Abstract
Exosomes are small membrane vesicles that are secreted by a multitude of cell types. The exosomes derived from dendritic cells (Dex), tumor cells (Tex), and malignant effusions demonstrate immunomodulatory functions, and are even under clinical trial for cancer treatments. In this study we report the phase I clinical trial of the ascites-derived exosomes (Aex) in combination with the granulocyte–macrophage colony-stimulating factor (GM-CSF) in the immunotherapy of colorectal cancer (CRC). The Aex isolated by sucrose/D2O density gradient ultracentrifugation are 60–90-nm vesicles that contain the diverse immunomodulatory markers of exosomes and tumor-associated carcinoembryonic antigen (CEA). Totally 40 patients (HLA-A0201+CEA+) with advanced CRC were enrolled in the study, and randomly assigned to treatments with Aex alone or Aex plus GM-CSF. Patients in both groups received a total of four subcutaneous immunizations at weekly intervals. We found that both therapies were safe and well tolerated, and that Aex plus GM-CSF but not Aex alone can induce beneficial tumor-specific antitumor cytotoxic T lymphocyte (CTL) response. Therefore, our study suggests that the immunotherapy of CRC with Aex in combination with GM-CSF is feasible and safe, and thus can serve as an alternative choice in the immunotherapy of advanced CRC.
Introduction
Exosomes are a type of 60–90-nm small vesicles secreted by many types of cells, including dendritic cells (DCs) and tumor cells.1,2,3,4,5,6 It has been shown that exosomes are highly enriched in major histocompatibility complex (MHC) class II molecules, MHC class I, heat shock proteins (HSPs), adhesion molecules, and other functional cellular components.7,8 Exosomes produced by numerous cell types can be defined by three major criteria: a size of 60–90 nm in diameter, a density of 1.13–1.21 g/dl in a sucrose gradient and an endocytic origin.1,2,3,4 It has been demonstrated previously that exosomes play functional roles in eradication of obsolete proteins, antigen presentation, or act as “Trojan horses” for viruses or prions.1,2,3,4
Recently, exosomes have been the focus of immunologists because exosomes derived from either DC (Dex) or tumor cells (Tex) can serve as a kind of cell-free vaccine and can induce potent antitumor immunity in animal models.5,6 Dex are able to present antigens to T cells (directly and indirectly) and stimulate antigen-specific T-cell responses.5,9,10,11,12,13,14,15 When used as a tumor vaccine, Dex loaded with acid-eluted tumor peptides can eradicate established tumors in mice.5 Most tumor cells constitutively release exosomes (Tex) in the extracellular milieu. In mice, it has been revealed that Tex can also serve as antigen delivery systems and can prevent autologous tumor development in a CD4+ and CD8+ T cell–dependent manner.3,6 However, the isolation of autologous Tex may require the in vitro culture of primary tumor cells derived from cancer patients, which may be inconvenient in clinical trials. The findings by Andre et al. suggest that exosomes can be isolated from malignant effusions of melanoma patients and can deliver melanoma antigen recognized by T cells 1 (Mart-1) and tumor antigens to DC for cross presentation to specific cytotoxic T lymphocytes (CTLs).16 Most recently, the exosomes derived from malignant effusions of cancer patients have also been prepared and are ready for clinical trial.17,18,19 Thus, Aex may be used as cell-free tumor vaccine in the immunotherapy of cancer.
Dex have been tested for their safety and efficiency in clinical trials of patients with metastatic melanoma and advanced nonsmall cell lung cancer, which suggests that Dex derived from good manufacturing procedures (GMPs) grade are safe, feasible, and efficient in induction of antigen-specific T-cell responses.20,21 Moreover, the oligodeoxynucleotide CpG, double-stranded RNA, and lipopolysaccharide have been used as adjuvants in the Dex-mediated immunotherapy.15,22,23 Previously we, and others, have demonstrated that Tex modified with interleukin-18 (IL-18), IL-2, or superantigen, and Tex isolated after heat stress can elicit potent antitumor immunity in mice models.24,25,26,27,28 It has been extensively demonstrated that the granulocyte–macrophage colony-stimulating factor (GM-CSF), when used as adjuvant, can significantly increase the efficiency of antitumor immunity induction.29,30 Therefore, we evaluated whether ascites-derived exosomes (Aex) combined with GM-CSF (Aex plus GM-CSF) can be considered as a promising vaccine for cancer immunotherapy. We find that the Aex derived from colorectal cancer (CRC) patients in combination with GM-CSF can efficiently induce potent carcinoembryonic antigen (CEA)-specific antitumor immunity in advanced CRC patients, suggesting that Aex plus GM-CSF can serve as a choice in clinical immunotherapy of CRC.
Results
Patient characteristics and treatment schedule
From January 2006 to February 2007, totally 54 CRC patients were eligible according to the enrollment criteria. Forty patients (seventeen females and twenty-three males), who had assessable advanced CRC (pathologically diagnosed as stage III or IV as per the American Joint Committee on Cancer) and matched all the criteria (e.g., HLA-A*0201+CEA+ and malignant effusions), were enrolled in this trial. These patients were randomly assigned to treatments with autologous Aex alone (groups A–D, corresponding to 100, 200, 300, and 500 μg doses, respectively) or Alex (groups E–H, corresponding to 100, 200, 300, and 500 μg doses, respectively) plus 50 μg GM-CSF (Table 1 ). The mean age of all patients was 52.7 years. The mean age of all eight groups was as follows: group A, 52.8 years; group B, 51.4 years; group C, 52.8 years; group D, 53.0 years; group E, 50.2 years; group F, 54.2 years; group G, 55.6 years; group H, 51.8 years. Thirty-seven patients completed four vaccinations at weekly intervals. Three patients left the treatment, among whom two patients (A2 and H1) had progressive disease and one patient (C5) had serious adverse events unrelated to the vaccine (Table 1). During the treatment schedule, all the adverse events were recorded after the first vaccination. Also the antitumor immune responses were evaluated 2 weeks after the last vaccination.
Table 1.
Patient baseline characteristics and treatments
| Patient no. | Age (years)/ sex | AJCC status | Primary tumor sites | Sites of metastates | Previous therapy (cycles) | Aex dose (µg) | Vaccination (times) | |
|---|---|---|---|---|---|---|---|---|
| Aex | A1 | 36/M | IV | Colon | Liver | F4a (12) | 100 | 4 |
| A2 | 56/M | IV | Rectum | Liver, lung, bone | F4 (12) + 40 Gy | 100 | 3 | |
| A3 | 45/F | III | Colon | No | Surg + F4 (12) | 100 | 4 | |
| A4 | 60/F | IV | Colon | Liver, lung | De-Gb (12) + 40 Gy | 100 | 4 | |
| A5 | 67/M | IV | Colon | Omentum | F4 (12) | 100 | 4 | |
| B1 | 50/F | IV | Colon | Liver, LN | F4 (12) + 45 Gy | 200 | 4 | |
| B2 | 55/M | IV | Colon | Liver | F4 (12) + 45 Gy | 200 | 4 | |
| B3 | 42/F | IV | Colon | Lung | F6c (12) + 45 Gy | 200 | 4 | |
| B4 | 61/M | IV | Colon | Liver, spleen | F4 (12) + 50 Gy | 200 | 4 | |
| B5 | 49/M | IV | Rectum | Peritoneum | Fd (6) | 200 | 4 | |
| C1 | 37/M | IV | Colon | Mesenterium | F (6) | 300 | 4 | |
| C2 | 69/M | IV | Colon | Liver, LN | XELOXe (6) + 30 Gy | 300 | 4 | |
| C3 | 57/F | III | Rectum | No | Surg + F4 (12) | 300 | 4 | |
| C4 | 54/F | IV | Rectum | Liver | F4 (12) + 50 Gy | 300 | 4 | |
| C5 | 47/M | IV | Colon | Liver, lung, bone, LN | F6 (12) + 45 Gy | 300 | 2 | |
| D1 | 59/M | IV | Colon | Omentum | F4 (12) | 500 | 4 | |
| D2 | 62/F | III | Colon | No | Surg + F4 (12) | 500 | 4 | |
| D3 | 45/M | IV | Colon | Lung, liver | F6 (12) + 30 Gy | 500 | 4 | |
| D4 | 48/F | IV | Colon | Lung | F4 (12) + 40 Gy | 500 | 4 | |
| D5 | 51/M | IV | Colon | Liver | F6 (12) + 40 Gy | 500 | 4 | |
| E1 | 39/M | IV | Rectum | Lung | F6 (12) + F (4) + 50 Gy | 100 | 4 | |
| E2 | 55/M | IV | Colon | Liver | F4 (12) | 100 | 4 | |
| Aex + GM-CSF | E3 | 42/F | IV | Colon | Liver, spleen, ovaries | F (12) + 50 Gy | 100 | 4 |
| E4 | 65/F | IV | Colon | Liver | F4 (12) + 45 Gy | 100 | 4 | |
| E5 | 50/M | IV | Colon | Omentum | F4 (12) | 100 | 4 | |
| F1 | 58/F | IV | Colon | Lung, liver | F4 (12) + 45 Gy | 200 | 4 | |
| F2 | 53/M | IV | Rectum | Liver, omentum | F4 (12) + 45 Gy | 200 | 4 | |
| F3 | 49/F | IV | Colon | Lung, liver, bone | F4 (12) + F (6) + 45 Gy | 200 | 4 | |
| F4 | 51/F | IV | Colon | Omentum | F6 (12) | 200 | 4 | |
| F5 | 60/M | III | Colon | Liver | Capecitabinef (6) | 200 | 4 | |
| G1 | 66/M | IV | Colon | Bone, INa | De-G (12) + 40 Gy | 300 | 4 | |
| G2 | 64/F | IV | Colon | Liver, spleen, pelvis | F4 (12) | 300 | 4 | |
| G3 | 51/F | IV | Colon | Omentum | AIOg (6) | 300 | 4 | |
| G4 | 50/M | IV | Colon | Liver | F4 (12) | 300 | 4 | |
| G5 | 47/M | IV | Colon | Lung, liver | F6 (12) + 45 Gy | 300 | 4 | |
| H1 | 40/M | IV | Colon | Lung, liver, LN | F4 (12) + F (12) + 30 Gy | 500 | 3 | |
| H2 | 46/M | IV | Colon | Liver | F4 (12) + 40 Gy | 500 | 4 | |
| H3 | 62/F | IV | Colon | Omentum | XELOX (6) | 500 | 4 | |
| H4 | 56/F | IV | Colon | Lung | F4 (12) + 40 Gy | 500 | 4 | |
| H5 | 55/M | IV | Rectum | Lung, liver, bone | F4 (12) | 500 | 4 |
Abbreviations: Aex, ascites-derived exosomes; AJCC, American Joint Committee on Cancer; F, female; GM-CSF, granulocyte-macrophage colony-stimulating factor; LN, lymph node; M, male; Surg, surgery.
F4 stands for FOLFOX4 regimen; oxaliplatin (85 mg/m2, IV on day 1); leucovorin (200 mg/m2, IV on days 1 and 2); 5-fluorouracil (400 mg/m2, IV on days 1 and 2); and 5-fluorouracil (600 mg/m2, continuous infusion (CI on days 1 and 2). The regimen was repeated every 2 weeks. bDe-G stands for De-Gramont regimen; leucovorin (400 mg/m2 IV on days 1 and 2) and 5-fluorouracil (400 mg/m2, IV on days 1 and 2, plus 600 mg/m2 5-fluorouracil via CI on days 1 and 2). The regimen was repeated every 2 weeks. cF6 stands for FOLFOX6 regimen: oxaliplatin (100 mg/m2, IV on day 1); leucovorin (400 mg/m2, IV on day 1) and 5-fluorouracil (400 mg/m2 (IV) plus 600 mg/m2 5-fluorouracil bolus on days 1 and 2,400-3,000 mg/m2 5-fluorouracil via CI over 46 hours). The regimen was repeated every 2 weeks. dF stands for FOLFIRI regimen (Douillard); irinotecan (180 mg/m2, IV on day 1); leucovorin (200 mg/m2 IV on days 1 and 2); and 5-fluorouracil (400 mg/m2, IV on days 1 and 2, plus 600 mg/m2 5-fluorouracil via CI >22 hours on days 1 and 2). This regimen was repeated every 2 weeks. eXELOX regimen: oxaliplatin (130 mg/m2, IV on day 1); capecitabine (1,000 mg/m2, PO (bid, total dose was ~2,000 mg/m2/day) on days 1-14). This regimen was repeated every 3 weeks. ‘Capecitabine regimen: capecitabine (1,000 mg/m2, PO (bid, total dose was ~2,000 mg/m2/day) on days 1-14). This regimen was repeated every 3 weeks. gAIO regimen. leucovorin (500 mg/m2, IV once per week); and 5-fluorouracil (2,000 mg/m2, CI once per week). This regimen was repeated every 3 weeks.
Characterization of Aex derived from CRC patients
According to the method previously described by Navabi and Andre, exosomes were successfully prepared from malignant ascites of the enrolled 40 CRC patients (HLA-A*0201+CEA+) by sucrose/D2O gradient ultracentrifugation.16,18 To examine the isolated products, we performed electron microscopy and Western blot to confirm the morphology and protein components. We found that the isolated Aex were universal membrane vesicles with the diameter ∼60–100 nm (Figure 1a ). Western blotting analysis demonstrated that Aex were enriched in MHC molecules (MHC-I and MHC-II), HSPs (including HSC70, HSP70, and HSP90), CD80, ICAM-1, CD71, and LAMP-3 (Figure 1b). Notably, the typical CEA of CRC was also detected in the Aex. Because the capacity of exosomes in stimulating antitumor immune responses is largely dependent on the levels of MHC molecules and HSPs,3,4 we examined the expression of MHC-I and HSC70 as quality control standards for all the Aex preparations. Most of the prepared autologous Aex contained high levels of MHC-I and HSC70 (Figure 1c). These results (morphology, density, and protein components) suggested that the Aex prepared by us were exosomes.
Figure 1.
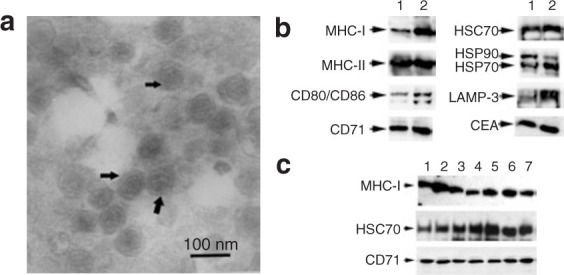
Characterization of Aex. (a) Electron microscopy assay of the isolated Aex. The data were representative of Aex derived from patient H5. Bar = 100 nm. (b) Western blot assay of the protein markers in Aex derived from patient H5. Thirty microgram of cell lysates derived from the ascites (lane 1) or Aex (lane 2) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and examined by Western blot. (c) Western blot assay of major histocompatibility complex class I (MHC-I), HSC70, and CD71 contained in Aex derived from representative patients. Lanes 1–7 correspond to Aex (30 μg per lane) derived from patients A1, B1, C1, D1, E1, F1, and G1, respectively.
Safety and early clinical outcome
Generally, both the therapies were safe and well tolerated. A total of 79 adverse events were recorded throughout the treatments in all the groups (Table 2 ). The most frequently reported adverse events causally related to the use of Aex or Aex plus GM-CSF were mild (grade 1–2) in severity and included injection site reactions (37 events in groups A–D and 42 events in groups E–H) including erythema, pruritus, or pain, fever (patient H2), nausea (patient H4), fatigue (patients D1, G2, and H5) (Table 2). There were no significant hepatic, renal, pulmonary, cardiac, hematologic, or neurologic toxicities attributable to the treatments. No clinical manifestations of autoimmune reactions were observed. No significant changes in temperature and blood pressure were recorded. After the last immunization, the status of CRC patients was followed. No detectable therapeutic response was observed in groups A–D, which were treated with Aex (100–500 μg per vaccination) alone. However, one case with a stable disease (patient G1 who was treated with 300 μg Aex plus 50 μg GM-CSF) and one case with a minor response (patient H3 who was treated with 500 μg Aex plus 50 μg GM-CSF) were observed in the Aex plus GM-CSF group (data not shown).
Table 2.
Adverse events observed during the treatments
| Adverse eventsa (gradeb) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Patient number | Erythema | Pain | Pruritus | Fatigue | Nausea | Fever | Total | |
| Aex | A1 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 |
| A2 | +(1-2) | +(1-2) | 0 | 0 | 0 | 0 | 2 | |
| A3 | 0 | 0 | +(1-2) | 0 | 0 | 0 | 1 | |
| A4 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| A5 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| B1 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| B2 | 0 | +(1-2) | 0 | 0 | 0 | 0 | 1 | |
| B3 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| B4 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| B5 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| C1 | +(1-2) | +(1-2) | 0 | 0 | 0 | 0 | 2 | |
| C2 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| C3 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| C4 | +(1-2) | +(1-2) | 0 | 0 | 0 | 0 | 2 | |
| C5 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| D1 | +(1-2) | 0 | +(1-2) | +(1-2) | 0 | 0 | 3 | |
| D2 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| D3 | +(1-2) | +(1-2) | +(1-2) | 0 | 0 | 0 | 3 | |
| D4 | +(1-2) | +(1-2) | 0 | 0 | 0 | 0 | 2 | |
| D5 | +(1-2) | +(1-2) | +(1-2) | 0 | 0 | 0 | 3 | |
| 18 | 7 | 11 | 1 | 0 | 0 | 37c | ||
| Aex + GM-CSF | E1 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 |
| E2 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| E3 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| E4 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| E5 | 0 | +(1-2) | 0 | 0 | 0 | 0 | 1 | |
| F1 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| F2 | +(1-2) | +(1-2) | 0 | 0 | 0 | 0 | 2 | |
| F3 | +(1-2) | 0 | 0 | 0 | 0 | 0 | 1 | |
| F4 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| F5 | +(1-2) | +(1-2) | +(1-2) | 0 | 0 | 0 | 3 | |
| G1 | +(1-2) | +(1-2) | +(1-2) | 0 | 0 | 0 | 3 | |
| G2 | +(1-2) | 0 | 0 | +(1-2) | 0 | 0 | 2 | |
| G3 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| G4 | +(1-2) | +(1-2) | 0 | 0 | 0 | 0 | 2 | |
| G5 | +(1-2) | 0 | +(1-2) | 0 | 0 | 0 | 2 | |
| H1 | +(1-2) | +(1-2) | +(1-2) | 0 | 0 | 0 | 3 | |
| H2 | +(1-2) | +(1-2) | 0 | 0 | 0 | +(1-2) | 3 | |
| H3 | +(1-2) | +(1-2) | +(1-2) | 0 | 0 | 0 | 3 | |
| H4 | +(1-2) | 0 | +(1-2) | 0 | +(1-2) | 0 | 3 | |
| H5 | +(1-2) | 0 | +(1-2) | +(1-2) | 0 | 0 | 3 | |
| 19 | 8 | 11 | 2 | 1 | 1 | 42d | ||
Abbreviations: Aex, ascites-derived exosomes; GM-CSF, granulocyte-macrophage colony-stimulating factor.
The adverse events were recorded from the day of the first immunization of 2 weeks after the last immunization. Toxicity was assessed throughout the study using the National Cancer Institute Common Toxicity Criteria version 2.0. cThe total adverse events in the Aex-treated groups were summarized and presented. dThe total adverse events in the Aex + GM-CSF-treated groups were summarized and presented.
Antitumor immune responses elicited by the treatments
Previously it has been shown that Dex, Tex, and Aex can induce antigen-specific T-cell responses and tumor growth regression.5,6,9,10,11,12,13,14,15,16 The above results indicated that Aex contained plenty of immune molecules as well as CEA, and the Aex plus GM-CSF treatments could improve the clinical outcomes of CRC patients to some extent. Therefore, we went further to examine whether the immunotherapies with Aex or Aex plus GM-CSF induced systemic antigen-specific antitumor immunity both in vitro and in vivo. First we performed the antigen recall experiments by examining the delayed-type hypersensitivity (DTH) response in CRC patients 2 weeks after the last immunization. We found that Aex immunization at a dose of 300 or 500 μg could efficiently induce DTH responses [100% in groups C (4/4), D (5/5), G (5/5), and H (4/4)] in the presence or absence of GM-CSF adjuvants (Table 3 ). However, when lower doses of Aex (200 μg per immunization) were used, GM-CSF could significantly increase the efficiency of Aex in the induction of DTH, as evidenced by the differences between the DTH response rate of group B (1/5, 20%) and that of group F (3/5, 60%) (Table 3). When a dose of 100 μg of Aex was used, poor DTH responses were recorded [25% (1/4) in group A versus 20% (1/5) in group E] (Table 3). The detection of DTH responses suggested that Aex could potentially induce systemic antitumor responses in vivo.
Table 3.
Immunological assessment of the treatments
| CTL induction |
DTH induction (mmd) |
|||||
|---|---|---|---|---|---|---|
| Patient number | CD8+ tetramer+ lymphocytesa | Cytotoxicityb | IFN-γ releasec (pg/ml) | Baseline | Post-treatments | |
| Aex | A1 | ND | ND | ND | <2 | <2 |
| A2 | ND | ND | ND | <2 | ND | |
| A3 | ND | ND | ND | <2 | <2 | |
| A4 | ND | ND | ND | <2 | <2 | |
| A5 | 0.04 | ND | ND | <2 | <2 | |
| B1 | ND | ND | ND | <2 | <2 | |
| B2 | ND | ND | ND | <2 | <2 | |
| B3 | 0.02 | ND | ND | <2 | 4 | |
| B4 | ND | ND | ND | <2 | <2 | |
| B5 | ND | ND | ND | <2 | <2 | |
| C1 | 2.71 | 34.1 (10.3) | 1,109 (239) | 3 | 13 | |
| C2 | 0.03 | ND | ND | <2 | 6 | |
| C3 | 0.04 | ND | ND | <2 | 8 | |
| C4 | 0.02 | ND | ND | <2 | 6 | |
| C5 | ND | ND | ND | <2 | ND | |
| D1 | 0.03 | ND | ND | <2 | 3 | |
| D2 | 0.02 | ND | ND | 3 | 3 | |
| D3 | 1.53 | 29.3 (6.5) | 1,007 (225) | 3 | 9 | |
| D4 | 0.04 | ND | ND | <2 | 3 | |
| D5 | ND | ND | ND | <2 | 3 | |
| Aex + GM-CSF | E1 | ND | ND | ND | <2 | <2 |
| E2 | 2.01 | 31.9 (12.3) | 1,331 (227) | 3 | 12 | |
| E3 | ND | ND | ND | <2 | <2 | |
| E4 | ND | ND | ND | <2 | <2 | |
| E5 | ND | ND | ND | <2 | <2 | |
| F1 | 1.31 | 30.7 (8.4) | 1,100 (250) | 3 | 12 | |
| F2 | 0.04 | ND | ND | <2 | 3 | |
| F3 | ND | ND | ND | <2 | <2 | |
| F4 | 1.12 | 16.8 (4.3) | 776 (258) | <2 | 6 | |
| F5 | ND | ND | ND | <2 | <2 | |
| G1 | 4.85 | 50.2 (5.3) | 1,651 (253) | 6 | 18 | |
| G2 | 3.15 | 43.3 (7.7) | 1,522 (289) | 6 | 9 | |
| G3 | 2.75 | 34.7 (9.9) | 1,203 (241) | <2 | 16 | |
| G4 | 1.70 | 25.6 (4.2) | 985 (251) | <2 | 6 | |
| G5 | 0.03 | 2.2 (5.1) | ND | <2 | 5 | |
| H1 | ND | ND | ND | <2 | ND | |
| H2 | 0.92 | 17.8 (4.1) | 801(264) | <2 | 7 | |
| H3 | 1.45 | 28.7 (3.4) | 1,105 (233) | 4 | 14 | |
| H4 | 1.23 | 19.1 (6.8) | 907 (247) | <2 | 8 | |
| H5 | 0.01 | ND | ND | <2 | 3 | |
Abbreviations: Aex, ascites-derived exosomes; CTL, cytotoxic T lymphocytes; DTH, delayed-type hypersensitivity; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; ND, not determined.
The results were presented as mean percentage of CD8+ tetramer (CAP-1)+ cells to that of total emigrated cells. bThe cytotoxicity was evaluated at the effector (DTH site-derived cultured cells) to target (SW480 cells) ratio of 50:1. The results were presented as mean percentage of cell lysis. The cytotoxicity against LoVo cells were used as control and presented as mean percentage of cell lysis in parentheses. cCD8+ T lymphocytes derived from DTH site-derived cultured cells were cocultured with CAP-1-pulsed T2 cells or Ssp-1-pulsed T2 cells (in parentheses) for 24 hours. IFN-γ in the coculture supernatant was measured by enzyme-linked immunosorbent assay were presented as mean level of IFN-γ. dThe DTH responses before the treatments (baseline) and 2 weeks after the treatments (post-treatments) against autologous Aex were evaluated. The results presented here were for diameter in millimeter of the response sites.
To further determine whether the antitumor immune response was specific for CRC, we examined the CTL response by tetramer staining, interferon-γ (IFN-γ) release assay, and cytotoxicity assay after isolating CD8+ T cells from the DTH response sites. The HLA-A*0201-restricted CEA peptide CAP-1 (YLSGANLNL) and the irrelevant HLA-A*0201-restricted coronavirus SARS-CoV spike protein peptide SSp-1 (RLNEVAKNL) were used in these assays. For the evaluation of the tetramer tests, we established a ratio of percentages (of CD8+tetramer+ cells specific to CAP-1 to that specific to SSp-1) of more than twofold as a positive one. We found that Aex alone at all the doses (100–500 μg) could not efficiently induce HLA-A*0201-restricted and CAP-1-specific CTL production (2 of the 10 tested patients are positive) while the GM-CSF combination could significantly (20% in the Aex group versus 76.9% in the Aex plus GM-CSF group, P < 0.01) promote the CTL production (10 of the 13 tested patients are positive) (Table 3). For the examination of CTL toxicity against CRC, we used SW480 cells (HLA-A2+CEA+) or LoVo cells (HLA-A2–CEA+) as targets, and we established a ratio of the percentage of specific lysis against SW480 to that against LoVo, when more than twofold as a positive one. We found that the Aex alone or the Aex plus GM-CSF treatments could both efficiently induce the Aex-specific CTL toxicity (Table 3). However, the GM-CSF could promote the induction of CTL cytotoxicity by Aex (Figure 2b and Table 3). For the evaluation of CAP-1-specific IFN-γ release, we used CAP-1-pulsed T2 cells as stimulators and SSp-1-pulsed T2 cells as negative control. We found that the Aex alone or the Aex plus GM-CSF treatments could both efficiently induce the CEA (CAP-1 peptide)-specific IFN-γ release of CD8+ T lymphocytes (Figure 2c and Table 3). Therefore, generally the GM-CSF could efficiently promote the induction of CEA-specific CTL production. Our data suggested that Aex plus GM-CSF treatment was more efficient than Aex alone in the induction of tumor antigen (CEA)-specific CTL response, which may favor a beneficial clinical outcome in CRC patients.
Figure 2.
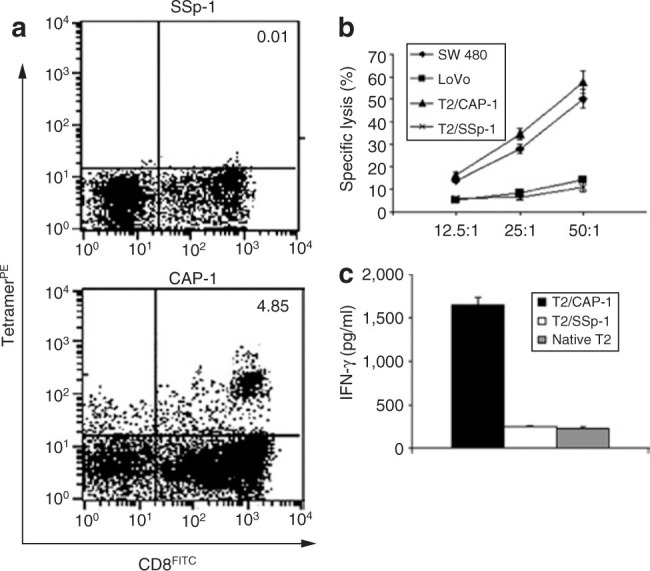
Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocyte (CTL) by the treatments. (a) Tetramer tests. Representative results derived from triplicate samples were shown. Delayed-type hypersensitivity (DTH) sites–derived emigrated cells were stained with phycoerythrin-labeled HLA-A*0201 tetramers (CAP-1 or SSp-1-specific) and fluorescein isothiocyanate (FITC)-conjugated CD8 monoclonal antibody and finally analyzed by flow cytometry. Numbers indicated for percentages of cells with positive staining. (b) Cytotoxicity assay. DTH site–derived cultured cells were cocultured with 51Cr-labeled SW480 cells, LoVo cells, T2 cells pulsed with CAP-1 (T2/CAP-1), or SSp-1 (T2/SSp-1) peptide. Specific cytotoxicity was evaluated by 51Cr release assay. Results were presented as the mean percentage of specific lysis ± SD of triplicate samples. (c) Interferon-γ (IFN-γ) release assay. CD8+ T cells isolated from the DTH site–derived cultured cells were cocultured with native T2 cells or T2/CAP-1 or T2/SSp-1 for 24 hours. IFN-γ level in the culture supernatant was determined by enzyme-linked immunosorbent assay, and the results were presented as mean ± SD of triplicate samples. Data presented here were the results derived from patient G1 who was treated with 300 μg Aex plus 50 μg granulocyte–macrophage colony-stimulating factor.
Discussion
CRC is one of the common gastrointestinal diseases encountered in clinical practice. Malignant ascites may be present when CRC cells metastase and are seeded in the peritoneal cavity. Patients with malignant ascites are always correlated with poor prognosis in clinical practice.31 However, isolation of exosomes contained in effusions and the induction of antitumor immune responses by Aex may be beneficial in clinical therapy of CRC patients with ascites. Immunotherapy of CRC with malignant ascites has not been performed in clinical trials. In this study, we have assessed the feasibility, safety, and efficiency of Aex plus GM-CSF in the treatment of CRC. We have demonstrated that Aex plus GM-CSF treatment is more efficient than Aex alone in the induction of tumor-specific systemic antitumor immunity and CTL responses. The treatment protocol tested in this study causes grade I or II toxicity in CRC patients, suggesting that the successfully isolated Aex can be safely applied in clinical trials. Therefore, our phase I clinical trial data suggest that the Aex from CRC patients can be selected to induce antitumor immunity, and the presence of GM-CSF as adjuvant can significantly promote the efficiency of the vaccine Aex.
We show in this study that Aex contain CEA, MHC molecules, and HSPs, which may be recognized by epidermic antigen-presenting cells and, in turn, stimulate the activation of T lymphocytes. Our in vivo DTH tests have confirmed that Aex alone are sufficient in the induction of systemic anti-Aex immunity, suggesting that the Aex alone are immunogenic. Our detection of CTL responses in the DTH sites–derived cultured cells indicates that Aex can activate CD8+ CTL and may elicit tumor antigen (e.g., CEA)-specific antitumor immunity. However, it is surprising that Aex alone, even at doses >200 μg, are rather inefficient in the induction of tumor-specific CTL responses, which may be due to the poor immunogenecity of Tex. Both Dex and Tex have been explored as cancer vaccines.5,6,20,21 The studies show that both Tex and Dex are efficient in the induction of tumor-specific immunity and the regression of tumor progression.5,6 Although Dex are a potent vaccine in tumor immunotherapy, controversial conclusions regarding the efficiency of Tex in tumor immunotherapy have been drawn.32,33,34,35,36 There is evidence suggesting that Tex may be a kind of tumor escape mechanism and can induce tolerance of the host to tumor antigens.32,33,34 Interestingly, the immunosuppressive cytokines, including IL-10 and tumor growth factor-β, have been detected in the Tex.32 Therefore, Tex may be of poor immunogenicity in clinical patients. However, exosomes derived from malignant effusions of melanoma patients are efficient in delivering melanoma antigens to autologous DC in vitro, suggesting that Aex may need the assistance of competent DC to present the antigens contained in Aex (either as an MHC–antigen peptide complex or as HSP-chaperoned antigens) to T cells in vivo.16 The malignant effusions, ascites, used in our study may also contain DC, T cells, methothelial cells in except of CRC cells. Therefore, the Aex we have tested may be a mixture of exosomes derived from various origins. Because exosomes derived from different origins are similar in morphology, gradient density, and protein components,1,2 it is hard to speculate which kind of exosomes are involved in the observed immune responses and clinical outcomes. However, based on the previous studies,16,18,19 the detection of CEA in Aex and the cytology examination of peritoneal cast-off cells (data not shown), we suggest here that the major exosomes were derived from CRC cells seeded in the peritoneal cavity, and the exosomes derived from DC, T cells, and methothelial cells may also contribute to the induction of host immune response against CRC. Further examination of specific markers for these cells may help to answer this question.
Results of our previous work have shown that exosomes prepared from heat-stressed CEA-positive tumor cells can induce and enhance HLA-A*0201-restricted and CEA-specific CTL response in vivo and in vitro because these exosomes accumulate HSPs and MHC-I molecules.24,26 It has also been demonstrated by the others that an exosome-based cancer vaccine can enhance the host immune responses against tumors in combination with CpG oligodeoxynucleotides or double-stranded RNA.22,23 GM-CSF has potential properties as a vaccine adjuvant and has been widely used as adjuvant in clinical trials of cancer vaccines.29,30 GM-CSF is a factor that can mediate the maturation and function of DC, and can increase the expression of MHC molecules and costimulators.37,38 Studies have shown that tumor cells either transfected with GM-CSF genes or mixed with GM-CSF biodegradable capsules are able to induce specific immune responses in vitro and in vivo.39,40 In this study, we selected a 50 μg dose of GM-CSF as adjuvant because the GM-CSF may enhance the vaccine-induced immune response at low doses (range 40–80 μg) but suppress immune function at higher doses (100–500 μg).41,42 Therefore, it can be expected that GM-CSF co-administration with Aex may lead to the enhancement of the efficiency of the Aex vaccine by promoting antigen presentation and T-cell activation, which is verified by our findings that the co-administration of Aex with GM-CSF is superior to Aex alone in inducing HLA-A*0201-restricted and CEA-specific CTL responses. Therefore, the immunogenic potential and efficiency of Aex still remain to be investigated in clinical trials. Trials with Aex in combination with other adjuvants may improve the efficiency of Aex in CRC immunotherapy.
To sum up, our study has shown that Aex is safe, nontoxic, and tolerable when used as a cancer vaccine, and GM-CSF is a positive adjuvant in the induction of antitumor immune responses in Aex-mediated immunotherapy. Our study may suggest that Aex in combination with GM-CSF may be an alternative choice for CRC immunotherapy in the future. Further characterization of Aex (e.g., the detection of immunosuppressive cytokines as quality control, the exact cellular origin of exosomes in Aex) and the optimization of the treatment plan will greatly increase the clinical outcomes of Aex-based immunotherapy.
Materials and Methods
Patients. This phase I clinical protocol was approved by the ethics committee and institutional review board of the Fourth Hospital Affiliated to Guangxi Medical University and conducted in compliance with the Helsinki Declaration. To be eligible, patients had to fulfill the following inclusion criteria: (i) age ≥18 years, (ii) histological diagnosis of CRC, (iii) an Eastern Cooperative Oncology Group performance status ≤3, (iv) HLA-A*0201+, (v) CEA+ in serum, (vi) malignant peritoneal effusions without hemorrhage and with a protein concentration >30 g/l. Exclusion criteria included (i) pregnancy or lactation, (ii) neutrophil count ≤0.5 × 109/l or a platelet count ≤20 × 109/l, (iii) anti-HIV antibodies (Abs), (iv) an active infection, (v) contraindications for leukapheresis, and (vi) chemotherapy or radiotherapy undertaken within the last 4 weeks before the first dose. All the patients with pathologically diagnosed CRC were unresponsive to or reluctant to accept the chemotherapy and radiotherapy.
Materials and cell lines. The primary Abs used in this study included the anti-human CEA (BD Biosciences, Becton Dickinson, CA) and the Abs against HSC70, HSP70, HSP90, CD71, LAMP-3, MHC-I, and MHC-II (Santa Cruz Biotechnology, Santa Cruz, CA). The horseradish peroxidase–coupled secondary Abs were from Santa Cruz Biotechnology. Human GM-CSF was purchased from Schering-Plough China (Shanghai, China). Roswell Park Memorial Institute medium-1640, fetal bovine serum were purchased from Hyclone (Logan, UT). Recombination human IL-2 was purchased from Sigma (St. Louis, MO). Human IFN-γ enzyme-linked immunosorbent assay kit was purchased from R&D systems (Minneapolis, MN). 51Cr sodium chromate was purchased from Amersham Pharmacia Biotech (Arlington Heights, IL). The CEA peptide CAP-1 and coronavirus SAS-CoV spike protein peptide SSp-1 were synthesized by GL Biochem (Shanghai, China), the purity of which was >95%. The phycoerythrin-labeled HLA-A*0201/CAP-1-tetramers were from ProImmune (Oxford BioBusiness Centre, Littlemore Park, Oxford, UK). The phycoerythrin-labeled HLA-A*0201/SSp-1-tetramers were a kind gift from Dr. X. Cao (Institute of Immunology, Second Military Medical University, P.R. China).24 The human colon adenocarcinoma SW480 (HLA-A2+CEA+) and LoVo (HLA-A2–CEA+) cell lines, and the transporter associated with antigen processing (TAP)-deficient T2 cell lines were obtained from American Type Culture Collection (Manassas, VA) and maintained with defined culture medium according to the supplier's specifications.
Preparation of exosomes from ascites of patients. The Aex were prepared according to a modified method previously described under GMP conditions.16,18 Briefly, 800 ml of malignant ascites from the enrolled patients were collected in a bag containing anticoagulant. Then, cell debris and protein aggregates of the ascites were removed by preliminary centrifugation at 300g. After centrifugations successively at 800g for 30 minutes, 10,000g for 30 minutes, and 100,000g for 1 hour, the pellet was collected, resuspended in phosphate-buffered saline (PBS) and centrifuged at 90,000g for 1.25 hours to remove irrelevent cell debris. After ultracentrifugation on 30% sucrose/D2O density gradient at 100,000g for 2 hours, Aex contained in sucrose were collected and resuspended in PBS, then ultracentrifuged at 100,000g for 1 hour. Finally, the Aex pellet was resuspended in PBS and stored for use at –80 °C. All the Aex were determined for protein concentration by Bradford assay (BioRad). Endotoxin of all exosomes is free as confirmed by Limulus amebocyte lysate assay.
Electron microscopy. The purified Aex were fixed for 1 hour in 4% paraformaldehyde and washed once with PBS. Then, the pellets were fixed in 2.5% glutaraldehyde, loaded on Formwar-/carbon-coated EM grids, postfixed in 1% glutaraldehyde, and contrasted successively in 2% methycellulose/0.4% uranyl acetate (pH 4.0) as described previously.24 Observations were made with Philips EM410 electron microscopy (Eindhoven, the Netherlands).
Western blotting. Analysis of Western blotting was done according to the protocol described previously by us.24 Briefly, same amount (30 μg) of Aex or whole cell lysate was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, transferred to nitrocellulose membranes, incubated with specific primary Abs at the supplier's recommended dilutions, and finally followed by horseradish peroxidase–coupled secondary Abs and chemiluminescence detection.
Randomization and treatment plan. Forty patients with advanced CRC were enrolled after giving written informed consent. These patients were randomly assigned to eight groups. Patients in groups A–D received 100, 200, 300, and 500 μg autologous Aex (in 100 μl PBS) subcutaneous immunization, respectively, in the forearm while patients in groups E–G received 100, 200, 300, and 500 μg autologous Aex plus 50 μg GM-CSF (in 100 μl PBS) subcutaneous immunization, respectively, in the forearm. The enrolled patients received a total of four immunizations at weekly intervals.
Clinical monitoring. In this study, assessment of the CRC patients was performed at baseline and 2 weeks after a course of vaccination (28 days) according to the RECIST criteria. Tumor progression (progressive disease): ≥20% increase of target lesions or the appearance of new lesions; partial responses: ≥30% decrease of target lesions; complete responses: the disappearance of target tumor. On day 28 of the first course of treatment, toxicity was re-evaluated by the treating physician and response to therapy was assessed using the WHO criteria. Toxicity was assessed throughout the study using the National Cancer Institute Common Toxicity Criteria version 2.0.
DTH responses. Analysis of specific immune response was performed by post-treatment DTH reactions.43 Briefly, 2 weeks after the last vaccination, 100 μg Aex were injected intradermally in the lateral limb of the vaccine immunization sites. After 48 hours, the maximum diameter (in millimeter) of induration was measured with a caliper.
DTH site–derived tissue culture and CD8+ T cells isolation. This procedure mainly followed a modified protocol described by de Vries et al.43 Briefly, punch biopsies derived from positive DTH sites (>2 mm) were cut into pieces, and leukocytes emigrating from these tissue pieces (DTH site–derived cells) were cultured in Roswell Park Memorial Institute medium-1640 containing 10% fetal bovine serum and 100 U/ml IL-2. Half of the medium was replaced by fresh IL-2-containing the culture medium every 5 days. After 2 weeks of culture, the cells were examined for CTL response. In order to test specific IFN-γ release and evaluate CTL production, CD8+ T lymphocytes were purified by using CD4+ cell-negative depletion.
Tetramer test. The tetramer test was conducted according to our previously described method.24 DTH site–derived cells were stained with phycoerythrin-labeled HLA-A*0201 tetramers (CAP-1 or SSp-1-specific) and fluorescein isothiocyanate–conjugated anti-CD8 for 40 minutes at room temperature. After washing, the samples were analyzed by flow cytometry (Beckman Epics XL).
IFN-γ release assay. Specific IFN-γ release assay was conducted as described.24 Briefly, T2 cells pulsed with the indicated peptide were used as stimulator cells, CD8+ T lymphocytes were prepared as effector cells from the DTH sites–derived cells. 5 × 104 effector cells and 1 × 104 stimulator cells were cocultured in 96-well microplates. After 24 hours, supernatants were collected and tested for IFN-γ release by enzyme-linked immunosorbent assay according to the manufacturer's protocol.
Cytotoxicity assessment. Cytotoxicity assessment was done according to a standard 51Cr release assay described.26 T2 cells pulsed with CAP-1 or SSp-1, and SW480 cells or LoVo cells were labeled with 51Cr sodium chromate in triplicate for 90 minutes at 37 °C, extensively washed three times, and were used as target cells. Effector cells (DTH site–derived cells) were cocultured with target cells at a different ratio in a final volume of 200 μl for 4 hours. Then 100 μl supernatants were harvested for measuring the chromate release. The proportion of specific cytotoxicity was determined according to formula: [(counts/minute of experimental 51Cr release – counts/minute of the spontaneous 51Cr release)/(counts/minute of the maximal 51Cr release – counts/minute of the spontaneous 51Cr release)] × 100%. Counts from targets incubated with medium alone were cited as spontaneous release, and counts from targets incubated with 5% Triton X-100 were cited as maximal release. The spontaneous release was always <15% of maximal release.
Statistical analysis. Results are expressed as the means ± SEM or the means ± SD. Comparisons between two groups were conducted by Student's t-test. Multiple comparisons were done with a one-way analysis of variance followed by Fisher's least significant difference analysis. Pairwise comparisons were done by performing the nonparametric Mann–Whitney U test. Most of the experiments were repeated at least two times. P values of ≤0.05 were considered significant.
Acknowledgments
This work was supported by grants from “Nature Science Foundation of Liuzhou, Guangxi, P. R. China” (20000231). We thank Lanqing Huang (the Johns Hopkins University) for critical reading of this article. We declared no potential conflicts of interest.
Footnotes
published online 5 February 2008
References
- 1.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 3.Chaput N, Taïeb J, Schartz NE, André F, Angevin E, Zitvogel L. Exosome-based immunotherapy. Cancer Immunol Immunother. 2004;53:234–239. doi: 10.1007/s00262-003-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A. Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol. 2006;80:471–478. doi: 10.1189/jlb.0206094. [DOI] [PubMed] [Google Scholar]
- 5.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 6.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 7.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 8.Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 9.Kovar M, Boyman O, Shen X, Hwang I, Kohler R, Sprent J. Direct stimulation of T cells by membrane vesicles from antigen-presenting cells. Proc Natl Acad Sci USA. 2006;103:11671–11676. doi: 10.1073/pnas.0603466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci USA. 2003;100:6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 12.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 13.Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 14.André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 15.Chaput N, Schartz NE, André F, Taïeb J, Novault S, Bonnaventure P. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 16.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 17.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31:114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 18.Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B. Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis. 2005;35:149–152. doi: 10.1016/j.bcmd.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams M, Navabi H, Croston D, Coleman S, Tabi Z, Clayton A. The rationale for combined chemo/immunotherapy using a Toll-like receptor 3 (TLR3) agonist and tumour-derived exosomes in advanced ovarian cancer. Vaccine. 2005;23:2374–2378. doi: 10.1016/j.vaccine.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 24.Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11:7554–7563. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol. 2006;36:1598–1607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- 26.Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8+ CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med. 2006;84:1067–1076. doi: 10.1007/s00109-006-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med. 2007;85:511–521. doi: 10.1007/s00109-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol. 2007;133:389–399. doi: 10.1007/s00432-006-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber J, Sondak VK, Scotland R, Phillip R, Wang F, Rubio V. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected stage II melanoma. Cancer. 2003;97:186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 30.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–949. doi: 10.1093/annonc/mdl499. [DOI] [PubMed] [Google Scholar]
- 32.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 33.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 34.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 36.Whiteside TL. Tumour-derived exosomes or microvesicles: another mechanism of tumour escape from the host immune system? Br J Cancer. 2005;92:209–211. doi: 10.1038/sj.bjc.6602360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markowicz S, Engleman EG. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. J Clin Invest. 1990;85:955–961. doi: 10.1172/JCI114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994;152:5208–5219. [PubMed] [Google Scholar]
- 39.Ferrome CR, Perales MA, Goldberg SM, Somberg CJ, Cymerman DH, Gregor PD. Adjuvanticity of plasmid DNA encoding cytokine fused to immunoglobulin Fc domains. Clin Cancer Res. 2006;12:5511–5519. doi: 10.1158/1078-0432.CCR-06-0979. [DOI] [PubMed] [Google Scholar]
- 40.Chavan R, Marfatia KA, An IC, Garber DA, Feinberg MB. Expression of CCL20 and granulocyte-macrophage colony-stimulating factor, but not Flt3-L, from modified vaccinia virus ankara enhances antiviral cellular and humoral immune responses. J Virol. 2006;80:7676–7687. doi: 10.1128/JVI.02748-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2006;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Bouton-Verville H, Holmes LM, Burgin KE, Jakubchak S, Yu X. Inhibition or promotion of tumor growth by granulocyte-macrophage colony stimulating factor derived from engineered tumor cells is dose-dependent. Anticancer Res. 2004;24:2717–2721. [PubMed] [Google Scholar]
- 43.de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]


