaques can be facilitated by introducing genes such as herpes simplex virus thymidine kinase [35], green fluorescent protein [36], or β-galactosidase [37] into the E1 domain, with the former allowing for selection of recombinant virus by ganciclovir and the latter for their identification by fluorescence or upon staining.
Types of adenoviral vectors: Deletions
The adenoviral capsid allows some latitude regarding the length of genome that can be packaged efficiently. Foreign sequences of up to 1.8 kb can be inserted into the adenoviral genome without necessitating deletions. Nevertheless, to increase the permitted size of the insert and to modify the biology of adenoviral vectors, vaccine constructs are generally based on adenoviral vectors with deletions of transcription units. The initial adenoviral vaccine vectors were deleted of E3, which encodes gene products that are nonessential for virus replication [38]. In these vectors, which accommodate an additional 3.5 kb of foreign sequence, the E3 promoter or the more potent immediate early promoter of CMV drives expression of the transgene product. E3-deleted adenoviral vectors are replication-competent and thus less predictable than replication-defective vectors. This raises concern about potential toxicity especially if they are used in immunocompromised patients, children, or the elderly. It has been argued that replication-competent adenoviruses achieve higher antigenic loads compared to replication-defective vectors and thus result in higher and more sustained transgene product-specific immune responses [39]. A direct side-by-side comparison of the efficacy of replication-defective vs replication-competent adenoviral vaccine carriers has not yet been conducted in a species that permits replication of E3-deleted vectors containing E1 thus one can only speculate on their potential advantage as vaccine carriers. E1-deleted, replication-competent adenoviral vectors encode all of the remaining adenoviral antigens in addition to the transgene product, i.e., the vaccine antigen. The adaptive immune response will thus be directed toward a multitude of different antigens, which can negatively affect the desired immune response to the vaccine antigen through competition for MHC binding sites or cytokines needed for activation, differentiation, and proliferation of T and B cells. Furthermore, adenoviruses cause in most cell types lytic infections and, once the viral progeny has been assembled, result in apoptotic death of the infected cells. This shortens the duration of antigen presentation by individual infected cells, which in turn could have a negative effect on induction of CD8+ T cells, which are stimulated primarily by peptides derived from de novo synthesized antigens, while potentially favoring activation of CD4+T cells, which are induced by peptides derived from lysosomal cleavage of proteins taken up by phagocytosis or pinocytosis. Although this has not been tested in depth for E3-deleted adenoviral vectors, our experience with poxvirus vectors indicates that replication competence does not necessarily translate into a stronger transgene product-specific immune response. Poxvirus vectors rapidly produce large amounts of transgene products; nevertheless, they induce, compared to E1-deleted adenoviral vectors expressing the same antigen, only modest B and CD8+ T cell responses to the transgene product [40], [41].
Most vectors currently used as vaccine carriers are deleted in E1. This not only renders the vectors replication-defective but also allows for sustained antigen presentation by reducing the vectors' ability to induce death of the infected cells. E1 is essential for viral replication and therefore has to be provided in trans during production of the vectors. To incorporate larger foreign sequences, vectors deleted of E1 and E3 have been developed [40], [42] and they accommodate up to 7.5 kb of foreign DNA. Vectors with additional deletions have thus far been explored mainly for gene therapy. Such vectors include those deleted of E1 and E4 [43]. E4-deleted vectors produce fewer late gene products compared to E1-only-deleted vectors and thus reduce induction of vector-specific immune responses. One would also expect that in the presence of preexisting adenovirus-specific CD8+ T cells, E4-deleted vectors would provide lower amounts of target antigens for T-cell-mediated destruction of antigen-presenting cells and thus potentially increase the duration of presentation of the vaccine antigen and in consequence the magnitude of the antigen-specific immune response, as remains to be tested. Furthermore, deletion of E4 minimizes outgrowth of replication-competent virus in packaging cell lines [44]. E1-and E2A-deleted vectors have been constructed but resulted in 30-to 40-fold lower yields compared to E2A-containing constructs [45]. Vectors deleted of E1, E2A, E4, and E3 [46], [47] and vectors lacking all viral genes have been developed for gene therapy. The latter type of constructs, commonly referred to as gutted vectors, can be propagated only in the presence of helper viruses, which commonly contaminate the vector batches [48]. Furthermore, gutted adenoviral constructs can currently not be propagated to quantities needed for clinical development of vectors destined for mass vaccination.
Adenoviral vectors with deletions other than of E3 can be grown only on cell lines that supply the deleted transcription units in trans. HEK 293 cells [49], which carry the left-side AdHu5 genome, including E1, the left-handed ITR, cis-acting packaging sequences, and protein IX sequences, are most commonly used for propagation of E1-deleted AdHu5 vectors. Growth of AdHu5 vectors on HEK 293 cells can lead to homologous recombination followed by outgrowth of replication-competent adenovirus (RCA). This common problem is at least in part circumvented in an alternative packaging cell line, i.e., PER.C6 cells [50]. PER.C6 cells contain the AdHu5 E1A and E1B sequences under the control of the human phosphoglycerate kinase promoter. Lack of overlap with sequences outside the E1 transcription units diminishes homologous recombination and thus outgrowth of RCA. The E1-deletion in adenoviral vectors of the chimpanzee serotypes such as C68 can be transcomplemented by E1 of AdHu5 virus and such vectors can be grown on HEK 293 cells [12]. Lack of sequence homology in the E1-flanking regions prevents homologous recombination and hence outgrowth of RCA from E1-deleted chimpanzee adenoviral vectors. Other human adenoviral vectors, such as those based on AdHu35 virus, are not transcomplemented by the E1 of AdHu5 virus and thus necessitate modifications of available packaging cell lines, such as insertion of the AdHu35 E1B gene into cell lines that carry the E1 of AdHu5 virus [16]. Alternatively, construction of other serotypes of adenoviral vectors, in which the endogenous E4 ORF6 (which binds to E1B) is replaced by that of AdHu5 virus, may allow for their propagation on available cell lines that provide the E1 of AdHu5 virus in trans [51]. Propagation of adenoviral vectors with additional deletion in either E4 or E2 requires that the deleted genes be provided by the packaging cell lines [46], [47] or by a helper virus [48].
Which deletion is most suitable for an adenoviral vaccine carrier will most likely depend on a number of variables such as type and size of the insert and potentially the serotype of adenovirus that is being vectored as well as growth characteristics of individual constructs [52].
Now that adenoviral vaccine carriers are progressing toward clinical trials, a more thorough analysis of the effects of various deletions on growth properties, genome stability, and capsid stability seems warranted. Although packaging allows for some latitude in the size of the adenoviral genome, it has been shown for AdHu5 virus that overly long inserts cause genetic instability of vectors [52]. It remains to be assessed more carefully for AdHu5 as well as other less well characterized serotypes of adenovirus to what degree genome size affects genetic stability, growth characteristics [53], and thermostability [54] of the constructs.
Altered Tropism
The tropism of adenoviral vectors can be altered by genetic engineering of the fiber knob. Replacing a large portion of the C-terminus of AdHu5 fiber with the σ1 protein of reovirus type 3, both of which share the structural feature of forming trimers through triple β spiral motifs and a head and tail morphology [55], results in stable vectors that use the reovirus receptor junctional adhesion molecule 1 for viral entry and thus show improved transduction of certain cell types, including dendritic cells, expressing this receptor [56]. AdHu5 vectors pseudotyped with fibers from adenoviruses of subgroups B, C, D, and F result in vectors with reduced tropism for myoblasts and endothelial cells (fibers from subgroups B and D) and increased tropism for dendritic cells (fibers from subgroup B, i.e., AdHu30 and AdHu35) in vitro and in vivo without affecting in mice the resulting transgene product-specific B or T cell responses [57].
In another report, the fiber of the subtype C AdHu5 virus was replaced with that of a subtype B fiber, i.e., the one derived from AdHu35 virus. Again, the pseudotyped vector has an increased ability to transduce dendritic cells and results in improved induction of CD8+ T cells in vitro or in vivo upon transfer of transduced dendritic cells. The effect of direct application of the pseudotyped vector on adaptive immunity was not investigated [58]. Insertion of RGD sequences into the fiber knob of AdHu5 vectors increases their efficiency in transducing dendritic cells. In a tumor model, these modified vectors were shown to be slightly more efficacious by inducing higher transgene product-specific CD4+and CD8+ T cell responses [59]. Coating adenovirus with an adaptor molecule that connects CAR with CD40L results in increased transduction of dendritic cells and enhances immune responses against a reporter protein approximately two-to fourfold [60]. Overall, the results of retargeting AdHu5 vectors to dendritic cells to enhance antigen presentation remain inconclusive and additional in vivo testing of such vectors is necessary to assess their utility as improved vaccine vehicles.
Testing of Vaccine-Induced Immune Responses
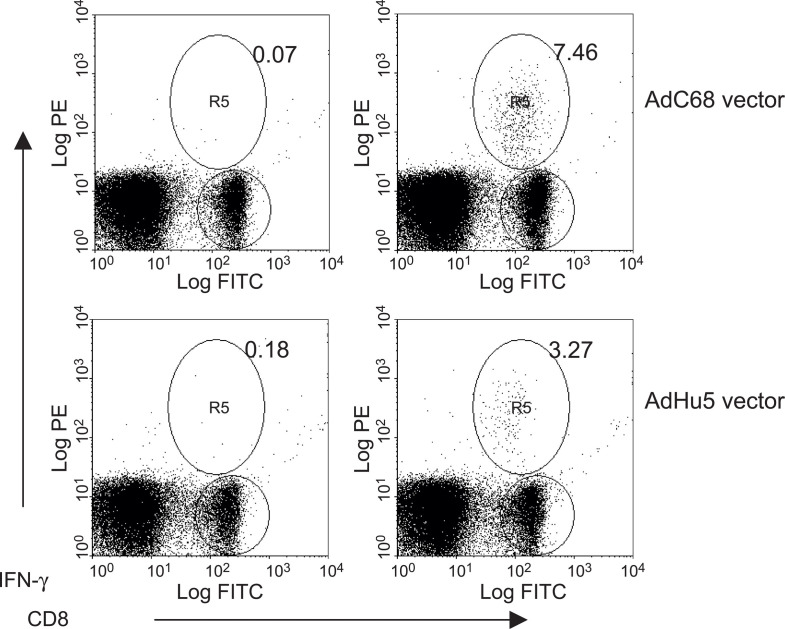
Quantitative methods to test for transgene product-specific CD8+ T cell frequencies, such as by intracellular cytokine staining shown in Fig. 3 , are now available [61], [62], [63], [64]. This allows for an accurate assessment of a vaccine's ability to induce T cell responses. Such quantitative methods combined with vaccine titrations and careful kinetics studies are especially warranted for models for which vaccine efficacy cannot be tested by challenge with a pathogen that causes significant morbidity or mortality in experimental animals, is not available. The use of such assays should also be a prerequisite for the evaluation of the potential benefits of vector modifications.
Fig. 3.
CD8+ T cell responses to Ad vectors. The graphs show the frequencies of transgene product-specific CD8+ T cells induced by adenoviral vectors of the human serotype 5 (bottom) and chimpanzee serotype 68 (top). Mice were injected intramuscularly with 1010 vp of vector. Ten days later, splenocytes were cultured for 5 h with the peptide carrying the immunodominant epitope of the transgene product (right) or an unrelated peptide (left) in presence of brefeldin. Cells were then stained with a FITC-labeled antibody to CD8 and, upon permeabilization of the membrane, with a PE-labeled antibody to interferon (IFN-γ). Cells were analyzed by two-color flow cytometry; data were analyzed by WimnDi software. Events were gated onto live cells. The percentages of double-positive cells (CD8 cell producing IFN-γ) over FITC-positive cells (CD8+ cells) were determined and are displayed in the upper right quadrants.
Immune responses to the transgene product of AdHu5 vectors
Gene therapists attempting to develop E1-deleted adenoviral vectors for replacement of faulty genes inadvertently pioneered the development of adenoviral vaccine carriers. Long-term expression of AdHu5 vector-encoded reporter proteins such as β-galactosidase was readily achieved in lungs or liver of immunodeficient mice. In contrast, in immunocompetent animals expression was transient and the AdHu5 vector-transduced cells were rapidly cleared within 7–10 days mainly through CD8+ T cells directed both against the transgene product and against antigens of adenovirus [65], [66]. The ability of the E1-deleted AdHu5 vectors to induce exceptionally potent transgene product-specific immune responses led to their use as a vaccine carrier for rabies virus glycoprotein [40], which is the sole antigen of rabies virus that induces virus-neutralizing antibodies known to provide protection against rabies virus challenge [67]. The vector termed Adrab.gp or AdHu5rab.gp induces rapidly, within a week, protective titers of neutralizing antibodies as well as rabies virus-specific CD8+and CD4+T cells [40]. E3-deleted replication-competent adenoviral vectors expressing the glycoprotein of a closely related strain of rabies virus perform comparatively poorly and require higher doses to induce protective antibody titers [68]. These studies were rapidly confirmed with other pathogens and antigens (Table 2 ) and by now E1-deleted AdHu5 vaccine vectors expressing antigens of HIV-1 are in clinical trials. All of the reports in experimental animal systems describe similar findings: AdHu5 vectors induce very potent transgene product-specific antibody and CD8+ T cell responses in rodents, dogs, and nonhuman primates. Transgene product-specific antibodies are mainly of the IgG2a isotype in mice although IgG1 is also induced, suggesting a mixed Th1/Th2 response that is predominated by Th1-type help. Adenoviral vectors do not induce overly potent transgene product-specific CD4+T cell responses. Although studies comparing the efficacy of adenoviral vectors to that of other subunit vaccines are still incomplete, available data show them to outperform poxvirus vectors, DNA vaccines, and alphavirus vectors with regard to induction of transgene product-specific antibody titers and CD8+ T cell frequencies [40], [41], [68], [69]. They induce protective immune responses quickly, the responses are long lived and can be induced in adults as well as in immunologically immature neonatal mice [70]. Antibody responses can be elicited upon systemic as well as mucosal, including oral, immunization [71], [72]. CD8+ T cells are primed by oral application of AdHu5 vectors but frequencies detected in the spleens are markedly lower than upon systemic application [73]. The exceptional immunogenicity of E1-deleted AdHu5 vectors is likely to reflect their ability to activate cells of the innate immune system, to transduce immature dendritic cells driving their maturation into antigen-presenting cells [23], to achieve long-lasting antigen presentation by not inducing apoptosis of the transduced cells, and to express high levels of transgene products driven in most vectors by the potent CMV promoter.
TABLE 2.
| Pathogen | Animal model | Insert | Immune response | References |
|---|---|---|---|---|
| Human immunodeficiency virus/simian immunodeficiency virus | Rodent, NHP, human | Gag, Pol, Nef, Env | CMI and antibody | [31], [73], [89], [90], [107], [108], [109] |
| Rabies virus | Rodent, dog | Glycoprotein | CMI and antibody | [40], [68], [70], [71], [93], [94], [110], [111], [112] |
| Dengue virus | Rodent | Envelope | CMI and antibody | [113] |
| Ebola virus | Rodent, NHP | Glycoprotein, nucleoprotein | CMI and antibody | [83], [114] |
| SARS–coronavirus | Rodent, NHP | Spike, nucleocapsid, membrane protein | CMI and antibody | [81] |
| Human papillomavirus | Rodent, NHP | L1, E5, E6, E7, | CMI and antibody | [41], [115], [116] |
| Hepatitis C virus | Rodent | E1, E2, core, NS3 | CMI and antibody | [42], [117], [118], [119], [120] |
| Hepatitis B virus | Rodent, dog, NHP | Surface antigen | Antibody | [121], [122] |
| Rotavirus | Rodent | VP7sc | Antibody | [123], [124] |
| Measles virus | Rodent | Nucleocapsid, hemagglutinin, fusion protein | CMI and antibody | [72], [125], [126], [127] |
| Respiratory syncytial virus | Rodent, dog, NHP | Glycoprotein | Antibody | [128], [129] |
| Cytomegalovirus | Rodent | Glycoprotein B | Antibody | [130], [131] |
| Herpes simplex 2 virus | Rodent | Glycoprotein B | CMI and antibody | [132], [133] |
| Epstein–Barr virus | Rodent | Latent membrane proteins 1 + 2, envelope glycoprotein | CMI and antibody | [134], [135] |
Abbreviations: CMI, cell-mediated immunity; NHP, nonhuman primate.
The number of different antigens that have been expressed by AdHu5-based vaccines, some of which are listed in Table 2, is by now too high for a detailed review. We will thus restrict this review to a limited number of viral pathogens that provide unique challenges to vaccine development that may be met by adenoviral vectors.
Most vaccines prevent infections through the induction of neutralizing antibodies against a pathogen's surface antigens. AdHu5 vectors induce protective neutralizing antibody titers against the transgene product very rapidly after a single application as was shown initially with the rabies virus glycoprotein [40]. This feature is particularly useful for postexposure vaccination or to combat infectious agents that cause infrequent but rapidly spreading outbreaks associated with high mortality.
Rabies vaccination is in most cases given to humans after exposure. This is feasible as the virus has a lengthy albeit varied incubation period of minimally 10 days to maximally several decades. In most cases rabies virus is transmitted to humans by bites from rabid animals, which unlike more discreet exposures to other pathogens directs the patient's attention to seek medical help. Currently used rabies vaccines based on inactivated virus require several booster immunizations (3–5 for tissue culture-grown vaccines, 14–21 for brain tissue-derived vaccines) to elicit protective titers of rabies virus-neutralizing antibodies. In the case of severe exposure, active immunization has to be combined with passive immunization with a rabies immunoglobulin (RIG) of human or equine origin. RIG is in short supply and expensive and thus underutilized [74]. Despite highly effective treatment regimens, rabies still claims in India alone a reported 35,000 deaths annually (the actual number is presumed to be significantly higher) with half of those in children under the age of 14. A single-dose rabies vaccine able to elicit protection rapidly would be expected to reduce the need for RIG, increase the patient's willingness to comply with treatment after a suspected exposure, and thus decrease mortality, which for symptomatic rabies infections is close to 100%. The AdHu5rab.gp vaccine induces in rodents, a suitable preclinical model to assess the efficacy of rabies vaccines, protective antibody titers within 7 days after a single intramuscular or subcutaneous application of a moderate dose of vector [40].
Protective titers to rabies virus can also be induced by intranasal application of the AdHu5 vector [40]. Intranasal application is not only more convenient than vaccine application by injection but also favors induction of transgene product-specific antibodies of the IgA isotype, which upon secretion at mucosal surfaces are best suited to prevent invasion of pathogens through the airways, the gastrointestinal tract, or the genital mucosa. Nevertheless, intranasal application of adenoviral vectors carries the potential risk of inadvertent transfer of vector into the nervous system. Reports have shown that upon intranasal application of adenovirus into mice the virus can infect the central nervous system [75]. Adenoviral vectors applied directly into the central nervous system have been shown previously to cause, albeit transient, histopathology consisting of localized necrosis, gliosis, malacia, and astrocytosis [76]. Additional toxicity studies in nonhuman primates are required to elucidate further the potential neurotoxicity of intranasally applied adenoviral vectors.
Oral application of the AdHu5rab.gp vaccine diluted in buffered saline induces protective antibody titers in adult as well as neonatal mice. Nevertheless, this route of application requires a significant dose escalation. This may be circumvented by formulations such as those previously used for oral vaccination of military personnel with live adenovirus vaccines against serotypes 4 and 7 [77], [78].
Oral immunization with adenoviral vaccine carriers may be particularly desirable to limit natural outbreaks in less developed countries. In the winter of 2002/2003 China reported a cluster of cases of severe acute respiratory syndrome (SARS) with a suspected infectious etiology [79]. Similar cases were soon reported from other parts of Asia, Europe, and Canada. The causative agent was identified as a coronavirus [80] that had crossed from animals to humans. Although the outbreak was rapidly contained through isolation of patients and their contacts, the high morbidity and mortality of SARS that may resurface from its yet to be identified animal reservoir makes it imperative to develop a vaccine that induces protective immunity rapidly after a single dose. AdHu5 vectors expressing the spike protein, the membrane protein, and the nucleoprotein of SARS virus were shown to induce virus-neutralizing antibodies and T cell responses in nonhuman primates [81].
A vaccine to Ebola virus, a filovirus that causes hemorrhagic fever with a high person-to-person transmission rate and mortality rates ranging from 25 to 90%, is currently not available. Antibodies directed to the viral glycoprotein are likely to provide protection against an Ebola virus infection [82]. AdHu5 vaccines expressing the Ebola glycoprotein have been developed and have shown efficacy in nonhuman primates [83]. Ebola virus is considered a potential weapon of mass destruction and the increasing threat of intentional release of such agents by terrorist groups or rogue nations is driving development of new and improved vaccines. Adenoviral vaccine carriers due to their ease of production, their potential for mucosal administration, and the speed with which they induce protective titers of antibodies may become valuable tools to contain morbidity from Ebola virus or other viral weapons introduced into a susceptible population.
A vaccine to HIV-1 remains elusive. Initial attempts to develop vaccines that generate broadly cross-reactive neutralizing antibodies to the human immunodeficiency virus (HIV) -1 failed [84] and most vaccines currently under development focus on stimulating T cells, most notably CD8+ T cells, against conserved sequences of HIV-1 [85]. AdHu5 vaccines expressing individual antigens of HIV-1 or SIV such as the envelope protein [86], [87], [88] or Gag [89], [90] or mixtures of antigens given either combined as a cocktail of vectors [91] or as fusion polypeptides by a single vector [92] were shown to induce potent CD8+ T cell responses in mice and nonhuman primates. In rhesus macaques a Gag-expressing AdHu5 vector provided protection against challenge with a pathogenic chimeric simian human immunodeficiency virus by preventing CD4+T cell loss and containing viral load [90].
The Effect of Preexisting Immunity on AdHu5-Based Vaccines
Although preclinical testing of AdHu5 vectors in experimental animals yielded uniformly promising results, these vectors are destined to encounter problems in human subjects who unlike experimental animals are preexposed to AdHu5 virus and in many cases, especially in children and young adults, still harbor residual wild-type virus in their lymphatic tissues that through some limited transcription may maintain the adaptive immune response against antigens of adenovirus at a heightened stage of activation.
Preexisting immunity due to natural infections results in sustained virus-neutralizing antibody titers, a major handicap for the successful use of common serotypes of adenovirus, including AdHu5 and AdHu2, as carriers for gene replacement therapy or vaccine carriers in a human population. Neutralizing antibodies even if present at moderate titers reduce uptake of the adenoviral vectors by cells, including antigen-presenting cells [31], and thus expression of the transgene product, which in turn impacts the resulting transgene product-specific immune response, as was shown initially in experimental animals [31], [93] and then in humans [51]. This, in theory, may be overcome by increasing the dose of vector, an approach that is limited by the vector's toxicity. Neutralizing antibody titers to adenovirus vary tremendously between individuals. In general, they are higher in children and young adults and decrease with age. The use of common human serotypes of adenovirus for vaccination either would require prior testing of each individual for titers of neutralizing antibodies and subsequent adjustment of the vaccine dose contingent on the magnitude of such titers or would result in unpredictable immune responses. Circumventing the impact of adenovirus-specific neutralizing antibodies is currently a major scientific focus of adenoviral vector vaccinologists. As already pointed out, dose escalation is unlikely to be a realistic option for use in humans due to the associated toxicity. Studies in AdHu5-preexposed nonhuman primates showed that a 1000-fold increase in dose is needed to achieve frequencies of transgene product-specific CD8+ T cells comparable to those obtained in animals that had not been preexposed [69]. Other efforts are focused on combining AdHu5 vectors with other vaccine carriers in prime–boost regimens. The repeated use of AdHu5 vectors has been tested [89], but in general does not afford a significant booster effect due to the induction of adenovirus-neutralizing antibodies after the first application of the vector. DNA vaccines used for priming have been shown to increase adaptive immune responses to the transgene product of an AdHu5 vaccine while reducing induction of antibody responses to the adenoviral antigens [94], [95]. Nevertheless, DNA vaccines have thus far performed poorly in clinical trials [96] and their effectiveness for priming of humans is currently unknown. Poxvirus vectors combined sequentially with adenoviral vectors result in very strong immune responses in experimental animals [31], and clinical trials combining an AdHu5 vector for HIV-1 antigens with an ALVAC vector are contemplated. Nevertheless, experimental animals preexposed to AdHu5 virus develop a dampened immune response to prime–boost regimens that incorporate an AdHu5 vaccine carrier [31].
AdHu5 vectors coated with PEG [97], a multivalent hydrophilic polymer based on poly- [N- (2-hydroxypropyl)methacrylamid] [98] or encapsulated into alginate microspheres [99] can circumvent the effects of virus-neutralizing antibodies as was shown by improved transduction of cells in mice with circulating AdHu5-neutralizing antibodies. Nevertheless, as neither of these studies addressed the effects of their additives on the induction of adaptive immune responses, the usefulness of such modifications for transfer of immunogens remains unknown.
The viral hexon is the main albeit not exclusive target for adenovirus-neutralizing antibodies. Hexon swaps have been tried and succeeded when the AdHu5 hexon was replaced with that of an adenovirus from the same subgroup, i.e., subgroup C. Replacement of the AdHu5 hexon with the hexon of AdHu12 (subgroup A) resulted in a vector that could be rescued although the vector performed poorly in vivo. Attempts to replace the AdHu5 hexon with those from subgroups B, D, or E viruses failed. Most of the chimeras show retarded growth and lower levels of transgene product expression compared to the original vector. One of the vectors in which the AdHu5 hexon was replaced by the AdHu6 hexon was tested in mice for interference of transgene product-specific B cell activation in the presence of AdHu5-neutralizing antibodies. The hexon modification could rescue the antibody response, which nevertheless remained significantly (∼100-fold) below that achieved in mice that had not been preexposed to AdHu5 virus [100].
Oral immunization with an AdHu5 vector was shown to overcome preexisting neutralizing antibodies to AdHu5 virus [71]. Nevertheless, as already pointed out, although oral immunization induces adequate transgene product-specific antibody titers, this route of immunization is thus far suboptimal for stimulating the potent transgene product-specific CD8+ T cell responses elicited by systemic administration of AdHu5 vectors.
Alternative adenovirus serotypes
Adenoviral recombinants from other species were shown to circumvent vector-specific neutralizing antibodies in gene therapy experiments [101]. Our efforts focused on several alternative adenoviral vaccine carriers based on viruses that were isolated from chimpanzees [12], [102], assuming that these adenoviral serotypes would closely resemble the well-characterized human serotypes [12] with regard to growth characteristics (which influences vaccine yields), tropism, and receptor utilization (both of which influence immunogenicity) and transcomplementation with E1 derived from AdHu5 virus. The simian E1-deleted vectors tested thus far, i.e., AdC68 (also termed AdPan9), AdC7 (AdPan7), and AdC6 (AdPan6), indeed show comparable yields upon propagation on HEK 293 cells, indicating that they could grow in the presence of E1 of AdHu5 virus. Using a heterologous E1 for transcomplementation has an added advantage: the sequences flanking the E1 gene in adenovirus show limited homology between different serotypes. The risk of contamination of E1-deleted adenoviral vaccine stocks with replication-competent virus arising upon homologous recombination of the vaccine with the E1 of the packaging cell line is thus virtually absent if both are derived from different serotypes. Vectors of one of these viruses, i.e., the AdC68 virus, have been tested extensively. According to its sequence, the Ad68 virus is most closely related to the subgroup E of human adenoviruses [12]. At the amino acid level the AdC68 virus was shown to have ∼90% sequence homology with the known sequences of adenovirus of the human serotype 4 (which has thus far only partially been sequenced). Humans do not carry neutralizing antibodies to AdC68 virus, while 80% of the tested chimpanzee sera have such antibodies, demonstrating that this virus is indeed prevalent in this species. Similar to AdHu5 virus, the AdC68 virus gains entry into the cells through the CAR [19] and should thus have a tropism similar to that of the well-studied human virus. The AdC7 virus shows partial cross-reactivity for virus-neutralizing antibodies to AdC68 virus and thus appears to belong to the same serotype, while AdC6 virus is not cross-neutralized by antibodies to AdC68 virus. All of these vectors were tested for transgene product expression in different cell types using, for example, green fluorescent protein as the transgene product [12]; vectors expressing the rabies virus glycoprotein were tested for stimulation of antibody responses [93] and vectors expressing a codon-optimized Gag of HIV-1 were tested for stimulation of CD8+ T cells [31]. Adaptive immune responses were assessed in naïve mice as well as in mice preexposed to common human serotypes of adenovirus, most notably AdHu5 virus. Transgene expression achieved by the simian vectors is comparable to that by AdHu5 vectors in some cell types, especially cells known to express CAR. In other cell types, including mouse and human dendritic cells, the chimpanzee-origin vectors express 8-to 15-fold less transgene product. Upon infection of dendritic cells the simian vectors induce production of high levels of type I interferon, which is not observed when dendritic cells are transduced with a comparable dose of an AdHu5 vector [103]. The simian vectors expressing the rabies virus glycoprotein induce upon intramuscular or subcutaneous application virus-neutralizing antibodies that protect mice from challenge with rabies virus; nevertheless titers are lower than those achieved with comparable doses of an AdHu5 vector. The simian vectors elicit upon systemic immunization an antibody response to the transgene product that is nearly exclusively of the IgG2a isotype, while AdHu5 vectors induce mainly IgG2a antibodies but also readily detectable levels of IgG1, indicative of a more balanced Th1/Th2 response [93]. Upon intranasal application both the simian vectors and the AdHu5 vectors induce comparable titers of antibodies in sera and at mucosal surfaces [93]. The simian vectors stimulate potent transgene product-specific CD8+ T cell responses in mice that are slightly superior to those achieved with an AdHu5 vector [31], [73]. In nonhuman primates, prime–boost regimens with either two heterologous chimpanzee-origin vectors or the AdHu5 vectors followed by a chimpanzee origin vector result in exceptionally high frequencies of transgene product (i.e., Gag of HIV-1) -specific CD8+ T cell responses, indicating that these vectors are also highly immunogenic in larger species [102].
Most importantly, at least in mice the transgene product-specific B cell responses elicited by the chimpanzee-origin vectors are not impaired by preexisting immunity to common human serotypes of adenovirus [71], while transgene product-specific CD8+ T cell responses that come up with a slight delay compared to those elicited by AdHu5 vectors are only slightly reduced in mice preexposed to AdHu5 virus. This is caused by CD8+ T cells, which cross-react between adenoviral serotypes of simian and human origin [31] and presumably eliminate some of the transduced antigen-presenting cells, thus reducing the antigenic load.
AdHu35 virus, which has a lower seroprevalence in humans than AdHu5 virus, has been vectored as an E1-deleted vaccine construct [104], [105], [106]. The vectors were shown to have a distinct biodistribution in mice (which do not express the vector's main receptor [20]) upon intravenous injection; while AdHu5 vectors strongly favor transduction of liver cells, this is not observed upon application of AdHu35 vectors. Neutralizing antibodies to AdHu5 virus do not inhibit transduction of cells in vitro or in vivo by AdHu35. E1-and E3-deleted AdHu35 vectors expressing Gag of SIVmac239 stimulate in mice [106] a Gag-specific CD8+ T cell response. Titration of the vector in comparison to an AdHu5 vector expressing the same transgene product showed that the AdHu35 vector has to be used at an approximately 10-fold higher dose than the AdHu5 vector to achieve comparable frequencies of transgene product-specific CD8+ T cells. The AdHu5 vector but not the AdHu35 vector induces a detectable transgene product-specific B cell response. As expected, preexisting immunity to AdHu5 virus strongly reduces the Gag-specific CD8+ T cell response to the AdHu5 vector without affecting that to the AdHu35 vector. An effect of low levels of AdHu35-neutralizing antibodies found in 25% of humans in the United States and 10% of human sera from Japan and Europe has not yet been investigated, nor has the vector been tested thoroughly for induction of transgene product-specific immune responses in animals expressing CD46 such as transgenic mice.
Conclusions
Outbreaks of new infectious diseases such as SARS and the continuing threat of bioterrorism have increased the pace of research for development of novel vaccines. It is our belief that due to the versatility and variety of adenovirus serotypes, they will be valuable tools for developing vaccines against new pathogens and against those to which vaccines have yet to be discovered. While there are some obstacles that have been identified in the use of vaccine carriers based on recombinant adenovirus, extensive knowledge of the biology of adenovirus has enabled researchers to develop methods to overcome these issues as was discussed in the text. Last, as is evidenced in this report, adenoviruses have moved to the forefront of vaccinology and are showing substantial promise as vehicles for antigen delivery for a number of vaccines currently being developed, including HIV.
Acknowledgments
We thank Dr. Roger Burnett for helpful suggestions and Christina Cole for manuscript preparation.
References
- 1.Branton P.E. Early gene expression. In: Seth P., editor. Adenoviruses: Basic Biology to Gene Therapy. Landes Bioscience; Georgetown, TX: 1999. pp. 39–55. [Google Scholar]
- 2.Stracker T.H., Carson C.T., Weitzman M.D. Adenovirus oncoproteins inactivate the Mre11-NBS1 DNA repair complex. Nature. 2002;418:348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- 3.Mathews M.B., Shenk T. Adenovirus virus-associated RNA and translation control. J. Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargent K.L., Meulenbroek R.A., Parks R.J. Activation of adenoviral gene expression by protein IX is not required for efficient virus replication. J. Virol. 2004;78:5032–5037. doi: 10.1128/JVI.78.10.5032-5037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Imperiale M.J. Requirement of the adenovirus IVa2 protein for virus assembly. J. Virol. 2003;77:3586–3594. doi: 10.1128/JVI.77.6.3586-3594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer J., Ketner G. Adenovirus late gene expression. In: Seth P., editor. Adenoviruses: Basic Biology to Gene Therapy. Landes Bioscience; Georgetown, TX: 1999. pp. 69–76. [Google Scholar]
- 7.Rux J.J., Burnett R. Adenovirus capsid proteins. In: Seth P., editor. Adenoviruses: Basic Biology to Gene Therapy. Landes Bioscience; Georgetown, TX: 1999. pp. 5–15. [Google Scholar]
- 8.Kojaoghlanian T., Flomenberg P., Horwitz M.S. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 9.Pham T.T., Burchette J.L., Jr., Hale L.P. Fatal disseminated adenovirus infections in immunocompromised patients. Am. J. Clin. Pathol. 2003;120:575–583. doi: 10.1309/AWXD-GNC5-D70E-N7YT. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.J. Genome type analysis of adenovirus types 3 and 7 isolated during successive outbreaks of lower respiratory tract infections in children. J. Clin. Microbiol. 2003;41:4594–4599. doi: 10.1128/JCM.41.10.4594-4599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillis W.D., Goodman R. Serologic classification of chimpanzee adenoviruses by hemagglutination and hemagglutination inhibition. J. Immunol. 1969;103:1089–1095. [PubMed] [Google Scholar]
- 12.Farina S.F. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath J., Palkonyay L., Weber J. Group C adenovirus DNA sequences in human lymphoid cells. J. Virol. 1986;59:189–192. doi: 10.1128/jvi.59.1.189-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnett C.T., Erdman D., Xu W., Gooding L.R. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 2002;76:10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNees A.L., Garnett C.T., Gooding L.R. The adenovirus E3 RID complex protects some cultured human T and B lymphocytes from Fas-induced apoptosis. J. Virol. 2002;76:9716–9723. doi: 10.1128/JVI.76.19.9716-9723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogels R. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seth P. Entry of adenoviruses into cells. In: Seth P., editor. Adenoviruses: Basic Biology to Gene Therapy. Landes Bioscience; Georgetown, TX: 1999. pp. 31–35. [Google Scholar]
- 18.Bergelson J.M. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 19.Cohen C.J. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 2002;83:151–155. doi: 10.1099/0022-1317-83-1-151. [DOI] [PubMed] [Google Scholar]
- 20.Gaggar A., Shayakhmetov D.M., Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 21.Short J.J. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology. 2004;322:349–359. doi: 10.1016/j.virol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R., Janeway C., Jr. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 24.Schnell M.A. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 25.Morelli A.E. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J. Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinier-Frenkel V. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J. Biol. Chem. 2003;278:37175–37182. doi: 10.1074/jbc.M303496200. [DOI] [PubMed] [Google Scholar]
- 27.Philpott N.J., Nociari M., Elkon K.B., Falck-Pedersen E. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA. 2004;101:6200–6205. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong S.S., Habib N.A., Franqueville L., Jensen S., Boulanger P.A. Identification of adenovirus (Ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative Ad (Addl1520) for treatment of liver tumors. J. Virol. 2003;77:10366–10375. doi: 10.1128/JVI.77.19.10366-10375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive M., Eisenlohr L., Flomenberg N., Hsu S. P. Flomenberg. The adenovirus capsid protein hexon contains a highly conserved human CD4+T-cell epitope. Hum. Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald J.C. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 32.Haj-Ahmad Y., Graham F.L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J. Virol. 1986;57:267–274. doi: 10.1128/jvi.57.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh-Choudhury G., Haj-Ahmad Y., Brinkley P., Rudy J., Graham F.L. Human adenovirus cloning vectors based on infectious bacterial plasmids. Gene. 1986;50:161–171. doi: 10.1016/0378-1119(86)90321-5. [DOI] [PubMed] [Google Scholar]
- 34.Worobey M. Origin of AIDS: contaminated polio vaccine theory refuted. Nature. 2004;428:820. doi: 10.1038/428820a. [DOI] [PubMed] [Google Scholar]
- 35.Imler J.L. An efficient procedure to select and recover recombinant adenovirus vectors. Gene Ther. 1995;2:263–268. [PubMed] [Google Scholar]
- 36.Davis A.R., Wivel N.A., Palladino J.L., Tao L., Wilson J.M. Construction of adenoviral vectors. Mol. Biotechnol. 2001;18:63–70. doi: 10.1385/MB:18:1:63. [DOI] [PubMed] [Google Scholar]
- 37.Moraes M.P., Mayr G.A., Grubman M.J. pAd5-Blue: direct ligation system for engineering recombinant adenovirus constructs. Biotechniques. 2001;31:1056. doi: 10.2144/01315st05. [DOI] [PubMed] [Google Scholar]
- 38.Saito I., Oya Y., Yamamoto K., Yuasa T., Shimojo H. Construction of nondefective adenovirus type 5 bearing a 2.8-kilobase hepatitis B virus DNA near the right end of its genome. J. Virol. 1985;54:711–719. doi: 10.1128/jvi.54.3.711-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J. Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen. J. Virol. 2003;77:8354–8365. doi: 10.1128/JVI.77.15.8354-8365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang Z.Q., Yang Y., Wilson J.M., Ertl H.C. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219:220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]
- 41.He Z. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000;270:146–161. doi: 10.1006/viro.2000.0271. [DOI] [PubMed] [Google Scholar]
- 42.Makimura M. Induction of antibodies against structural proteins of hepatitis C virus in mice using recombinant adenovirus. Vaccine. 1996;14:28–36. doi: 10.1016/0264-410x(95)00161-s. [DOI] [PubMed] [Google Scholar]
- 43.Mizuguchi H., Kay M.A. A simple method for constructing E1-and E1/E4-deleted recombinant adenoviral vectors. Hum. Gene Ther. 1999;10:2013–2017. doi: 10.1089/10430349950017374. [DOI] [PubMed] [Google Scholar]
- 44.Yeh P. Efficient dual transcomplementation of adenovirus E1 and E4 regions from a 293-derived cell line expressing a minimal E4 functional unit. J. Virol. 1996;70:559–565. doi: 10.1128/jvi.70.1.559-565.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H., O'Neal W., Morral N., Beaudet A.L. Development of a complementing cell line and a system for construction of adenovirus vectors with E1 and E2a deleted. J. Virol. 1996;70:7030–7038. doi: 10.1128/jvi.70.10.7030-7038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lusky M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J. Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorziglia M.I. Generation of an adenovirus vector lacking E1, E2a, E3, and all of E4 except open reading frame 3. J. Virol. 1999;73:6048–6055. doi: 10.1128/jvi.73.7.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinwaerder D.S., Carlson C.A., Lieber A. Generation of adenovirus vectors devoid of all viral genes by recombination between inverted repeats. J. Virol. 1999;73:9303–9313. doi: 10.1128/jvi.73.11.9303-9313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 50.Fallaux F.J. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- 51.ShiverJ.W., Keystone Symposia, Banff, 2003. In Keystone Symposia 2003.
- 52.Bett A.J., Prevec L., Graham F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youil R. Comparative analysis of the effects of packaging signal, transgene orientation, promoters, polyadenylation signals, and E3 region on growth properties of first-generation adenoviruses. Hum. Gene Ther. 2003;14:1017–1034. doi: 10.1089/104303403766682278. [DOI] [PubMed] [Google Scholar]
- 54.Ugai H. Stability of a recombinant adenoviral vector: optimization of conditions for storage, transport and delivery. Jpn. J. Cancer Res. 2002;93:598–603. doi: 10.1111/j.1349-7006.2002.tb01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chappell J.D., Prota A.E., Dermody T.S., Stehle T. Crystal structure of reovirus attachment protein sigma1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 2002;21:1–11. doi: 10.1093/emboj/21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mercier G.T. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 2004;101:6188–6693. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mercier S. Adenovirus fibre exchange alters cell tropism in vitro but not transgene-specific T CD8+immune responses in vivo. J. Gen. Virol. 2004;85:1227–1236. doi: 10.1099/vir.0.79846-0. [DOI] [PubMed] [Google Scholar]
- 58.Rea D. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 2001;166:5236–5244. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- 59.Worgall S. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J. Virol. 2004;78:2572–2580. doi: 10.1128/JVI.78.5.2572-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereboev A.V. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol. Ther. 2004;9:712–720. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Taguchi T. Detection of individual mouse splenic T cells producing IFN-gamma and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J. Immunol. Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 62.Labalette-Houache M., Torpier G., Capron A., Dessaint J.P. Improved permeabilization procedure for flow cytometric detection of internal antigens: analysis of interleukin-2 production. J. Immunol. Methods. 1991;138:143–153. doi: 10.1016/0022-1759(91)90162-9. [DOI] [PubMed] [Google Scholar]
- 63.Altman J.D. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 64.Crawford F., Kozono H., White J., Marrack P., Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y., Li Q., Ertl H.C., Wilson J.M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y., Ertl H.C., Wilson J.M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 67.Xiang Z.Q., Knowles B.B., McCarrick J.W., Ertl H.C. Immune effector mechanisms required for protection to rabies virus. Virology. 1995;214:398–404. doi: 10.1006/viro.1995.0049. [DOI] [PubMed] [Google Scholar]
- 68.Prevec L., Campbell J.B., Christie B.S., Belbeck L., Graham F.L. A recombinant human adenovirus vaccine against rabies. J. Infect. Dis. 1990;161:27–30. doi: 10.1093/infdis/161.1.27. [DOI] [PubMed] [Google Scholar]
- 69.Casimiro D.R. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y., Xiang Z., Pasquini S., Ertl H.C. The use of an E1-deleted, replication-defective adenovirus recombinant expressing the rabies virus glycoprotein for early vaccination of mice against rabies virus. J. Virol. 1997;71:3677–3683. doi: 10.1128/jvi.71.5.3677-3683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiang Z.Q. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 2003;77:10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharpe S. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology. 2002;293:210–216. doi: 10.1006/viro.2001.1281. [DOI] [PubMed] [Google Scholar]
- 73.Pinto A.R., Fitzgerald J.C., Gao G.P., Wilson J.M., Ertl H.C. Induction of CD8+ T cells to an HIV-1 antigen upon oral immunization of mice with a simian E1-deleted adenoviral vector. Vaccine. 2004;22:697–703. doi: 10.1016/j.vaccine.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 74.Wilde H., Briggs D.J., Meslin F.X., Hemachudha T., Sitprija V. Rabies update for travel medicine advisors. Clin. Infect. Dis. 2003;37:96–100. doi: 10.1086/375605. [DOI] [PubMed] [Google Scholar]
- 75.Lemiale F. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 2003;77:10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith J.G. Intracranial administration of adenovirus expressing HSV-TK in combination with ganciclovir produces a dose-dependent, self-limiting inflammatory response. Hum. Gene Ther. 1997;8:943–954. doi: 10.1089/hum.1997.8.8-943. [DOI] [PubMed] [Google Scholar]
- 77.Gurwith M.J. Current use and future directions of adenovirus vaccine. Semin. Respir. Infect. 1989;4:299–303. [PubMed] [Google Scholar]
- 78.Top F.H., Jr. Control of adenovirus acute respiratory disease in U.S. Army trainees. J. Yale, Biol. Med. 1975;48:185–195. [PMC free article] [PubMed] [Google Scholar]
- 79.Tsang K.W. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 80.Drosten C. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 81.Gao W. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson J.A. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan N.J. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burton D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 85.McMichael A.J., Hanke T. HIV vaccines 1983–2003. Nat. Med. 2003;9:874–880. doi: 10.1038/nm0703-874. [DOI] [PubMed] [Google Scholar]
- 86.Vinner L. Immunogenicity in Mamu-A*01 rhesus macaques of a CCR5-tropic human immunodeficiency virus type 1 envelope from the primary isolate (Bx08) after synthetic DNA prime and recombinant adenovirus 5 boost. J. Gen. Virol. 2003;84:203–213. doi: 10.1099/vir.0.18589-0. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida T. Activation of HIV-1-specific immune responses to an HIV-1 vaccine constructed from a replication-defective adenovirus vector using various combinations of immunization protocols. Clin. Exp. Immunol. 2001;124:445–452. doi: 10.1046/j.1365-2249.2001.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruce C.B. Replication-deficient recombinant adenoviruses expressing the human immunodeficiency virus Env antigen can induce both humoral and CTL immune responses in mice. J. Gen. Virol. 1999;80:2621–2628. doi: 10.1099/0022-1317-80-10-2621. [DOI] [PubMed] [Google Scholar]
- 89.Casimiro D.R. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003;77:7663–7668. doi: 10.1128/JVI.77.13.7663-7668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiver J.W. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 91.Patterson L.J., Malkevitch N., Zhao J., Peng B., Robert-Guroff M. Potent, persistent cellular immune responses elicited by sequential immunization of rhesus macaques with Ad5 host range mutant recombinants encoding SIV Rev and SIV Nef. DNA Cell Biol. 2002;21:627–635. doi: 10.1089/104454902760330165. [DOI] [PubMed] [Google Scholar]
- 92.Patterson L.J. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus–SIV multigene vaccine priming and subunit boosting. J. Virol. 2004;78:2212–2221. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiang Z. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiang Z.Q., Pasquini S., Ertl H.C. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J. Immunol. 1999;162:6716–6723. [PubMed] [Google Scholar]
- 95.Yang Z.Y. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacGregor R.R. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 97.O'Riordan C.R. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 98.Fisher K.D., Stallwood W., Ulbrich K., Mautner V., Seymour L.W. Protection and retargeting of adenovirus using a multifunctional hydrophilic polymer. Mol. Ther. 2000;1S:57. [Google Scholar]
- 99.Sailaja G., HogenEsch H., North A., Hays J., Mittal S.K. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Youil R. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 2002;13:311–320. doi: 10.1089/10430340252769824. [DOI] [PubMed] [Google Scholar]
- 101.Hofmann C. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reyes-Sandoval A. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 2004;78:7392–7399. doi: 10.1128/JVI.78.14.7392-7399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varnavski A.N., Schlienger K., Bergelson J.M., Gao G.P., Wilson J.M. Efficient transduction of human monocyte-derived dendritic cells by chimpanzee-derived adenoviral vector. Hum. Gene Ther. 2003;14:533–544. doi: 10.1089/104303403764539323. [DOI] [PubMed] [Google Scholar]
- 104.Gao W., Robbins P.D., Gambotto A. Human adenovirus type 35: nucleotide sequence and vector development. Gene Ther. 2003;10:1941–1949. doi: 10.1038/sj.gt.3302097. [DOI] [PubMed] [Google Scholar]
- 105.Sakurai F., Mizuguchi H., Yamaguchi T., Hayakawa T. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol. Ther. 2003;8:813–821. doi: 10.1016/s1525-0016(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 106.Barouch D.H. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 107.Lubeck M.D. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 108.Pinto A.R. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J. Immunol. 2003;171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- 109.Cohen J. AIDS research: Merck reemerges with a bold AIDS vaccine effort. Science. 2001;292:24–25. doi: 10.1126/science.292.5514.24. [DOI] [PubMed] [Google Scholar]
- 110.Xiang Z., Li Y., Gao G., Wilson J.M., Ertl H.C. Mucosally delivered E1-deleted adenoviral vaccine carriers induce transgene product-specific antibody responses in neonatal mice. J. Immunol. 2003;171:4287–4293. doi: 10.4049/jimmunol.171.8.4287. [DOI] [PubMed] [Google Scholar]
- 111.Xiang Z., Ertl H.C. Induction of mucosal immunity with a replication-defective adenoviral recombinant. Vaccine. 1999;17:2003–2008. doi: 10.1016/s0264-410x(98)00449-6. [DOI] [PubMed] [Google Scholar]
- 112.Vos A. Immunogenicity of an E1-deleted recombinant human adenovirus against rabies by different routes of administration. J. Gen. Virol. 2001;82:2191–2197. doi: 10.1099/0022-1317-82-9-2191. [DOI] [PubMed] [Google Scholar]
- 113.Jaiswal S., Khanna N., Swaminathan S. Replication-defective adenoviral vaccine vector for the induction of immune responses to dengue virus type 2. J. Virol. 2003;77:12907–12913. doi: 10.1128/JVI.77.23.12907-12913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.Y., Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 115.Tobery T.W. Effect of vaccine delivery system on the induction of HPV16L1-specific humoral and cell-mediated immune responses in immunized rhesus macaques. Vaccine. 2003;21:1539–1547. doi: 10.1016/s0264-410x(02)00679-5. [DOI] [PubMed] [Google Scholar]
- 116.Liu D.W. Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth. J. Virol. 2000;74:9083–9089. doi: 10.1128/jvi.74.19.9083-9089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park S.H. Efficient induction of T helper 1 CD4+T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine. 2003;21:4555–4564. doi: 10.1016/s0264-410x(03)00499-7. [DOI] [PubMed] [Google Scholar]
- 118.Matsui M., Moriya O., Akatsuka T. Enhanced induction of hepatitis C virus-specific cytotoxic T lymphocytes and protective efficacy in mice by DNA vaccination followed by adenovirus boosting in combination with the interleukin-12 expression plasmid. Vaccine. 2003;21:1629–1639. doi: 10.1016/s0264-410x(02)00704-1. [DOI] [PubMed] [Google Scholar]
- 119.Arribillaga L. Vaccination with an adenoviral vector encoding hepatitis C virus (HCV) NS3 protein protects against infection with HCV-recombinant vaccinia virus. Vaccine. 2002;21:202–210. doi: 10.1016/s0264-410x(02)00456-5. [DOI] [PubMed] [Google Scholar]
- 120.Seong Y.R. Immunogenicity of the E1E2 proteins of hepatitis C virus expressed by recombinant adenoviruses. Vaccine. 2001;19:2955–2964. doi: 10.1016/s0264-410x(00)00534-x. [DOI] [PubMed] [Google Scholar]
- 121.Hung P.P. Adenovirus vaccine strains genetically engineered to express HIV-1 or HBV antigens for use as live recombinant vaccines. Nat. Immun. Cell Growth Regul. 1990;9:160–164. [PubMed] [Google Scholar]
- 122.Lubeck M.D. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc. Natl. Acad. Sci. USA. 1989;86:6763–6767. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu Z.Z., Krougliak V., Prevec L., Graham F.L., Both G.W. Investigation of promoter function in human and animal cells infected with human recombinant adenovirus expressing rotavirus antigen VP7sc. J. Gen. Virol. 1995;76:1971–1980. doi: 10.1099/0022-1317-76-8-1971. [DOI] [PubMed] [Google Scholar]
- 124.Both G.W. Protective immunity to rotavirus-induced diarrhoea is passively transferred to newborn mice from naive dams vaccinated with a single dose of a recombinant adenovirus expressing rotavirus VP7sc. Virology. 1993;193:940–950. doi: 10.1006/viro.1993.1203. [DOI] [PubMed] [Google Scholar]
- 125.Fooks A.R. Oral or parenteral administration of replication-deficient adenoviruses expressing the measles virus haemagglutinin and fusion proteins: protective immune responses in rodents. J. Gen. Virol. 1998;79:1027–1031. doi: 10.1099/0022-1317-79-5-1027. [DOI] [PubMed] [Google Scholar]
- 126.Fooks A.R. High-level expression of the measles virus nucleocapsid protein by using a replication-deficient adenovirus vector: induction of an MHC-1-restricted CTL response and protection in a murine model. Virology. 1995;210:456–465. doi: 10.1006/viro.1995.1362. [DOI] [PubMed] [Google Scholar]
- 127.Alkhatib G., Briedis D.J. High-level eucaryotic in vivo expression of biologically active measles virus hemagglutinin by using an adenovirus type 5 helper-free vector system. J. Virol. 1988;62:2718–2727. doi: 10.1128/jvi.62.8.2718-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsu K.H. Immunogenicity of recombinant adenovirus-respiratory syncytial virus vaccines with adenovirus types 4, 5, and 7 vectors in dogs and a chimpanzee. J. Infect. Dis. 1992;166:769–775. doi: 10.1093/infdis/166.4.769. [DOI] [PubMed] [Google Scholar]
- 129.Connors M. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia–RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 130.Shanley J.D., Wu C.A. Mucosal immunization with a replication-deficient adenovirus vector expressing murine cytomegalovirus glycoprotein B induces mucosal and systemic immunity. Vaccine. 2003;21:2632–2642. doi: 10.1016/s0264-410x(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 131.Berencsi K. Murine cytotoxic T cell response specific for human cytomegalovirus glycoprotein B (gB) induced by adenovirus and vaccinia virus recombinants expressing gB. J. Gen. Virol. 1993;74:2507–2512. doi: 10.1099/0022-1317-74-11-2507. [DOI] [PubMed] [Google Scholar]
- 132.Gallichan W.S., Rosenthal K.L. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 1998;177:1155–1161. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 133.Gallichan W.S., Rosenthal K.L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 134.Ragot T., Finerty S., Watkins P.E., Perricaudet M., Morgan A.J. Replication-defective recombinant adenovirus expressing the Epstein–Barr virus (EBV) envelope glycoprotein gp340/220 induces protective immunity against EBV-induced lymphomas in the cottontop tamarin. J. Gen. Virol. 1993;74:501–507. doi: 10.1099/0022-1317-74-3-501. [DOI] [PubMed] [Google Scholar]
- 135.Duraiswamy J. Induction of therapeutic T-cell responses to subdominant tumor-associated viral oncogene after immunization with replication-incompetent polyepitope adenovirus vaccine. Cancer Res. 2004;64:1483–1489. doi: 10.1158/0008-5472.can-03-2196. [DOI] [PubMed] [Google Scholar]