Abstract
Small-scale biogas digesters are widely promoted worldwide as a sustainable technology to manage livestock manure. In Vietnam, pig slurry is commonly applied to biogas digesters for production of gas for electricity and cooking with the effluent being used to fertilize field crops, vegetables and fish ponds. Slurry may contain a variety of zoonotic pathogens, e.g. Salmonella spp., which are able to cause disease in humans either through direct contact with slurry or by fecal contamination of water and foods. The objective of this study was to evaluate the survival of Salmonella spp. and the fecal indicator bacteria, enterococci, E. coli, and spores of Clostridium perfringens in biogas digesters operated by small-scale Vietnamese pig farmers. The serovar and antimicrobial susceptibility of the Salmonella spp. isolated were also established. The study was conducted in 12 farms (6 farms with and 6 farms without toilet connected) located in Hanam province, Vietnam. Sampling of pig slurry and biogas effluent was done during two seasons. Results showed that the concentration of enterococci, E. coli, and Clostridium perfringens spores was overall reduced by only 1–2 log10-units in the biogas digesters when comparing raw slurry and biogas effluent. Salmonella spp. was found in both raw slurry and biogas effluent. A total of 19 Salmonella serovars were identified, with the main serovars being Salmonella Typhimurium (55/138), Salmonella enterica serovar 4,[5],12:i:- (19/138), Salmonella Weltevreden (9/138) and Salmonella Rissen (9/138). The Salmonella serovars showed similar antimicrobial resistance patterns to those previously reported from Vietnam. When promoting biogas, farmers should be made aware that effluent should only be used as fertilizer for crops not consumed raw and that indiscriminate discharge of effluent are likely to contaminate water recipients, e.g. drinking water sources, with pathogens. Relevant authorities should promote safe animal manure management practices to farmers and regulations be updated to ensure food safety and public health.
Keywords: Biogas, Effluent, Salmonella, Fecal indicators, Food safety, Vietnam
Introduction
Vietnamese pig production is rapidly increasing due to a human population and economy that are growing resulting in increased consumer demands for pork meat. In Vietnam, the per capita consumption of pork is expected to increase from 37.8 kg in 2012 to 48.7 kg by 2020 and pig production is projected to increase from 31.2 million pigs in 2012 to 34.8 million in 2020 (Stuart, 2012). In consequence, Vietnam is facing huge challenges managing large volumes of pig manure in an environmental sustainable manner (Huynh et al., 2006). Through surface run-off and seepage nitrate and other nutrients originating from manure may contaminate water sources including aquifers and wells used for extracting drinking water (Burton and Turner, 2003). Pig and other livestock manure may also contain a range of different zoonotic pathogenic bacteria, e.g. Salmonella spp., E. coli, Campylobacter spp., Yersinia spp.; parasites, e.g. Ascaris spp., Taenia spp. and protozoan parasites; and vira, e.g. Hepatitis E and exotic viruses like Nipah, that can cause disease in humans through direct exposure or comsumption of contaminated water or food (Guan and Holley, 2003, Herremans et al., 2007, Spencer and Guan, 2004). Pig manure is commonly used as crop fertilizer and Salmonella spp. and other pathogens frequently found in pig wastes (Hutchison et al., 2005a) may then contaminate the environment and crops (Baloda et al., 2001).
Vietnamese farmers have different ways to manage manure with most small-scale pig farms composting manure in heaps or digesting it in biogas systems. Solid pig manure is normally mixed with straw, ash or lime, then covered by mud or plastic and stored for a few months before use as fertilizer (Tuan et al., 2006, Vu et al., 2007). In farms integrating pig and fish production, both solid and liquid manure is discharged directly into fish ponds to fertilize growth of plankton and other organisms that is eaten by the fish (Vu et al., 2007). Large-scale farms store slurry mainly in lagoons but may also operate biogas digesters, or occasionally discharge the slurry into aquatic recipients.
Biogas digestion is an anaerobic process that generates little heat. The digestion is typically done at mesophilic (35–40 °C) or at thermophilic temperatures (50–55 °C) when systems are heated. Mesophilic digestion is associated with limited pathogen reduction which is in contrast to the thermophilic conditions where most pathogens are inactivated (Sari et al., 2011, Teodorita et al., 2008). In Vietnam, the use of biogas digesters to manage and treat livestock manure has grown fast since 2003 where main efforts were initiated to promote the technology (Khai and Luong, 2010). By the end of 2012, a total of 125,000 biogas units were built in Vietnam with support from the government as well as non-governmental organizations. Several designs of biogas digesters, e.g. fixed dome and plastic tube biogas digesters, have been developed addressing the needs of different livestock farming systems with the vast majority of biogas systems being small-scale digesters receiving pig manure from up to 20 fattening pigs (Biogas Program, 2012, Khai and Luong, 2010).
Biogas digestion of livestock manure provides renewable energy, potent fertilizer and a potential reduction in pollution of the environment if managed correctly (Lantz et al., 2007, Sommer et al., 2003). In Vietnam, biogas systems not only provide gas for cooking but also improve the household environment by reducing problems with odor and flies (Huong et al., 2014). Traditionally, Vietnamese households have used solid animal manure to fertilize crops in fields and gardens. With many households now operating biogas digesters, they have shifted to the use of biogas effluent replacing raw or composted manure as a fertilizer of crops and vegetable (Chau, 1988, Francese et al., 2000); fish ponds and even as an animal food additive (Ngoc and Nhai, 2008, Tuyen et al., 2011). When building new houses, many Vietnamese pig farmers have installed new flush toilets that are connected to the biogas digesters (Huong et al., 2014). This is of concern since biogas effluent will most likely contain not only pathogens originating from pigs, but also a range of human pathogens that may be transmitted through contaminated water and foods when using biogas effluent as crop fertilizer or discharged indiscrinately into the external environment.
The objective of this study was therefore to evaluate the survival of Salmonella spp. and fecal bacterial indicators in biogas digesters operated by small-scale Vietnamese pig farmers and establish the serovar and antimicrobial susceptibility of the Salmonella spp. isolated.
Materials and methods
Selection and manure management practices of small-scale pig farms operating biogas digesters in Hanam province
The study was conducted and manure samples collected in the summer (June to August, 2011) and winter (December 2011 to February 2012), in Hoang Tan and Nhat Tan communes located in Kim Bang district, Ha Nam province in Vietnam. Lists of pig farmers with biogas digesters in the two communes were provided by the Department of Agriculture in Kim Bang district and the Center for Rural Water Supply and Environmental Sanitation of Hanam province. Farms were stratified by commune and whether their toilet was connected to the biogas unit or not. Eleven farms were selected from each stratum in Hoang Tay and 10 farms in Nhat Tan for questionnaire interviews. The questionnaire interview was done by first author of the person who managed manure (husband or wife) to investigate the current situation and hygiene aspect of manure management by farmers with biogas unit installed in Hoang Tay and Nhat Tan commune. The questionnaire interview collected information on the animal husbandry practices, management of the biogas digester, household toilet facilities as well as disposal and use of biogas effluent. In addition, farmers were asked whether health, fly and smell problems did change after installing biogas.
Collection of slurry and biogas effluent
Among the interviewed households that had an input tank connected to their biogas digester, six pig farms were randomly selected in each commune (three farms with toilet connected to the biogas digester and three farms without toilet connected to the digester) for microbiological analysis. Sampling was done during summer (June to August, 2011) and winter (December to February, 2012). In each season, households were visited six times. At each visit to the farm, one raw slurry sample was collected from the inlet tank placed before the biogas digester and one effluent sample was collected from the compensation tank of the biogas unit located after the biogas digester (Fig. 1 ). In total, 144 raw slurry and 144 effluent samples were collected. A sterilized 70-cm long spoon was used to mix the content of each tank thoroughly before sampling. About 1000 g of samples were collected from different parts of the tanks and poured into clean plastic buckets and approximately 200 g sample was transferred to a sterile glass bottle with screw cap. Samples were collected and kept in an insulated box with cooling elements and transported to the laboratory of the National Institute of Veterinary Research, Hanoi for bacteriological analysis which was initiated on the day of sampling. Samples were analyzed for E. coli, Enterococcus spp., spores of Clostridium perfringens and Salmonella spp.
Fig. 1.
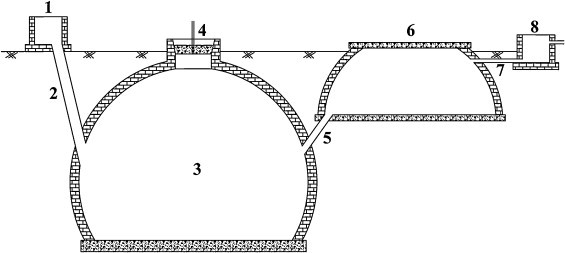
Schematic illustration of the biogas digester system operated by Vietnamese small-scale pig farmers (1: inlet tank; 2: inlet pipe; 3: digester tank; 4: gas collection pipe; 5: outlet pipe; 6: compensation tank; 7: overflow pipe; 8: effluent tank).
Measurement of temperature, ammonia and pH
Manure samples from the biogas digester tank were collected during 12 sampling events for temperature, ammonium and pH analysis. Using handmade sampling equipment consisting of a plastic pipe with a diameter of 10 cm, a length of 2 m and connected to an inox stick with a length of 2.2 m, samples were collected inside the digester tank through the inlet pipe (Fig. 1). The sampler was cover by a lid to ensure that no manure from the input tank and input pipe would mix with the slurry in the digester tank. When the sampler reached the bottom of the digester tank, the lid was opened by pressuring with the inox stick. The lid remained open for one minute to allow enough slurry to be collected. The digestate in the sampler was poured into a clean sterile plastic bucket, mixed thoroughly and temperature measured directly by a normal hand-held thermometer. Approximately 500 g of digestate was transferred to a sterile 1000 mL glass bottle with screw lead. Samples were kept in an insulated box with cooling elements and transported to the laboratory of the National Institute of Soil and Fertilizer, Hanoi for pH and NH4 analysis. pH was measured using a Hanna HI 8424 machine according to the Vietnamese standard method TCVN 6492-1999 (ISO 10523-1994) and NH4 was mesuared according to TCVN 5988-1995 (ISO 5664-1984), and NH3 concentrations were estimated as described by Christensen and Sommer (2013).
Enumeration of E. coli, Enterococcus spp. and Cl. perfringens spores
For the E. coli and enterococci analysis, a 10 g sub-sample (raw slurry and biogas effluent) was weighed in a sterile stomacher bag, containing 90 mL of Maximum Recovery Dilution solution (MRD) (CM 0733, Oxoid, UK). The sample was homogenized in a stomacher (Seward, West Sussex, UK) at 3000 rpm for 1 min and appropriate series of tenfold dilutions was prepared in MRD. Volumes of 100 μL of each dilution were surface spread onto Brilliant E. coli/coliform Selective Agar (CM 1046, Oxoid, UK) and Slanetz & Bartley agar plates (CM 0377, Oxoid, UK). For the E. coli analysis, Brilliant E. coli/coliform Selective Agar plates were incubated at 37 °C for 24 h and purple colonies were counted. Enterococcus spp. were determined as typical red–maroon colonies on Slanetz & Bartley agar following incubation at 44 °C for 48 h (DS 2401, 1999).
Enumeration of Cl. perfringens spores was done according to a method described by the Danish Standard (DS 2256, 1983). Briefly, samples were heat treated in a water bath at 80 °C for 5 min and one gram of sample was mixed with 9 mL of dilution solution (3 g K2HPO4 and 1 g KH2PO4 in 1000 mL distilled water) and further tenfold serial diluted. One mL of each sample dilution was transferred to a glass tube with 10 mL melted agar containing ammonium iron (II) sulphate and sodium sulphite. For each glass tube, 4 mL dilution liquid was added as s cover layer. The glass tubes were incubated at 48 °C for 24 h. Black colonies with a diameter over 1 mm were counted as Cl. perfringens.
Salmonella spp. isolation and serotyping
Volumes of 25 g of raw slurry or biogas effluent were weighed and added to a sterile stomacher bag containing 225 mL of Buffered Peptone Water (CM0509, Oxoid, UK). The sample was homogenized in a stomacher (Seward, West Sussex, UK) for 2 min at 3000 rpm and incubated for 18–24 h at 37 °C. Subsequently, 100 μL and 1 mL of pre-enriched cultures were transferred to Modified Semi-solid Rappaport-Vassiliadis (MSRV) medium (CM0910, Oxoid, UK) and Muller Kauffmann Tetrathionate broth (CM0343, Oxoid, UK) incubated at 42 °C for 24 h and 37 °C for 24 h, respectively. One loopful from each of the enriched broths were streaked on both Rambach agar (107500, Merck, Darmstadt, Germany) plates and Xylose Lysine Desoxycholate (XLD) agar (CM0469, Oxoid, UK) and incubated at 37 °C for 24 h. Presumptive Salmonella spp. colonies on Ramback agar (red color) and on XLD agar (black color) was confirmed on Kligner agar (CM0033, Oxoid, UK). All isolates were confirmed as Salmonella spp. by slide agglutination using polyvalent O and H antisera (Biorad, France). Isolates were further characterized by serotyping according to the Kauffman-White scheme (Popoff and Le Minor, 1997) at the Department of Medical Sciences, Ministry of Public Health in Bangkok, Thailand.
Antimicrobial susceptibility of Salmonella spp. isolates
MICs (Minimum Inhibitory Concentration) of Salmonella spp. isolates were determined by the broth microdilution method using the Sensititre system (code EUMVS2; Trek Diagnostic Systems, Ohio, USA). Salmonella spp. isolates were assayed for susceptibility to 14 antimicrobials, including ampicillin (AMP, 0.5–32 μg/mL), cefotaxime (FOT, 0.06–4 μg/mL), ceftazidime (TAZ, 0.25–16 μg/mL), chloramphenicol (CHL, 2–64 μg/mL), ciprofloxacin (CIP, 0.008–8 μg/mL), colistin (COL, 2–4 μg/mL), florfenicol (FFN, 2–64 μg/mL), gentamicin (GEN, 0.25–32 μg/mL), kanamycin (KAN, 4–128 μg/mL) and nalidixic acid (NAL, 4–64 μg/mL) streptomycin (STR, 2–128 μg/mL), sulfamethoxazole (SMX, 8–1024 μg/mL), tetracycline (TET, 1–64 μg/mL) and trimethoprim (TMP, 0.5–32 μg/mL). Escherichia coli ATCC 25922 was used as quality control. The Sensititre panels were read by Sensititre SensiTouch reader (Trek Diagnostic System, Ohio, USA) and for all antimicrobials breakpoint guideline values were interpreted according to the Clinical and Laboratory Standards Institute (CLSI, 2013a) except for florfenicol (CLSI, 2013b), colistin (EUCAST, 2013) and streptomycin (NARMS, 2011).
Statistical analyses
Comparison of manure management practices as shown in categorical responses from the questionnaire interviews of farmers who had toilet connected to the biogas digester and those who did not were done based on Chi-square tests on cross tabulations. Similar comparisons of quantitative responses obtained from the questionnaire were done using Mann–Whitney U-test. The bacteria concentration did not follow a normal distribution and were therefore logarithmically transformed (base 10) after adding 1 to accommodate concentrations below the limit of detection (zero counts). Comparisons of the concentration of E. coli, Enterococcus spp. and spores of Cl. perfringens between sampling seasons, commune and households with toilet connected to the biogas digester were done using linear regression adjusting for clustering within farms. Each factor was tested alone and finally jointly in multi-variable tests. Interaction terms between each pair of factors were tested one by one in models adjusting for the main effects of both factors and manure type (slurry/effluent). Presence/absence of Salmonella spp. was analyzed in a similar manner using logistic regression.
Hydraulic retention time (HRT) was calculated based on estimated volumes of water and manure inputs and the volume of the digester. HRT, pH, temperature and NH3 concentration as predictors of the reduction in bacterial concentrations [log10(X Slurry + 1) − log10(X Effluent + 1)] were tested individually using linear regression adjusting for clustering within farms (X is the concentration of bacteria or spores). All statistical analyes were done using Stata version 12 (Stata Corp, USA). p values <0.05 were taken to indicate significant differences.
Results
Manure management practices
The questionnaire interview was conducted at 22 farms in Hoang Tay and 20 farms in Nhat Tan commune to collect information about manure management practices on farms with brick dome type of biogas digesters (Fig. 1). Twenty-one farms had their toilet connected to the biogas unit and toilets at 21 farms were connected to a septic tank (Table 1 ). In average, four (1–7) people lived at each farm. The house, pig pen and housing facilities for other animals, e.g. chickens, were located close together due to limited space and only 9/42 farms had an effluent tank installed (Fig. 1 and Table 1). The digester tank was located underground inside the pigpen area at some farms. Farmers said that the volume of the digester tank was mainly based on available farmland, household economy and ranged from 7.5 to 14 m3 in Hoang Tay and 6 to 20 m3 in Nhat Tan communes (Table 1).
Table 1.
Farm information and manure management practices by small-scale pig farmersa with biogas digesters in Hoang Tay and Nhat Tan commune. Range values are shown in brackets.
| Hoang Tay (N = 22) | Nhat Tan (N = 20) | |||
|---|---|---|---|---|
| Toilet connected to biogas digester |
Toilet connected to biogas digester |
|||
| Yes (n = 11) | No (n = 11) | Yes (n = 10) | No (n = 10) | |
| Number of persons living at farms | 4 (2–7) | 4 (1–7) | 4.5 (3–7) | 3.6 (2–6) |
| Number of sows per farm | 2.4 (0–6) | 6.2 (2–20) | 0.9 (0–3) | 9.9 (0–40) |
| Number of fattening pigs per farm | 4.6 (0–22) | 18.4 (0–62) | 16.5 (5–26) | 41.6 (8–150) |
| Number of piglets per farm | 8.3 (0–35) | 27.6 (0–90) | 0.5 (0–5) | 24.5 (0–130) |
| Biogas volume (m3) | 10.1 (7.5–13) | 12.1 (10–14) | 11.1 (6–17) | 13.9 (7.5–20) |
| Farms using disinfectants in pigpen | 10 | 10 | 8 | 6 |
| Effluent tank present | 1 | 2 | 2 | 4 |
| Farmers applying protective measures when handling manureb | 6 | 10 | 9 | 8 |
| Farmers applying protective measures when handling effluentb | 7 | 6 | 3 | 6 |
| Farmers using personal hygiene activity after handling manure/effluentc | 9 | 11 | 8 | 10 |
| Farmers experienced reduced fly problems | 11 | 11 | 9 | 10 |
| Farmers experienced reduced smell problems | 11 | 11 | 10 | 10 |
Farmers include one per farm (either husband or wife).
Boots, clothes, gloves, and mask.
Shower, wash hand with water, wash hand with soap.
Farmers stated that the main reason for installing a biogas digester was the gas produced and reduced problems with bad smells and flies. The gas was used for lighting and cooking purposes with volumes produced exciding farmer’ needs. Excess amount of gas were either discharged to the environment through a pipe or shared with neighbors. All 42 farms used pig manure as the main organic input to the digester irrespectively of having a toilet connected or not. Some farmers with high number of pigs removed solid manure when cleaning the pig pen to fertilize crops or fish ponds. The liquid digestate from the compensation and effluent tank (Fig. 1) was used by farmers in Hoang Tay (16/22) and Nhat Tan (7/20) to fertilize field and garden crops, including fruit trees, as well as fish ponds. Vegetables fertilized included those consumed raw, i.e. coriander, salad, carrots and cucumber. Several farmers did only partly utilize the effluent as fertilizer and discharged the remaining effluent directly to the canals, lakes and occasionally the public sewer system.
The few farms (3/42) that experienced diarrhea problems in piglets during the past month treated the animals with amoxicillin, enrofloxcacin or norfloxcaxin following instructions shown on the package lables. Tetracycline, sulfonamides or ampicillin was mixed with pelleted pig feed as some farmers (7/42) stated that this enhanced growth. Most farms (34/42) in the two communes used disinfectants such as chloramine and iodine solutions weekly to disinfect the pig pens after cleaning the pens with water. Thus, slurry containing such disinfectants was continuously supplied to the biogas units. Powdered lime was typically used as a disinfectant between batches of pigs after which the organic waste containing the powdered lime was removed. Soap and other chemicals were also used to disinfect toilets (19/21) that were connected to a biogas digester.
It was observed that 15 and 10 farmers with toilet connected to the biogas unit used protective measures like clothes, boots, gloves and masks when handling manure and effluent compared to 18 and 12 farms without toilet connected to the biogas unit, respectively. Most (38/42) farmers did perform hygiene activities, e.g. showering, washing hands with water, washing hands with soap, after handling manure and effluent (Table 1).
Almost 100% of farmers in Hoang Tay and Nhat Tan stated that the biogas units reduced fly and bad odor problems. Such benefits were found very important as bad odor would cause social problems with nearby neighbors, but was also perceived to directly affect the farmer's health, e.g. cause respiratory problems and transmit pathogens. Compared with untreated slurry, the farmers said that biogas slurry attracted less flies which was also confirmed by observations in the field.
Fecal bacterial indicators in slurry and effluent
Slurry and effluent were analyzed for the presence and concentration of fecal indicator bacteria, i.e. E. coli, Enterococcus spp. and Cl. perfringens spores during two seasons (Fig. 2 ). Differences in concentrations of the three indicator bacteria in slurry and effluent by commune, season and toilet connected to biogas are shown in Fig. 2. In general, a 1–2 log unit reduction was seen for the bacterial indicators.
Fig. 2.
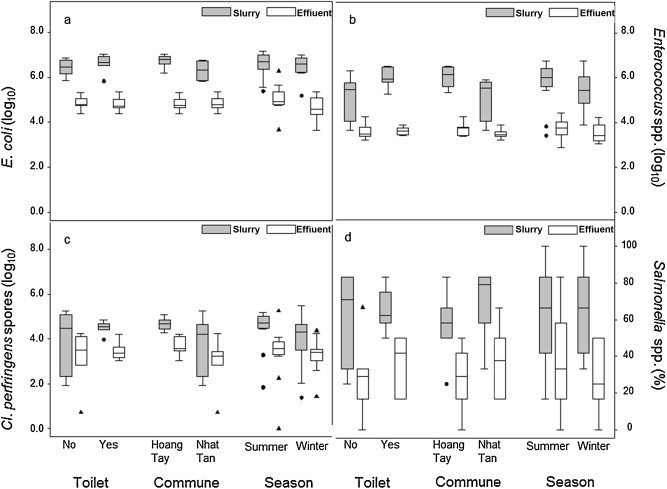
Box plots showing concentration of fecal indicator bacteria (a–c) and prevalence of Salmonella spp. (d) in slurry and biogas effluent illustrated by season, commune and toilet connected. The concentration of bacteria or spores detected in slurry and biogas effluent is presented as cfu/mL or pfu/mL. The horizontal line within the box shows the median, the box the interquartile range and the whiskers show the adjacent values (i.e. up to 1.5 times the interquartile range below the 25th percentile or above the 75th percentile). Values outside this range are shown by markers.
The concentration of all three bacteria differed significantly among farms (p < 0.001). The concentration of Enterococcus spp. varied greatly in slurry and although values in effluent on average were lower, the concentration in effluent could be quite high, i.e. up to 5.23 log units. In a test with a single variable, Nhat Tan commune had lower concentrations (0.59 log units) than in Hoang Tay commune (p < 0.05), i.e. slurry and effluent samples combined. In effluent, the concentration of Enterococcus spp. was 1.95 log unit lower (p < 0.001) than that in slurry. In a multi-variable model, the effect of commune remained significant (p < 0.05), farms with toilet connected had higher enterococci concentrations than farms with no toilet connected (0.87 log unit; p < 0.05). The enterococci concentration in effluent was on average 2.37 log unit less than those in slurry, but there was a significant interaction between toilet and manure type, i.e. concentration of Enterococcus spp. in effluent from farms with toilet connected was 0.84 log unit higher (p < 0.05) than those in effluent from farms without toilet connected.
For E. coli and Cl. perfringens, the only significant predictor was manure type. E. coli concentrations in effluent was on average 1.69 log unit lower than that in slurry and Cl. perfringens in effluent was 0.91 log unit lower than concentrations found in slurry. For Enterococcus spp., the effluent had on a single occasion higher concentrations than slurry, while for Cl. perfringens and E. coli this was observed on 11 (7.6%) and 6 (4.2%) occasions, respectively.
Temperature, pH, NH3, HRT and reduction of bacterial indicators
Temperature, pH, NH3 concentration were not significant predictors of reductions in E. coli, Enterocuccus spp. and spores of Cl. perfringens in slurry as compared with effluent, while the estimated hydraulic retention time (HRT) was positively related to the reduction of E. coli (p < 0.05).
The estimated HRT differed significantly (p < 0.01) between summer (mean ± sd: 13.5 ± 1.8 days; range: 6.3–28.9 days) and winter (43.2 ± 7.9 days; 28.2–124.2 days). This was due to use of larger quantities of water to clean and cool the pig pens/pigs during summer, where water consumption was 2–7 times higher than in the winter. The temperature in the digester tank, which was always placed underground, was measured to 20.5 ± 2.5 °C and 29.5 ± 1.5 °C during winter and summer, respectively. The pH of biogas effluent varied significantly among farms (p < 0.001), while there was no difference between seasons; mean pH scores for individual farms ranged from 6.8 to 7.7 with overall total pH scores varying from 6.0 to 8.8. Overall, NH3 (mg/L) values ranged from 0.14 to 355.29 mg/L. Median NH3 concentration was 3.25 mg/L. Only on two occasions were concentrations of NH3 above 100 mg/L recorded (i.e. 198.80 and 355.30 mg/L) and excluding those two scores the maximum was 66.14 mg/L. Regression analysis showed that NH3 (mg/L) values differed significantly among farms (p < 0.01) but did not differ between seasons and the interaction between these two factors was not significant. Mean values of NH3 for individual farms (both seasons combined) ranged from 2.47 to 57.9 mg/L or 14.10 mg/L if excluding the two highest values, which were both, recorded from one farm.
Salmonella spp. isolation, serovars and antimicrobial resistance
In general, Salmonella spp. was often found in both slurry and biogas effluent. The presence of Salmonella spp. was analyzed in 288 slurry and effluent samples with 47.9% (138/288) of the samples being positive. The mean Salmonella concentration in positive slurry and effluent samples were 105 cfu/25 g and 103 cfu/25 g in the summer season and 104 cfu/25 g and 102 cfu/25 g in the winter season, respectively. Salmonella spp. was present significantly more (p < 0.001) in slurry samples (63.2%) compared to effluent samples (32.6%) (Fig. 3 ). The odds of detecting Salmonella spp. among farms also differed significantly (p < 0.01). The difference of Salmonella spp. in slurry and effluent by commune, season and toilet connected to biogas are shown in Fig. 2. However, these three factors provided no prediction for presence of Salmonella spp. Only slurry and effluent was significant (p < 0.001), i.e. the odds of finding Salmonella spp. in effluent was 0.28 of that in slurry.
Fig. 3.
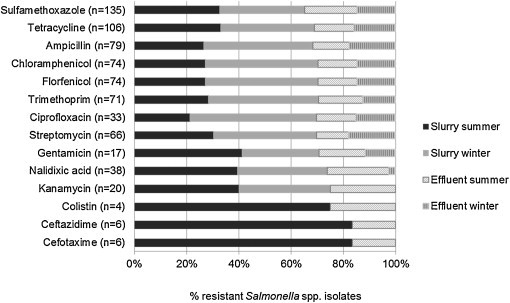
Distribution of antimicrobial-resistant Salmonella spp. by source (slurry, biogas effluent) and season (summer, winter), n = number of Salmonella spp. isolates that are resistant to a specific antimicrobial.
Nineteen serovars of Salmonella spp. were detected in slurry and effluent (Table 2 ). The most frequently serovars was Salmonella Typhimurium (55/138), Salmonella 4,[5],12:i:- (19/138), Salmonella Weltevreden (9/138) and Salmonella Rissen (9/138). Salmonella Typhimurium and Salmonella 4,[5],12:i:- were found in both slurry and biogas effluent in 11/12 farms and 10/12 farms, respectively. Specially, Salmonella 4,[5],12:i:- was detected more often during winter time (15/19) compared to summer time (4/19). Salmonella Anatum, Salmonella Cerro, Salmonella Derby, and Salmonella Newport were only found in biogas effluent. Salmonella Cerro was only detected from a farm with toilet connected to the biogas digester while Salmonella Derby and Salmonella Newport were isolated in biogas effluent on farms without toilet connected. In addition, one Salmonella Anatum isolate and the Salmonella Cerro was found only in effluent in farms with toilet connected indicating that these isolates might originate from human excreta.
Table 2.
Salmonella serovars isolated from pig manure and biogas effluent.
| Salmonella serovars (total no. of isolates) | No. of farms | Farms without toilet connected to biogas |
Farms with toilet connected to biogas |
||
|---|---|---|---|---|---|
| Slurry | Effluent | Slurry | Effluent | ||
| Salmonella Albany (8) | 4 | 3 | 3 | ||
| Salmonella Anatum (2) | 2 | 1 | 1 | ||
| Salmonella Bareilly (7) | 5 | 2 | 2 | 3 | |
| Salmonella Cerro (1) | 1 | 1 | |||
| Salmonella Corvallis (1) | 1 | 1 | |||
| Salmonella Derby (2) | 2 | 2 | |||
| Salmonella Enteritidis (1) | 1 | 1 | |||
| Salmonella Hadar (2) | 1 | 1 | |||
| Salmonella Indiana (8) | 4 | 1 | 1 | 3 | 1 |
| Salmonella Litchfield (1) | 1 | 1 | |||
| Salmonella London (2) | 1 | 1 | 1 | ||
| Salmonella Newport (1) | 1 | 1 | |||
| Salmonella Rissen (9) | 5 | 2 | 1 | 2 | 2 |
| Salmonella Typhimurium (55) | 11 | 5 | 4 | 6 | 5 |
| Salmonella Weltevreden (9) | 6 | 2 | 1 | 2 | 1 |
| Salmonella 1,4,[5],12:i:- (1) | 1 | 1 | |||
| Salmonella 4,[5],12:b:- (7) | 7 | 3 | 2 | 2 | |
| Salmonella 4,[5],12:i:- (19) | 10 | 6 | 2 | 4 | 4 |
| Salmonella 8,20:i:- (2) | 2 | 2 | |||
The distribution of MIC values irrespectively of serovar is shown in Table 3 . Salmonella spp. showed resistance to a several antimicrobials tested including SMX (97.8%), TET (76.8%) and AMP (57.2%) A large proportion of Salmonella spp. isolates were found sentitive to TAZ (95.7%), FOT (91.3%) and GEN (82.6%) while intermediate resistances were observed to COL (97.1%), FFN (42.0%) and CIP (38.4%). Salmonella spp. was also found to resistant to the critically important antimicrobials CIP (23.9%), FOT (4.3%) and TAZ (4.3%). Resistance to FOT, TAZ and COL was only found during the summer season (Fig. 3). The two Salmonella Typhimurium isolates and one Salmonella 4,[5],12:i:- isolate resistant to all 14 antimicrobials were only detected in slurry during summer sampling while Salmonella spp. resistant to 11 or less antimicrobials were found in effluent in both summer and winter sampling. Several of the other Salmonella serovars, i.e. Salmonella Albany, Salmonella Derby, Salmonella Enteritidis, Salmonella Indiana, Salmonella London and Salmonella 1,4,[5],12:i:-, showed resistance to five or more antimicrobials. One strain of Salmonella Albany detected in biogas effluent was resistant to all of the tested antimicrobials. Elleven Salmonella serovars were resistant to less than three antimicrobials, primarily SMX and TET.
Table 3.
Distribution of MIC values to antimicrobials among Salmonella spp. isolated (n = 138) from pig slurry and biogas effluent.
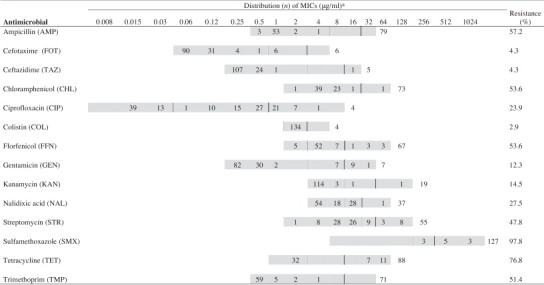
aThe shaded areas indicate the range of dilutions tested for each antimicrobial. Single vertical bars indicate the breakpoints for susceptibility, while double vertical bars indicate the breakpoints for resistance. Numbers in the un-shaded area indicate the number of isolates with MICs greater than the highest tested concentrations. Numbers listed for the lowest tested concentrations represent the number of isolates with MICs equal to or less than the lowest tested concentration.
Multi (≥3) antimicrobial resistance patterns among the two most common Salmonella serovars, i.e. Salmonella Typhimurium and Salmonella 4,[5],12:i:-, are shown in Table 4 . Salmonella Typhimurium was resistant to 1–14 antimicrobials where as Salmonella 4,[5],12:i:- was resistant to 4–14 antimicrobials. All of the Salmonella 4,[5],12:i:- were resistant to at least AMP, TET, and SMX but the majority of isolates (n = 10) were additional resistant to STR.
Table 4.
Multi-drug (≥3) resistance patterns of Salmonella Typhimurium and Salmonella 4,[5],12:i:-.
| Multi-drug resistance patterns | S. Typhimurium (n = 55) | S. 4,[5],12:i:- (n = 19) |
|---|---|---|
| N (%) | N (%) | |
| STR, SMX, TET | 8 (14.5) | |
| AMP, STR, SMX, TET | 10 (52.6) | |
| AMP, CHL, FFN, SMX, TMP | 1 (1.8) | |
| AMP, STR, SXM, TET, TMP | 1 (5.3) | |
| AMP, CHL, FFN, SMX, TET, TMP | 8 (14.5) | |
| AMP, CHL, FFN, STR, SMX, TET | 1 (1.8) | |
| AMP, CHL, CIP, FFN, NAL, SMX, TET | 1 (1.8) | |
| AMP, CHL, FFN, GEN, STR, SXM, TET | 1 (5.3) | |
| AMP, CHL, FFN, STR, SMX, TET, TMP | 12 (21.8) | 1 (5.3) |
| AMP, CHL, CIP, FFN, STR, SMX, TET, TMP | 12 (21.8) | |
| AMP, CHL, FFN, KAN, NAL, SMX, TET, TMP | 1 (5.3) | |
| AMP, CHL, CIP, FFN, KAN, NAL, SMX, TET, TMP | 3 (5.4) | 1 (5.3) |
| AMP, CHL, CIP, FFN, NAL, STR, SMX, TET, TMP | 1 (1.8) | |
| AMP, CHL, CIP, FFN, GEN, KAN, NAL, SMX, TET, TMP | 4 (7.3) | 1 (5.3) |
| AMP, CHL, CIP, FFN, GEN, KAN, NAL, STR, SMX, TET, TMP | 1 (1.8) | 2 (10.5) |
| AMP, FOT, TAZ, CHL, CIP, COL, FFN, GEN, KAN, NAL, STR, SMX, TET, TMP | 2 (3.6) | 1 (5.3) |
Abbreviation: Ampicillin (AMP), cefotaxime (FOT), ceftazidime (TAZ), chloramphenicol (CHL), ciprofloxacin (CIP), colistin (COL), florfenicol (FFN), gentamicin (GEN), kanamycin (KAN), nalidixic acid (NAL), streptomycin (STR), sulfamethoxazole (SMX), tetracycline (TET), and trimethoprim (TMP).
Discussion
The present study demonstrated that treatment of pig slurry in biogas digesters only reduced the concentration of the fecal indicator bacteria Enterococcus spp., E. coli and spores of Cl. perfringens by 1–2 log units when comparing the bacterial concentrations in slurry and in biogas effluent. This is in agreement with reported results from other studies on the bacterial reduction capacity of biogas digesters in Vietnam (Kobayashi et al., 2003, Son et al., 2008) as well as other parts of the world (Bagge et al., 2005, Massé et al., 2011, Pourcher et al., 2007, Ram et al., 2009) The prevalence of Salmonella was 63.2% in raw pig slurry and was reduced to 32.6% in effluent samples. Salmonella spp. is commonly found in pig manure and may therefore be found in composted manure or other manure by-products (Létourneau et al., 2010). A study in Vietnam on raw septic sludge and mesophillic treated septic sludge found that 70% and 60% of samples were positive for Salmonella spp., respectively (Yen-Phi et al., 2010) which suggests that human excreta is a likely source of Salmonella spp. when used in biogas digesters. Pourcher et al. (2007) showed in France that Salmonella was detected in raw pig manure (60%) and in treated manure after biogas digestion (20%).
There are a number of rules and guidelines on treatment and hygienic quality requirements for biosolids, i.e. animal manure and biogas effluent, to be applied on agricultural land (NSWEPA, 2000, NZWWA, 2003, UKDoE, 1996, USEPA, 2003). According to United States Environmental Protection Agency (EPA) part 503 rule (USEPA, 2003), Class A biosolids must contain less than 3 MPN/4 g of Samonella or less than 1000 MPN/g of E. coli before land application where as Class B biosolids must contain less than a geometric mean of two million fecal coliforms per g when applied to land. In Vietnam, there does not seem to exist any specific regulations on the hygienic quality of manure or biogas effluent to be applied to agricultural fields. However, regulations from the Ministry of Natural Resources and Environment states that water used to irrigate vegetables or plants eaten raw must contain less than 200 E. coli cfu/100 mL (QCVN, 2011). Regulations of biosecurity at pig farms in Vietnam states that wastewater, including liquid slurry, discharged to the external environment should not contain any Salmonella in 50 mL wastewater and less than 500 MPN fecal coliforms per 100 mL (QCVN, 2010). In our study, the mean Salmonella and E. coli concentrations in effluent samples were 103 cfu/25 g and 4.3 cfu log10/g in the summer season, respectively and 102 cfu/25 g and 4.8 cfu log10/g in the winter season, respectively. Thus, according to the EPA rules our biogas effluent should be classified as biosolids Class B with a range of restrictions on use, e.g. it cannot be used to fertilize crops for animal and human consumption. Also, the effluent does not fulfill the stipulated national rules for discharge into the environment. It should be noted that a main barrier for farmer's use of biogas liquid effluent and subsequent discharge into the environment is lack of adequate technologies to remove, transport and apply the effluent.
Anaerobic digestion in biogas systems is a potential sustainable technology to manage manure from small-scale pig farmers in Vietnam, but our results document that Salmonella spp. and other pathogens are likely to be present in effluent and subsequent transferred to fertilized crops, i.e. rice, maize, peanuts, potatoes and fruit trees, but also to vegetables consumed raw, i.e. coriander, salad, carrots and cucumber. In addition, the indiscriminate discharge of effluent by the farmers to the external environment, e.g. canals and household perimeter, may pollute aquatic recipients as well as drinking water sources, e.g. household wells. Low temperatures enhance survival of pathogens and mesophilic digestion is not a sufficient hygienic treatment method of biowaste due to possible survival of Salmonella and other pathogens (Gerba and Smith, 2005, Nicholson et al., 2005). Even at thermophillic conditions, Salmonella has been found in 55% of treated septic sludge samples as compared with 67% of septic sludge before treatment (Sahlström et al., 2004). Salmonella spp. have been reported to survive for an extended period of time in soil and water (Bicudo and Goyal, 2003, Guan and Holley, 2003) and can survive for several months in stored slurry (Hutchison et al., 2005b). Also, Salmonella spp. is relatively persistent in soil and can persist for up to 100 days in slurry applied to grass (Mawdsley et al., 1995), e.g. Salmonella Typhimurium was found to persist up to 231 days in soils amended with contaminated composts (Islam et al., 2004). On vegetables, Salmonella Typhimurium was detected for up to 63 days and 231 days on lettuce and parsley, respectively (Islam et al., 2004). Fresh produce has been implicated in several foodborne disease outbreaks involving Salmonella spp. (CDC, 2013, Harris et al., 2003) and recently a multistate outbreak of human Salmonella serotype 1,4,[5],12:i:- infections linked to alfalfa sprouts was reported (CDC, 2011). Information about similar disease outbreaks is not available for Vietnam. The use of human excreta in biogas digesters represents an increased risk of transfer of human pathogens when biogas effluent is used as crop fertilizer or discharged into the environment. Although we did only analyze for Salmonella spp., it is likely that other human viral and parasite pathogens will survive the anaerobic digestion and be present in biogas effluent. In particular parasites, e.g. protozoans and helminthes, are of concern as they are much more resistant to environmental stress compared to bacterial pathogens. Our microbiological findings and the use of biogas effluent to fertilize vegetables and other plants consumed raw suggests that such use practices represent actual food safety hazards. Further quantitative microbial risk assessment studies are needed to determine associated human health risk.
Guidelines from the Netherlands Development Organisation (SNV) (Biogas Program, 2012) suggest that the biogas digester should be fed at a ratio between manure and water of 1:3, but most farms did not meet that criteria, and in consequence, the manure was too diluted and the HRT low which partly explain the limited bacterial reduction. We also found that the farmer's house, the pig pen as well as cages for other animals were usually located closely together on limited land in densely populated areas which increases the risks for transmission of zoonotic pathogens. Farmers often raised high numbers of pigs in a limited area (Vu et al., 2007) which is different compared to Europe where the number of pigs per m2 is regulated (Burton and Turner, 2003). The farmers reported a broad use of disinfectants to clean pig pens and household toilets. However, it is currently unknown to what extent such use of disinfectants affects the microbial populations responsible for the methane production as well as the survival of animal and human pathogens present. Temperature in the biogas digester tank ranged from 18 to 23 °C (winter season) and 28 to 31 °C (summer season) which is low compared with biogas systems in Europe where digestion is often done in heated systems either at mesophilic (35–40 °C) or thermophilic temperatures (50–55 °C) (European Bioplastic, 2010, Teodorita et al., 2008). With the current practices of placing the biogas digester underground it will not be possible to increase temperature. However, there exist low-cost biogas digesters made of polyethylene, e.g. some are operating in southern Vietnam, which are placed on the ground (An, 2002). It remains to be determined if such exposure to sunlight will increase temperatures to levels, thermophilic conditions, needed to inactivate pathogens. Also, as winters are quite cold in northern Vietnam it is unlikely any thermophilic conditions can be achieved during the winter. Although solar panel heated biogas digesters have been developed such technology are not yet available for small-scale farmers at acceptable costs. Increased hydraulic retention time (HRT) will lead to increased pathogen die-off. However, although use of less water for cleaning and cooling pigs will increase HRT, it will not be possible to obtain any significant increased in HRT in the existing biogas digesters.
Salmonella spp. can survive pH ranges of 4.05–9.5, with a pH between 6.5 and 7.5 being optimal for growth (Cox, 2000). As we found pH in the biogas digesters to range from 6.0 to 8.0, only limited reduction of Salmonella spp. can be expected. The NH3 concentration in our study ranged from 0.14 to 355.29 mg/L. The formation of ammonia depends on the temperature and pH in the digestate, i.e. the higher the temperature and pH, the higher the formation will be of ammonia (Mata-Alvarez, 2003). The inactivating effect of ammonia has been shown for bacteria (Ottoson et al., 2008, Vinnerås et al., 2008), viruses (Emmoth et al., 2011), protozoa (Jenkins et al., 1998) and helminth eggs (Pecson and Nelson, 2005). Ammonia inactivates pathogens when a sufficient high NH3concentration is achieved (EU, 2011). Addition of 0.5% ammonia (4950 mg/L) to human excreata composted at 40 °C provided a 5 log10 reduction of Enterococcus, thermotolerant coliform, Salmonella and coliphages (Marcus, 2012). Vinnerås et al. (2003) reported that the addition of urea at a dosage of 30 g ammonia per kilogram (26,970 mg/L) to composted fecal waste resulted in more than 6 log10 reduction of E. coli, Enterococcus spp., and Salmonella spp. within three weeks and no viable Ascaris suum eggs were found after 50 days of treatment. Emmoth et al. (2011) showed that adding 0.5% (w/w) ammonia (4950 mg/L) to poultry by-product waste before use as fertilizer or as substrate in a biogas digester yielded a 1-log reduction (D value) within 3.0 h of enveloped and naked single-stranded RNA viruses such as coronavirus. In this study, we found a median NH3 concentration of 3.25 mg/L and only on two occasions were concentrations of NH3 above 100 mg/L recorded. With the low temperatures and pH values found in the Vietnamese biogas digesters in both winter and summer season, the NH3 concentration will not reach levels needed for any major inactivation of bacteria, vira and parasite pathogens.
Our study indicated that most farmers installed the biogas digester with support from different national and international, e.g. the SNV (Netherlands Development Organisation) programs (Biogas Program, 2012). Generally when farmers had to build new housing facilities and at the same time wanted to install a biogas digester, then the toilet was connected to the biogas digester. However, households already having toilets (flush system) connected to a septic tank did not connect such toilets to the biogas digester. Effluent was often discharged to the environment because farmers were not aware of the nutrient content and fertilizer value of biogas effluent and therefore mainly saw the effluent as a waste product. One reason stated by the farmers for discharging the biogas effluent to the environment was that equipment for transport of the effluent to the field is lacking and the distance from househoulds to the field is quite far. Thus, there is a need to find ways and technologies to transport the liquid effluent to the field.
A total of 19 Salmonella serovars were identified in both slurry and biogas effluent. Since we selected only one Salmonella isolate for serotyping from each sample it is likely that additional Salmonella serovars were present. All of isolated Salmonella serovars are potential pathogens and can cause gastroenteritis in humans. The most frequent isolated Salmonella serovars was Salmonella Typhimurium and Salmonella 4,[5],12:i:- which is a monophasic variant of Salmonella Typhimurium (EFSA, 2010, Soyer et al., 2009). In addition, Salmonella Weltevreden, Salmonella Rissen and Salmonella Albany were identified each with a prevalence of approximately 6%. Salmonella Typhimurium is a common cause of salmonellosis worldwide and has been reported to be the main species implicated in food contamination (NARMS, 2011). Salmonella 4,[5],12:i:- is currently considered an emerging epidemic serovar owing to the rapid increase in the prevalence of human outbreaks worldwide over a relatively short period of time (Agasan et al., 2002, Hopkins et al., 2010, Mossong et al., 2007, Switt et al., 2009, Tavechio et al., 2004) and to the increase in trade and consumption of pigs and pork products (Barco et al., 2012, Echeita et al., 1999, Hauser et al., 2010). Salmonella 4,[5],12:i:- is therefore of increasing public health concern (EFSA, 2010). In Vietnam, Salmonella spp. has been found in 49% of pig carcasses (Nguyen, 2007) and in 40–70% of raw pork meat (Thai et al., 2012, Van et al., 2007). Studies in Vietnam have reported that the most common Salmonella serovars found in human and animal feces, carcasses and meat were Salmonella Typhimurium, Salmonella Anatum, Salmonella Emek and Salmonella Rissen (Thai et al., 2012, Vo et al., 2006).
The level of antimicrobial resistance found in Salmonella spp. in the present study is similar with resistance patterns reported among Salmonella spp. isolated from pork meat, human and animal in Vietnam (Le et al., 2004, Ogasawara et al., 2008, Van et al., 2012, Vo et al., 2010). Our Salmonella isolates showed some resistance to ciprofloxacin (23.9%) which is often the first drug of choice for treatment of human salmonellosis (Giraud et al., 2006), including in Vietnam (Kinh, 2010). Resistance to multiple antimicrobials was further studied among Salmonella Typhimurium and Salmonella 4,[5],12:i:- (Table 4), which both are considered foodborne pathogens (Soyer et al., 2009). These multiple resistance patterns are similar to those reported in Salmonella Typhimurium isolated from pork meat collected from markets and supermakets in southern Vietnam (Van et al., 2007) and from pig carcasses in northern Vietnam (Ellerbroek et al., 2010, Thai et al., 2012).
Conclusions
In conclusion, the limited reduction of enterococci, E. coli and Clostridium perfringens spore concentrations (1–2 log10 units) and the presence of Salmonella spp. in biogas effluent represent a risk to human health especially due to the common practice of applying human excreta to the biogas digester and subsequent use of the effluent to fertilize vegetable crops, in particular those eaten raw.
The small-scale biogas digesters that are widespread and heavily promoted in Vietnam can be a sustainable technology to manage pig manure, but farmers have limited knowledge on safe animal manure management practices and should be made aware that biogas effluent should only be used as fertilizer for crops not consumed raw and that indiscriminate discharge of effluent are likely to contaminate water recipients and drinking water sources with pathogens. Regulations in Vietnam should be updated providing guidance on how safely to manage and use biogas effluent as a crop fertilizer, including means of treatment before use to high risk crops, i.e. vegetables consumed raw as well as measures to reduce occupational health risks.
Acknowledgements
This study was funded by the Danida-supported (Danish International Development Assistance) SUSANE (Sustainable, Sanitary and Efficient Management of Animal Manure for Plant Nutrition) project phase 2 (J.nr.104.Dan.8.L.722). We would like to thank Pham Thi Ngoc, Truong Thi Quy Duong and Vu Thi Kim Hue from Department of Veterinary Hygiene, National Institute of Veterinary Research in Vietnam for their support in sampling and processing. The technical assistance of Gitte Petersen from Department of Veterinary Disease Biology, Faculty of Health and Medical Sciences, University of Copenhagen is much appreciated.
References
- Agasan A., Kornblum J., Williams G., Pratt C.C., Fleckenstein P., Wong M., Ramon A. Profile of Salmonella enterica subsp. enterica (subspecies I) serotype 4,5,12:i:- strains causing food-borne infections in New York City. J. Clin. Microbiol. 2002;40:1924–1929. doi: 10.1128/JCM.40.6.1924-1929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B.X. Biogas technology in developing countries: Vietnam case study. In: Preston T.R., Khang D.N., editors. International Workshop – Research and Development on Use of Biodigesters in SE Asia Region; Ho Chi Minh City, Vietnam; 2002. [Google Scholar]
- Bagge E., Sahlstrom L., Albihn A. The effect of hygienic treatment on the microbial flora of biowaste at biogas plants. Water Res. 2005;39:4879–4886. doi: 10.1016/j.watres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Baloda S.B., Christensen L., Trajcevska S. Persistence of a Salmonella enterica serovar Typhimurium DT12 clone in a piggery and in agricultural soil amended with Salmonella-contaminated slurry. Appl. Environ. Microbiol. 2001;67:2859–2862. doi: 10.1128/AEM.67.6.2859-2862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco L., Mancin M., Ruffa M., Saccardin C., Minorello C., Zavagnin P., Lettini A.A., Olsen J.E., Ricci A. Application of the random forest method to analyse epidemiological and phenotypic characteristics of Salmonella 4,[5],12:i:- and Salmonella Typhimurium strains. Zoonoses Public Hlth. 2012;59:505–512. doi: 10.1111/j.1863-2378.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- Bicudo J.R., Goyal S.M. Pathogens and manure management systems: a review. Environ. Technol. 2003;24:115–130. doi: 10.1080/09593330309385542. [DOI] [PubMed] [Google Scholar]
- Biogas Program . 2012. Biogas Program for the Animal Husbandry Sector in Vietnam.http://biogas.org.vn/english/Home.aspx [Google Scholar]
- Burton C.H., Turner C. Lister and Durling Printers; Flitwick, Bedford, UK: 2003. Manure Management: Treatment Strategies for Sustainable Agriculture. [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2011. Multistate Outbreak of Human Salmonella I 4,[5],12:i:- Infections Linked to Alfalfa Sprouts (Final Update)http://www.cdc.gov/salmonella/i4512i-/ [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2013. Reports of Selected Salmonella Outbreaks Investigations.http://www.cdc.gov/salmonella/outbreaks.html [Google Scholar]
- Chau L.H. Livestock Research for Rural Development. CIPAV; Columbia: 1988. Biodigester effluent vernus manure from pigs or cattle as fertilizer for production of cassava foliage.http://www.lrrd.org/lrrd10/3/chau1.htm [Google Scholar]
- Christensen M.L., Sommer S.G. Manure characterisation and inorganic chemistry. In: Sommer S.G., Christensen M.L., Schmidt T., Jensen L.S., editors. Animal Manure Recycling: Treatment and Management. John Wiley & Sons, Ltd.; West Sussex, United Kingdom: 2013. pp. 41–65. [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; USA: 2013. M100S23: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; USA: 2013. VET01-S2: Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Second Informational Supplement. [Google Scholar]
- Cox J. Salmonella. In: Robinson R.K.B.C., Patel P.D., editors. Encyclopedia of Food Microbiology. Academic Press California; USA: 2000. pp. 1928–1937. [Google Scholar]
- DS 2256 . Dansk Standard; 1983. Determination of Clostridium perfringens in Water. [Google Scholar]
- DS 2401 . Dansk Standard; 1999. Environmental Quality – Enumeration of Enterococci – Colony Count on Solid Medium – Spread Plate Method. [Google Scholar]
- Echeita M.A., Aladuena A., Cruchaga S., Usera M.A. Emergence and spread of an atypical Salmonella enterica subsp. enterica serotype 4,5,12:i:- strain in Spain. J. Clin. Microbiol. 1999;37:3425. doi: 10.1128/jcm.37.10.3425-3425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA . European Food Safety Authority, Panel on Biological Hazards; 2010. Scientific Opinion on Monitoring and Assessment of the Public Health Risk of “Salmonella Typhimurium-like” Strains.http://www.efsa.europa.eu/en/efsajournal/doc/1826.pdf [Google Scholar]
- Ellerbroek L., Narapati D., Phu Tai N., Poosaran N., Pinthong R., Sirimalaisuwan A., Tshering P., Fries R., Zessin K.H., Baumann M., Schroeter A. Antibiotic resistance in Salmonella isolates from imported chicken carcasses in Bhutan and from pig carcasses in Vietnam. J. Food Prot. 2010;73:376–379. doi: 10.4315/0362-028x-73.2.376. [DOI] [PubMed] [Google Scholar]
- Emmoth E., Ottoson J., Albihn A., Belak S., Vinneras B. Ammonia disinfection of hatchery waste for elimination of single-stranded RNA viruses. Appl. Environ. Microbiol. 2011;77:3960–3966. doi: 10.1128/AEM.02990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EU . Official Journal of the European Communities; Brussels: 2011. Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption. [Google Scholar]
- EUCAST . European Committee on Antimicrobial Susceptibility Testing; Sweden: 2013. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 3.1. [Google Scholar]
- European Bioplastic . 2010. Anaerobic Digestion. Fact Sheet.http://en.european-bioplastics.org/ [Google Scholar]
- Francese A.P., Mathiesen A., Olesen T., Cordoba P.R., Sineriz F. Feeding approaches for biogas production from animal wastes and industrial effluents. World J. Microbiol. Biotechnol. 2000;16:147–150. [Google Scholar]
- Gerba C.P., Smith J.E. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 2005;34:42–48. [PubMed] [Google Scholar]
- Giraud E., Baucheron S., Cloeckaert A. Resistance to fluoroquinolones in Salmonella: emerging mechanisms and resistance prevention strategies. Microbes Infect. 2006;8:1937–1944. doi: 10.1016/j.micinf.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Guan T.Y., Holley R.A. Pathogen survival in swine manure environments and transmission of human enteric illness – a review. J. Environ. Qual. 2003;32:383–392. doi: 10.2134/jeq2003.3830. [DOI] [PubMed] [Google Scholar]
- Harris L.J., Farber J.N., Beuchat L.R., Parish M.E., Suslow T.V., Garrett E.H., Busta F.F. Outbreaks associated with fresh produce: incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003;2:78–141. [Google Scholar]
- Hauser E., Tietze E., Helmuth R., Junker E., Blank K., Prager R., Rabsch W., Appel B., Fruth A., Malorny B. Pork contaminated with Salmonella enterica serovar 4,[5],12:i:-, an emerging health risk for humans. Appl. Environ. Microbiol. 2010;76:4601–4610. doi: 10.1128/AEM.02991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans M., Vennema H., Bakker J., van der Veer B., Duizer E., Benne C.A., Waar K., Hendrixks B., Schneeberger P., Blaauw G., Kooiman M., Koopmans M.P. Swine-like hepatitis E viruses are a cause of unexplained hepatitis in the Netherlands. J. Viral Hepat. 2007;14:140–146. doi: 10.1111/j.1365-2893.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- Hopkins K.L., Kirchner M., Guerra B., Granier S.A., Lucarelli C., Porrero M.C., Jakubczak A., Threlfall E.J., Mevius D.J. Multiresistant Salmonella enterica serovar 4,[5],12:i:- in Europe: a new pandemic strain? Euro Surveill. 2010;15:19580. [PubMed] [Google Scholar]
- Huong L.Q., Madsen H., Anh le X., Ngoc P.T., Dalsgaard A. Hygienic aspects of livestock manure management and biogas systems operated by small-scale pig farmers in Vietnam. Sci. Total Environ. 2014;470/471:53–57. doi: 10.1016/j.scitotenv.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Hutchison M.L., Walters L.D., Avery S.M., Munro F., Moore A. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 2005;71:1231–1236. doi: 10.1128/AEM.71.3.1231-1236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison M.L., Walters L.D., Moore A., Avery S.M. Declines of zoonotic agents in liquid livestock wastes stored in batches on-farm. J. Appl. Microbiol. 2005;99:58–65. doi: 10.1111/j.1365-2672.2005.02585.x. [DOI] [PubMed] [Google Scholar]
- Huynh T.T.T., Aarnink A.J.A., Drucker A., Verstegen M.W.A. Pig production in Cambodia, Laos, Philippines, and Vietnam: a review. Asian J. Agric. Dev. 2006;4:69–90. [Google Scholar]
- Islam M., Morgan J., Doyle M.P., Phatak S.C., Millner P., Jiang X. Persistence of Salmonella enterica serovar typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog. Dis. 2004;1:27–35. doi: 10.1089/153531404772914437. [DOI] [PubMed] [Google Scholar]
- Jenkins M.B., Bowman D.D., Ghiorse W.C. Inactivation of Cryptosporidium parvum Oocysts by Ammonia. Appl. Environ. Microbiol. 1998;64:784–788. doi: 10.1128/aem.64.2.784-788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khai N.V., Luong N.G. Natural Science and Technology Publisher; Vietnam: 2010. Monograph of Biogas Technology. [Google Scholar]
- Kinh N.V. Global Anitibiotic Resistance Partnership – Vietnam Inaugural Workshop. The Center for Global Health Research; Washington, DC: 2010. Situation analysis: antibiotic use and resistance in Vietnam.http://www.cddep.org/sites/cddep.org/files/publication_files/VN_Report_web_1.pdf [Google Scholar]
- Kobayashi H., Khai L.T.L., Phan T.T., Yamasaki S., Taniguchi T. Prevalence of pathogenic Escherichia coli in a swine breeding environment in Can Tho province, Vietnam. Jpn. Agric. Res. Q. 2003;37:59–63. [Google Scholar]
- Lantz M., Svensson M., Björnsson L., Börjesson P. The prospects for an expansion of biogas systems in Sweden—incentives, barriers and potentials. Energ. Policy. 2007;35:1830–1843. [Google Scholar]
- Le T.A., Lejay-Collin M., Grimont P.A., Hoang T.L., Nguyen T.V., Grimont F., Scavizzi M.R. Endemic, epidemic clone of Salmonella enterica serovar typhi harboring a single multidrug-resistant plasmid in Vietnam between 1995 and 2002. J. Clin. Microbiol. 2004;42:3094–3099. doi: 10.1128/JCM.42.7.3094-3099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létourneau V., Duchaine C., Côté C., Letellier A., Topp E., Massé D. Presence of zoonotic pathogens in physico-chemically characterized manures from hog finishing houses using different production systems. Bioresour. Technol. 2010;101:4048–4055. doi: 10.1016/j.biortech.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Marcus H. Swedish University of Agricultural Sciences; 2012. Safe Retrieval of Nutrients to Improve Food Security.http://stud.epsilon.slu.se/5172/7/hjalmarsson_m_130110.pdf [Google Scholar]
- Massé D., Gilbert Y., Topp E. Pathogen removal in farm-scale psychrophilic anaerobic digesters processing swine manure. Bioresour. Technol. 2011;102:641–646. doi: 10.1016/j.biortech.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Mata-Alvarez J. IWA Publishing; UK: 2003. Fundamentals of the Anaerobic Digestion Process. [Google Scholar]
- Mawdsley J.L., Bardgett R.D., Merry R.J., Pain B.F., Theodorou M.K. Pathogens in livestock waste, their potential for movement through soil and environmental pollution. Appl. Soil Ecol. 1995;2:1–15. doi: 10.1016/0929-1393(94)00039-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossong J., Marques P., Ragimbeau C., Huberty-Krau P., Losch S., Meyer G., Moris G., Strottner C., Rabsch W., Schneider F. Outbreaks of monophasic Salmonella enterica serovar 4,[5],12:i:- in Luxembourg, 2006. Euro Surveill. 2007;12:E11–E12. doi: 10.2807/esm.12.06.00719-en. [DOI] [PubMed] [Google Scholar]
- NARMS . Center for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases; 2011. Human isolates final report. [Google Scholar]
- Ngoc T.T.B., Nhai L.T. 2008. Using Biodigested Effluent from Digester Fed with Pig Dung as Additional Pig Feed.http://www.biogas.org.vn/english/An-pham/Nam-2008.aspx [Google Scholar]
- Nguyen P.T. Chiang Mai University; 2007. Prevalence of Salmonella on Pig Carcasses at a Slaughterhouse in Hanoi, Vietnam. vphcap.vet.cmu.ac.th/file/THESIS/02nd-student/full/Phu%20Thai.pdf. [Google Scholar]
- Nicholson F.A., Groves S.J., Chambers B.J. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 2005;96:135–143. doi: 10.1016/j.biortech.2004.02.030. [DOI] [PubMed] [Google Scholar]
- NSWEPA . New South Wales Environment Protection Agency; Sydney: 2000. Environmental Guidelines: Use and Disposal of Biosolids Products. [Google Scholar]
- NZWWA . New Zealand Waste Water Association, Ministry for the Environment; Wellington: 2003. Guidelines for the Safe Application of Biosolids to Land in New Zealand. [Google Scholar]
- Ogasawara N., Tran T.P., Ly T.L., Nguyen T.T., Iwata T., Okatani A.T., Watanabe M., Taniguchi T., Hirota Y., Hayashidani H. Antimicrobial susceptibilities of Salmonella from domestic animals, food and human in the Mekong Delta, Vietnam. J. Vet. Med. Sci. 2008;70:1159–1164. doi: 10.1292/jvms.70.1159. [DOI] [PubMed] [Google Scholar]
- Ottoson J., Nordin A., von Rosen D., Vinneras B. Salmonella reduction in manure by the addition of urea and ammonia. Bioresour. Technol. 2008;99:1610–1615. doi: 10.1016/j.biortech.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Pecson B.M., Nelson K.L. Inactivation of Ascaris suum eggs by ammonia. Environ. Sci. Technol. 2005;39:7909–7914. doi: 10.1021/es050659a. [DOI] [PubMed] [Google Scholar]
- Popoff M.Y., Le Minor L. World Health Organization Collaborating Centre for Reference and Research on Salmonella, Pasteur Institute; Paris, France: 1997. Antigenic Formulas of the Salmonella Serovars. [Google Scholar]
- Pourcher A.M., Marti R., Thorigné A., Jégou B., Dabert P. Effect of anaerobic storage and aerobic digestion on micro-organisms in pig manure: cultural and molecular approaches. In: Aland A., editor. XIIIth International Congress on animal Hygiene 2007. The International Society for Animal Hygiene (ISAH); Tartu, Estonia; 2007. [Google Scholar]
- QCVN . Ministry of Agriculture and Rural Development; Hanoi, Vietnam: 2010. National Technical Regulation Conditions for Biosecurity of Pig Farms. [Google Scholar]
- QCVN . Ministry of Natural Resources and Environment; Hanoi, Vietnam: 2011. National Technical Regulation on Water Quality for Irrigated Agriculture. [Google Scholar]
- Ram C.P., Dev R.J., Nawa R.D., Amrit B.K. Evaluation of hygienic treatment of biowastes by anaerobic digestion in biogas plants. Nepal J. Sci. Technol. 2009;10:183–188. [Google Scholar]
- Sahlström L., Aspan A., Bagge E., Danielsson-Tham M.L., Albihn A. Bacterial pathogen incidences in sludge from Swedish sewage treatment plants. Water Res. 2004;38:1989–1994. doi: 10.1016/j.watres.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Sari L., Argo N., Mats E. 2011. Overview of Biogas Technology, Baltic Forum for Innovative Technologies for Sustainable Manure Management. WP6 Energy Potentials Baltic Manure. [Google Scholar]
- Sommer S.G., Génermont S., Cellier P., Hutchings N.J., Olesen J.E., Morvan T. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003;19:465–486. [Google Scholar]
- Son C.K., Dung T.T.M., Dung H.T., Luong L.M., Hau N.Y., Hung N.V. 2008. Assess Quality Of Bioslurry Under Biogas Program for Animal Husbandry Sector of Vietnam.http://www.biogas.org.vn/english/An-pham/Nam-2008.aspx [Google Scholar]
- Soyer Y., Moreno Switt A., Davis M.A., Maurer J., McDonough P.L., Schoonmaker-Bopp D.J., Dumas N.B., Root T., Warnick L.D., Grohn Y.T., Wiedmann M. Salmonella enterica serotype 4,5,12:i:-, an emerging Salmonella serotype that represents multiple distinct clones. J. Clin. Microbiol. 2009;47:3546–3556. doi: 10.1128/JCM.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.L., Guan J. Public health implications related to spread of pathogens in manure from livestock and poultry operations. Methods Mol. Biol. 2004;268:503–515. doi: 10.1385/1-59259-766-1:503. [DOI] [PubMed] [Google Scholar]
- Stuart L. 2012. Vietnamese Pig Industry Expanding to Meet Rising Pork Demand.http://www.wattagnet.com/Vietnamese_pig_industry_expanding_to_meet_rising_pork_demand.html [Google Scholar]
- Switt A.I., Soyer Y., Warnick L.D., Wiedmann M. Emergence, distribution, and molecular and phenotypic characteristics of Salmonella enterica serotype 4,5,12:i. Foodborne Pathog. Dis. 2009;6:407–415. doi: 10.1089/fpd.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavechio A.T., Ghilardi A.C., Fernandes S.A. “Multiplex PCR” identification of the atypical and monophasic Salmonella enterica subsp. enterica serotype 1,4,[5],12:i:- in São Paulo State, Brazil: frequency and antibiotic resistance patterns. Rev. Inst. Med. Trop. S. Paulo. 2004;46:115–117. doi: 10.1590/s0036-46652004000200012. [DOI] [PubMed] [Google Scholar]
- Teodorita A.S., Dominik R., Heinz P., Michael K., Tobias F., Silke V., Rainer J. University of Southern Denmark Esbjerg; Denmark: 2008. Biogas Handbook.http://www.lemvigbiogas.com/BiogasHandbook.pdf [Google Scholar]
- Thai T.H., Hirai T., Lan N.T., Yamaguchi R. Antibiotic resistance profiles of Salmonella serovars isolated from retail pork and chicken meat in North Vietnam. Int. J. Food Microbiol. 2012;156:147–151. doi: 10.1016/j.ijfoodmicro.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Tuan V.D., Porphyre V., Farinet J.L., Toan T.D. Composition of animal manure and co-products. In: Porphyre V., Coi N.Q., editors. Pig Production Development, Animal Waste Management and Environment Protection: A Case Study in Thai Binh Province, Northern Vietnam. PRISE Publications; France: 2006. pp. 127–143. [Google Scholar]
- Tuyen N.V., Hong P.T.L., Tuyet T.A., Khuyen T.D., Hoan V.Q. 2011. Utilization of Bioslurry for Comercial Fishpond.http://www.biogas.org.vn/english/An-pham/Nam-2011.aspx [Google Scholar]
- UKDoE . UK Department of the Environment; London: 1996. Code of Practice for Agriculture Use of Sewage Sludge. [Google Scholar]
- USEPA . U.S. Environmental Protection Agency; Cincinnati, OH 45268: 2003. Environmental Regulations and Technology – Control of Pathogens and Vector Attraction in Sewage Sludge. EPA/625/R-92/013 (Under 40 CFR Part 503) [Google Scholar]
- Van T.T., Moutafis G., Istivan T., Tran L.T., Coloe P.J. Detection of Salmonella spp. in retail raw food samples from Vietnam and characterization of their antibiotic resistance. Appl. Environ. Microbiol. 2007;73:6885–6890. doi: 10.1128/AEM.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T.T.H., Nguyen H.N.K., Smooker P.M., Coloe P.J. The antibiotic resistance characteristics of non-typhoidal Salmonella enterica isolated from food-producing animals, retail meat and humans in South East Asia. Int. J. Food Microbiol. 2012;154:98–106. doi: 10.1016/j.ijfoodmicro.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Vinnerås B., Holmqvist A., Bagge E., Albihn A., Jönsson H. The potential for disinfection of separated faecal matter by urea and by peracetic acid for hygienic nutrient recycling. Bioresour. Technol. 2003;89:155–161. doi: 10.1016/s0960-8524(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Vinnerås B., Nordin A., Niwagaba C., Nyberg K. Inactivation of bacteria and viruses in human urine depending on temperature and dilution rate. Water Res. 2008;42:4067–4074. doi: 10.1016/j.watres.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Vo A.T., Van Duijkeren E., Fluit A.C., Heck M.E., Verbruggen A., Maas H.M., Gaastra W. Distribution of Salmonella enterica serovars from humans, livestock and meat in Vietnam and the dominance of Salmonella Typhimurium phage type 90. Vet. Microbiol. 2006;113:153–158. doi: 10.1016/j.vetmic.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Vo A.T., Van Duijkeren E., Gaastra W., Fluit A.C. Antimicrobial resistance, class 1 integrons, and genomic island 1 in Salmonella isolates from Vietnam. PLoS ONE. 2010;5:e9440. doi: 10.1371/journal.pone.0009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T.K.V., Tran M.T., Dang T.T.S. A survey of manure management on pig farms in Northern Vietnam. Livest. Sci. 2007;112:288–297. [Google Scholar]
- Yen-Phi V.T., Rechenburg A., Vinneras B., Clemens J., Kistemann T. Pathogens in septage in Vietnam. Sci. Total Environ. 2010;408:2050–2053. doi: 10.1016/j.scitotenv.2010.01.030. [DOI] [PubMed] [Google Scholar]


