Highlights
-
•
One Health emphasizes the interdependence of human, animal, and environmental health.
-
•
Wildlife parasites are ubiquitous; how do we decide which are One Health issues?
-
•
We propose questions to help to prioritize wildlife parasites in a One Health context.
-
•
We suggest principles for taking action on wildlife parasites with One Health significance.
Keywords: parasitology, food security, wildlife, conservation, biodiversity
Abstract
One Health has gained a remarkable profile in the animal and public health communities, in part owing to the pressing issues of emerging infectious diseases of wildlife origin. Wildlife parasitology can offer insights into One Health, and likewise One Health can provide justification to study and act on wildlife parasites. But how do we decide which wildlife parasites are One Health issues? We explore toxoplasmosis in wildlife in the Canadian Arctic as an example of a parasite that poses a risk to human health, and that also has potential to adversely affect wildlife populations of conservation concern and importance for food security and cultural well-being. This One Health framework can help communities, researchers, and policymakers prioritize issues for action in a resource-limited world.
What is One Health?
Although there is no universally accepted definition of the term One Health [1], most would agree that One Health practitioners seek to collaboratively address shared challenges to the health of people, domestic animals, and wildlife (see Glossary). In 2004, the Wildlife Conservation Society (WCS) coined the term ‘One World, One Health’, partly in response to the recognition of wildlife as the probable source of the global outbreak of severe acute respiratory syndrome (SARS) 2, 3. The Manhattan principles of One Health laid out by the WCS sought to encourage coordinated responses to emerging zoonoses that also cared for the integrity of ecosystems and conservation of biodiversity (http://www.oneworldonehealth.org). Subsequently, many efforts have focused on predicting and mitigating ‘the next big’ emergence of zoonotic disease of wildlife origin 4, 5, such as the 2014 Ebola outbreak in West Africa.
Glossary.
Biodiversity: variability among living organisms at all ecological and taxonomic levels.
Biodiversity disease: a disease that has potential to worsen the conservation status of a wildlife species or population [51].
Determinants of health: the interacting biological, social, and environmental factors, as well as individual attributes and behaviors, that affect the capacity to cope with change and thrive [63].
Ecohealth: transdisciplinary action-research that strives for sustainable health of people, domestic animals, wildlife, and ecosystems, and that draws upon multiple types of knowledge from the natural, social, and health sciences, and from the humanities (http://www.ecohealth.net/aboutus_aimsscope.php) [6].
Emerging disease or pathogen: new, or newly detected (apparently emerging) disease; an increase in the distribution, incidence, transmission, diversity, or virulence of a disease or pathogen 4, 9.
Food security: according to the Food and Agriculture Organization of the United Nations: when all people, at all times, have access to sufficient, safe, and nutritious food to meet their dietary needs and food preferences for an active and healthy life [52]; for indigenous peoples, cultural food security is the ability to reliably access culturally important foods through traditional harvesting methods [54].
Interdisciplinary: synthetic attempt of mutual interaction between disciplines [64]; all activities which juxtapose, apply, combine, synthesize, integrate, or transcend parts of two or more disciplines [65].
Neglected diseases: chronic infectious diseases of people that primarily occur in developing countries, or in rural and poor urban areas of developed countries, and often exacerbate poverty (http://www.plosntds.org/static/scope).
One Health: a coordinated, collaborative, interdisciplinary and cross-sectoral approach that recognizes that the health statuses of humans, animals, and ecosystems are intimately connected [6].
Spillover/spillback: sensu lato, when pathogens transmit among ‘host compartments’ – in other words wildlife, domestic animals, and people [11]. When used unidirectionally the term spillover often refers to transmission from domestic animals to wildlife, or from wildlife to people, in which case it is distinguished from ‘spillback’ – when these diseases subsequently transmit back to domestic animals and/or people 17, 66.
Wildlife parasitology: the study of parasites of wildlife. It may include immunology, systematics, taxonomy, disease ecology, epizootiology, and host–parasite dynamics, as well as wildlife population biology and ecology. Wildlife may include free-ranging, semi-domesticated, and captive wildlife, both vertebrates and invertebrates. (http://www.journals.elsevier.com/international-journal-for-parasitology-parasites-and-wildlife/).
Who does One Health?
Despite its inclusiveness and interdisciplinary ethos, One Health, as currently practiced, almost exclusively involves the fields of veterinary medicine and public health, and this has led to a focus on disease transmission at the animal/human interface, and less on the environmental and socioeconomic aspects 6, 7. Even within the health community, there remains ‘a disconnect between human health and One Health efforts [that] has often impeded the translation of One Health from concept to reality’ [8]. To address the challenges posed by the environmental and socioeconomic determinants of health, including biodiversity loss, climate change, depletion of ecological services, conflicting resource uses and users, and an exponentially growing human population, we will need to engage a wider variety of potential collaborators in the environmental and social sciences. These collaborators bring different perspectives to studying and solving complex problems at the interface of human, animal, and environmental health; this could perhaps best be accomplished by integrating or adapting concepts from Ecohealth, with its foundation in environmental sustainability [6].
What is the interface between wildlife parasitology and One Health?
A scan of the literature since 2010 using the terms wildlife, parasitology, and ‘One Health’ suggests that the major intersections of One Health and wildlife parasitology include parasitic zoonoses, vectors and vector-borne diseases, emerging and transboundary infectious diseases, disease transmission at the wildlife/domestic animal/human interfaces, neglected diseases, global health, climate change, and food safety. More generally, issues within the scope of One Health include prevention and control of zoonotic disease, food safety and security, and antimicrobial resistance [6].
Wildlife parasitology, with its ecological approach to the diversity and complexity of parasite transmission among host compartments, trophic levels, and the environment, together with recognition that many neglected parasitic diseases are tied to socioeconomic risk factors, applied One Health thinking long before the concept of One Health became popular 9, 10, 11. Many of the examples in Calvin Schwabe's seminal work on ‘One Medicine’, the precursor to One Health, drew heavily on wildlife parasites to illustrate its points [12]. Wildlife parasitologists have contributed greatly to integrative health research that crosses disciplinary boundaries; one of the world's pre-eminent wildlife parasitologists, Dr. Robert Rausch, practiced One Health over his entire 60 year career, making ‘critical contributions to northern biology and the well-being of people in those sensitive environments on which increasingly fragile cultures remain dependent’ [13]. Wildlife parasitology has always recognized the importance of abiotic determinants of the survival and development of environmental stages of parasites, and effects of environmental change on host–parasite relationships are increasingly recognized 14, 15, 16, illustrating the importance of the environmental component in One Health.
Despite the original motivations and intentions of its founders, wildlife conservation and environmental sustainability have not often been the primary focus of One Health activities. Wildlife are frequently considered in One Health contexts as reservoirs of emerging diseases that threaten human health or food security 17, 18, and not as fellow inhabitants of a changing environment with shared risks. This has led to an anthropocentric, unidirectional concept of One Health (especially within human health) that has emphasized the flow, or spillover, of infectious diseases from animal sources to human recipients. Public perception of emerging diseases of wildlife, if not framed and communicated in a balanced manner, can pose a threat to wildlife conservation and therefore wildlife themselves [19].
Wildlife parasitology provides evidence and a theoretical foundation to argue that parasites are natural parts of ecosystems, and that understanding the ecological dynamics of a parasite shared among species is crucial for assessing and managing risks to one of the species, even if the rest of the system is unharmed. A deeper understanding of the fundamental ecological mechanisms may arise from applying methods and concepts from wildlife parasitology to other One Health problems. Parasites are ubiquitous in wildlife populations, and the presence of parasites does not necessarily mean that wildlife are unhealthy. Indeed, parasites can serve as indicators of high biodiversity and intact trophic relationships in healthy ecosystems [20]. Understanding the ecological system, as well as the social framework and underlying risk perceptions, may help in deciding when and how to act in response to the detection of a parasite in wildlife.
What should be included under the One Health ‘umbrella’?
One Health is not only a framework for surveillance or research, but also for action 1, 7. In a resource-limited environment for scientific research and public health programming, One Health can give an appealing motivation and justification for studying and managing wildlife parasites. This is why parasitologists are eager to be, and stay, under the One Health ‘umbrella’ [21]. However, as other authors have emphasized, few parasites have been the subject of One Health attention, in large part because of the focus on emerging diseases (generally of viral or bacterial origin) with public health or economic significance 4, 18, 21. In the following section we use the example of toxoplasmosis in wildlife in the Arctic to propose a set of questions to help communities, researchers, and policy-makers prioritize wildlife parasites from a One Health perspective. These guiding questions were modified from those proposed by other authors [22] to assist decision-makers in wildlife disease management and evolutionary ecological research (Box 1 ).
-
(i)
Does the parasite in wildlife represent a risk to human health?
Zoonotic parasites in wildlife have obvious relevance for One Health; however, a risk-based approach also considers the direction of transmission, the extent and scope of human exposure, the relative importance of wildlife as a source of human exposure, and whether there is evidence of clinical disease associated with the parasite in people. In our example, all genotypes of Toxoplasma gondii are considered zoonotic. The zoonotic potential and pathogenicity of ‘atypical’ strains of T. gondii common in wildlife is often unknown [11], and virulence varies among the different genotypes [23]. There is evidence of unusually high levels of human exposure to T. gondii in some regions of the Canadian Arctic. In Nunavik, northern Quebec east of Hudson's Bay, overall seroprevalence in the Inuit population is 60%, and as high as 87% in some individual communities 24, 25, 26. There are multiple routes of exposure possible for this parasite, including inadvertent consumption of oocysts shed in the feces of felids, ingestion of tissue cysts in other infected intermediate vertebrate hosts, and transplacental transmission [27]. Transmission of T. gondii is complex and enigmatic in the Arctic, where felid definitive hosts are scarce. Possible routes of transmission include oocysts shed in felid feces conducted via marine and freshwater routes from subarctic and temperate regions 28, 29, trophic, and vertical routes of transmission (Figure 1 ).
Harvesting and consuming wildlife has been identified as a risk factor for exposure to toxoplasmosis in several epidemiological studies in the eastern Canadian Arctic 24, 26, 30. In people, this parasite can cause transient ‘flu-like illness’ and, although controversial, has been linked to neurological or behavioral changes in latently infected, immunocompetent people 31, 32. The most severe manifestations of disease occur in immunocompromised people and women infected for the first time during pregnancy, including miscarriage, stillbirths, and fetal deformities 27, 33. Congenital toxoplasmosis has been documented in the Nunavik region of the Canadian Arctic [30], before implementation of monitoring and treatment of pregnant women in this region [34]. Therefore, there is evidence of human exposure, disease, and potential wildlife sources for T. gondii in the Canadian Arctic. However, further molecular and traditional epidemiological investigation will be necessary to determine if people are being exposed and developing clinical disease from harvesting and consumption of wildlife. Action in this instance could involve testing and treating high-risk human groups for toxoplasmosis, and enhanced surveillance through the addition of toxoplasmosis to the list of diseases that are notifiable to public health authorities in Canada.
-
(ii)
Does the parasite have potential to adversely affect wildlife populations of conservation concern?
We have deliberately avoided terminology such as ‘at risk’, ‘endangered’, or ‘threatened’, which are variably defined, and we recognize the significant scientific and social challenges in establishing whether or not a wildlife population is of concern. Legal categorization can lag ecological reality; concern for a consumed or economically-important species can differ from others; and the science of establishing thresholds for concern within a rapidly changing environment is an ongoing challenge. It is beyond the scope of this paper to define ‘concern’; instead we advise relying on the prevailing expertise and statutes. We emphasize the need to act quickly to address population-limiting factors such as pathogens, pollution, and habitat loss because recent findings demonstrate that – by the time a species is formally described as ‘at-risk’ – they rarely recover, at least in Canada [35]. In particular, harvested species and wildlife in Canada's North are among the least-likely species to be listed under the federal Species At Risk Act, with its accompanying protection, monitoring, and action plans 35, 36.
In addition, we recognize that definitive evidence for population level effects of parasites is challenging to establish in free-ranging wildlife. This has been achieved in only a handful of situations, usually involving an island population of wildlife or a semi-captive situation where monitoring and intervention can be accomplished 37, 38. Because these guiding questions can be used to triage potential One Health problems, and guide decision-makers towards priority issues (rather than to definitively establish cause–effect relationships), it is sufficient to relax criteria for demonstrating population-level effects for parasites. Instead, it is enough to establish the potential for effects based on the best available evidence (experimental infections, pathology in wildlife found dead of natural causes, presence of the parasite in a declining population and absence in stable or increasing populations, correlations between prevalence/intensity and host abundance, intervention studies, etc.). Although a parasite may not generally be considered to be population-limiting, it may have greater significance in a wildlife population experiencing the cumulative effects of other pathogens and stressors.
Evidence for effects of T. gondii is often limited to reports demonstrating pathology contributing to death in a wild animal 39, 40, or experimental infections of captive or semi-domesticated wildlife [41]. Clinical toxoplasmosis is rarely reported even in domestic animals, with the exception of abortion storms in sheep [33]. Toxoplasmosis has been detected in a wide range of terrestrial and marine mammals across the Canadian Arctic, including caribou, which are undergoing population declines around the circumpolar North 25, 42, 43, 44. Toxoplasmosis can cause clinical disease in marine mammals, and serological evidence and pathology have been reported in Arctic pinnipeds and beluga of the St. Lawrence estuary 45, 46, 47, 48. Perhaps the best available evidence that T. gondii has the potential to negatively affect wildlife populations comes from sea otters in California 39, 49. However, it is difficult to link a pathogen causally to a population decline in wildlife under siege from cumulative effects of many interacting stressors. T. gondii is only one of many pathogens and pollutants affecting Arctic wildlife; other stressors include hunting pressure, increased traffic through the Northwest Passage, and climate-driven changes affecting Arctic ecosystems and communities [50]. Compared to pathogens such as white-nose syndrome in bats and chytridiomycosis in amphibians, T. gondii cannot be considered a ‘biodiversity disease’ in most wildlife populations [51]. However, because it has potential to cause mortality or decreased reproductive success in wildlife, especially marine mammals, further surveillance and study are warranted to determine the effects of T. gondii in wildlife populations of conservation concern in the Arctic.
-
(iii)
Is the parasite perceived as a threat to people who rely on the health and sustainability of the wildlife resource?
This question recognizes that there is a complex relationship between people and wildlife that may include food security, economic, cultural, and spiritual benefits. Therefore, adverse effects of wildlife parasites are not limited to food-borne diseases. One Health is a living concept in Canada's North, where Inuit people are strongly connected to the land and wildlife, and these connections are crucial to their health, food security, and culture 52, 53, 54. However, Arctic ecosystems and traditional food consumption are undergoing marked changes (climatic and anthropogenic), and these changes may be occurring so rapidly that they exceed the ability of Arctic indigenous peoples (and wildlife) to adapt 50, 55, necessitating more effective and rapid means to translate research into action.
Wildlife populations harvested for food purposes are particularly important in the Canadian North, and this warrants consideration of both the food safety risks and the food security benefits of the wildlife resource. In the Canadian Arctic in 2008, about a third of Inuit regularly consumed wildlife such as fish, caribou, and marine mammals [55]. The importance of sustainable wildlife harvest in the north is highlighted by the fact that Inuit in the Canadian Arctic have levels of food insecurity 5–6-fold greater than the Canadian national average [56]. In recognition of these high levels of food insecurity, and the nutritional benefits of harvested wildlife, public health messaging in the Canadian Arctic continues to reinforce that consuming foods of wildlife origin is considered to be safe for the general population. While exposure to T. gondii has been documented in caribou, marine mammals, and geese in the Canadian North 25, 42, 57, the implications of this parasite on the availability and sustainability of foods of wildlife origin remains unknown, under current as well as future conditions. The environment is not static, and there has been much interest in the possible emergence of T. gondii as a result of climate change, hydrology, and pollution 29, 49, 58, 59.
Management responses to a parasite in wildlife can impact upon social capital, community trust, and access to resources. Even public health messaging designed to raise awareness about a wildlife parasite as a food safety issue can have undesirable outcomes if people perceive that their traditional food sources are unsafe or unsustainable. An effective management strategy for parasites of One Health significance in wildlife populations important for harvest requires community acceptance and action. This is greatly facilitated when communities are part of the decision-making as early in the process as possible, and when communities perceive a benefit in implementing practices that reduce disease transmission – a ‘win–win’ outcome for wildlife conservation and human health [22]. A win–win outcome in our example could involve offering a carcass-side test for T. gondii in harvested wildlife, such as the walrus-testing program for trichinellosis in Nunavik [60], which allows community members to make informed decisions about food consumption (species and organs), method of food preparation, and disposal of carcasses in a manner that does not facilitate transmission of the parasite.
Box 1. Should a wildlife parasite be prioritized based on a One Health perspective?
Below are three guiding questions to determine whether a wildlife parasite should be prioritized for action from a One Health perspective, modified from those proposed in [22]. Sub-questions may need to be answered to address the guiding question.
-
(i)
Does the parasite in wildlife represent a risk to human health?
Are there strains or genotypes of the parasite? Do they differ in zoonotic potential? Is wildlife a source of human exposure? What wildlife species, organs, and routes of exposure are important? What other sources exist (human to human, domestic animal to human, shared contaminated environment)? Is there evidence of human exposure? Is this higher than expected? Is there evidence of human disease? How severe is the disease in the general and high-risk human populations? Are there already mechanisms in place that limit human exposure?
-
(ii)
Does the parasite have potential to adversely affect wildlife populations of conservation concern?
Is this a biodiversity disease? Is the host species or population of conservation concern? Is there sufficient knowledge of the population trend of the host population? How strong is the evidence for individual and population level effects of the parasite? Is there evidence of widespread animal exposure? Is this higher than expected? How severe is the disease in the general and high-risk animal populations? How long has the parasite likely been present in this population? What is the importance of this parasite relative to other potential limiting factors? What other host species depend on this species/population? Are these key to ecosystem stability and function?
-
(iii)
Is the parasite perceived as a threat to people who rely on the health and sustainability of the wildlife resource?
What is the relationship between local people and this wildlife population (subsistence harvest, commodity production, ecotourism, cultural and spiritual significance, etc.)? What proportion of the human population relies on this wildlife population? What proportion of harvested wildlife does this species/population represent? Can harvesters switch to other prey species? Are these prey species likely to experience undesirable effects as a result? What stakeholders need to be engaged in the decision-making process about intervention? Are interventions culturally acceptable and enforceable? Do they place unusual hardship on a vulnerable subpopulation of people? If the parasite is zoonotic, how should public health messaging address risk-perception issues?
Figure 1.
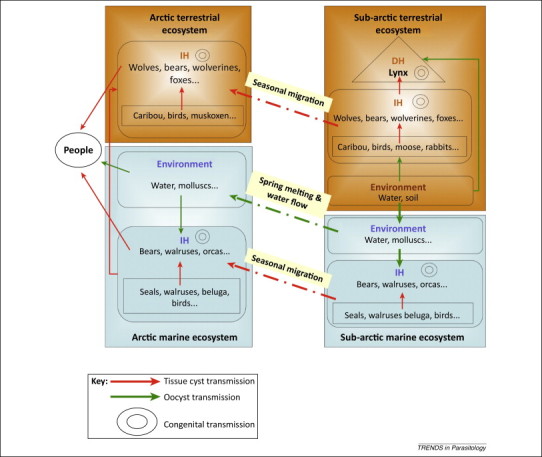
Complexities of transmission of Toxoplasma gondii in Arctic regions in the absence of felid definitive hosts (DH) that shed oocysts above the treeline. Complexities include the possibilities of parasite flow among terrestrial, marine, and freshwater ecosystems; south to north transport of tissue cysts in migratory wildlife intermediate hosts (IH); and congenital (transplacental) transmission, which is thought to occur in most mammalian hosts. Adapted with permission from Audrey Simon, PhD Thesis, Université de Montréal, 2012.
Concluding remarks
We suggest that prioritizing wildlife parasites in a One Health context involves consideration of whether parasites in wildlife represent a risk to human health (in terms of both exposure and development of disease) and whether they demonstrate potential to adversely affect wildlife populations of conservation concern, those key to ecosystem stability and function, or human communities who rely on wildlife. How the answers to the questions are weighed and integrated remains a judgment-call influenced by local laws, culture, and priorities. In Box 2 , we propose a set of principles and practices that may aid in taking action on a wildlife parasite from a One Health perspective. Issues likely to be successfully operationalized within a One Health framework have in common a perception of risk (i.e., the parasites are perceived to pose a real threat), there is something practical that can be done about it (perceived feasibility), and there is support from stakeholders at multiple levels (i.e., communities, policy-makers, and researchers), generally tied to a perceived benefit. The importance of public perception and balanced risk communication cannot be overemphasized when implementing management policies for a wildlife parasite with One Health significance [19].
Box 2. Suggested practices for managing and communicating risks associated with wildlife parasites within a One Health framework.
-
(i)Acknowledge complexity and uncertainty
-
•There may be multiple interdependent species affected by the parasite.
-
•New findings may truly be new (range expansion, novel species or strains), but could also be an apparent emergence due to increased sampling effort, enhanced diagnostic technologies, etc.
-
•For parasites with potential for population-level effects, consider the conservation status, ecological role, and value (nutritional, cultural, economic, and spiritual) to local communities.
-
•Hosts and parasites share the environment, and overall effects on parasite ecology as a result of environmental change are complex and potentially compensatory.
-
•
-
(ii)Anticipate unintended consequences
-
•Managing a single host–parasite system can have unforeseen effects, and this calls for a systems-level approach.
-
•Removal of a hazard could also affect benefits derived from a human–wildlife interaction.
- •
-
•
-
(iii)Recognize that healthy systems include parasites
-
•Parasites are natural parts of ecosystems. Finding parasites in wildlife does not necessarily mean that the wildlife is unhealthy or cannot be used or consumed.
-
•
-
(iv)Understand the context for risk communication and management
- •
-
•For zoonoses, work with community and public health officials to review the risks and benefits of altering the human–animal relationship.
-
•Embed risk-management recommendations in the socio-ecological system to develop a rich understanding of potential routes of exposure, mechanisms for risk reduction, and implications of actions for all affected species.
-
•Consult with community, technical experts, and authorities responsible for risk management when formulating recommendations.
One Health can serve as a framework to emphasize the interconnected nature of health, and provide justification for management actions that optimize benefits and minimize harm to people, wildlife, and the environment – ‘win–win’ outcomes [22]. Primum non nocere is a fundamental principle of the health professions, and the challenge for One Health practitioners is to apply this across multiple mandates and sometimes competing interests. This will be facilitated if practitioners of One Health continue to seek out partners outside the animal and human health disciplines to address a broad range of sociological, cultural, and ecological factors not traditionally within the purview of wildlife parasitologists. Wildlife is a social good that provides ecological services necessary for sustainable economies and human health 61, 62, and wildlife come along with a suite of parasites and pathogens. Not all require action, and resources are limited; therefore, we propose an integrated approach to making decisions on priorities for One Health investment that considers ecological, social, conservation, and public health concerns.
Acknowledgments
We recognize the valuable and thorough comments from reviewers, editors, and colleagues, particularly Doug Clark, Lydden Polley, and R.C. Andrew Thompson.
References
- 1.Leboef A. Institut Français des Relations Internationales; 2011. Making Sense of One Health; Cooperating at the Human–Animal–Ecosystem Health Interface (IFRI Health and Environment Reports No. 7) [Google Scholar]
- 2.Tu C. Antibodies to SARS coronavirus in civets. Emerg. Infect. Dis. 2004;10:2244–2248. doi: 10.3201/eid1012.040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruse H. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 2004;10:2067–2072. doi: 10.3201/eid1012.040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones K.E. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron A. Epidemiological interaction at the wildlife/livestock/human interface: can we anticipate emerging infectious diseases in their hotspots? A framework for understanding emerging diseases processes in their hot spots. In: Morand S., editor. New Frontiers of Molecular Epidemiology of Infectious Diseases. Springer; 2012. pp. 311–332. [Google Scholar]
- 6.Zinsstag J. Convergence of Ecohealth and One Health. Ecohealth. 2012;9:371–373. doi: 10.1007/s10393-013-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephen C., Karesh W.B. Is ‘One Health’ delivering results? OIE Sci. Tech. Rev. 2014;33:375–379. doi: 10.20506/rst.33.2.2301. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar M. One Health: a perspective from the human health sector. Rev. Sci. Tech. Off. Int. Épizooties. 2014;33:407–412. doi: 10.20506/rst.33.2.2299. [DOI] [PubMed] [Google Scholar]
- 9.Polley L. Navigating parasite webs and parasite flow: emerging and re-emerging parasitic zoonoses of wildlife origin. Int. J. Parasitol. 2005;35:1279–1294. doi: 10.1016/j.ijpara.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Hotez P.J. Neglected infections of poverty among the indigenous peoples of the arctic. PLoS Negl. Trop. Dis. 2010;4:e606. doi: 10.1371/journal.pntd.0000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson R.C.A. Parasite zoonoses and wildlife: One Health, spillover and human activity. Int. J. Parasitol. 2013;43:1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwabe C.W. 3rd edn. Williams and Wilkins; 1984. Veterinary Medicine and Human Health. [Google Scholar]
- 13.Hoberg E.P. Robert Lloyd Rausch – a life in nature and field biology: 1921-2012. J. Parasitol. 2014;100:547–552. doi: 10.1645/14-561.1. [DOI] [PubMed] [Google Scholar]
- 14.Patz J.A. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 15.Bradley C.A., Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutz S.J. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet. Parasitol. 2009;163:217–228. doi: 10.1016/j.vetpar.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Daszak P. Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 18.Taylor L.H. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buttke D.E. The role of one health in wildlife conservation: a challenge and opportunity. J. Wildl. Dis. 2015;51:1–8. doi: 10.7589/2014-01-004. [DOI] [PubMed] [Google Scholar]
- 20.Hudson P.J. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Robertson L.J. Keeping parasitology under the One Health umbrella. Trends Parasitol. 2014;30:369–372. doi: 10.1016/j.pt.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crozier G.K.D., Schulte-Hostedde A.I. The ethical dimensions of wildlife disease management in an evolutionary context. Evol. Appl. 2014;7:788–798. doi: 10.1111/eva.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dardé M.L. Toxoplasma gondii, ‘new’ genotypes and virulence. Parasite. 2008;15:366–371. doi: 10.1051/parasite/2008153366. [DOI] [PubMed] [Google Scholar]
- 24.Messier V. Seroprevalence of Toxoplasma gondii among Nunavik Inuit (Canada) Zoonoses Public Health. 2009;56:188–197. doi: 10.1111/j.1863-2378.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins E.J. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv. Parasitol. 2013;82:33–204. doi: 10.1016/B978-0-12-407706-5.00002-2. [DOI] [PubMed] [Google Scholar]
- 26.Goyette S. Seroprevalence of parasitic zoonoses and their relationship with social factors among the Canadian Inuit in Arctic regions. Diagn. Microbiol. Infect. Dis. 2014;78:404–410. doi: 10.1016/j.diagmicrobio.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Tenter A.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsay D. Sporulation and survival of Toxoplasma gondii oocysts in seawater. J. Eukaryot. Microbiol. 2003;50:687–688. doi: 10.1111/j.1550-7408.2003.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 29.Simon A. Hydrological modelling of Toxoplasma gondii oocysts transport to investigate contaminated snowmelt runoff as a potential source of infection for marine mammals in the Canadian Arctic. J. Environ. Manage. 2013;127:150–161. doi: 10.1016/j.jenvman.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 30.McDonald J.C. An outbreak of toxoplasmosis in pregnant women in northern Quebec. J. Infect. Dis. 1990;161:769–774. doi: 10.1093/infdis/161.4.769. [DOI] [PubMed] [Google Scholar]
- 31.Cook T.B. ‘Latent’ infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. J. Psychiatr. Res. 2015;60:87–94. doi: 10.1016/j.jpsychires.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Jones J.L. Neglected parasitic infections in the United States: toxoplasmosis. Am. J. Trop. Med. Hyg. 2014;90:794–799. doi: 10.4269/ajtmh.13-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubey J.P., Jones J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Lévesque B. Evaluation of the toxoplasmosis screening program among pregnant Nunavik (Canada) women between 1994–2003. Epidemiology. 2007;18:S32. [Google Scholar]
- 35.Favaro B. Trends in extinction risk for imperiled species in Canada. PLoS ONE. 2014;9:e113118. doi: 10.1371/journal.pone.0113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mooers A.O. Science, policy, and species at risk in Canada. BioScience. 2010;60:843–849. [Google Scholar]
- 37.Hudson P.J., editor. Ecology of Wildlife Diseases. Oxford University Press; 2001. [Google Scholar]
- 38.Irvine R.J. Parasites and the dynamics of wild mammal populations. Anim. Sci. 2006;82:775–781. [Google Scholar]
- 39.Miller M.A. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int. J. Parasitol. 2004;34:275–284. doi: 10.1016/j.ijpara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen K.K. Acute toxoplasmosis in three wild arctic foxes (Alopex lagopus) from Svalbard; one with co-infections of Salmonella enteritidis PT1 and Yersinia pseudotuberculosis serotype 2b. Res. Vet. Sci. 2005;78:161–167. doi: 10.1016/j.rvsc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Oksanen A. Experimental Toxoplasma gondii infection leading to fatal enteritis in reindeer (Rangifer tarandus) J. Parasitol. 1996;82:843–845. [PubMed] [Google Scholar]
- 42.Kutz S.J. Prevalence of Toxoplasma gondii antibodies in barren-ground caribou (Rangifer tarandus groenlandicus) from the Canadian Arctic. J. Parasitol. 2001;87:439–442. doi: 10.1645/0022-3395(2001)087[0439:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Vors L.S., Boyce M.S. Global declines of caribou and reindeer. Glob. Change Biol. 2009;15:2626–2633. [Google Scholar]
- 44.Elmore S.A. Toxoplasma gondii in people and wildlife of the circumpolar North. Vector-Borne Zoonotic Dis. 2012;12:1–9. doi: 10.1089/vbz.2011.0705. [DOI] [PubMed] [Google Scholar]
- 45.Mikaelian I. Toxoplasmosis in beluga whales (Delphinapterus leucas) from the St Lawrence Estuary: two case reports and a serological survey. J. Comp. Pathol. 2000;122:73–76. doi: 10.1053/jcpa.1999.0341. [DOI] [PubMed] [Google Scholar]
- 46.Dubey J.P. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 2003;116:275–296. doi: 10.1016/s0304-4017(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 47.Measures L.N. Seroprevalence of Toxoplasma gondii in Canadian Pinnipeds. J. Wildl. Dis. 2004;40:294–300. doi: 10.7589/0090-3558-40.2.294. [DOI] [PubMed] [Google Scholar]
- 48.Simon A. Spatio-temporal variations and age effect on Toxoplasma gondii seroprevalence in seals from the Canadian Arctic. Parasitology. 2011;1:1–7. doi: 10.1017/S0031182011001260. [DOI] [PubMed] [Google Scholar]
- 49.VanWormer E. Using molecular epidemiology to track Toxoplasma gondii from terrestrial carnivores to marine hosts: implications for public health and conservation. PLoS Negl. Trop. Dis. 2014;8:e2852. doi: 10.1371/journal.pntd.0002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cramer W., Yohe G. Detection and attribution of observed impacts. In: Field C.B., editor. Climate change 2014: Impacts, Adaptation and Vulnerability Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2014. pp. 979–1037. [Google Scholar]
- 51.Grogan L.F. Surveillance for emerging biodiversity diseases of wildlife. PLoS Pathog. 2014;10:e1004015. doi: 10.1371/journal.ppat.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan H. Food security in Nunavut, Canada: barriers and recommendations. Int. J. Circumpolar Health. 2006;65:416–431. doi: 10.3402/ijch.v65i5.18132. [DOI] [PubMed] [Google Scholar]
- 53.Lambden J. Traditional food attributes must be included in studies of food security in the Canadian Arctic. Int. J. Circumpolar Health. 2007;66:308–319. doi: 10.3402/ijch.v66i4.18272. [DOI] [PubMed] [Google Scholar]
- 54.Power E.M. Conceptualizing food security for Aboriginal people in Canada. Can. J. Public Health. 2008;99:95–97. doi: 10.1007/BF03405452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheikh N. Changing dietary patterns and body mass index over time in Canadian Inuit communities. Int. J. Circumpolar Health. 2011;70:511–519. doi: 10.3402/ijch.v70i5.17863. [DOI] [PubMed] [Google Scholar]
- 56.Rosol R. Prevalence of affirmative responses to questions of food insecurity: International Polar Year Inuit Health Survey, 2007–2008. Int. J. Circumpolar Health. 2011;70:488–497. doi: 10.3402/ijch.v70i5.17862. [DOI] [PubMed] [Google Scholar]
- 57.Elmore S.A. Toxoplasma gondii exposure in arctic-nesting geese: A multi-state occupancy framework and comparison of serological assays. Int. J. Parasitol. Parasites Wildl. 2014;3:147–153. doi: 10.1016/j.ijppaw.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller M.A. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- 59.Jensen S.K. The prevalence of Toxoplasma gondii in polar bears and their marine mammal prey: evidence for a marine transmission pathway? Polar Biol. 2010;33:599–606. [Google Scholar]
- 60.Larrat S. From science to action and from action to science: the Nunavik Trichinellosis Prevention Program. Int. J. Circumpolar Health. 2012;71:18595. doi: 10.3402/ijch.v71i0.18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deem S.L. Putting theory into practice: wildlife health in conservation. Conserv. Biol. 2001;15:1224–1233. [Google Scholar]
- 62.Aguirre A.A. Wild canids as sentinels of ecological health: a conservation medicine perspective. Parasit. Vectors. 2009;2:S7. doi: 10.1186/1756-3305-2-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephen C. Toward a modernized definition of wildlife health. J. Wildl. Dis. 2014;50:427–430. doi: 10.7589/2013-11-305. [DOI] [PubMed] [Google Scholar]
- 64.Huutoniemi K. Analyzing interdisciplinarity: typology and indicators. Res. Policy. 2010;39:79–88. [Google Scholar]
- 65.Miller R.C. Varieties of interdisciplinary approaches in the social sciences: a 1981 overview. Issues Integr. Stud. 1982;1:1–37. [Google Scholar]
- 66.Rhyan J.C., Spraker T.R. Emergence of diseases from wildlife reservoirs. Vet. Pathol. 2010;47:34–39. doi: 10.1177/0300985809354466. [DOI] [PubMed] [Google Scholar]
- 67.Artois M. Sustainable control of zoonotic pathogens in wildlife: how to be fair to wild animals? Rev. Sci. Tech. 2011;30:733–743. doi: 10.20506/rst.30.3.2069. [DOI] [PubMed] [Google Scholar]
- 68.Korsmo F.L., Graham A. Research in the North American North: action and reaction. Arctic. 2002;55:319–328. [Google Scholar]
- 69.Schnarch B. Ownership, control, access, and possession (OCAP) or self-determination applied to research. J. Aborig. Health. 2004;1:80–95. [Google Scholar]


