Highlights
-
•
Diagnosis of cryptosporidiosis is currently based on a variety of laboratory methods.
-
•
Typing of isolates supports disease investigations, but application is inconsistent.
-
•
To harmonise work, there is a need for consensus in multi-locus typing schemes.
-
•
Translation of genomic data into improved diagnostic and typing assays will facilitate future harmonisation.
Keywords: cryptosporidiosis, Cryptosporidium, detection, diagnosis, typing, subtyping
Abstract
The protozoan Cryptosporidium is a major public and animal health concern. Young children, immunocompromised people, and pre-weaning animals are especially vulnerable, but treatment options are limited and there is no vaccine. A laboratory diagnosis is required to confirm cases of cryptosporidiosis, and species and genotype determination is essential in distinguishing human from non-human sources, understanding transmission, and strengthening the epidemiological evidence for causative links in outbreaks. However, testing is not consistent, as demonstrated by investigation of a significant increase in cases in some European countries during 2012. Many methods employed are laborious and time-consuming; recent advances, translated into diagnostic assays, can improve testing and facilitate typing to support clinical and environmental investigations.
Why look for Cryptosporidium?
Cryptosporidium causes sporadic cases and outbreaks of gastroenteritis (cryptosporidiosis) through faeces-contaminated drinking and recreational water [1], food and beverages [2], person-to-person spread in households and institutions such as daycare settings [3], farmed animal contact [4], and in livestock, companion, and other animals [5] (Figure 1 ). Increased morbidity, mortality, and socio-economic implications 6, 7 are recognised by inclusion of Cryptosporidium in the Neglected Diseases Initiative of the World Health Organization (WHO) [8]. Cryptosporidiosis is a laboratory diagnosis because it is not pathognomonic – in other words, the acute signs and symptoms can be similar to other infectious and non-infectious causes of gastroenteritis 9, 10. In cases where other parasites are also suspected, or in populations where they are prevalent, multiple faecal samples with and without chemical preservation may be required [11] (Figure 2 ). Differential diagnosis enables provision of appropriate advice on treatment, control, and management, and at the population level it is required for the identification of outbreaks and for monitoring trends, risk factors, and interventions.
Figure 1.
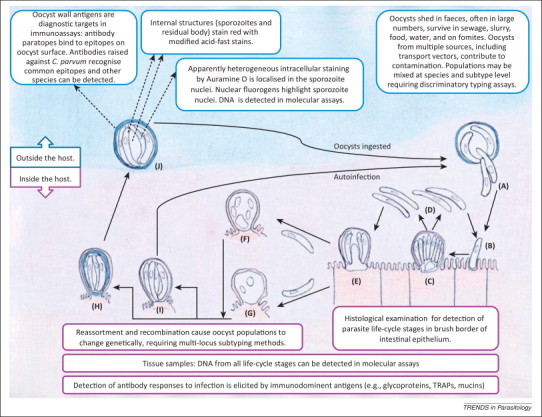
Schematic representation of the Cryptosporidium life-cycle and transmission emphasising elements exploited in diagnosis and detection. A population of sporulated oocysts is ingested by a host, and undergo excystation in the intestinal lumen. Four sporozoites are present in each oocyst, and emerge (A) to invade the microvilli at the brush border of the mucosal epithelium (B) and develop into trophozoites (not shown). Trophozoites undergo asexual division (merogony) to form eight merozoites within type I meronts (C), and release invasive merozoites (D) that invade adjacent host cells to form additional type I meronts or to form type II meronts (E). Merozoites released from type II meronts do not recycle but invade host cells to initiate the sexual stage. Microgametocytes released from microgamonts (F) fertilise the macrogamonts (G) to become zygotes. Most of the zygotes develop into oocysts with a thick, two-layered wall (H) but a minority only have a unit membrane surrounding the four sporozoites (I) and facilitate the autoinfective cycle that maintains infection without further ingestion of thick-walled oocysts. Sporulated, thick-walled oocysts containing four sporozoites (J) are released in faeces and transmit infection between hosts. Abbreviation: TRAPs, thrombospondin related adhesive proteins.
Figure 2.
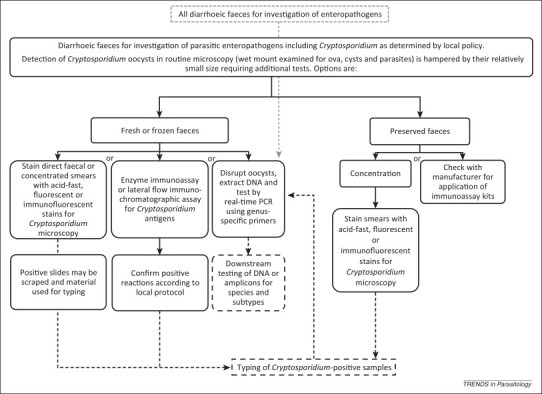
Workflow for Cryptosporidium diagnosis during investigation of gastroenteritis. Unbroken boxes represent adopted tests [11] (see: http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/); broken lines and boxes represent reference or specialist tests; broken, grey lines and box represents alternative workflow based on multi-pathogen diagnostic PCRs. The optimisation of automated nucleic acid extraction processes and application of multiplex, real-time PCR offers great potential for streamlining diagnostic workflows.
The ability of Cryptosporidium to break through multiple water-treatment barriers and cause large-scale outbreaks has had huge impact on the water industry and its regulation [12], and it has been used as a reference pathogen for the faecal-orally transmitted protozoa in the design and implementation of the WHO Guidelines for Drinking Water Quality 13, 14. Monitoring for oocysts is part of the surveillance to support water-safety plans [14].
During 2012 a significant but unexplained increase in the incidence of human cryptosporidiosis was reported by Fournet and colleagues in the UK, The Netherlands (NL), and Germany to the European Centre for Disease Control [15]. This report highlights difficulties in comparing data across countries, derived differently due to variations in laboratory testing and reporting. The lack of widespread, routine Cryptosporidium diagnosis and species identification, or a standardised subtyping scheme, impinges on investigation. These shortcomings also occur in water testing where only the genus is identified. Phylogenetic comparisons of isolates from cases and sources are not undertaken in most settings, impacting on source attribution and risk assessments for drinking water and other exposures [13].
Techniques for the detection, diagnosis, and characterisation of Cryptosporidium spp. from different sample types have been reviewed previously 16, 17, 18. However, not all have been delivered from research to diagnostic settings. Here we examine the current workflows and review advances that have been translated into clinical and environmental investigations.
Cryptosporidium, an uncertain taxonomy
Taxonomic positions are important in understanding the evolution, biology, pathogenesis, sources, and transmission of pathogens as well as focussing efforts for identifying targets for diagnosis and drug therapies. The taxonomy of Cryptosporidium (phylum Apicomplexa, class Coccidea, order Eimeriidae) was disputed, because of life-cycle (Figure 1), phylogenetic, and biological differences observed in comparison with other Eimeriidae (e.g., Cyclospora, Cystoisospora, and Eimeria) [19]. Traditionally, Apicomplexan species were defined by host-specificity, location of endogenous stages, and morphology. For Cryptosporidium spp., however, host-specificity is variable, and oocyst morphology distinguishes only those with smaller, near-spherical oocysts (4–6 μm in diameter) from those with larger, more oval oocysts (7 × 5 μm) (Table 1 ). Therefore, a combination of biological and genetic data is recommended for identifying and naming new species: (i) morphological features of the oocyst and demonstration of developmental stages; (ii) natural and experimental host range; (iii) detailed biological characterisation, including localisation and pathology associated with an individual host; (iv) differential diagnosis; (v) genetic characterisation at multiple loci, to include the small subunit ribosomal RNA (SSU rRNA) and other functional genes; (vi) deposition of definitive reference sequences and preserved material for further analyses; and (vii) compliance with International Committee for Zoonotic Nomenclature (ICZN) species-naming rules [20]. Where mainly genetic data are available, isolates are described as ‘genotypes’ [21]. The ICZN rules are open to interpretation, especially in the Principle of Priority, leading to conflicting opinions about renaming Cryptosporidium spp. (e.g., Cryptosporidium parvum as Cryptosporidium pestis) [22]. To avoid confusion and promote stability, here we use C. parvum for the species infective to calves and humans [21].
Table 1.
Cryptosporidium species and their association with clinical disease
| Cryptosporidium species and genotypesa | Mean oocyst dimensions (μm)b | Major host(s) | Association with human cryptosporidiosis [18] | Association with animal cryptosporidiosis 5, 21, 23 | Additional key Refs |
|---|---|---|---|---|---|
| C. andersoni | 7.4 × 5.5 | Cattle | Rarely associated; individual reports from UK, Australia, and Malawi. | Usually seen in yearlings and adult cattle; not associated with diarrhoea but linked to reduced milk-yields and weight gain. | [63] |
| C. baileyi | 6.2 × 4.6 | Chickens, other birds | No association. | Many different bird species can be affected, mainly by infection in the upper respiratory tract which can be fatal; also linked to renal and ocular disease. Severe outbreaks and mortalities in poultry units. | |
|
C. bovis (previously bovine B genotype) |
4.9 × 4.6 | Cattle | Rarely associated; individual reports from Australia and India of cases reporting cattle contact. | Shedding is seen mainly in weaned and sometimes pre-weaning calves but infection is considered non-pathogenic. | 63, 64 |
|
C. canis (previously dog genotype) |
5.0 × 4.7 | Dog | Epidemiologically linked to diarrhoea in children in a shanty town in Lima, Peru; occasional sporadic cases in various, especially non-industrialised, countries; some cases report contact with dogs. | Diarrhoea usually seen in puppies and younger dogs; asymptomatic shedding of oocysts has been reported in older and immune- competent dogs. | |
|
C. cuniculus (previously rabbit genotype) |
5.6 × 5.4 | Rabbit, humans | Caused a waterborne outbreak in UK; occasional, seasonal sporadic cases in UK, individual reports from France and children in Nigeria. | Asymptomatic shedding of oocysts in natural and experimental infections in rabbits. | 65, 66, 67 |
|
C. fayeri (previously marsupial genotype I) |
4.9 × 4.3 | Marsupials | Rarely associated; individual report from Australia of a person in contact with marsupials. | Oocyst shedding is not associated with diarrhoea and no clinical signs are described. | [63] |
| C. felis | 4.6 × 4.0 | Cat | Epidemiologically linked to diarrhoea in children in a shanty town in Lima, Peru; occasional sporadic cases in various countries including developed countries, especially immunocompromised people and those with cat contact. | More common in kittens and younger cats and is then associated with diarrhoea. Often only low numbers of oocysts detectable. | [68] |
| C. fragile | 6.2 × 5.5 | Black spined toad | No association. | Infection appears to be host-specific because infections of four amphibian and one fish species failed. | |
| C. galli | 8.3 × 6.3 | Chicken | No association. | Infection of the proventriculus can lead to diarrhoea and fatalities; shedding can be prolonged and is not necessarily associated with diarrhoea. | |
|
C. hominis (previously referred to as C. parvum human genotype, genotype I, and genotype H) |
4.9 × 5.2 | Humans | Common cause of diarrhoeal disease in sporadic cases and outbreaks. Infectivity data from experimental infections in adults extrapolated to dose–response indicate that ingestion of a single oocyst carries a discrete probability of infection. Transmitted either directly person-to-person (especially in daycare centres, household contacts, toileting, or nappy changing), or indirectly via contaminated drinking water, recreational water, food, or fomites. High parasite genetic heterogeneity in non-industrialised countries; more homogenous in industrialised countries. Children in Brazil shed more oocysts, had more frequent presence of faecal lactoferrin, and greater growth shortfalls than those infected with C. parvum, even in the absence of symptoms. HIV-positive persons in Lima, Peru, experienced more severe symptoms than those infected with other species. High virulence was evident within gp60 subtype family Id but was absent in subtype families Ia and Iec. | There is no defined animal host for this species, although low density of the parasite has been detected occasionally in cattle, sheep and goat faeces. Experimental infection can be maintained in piglets and calves. Immunosuppressed Mongolian gerbils provide an asymptomatic small mammal model for infection. |
[13] |
| C. macropodum (previously marsupial genotype II) | 5.4 × 4.9 | Eastern grey kangaroo | No association. | Infection is limited to marsupials, which can excrete large numbers of oocysts without any clinical signs. | [63] |
| C. meleagridis | 5.2 × 4.6 | Homoeo-thermic birds; mammals | Infectivity data from experimental infections in adults indicate mild illness. Sporadic cases are reported more frequently in some populations, for example infections are as common as for C. parvum (10–20% of cryptopsoridiosis cases) in Bangkok, Thailand and Lima, Peru; travel to endemic countries; possible outbreak on a farm in Sweden. | Associated with enteritis, diarrhoea and death in birds. Severe outbreaks and mortalities reported in poultry units and game birds. Asymptomatic infections and growth reductions have also been reported. Readily transmissible between birds and mammals. | 33, 68, 69 |
| C. molnarid | 4.7 × 4.5 | Sea bream | No association. | Infection is seasonal, mostly in younger fish in the spring. | [70] |
| C. muris | 7.0 × 5.0 | Rodents | Rarely associated; individual reports from various developing countries. | Clinical signs are not usually reported; infection and oocyst shedding has been reported in other animal species. | |
|
C. parvum (also sometimes previously called bovine genotype, genotype II, and genotype B) |
5.0 × 4.5 | Humans, mammals | Common in sporadic cases and outbreaks; zoonotic transmission, either directly animal-to-person through recreational or occupational farm animal contact (especially young ruminants), or indirectly through contaminated drinking water, recreational water, environmental contact, food, or fomites. Person-to-person spread also occurs. Infectivity data from experimental infections in adults extrapolated to dose–response indicate that ingestion of a single oocyst carries a discrete probability of infection. The infectious dose with C. parvum in neonatal ruminants is similar. High heterogeneity; some subtypes (e.g., gp60 IIc) appear host-adapted to humansc. | Common cause of diarrhoea in pre-weaning calves, lambs, and goats; infection can be fatal. Also reported in foals, alpaca, llama. Occasional respiratory symptoms. Disease is age-dependent, where older animals are usually asymptomatic. Some subtypes (e.g., gp60 IIc) have no defined animal host. Asymptomatic infectivity models include neonatal, immunosuppressed and immunocompromised mice. |
13, 71 |
|
C. ryanae (previously deer-like genotype) |
3.7 × 3.2 | Cattle | No association. | Infection is host-specific and shedding is seen in weaned calves but is thought to be asymptomatic. | |
| C. scophthalmid | 4.4 × 3.9 | Turbot | No association. | Infection occurs in the intestine and is seen seasonally in younger fish; has been linked to reduced growth rates. | [72] |
| C. scrofarum (previously pig genotype II) | 5.2 × 4.8 | Pig | Rarely associated; individual report from Czech Republic involving contact with pigs. | Infection is seen in pigs aged more than 6 weeks resulting in low levels of oocyst shedding and no association with diarrhoea. | [73] |
| C. serpentis | 6.2 × 5.3 | Reptiles | No association. | Infection is common. In snakes, infection manifests as anorexia, persistent postprandial regurgitation, lethargy, mid-body swelling, and chronic weight-loss, which is usually protracted and almost always fatal. In lizards infection is usually asymptomatic. | |
|
C. suis (previously pig genotype I) |
4.6 × 4.2 | Pig | Rarely associated; individual reports from UK and Peru involving contact with pigs. | Infection is usually seen in pre-weaning pigs but is not associated with diarrhoea. | [73] |
| C. tyzzeri (previously mouse genotype I) | 4.6 × 4.2 | Mice | Rarely associated; individual report from Czech republic involving contact with wild mice. | Infection is usually most intense in the ileum but no clinical signs were recorded in experimental infections. | 74, 75 |
|
C. ubiquitum (previously cervine genotype) |
5.0 × 4.7 | Various mammals | Sporadic cases in various countries, especially developed countries, possibly involving untreated water supplies contaminated by animal hosts in the catchment. | Broad host-range and a common parasite of weaned lambs. No association of oocyst shedding and clinical symptoms in experimentally infected lambs. | |
| C. viatorum | 5.4 × 4.7 | Humans | Sporadic cases emerging in the UK and Sweden are linked to visits to the Indian subcontinent, South America, and Kenya. | No animal host is known for this species. | 76, 77 |
|
C. varanii (syn. C. saurophilum) |
4.8 × 4.7 | Reptiles | No association. | Infection is seen in lizards and snakes. Clinical signs include anorexia, progressive weight-loss, abdominal swelling, and death, particularly in young animals. | |
| C. wrairi | 5.4 × 4.6 | Guinea pig | No association. | Disease not described. | [78] |
|
C. xiaoi (previously Cryptosporidium bovis-like genotype or C. bovis from sheep) |
3.9 × 3.4 | Sheep | No association. | In sheep, asymptomatic carriage; no association of oocyst shedding and clinical symptoms in experimentally infected lambs. Infection in goat kids can be associated with diarrhoea. | [79] |
| Chipmunk genotype I | Not reported | Chipmunk; possibly other Sciuridae | Rarely associated; individual reports from the USA, France, Sweden. | No association of clinical signs with shedding oocysts. | |
| Horse genotype | 4.6 × 4.2 | Horses | Rarely associated; individual reports from the UK and USA | No association of oocyst shedding with diarrhoea. | [80] |
| C. hominis monkey genotype | Not reported | Monkey, human | Rarely associated; individual reports from UK and Malawi. | No association of oocyst shedding with diarrhoea recorded. | |
| Skunk genotype | Not reported | Skunk; possibly other mustelids | Rarely associated; an individual report from the UK where skunks are not found outside zoos. | No association of oocyst shedding with diarrhoea recorded. |
Only those genotypes found in humans have been included here.
From the original papers describing the species or genotype.
See [18] for an explanation of gp60 subtyping and nomenclature.
Currently, Cryptosporidium is the only genus in the Family Cryptosporidiidae, but piscine species show considerable genetic distance, ultrastructural and developmental differences in comparison with other Cryptosporidium species, and a new genus, Piscicryptosporidium, has been proposed, pending study of additional piscine isolates [81].
Cryptosporidium species: infection and disease in relation to diagnosis
At time of writing 26 Cryptosporidium species have been named (Table 1). There is good evidence for six as important causes of human cryptosporidiosis: most commonly Cryptosporidium hominis and C. parvum, followed by Cryptosporidium meleagridis and occasionally Cryptosporidium cuniculus, Cryptosporidium felis, and Cryptosporidium canis [18]. Infection is transmitted by oocysts and acquired mainly by the faecal–oral route, with very rare instances of respiratory infection following inhalation or aspiration [6]. Despite occasional reports in livestock, C. hominis appears to be transmitted anthroponotically. Immunocompromised patients have provided sentinel alert to some new infections [23]. In animals, the clinically and economically important gastrointestinal species are C. parvum (pre-weaning ruminants), C. meleagridis (birds), C. canis (puppies), C. felis (kittens), Cryptosporidium serpentis (snakes), and Cryptosporidium varanii (reptiles) [5]. Cryptosporidium baileyi is also associated with respiratory disease in birds [5]. The clinical significance of other species or genotypes in detected faeces is doubtful if disease is not described or tissue infection has not been demonstrated.
Human cryptosporidiosis (recently reviewed, [24]) is usually characterised by acute symptoms of watery diarrhoea accompanied by abdominal cramps, vomiting, low-grade fever, and loss of appetite. These indicate the pathogenesis with infection mainly of the small bowel (Figure 1), causing malabsorption and some elements of inflammation. The incubation period (normal range 3–12 days, usually 5–7 days) is dose-dependent. Cryptosporidiosis is self-limiting in immunocompetent people, and the symptoms vary in severity (mild to severe); duration can be for up to 1 month during which time relapse occurs in about one third of cases. Severity varies partially with the general health of the patient, and those experiencing mild, self-limiting diarrhoea will be unlikely to seek medical attention and obtain a diagnosis (Table 2 ).
Table 2.
Specimen types and applications of Cryptosporidium diagnostic tests
| Specimen type | Relevant patient or animal group | Sample preparation and standard test options | Appropriate number of specimens | Test availability; key findings |
|---|---|---|---|---|
| Direct detection of parasite | ||||
| Faeces (the most commonly examined specimen). | Patients/animals with diarrhoea. Local policy may not include examination specifically for Cryptosporidium in all samples, even if a request for ‘ova, cysts, and parasites’ is made. Common selection criteria for examination include: • Young age (for farmed animals, usually <1 month of age); • Recent travel to an endemic country; • Other risk factor; for example farm visits, local outbreak; immunocompromise. Clinicians should familiarise themselves with local laboratory practice, and specify Cryptosporidium on the request form to ensure that appropriate testing is carried out. |
Fresh, frozen or preserved faeces [e.g., in 10% formalin, merthiolate–iodine–formaldehyde; sodium acetate–acetic acid–formalin; other preservatives: e.g., polyvinyl alcohol (PVA) may optimise detection of other parasites, especially for the morphological examination of trophozoites, and preservation in different vials may be required]. Preservation pros: • Preserves other parasite morphology or stages. • Allows transport at high ambient temperature. • Allows prolonged storage. Preservation cons: • May impede PCR analysis, Strongyloides larvae concentration, and examination for trophozoites of other protozoans of interest. • Many immunoassays cannot be performed on PVA preserved samples. • Preserved faeces require concentration by a recognised method before microscopy. Some faeces may need additional processing, for example very liquid stools may be concentrated, high-fat faeces may require de-fatting, mucoid samples may need treatment with KOH or dithiothreitol, high-fibre faeces may need sieving to remove fibres. Test faeces or concentrate as shown in Figure 2. |
For farm animals, ideally, collect faecal samples from a representative number of affected animals in the herd/flock. Repeat sampling may be required if samples are negative and Cryptosporidium is still suspected because shedding can be intermittent: • Ideally, for humans, three stool specimens collected over no more than a 10 day period, usually collected every other day, before being deemed negative. • For animals, 2–3 consecutive negative faeces before confirmed to have cleared the infection. |
Available locally. Prevalence among patients with gastrointestinal symptoms is 1–4% in Europe and North America and 3–20% in Africa, Asia, Australia, South and Central America [82]. Incidence rates may be underestimated because not all infections are symptomatic and persons with symptoms do not always seek medical attention, laboratory diagnosis may not be sought, laboratories may not include Cryptosporidium testing, and case reports or notifications may not be complete. In the UK, where most laboratories test all diarrhoeic stools for Cryptosporidium, the ratio of disease rates in the community and presenting to general practice relative to the rate of reported diagnoses to national surveillance) has been estimated to be 8.2 (95% CI 2.1–31.7) [42]. One of the most frequent causes of calf diarrhoea [25]. |
| Bowel content collected postmortem. | Animals that died with signs of diarrhoea. | As above. | As discussed with clinicians. Sampling guided by gross pathology. |
Available locally. |
| Small bowel scrapings or aspirates. | Severely immunocompromised patients with persistent idiopathic gastrointestinal symptoms. Animals that died with clinical signs consistent with cryptosporidiosis. |
Prepare a smear on a well slide and stain with immunofluorescent stains for oocysts and/or extract DNA and perform PCR. | As discussed with clinicians. | May be available locally; more likely refer for specialist testing. |
| Small bowel biopsy or tissue sections collected postmortem. | As above. | Examine haematoxylin and eosin (H&E) histology sections for endogenous stages and pathology consistent with cryptosporidiosis and/or extract DNA and perform PCR. DNA quality from fixed tissue sections is reduced but can be used for PCR. | As discussed with clinicians. Sampling guided by gross pathology. |
As above. |
| Tracheal scrape collected postmortem. | Birds that died with clinical signs consistent with respiratory cryptosporidiosis. | As above. | As above. | As above. |
| Bile from endoscopic retrograde cholangio-pancreatography. | Severely immunocompromised patients with symptoms of cholangitis whose stool test is negative for Cryptosporidium. | Prepare a smear on a well slide and stain with immunofluorescent stains for oocysts and/or extract DNA and perform PCR. | As discussed with clinicians. | As above. |
| Liver biopsy, preferably in saline. | Severely immunocompromised patients with symptoms of liver disease. | Examine H&E stained sections for endogenous stages and pathology consistent with infection and/or extract DNA and perform PCR. DNA quality from fixed tissue sections is reduced but can be used for PCR. | As discussed with clinicians. Sampling specific lesions can be ultrasound or computerised tomography scan guided. |
As above. |
| Sputum or bronchoalveolar lavage. | Severely immunocompromised patients with unexplained respiratory symptoms. | May require pretreatment with dithiothreitol. Prepare a smear on a well slide and stain with immunofluorescent stains for oocysts and/or extract DNA and perform PCR. | As discussed with clinicians. | As above. |
| Antral washout. | Severely immunocompromised patients with unexplained sinusitis. | Prepare a smear on a well slide and stain with immunofluorescent stains for oocysts and/or extract DNA and perform PCR. | As above. | As above. |
| Indirect detection of infection | ||||
| Blood serum. | Human populations for seroconversion, prevalence or sero-epidemiological analysis. | Large and mini-format Western blot, enzyme immunoassay (EIA), or microsphere assay incorporating recombinant proteins (e.g., 15/17 kD and 27 kD) for detection of Cryptosporidium-specific IgG and IgM and sometimes IgA. | Depends on study design. | Specialist test for research purposes. Strong IgG responses elicited to the 15/17 kD antigen occur within 6 months of infection; response to 27 kD antigen is usually much weaker but is detectable for longer [83]. |
| Oral fluid. | Populations for seroconversion, prevalence, or sero-epidemiological analysis. | Dissociation-enhanced lanthanide fluorescence immunoassay (DELFIA) or microsphere assays incorporating recombinant proteins for detection of Cryptosporidium-specific IgG and IgA. | Depends on study design. | A specialist test is under development for population monitoring [50]. Anti-Cryptosporidium IgG seroconversion reported in infected children [84]; detection of anti-Cryptosporidium IgG in oral fluid has been associated with recent diarrhoea [85] |
| Faeces/stool. | Indication of active infection. | Copro-antibody EIA for detection of Cryptosporidium-specific IgA. | Depends on study design. | Used for research purposes. |
In pre-weaning ruminants, C. parvum is usually associated with self-limiting gastroenteritis, characterised by profuse watery diarrhoea and signs of depression, dehydration, anorexia, and weakness, and in extreme cases it can be fatal [5]. The parasite is widely endemic and is one of the most frequently diagnosed causes of diarrhoea in pre-weaning calves [25]. Multiple pathogens can be involved in neonatal diarrhoea (rotavirus, coronavirus, pathogenic strains of Escherichia coli and Salmonella spp.), and cryptosporidiosis is confirmed by finding significant numbers of oocysts in diarrhoeic faeces in their absence [25]. However, coinfection may modulate the clinical presentation, and has been widely speculated to lead to more severe cryptosporidiosis [5], although experimental data to support this are still lacking. Infection with C. felis is often asymptomatic in cats but those also infected with the feline leukaemia virus are more likely to exhibit clinical signs [5]. A very different clinical spectrum may also be seen for example in birds where C. baileyi and C. meleagridis can also cause respiratory disease, requiring investigation of different sample types (Table 2).
The gut epithelium generally recovers after resolution of symptoms, but there are some indications that long-term sequelae may arise. For example, C. hominis, but not C. parvum, was associated with joint pain, eye pain, headaches, and fatigue during the 2 months following human infection [26]. Although a study of intestinal mucosal biopsies and serology from patients with inflammatory bowel disease (IBD) did not support a major role for Cryptosporidium in its pathogenesis [27], infection can trigger IBD flare, and inclusion of a test for Cryptosporidium is indicated in IBD patients seeking medical intervention for sudden exacerbation of digestive symptoms [28]. Experimental infection of rats with C. parvum triggers long-term pathological changes in the small intestine similar to those found in patients with irritable bowel syndrome (IBS) [29], supporting anecdotal reports of association. If a postinfectious (PI) association is found with Cryptosporidium for IBS (PI-IBS), reassurance could be provided to diagnosed patients that their altered bowel habit is consistent with PI-IBS and is likely to resolve without need for medical intervention. Potential impacts include reduction in use of health services (e.g., consultations and investigations).
In malnourished children, who are effectively immunocompromised, infection has been reported to cause substantial morbidity even for those with no acute symptoms [6]. Patients with severe immunodeficiency may suffer from chronic, severe and intractable cryptosporidiosis with significant mortality (reviewed in [30]). At most risk are those with primary or secondary T cell immunodeficiencies, notably untreated advanced HIV infection, lymphoma, and leukaemia (especially children). In this patient group, complications include pancreato-biliary infection, which may lead to sclerosing cholangitis and liver cirrhosis, and respiratory cryptosporidiosis (Table 2). Rapid detection of carriage in high-risk groups could help to limit clinical sequelae [31]. The findings of a recent study indicate that even low doses of C. parvum infect dexamethasone-treated, severe combined immunodeficiency (SCID) mice, leading to digestive adenocarcinoma, implying that severely immunocompromised humans may also be susceptible [32], which highlights the need for vigilance in diagnosis.
In otherwise healthy adults, no significant difference in acute symptoms caused by C. parvum, C. hominis, and C. meleagridis has been found [33]. However, species- and subtype-related differences in clinical manifestations have been reported in children and immunocompromised patients, indicating more severe disease with C. hominis, and some subtypes in particular, than with C. parvum (Table 1) [18]. A combination of parasite- and host-related factors influence the outcome of infection. Pathogenicity and virulence have been reviewed recently [34], and the reader is referred to reviews of host genetic susceptibility [35], host cell [36] and immune responses [37].
Asymptomatic carriage of Cryptosporidium occurs, and in humans has been mainly investigated in young children, including those in industrialized countries where carriage rates of up to 3.8% have been reported [38]. A study of children in daycare in the UK used highly-sensitive immunomagnetic separation (IMS) and direct immunofluorescence microscopy (IFM), and reported concentrations of 13367 C. hominis oocysts per gram (opg), 3471 C. ubiquitum opg, and 56 skunk genotype opg [38]. Only the C. hominis-positive sample contained sufficient oocysts for detection by some current methods (Figure 2), and low parasite density challenges most diagnostic assays (Box 1 ). This is also an issue for samples obtained late in an infection when oocyst numbers may be in decline or where partial immunity may result in few oocysts being shed [31].
Box 1. Attributes of current Cryptosporidium faecal diagnostic assays.
-
•
Selection criteria include resources (staff time, expertise, grade, finances, specialist equipment), desired turnaround time, the population being tested, desired sensitivity and specificity, and the need to identify other pathogens.
-
•
Preservatives can inhibit PCR and must be removed by washing; this may not be possible if the preservative has penetrated the oocysts.
-
•
Faeces can be concentrated by sedimentation using modified formol–ethyl acetate or solvent-free techniques, but significantly fewer parasites and larger deposits have been reported in a solvent-free system, and low-density parasites might be missed [86]. Conventional flotation (e.g., zinc sulphate, saturated sodium chloride or sucrose) results in cleaner preparations but low-density oocysts may be missed.
-
•
Using fluorescent stains (e.g., Auramine O) oocyst observation is easier and less subjective, and slides can be scanned at lower magnification than acid-fast stained slides, but a fluorescence microscope is needed.
-
•
IFM is less subjective than other microscopy stains and increases sensitivity and specificity, although some notable cross-reactions (e.g., algae) may occur in food, water, and environmental testing, necessitating observation of confirmatory features: internal contents and sporozoites by differential interference contrast and sporozoite nuclei by staining with a nuclear fluorogen.
-
•
EIAs can be automated and are more sensitive than acid-fast microscopy. Immunochromatographic lateral flow (ICLF) is less sensitive, but requires little skill and can be performed anywhere as a rapid test, for example pen-side. ICLF and EIA kits may include other pathogenic protozoa in a single test (e.g., Giardia [87]), and additionally for humans, Entamoeba histolytica [88].
-
•
Cryptosporidium species antigen variability may compromise immunodiagnostic tests based on antibodies raised against Cryptosporidium parvum; there is some evidence that Cryptosporidium felis may be less well detected [89].
-
•
Conventional and real-time PCR assays have been described, but real-time PCR has been reported as the most sensitive and specific test for Cryptosporidium in diarrhoeic calf samples [90].
-
•
Molecular assays are underpinned by efficient DNA extraction but oocysts may be present at low density in complex matrices (faeces, slurry, food, water) that may contain PCR inhibitors. Oocysts in faeces or semi-purified suspensions need first to be disrupted by procedures such as bead beating, freeze–thaw, cycles or enzymatic digestion [91]. Automated DNA extraction procedures can be used [48].
-
•
PCR conditions must include mitigation of potentially inhibitory substances (e.g., heme, bilirubin, bile salts, or complex carbohydrates). PCR primers and conditions need to be selected carefully to amplify all Cryptosporidium species of interest and to avoid non-specific amplification.
There is no gold-standard diagnostic test. For comparison of sensitivity and specificity, either one must be nominated or comparison tests such as McNemar's or a Bayesian model must be applied [90]. The outcome depends not only on the test properties but also the nature of the samples (e.g., consistency, parasite density), the statistical power of the study, and skill in performing assays. Nevertheless, when performed under diagnostic conditions, the general consensus is as shown below (Figure I ).
Supportive therapy to prevent dehydration and restore electrolyte balance may be needed, but specific drug therapy is not usually required for the immunocompetent. Young children and immunocompromised patients may not resolve symptoms, and nitazoxanide is licensed by the US Food and Drug Administration (FDA) for treatment of cryptosporidiosis in immunocompetent individuals over 1 year of age; the latest Cochrane review confirmed the absence of evidence for effective agents for immunocompromised patients [39], which remains a key challenge. Immune reconstitution is most effective but not always possible. Halofuginone is licensed for the treatment and control of cryptosporidiosis in calves. However, this drug is toxic at twice the recommended dose and is contraindicated for use in diarrhoeic animals. A recent meta-analysis found that although it provided beneficial prophylactic use, there were insufficient data to establish a therapeutic effect [40]. However, a study in Ireland showed that halofuginone was effective in reducing clinical signs and environmental contamination with oocysts [41]. Screening for parasite clearance following treatment may require the more sensitive assays (Box 1, Figure I).
Figure I.
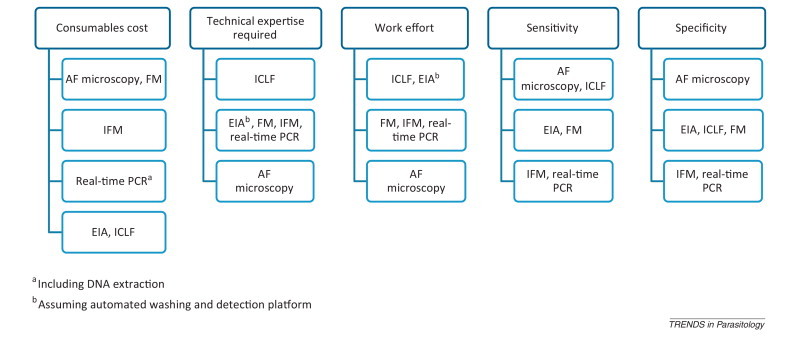
Relative attributes of features of diagnostic assays, ranked low to high. Abbreviations: AF, acid fast; EIA, enzyme immunoassay; FM, fluorescence microscopy; ICLF, immunochromatographic lateral flow; IFM, immunofluorescence microscopy.
Recent advances incorporated in clinical diagnosis
To diagnose enteric cryptosporidiosis a faecal sample must be tested, requiring a series of events to occur in the clinical consultation, specimen requesting, sampling, submission, testing and reporting process [42]. Cryptosporidium should be suspected in all patients who present with acute gastroenteritis, most especially young children or if the symptoms are prolonged (>3 days) [43], and in all neonatal ruminant diarrhoea; samples are also likely to include postmortem examinations of animals that died. Detection is very difficult in routine parasitological examinations (Figure 2) and, as with most pathogens, specific testing is often selective according to local policy [44].
The diagnostic trend during the last decade has been towards greater use of enzyme immunoassays (EIA) for detecting oocyst antigens [44], but there are no immunological tools that distinguish between oocysts of different species. One approach to overcoming this has been the commercial development of a PCR-EIA that identifies Cryptosporidium spp., C. hominis and C. parvum [45]. However, molecular diagnostic assays are being implemented in diagnostic laboratories, especially in virology and some aspects of bacteriology, and are emerging for enteric protozoa. Real-time PCR probe-based assays for the detection of multiple protozoa have been described, such as for Cryptosporidium spp., Giardia lamblia, Entamoeba histolytica, and Dientamoeba fragilis, with a testing algorithm for microscopy based on clinical information, which could be adapted to specific settings depending on the likelihood of involvement of other parasites [46]. The replacement of probes with carboxylated Luminex microspheres provides an alternative detection system, as demonstrated for several parasites [47]. An assay based on multiplex-tandem PCR (MT-PCR) describes the simultaneous detection of bacterial pathogens in Cryptosporidium-positive stools, and the authors suggest that the increased amplification efficiency would also be useful for water samples [48]. Commercial multiplex PCR-based assays for comprehensive panels of gastrointestinal pathogens are becoming available, utilising robotic platforms for DNA extraction and assay set-up. Although offering streamlined and high testing capacity, investigation of a wide range of pathogens in all samples increases the number of positive findings; this may not always be warranted clinically but may shed light on the importance of infection in patients previously not investigated, and of coinfections. Pathogen panels should ideally be tailored for the population under investigation. Downstream testing of extracted DNA for further species/genotype discrimination of Cryptosporidium-positive samples either in the same, or more likely a reference, laboratory could be readily implemented for epidemiological and outbreak investigations (Figure 2).
Thus, a variety of methods are used for Cryptosporidium diagnosis, as Fournet and colleagues discovered in their 2012 investigation: fluorescence microscopy (FM) using Auramine O (UK) or acid fast (AF) modified Ziehl–Neelsen staining (NL, UK, and Germany), EIA (NL, UK, and Germany), and multiplex PCR (NL) [15]. Species determination was performed routinely on a higher proportion of cases in the UK than the NL and not at all in Germany. Subtyping a proportion of the predominant C. hominis isolates at the gp60 locus [18] revealed mainly subtype IbA10G2, which predominates in these countries, and multilocus genotyping is underway. The investigation highlights the need for wider application of molecular epidemiology across the European Union.
Detection in food, water and environmental samples
Testing non-clinical samples for Cryptosporidium supports risk assessment and surveillance, and with the application of typing assists in the investigation of possible sources of contamination and routes of transmission, and provides microbiological evidence in outbreak investigations. In the USA, classification of waterborne outbreaks according to the strength of epidemiological, clinical, and environmental data now reflects the increasing use of molecular characterization of pathogens both in clinical and environmental samples [49]. However, outbreak investigations focus on specific incidents, and the contamination event will usually have passed some time before recognition of an outbreak. For understanding Cryptosporidium spp. transmission, baseline data need to be generated by well-structured sample surveys. Interventions such as those implemented to reduce waterborne disease need to be monitored for effectiveness, and one strategy is to compare population exposure. Various serological methods have been investigated for Cryptosporidium antibodies (Table 2), which are moving in focus towards multiplex platforms to facilitate monitoring for a range of pathogens [50].
Standard methods for the detection and enumeration of Cryptosporidium in water are based on principles of large-volume sampling (10–1000 L), concentration by filtration and elution from the filter (or by flocculation for volumes <20 L), separation of oocysts from other particulates by IMS, and detection by IFM incorporating a nucleic acid stain as an identification aid ([51] for example). Alternative methods such as continuous-flow centrifugation have been shown to provide at least equivalent recovery efficiencies [51]. A similar sequence of oocyst recovery and detection from surface eluates of fresh food produce 2, 17 forms the basis of a method currently being considered as an International Organization for Standardization (ISO) standard. However, the process is time-consuming and expensive. The production of automated, miniaturised systems has been sought as an alternative to laboratory testing of water samples, based on a variety of detection technologies including optical, mass, electrical, surface plasmon resonance, and nucleic acid detection. However, few have been performance-tested in the field, and a key step is sample preparation, still requiring IMS to deliver oocysts to the system [52].
Molecular detection of Cryptosporidium has not yet been adopted for regulatory purposes partly because only oocysts containing sporozoite DNA are detected, and no reliably quantitative method has been validated to replace oocyst counts. The small number of oocysts present in most water samples is challenging. One risk-assessment approach is to screen samples for a range of protozoa using universal primers, with fluorescent melt-curve analysis differentiating between genera [53]. Although a potentially valuable screening tool, the detection of less-abundant targets in field samples requires validation.
Standard methods provide no information about the species, virulence, or viability of oocysts; Cryptosporidium spp. need to be identified in water monitoring otherwise all oocysts detected must be assumed to present a public health risk. Sequencing DNA from nested PCR of the SSU rRNA gene, using a repetitive and limiting PCR template approach to improve analytical sensitivity and differentiation of multiple species or genotypes in a single sample, provides the benchmark [54]. However, this is technically demanding, and is outwith the scope of most water-testing laboratories. A simplified approach is being validated using single-round, conventional, or real-time PCR of the SSU rRNA and hsp70 genes to differentiate the main human pathogens C. hominis, C. parvum, and C. meleagridis from other species commonly found in the environment (http://waterrf.org/Pages/Projects.aspx?PID=4099). A non-PCR-based method is loop-mediated isothermal amplification (LAMP), based on autocycling strand-displacement DNA synthesis by Bst polymerase, and this may overcome the inhibitory potential of some samples and has been reported to detect organisms at relatively low concentrations in water samples [55], although it has not yet been subjected to independent evaluation.
Impact of genomics on detection, diagnosis, and typing
Molecular biology had a vast impact on the understanding of the species concept within the genus Cryptosporidium, resulting in a dramatic increase in species numbers and genotypes [21]. As a result, there is a need to understand the level of within-species genetic variation such that selected DNA sequences can be indentified as references. This is particularly important because a single locus (the SSU rRNA gene) is often used as species marker; confidence in species determination and subtyping is greatly increased by using multiple markers that are spread throughout the genome [56]. Phylogenetic analysis has proven to be an essential tool for determining species status; this approach provides more information than is achieved through individual sequence comparisons (such as BLAST searches) because more weight is given to informative mutations that may be species- or group-specific 20, 54. Use of reference sequences will help to reduce the confusion caused by sequences deposited in databases that may have been falsely attributed to a species due to limited understanding of species complexity at the time of submission. This is a considerable problem for a genus in which species numbers are still increasing.
Comparative analyses of the published C. parvum and C. hominis genomes (http://cryptodb.org) have revealed new possible diagnostic targets, for example a new family of telomerically-encoded Cryptosporidium proteins, the C. parvum-specific Cops-1 and the C. hominis-specific Chos-1 [34]. The relatively small Cryptosporidium genome (9.1–9.2 Mb) combined with ever-reducing sequencing costs will allow more Cryptosporidium genomes to be sequenced, and from different species and from multiple isolates of the same species [57]. Publication of new genomes will allow detailed comparisons, resulting in identification of more species-specific markers, polymorphic mini- and micro-satellite markers, and single-nucleotide polymorphisms (SNPs) [58]. These markers can be exploited to develop improved PCR-based diagnostics utilising the more informative parts of the Cryptosporidium genome [16]. The SNPs may also be used in attempts to identify virulence genes or factors involved in host-specificity [58]. The increasing amount of genomic data will undoubtedly lead to further development of microarrays for detecting variation within parasite populations. However, this is a longer-term development and currently there is much focus on the use of mini- and micro-satellite markers. Generally, these are very variable because they are commonly in noncoding regions of the genome and are subject to fewer functional restraints that impede the accumulation of mutations. However, many Cryptosporidium satellite markers were identified before genome sequences became available, and are mostly within coding regions [59]. These satellite markers have been used successfully for studying Cryptosporidium population dynamics to evaluate transmission routes and zoonotic potential 59, 60, 61, 62. There is a need not only for the further development of subtyping methods for epidemiological purposes, but also for their standardisation. A recent review revealed that 55 markers have been used in various combinations on different platforms to investigate variation within C. parvum and C. hominis [59]. Although multi-locus typing is a potentially powerful tool, global meta-analysis and comparisons are impossible if different methods are used, and there is a need for standardisation. Assessment of existing markers and suggestions for taking these forward in independent validation studies for harmonisation of typing schemes have been published recently by Robinson and Chalmers [59]. Typing tools developed based on Cryptosporidium genome analyses, if harmonised, will also have the potential to help with outbreak investigations, source attribution, investigations into the fate and transport of oocysts, persistence within hosts, studies into the role of strain-specific immunity, and the identification of virulence factors and genes involved in host specificity.
Concluding remarks
Cryptosporidium presents many challenges for detection and diagnosis, and the use of different diagnostic methods and the inconsistent application of typing techniques can make direct comparisons difficult or even impossible between clinical, veterinary, and environmental testing or between different regions or countries. Adoption of standardized techniques, where possible, for diagnosis and typing would allow better correlations between animal, human, and environmental data at national and international levels. This would aid source-tracking of outbreaks and enable better understanding of transmission of the parasite.
The absence of a gold-standard diagnostic test for comparison of sensitivity and specificity can be overcome either by nominating one or using appropriate statistical tests for comparison (Box 1). Small numbers of parasites may be clinically significant, and one of the challenges faced by scientists and clinicians working with Cryptosporidium is to increase the sensitivity of existing methods; this applies particularly to molecular detection and typing from water samples where the ultimate goal is to reliably speciate and multi-locus-type samples containing single oocysts.
Expected advances for clinical diagnosis of Cryptosporidium infections may come from the direct application of next-generation sequencing to faecal samples because this platform will allow the detection of multiple pathogens and their variants based directly on the presence of their DNA. More imminent is the implementation of molecular diagnostics. However, adopting these new technologies in a clinical laboratory involves significant changes to the current workflows, as depicted in Figure 2.
From the research community, the availability of genome sequences for different Cryptosporidium species and multiple genome sequences for the most clinically and economically important species is imminent. These genome datasets will facilitate the development of better diagnostic and typing tools at inter- and intra-species level, and efforts must be made to ensure that technological advances are also implemented in low-income countries where the burden of cryptosporidiosis is highest.
References
- 1.Baldursson S., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Robertson L.J., Chalmers R.M. Foodborne cryptosporidiosis: is there really more in Nordic countries? Trends Parasitol. 2013;29:3–9. doi: 10.1016/j.pt.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Artieda J. Outbreak of cryptosporidiosis in a child day-care centre in Gipuzkoa, Spain, October to December 2011. Euro Surveill. 2012;17:pii=20070. doi: 10.2807/ese.17.05.20070-en. [DOI] [PubMed] [Google Scholar]
- 4.Gormley F.J. Zoonotic cryptosporidiosis from petting farms, England and Wales, 1992-2009. Emerg. Infect. Dis. 2011;17:151–152. doi: 10.3201/eid1701.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santín M. Clinical and subclinical infections with Cryptosporidium in animals. N. Z. Vet. J. 2013;61:1–10. doi: 10.1080/00480169.2012.731681. [DOI] [PubMed] [Google Scholar]
- 6.Shirley D.A. Burden of disease from cryptosporidiosis Curr. Opin. Infect. Dis. 2012;25:555–563. doi: 10.1097/QCO.0b013e328357e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Featherstone C.A. Cryptosporidium species in calves submitted for postmortem examination in England and Wales. Vet. Rec. 2010;167:979–980. doi: 10.1136/vr.c3948. [DOI] [PubMed] [Google Scholar]
- 8.Savioli L. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006;22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Sreiner T.S., Mandell G.L. Principles and syndromes of enteric infection. In: Mandell G.L., editor. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th edn. Churchill Livingstone; 2009. pp. 1335–1351. [Google Scholar]
- 10.Ferreira J. Cryptosporidiosis masquerading as a Crohn's flare. Inflamm. Bowel Dis. 2011;17:E133–E134. doi: 10.1002/ibd.21811. [DOI] [PubMed] [Google Scholar]
- 11.Garcia L.S. ASM Press; 2007. Diagnostic Medical Parasitology. [Google Scholar]
- 12.Chalmers R.M. Waterborne cryptosporidiosis outbreaks. Ann. Ist. Super. Sanità. 2012;48:429–446. doi: 10.4415/ANN_12_04_10. [DOI] [PubMed] [Google Scholar]
- 13.Medema G. World Health Organization; 2009. Risk Assessment of Cryptosporidium in Drinking-Water. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . 4th edn. WHO; 2011. Guidelines for Drinking-Water Quality. [Google Scholar]
- 15.Fournet N. Simultaneous increase of Cryptosporidium infections in the Netherlands, the United Kingdom and Germany in late summer season, 2012. Euro Surveill. 2013;18:pii=20348. [PubMed] [Google Scholar]
- 16.Jex A. Cryptosporidium – biotechnological advances in the detection, diagnosis and analysis of genetic variation. Adv. Parasitol. 2008;77:141–173. doi: 10.1016/j.biotechadv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Smith H., Nichols R.A. Cryptosporidium: detection in water and food. Exp. Parasitol. 2010;124:61–79. doi: 10.1016/j.exppara.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Barta J.R., Thompson R.C.A. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 2006;22:463–468. doi: 10.1016/j.pt.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Jirku New species of Cryptosporidium Tyzzer, 1907 (Apicomplexa) from amphibian host: morphology, biology and pylogeny. Folia Parasitol. 2008;55:81–94. [PubMed] [Google Scholar]
- 21.Fayer R. Taxonomy and species delimitation in Cryptosporidium. Exp. Parasitol. 2010;124:90–97. doi: 10.1016/j.exppara.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Xiao L. Cryptosporidium tyzzeri and Cryptosporidium pestis: which name is valid? Exp. Parasitol. 2012;130:308–309. doi: 10.1016/j.exppara.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Xiao L., Feng Y. Zoonotic cryptosporidiosis. FEMS Immunol. Med. Microbiol. 2008;52:1–15. doi: 10.1111/j.1574-695X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers R.M., Davies A.P. Minireview: clinical cryptosporidiosis. Exp. Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz I. Calf health from birth to weaning. II. Management of diarrhoea in pre-weaned calves. Ir. Vet. J. 2011;64:9. doi: 10.1186/2046-0481-64-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter P.R. Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin. Infect. Dis. 2004;39:504–510. doi: 10.1086/422649. [DOI] [PubMed] [Google Scholar]
- 27.Chen W. Cryptosporidium parvum in intestinal mucosal biopsies from patients with inflammatory bowel disease. Am. J. Gastroenterol. 2001;96:3463–3464. doi: 10.1111/j.1572-0241.2001.05359.x. [DOI] [PubMed] [Google Scholar]
- 28.Colussi O. Acute cryptosporidiosis as a cause of sudden recurrence of digestive symptoms in patients with Crohn's disease. J. Crohns Colitis. 2010;4:669–670. doi: 10.1016/j.crohns.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Khaldi S. Cryptosporidium parvum isolate-dependent postinfectious jejunal hypersensitivity and mast cell accumulation in an immunocompetent rat model. Infect. Immun. 2009;77:5163–5169. doi: 10.1128/IAI.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter P.R., Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002;15:145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLauchlin J. Polymerase chain reaction-based diagnosis of infection with Cryptosporidium in children with primary immunodeficiencies. Pediatr. Infect. Dis. J. 2003;22:329–334. doi: 10.1097/01.inf.0000059402.81025.cd. [DOI] [PubMed] [Google Scholar]
- 32.Benamrouz S. Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS ONE. 2012;7:e51232. doi: 10.1371/journal.pone.0051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chappell C.L. Cryptosporidium meleagridis: infectivity in healthy adult volunteers. Am. J. Trop. Med. Hyg. 2011;85:238–242. doi: 10.4269/ajtmh.2011.10-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouzid M. Cryptosporidium pathogenicity and molecular virulence factors. Clin. Microbiol. Rev. 2013;26:115–134. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores J., Okhuysen P.C. Host factors – genetics of susceptibility to infection with enteric pathogens. Curr. Opin. Infect. Dis. 2009;22:471–476. doi: 10.1097/QCO.0b013e3283304eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y-L. Cell sorting-assisted microarray profiling of host cell response to Cryptosporidium parvum infection. Infect. Immun. 2010;78:1040–1048. doi: 10.1128/IAI.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borad A., Ward H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010;5:507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies A.P. Asymptomatic carriage of protozoan parasites in children in day care centers in the United Kingdom. Paediatr. Infect. Dis. J. 2009;28:838–840. doi: 10.1097/INF.0b013e31819d646d. [DOI] [PubMed] [Google Scholar]
- 39.Abubakar I. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2007;63:387–393. doi: 10.1111/j.1365-2125.2007.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverlås C. Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis. Prev. Vet. Med. 2009;91:73–84. doi: 10.1016/j.prevetmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 41.De Waele V. Control of cryptosporidiosis in neonatal calves: use of halofuginone lactate in two different calf rearing systems. Prev. Vet. Med. 2010;96:143–151. doi: 10.1016/j.prevetmed.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam C.C. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerrant R.L. Infectious Diseases Society of America. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 2001;32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 44.Chalmers R.M. Clinical laboratory practices for the detection and reporting of Cryptosporidium in community cases of diarrhoea in the United Kingdom, 2008. Euro Surveill. 2010;15:pii=519731. doi: 10.2807/ese.15.48.19731-en. [DOI] [PubMed] [Google Scholar]
- 45.Savin Assessment of cryptodiag for diagnosis of cryptosporidiosis and genotyping Cryptosporidium species. J. Clin. Microbiol. 2008;46:2590–2594. doi: 10.1128/JCM.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruijnesteijn van Coppenraet L.E. Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy. Clin. Microbiol. Infect. 2009;15:869–874. doi: 10.1111/j.1469-0691.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 47.Taniuchi High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am. J. Trop. Med. Hyg. 2011;2:332–337. doi: 10.4269/ajtmh.2011.10-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jex A.R. Detection of diarrhoeal pathogens in human faeces using an automated, roboticplatform. Mol. Cell. Probes. 2012;26:11–15. doi: 10.1016/j.mcp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control Surveillance for waterborne disease outbreaks and other health events associated with recreational water – United States, 2007–2008 and surveillance for waterborne disease outbreaks associated with drinking water – United States, 2007–2008. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1–75. [PubMed] [Google Scholar]
- 50.Lammie P.J. Development of a new platform for neglected tropical disease surveillance. Int. J. Parasitol. 2012;42:797–800. doi: 10.1016/j.ijpara.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Environmental Protection Agency . US EPA; 2005. Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. [Google Scholar]
- 52.Bridle H. Detection of Cryptosporidium in miniaturised fluidic devices. Water Res. 2012;46:1641–1661. doi: 10.1016/j.watres.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Lalonde L.F., Gajadhar A.A. Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. J. Parasitol. 2012;97:725–730. doi: 10.1645/GE-2706.1. [DOI] [PubMed] [Google Scholar]
- 54.Ruecker N.J. Molecular and phylogenetic approaches for assessing sources of Cryptosporidium contamination in water. Water Res. 2012;46:5135–5150. doi: 10.1016/j.watres.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 55.Bakheit M. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet. Parasitol. 2008;25:11–22. doi: 10.1016/j.vetpar.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Widmer G., Lee Y. Comparison of single- and multilocus genetic diversity in the protozoan parasites Cryptosporidium parvum and C. hominis. Appl. Environ. Microbiol. 2010;76:6639–6644. doi: 10.1128/AEM.01268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widmer G., Sullivan S. Genomics and population biology of Cryptosporidium species. Parasite Immunol. 2012;34:61–71. doi: 10.1111/j.1365-3024.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Widmer G. Comparative genome analysis of two Cryptosporidium parvum isolates with different host range. Infect. Genet. Evol. 2012;12:1213–1221. doi: 10.1016/j.meegid.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson G., Chalmers R.M. Assessment of polymorphic genetic markers for multi-locus typing of Cryptosporidium parvum and Cryptosporidium hominis. Exp. Parasitol. 2012;132:200–215. doi: 10.1016/j.exppara.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Morrison L.J. The population structure of the Cryptosporidium parvum population in Scotland: a complex picture. Infect. Genet. Evol. 2008;8:121–129. doi: 10.1016/j.meegid.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanriverdi S. Inferences about the global population structures of Cryptosporidium parvum and Cryptosporidium hominis. Appl. Environ. Microbiol. 2008;74:7227–7234. doi: 10.1128/AEM.01576-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drumo R. Evidence of host-associated populations of Cryptosporidium parvum in Italy. Appl. Environ. Microbiol. 2012;78:3523–3529. doi: 10.1128/AEM.07686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan U., Power M. Cryptosporidium species in Australian wildlife and domestic animals. Parasitology. 2012;20:1–16. doi: 10.1017/S0031182012001151. [DOI] [PubMed] [Google Scholar]
- 64.Khan S.M. Molecular characterization and assessment of zoonotic transmission of Cryptosporidium from dairy cattle in West Bengal, India. Vet. Parasitol. 2010;171:41–47. doi: 10.1016/j.vetpar.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 65.The ANOFEL Cryptosporidium National Network Laboratory-based surveillance for Cryptosporidium in France, 2006–2009. Euro Surveill. 2010;15:pii=19642. [PubMed] [Google Scholar]
- 66.Molloy S.F. Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am. J. Trop. Med. Hyg. 2010;82:608–613. doi: 10.4269/ajtmh.2010.09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson G. Re-description of Cryptosporidium cuniculus Inman and Takeuchi, 1979 (Apicomplexa: Cryptosporidiidae): morphology, biology and phylogeny. Int. J. Parasitol. 2010;40:1539–1548. doi: 10.1016/j.ijpara.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Elwin K. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000 to 2008. Epidemiol. Infect. 2011;140:673–683. doi: 10.1017/S0950268811000860. [DOI] [PubMed] [Google Scholar]
- 69.Silverlås C. Zoonotic transmission of Cryptosporidium meleagridis on an organic Swedish farm. Int. J. Parasitol. 2012;42:963–967. doi: 10.1016/j.ijpara.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Sitjà-Bobadilla A. Epidemiology of Cryptosporidium molnari in Spanish gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.) cultures: from hatchery to market size. Appl. Environ. Microbiol. 2005;71:131–139. doi: 10.1128/AEM.71.1.131-139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blewett D.A. Infective dose size studies on Cryptosporidium parvum using gnotobiotic lambs. Water Sci. Technol. 1993;27:61. [Google Scholar]
- 72.Alvarez-Pellitero P. Host and environmental risk factors associated with Cryptosporidium scophthalmi (Apicomplexa) infection in cultured turbot, Psetta maxima (L.) (Pisces, Teleostei) Vet. Parasitol. 2009;165:207–215. doi: 10.1016/j.vetpar.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Němejc K. Occurrence of Cryptosporidium suis and Cryptosporidium scrofarum on commercial swine farms in the Czech Republic and its associations with age and husbandry practices. Parasitol. Res. 2013;112:1143–1154. doi: 10.1007/s00436-012-3244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren X. Cryptosporidium tyzzeri n. sp. (Apicomplexa: Cryptosporidiidae) in domestic mice (Mus musculus) Exp. Parasitol. 2012;130:274–281. doi: 10.1016/j.exppara.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Rasková V. Case of human cryptosporidiosis caused by Cryptosporidium tyzzeri and C. parvum presumably transmitted from wild mice. J. Clin. Microbiol. 2013;51:360–362. doi: 10.1128/JCM.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elwin K. Cryptosporidium viatorum n. sp. (Apicomplexa: Cryptosporidiidae) among travellers returning to the United Kingdom from the Indian Subcontinent. Int. J. Parasitol. 2012;42:675–682. doi: 10.1016/j.ijpara.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Insulander M. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol. Infect. 2012;9:1–12. doi: 10.1017/S0950268812001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv C. Cryptosporidium spp. in wild, laboratory, and pet rodents in China: prevalence and molecular characterization. Appl. Environ. Microbiol. 2009;75:7692–7699. doi: 10.1128/AEM.01386-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rieux A. Molecular characterization of Cryptosporidium spp. in pre-weaned kids in a dairy goat farm in western France. Vet. Parasitol. 2013;192:268–272. doi: 10.1016/j.vetpar.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Burton A.J. The prevalence of Cryptosporidium, and identification of the Cryptosporidium horse genotype in foals in New York State. Vet. Parasitol. 2010;174:139–144. doi: 10.1016/j.vetpar.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 81.Palenzuela O. Molecular characterization of Cryptosporidium molnari reveals a distinct piscine clade. Appl. Environ. Microbiol. 2010;76:7646–7649. doi: 10.1128/AEM.01335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Current W.L., Garcia L.S. Cryptosporidiosis. Clin. Microbiol. Rev. 1991;4:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Priest J.W. Multiplex assay detection of immunoglobulin G antibodies that recognize Giardia intestinalis and Cryptosporidium parvum antigens. Clin. Vaccine Immunol. 2010;17:1695–1707. doi: 10.1128/CVI.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griffin S. Development of a multiplex microsphere immunoassay for the quantitation of salivary antibody responses to selected waterborne pathogens. J. Immunol. Methods. 2011;364:83–93. doi: 10.1016/j.jim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 85.Egorov A. Recent diarrhea is associated with elevated salivary IgG responses to Cryptosporidium in residents of an Eastern Massachusetts community. Infection. 2010;38:117–123. doi: 10.1007/s15010-009-9323-4. [DOI] [PubMed] [Google Scholar]
- 86.Saez A.C. Comparison between the Midi Parasep and Midi Parasep Solvent Free (SF) faecal parasite concentrators. J. Clin. Pathol. 2011;64:901–904. doi: 10.1136/jcp.2011.090639. [DOI] [PubMed] [Google Scholar]
- 87.Budu-Amoako E. Occurrence of Cryptosporidium and Giardia on beef farms and water sources within the vicinity of the farms on Prince Edward Island, Canada. Vet. Parasitol. 2012;28:1–9. doi: 10.1016/j.vetpar.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 88.Goñi P. Evaluation of an immunochromatographic dip strip test for simultaneous detection of Cryptosporidium spp, Giardia duodenalis, and Entamoeba histolytica antigens in human faecal samples. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:2077–2082. doi: 10.1007/s10096-012-1544-7. [DOI] [PubMed] [Google Scholar]
- 89.Agnamey P. Evaluation of four commercial rapid immunochromatographic assays for detection of Cryptosporidium antigens in stool samples: a blind multicenter trial. J. Clin. Microbiol. 2011;49:1605–1607. doi: 10.1128/JCM.02074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Waele V. Age-stratified Bayesian analysis to estimate sensitivity and specificity of four diagnostic tests for detection of Cryptosporidium oocysts in neonatal calves. J. Clin. Microbiol. 2011;49:76–84. doi: 10.1128/JCM.01424-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elwin K. A comparison of two approaches to extracting Cryptosporidium DNA from human stools as measured by a real-time PCR assay. J. Microbiol. Methods. 2012;89:38–40. doi: 10.1016/j.mimet.2012.02.006. [DOI] [PubMed] [Google Scholar]


