Summary
Background
Middle East respiratory syndrome coronavirus (MERS-CoV) infection is associated with high mortality and has no approved antiviral therapy. We aimed to compare ribavirin and interferon alfa-2a treatment for patients with severe MERS-CoV infection with a supportive therapy only.
Methods
In this retrospective cohort study, we included adults (aged ≥16 years) with laboratory-confirmed MERS-CoV infection and pneumonia needing ventilation support, diagnosed between Oct 23, 2012, and May 1, 2014, at the Prince Sultan Military Medical City (Riyadh, Saudi Arabia). All patients received appropriate supportive care and regular clinical and laboratory monitoring, but patients diagnosed after Sept 16, 2013, were also given oral ribavirin (dose based on calculated creatinine clearance, for 8–10 days) and subcutaneous pegylated interferon alfa-2a (180 μg per week for 2 weeks). The primary endpoint was 14-day and 28-day survival from the date of MERS-CoV infection diagnosis. We used χ2 and Fischer's exact test to analyse categorical variables and the t test to analyse continuous variables.
Findings
We analysed 20 patients who received ribavirin and interferon (treatment group; initiated a median of 3 days [range 0–8] after diagnosis) and 24 who did not (comparator group). Baseline clinical and laboratory characteristics were similar between groups, apart from baseline absolute neutrophil count, which was significantly lower in the comparator group (5·88 × 109/L [SD 3·95] vs 9·88 × 109/L [6·63]; p=0·023). 14 (70%) of 20 patients in the treatment group had survived after 14 days, compared with seven (29%) of 24 in the comparator group (p=0·004). After 28 days, six (30%) of 20 and four (17%) of 24, respectively, had survived (p=0·054). Adverse effects were similar between groups, apart from reduction in haemoglobin, which was significantly greater in the treatment group than in the comparator group (4·32 g/L [SD 2·47] vs 2·14 g/L [1·90]; p=0·002).
Interpretation
In patients with severe MERS-CoV infection, ribavirin and interferon alfa-2a therapy is associated with significantly improved survival at 14 days, but not at 28 days. Further assessment in appropriately designed randomised trials is recommended.
Funding
None.
Introduction
Since it was first described in September, 2012, 855 cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection have been confirmed, 333 of which were fatal.1, 2 Cases occur sporadically, as community clusters or as hospital outbreaks, and range in severity from asymptomatic or mild illness to rapidly progressive and fatal disease.2, 3, 4, 5, 6 The management of patients with MERS-CoV infection consists of a combination of supportive measures, antimicrobial therapy for any associated bacterial or viral infections, and strict implementation of appropriate infection control precautions.7 So far, no antiviral therapy has been approved for the treatment of patients with MERS-CoV infection.8
Several therapeutic interventions for coronavirus were investigated during the large multinational outbreak of severe acute respiratory syndrome (SARS) in 2003.9, 10 Reviews of the available scientific literature suggest that a combination of ribavirin and interferon might be of benefit in patients with severe MERS-CoV infection.8, 11, 12 Furthermore, this combination was shown to inhibit MERS-CoV in cell culture and seemed to improve outcomes in an animal study.13, 14 Both agents are associated with substantial potential adverse effects and hence their clinical use should be carefully balanced against any potential harm.8
We aimed to assess outcomes of a treatment programme for patients with severe MERS-CoV infection that consisted of oral ribavirin and subcutaneous pegylated interferon alfa-2a. We report the results and outcomes in patients given treatment in accordance with this protocol by comparison with a historical group who received supportive therapy only.
Methods
Study design and participants
This single-centre, retrospective cohort study included individuals who were diagnosed with laboratory-confirmed MERS-CoV infection between Oct 23, 2012, and May 1, 2014, at the Prince Sultan Military Medical City (Riyadh, Saudi Arabia). Eligible patients were those aged 16 years or older with severe pneumonia needing invasive or non-invasive ventilation. No exclusion criteria were applied at this stage.
MERS-CoV infection was diagnosed by RT-PCR testing of respiratory tract samples for MERS-CoV upE, ORF 1b, and N genes.15 All RT-PCR tests for MERS-CoV were done at the Saudi Ministry of Health Regional Laboratory in Jeddah and Riyadh, Saudi Arabia. Pneumonia was defined as new, otherwise unexplained, lower respiratory tract symptoms such as cough or shortness of breath with at least one systemic feature such as fever or chills, and new focal chest signs on examination, in addition to new or progressive pulmonary infiltrates on chest radiograph.16
From Sept 16, 2013, all eligible patients were offered treatment with oral ribavirin and subcutaneous pegylated interferon alfa-2a after informed written consent had been obtained from the patients themselves or their next of kin. The treatment protocol was approved by Pharmacy and Therapeutics Committee. The study was approved by the Research Ethics Committee at the Prince Sultan Military Medical City to allow retrospective access to patients' records and files.
Procedures
Pegylated interferon alfa-2a (Pegasys; Roche Pharmaceuticals, Basel, Switzerland) was given by subcutaneous injection at a dose of 180 μg per week for 2 weeks. The dose of oral ribavirin (Copegus; Roche Pharmaceuticals) was adjusted according to calculated creatinine clearance and continued for 8–10 days.12 Patients with a creatinine clearance of greater than 0·833 mL/sec/m2 received a 2000 mg loading dose, followed by 1200 mg every 8 h for 4 days then 600 mg every 8 h for 4–6 days; those with a creatinine clearance of 0·333–0·833 mL/sec/m2 received a 2000 mg loading dose, followed by 600 mg every 8 h for 4 days then 200 mg every 6 h for 4–6 days; and those with a creatinine clearance of <0·333 mL/sec/m2 or on dialysis received a 2000 mg loading dose, followed by 200 mg every 6 h for 4 days then 200 mg every 12 h for 4–6 days. Patients did not receive ribavirin and interferon alfa-2a therapy if they were diagnosed before Sept 16, 2013, or if they declined consent. All patients received appropriate supportive care such as supplementary oxygen, vasopressor therapy, and renal replacement as needed. Hydrocortisone 200 mg daily was given to patients with refractory septic shock and continued until vasopressor therapy was no longer needed.17 In addition to regular clinical monitoring, renal function, liver enzymes, and blood count were assessed at baseline and daily throughout the treatment course. Conscious patients were monitored for any clinical signs of depression or acute confusion.
Patients who received ribavirin and interferon alfa-2a therapy were classified as being in the treatment group and those who did not made up the comparator group. Two investigators, ASO and KB, both of whom were masked to group allocation and the patients' clinical outcomes, compared baseline characteristics of the two groups.
Outcomes
The primary endpoints for the study were 14-day and 28-day survival from the date of MERS-CoV infection was diagnosis.
Statistical analysis
We used χ2 and Fischer's exact tests for categorical variables, whereas we used the student's t test for continuous variables to assess the differences in means of the two groups. The log-rank test was used for assessing survival differences between the two groups. Our cutoff for statistical significance was 0·05. The graphical and statistical tests suggested that the proportional-hazard assumption was not violated. We did statistical analyses using Microsoft Excel 2011 and Stata Statistical Software, Release 12.
Role of funding source
No external funding was received for this study. ZM had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
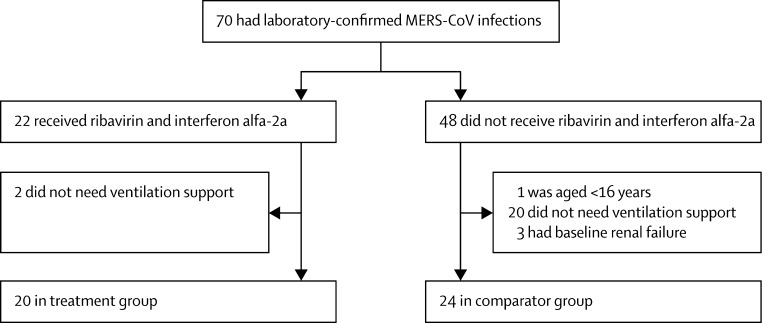
70 individuals were diagnosed with MERS-CoV infection between Oct 23, 2012, and May 1, 2014. Baseline characteristics were generally similar between patients who received ribavirin and interferon alfa-2a therapy and those who did not (table 1 ), with the exception that end-stage renal failure was present in three patients who did not receive study treatment and in none who did. After excluding ineligible patients, 44 patients were included in the study: 20 in the treatment group and 24 in the comparator group (figure 1 ). The mean age of all 44 patients was 65·5 years (SD 18·2), and 32 (73%) were men (table 1). The median number of comorbidities was three (range 0–6). Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 27 (SD 10·28) and mean Sequential Organ Failure Assessment (SOFA) score was 11 (4·32).
Table 1.
Baseline characteristics on day of diagnosis of Middle East respiratory syndrome coronavirus infection, by patient group
| Treatment group (n=20) | Comparator group (n=24) | p value | |
|---|---|---|---|
| Men | 16 (75%) | 16 (67%) | 0·323 |
| Age, years | 67·4 (18·5) | 64·0 (18·1) | 0·54 |
| Critical-care support | 20 (100%) | 24 (100%) | .. |
| Congestive heart failure | 5* (26%) | 7 (29%) | 0·84 |
| Dementia | 1 (5%) | 1 (4%) | 1·00 |
| Chronic obstructive pulmonary disease | 2 (10%) | 4 (17%) | 0·67 |
| Asthma | 1 (5%) | 0 | 0·45 |
| Rheumatological disease | 1 (5%) | 1 (4%) | 1·00 |
| Chronic liver disease | 0 | 3 (13%) | 0·24 |
| Diabetes mellitus | 14 (70%) | 16 (67%) | 0·81 |
| Hemiplegia | 5* (26%) | 6 (25%) | 1·00 |
| Chronic kidney disease | 4* (21%) | 7 (29%) | 0·73 |
| Malignant disorder | 1 (5%) | 1 (4%) | 1·00 |
| HIV infection | 0 | 0 | .. |
| Obesity | 1† (8%) | 4* (21%) | 0·63 |
| Number of comorbidities | 2·50 (1·4) | 2·92 (1·7) | 0·4 |
| Immunosuppressive therapy | 1 (5%) | 4‡ (18%) | 0·66 |
| Haemoglobin (g/L) | 11·46 (2·80) | 10·28 (2·20) | 0·124 |
| Peripheral white cell count (×109/L) | 7·86 (4·50) | 10·96 (6·83) | 0·090 |
| Absolute neutrophil count (×109/L) | 5·88 (3·95) | 9·88 (6·63) | 0·023 |
| Lymphocyte count (×109/L) | 1·53 (2·88) | 1·00 (0·59) | 0·380 |
| Platelet count (×109/L) | 190·85 (90·55) | 207·17 (142·03) | 0·660 |
| Alanine transaminase (IU/L) | 35·75 (28·50) | 35·63 (40·52) | 0·991 |
| Aspartate transaminase (IU/L) | 689·63 (2707·43) | 63·91 (53·52) | 0·273 |
| Serum bilirubin (μmol/L) | 18·45 (25·17) | 38·20 (80·97) | 0·301 |
| Alkaline phosphatase (IU/L) | 115·10 (106·13) | 183·13 (159·34) | 0·111 |
| Serum creatinine (μmol/L) | 133·75 (81·72) | 142·38 (73·18) | 0·714 |
| Serum albumin (g/L) | 26·95 (4·72) | 26·39 (5·02) | 0·710 |
| APACHE II score | 25·31 (11·40) | 27·94 (9·20) | 0·479 |
| SOFA score | 10·38 (4·15) | 12·38 (4·38) | 0·195 |
Data are number (%) or mean (SD). APACHE II=Acute Physiology and Chronic Health Evaluation II. SOFA=Sequential Organ Failure Assessment.
Only 19 patients were assessed.
Only 13 patients were assessed.
Only 22 patients were assessed.
Figure 1.
Study profile
Mean blood indices on the day of MERS-CoV diagnosis include haemoglobin 10·81 g/dL (SD 2·53 g/dL), peripheral white cell count 9·55 × 109/L (6·03 × 109/L), absolute neutrophil count 8·06 × 109/L (5·87 × 109/L), lymphocyte count 1·24 × 109/L (1·98 × 109/L), platelets 199·75 × 109/L (120·33 × 109/L), alanine transaminase 35·68 IU/L (35·17 IU/L), aspartate transaminase 346·98 IU/L (1821·82 IU/L), alkaline phosphatase 152·2 IU/L (140·46 IU/L), bilirubin 29·22 μmol/L (62·34 μmol/L), serum creatinine 138·45 μmol/L (76·38 μmol/L), and serum albumin 26·65 g/L (4·83 g/L). Overall, 41 (93%) of 44 patients needed invasive ventilation, whereas three patients (7%) needed bilevel positive airway pressure. 22 (50%) of all patients needed renal replacement therapy. All patients received broad-spectrum antibacterial therapy and 33 (75%) also received oseltamivir. In the treatment group, ribavirin and pegylated interferon alfa-2a were started within a median of 3 days (range 0–8) from diagnosis of MERS-CoV infection.
Mean absolute neutrophil count was significantly lower in the treatment group than in the comparator group (table 1); however, no other statistically significant differences in baseline characteristics or support measures were noted between the two groups (Table 1, Table 2 ).
Table 2.
Support measures offered during the course of Middle East respiratory syndrome coronavirus infection, by patient group
| Treatment group (n=20) | Comparator group (n=24) | p value | |
|---|---|---|---|
| Invasive ventilation | 19 (95%) | 22 (92%) | 1·00 |
| Extracorporeal membrane oxygenation | 2 (10%) | 2 (8%) | 1·00 |
| Prone positioning | 4* (22%) | 1† (5%) | 0·18 |
| Renal replacement therapy | 12 (60%) | 10 (42%) | 0·23 |
| Vasopressor therapy | 14 (70%) | 18 (75%) | 0·71 |
| Immunoglobulin therapy | 0 | 0 | .. |
| Packed red blood cell transfusion | 10† (53%) | 9 (38%) | 0·32 |
| Corticosteroid therapy | 11† (58%) | 12 (50%) | 0·71 |
| Number of antibacterial therapy agents | 5·5 (2·5) | 4·58 (1·5) | 0·134 |
| Oseltamivir therapy | 17 (85%) | 16 (67%) | 0·16 |
Data are number (%) or mean (SD).
Only 18 patients were assessed.
Only 19 patients were assessed.
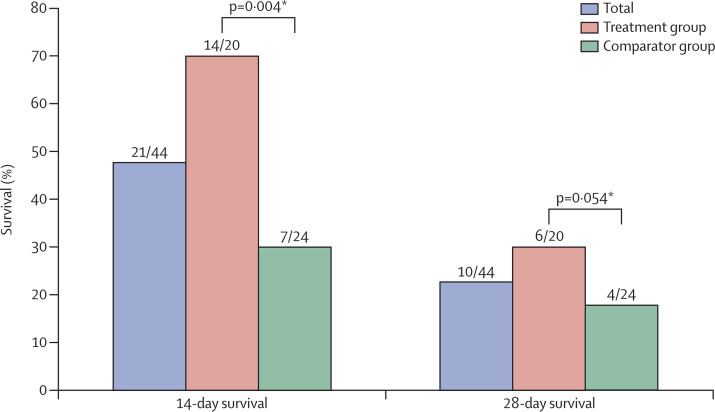
Of all 44 patients, 21 (48%) were still alive 14 days after diagnosis of MERS-CoV infection, whereas at 28 days only ten (23%) had survived. 14 (70%) of 20 patients in the treatment group were alive 14 days after diagnosis, compared with seven (29%) of 24 in the comparator group (p=0·004). However, six (30%) of 20 patients in the treatment group survived up to 28 days from diagnosis of MERS-CoV infection, whereas four (17%) of 24 did in the comparator group (p=0·054; figure 2 ).
Figure 2.
Survival from date of diagnosis of MERS-CoV infection by patient group
*Log-rank test.
Ribavirin and pegylated interferon therapy was well tolerated by the treatment group with no premature discontinuation secondary to adverse effects. However, the mean drop in haemoglobin over the treatment course was significantly greater in the treatment group (4·32 g/L [SD 2·47]) than in the comparator group (2·14 g/L [1·90]; p=0·002). Mean minimum absolute neutrophil count was also significantly lower in the treatment group (2·99 × 109/L [SD 1·87]) compared with the comparator group (4·42 × 109/L [1·89]; p=0·017). No other significant differences in laboratory indices between the two groups were noted during the treatment period (table 3 ). Depression or acute confusion was not diagnosed in any of the patients in either group.
Table 3.
Laboratory indices throughout treatment course, by patient group
| Treatment group (n=20) | Comparator group (n=24) | p value | |
|---|---|---|---|
| Haemoglobin, minimum (g/L) | 7·15 (2·13) | 8·13 (1·78) | 0·101 |
| Haemoglobin change, maximum (g/L) | −4·32 (2·47) | −2·14 (1·90) | 0·002 |
| Peripheral white cell count, minimum (×109/L) | 5·04 (3·36) | 5·71 (2·08) | 0·421 |
| Absolute neutrophil count, minimum (×109/L) | 2·90 (1·87) | 4·42 (1·89) | 0·017 |
| Minimum lymphocyte count, minimum (×109/L) | 1·07 (2·55) | 0·53 (0·29) | 0·310 |
| Platelet count, minimum (×109/L) | 117·80 (62·73) | 113·92 (59·28) | 0·834 |
| Alanine transaminase, maximum (IU/L) | 139·35 (189·63) | 152·75 (269·03) | 0·852 |
| Aspartate transaminase, maximum (IU/L) | 1195·84 (2890·29) | 859·87 (1650·26) | 0·639 |
| Serum bilirubin, maximum (μmol/L) | 70·95 (134·21) | 59·50 (98·33) | 0·746 |
| Alkaline phosphatase, maximum (IU/L) | 255·65 (243·97) | 398·17 (430·52) | 0·196 |
| Serum creatinine, maximum (μmol/L) | 310·70 (161·04) | 329·17 (223·75) | 0·759 |
| Serum albumin, minimum (g/L) | 21·05 (2·81) | 21·04 (4·36) | 0·995 |
Data are mean (SD).
Discussion
Effective treatment interventions for patients with severe MERS-CoV infection are still urgently needed. In critically ill patients with severe MERS-CoV infection, our study shows that ribavirin and pegylated interferon alfa-2a therapy is associated with a significant 14-day survival benefit compared with standard treatment. 28-day survival also seemed to improve with ribavirin and pegylated interferon alfa-2a therapy, but the difference between groups was not significant (panel ). The loss of a significant survival difference over time might be partly explained by most patients in our cohort having several comorbidities with high APACHE II and SOFA scores. Mortality is known to be very high in patients with severe MERS-CoV infection who need critical-care support.24 Therefore, long-term survival benefit, if present, might be difficult to show in smaller studies.
Panel. Research in context.
Systematic review
We searched PubMed for reports published in English any time before June 24, 2014, with the search term “[(MERS-CoV OR HCoV-EMC OR novel coronavirus) AND (therapy OR interferon OR ribavirin]”. We found one animal study,14 two small case series in human beings,18, 19 and several in-vitro studies.13, 20, 21, 22, 23 The data suggested that combination therapy with ribavirin and interferon alfa could have potential benefits for patients with severe MERS-CoV infection.
Interpretation
This is, to our knowledge, the largest clinical study done so far assessing the potential benefit and safety of combination therapy with pegylated interferon alfa-2a plus ribavirin in patients with severe MERS-CoV infection. Because we noted a significant 14-day survival benefit in patients who received the combination compared with those who received supportive therapy only, but no survival benefit at 28 days, we recommend further assessment in appropriately designed randomised clinical trials to provide further information about the role of this combination in the treatment of patients with severe MERS-CoV infection.
Treatment with ribavirin and interferon was well tolerated in our study. The only adverse event that was significantly worse in the treatment group was mean decrease in haemoglobin (4.32 g/L in the treatment group compared with 2·14 g/L in the comparator group). Anaemia is a well recognised complication of ribavirin therapy and was noted previously in studies investigating the role of ribavirin in the treatment of SARS coronavirus infection.25, 26 Of note, receipt of packed red blood cells was not significantly different between the treatment and comparator groups in our study. Furthermore, no treatment discontinuations occurred as a result of anaemia. Therefore, the risk of ribavirin-associated anaemia—although substantial and in need of careful monitoring—might not hinder the use of ribavirin for patients with severe MERS-CoV infection, especially if a survival benefit can be confirmed. Baseline absolute neutrophil count was significantly lower in the treatment group and therefore a significantly lower minimal absolute neutrophil count during the course of the illness is not surprising.
Several investigators showed that interferon α has useful in-vitro activity against MERS-CoV.13, 20, 26 However, when compared with interferon α and interferon γ, interferon β seems to have the most potent inhibitory in-vitro activity against MERS-CoV.21 Ribavirin has slight anti-MERS-CoV activity in vitro when used alone or in combination with interferon α.13, 22 Mycophenolic acid is another compound that exhibits significant in-vitro activity against MERS-CoV.21 Of 290 compounds screened, 60 were active in cell culture against MERS-CoV.23 Although only the combination of interferon α plus ribavirin has so far undergone in-vivo assessment against MERS-CoV,14 many others are potential candidates for further clinical assessment.
One of the limitations of our study is its small size. However, only two previous reports of clinical use of ribavirin and interferon for MERS-CoV infection have been published.18, 19 In a retrospective report, five patients with severe MERS-CoV infection, all of whom had significant comorbidities and needed mechanical ventilation, received a combination of ribavirin and pegylated interferon alfa-2b a median of 19 days after admission. None of the patients survived and the investigators concluded that late commencement of therapy might not be beneficial.18 In another report,19 a patient with severe MERS-CoV infection received ribavirin and interferon therapy with good clinical response and no significant adverse effects. Our study, albeit small, is the largest clinical investigation so far to assess the use of this combination in the treatment of patients with severe MERS-CoV infection. Although baseline characteristics of our treatment and comparator groups seem to be reasonably balanced, substantial differences might not be apparent because of the small number of patients in the study.
Our study is also limited by its retrospective, non-randomised nature. Inevitably, selection and unmeasured confounding bias cannot be completely excluded. Undoubtedly, new interventions should ideally be assessed in randomised, controlled clinical trials. However, such an approach is generally accepted to not always be practically feasible in the context of an emerging and relatively uncommon disease such as MERS-CoV infection.8 We carefully selected our comparator group, ensuring that the two cohorts were matched as closely as possible in their clinical characteristics and treatment interventions other than the receipt of ribavirin and interferon. We removed three individuals who had outlying baseline serum creatinine from the comparator group to minimise the risk of any spurious conclusions driven by clinical characteristics that might be potentially detrimental to clinical outcome. Clinical outcomes for each individual were masked from investigators who selected patients and did matching assessments. The absence of serial viral load measurement in lower respiratory tract samples in our study makes it impossible to show any association between temporal viral load changes and antiviral therapy. Such measurements should be included in any future clinical studies exploring the therapeutic benefit of any antiviral intervention for patients with MERS-CoV infection.
Severe MERS-CoV is associated with poor overall survival. Treatment with oral ribavirin and subcutaneous pegylated interferon alfa-2a is associated with significantly improved survival at 14 days, but not at 28 days. The combination is associated with significant falls in haemoglobin, but no other significant adverse effects were noted. Treatment with ribavirin and pegylated interferon might be considered in patients with severe MERS-CoV infection, provided that adequate monitoring and assessment can be ensured. Further assessment, including in patients with less severe MERS-CoV infection, in appropriately designed randomised trials, is recommended.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on Month date, 2014
Acknowledgments
Acknowledgments
We thank staff of Prince Sultan Military Medical City, Riyadh, Saudi Arabia, for the clinical care given to the patients and for facilitating access to the relevant medical records.
Contributors
This study was initiated and designed by ASO, MMS, ZAM, and AMA. ASO, MMS, AB, MA-M, AYA, GAA, MMA, ZAM, and AMA obtained and collated patient data. KB undertook all statistical analyses for the study. ASO and KB prepared all tables and figures. ASO, MMS, ZAM, and AMA wrote the first draft of the manuscript and all authors reviewed and contributed to subsequent drafts and the final report.
Declaration of interests
ASO has received consultancy fees from Gilead, Pfizer, MSD, and ViiV; payment for lectures from Pfizer, MSD, GlaxoSmithKline, and Sanofi-Aventis; and sponsorship to attend international meetings and conferences from MSD, Pfizer, Biopharma, Bristol-Myers Squibb, and Janssen-Cilag. AB has received travel funding to attend an international meeting from Pfizer. GAA has received travel funding to attend an international meeting from Edwards Lifesciences. All other authors declare no competing interests.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control Severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV)–11th update. August 21, 2014. http://www.ecdc.europa.eu/en/publications/Publications/Middle-East-respiratory-syndrome-coronavirus-Saudi%20Arabia-Qatar-Jordan-Germany-United-Kingdom.pdf (accessed Sept 17, 2014).
- 3.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA, Albarrak AM. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J InfectDis. 2013;17:e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assiri A, McGeer A, Perl TM. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi YM, Arifi AA, Balkhy HH. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 7.WHO Clinical management of severe acute respiratory infections when novel coronavirus is suspected: what to do and what not to do. February 11, 2013. http://www.who.int/csr/disease/coronavirus_infections/InterimGuidance_ClinicalManagement_NovelCoronavirus_11Feb13.pdf (accessed June 12, 2014).
- 8.Public Health England. International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Clinical decision making tool for treatment of MERS-CoV v.1.1. July 29, 2013. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317139281416 (accessed June 14, 2014).
- 9.Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 10.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCloskey B, Zumla A, Stephens G, Heymann DL, Memish ZA. Applying lessons from SARS to a newly identified coronavirus. Lancet Infect Dis. 2013;13:384–385. doi: 10.1016/S1473-3099(13)70082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momattin H, Mohammed K, Zumla A, Memish ZA, Al-Tawfiq JA. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)--possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis. 2013;17:e792–e798. doi: 10.1016/j.ijid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falzarano D, de Wit E, Rasmussen AL. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman VM, Muller MA, Costabel U. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:20334. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 16.US Centers for Disease Control and Prevention National Healthcare Safety Network Surveillance definitions for specific types of infections. Jan 1, 2014. http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed June 14, 2014).
- 17.Dellinger RP, Levy M, Rhodes A. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intens Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Ribavirin and interferon (IFN)-alpha-2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus (MERS-CoV): a preliminary report of two cases. [DOI] [PubMed]
- 20.de Wilde AH, Raj VS, Oudshoorn D. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J Gen Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart BJ, Dyall J, Postnikova E. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JF, Chan KH, Kao RY. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyall J, Coleman CM, Hart BJ. Repurposing of clinically developed drugs for treatment of Middle East Respiratory Coronavirus Infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Antivir Ther. 2014 doi: 10.3851/IMP2792. published online May 15. [DOI] [Google Scholar]
- 24.Al-Tawfiq JA, Hinedi K, Ghandour J. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles SR, Phillips EJ, Dresser L, Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis. 2003;37:1139–1142. doi: 10.1086/378304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung JJ, Wu A, Joynt GM. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]