Abstract
Organoids are multicellular culture systems that replicate tissue architecture and function, and are increasingly used as models of viral, bacterial, and protozoan infections. Organoids have great potential to improve our current understanding of helminth interactions with their hosts and to replace or reduce the dependence on using animal models. In this review, we discuss the applicability of this technology to helminth infection research, including strategies of co-culture of helminths or their products with organoids and the challenges, advantages, and drawbacks of the use of organoids for these studies. We also explore how complementing organoid systems with other cell types and components may allow more complex models to be generated in the future to further investigate helminth–host interactions.
Keywords: organoids, helminths, excretory/secretory, models, host–parasite interaction
Organoids to Mimic Host Interactions with Pathogens
Organoids are in vitro multicellular clusters containing various differentiated cell types, capable of self-renewal and organization, and exhibiting some level of functionality and architecture of the tissue of origin [1,2]. Organoids have transformed biomedical research in the past 10 years. Part of this revolution has been in their use for a better understanding of host–pathogen interactions. Organoid models have been used to investigate viral, bacterial, and protozoan parasite infections [1,3, 4, 5, 6].
Helminth infections affect millions of people and livestock, resulting in a major social and economic burden worldwide [7,8]. Despite their impact, research into helminth infections relies heavily on the use of animals and is limited by a lack of models that more closely reproduce the interaction of the parasites with their specific hosts [9]. In vivo model organisms for human and livestock helminth infection have provided invaluable data on the immune response to helminths, parasite genetics, and transmission. Nevertheless, these models are expensive and complex, hindering the study of host–parasite interactions at the molecular level. Moreover, model organisms cannot fully recapitulate human helminth infections. Entirely new systems are therefore needed that allow the processes of invasion and colonization of human helminths to be studied, and that open up new possibilities for testing new drug targets and identifying vaccine candidates. Organoids from different organs infected by helminths during their life cycle (including gut, lung, bladder, and liver) have enormous potential as novel models for studying the interactions of helminths and their products with their hosts. Nevertheless, the size and complexity of helminths pose challenges on the adaptability of organoid cultures for their use in research. Here, we discuss the applicability of this technology. From our recent investigations into the interactions of whipworm larvae with caecal epithelium and evaluations of the host response to excretory/secretory (ES) products (see Glossary), we explore the advantages and pitfalls of using organoid systems.
The implementation of organoid models promises major new avenues for developing new therapeutics and vaccines against helminths, for understanding the anti-inflammatory effects of helminth infections, and for using helminths or their products as alternative therapies to treat inflammatory diseases.
Organoids: What, Whence, and Where to Infection Biology
Organoids can be derived from either primary tissue stem cells or embryonic or induced pluripotent stem cells (PSCs) when cultured in conditions that resemble those of the stem cell niche (SCN) for a specific organ [1,10,11]. The SCN is a specialized, dynamic and restricted microenvironment where the stem cells reside, and it is composed of physical and cellular components [12,13]. The physical niche comprises the extracellular matrix (ECM), the shape and arrangement of cells and mechanical forces, and the cellular niche refers to the resident immune and stromal cells embedded in the ECM that provide signaling clues to maintain stem cell division and differentiation [12,14,15].
Current organoid culture conditions combine an ECM support with exogenous growth factors and morphogens (both promoters and inhibitors) that direct the division and differentiation of daughter cells into the multiple cellular populations present in the targeted organ [1,2,10,11,16]. Organoids derived from embryonic or induced PSCs additionally require germ layer- and lineage-specific-factors to direct their differentiation into several endoderm-, mesoderm-, and ectoderm-derived tissues [1,2,10,11].
Organoid systems were initially developed to recreate murine and human small intestine and colon epithelia, and involved the use of specific culture media cocktails to recreate the SCN conditions for each intestinal segment in the specific species [10,11,17]. Subsequently, organoids have been developed for a great variety of tissues and organs, including stomach, esophagus, liver, lung, pancreas, prostate, brain, kidney, mammary gland, ovary, lingual, taste bud, salivary gland, testis, endometrium, Fallopian tube, lymph nodes, blood vessels, skin, inner ear, and retina [1,2,11,18, 19, 20, 21]. Moreover, intestinal, mammary, keratinocyte, and liver organoids from other animal models already exist, including those of bovine, porcine, ovine, chicken, feline, and canine origin [22].
Currently, organoids are used as models of different pathologies, including infectious diseases caused by viruses, bacteria, and protozoans [3, 4, 5]. Viral infection studies with organoid systems have included: norovirus, rotavirus, enteric adenovirus, and coronavirus invasion of intestinal organoids [3, 4, 5, 6]; herpes simplex virus 1 [23] and cytomegalovirus [24] infection of cerebral organoids; Zika virus infection of cerebral [4,5] and human testicular organoids [25]; human airway organoids to model pathology and assess infectivity of emerging influenza [26], parainfluenza [27] and respiratory syncytial viruses [28]; BK virus infection in human kidney tubuloids [29]; and human liver organoids to study infection with hepatitis B and its related tumorigenesis [30].
Organoid models of bacterial pathogenesis include enterohaemorrhagic, enteroaggregative, and enteropathogenic Escherichia coli, Vibrio cholerae, Salmonella, Clostridium difficile, and Shigella infecting intestinal organoids of mouse, bovine, porcine, and human origin [3, 4, 5, 6,31], as well as Helicobacter pylori colonizing gastric (stomach) organoids [4, 5, 6]. In addition, the involvement of bacteria in adenocarcinoma formation in gallbladders has been studied using murine gallbladder organoids infected with Salmonella [4,5]. Infection with the uropathogen Enterococcus faecalis has been studied in human urothelial organoids [32]. More recently, Fallopian tube organoids have served as a model to study the long-term impact of Chlamydia trachomatis infections in the human epithelium that may contribute to the development of ovarian cancer [18].
Organoids are also gaining in popularity to model protozoal infections. In particular, Toxoplasma gondii infects bovine and porcine small intestinal organoids [31]. Furthermore, the entire life cycle of Cryptosporidium parvum can now be modelled in murine and human small intestinal [33,34] and lung organoids [34]. Lung organoids have successfully recapitulated respiratory tract infections with C. parvum occurring in immune-competent and -deficient individuals [34].
Organoids as a Tool for Studying Helminth Infections
Research on helminth infections has largely focused on the use of model organisms that have helped us to understand the observations made in patient samples collected in the field (Table 1 ) [35]. In recent years, organoids have started to be exploited as a tool to indirectly investigate the effects on the intestinal epithelia of the immune responses triggered by intestinal helminth infections. Specifically, mouse small intestinal organoids have served as an experimental system to demonstrate the expansion of tuft cells as a consequence of ex vivo stimulation with interleukin (IL) 13. IL-13 is secreted by innate lymphoid cells in response to stimulation with IL-25, a cytokine produced by tuft cells during the early response to in vivo infection with the intestinal dwelling nematodes Nippostrongylus brasiliensis, Heligmosomoides polygyrus, and Trichinella spiralis [36, 37, 38, 39]. These findings add to the evidence that helminth infections influence stem-cell proliferation and differentiation towards specific epithelial cellular populations, including the goblet cell and the tuft cell compartments that are key for worm expulsion [38,40]. Comparisons of organoids generated from uninfected and H. polygyrus-infected mice provided further evidence of the impact of helminth infections on stem cell fate within the intestinal crypts. Larval invasion of the submucosal tissue breaches the epithelial barrier, and results in a switch of stem cell phenotype from Lgr5+ to Sca1+, the latter resembling a fetal-like, proliferative, or wound-healing intestinal epithelium profile [41].
Table 1.
Selection of Human and Livestock Helminth Infections and Life Cycle Stages with Potential to Be Studied Using Organoids
| Organ | Helminth | Host | Life cycle stage relevant to organoid models |
|---|---|---|---|
| Stomach (abomasum) | Haemonchus contortus (barber's pole worm) | Sheep | L3 larvae invade epithelium of abomasum and mature to adults that remain attached |
| Small intestine | Ascaris lumbricoides; A. suum (roundworm) | Human; pig | L3 larvae hatch from ingested eggs, invade epithelium, L4 larvae/adults in lumen |
| Toxocara canis, T. cati (roundworm) | Human, dog, cat, rodents, rabbit, birds | L2 larvae hatch from ingested eggs, invade epithelium, L3/L4/ L5 and adults in lumen | |
| Heligmosomoides polygyrus | Mouse | Ingested L3 larvae invade epithelium and submucosa; re-emerge as adults into lumen | |
| Trichinella spiralis (pork worm) | Human, pig, mouse | L1 larvae invade epithelium and mature to adults | |
| Necator americanus, Ancylostoma duodenale (hookworms); Nippostrongylus brasiliensis | Human; mouse, rat | L4 larvae/adult worms in lumen (L3 enter through skin) | |
| Strongyloides stercoralis (thread worm); S. venezuelensis, S. ratti | Human, dog; mouse, rat | L4 larvae/adult worms in lumen (L3 enter through skin) | |
| Taenia saginata, T. solium (tapeworms); T. taeniaeformis, T. crassiceps | Human, pig, ruminants; mouse, rat | Ingested larvae (cysticerci) attach to epithelium, grow to adults | |
| Echinococcus granulosus, E. multilocularis (tapeworms) | Human, dog, cat, cattle, horse, sheep, pig, rodents | Eggs ingested by the first host hatch releasing oncospheres (larvae) that penetrate the epithelium and submucosa. Cysts and protoscolices that are ingested by a second host attach to epithelium and develop into adults |
|
| Fasciola hepatica, F. gigantica (liver fluke) | Human, sheep, cattle, mouse | Newly excisted juvenile (larvae) penetrate the intestinal wall of the duodenum into peritoneal cavity | |
| Small and large intestine | Schistosoma mansoni, S. japonicum (blood fluke) | Human, mouse | Adults in mesenteric veins produce eggs which transit intestinal wall to lumen |
| Large intestine | Trichuris trichiura; T. muris; T. suis (whipworm) | Human; mouse; pig | L1 larvae invade caecal/large intestinal epithelium and mature to adults |
| Skin |
N. americanus, A. duodenale; N. brasiliensis |
Human; mouse, rat | Free-living L3 larvae penetrate unbroken skin |
| S. stercoralis; S. venezuelensis, S. ratti | Human, dog; mouse, rat | Free-living L3 larvae penetrate unbroken skin | |
|
S. mansoni, S. haematobium, S. japonicum |
Human, mouse | Free-swimming cercariae in freshwater penetrate unbroken skin | |
| Lung | T. canis, T. cati | Human, dog, cat, cattle, horse, sheep, pig, rodents | L2/L3 larvae transit lung where may encapsulate or migrate through trachea and oesophagus to gut |
|
N. americanus, A. duodenale; N. brasiliensis; Ascaris spp |
Human; mouse, rat; pig | Developing L3/L4 stages transit lung, migrate through trachea and oesophagus to gut | |
| S. stercoralis; S. venezuelensis, S. ratti | Human, dog; mouse, rat | L3 larvae transit lung, migrate through trachea and oesophagus to gut | |
|
S. mansoni, S. haematobium, S. japonicum |
Human, mouse | Schistosomulae (larvae) develop prior to migration to vascular niche | |
| E. granulosus | Human, dog, cat, cattle, horse, sheep, pig, rodents | Oncospheres (larvae) circulate to the lungs where they develop into cysts and protoscolisces | |
| Liver | T. canis, T. cati | Human, dog, cat, cattle, horse, sheep, pig, rodents | L2 larvae transit liver where may encapsulate or migrate to the lung |
| S. mansoni, S. japonicum | Human, mouse | Eggs frequently trapped in liver, pathogenic | |
| E. granulosus, E. multilocularis | Human, dog, cat, cattle, horse, sheep, pig, rodents | Oncospheres (larvae) circulate to the liver where they develop into cysts and protoscolisces | |
| F. hepatica, F. gigantica | Human, sheep, cattle, mouse | Migrating juvenile and adults | |
| Brain | T. solium, Mesocestoides corti | Human, mouse | Cysts form in brain |
| Bladder | S. haematobium | Human, mouse | Eggs from adults breach barrier to reach urinary tract |
| Lymphatics/blood vessels | Brugia malayi (and other lymphatic filariae) | Human | Adults live in lymphatic system, microfilariae in peripheral blood |
Organoids have also been utilized in experiments that aim to characterize the interactions and intestinal epithelial responses to ES products of various nematodes. In particular, stimulation of murine small intestine organoids with ES products and extracts of T. spiralis showed that sensing of parasitic products by tuft cell receptors results in Ca2+ responses [39]. Moreover, imaging experiments of murine small intestine and colon organoids, microinjected with exosome-like extracellular vesicles (EVs) present in the ES of N. brasiliensis and Trichuris muris, respectively, showed their internalization by host epithelial cells [42,43]. A similar approach has been taken to visualize the uptake of Ascaris suum EVs cocultured with canine intestinal organoids [44]. However, to date, an organoid system has not been exploited to study direct interactions (and the molecular basis of those interactions) between host epithelia and live helminths.
Various life cycle stages of helminths infect organs for which organoids of human, murine, porcine, and canine origin exist (Table 1). However, the use of organoids is currently restricted to investigations of the interactions with invasive parasitic stages or ES products from different life cycle stages, because co-cultures with established adult stages would require long-term culture and, therefore, cannot yet be recreated (discussed later).
Comparing Organoids with Other Models to Study Helminths: In Vitro, Ex Vivo, and In Vivo
In vitro models for helminth infections are based mainly on the use of cell lines. In particular, some host interactions with intestinal helminths have been modelled with commonly used cell lines (T84, Caco-2, and HT-29) that enable small intestine and colon epithelial function to be studied [6]. For high-throughput screening, biobanking and genetic manipulation, cell lines are excellent tools. However, they are generated by immortalization of cancerous cells, severely limiting their use as systems to understand physiological processes in normal/healthy tissues [2,6].
To date, epithelial and fibroblast cell lines of diverse origin (small intestine, colon, kidney, lung, among others) have been used to study the invasion and early colonization of T. spiralis [45,46]. Prolonged cultures of L1 larvae of T. spiralis in Caco-2 cells sustain moulting, ecdysis, development to adulthood and reproduction of the parasite [47]. T. spiralis also invades 2D cultures of mouse embryonic small intestinal cells [48].
Cell lines and primary small intestine and colonic epithelial cells have also been used to study the responses of epithelial cells to H. polygyrus [49], T. muris [50,51], T. spiralis [39], and Ascaris ES products and EVs [52]. While the use of cellular preparations ex vivo allows experiments to be performed with the different cell populations present in the tissue, the isolation procedures disrupt the architecture and polarization of the cells in the tissue, making it difficult to interpret the results. In addition, ex vivo cultures are short-lived, restricting their use to experiments that evaluate only very early interactions.
Organoids are an excellent alternative, bridging in vitro, ex vivo, and in vivo models (Table 2 ). Organoids derived from a single individual can be expanded and cryopreserved as individual lines, allowing several experiments to be performed using the same starting material [1,10]. Moreover, organoids can be grown in a 2D and 3D conformation, and, in contrast to traditional cell lines, they retain organ specificity and maintain genome stability, allowing their biobanking [2,6]. These features make them amenable to high-throughput screening, although it is important to consider that experiments using organoids are currently subject to variability due to inherent heterogeneity in size, shape, structural organization and functional capacity, and batch variability of both the ECM and growth factors used in culture [3,16,53].
Table 2.
Advantages and Drawbacks of Organoids to Study Helminth–Host Interactions
| Advantages | Disadvantages |
|---|---|
| Experimental | |
|
|
| Technical | |
|
|
Compared with animal models, organoids are much less complex because they lack vascularization, innervation, interactions with other cells present in the organ, and microbiota. However, organoids do enable aspects of human physiology and disease to be studied that cannot be modelled in animals due to significant interspecies differences [2]. Most helminths are host-restricted and, in such cases, animal models are of limited use in the understanding of helminth pathogenesis. Organoids derived from the specific host could overcome this issue by providing a model with increased relevance for the specific infection; for instance, using human organoids and the human infective nematode Trichuris trichiura, which is currently experimentally intractable, could replace some studies in mice using T. muris.
The multicellular composition of organoids is critical for studying the varying roles of specific cell types in colonization, replication, transmission, host damage, and clearance of pathogens. These studies are not achievable with cell lines that often present only one or two types of cells present in the organ of interest [6]. Moreover, 'wild-type' organoids can be genome-edited using lentiviral expression systems or CRISPR/Cas9, a process that, in animal models, requires embryo mutagenesis and breeding. An additional advantage of organoids is that they can be generated from patient-derived stem cells, allowing the long-term impact of pathogen infections on histological features and physiological phenotypes to be studied.
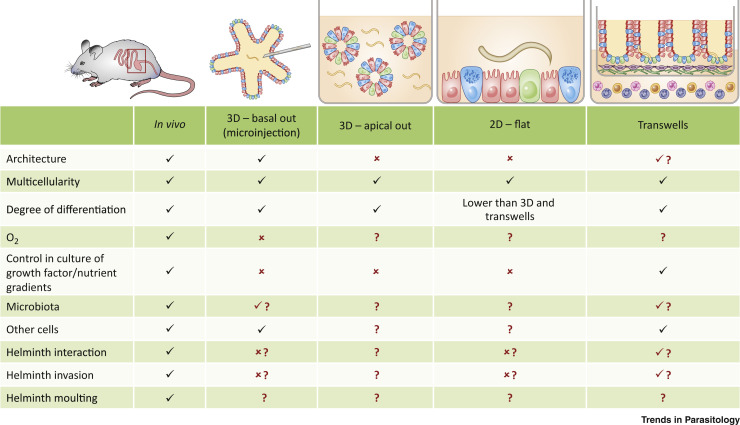
The use of organoids in experiments with live helminth parasites requires careful consideration of the different components of the in vivo host–parasite interaction that this system can potentially recreate (Figure 1 , Key Figure) [3]. Those include specific host molecular structures sensed by helminths that are defined by tissue architecture, polarization, and multicellularity; environmental clues such as the presence of commensal microbiota, gas exchange, and nutrient gradients; and physiologically relevant biomechanical forces (e.g., fluid shear, stretch, compression) [3,6]. In addition, it is crucial to consider the life cycle stage of the parasite to be used in organoid studies as this impacts the mechanistic and physiological experimental conditions to be used. Next, we discuss our own insights on the use of intestinal organoids to model host–helminth interactions.
Figure 1.
Key Figure. Comparison of In Vivo Models and Organoid Systems on the Degree of Modelling of the Components of the In Vivo Host–Helminth Interaction
In vivo models allow a holistic recreation of the conditions required for parasite infection, including tissue architecture, oxygenation, multicellularity, cell differentiation and growth factor and nutrient gradients, colonization by local microbiota, and interactions with other cells that create a unique microenvironment hosting the parasite. Organoids can be grown in 2D and 3D conformations mimicking to some extent the components of the in vivo host–parasite interaction. '✓': fully recreated; '✗': not modelled; '?': condition modelling not known or investigated; '✓?': conditions that have been recreated to certain extent; '✗?': those that have not been successfully modelled by the authors, but are still to be tested in work with other helminths.
How 2D and 3D Conformations of Organoids Recreate In Vivo Conditions
Organoids can be utilized in their 3D conformation, when grown embedded in an ECM, or as 2D monolayers grown over plasticware or permeable supports/transwells coated with ECM [3, 4, 5, 6,54,55]. The 2D models require numerous 3D organoids to be dissociated into single cells. The resulting single cells are then grown as a monolayer that requires further culturing before being differentiated [3, 4, 5, 6,54,55]. In our experience, when the cells of intestinal organoids are cultured over plasticware they differentiate into the major cell types of the organ, but do not present any organization. In contrast, cultures grown on permeable supports/transwells have a more physiologically relevant appearance to those grown on conventional plasticware [3], and promote the differentiation of cells and their organization in a specific conformation that, to an extent, resembles intestinal crypts. Moreover, culturing on permeable supports/transwells allows the independent control of apical and basolateral surfaces, as cells grown on these conditions polarize [6,54,55]. This permits the creation of nutrient/growth factor gradients and an air–liquid interface that promotes cell differentiation, and the co-culture with other cell types. Nevertheless, organoids grown in a 2D fashion lack any semblance of the organ architecture and self-organization that characterize 3D organoids and in vivo conditions (Figure 1).
A recent publication has shown that polarity of 3D gastrointestinal organoids can be controlled [56]. When organoids are cultured embedded in ECM they present a basal-out conformation with the apical side facing the lumen of the organoid. This can be reversed to an apical-out conformation by ECM removal and culture in suspension. The barrier function and major cell types are still maintained, and the conformation facilitates the exposure of the apical side to nutrients, drugs, and pathogens [56].
New engineering-based approaches for control over organoid composition, size, spatial organization, luminal accessibility, chemical gradients, and biomechanical forces are being developed [1,3,15,53,57,58]. These include 3D-bioprinting, chemically programmed assembly and defined ECM, microwell arrays, droplet-based microfluidics, peristaltic pumps, and bioreactors [3,15,16,58,59]. These approaches offer increasing control over physiological conditions and will enable a better understanding of how tissue architecture influences the cellular responses during helminth infection.
Strategies of Co-culture of Helminths or Their Products with Organoids
The dimensional conformation in which organoids are grown determines the accessibility and methods used for their co-culture with pathogens or their products [5]. Moreover, the size of the life stage of the helminth of interest impacts the strategy of delivery to be utilized. Sizes range from a few hundred micrometers in the eggs and infective stages (L1/L3 larvae, cercariae) up to meters in the adults of some cestodes. Thus, a combination of parasite size and organoid dimensional conformation determines the extent to which the co-culture/infection can be controlled, both in terms of the number of parasites and concentration of ES products, as well as the conditions required for potential hatching of eggs, or activation of larvae/adults and their invasion (such as oxygen levels, presence of host molecular structures, cellular polarization/architecture, and microbiota). For pathogens other than helminths, there are no reports on the impact of the dimensional conformation and architecture of organoids on the interactions with the host cells. Indeed, the life cycle of the protozoan parasite C. parvum can be replicated both in 3D and 2D organoids [33,34].
3D Organoids
Until recently, basal-out 3D organoids were the only approach available to perform studies on host–pathogen interactions while preserving the cellular architecture of the organoids. Basal-out 3D organoids have restricted luminal access, thus requiring delivery of parasites or their products directly into the lumen of organoids by microinjection. Microinjection is technically challenging, slow, and labor-intensive [3]. This technique poses problems with delivery depending on organoid size, as the number of cells per organoid and luminal volumes vary, making it difficult to perform precise quantitative experiments [3,5,6]. The volume injected also depends on the accumulation of mucus and cell debris within the enclosed organoid lumen, which restricts access to the apical surface of the epithelia [56]. In addition, microinjection can result in spillage of the cargo into the basolateral space that can lead to confounding results [56].
For microinjection, it is important to consider the diameter of the injecting needle needed to penetrate the organoid without causing major damage. Therefore, in the case of live helminth microinjection, the diameter of the needle limits the life stage of the parasite that can be injected. In our experience, microinjection is viable for ES products and larvae only (eggs and adults are too large), and is not amenable to high-throughput approaches because larvae often block the microinjecting needle. Upon microinjection of the parasites, it is important to consider the luminal conditions in the organoid, as low levels of oxygen (varying from approximately 3–10% [60]) and accumulation of dead cells can impact parasite ability to survive and invade (Figure 1).
Apical-out 3D gastrointestinal organoids offer the possibility of co-culturing with parasites or their products in suspension, potentially offering greater control over delivery, a key factor for quantitative experiments [56]. Nevertheless, when using apical-out 3D organoids the conditions required for parasite invasion need additional consideration; for instance, the nematode T. spiralis requires a semi-solid medium to invade host cells in vitro [45,46]. Furthermore, apical-out 3D organoids lack a lumen, they release mucus into the culture media, and do not seem to recreate the epithelial architecture as budding structures are missing [56]. All of these features may impact on parasite invasion and active infection.
2D Organoid Cultures
While 2D organoid cultures lack the cellular architecture that may have an important role on helminth invasion [5], they enable apical and quantitative exposure to parasites and their products to a layer of cells that, in the case of gastrointestinal organoids, is polarized and that produces and maintains a mucus layer. An additional advantage of this system is the better oxygenation of the culture, compared with 3D organoids where the low concentrations of oxygen in the lumen may be detrimental to parasite viability. Moreover, permeable supports/transwells permit access to the basolateral compartment, so that growth factor or nutrient gradients can be established, and other cells can be co-cultured, such as immune cells, to better reproduce the native microenvironment. Unfortunately, a current major limitation of this system is that live imaging of the transwells is currently not possible without disassembling the permeable support [56].
In theory, 2D organoid cultures allow interactions to be studied with many different life stages of helminth parasites and their ES products. However, it is unlikely that adult parasites will actively infect cells that, under natural conditions, would be invaded by larval stages (with subsequent moult and then development into adults in tissue). Moreover, upon successful infection of both 2D and 3D organoids by helminth larvae, it is implausible that these systems can support moulting of parasites to reach adult stage; the time interval for moulting exceeds the 48–72 h interval for splitting organoid cultures required for their culture maintenance. Moreover, in the case of 3D organoids, they grow to only a few millimeters in size due to the limitations of passive diffusion of oxygen and nutrients, and their luminal space may constrain the size of the parasite that can be contained (Figure 1).
Consequently, current organoid systems to study interactions of live helminths with their host through their life cycle may be restricted to specific parasitic stages. In the future, advances in engineering-based approaches for organoids, including vascular systems to promote extended organoid growth and function [16,53], will potentially support the development of long-term organoid cultures that could support in vitro life cycles of helminth parasites.
Complementing the System for Future Applications
While organoids are more physiological and complex models than cell lines, they are still reductionistic systems with consequent limitations on the interpretation of results obtained with them. In the future, complementation of organoids with the tissue components that they currently lack will be required to better mimic the host–helminth interactions regulating infection in the native microenvironment [6]. Those components include the SCN, the commensal microbiota of the specific organ, and chemical gradients and physical/mechanical forces [1,3,53].
For instance, the intestinal SCN influences stem cell behavior to govern intestinal homeostasis under physiological and pathological conditions [13] and plays a pivotal role in responses to infection [41,61]. The intestinal microbiota and their by-products shape the architecture of the intestinal epithelia [62,63], and influence the intestinal SCN renewal and differentiation, through stimulation of stromal, epithelial, and immune cells [5,63]. Moreover, during infection with intestinal pathogens, the microbiota influences their establishment in the tissue and the development of immune responses [40,64].
Hence, improving the state-of-the-art culture of intestinal organoids by complementation with stromal (including fibroblasts, myofibroblasts, telocytes, pericytes, neural, endothelial, and smooth muscle cells) and immune cells, and microbiota in vitro, will illuminate the intricate interactions of these components during infection with intestinal helminths (Figure 1).
Co-culture of organoids, both in 2D and 3D conformation, with other components of the intestinal SCN has been described with interesting results. Fibroblasts promote the growth of intestinal and esophageal organoids [65, 66, 67]. Similarly, neural cells induce stem cell division of gastric [68] and intestinal [69,70] organoids, while also supporting tuft cell survival [69]. Co-culture with immune cells includes: (i) intraepithelial lymphocytes that proliferate and incorporate in the organoid epithelium [71]; (ii) macrophages that enhance barrier function and maturity of enteroid monolayers and respond to infections with E. coli [72]; (iii) lamina propria lymphocytes that mediate commensal protection of the epithelium [73]; and (iv) dendritic cells that alter stem cell fate by inhibiting secretory lineage and drive absorptive lineage [74]. The anaerobic nature of the majority of the members of the intestinal microbiota complicates its co-culture in 2D organoid systems that grow under normal oxygen concentrations [5,75]. However, introduction of some oxygen-tolerant microbiota members (and their components and by-products), in organoid cultures and other intestinal models, has allowed their influence on the intestinal SCN to be studied [62,73,75].
Co-cultures of induced PSC-derived organoids and other cells, such as vascular and neural cell types, to simulate embryonic organogenesis, results in their own 3D self-organization promoting higher order function [53]. However, for organoids of other origin, permeable supports/transwell systems and novel microengineered culture scaffolds have the potential to increase the complexity of the organoid models by allowing stromal cells to be added, such as fibroblasts and nerves, as separate matrix layers in a sandwich-style model [6,58,59,65,67]. These systems also permit the creation of air and nutrient gradients and the co-culture with immune cells to address their interactions with intestinal epithelial cells during infection (Figure 1).
Complementation of organoid cultures from other organs with their own SCN and tissue components will equally be crucial in the implementation of organoid systems as models to study helminth infections. Recent models include spatial separation of different cell types, microchannels, and fluidic systems that mimic perfusion, oxygen and nutrient supply, and mechanical cues simulating the tissue-specific microenvironment and further promoting organoid maturation and self-organization [16,53]. In the future, more sophisticated modelling systems such as organ-on-chip approaches will integrate organoids [16] and provide more complex tools to recreate and dissect the molecular mechanisms governing host–helminth interactions.
Concluding Remarks
The use of organoids in helminth research is in its infancy, but it offers an exciting approach to complement other experimental systems to better understand the interactions of helminth parasites with their specific hosts at a molecular level. The opportunity that organoids provide for working with human or livestock material, and overcoming the limitations of model organisms, is also invaluable. Moreover, the features of organoids that allow expansion and cryopreservation of organoid lines will certainly promote collaborations among laboratories studying diverse helminths, having a substantial impact in reducing the number of animals used in research and in the fight against the neglected diseases caused by helminth parasites.
While the use of organoids requires experimental fine-tuning to recreate and promote the invasion and colonization by helminth parasites, the field is quickly evolving and providing new engineering solutions to better mimic the organs that are naturally targeted (see Outstanding Questions). At present, most of the developments are focused on organoids derived from gastrointestinal tissues and will benefit investigations on helminths that reside in those organs; nevertheless, we foresee a broad application of organoids derived from other organs in the study of helminth–host interactions. Importantly, interacting molecules at the host–helminth interface will provide good candidates for novel antiparasitic interventions such as vaccine development or targets for drug discovery – and the organoid system provides an experimental set-up suitable for screening approaches. Drug discovery not only includes those to treat helminth infections, but also medicaments that target chronic noninfectious diseases resulting from a dysregulated immune system, such as inflammatory bowel disease (IBD), for which alternative therapies with helminth-derived compounds are currently being developed [43,76].
Outstanding Questions.
Which other variables (e.g., mucus composition and hormone levels) affect the establishment of infection and colonization by helminths and need to be considered when recreating these processes in organoids?
Are organoids from specific genetic backgrounds required to reproduce infections with certain helminths?
Can organoids support the growth and moulting of helminths that characterize their life cycle stages? What are the constraints limiting longer term organoid culture, and how can these be rectified to accommodate the development of parasites through their life cycle?
Will the integration of organoids derived from different organs such as the lung, liver, and intestine (body-on-a-chip) sustain complex helminth life cycles, for example, hookworms and schistosomes?
To which scale can organoids sustain the screening of new drugs for antihelminth therapy and the testing of the anti-inflammatory effects of helminths on chronic non-infectious diseases?
Can we develop organoids from helminth tissue to further explore the fundamental biology of parasites?
Acknowledgments
We thank Jose A. Dianes-Santos for design of graphic illustrations.
Glossary
- Excretory/secretory (ES) products
soluble mediators released by helminths, either actively exported through secretory pathways or diffused or leaked from the parasite soma as a consequence of physiological process such as digestion. ES products may interact with host tissues and are involved in parasite invasion and modulation of host immune responses.
- Extracellular matrix (ECM)
3D network of extracellular hydrated macromolecules, such as collagen and glycoproteins, present within all tissues and organs. ECM provides essential physical scaffolding for cells, together with the biochemical and biomechanical support required for tissue morphogenesis, differentiation, and homeostasis.
- Extracellular vesicles (EVs)
lipid bilayer-enclosed nanoparticles released by cells and carrying complex cargoes, including proteins, lipids, and nucleic acids involved in intercellular, interorganismal, and even interspecies communication.
- Goblet cells
a mucus-producing cell population of the intestinal and bronchial tract epithelium.
- Inflammatory bowel disease (IBD)
chronic inflammatory disorder of the gastrointestinal tract comprising two main clinical forms: Crohn's disease and ulcerative colitis. IBD is associated with multiple pathogenic factors, including genetic variants conferring susceptibility, a dysregulated immune response, abnormal gut microbiota, and environmental changes.
- Microbiota
all the microorganisms residing in a specific niche such as the human gut.
- Microinjection
a method of delivery of substances into a tissue, organoid, or a single cell by injection under the microscope using a glass micropipette.
- Organ-on-chip
microfabricated in vitro cell culture devices that model functional units of organs. They are composed of multiple, individually reachable microchambers where various cell types are grown and that are connected through microfluidics that allow the control of the culture microenvironment.
- Pericytes
mural cells of the vasculature that wrap around the endothelial cells that line the capillaries, arterioles and venules throughout the body.
- Permeable support/transwell
cell culture device, also known as cell culture inserts, composed of a porous membrane which hangs in the centre of an outer well and provides independent access to both sides of a monolayer.
- Pluripotent stem cells (PSCs)
stem cells with the capacity to differentiate into any type of cell of all three germ layers: ectoderm, endoderm, and mesoderm. They include both embryonic and induced PSCs. Embryonic PSCs are derived from the inner cell mass of the blastocyst of an early-stage preimplantation embryo. Induced PSCs refer to those derived from adult somatic cells that are reprogrammed to an embryonic stem-cell-like state.
- Primary tissue stem cells
stem cells present in organs from adult organisms and with the capacity to divide and differentiate into the different cell types present in the organ of origin.
- Stromal cells
group of connective tissue cells of an organ that provide support, structure, and anchoring of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes.
- Telocyte
a particular type of stromal cell presenting extremely long and thin prolongations named telopodes.
- Tubuloids
primary kidney tubular epithelial organoids representing proximal as well as distal nephron segments.
- Tuft cells
chemosensory cells with blunt apical microvilli, found in the thymus and hollow organs or tubes lined by an epithelium such as the urinary tract, the digestive and respiratory systems.
References
- 1.Fatehullah A., et al. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 2.Li M., Izpisua Belmonte J.C. Organoids – preclinical models of human disease. N. Engl. J. Med. 2019;380:569–579. doi: 10.1056/NEJMra1806175. [DOI] [PubMed] [Google Scholar]
- 3.Barrila J., et al. Modeling host–pathogen interactions in the context of the microenvironment: three-dimensional cell culture comes of age. Infect. Immun. 2018;86 doi: 10.1128/IAI.00282-18. e00282–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta D., Clevers H. Organoid culture systems to study host–pathogen interactions. Curr. Opin. Immunol. 2017;48:15–22. doi: 10.1016/j.coi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta D., et al. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 6.In J.G., et al. Human mini-guts: new insights into intestinal physiology and host–pathogen interactions. Nat. Rev. Gastroenterol. Hepatol. 2016;13:633–642. doi: 10.1038/nrgastro.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethony J., et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 8.Charlier J., et al. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30:361–367. doi: 10.1016/j.pt.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Aebischer T., et al. Editorial: parasite infections: from experimental models to natural systems. Front. Cell Infect. Microbiol. 2018;8:12. doi: 10.3389/fcimb.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Date S., Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu. Rev. Cell Dev. Biol. 2015;31:269–289. doi: 10.1146/annurev-cellbio-100814-125218. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 12.Meran L., et al. Intestinal stem cell niche: the extracellular matrix and cellular components. Stem Cells Int. 2017;2017:7970385. doi: 10.1155/2017/7970385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voog J., Jones D.L. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoshkes-Carmel M., et al. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murrow L.M., et al. Dissecting the stem cell niche with organoid models: an engineering-based approach. Development. 2017;144:998–1007. doi: 10.1242/dev.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S.E., et al. Organoids-on-a-chip. Science. 2019;364:960–965. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 18.Kessler M., et al. Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nat. Commun. 2019;10:1194. doi: 10.1038/s41467-019-09144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wimmer R.A., et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565:505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J., et al. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. 2018;22:242–254. doi: 10.1016/j.celrep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenti E., et al. Therapeutic regeneration of lymphatic and immune cell functions upon lympho-organoid transplantation. Stem Cell Rep. 2019;12:1260–1268. doi: 10.1016/j.stemcr.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Augustyniak J., et al. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. 2018;39:1610–1622. doi: 10.1002/jat.3815. [DOI] [PubMed] [Google Scholar]
- 23.D'Aiuto L., et al. Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J. Virol. 2019;93 doi: 10.1128/JVI.00111-19. e00111-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown R.M., et al. Human cytomegalovirus compromises development of cerebral organoids. J. Virol. 2019 doi: 10.1128/JVI.00957-19. Published online September 13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strange D.P., et al. Human testicular organoid system as a novel tool to study Zika virus pathogenesis. Emerg. Microbes Infect. 2018;7:82. doi: 10.1038/s41426-018-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J., et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc. Natl Acad. Sci. U. S. A. 2018;115:6822–6827. doi: 10.1073/pnas.1806308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porotto M., et al. Authentic modeling of human respiratory virus infection in human pluripotent stem cell-derived lung organoids. mBio. 2019 doi: 10.1128/mBio.00723-19. Published online May 7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachs N., et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38:e100300. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutgens F., et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 2019;37:303–313. doi: 10.1038/s41587-019-0048-8. [DOI] [PubMed] [Google Scholar]
- 30.De Crignis E., et al. Human liver organoids; a patient-derived primary model for HBV infection and related hepatocellular carcinoma. bioRxiv. 2019 doi: 10.1101/568147. Published online March 5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derricott H., et al. Developing a 3D intestinal epithelium model for livestock species. Cell Tissue Res. 2019;375:409–424. doi: 10.1007/s00441-018-2924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horsley H., et al. A urine-dependent human urothelial organoid offers a potential alternative to rodent models of infection. Sci. Rep. 2018;8:1238. doi: 10.1038/s41598-018-19690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilke G., et al. A stem-cell-derived platform enables complete cryptosporidium development in vitro and genetic tractability. Cell Host Microbe. 2019;26:123–134 e8. doi: 10.1016/j.chom.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo I., et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018;3:814–823. doi: 10.1038/s41564-018-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inclan-Rico J.M., Siracusa M.C. First responders: innate immunity to helminths. Trends Parasitol. 2018;34:861–880. doi: 10.1016/j.pt.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerbe F., et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howitt M.R., et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Moltke J., et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo X.C., et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl Acad. Sci. U. S. A. 2019;116:5564–5569. doi: 10.1073/pnas.1812901116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grencis R.K. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu. Rev. Immunol. 2015;33:201–225. doi: 10.1146/annurev-immunol-032713-120218. [DOI] [PubMed] [Google Scholar]
- 41.Nusse Y.M., et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature. 2018;559:109–113. doi: 10.1038/s41586-018-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichenberger R.M., et al. Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication. J. Extracell. Vesicles. 2018;7:1428004. doi: 10.1080/20013078.2018.1428004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eichenberger R.M., et al. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. Immunol. 2018;9:850. doi: 10.3389/fimmu.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandra L., et al. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biol. 2019;17:33. doi: 10.1186/s12915-019-0652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ManWarren T., et al. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect. Immun. 1997;65:4806–4812. doi: 10.1128/iai.65.11.4806-4812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C.K., et al. Production of proinflammatory cytokines and inflammatory mediators in human intestinal epithelial cells after invasion by Trichinella spiralis. Infect. Immun. 1998;66:2200–2206. doi: 10.1128/iai.66.5.2200-2206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gagliardo L.F., et al. Molting, ecdysis, and reproduction of Trichinella spiralis are supported in vitro by intestinal epithelial cells. Infect. Immun. 2002;70:1853–1859. doi: 10.1128/IAI.70.4.1853-1859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren H.J., et al. Normal mouse intestinal epithelial cells as a model for the in vitro invasion of Trichinella spiralis infective larvae. PLoS One. 2011;6:e27010. doi: 10.1371/journal.pone.0027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coakley G., et al. Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep. 2017;19:1545–1557. doi: 10.1016/j.celrep.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowcutt R., et al. A role for the pattern recognition receptor Nod2 in promoting recruitment of CD103+ dendritic cells to the colon in response to Trichuris muris infection. Mucosal Immunol. 2014;7:1094–1105. doi: 10.1038/mi.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.deSchoolmeester M.L., et al. The innate immune responses of colonic epithelial cells to Trichuris muris are similar in mouse strains that develop a type 1 or type 2 adaptive immune response. Infect. Immun. 2006;74:6280–6286. doi: 10.1128/IAI.01609-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakobsen S.R., et al. Effects of Ascaris and Trichuris antigens on cytokine production in porcine blood mononuclear and epithelial cells. Vet. Immunol. Immunopathol. 2019;211:6–9. doi: 10.1016/j.vetimm.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Takebe T., Wells J.M. Organoids by design. Science. 2019;364:956–959. doi: 10.1126/science.aaw7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozuka K., et al. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep. 2017;9:1976–1990. doi: 10.1016/j.stemcr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon C., et al. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7:818–828. doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Co J.Y., et al. Controlling epithelial polarity: a human enteroid model for host–pathogen interactions. Cell Rep. 2019;26:2509–2520 e4. doi: 10.1016/j.celrep.2019.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim R., et al. Formation of arrays of planar, murine, intestinal crypts possessing a stem/proliferative cell compartment and differentiated cell zone. Lab. Chip. 2018;18:2202–2213. doi: 10.1039/c8lc00332g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., et al. Formation of human colonic crypt array by application of chemical gradients across a shaped epithelial monolayer. Cell Mol. Gastroenterol. Hepatol. 2018;5:113–130. doi: 10.1016/j.jcmgh.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., et al. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol. Gastroenterol. Hepatol. 2017;4:165–182 e7. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okkelman I.A., et al. Live cell imaging of mouse intestinal organoids reveals heterogeneity in their oxygenation. Biomaterials. 2017;146:86–96. doi: 10.1016/j.biomaterials.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 61.Haber A.L., et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos A.J.M., et al. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 2018;28:1062–1078. doi: 10.1016/j.tcb.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peck B.C.E., et al. Gut microbial influences on the mammalian intestinal stem cell niche. Stem Cells Int. 2017;2017:5604727. doi: 10.1155/2017/5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapin A., Harris N.L. Helminth–bacterial interactions: cause and consequence. Trends Immunol. 2018;39:724–733. doi: 10.1016/j.it.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Kalabis J., et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat. Protoc. 2012;7:235–246. doi: 10.1038/nprot.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ootani A., et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pastula A., et al. Three-dimensional gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the gastrointestinal stem cell niche. Stem Cells Int. 2016;2016:3710836. doi: 10.1155/2016/3710836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao C.M., et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westphalen C.B., et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Workman M.J., et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nozaki K., et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol. 2016;51:206–213. doi: 10.1007/s00535-016-1170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noel G., et al. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host–pathogen interactions. Sci. Rep. 2017;7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hou Q., et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ihara S., et al. Adhesive interactions between mononuclear phagocytes and intestinal epithelium perturb normal epithelial differentiation and serve as a therapeutic target in inflammatory bowel disease. J. Crohns Colitis. 2018;12:1219–1231. doi: 10.1093/ecco-jcc/jjy088. [DOI] [PubMed] [Google Scholar]
- 75.Baker K. Organoids provide an important window on inflammation in cancer. Cancers (Basel) 2018;10:158. doi: 10.3390/cancers10050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sobotkova K., et al. Helminth therapy – from the parasite perspective. Trends Parasitol. 2019;35:501–515. doi: 10.1016/j.pt.2019.04.009. [DOI] [PubMed] [Google Scholar]