Highlights
-
•
We developed new primers to improve genotyping of HAdV D.
-
•
Six out of seven HAdV species from at least 13 HAdV types were identified.
-
•
Young children (<5 years old) were more likely to be positive for HAdV.
-
•
Co-infections with other gastrointestinal or respiratory viruses were common.
-
•
A HAdV surveillance system is required to monitor circulating species and types.
Keywords: Human adenovirus, Molecular epidemiology, Phylogeny, Respiratory virus, Gastroenteritis virus
Abstract
Background
Human adenoviruses (HAdVs) can cause respiratory tract infections, conjunctivitis, diarrhoea and outbreaks have been reported. However, little is known about the disease burden and the molecular epidemiology of HAdV.
Objectives
To retrospectively perform a molecular characterization of HAdV positive samples received at Statens Serum Institut during the period 2011–2016 and to compare this with demographic information, geographic location, sample collection date and type and co-infection with other viral pathogens.
Study design
152 HAdV positive samples were genotyped by Sanger sequencing of a fragment of the hexon gene using published primers along with a newly developed primer set for enhanced genotyping of HAdV D. Phylogenetic analysis was used for genotyping and genotypes were compared with epidemiological information. In addition, HAdV burden and co-infection was evaluated for samples tested in laboratory analysis packages.
Results
Six out of seven HAdV species were identified and represented by 13 types. Young children (<5 years old) were more likely to be positive for HAdV and co-infections with other gastrointestinal or respiratory viruses were common. Possible outbreaks of ocular infections due to HAdV D could not be confirmed.
Conclusion
A diverse set of HAdV species were circulating in Denmark in the study period and although possible transmission clusters were identified, this could not be verified with current genotyping methods Young children were commonly affected by HAdV infection and co-infections with other viral pathogens were frequent suggesting a possible underestimation of the real HAdV burden.
1. Background
Human adenoviruses (HAdVs) consist of seven different species (A to G) subdivided into 67 HAdV types [1], [2]. HAdV typing is performed by PCR amplification and sequencing of a hypervariable region of the hexon gene, coding for one of three HAdV capsid proteins. HAdV can cause a wide range of diseases, namely febrile respiratory tract infections, conjunctivitis, diarrhoea and more rarely haemorrhagic cystitis and meningitis. Recent studies identified HAdV among 6% to 20% of hospitalised or emergency room admitted children [3], [4], [5], [6] presenting with lower respiratory tract infections and 10% and 23% of children admitted with acute gastroenteritis [7], [8]. HAdV species have been associated with different clinical manifestations [9]: gastroenteritis with HAdV species F, keratoconjunctivitis with species D and respiratory disease with species C and E. Species A and B (type 1) have been associated with gastroenteritis, respiratory infections, and keratoconjunctivitis. In particular, HAdV-B7 has been associated with severe respiratory disease [5], [10].
HAdV transmission occurs either through aerosols, respiratory secretions, person-to-person contact, contaminated fomites, or through the faecal-oral route. Non-symptomatic infected individuals can keep emitting viral particles in the environment for weeks through their feces, notably species B and C [11], [12], [13]. HAdVs are non-enveloped viruses, which make them more resistant to lipid disinfectants and provide them with a prolonged capacity to survive in the environment [14], [15]. All these factors contribute to the outbreak potential of HAdVs. The potential for outbreaks of respiratory disease has led to the development of a vaccine to be used among US military recruits [16], [17].
Although the proportion of HAdV infections among children <5 years hospitalised for gastroenteritis was estimated to be 11.2% in a survey conducted over a 12-month period in Denmark [7], no systematic surveillance of HAdV infections or typing exist currently in Denmark.
2. Objectives
Our objectives were to report demographic characteristics of HAdV cases identified in Denmark over the 2011–2016 period, to report the association between HAdV species and sample types (as a proxy for the type of infection) and to estimate the prevalence of HAdV infections in samples simultaneously analysed for a broad range of viruses in respiratory and gastrointestinal multiplex assays. The genotyping analysis of HAdV positive samples were used to: i) characterize which HAdV species and types circulated in Denmark in study period and ii) to investigate whether phylogenetic, geographical and temporal clusters occurred in the study period and whether the current methods for HAdV genotyping might be sufficient for outbreak investigations. Together, this knowledge was used as an evidence-based platform for an evaluation of the added value of a laboratory-based surveillance system for HAdV.
3. Study design
3.1. Patient samples
Feces, eye or respiratory samples received at the Statens Serum Institut for primary (real time PCR targeting F/non-F species in feces or non-feces, respectively) differential diagnosis of HAdV infections [18], [19] between January 2011 and September 2016 were retrospectively selected for this study. Type of sample material, demographic information (age and sex) and geographical location were available from most samples. One sample per patient was genotyped.
3.2. Genotyping of HAdV and phylogenetic analyses
Nucleic acids were extracted from samples using the MagNA Pure LC Total Nucleic Acid Isolation Kit on the MagNa Pure 96/32 (Roche Diagnostics). A fragment of the hexon gene was amplified using AmpliTaq DNA (Applied Biosystems™) and the following nested-PCR primers: PCR1-F 5′-GATGCCGCAGTGGKCKTACATG-3′, PCR1-R 5′-GCTTACAAYTCNCTSGCT-3′, and PCR2-F 5′-GACGCYTCGGAGTACCTGAG-3′ [13], PCR2-R 5′- GGCYAGCACNTACTTTGACATYCG −3′. Cycling conditions were as follows for both nested PCR reactions: initial denaturation at 95 °C for 3 min followed by 40 cycles of: 20 s at 95 °C, 30 s at 55 °C, 30 s at 72 °C. PCR products were purified using exo-SAP IT (GE Healthcare, Buckinghamshire, UK) prior to Sanger sequencing. Sequences were assembled in BioNumerics v7.6 (Applied Maths, Belgium) and Geneious and subsequently aligned against HAdV reference using MAFFT [20]. Phylogenetic trees were constructed in MEGA6 [21] using the Neighbor Joining method (Jukes Cantor model, using the ‘partial deletion’ option for handling gaps and 1000 bootstrap replicates). Species identification was based on the phylogenetic analysis.
Further subtyping of samples belonging to species D was performed with a nested-PCR with primers amplifying a larger fragment of the hexon gene: PCR1-D-F 5′-TACAAGGCGCGMTTCA-3′, PCR1-D-R 5′-CAGGTTGGCCTGVAGGTT-3′, PCR2-D-F 5′-ACAACCGGGTGCTAGACATGG-3′, PCR2-D-R 5′-TCVACHGCAGARTTCCACAT-3′. The following cycling conditions were used: initial denatureation at 95 °C for 3 min followed by 40 cycles of: 10 s at 95 °C, 10 s annealing at 55 °C (PCR1) or 61 °C (PCR2), 80 s at (PCR1) or 60 s (PCR2) at 72 °C (PCR1). Sequences were analysed as described above. All sequences were deposited in GenBank under the accession numbers MG199243 to MG199441.
4. Results
4.1. HAdV infection and patient sex and age
During the study period (2011-16), HAdV was identified in 664 samples out of ∼13,000 clinical samples screened for HAdV. 594 HAdV positive samples out of 12934 samples (Table 1 ) were included in the study (no more than one sample per patient). Feces (45%) and respiratory specimen (28%) were the most common sample types. No difference in sex distribution was observed between HAdV positive and negative individuals across all sample types, except for a higher proportion of HAdV positive eye samples from males (Table 1). The median age of HAdV positive individuals was lower than that of HAdV negative individuals across the different sample types. In particular, the median age of HAdV positive patients was 1.5 years for those who had a feces specimen tested and 3.5 years old for those who had a respiratory specimen tested. Overall children <5 years old were 5 (95% CI = 4.3-7.6) and 15 (95% CI = 7.0-33.9) times more likely to be tested positive for HAdV, in respiratory and feces samples respectively, than other individuals in this sample set.
Table 1.
Demographic characteristics of patients tested for HAdV in feces, respiratory, eye and other-unknown samples, January 2011- September 2016.
| Samples tested | Feces |
Respiratory |
Eye |
Other – Unknown |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAdV + | HAdV- | p* | HAdV + | HAdV- | p* | HAdV + | HAdV- | p* | HAdV + | HAdV- | p* | |
| No. cases (%) | 54 (0.9) | 5804 (99.1) | 236 (6.5) | 3417 (93.5) | 139 (12.5) | 971 (87.5) | 165 (7.1) | 2148 (92.9) | ||||
| Sex male (%) | 31 (57.4) | 2838 (49.0) | 0.2 | 122 (53.5) | 1683 (49.3) | 0.2 | 68 (48.9) | 380 (39.1) | 0.03 | 74 (45.1) | 1074 (50.1) | 0.2 |
| Median age, years [IQR] | 1.5 (1.0–2.9) | 29.6 (5.6–55.3) | <0.001 | 3.5 (1.0–28.5) | 44 (15–64) | <0.001 | 36 (29–49) | 41 (22–57) | 0.04 | 4 (1–26.5) | 31 (7–51) | <0.001 |
Note: * Chi square test for comparison of proportions (sex) and non parametric test of median for median age.
4.2. HAdV species and types and associations with sample material type
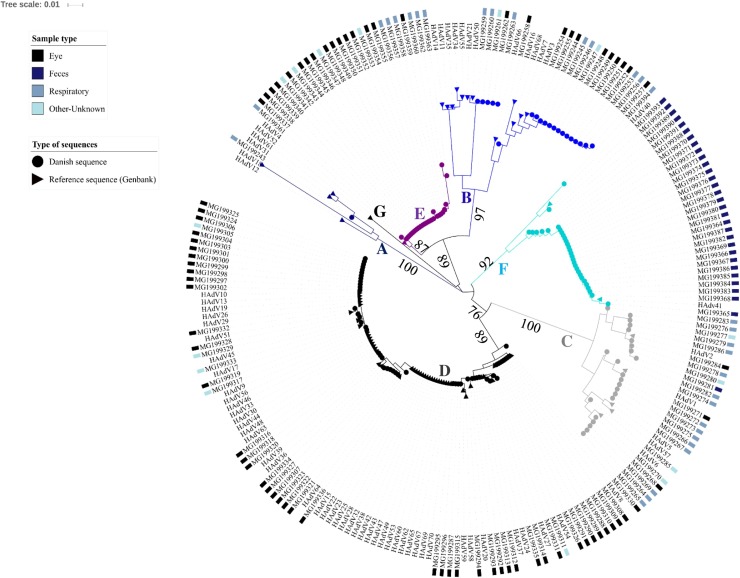
257 samples from 257 patients were available for genotyping and a HAdV species was identified in 152 samples through phylogenetic analyses (Fig. 1 ).
Fig. 1.
Phylogenetic analysis of HAdV hexon partial sequences.
Scale: nucleotide substitutions per site. Danish isolates are annotated with the type of sample that was collected. Bootstrap values for the main branches are indicated on the tree.
An association between HAdV species and sample types was observed (Table 2 ): HAdV species D, E and B were most frequently identified from eye specimens and species C from respiratory specimens (p < 0.001 for all). Species F was only detected once in a respiratory sample, species G was not identified in this sample set, and species A in one respiratory sample only. As expected from the analysis of the population affected by HAdV presented above and the distribution of HAdV species among sample types, the median age of infected individuals was different (p < 0.001). However, it cannot be excluded that some species were not identified due to the exclusion of negative samples in the initial diagnostics (See 3.1).
Table 2.
HAdV species identified and patients characteristics, SSI, Denmark, 2011-16.
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| No. cases | 1 | 20 | 23 | 50 | 27 | 31 |
| Sex male (%) | 1 (100) | 12 (60) | 12 (52) | 34 (68) | 10 (37) | 19 (63) |
| Median age (IQR) | 0.5 | 29 (15–55) | 4 (0.7–37) | 40 (12–67) | 32 (13–49) | 2 (1–28) |
| Sample type − no. | ||||||
| Feces | – | – | 1 | – | – | 30 |
| Eye swab | – | 11 | 3 | 45 | 16 | – |
| Respiratory | 1 | 7 | 15 | – | 7 | 1 |
| Unknown – other | – | 2 | 4 | 5 | 4 | – |
4.3. HAdV prevalence in analysis packages
We then estimated the prevalence of HAdV infections and compared it to that of other viruses in our sample set. For this analysis, we limited the sample set to the samples which were tested simultaneously in laboratory analysis packages including both HAdV and a defined set of respiratory and gastrointestinal viruses. This approach was taken in order to limit potential biases (overestimation of HAdV prevalence) in samples that would have been tested only for HAdV. The sample set comprised 5858 feces samples and 1784 respiratory samples. In respiratory specimens, the package consisted of Rhinovirus, Enterovirus, Influenza, Respiratory Syncytial Virus (RSV), Parainfluenza, Metapneumovirus, Coronavirus and Parechovirus. In the feces samples, viruses included in the laboratory analysis package were Norovirus GGI and GGII, Rotavirus, Sapovirus and Astrovirus. Overall the estimated prevalence of HAdV infections in the selected sample set was low at 0.9% (n = 54) and 3.5% (n = 62) among feces and respiratory specimens, respectively. Norovirus was the most frequently detected viral pathogen in feces specimens and Rhinovirus-Enterovirus in respiratory specimens (Table 3 a and b). In 16 feces samples (28%), HAdV was found together with one or more other virus (Norovirus, n = 6, Astrovirus, n = 5, Rotavirus, n = 3 and Sapovirus, n = 1), whereas samples containing HAdV alone accounted for 72%. In most HAdV positive respiratory samples (n = 38, 61%), a co-infection was found (Rhinovirus-Enterovirus, n = 27, RSV, n = 9, Coronavirus, n = 6, Parainfluenza virus, n = 3, Influenza, n = 2, Parechovirus, n = 1, Metapneumovirus, n = 1).
Table 3.
Proportions of virus positive among feces (a) and respiratory specimens (b) tested in laboratory analysis packages, SSI, Denmark, January 2011– September 2016.
| (a) | |
|---|---|
| Virus | Number (%) |
| Norovirus | 829 (14.2) |
| Rotavirus | 195 (3.3) |
| Sapovirus | 196 (3.4) |
| Astrovirus | 82 (1.4) |
| Adenovirus | 54 (0.9) |
| (b) | |
|---|---|
| Virus | Number (%) |
| Rhino-/Enterovirus | 264 (14.8) |
| Influenza | 129 (7.2) |
| RSV | 109 (6.1) |
| Parainfluenza | 63 (3.5) |
| Adenovirus | 62 (3.5) |
| Metapneumovirus | 53 (3.0) |
| Coronavirus | 47 (2.6) |
| Parechovirus | 5 (0.3) |
4.4. HAdV types circulating in Denmark in the 2011–2016 period and geographical clusters
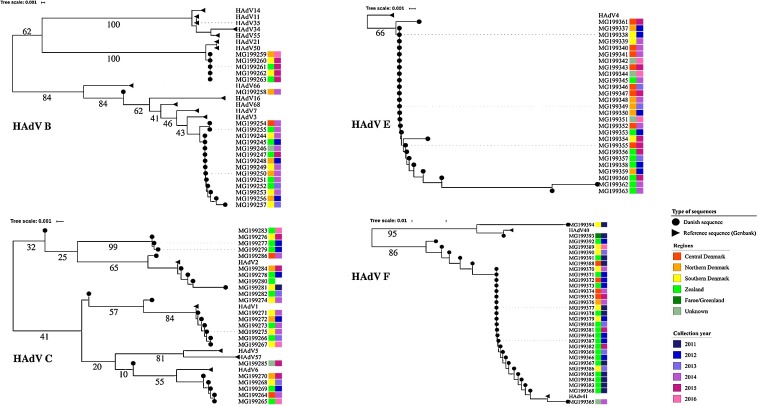
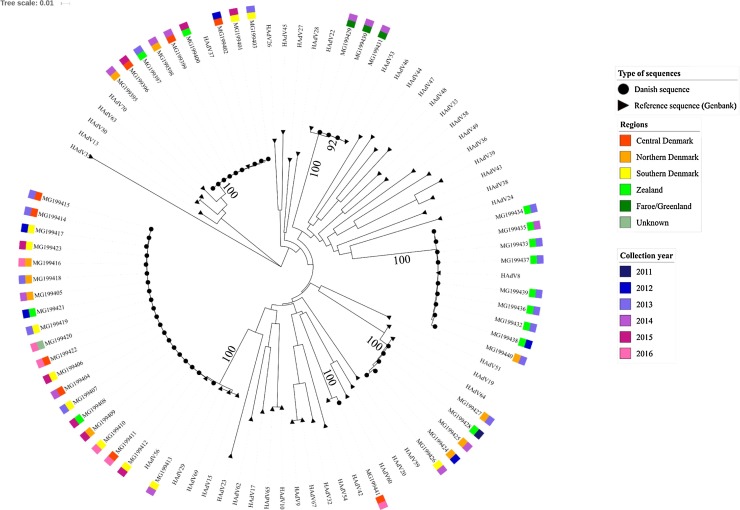
Through analysis of the short hexon gene fragment, it was possible to assign 95 of 102 (93%) samples into 13 different HAdV types (Fig. 1, Fig. 2 ). The frequency of each type as well as sample types screened are indicated in Table 4 . Isolates belonging to species B were observed in all regions from Denmark, however species B isolates belonging to the cluster of HAdV-B3 and B7 were mainly collected in 2013-14, and those belonging to the cluster of HAdV-B50 and B21 in 2015-16 (Fig. 2). Species C and E isolates were identified from the different Danish regions and across the study time period. All but two HAdV-F clustered with genotype 41 and were mainly isolated in 2011–12 (n = 18). Overall, most HAdV-F isolates originated from two regions (Zealand, n = 17 and Southern Denmark, n = 6). Since the short sequences did not provide enough discrimination power to distinguish most samples assigned to species D, these samples were further characterized by sequencing and phylogenetic typing analysis of a longer fragment of the hexon gene (Fig. 3 ). This improved the typing resolution and allowed classification of 47 of the 50 sequences into six genotypes. Type 56 and 37 were identified in samples from all regions (but Faroe Islands and Greenland), in the years spanning 2012–2016 and 2012–2015, respectively. Type 53 was only found in Faroe Islands and type 8 was mainly identified in samples from the region of Zealand, suggesting geographically restricted transmission. Clusters of two to three HAdV type 8 sequences (Fig. 3) were investigated for potential outbreaks as samples originated from the same location and were collected less than 30 days apart. However, phylogenetic analyses of the long hexon sequences from these samples did not permit for discrimination between them and other HAdV type 8 sequences with different temporal or geographical origins. Type 64 was found across multiple Danish regions and years, whereas type 42 was only found in a single sample from the region of Central Denmark.
Fig. 2.
Subtrees for HAdV sequences belonging to species A, B, C and F based on the analysis of HAdV hexon partial sequences.
Scale: nucleotide substitutions per site. Danish isolates are annotated by sample collection years and region of origin. Genbank sequences have no color bars. Bootstrap values for the main branches are indicated on the tree.
Table 4.
HAdV genotypes identified per sample type, SSI, Denmark, 2011-16.
| HAdV species and genotypes | Sample type (number of samples) | Year(s) |
|---|---|---|
| Species A | ||
| HAdV-31/HAdV-61 * | respiratory (1) | 2013 |
| Species B | ||
| HAdV-21/HAdV-50 * | eye (1), respiratory (3), other-unknown (1) | 2013, 2015–16 |
| HAdV-3 | eye (9), respiratory (4), other-unknown (1) | 2012–15 |
| Undetermined | eye (1) | 2014 |
| Species C | ||
| HAdV-1 | eye (1), respiratory (7) | 2012–14, 2016 |
| HAdV-2 | eye (1), feces (1), respiratory (5), other-unknown (2) | 2011–12, 2014–16 |
| HAdV-6 | eye (1), respiratory (3), other-unknown (2) | 2012–16 |
| Species D | ||
| HAdV-8 | eye (9) | 2012–14 |
| HAdV-37 | eye (8), other-unknown (1) | 2012–15 |
| HAdV-42 | eye (1) | 2016 |
| HAdV-53 | eye (3) | 2014 |
| HAdV-56 | eye (16), other-unknown (4) | 2012–16 |
| HAdV-64 | eye (5) | 2011–14 |
| Undetermined ** | eye (3) | 2012, 2015 |
| Species E | ||
| HAdV-4 | eye (16), respiratory (7), other-unknown (4) | 2012–16 |
| Species F | ||
| HAdV-40 | feces (1), respiratory (1) | 2011 |
| HAdV-41 | feces (29) | 2011–16 |
Note: * For samples for which a type could not be determined with certainty, the closest types are indicated when clustering was observed in phylogenetic analyses. ** Amplification of the longer hexon D fragment failed for these samples.
Fig. 3.
Phylogenetic analysis of HAdV species D using a long fragment of the hexon gene.
Scale: nucleotide substitutions per site. Danish isolates are annotated by sample collection years and region of origin. Genbank sequences have no color bars. Bootstrap values for the main branches are indicated on the tree.
5. Discussion
This study showed a low overall prevalence of HAdV infections at 0.9% and 3.5% in gastrointestinal and respiratory samples respectively but highlighted that young children were more frequently infected. In addition, HAdV infected patients were frequently co-infected with other viruses known to infect similar age groups (e.g. enteroviruses and RSV in young children with respiratory infections).
We show that 6 of 7 known HAdV species have been circulating in Denmark in the study period. Eye swabs and feces samples were the most commonly available samples which resulted in the identification of a large number of HAdV species D and species F. Type 41 was the most common species F strain circulating in Denmark. Of the six types belonging to species D, five types were identified in Denmark, and one additional type was identified in samples from the Faroe Islands. Sequencing a longer fragment of the hexon gene improved the classification of HAdV D from to type level for a total of 142 of 152 (93%) samples. To perform typing of HAdV it would therefore be advisable to have species specific primers for typing of some HAdV species after an initial and broad screening is performed with the primers capable of detecting all species. We here provide such additional primers for HAdV D.
Due to the absence of preventive measures against HAdV (antivirals, common vaccine, antiseptics), it is important to investigate if outbreaks are common in order to assess both the possible public health burden of HAdV and to evaluate whether current molecular methods are sufficient for outbreak identifications and subsequent intervention strategies. Species D type 8, identified in this study, has previously been associated with outbreaks of keratoconjunctivitis [22], [23]. It was therefore investigated if possible outbreaks could be observed in the study period through a combined phylogenetic, geographical and temporal clustering. Although geographical and partial temporal clustering of type 8 was observed and indicated a possible outbreak on Zealand in 2013 the phylogenetic resolution, even with the larger hexon gene fragment, was not high enough for a clear distinction between sequences from Zealand in 2013 and other type 8 sequences with different geographical and temporal origins. Therefore larger parts or whole genome sequencing approaches might be required to differentiate between sequences originating from an outbreak and sporadic cases. Since HAdVs genome consists of dsDNA, it might also be far less variable than other (ssRNA) viruses, for instance norovirus, where molecular methods are commonly used to identify point-source outbreaks [24], [25]. Systematic surveillance and reporting of HAdV infections and virus genotyping would provide critical information on HAdV types commonly in circulation and their genetic diversity and also allow for the identification of clusters of infections likely to be outbreaks.
Furthermore, associations between HAdV types and severe clinical manifestations are still poorly described due to the gap in the literature presenting HAdV subtyping outcomes. A few other European countries have set up HAdV surveillance systems. As examples, in the Netherlands, virological sentinel surveillance collects weekly numbers of HAdV positive samples from laboratories serving primary care, hospitals and long-term care facilities. In Scotland, HAdV surveillance is done as part of a routine respiratory screen both from hospital samples all year-round and from general practitioner samples during the influenza season as part of the sentinel swabbing scheme for influenza-like illness. In Germany, the surveillance system only covers epidemic keratoconjunctivitis. These surveillance systems mainly rely on existing infrastructure but have enabled countries to monitor trends and also, in the example of Germany, to investigate outbreaks [26]. Denmark could also take advantage of existing surveillance systems such as the Influenza sentinel surveillance system and the enhanced enterovirus surveillance system [27], which focus on detection and typing of viral respiratory infections including adenoviruses.
Limitations: As regions implemented HAdV laboratory testing, the number of samples received at SSI decreased over the years, limiting interpretation of observed HAdV diversity. Samples sent in for analysis at SSI may also not be representative of all HAdV samples processed in Denmark. Lastly, the diagnostic PCR used on stool samples primarily targeted species D, making it unlikely to detect other species.
In summary, we demonstrate that young children were more likely to be infected with HAdV and that co-infections with other viral pathogens are common, as previously found [28], which suggest a possible underestimation of the real HAdV burden. Our study also shows the diversity of HAdV circulating in Denmark and the possible existence of clusters of transmission. In the absence of a surveillance system aimed at detection and reporting HAdV infections it is currently not possible to determine if outbreaks of HAdV have occurred in Denmark during the study period.
We recommend to further investigate the contribution of HAdV to the burden of respiratory and diarrheal disease in young children by conducting prospective cohort studies with regular sampling schemes irrespective of symptoms and of respiratory material as well as feces from children. The implementation of a laboratory based surveillance system for HAdV, including genotyping, could then be considered.
Conflict of interest
None to declare.
Funding
None.
Ethical approval
Not required.
Acknowledgments
The authors would like to acknowledge Dr Louise Coole for her critical review of this manuscript and Janine Thoulass, Roan Pijnacker and Diogo Marques, fellows from the European Programme for Intervention Epidemiology Training (EPIET) for their feedback on human adenovirus surveillance in Germany, the Netherlands and Scotland.
References
- 1.Davison A.J., Benko M., Harrach B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003;84(Pt 11):2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 2.Ghebremedhin B. Human adenovirus: viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. (Bp) 2014;4(1):26–33. doi: 10.1556/EuJMI.4.2014.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cebey-Lopez M., Herberg J., Pardo-Seco J., Gomez-Carballa A., Martinon-Torres N., Salas A. Viral co-infections in pediatric patients hospitalized with lower tract acute respiratory infections. PLoS One. 2015;10(9):e0136526. doi: 10.1371/journal.pone.0136526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esposito S., Zampiero A., Bianchini S., Mori A., Scala A., Tagliabue C. Epidemiology and clinical characteristics of respiratory infections due to adenovirus in children living in milan, Italy,during 2013 and 2014. PLoS One. 2016;11(4):e0152375. doi: 10.1371/journal.pone.0152375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C., Xiao Y., Zhang J., Ren L., Li J., Xie Z. Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007–2012. BMC Infect. Dis. 2015;15:408. doi: 10.1186/s12879-015-1126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter J., Panayiotou C., Tryfonos C., Koptides D., Koliou M., Kalogirou N. Aetiology of acute respiratory tract infections in hospitalised children in Cyprus. PLoS One. 2016;11(1):e0147041. doi: 10.1371/journal.pone.0147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer T.K., Rungoe C., Jensen C.S., Breindahl M., Jorgensen T.R., Nielsen J.P. The burden of rotavirus disease in Denmark 2009–2010. Pediatr. Infect. Dis. J. 2011;30(7):e126–e129. doi: 10.1097/INF.0b013e3182145277. [DOI] [PubMed] [Google Scholar]
- 8.La RG Della LS, Petricca S., Iaconelli M., Donia D., Saccucci P. Genetic diversity of human adenovirus in children with acute gastroenteritis, Albania, 2013–2015. BioMed Res. Int. 2015;2015:142912. doi: 10.1155/2015/142912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014;27(3):441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott M.K., Chommanard C., Lu X., Appelgate D., Grenz L., Schneider E. Human adenovirus associated with severe respiratory infection, Oregon, USA, 2013–2014. Emerg. Infect. Dis. 2016;22(6):1044–1051. doi: 10.3201/eid2206.151898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt C.D., Kim H.W., Rodriguez W.J., Arrobio J.O., Jeffries B.C., Stallings E.P. Adenoviruses and pediatric gastroenteritis. J. Infect. Dis. 1985;151(3):437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- 12.Fox J.P., Hall C.E., Cooney M.K. The Seattle virus watch: VII. observations of adenovirus infections. Am. J. Epidemiol. 1977;105(4):362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- 13.Moyo S.J., Hanevik K., Blomberg B., Kommedal O., Nordbo S.A., Maselle S. Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; a case control study. BMC Infect. Dis. 2014;14:666. doi: 10.1186/s12879-014-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grondahl-Rosado R.C., Yarovitsyna E., Trettenes E., Myrmel M., Robertson L.J. A one year study on the concentrations of norovirus and enteric adenoviruses in wastewater and a surface drinking water source in Norway. Food Environ. Virol. 2014;6(4):232–245. doi: 10.1007/s12560-014-9161-5. [DOI] [PubMed] [Google Scholar]
- 15.Ogorzaly L., Walczak C., Galloux M., Etienne S., Gassilloud B., Cauchie H.M. Human adenovirus diversity in water samples using a next-generation amplicon sequencing approach. Food Environ. Virol. 2015;7(2):112–121. doi: 10.1007/s12560-015-9194-4. [DOI] [PubMed] [Google Scholar]
- 16.Kajon A.E., Hang J., Hawksworth A., Metzgar D., Hage E., Hansen C.J. Molecular epidemiology of adenovirus type 21 respiratory strains isolated from US military trainees (1996–2014) J. Infect. Dis. 2015;212(6):871–880. doi: 10.1093/infdis/jiv141. [DOI] [PubMed] [Google Scholar]
- 17.Kajon A.E., Ison M.G. Severe infections with human adenovirus 7d in 2 adults in family, illinois, USA, 2014. Emerg. Infect. Dis. 2016;22(4):730–733. doi: 10.3201/eid2204.151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logan C., O'Leary J.J., O'Sullivan N. Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. J. Clin. Microbiol. 2006;44(9):3189–3195. doi: 10.1128/JCM.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Alphen L.B., Dorleans F., Schultz A.C., Fonager J., Ethelberg S., Dalgaard C. The application of new molecular methods in the investigation of a waterborne outbreak of norovirus in Denmark, 2012. PLoS One. 2012;9(9):e105053. doi: 10.1371/journal.pone.0105053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6. Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki K., Ishiko H., Konno T., Shimada Y., Hayashi A., Kaneko H. Epidemic keratoconjunctivitis due to the novel hexon-chimeric-intermediate 22, 37/H8 human adenovirus. J. Clin. Microbiol. 2008;46(10):3259–3269. doi: 10.1128/JCM.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelmann I., Madisch I., Pommer H., Heim A. An outbreak of epidemic keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin. Infect. Dis. 2006;43(7):e64–e66. doi: 10.1086/507533. [DOI] [PubMed] [Google Scholar]
- 24.Fonager J., Stegger M., Rasmussen L.D., Poulsen M.W., Ronn J., Andersen P.S. A universal primer-independent next-generation sequencing approach for investigations of norovirus outbreaks and novel variants. Sci. Rep. 2017;7(1):813. doi: 10.1038/s41598-017-00926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller L., Rasmussen L.D., Jensen T., Schultz A.C., Kjelso C., Barnadas C. Series of norovirus outbreaks caused by consumption of green coral lettuce, Denmark, april 2016. PLoS Curr. 2016:8. doi: 10.1371/currents.outbreaks.115761d5d6de6a8bc7dd4b41f0f5f142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adlhoch C., Schoneberg I., Fell G., Brandau D., Benzler J. Increasing case numbers of adenovirus conjunctivitis in Germany. Euro Surveill. 2010;15(45) (2010) [PubMed] [Google Scholar]
- 27.Barnadas C., Midgley S.E., Skov M.N., Jensen L., Poulsen M.W., Fischer T.K. An enhanced Enterovirus surveillance system allows identification and characterization of rare and emerging respiratory enteroviruses in Denmark, 2015–16. J. Clin. Virol. 2017;93:40–44. doi: 10.1016/j.jcv.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee A., De P., Manna B., Chawla-Sarkar M. Molecular characterization of enteric adenovirus genotypes 40 and 41 identified in children with acute gastroenteritis in Kolkata, India during 2013–2014. J. Med. Virol. 2017;89(4):606–614. doi: 10.1002/jmv.24672. [DOI] [PubMed] [Google Scholar]