Highlights
-
•
Nasal aspirates were subjected to real-time PCR to detect 16 respiratory viruses.
-
•
One or more viruses were detected in 83% of specimens.
-
•
Rhinoviruses were the most frequently detected viruses.
-
•
Seasonal distribution was seen for each virus.
-
•
The clinical severity did not differ among main respiratory viral infections.
Abbreviations: PCR, polymerase chain reaction; RS virus, respiratory syncytial virus
Keywords: Respiratory virus, Real-time PCR, Multiple infection, Human rhinovirus, Clinical severity
Abstract
Background
Using the polymerase chain reaction (PCR) method it is possible to detect uncultivable viruses and discover multiple viral infections. However, the clinical importance of these findings in relation to symptoms is not known.
Objectives
The seasonal fluctuations of respiratory viruses and the clinical outcomes of single infections and dual infections were investigated.
Study design
Nasal aspirate samples were obtained from outpatients and inpatients of a children’s hospital and these samples were subjected to real-time PCR to detect 16 respiratory viruses. Seasonal variations of the 16 viruses and the clinical outcomes such as wheezing, the need for oxygenation and prolonged hospitalization of patients with single viral infections and multiple infections were determined for the 5 most often detected viruses.
Results
Among 512 specimens analyzed, one or more viruses were detected in 424 (83%) specimens. Two or more viruses were detected in 160 samples (31% of all samples). The epidemic peaks of the viruses did not coincide with each other. Rhinoviruses were the most frequently detected viruses and their coinfection rates were also higher. However, the disease severity in the lower respiratory tract did not differ in most respiratory viral infections regardless of whether there was single infection or dual infection with a rhinovirus and other respiratory virus.
Conclusions
Seasonal distribution was seen for each virus. There were no significant differences in clinical symptoms in the children studied. Because the infection of rhinoviruses is the common occurrence in children, it is hypothesized that the factors related to disease severity are mainly the underlying conditions of the children.
1. Background
Respiratory tract infections are frequently seen in children and a significant number of these infections are caused by viral pathogens [1], [2]. Especially for infants, viral respiratory infections carry a high risk for severe symptoms resulting in hospitalization. There is a strong correlation between viral bronchiolitis in infants and wheezing later in childhood [3]. However, most children show mild symptoms during viral respiratory infections involving only the nose and upper respiratory passages. Moreover, clinically useful antivirals do not exist for most such viruses and it is thought that for viral respiratory infections it is not necessary to examine the pathogen.
Recently, nucleic acid amplification tests such as PCR are increasingly being used to diagnose viral respiratory tract infections. Several studies have shown that most common respiratory viruses have epidemic seasons in many areas [4], [7]. PCR makes it possible to detect uncultivable viruses such as human bocavirus and rhinovirus C and discover concurrent viral infections. However, the clinical importance of these findings with regard to symptoms is not known. Some reports indicate that human “classical” subtypes of coronavirus, OC43, NL63, 229E and HKU-1, have low impacts on respiratory health [8], [9].
2. Objectives
In this study, separate real-time PCR assays were used to detect 16 respiratory viruses in nasal aspirates taken from pediatric patients and we investigated the seasonal fluctuations of the respiratory viruses. We also compared the clinical outcomes such as wheezing, the need for oxygenation and prolonged hospitalization, for patients with single and multiple viral infections.
3. Study design
3.1. Patients and samples
From week seventeen 2013 to week sixteen 2014, nasal aspirate samples were obtained from outpatients and inpatients of a children’s hospital. Their symptoms were systematically recorded by the attending physicians. Written informed consent was obtained from the parents. Of the 513 samples obtained, 1 specimen was excluded because of withdrawal of approval.
The median age of the patients was 1y (range 0–14 years). Age groups were: 0 year 35.9% (n = 184), 1 year 32.4% (n = 166), 2 years 11.9% (n = 61), 3years 7.2% (n = 37), 4 years 5.6% (n = 30), and ≥5 years 6.8% (n = 35). The proportion of females was 41.4%.
3.2. Molecular analysis
Each sample was amplified using primers and probes specific for each of the targets as previously described [10]. Briefly, nucleic acids were extracted from 200 μL specimens using the Magtration System with a MagDEA viral DNA/RNA 200 kit (Precision System Science Co., Ltd., Chiba, Japan) with a 50 μL elution volume. RT reactions were performed using a ReverTra Ace qPCR RT kit (Toyobo Co., Ltd., Osaka, Japan) following the manufacturer’s instructions. The cDNA was then amplified using Realtime PCR Master Mix (Toyobo) with a total volume of 25 μL. The sensitivity of each of the real-time PCR methods was reported previously [10]. Enteroviruses and rhinoviruses were genotyped by direct sequencing. Amplification of the VP4/VP2 region of the enterovirus or rhinovirus for typing was performed with semi-nested RT-PCR as previously described [11]. The purified PCR products were subjected to direct sequencing with a BigDye Terminator v1.1 kit as per the manufacturer’s instructions (Applied Biosystems, CA, USA). Sequence analysis was performed using the DNADynamo program (Blue Tractor Software, UK). Using MEGA5.2 (Tamura et al., 2011, Ver5.2.2), we employed the neighbor-joining method [12] to construct phylogenetic trees from the VP4/VP2 region (420 nt) sequences of prototype isolates of each rhinovirus type commonly used in epidemiologic studies of human rhinoviruses retrieved from GenBank [13], [14], [15] and new types proposed previously [13], [16], [17]. Genotypes were assigned on the basis of their clustering with known prototype reference strains.
3.3. Statistics
The Kruskal–Wallis test, Mann–Whitney U-test and Fisher’s exact test were used for comparisons. For all analyses, a p-value of less than 0.05 was considered significant. Statistical analysis was performed using SPSS v16.0 (SPSS Inc., Tokyo, Japan).
4. Results
4.1. Real-time PCR detection
Among the 512 specimens analyzed, one or more viruses were detected in 424 (83%) specimens (Table 1 ). Two or more viruses were detected in 160 samples (31% of all samples). Only one specimen included 5 distinct viruses (human metapneumovirus, Coxsackievirus type B5, rhinovirus C, bocavirus and influenza virus type A).
Table 1.
The monthly variation of viruses detected in nasal aspirates during the study period.
| Virusa | Month |
Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013-April | May | June | July | August | September | October | November | December | 2014-January | February | March | April | n (%) | |
| Parainfluenzavirus 1 | 0 | 0 | 4 | 4 | 2 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 16 (3.1) |
| Parainfluenzavirus 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.2) |
| Parainfluenzavirus 3 | 5 | 17 | 9 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 41 (8.0) |
| Parainfluenzavirus 4 | 0 | 0 | 1 | 3 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 (2.7) |
| RSvirus | 2 | 3 | 3 | 3 | 3 | 6 | 8 | 7 | 5 | 0 | 2 | 8 | 2 | 52 (10.2) |
| human Metapneumovirus | 3 | 4 | 1 | 5 | 2 | 0 | 0 | 0 | 0 | 1 | 14 | 21 | 17 | 68 (13.3) |
| Enterovirus/Rhinovirus | 6 | 27 | 21 | 24 | 13 | 15 | 19 | 22 | 20 | 5 | 13 | 16 | 18 | 219 (42.8) |
| human Bocavirus | 4 | 20 | 9 | 4 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 10 | 12 | 64 (12.5) |
| human Parechovirus | 0 | 2 | 0 | 2 | 3 | 3 | 3 | 3 | 2 | 0 | 1 | 0 | 0 | 19 (3.7) |
| Adenovirus | 2 | 15 | 10 | 6 | 4 | 2 | 5 | 9 | 10 | 3 | 4 | 6 | 10 | 86 (16.8) |
| human Coronavirus OC43 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 8 (1.6) |
| human Coronavirus NL63 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 1 | 8 (1.6) |
| human Coronavirus 229E | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 4 (0.8) |
| human Coronavirus HKU-1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 8 (1.6) |
| Influenza virus type A | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 5 | 2 | 0 | 11 (2.1) |
| Influenza virus type B | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 6 | 12 (2.3) |
| Total positive viruses | 23 | 93 | 63 | 59 | 39 | 29 | 38 | 43 | 43 | 14 | 46 | 71 | 70 | 631 |
| Enterovirus | 0 | 3 | 1 | 1 | 3 | 5 | 4 | 3 | 2 | 1 | 2 | 0 | 0 | 25 (4.9) |
| Rhinovirus A | 6 | 15 | 7 | 20 | 7 | 10 | 9 | 5 | 9 | 4 | 2 | 11 | 8 | 113 (22.1) |
| Rhinovirus B | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 8 (1.6) |
| Rhinovirus C | 0 | 7 | 7 | 3 | 3 | 0 | 5 | 14 | 9 | 0 | 9 | 4 | 10 | 71 (13.9) |
| EV/RV Untyped | 0 | 2 | 4 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 8 (1.6) |
| Samples with 1 virus | 3 | 31 | 22 | 24 | 15 | 17 | 28 | 21 | 16 | 8 | 21 | 19 | 27 | 262 (51.2) |
| Samples with 2 viruses | 7 | 22 | 17 | 10 | 8 | 3 | 5 | 7 | 11 | 3 | 6 | 8 | 11 | 118 (23.0) |
| Samples with 3 or more viruses | 2 | 6 | 2 | 5 | 3 | 2 | 0 | 3 | 2 | 0 | 4 | 8 | 7 | 44 (8.6) |
| Total samples | 15 | 69 | 45 | 46 | 32 | 26 | 38 | 37 | 36 | 20 | 38 | 55 | 55 | 512 (100) |
RS virus, respiratory syncytial virus.
Multiple viruses included.
Rhinoviruses were found most often (n = 192, 37.5% of all samples and 45.3% of positive samples) followed by adenoviruses (n = 86, 16.8% of all samples) and human metapneumovirus (n = 68, 13.3%).
4.2. Seasonal distribution
Influenza virus types A and B were detected in the winter and human metapneumovirus was detected during the spring months. Human bocavirus and parainfluenza virus type 3 were found during the spring and early summer. Parainfluenza virus type 1 and parechovirus were detected mainly in the summer. The detection of RS virus increased in the autumn.
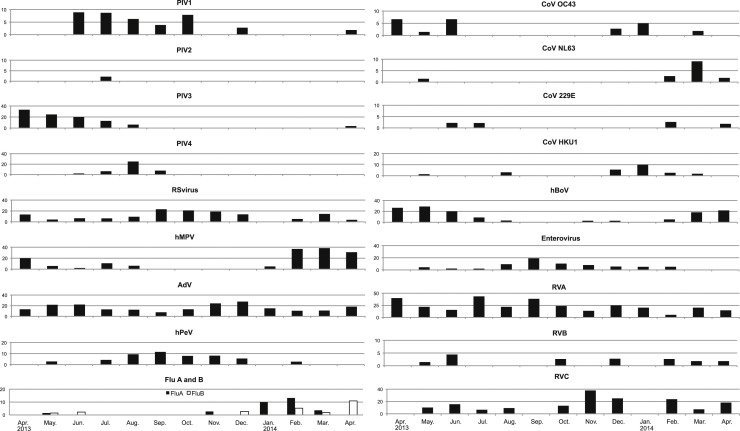
Genetically conserved regions of both enteroviruses and rhinoviruses were detected by real-time PCR all year round with a high proportion of positive samples. Genotyping revealed the presence of enteroviruses in the summer and a decrease in rhinovirus A in the winter. On the other hand, rhinovirus C was detected in the winter months. Adenoviruses were detected mainly in the summer and winter (Fig. 1 ).
Fig. 1.
Monthly prevalences of detection of 19 respiratory viruses/serotypes by real-time PCR assay from week 17, 2013 to week 16, 2014. (percent positive).
Note that the scale on the vertical axis differs between viruses.
PIV, parainfluenza viruses; RS virus, respiratory syncytial virus; hMPV, human metapneumovirus; AdV, adenovirus; hPeV, human parechovirus, Flu A and B, influenzavirus type A and type B;
CoV, coronavirus; hBoV, human bocavirus; RVA, rhinovirus A; RVB, rhinovirus B; RVC, rhinovirus C.
4.3. Multiple infections
Next, we evaluated the prevalence of multiple infections by the viruses. For the human bocavirus, parechovirus and rhinoviruses A and C, the rates of coinfection were high compared with other respiratory viruses. Rhinoviruses were the most frequently detected viruses and their coinfection rates were also higher than those of the other viruses. Therefore, we compared the clinical symptoms caused by five types of viruses, adenoviruses, human bocavirus, RS virus, parainfluenza virus type 3, and human metapneumovirus, which were detected most often after rhinoviruses, and rhinovirus single infections and symptoms in cases with dual infections including rhinoviruses.
There was no significant difference between the number of days of hospitalization caused by rhinoviruses and the other five viruses. The number of days in the hospital of patients in whom RS virus was detected was longer than that of patients infected with human metapneumovirus (Table 2 ).
Table 2.
Asymptotic p-values of duration of hospitalization caused by single infection with each of six viruses.
| PIV3 | RSV | hMPV | hBoV | AdV | RV | |
|---|---|---|---|---|---|---|
| No. of single detections (% of total) | 15 (36.6) | 26 (50) | 37 (54.4) | 12 (18.8) | 24 (27.9) | 98 (49.0) |
| Duration of hospitalization, median number of days | 8 | 9 | 7 | 7.5 | 7.5 | 8 |
| PIV3 | 0.310 | 0.576 | 0.901 | 0.930 | 0.864 | |
| RSV | 0.048* | 0.408 | 0.618 | 0.150 | ||
| hMPV | 0.661 | 0.440 | 0.599 | |||
| hBoV | 0.747 | 0.961 | ||||
| AdV | 0.685 |
Mann–Whitney U test. asymptotic significance (2-tailed).
p < 0.05. No other significant between-group differences.
For the five viruses discussed above, we compared the number of hospitalization days of the cases with single infections by the each 5 viruses with those having dual infections with a rhinovirus and those with dual infection with a virus other than a rhinovirus. The number of days of hospitalization of the children with parainfluenza virus type 3 infection alone was shorter than for children with paranfluenza virus and rhinovirus dual infection. On the other hand, children with infection by human metapneumovirus alone spent fewer days in the hospital than those with dual infections by human metapneumovirus and a respiratory virus other than a rhinovirus (Table 3 ).
Table 3.
Comparison of single and dual infections with and without rhinoviruses by duration of hospitalization.
| PIV3 | RSV | hMPV | hBoV | Ad | |
|---|---|---|---|---|---|
| Total number of detections | 41 | 52 | 68 | 64 | 86 |
| No. of single detections (% of total) | 15 (36.6) | 26 (50) | 37 (54.4) | 12 (18.8) | 24 (27.9) |
| Duration of hospitalization, median no. of days | 8 | 9 | 7 | 7.5 | 7.5 |
| Dual detection with rhinovirus: No. (%) | 6 (14.6) | 10 (19.2) | 7 (10.3) | 8 (12.5) | 18 (20.9) |
| Duration of hospitalization, median no. of days | 12 | 8 | 7 | 7.5 | 9 |
| asymptotic significance (2-tailed) | 0.030* | 0.886 | 0.742 | 0.907 | 0.099 |
| Dual detection with other respi. Virus: No. (%) | 11 (26.8) | 9 (17.3) | 8 (11.8) | 21 (32.8) | 19 (22.1) |
| Duration of hospitalization, median no. of days | 8 | 9 | 10 | 9 | 9 |
| asymptotic significance (2-tailed) | 0.213 | 0.338 | 0.009* | 0.384 | 0.094 |
| Dual detection with rhinovirus vs. dual detection with other respiratory virus | 0.245 | 0.432 | 0.144 | 0.431 | 0.939 |
| Asymptotic significance (2-tailed) |
Mann–Whitney U test.
p < 0.05. No other significant between-group differences.
We next compared the requirement for oxygenation and the presence of wheezing of the children with single infections and dual infections with a rhinovirus or other respiratory virus. The patients with dual infections with an adenovirus and rhinovirus needed significantly more oxygenation than those with an adenovirus infection or dual infection with an adenovirus and other respiratory virus. However, the severity of the lower respiratory tract disease for which the requirement of oxygenation was assumed and the presence of wheezing as an index did not differ among most respiratory viral infections, regardless of whether they were single infections or dual ones with a rhinovirus and other respiratory virus (Table 4 ).
Table 4.
Correlations between coinfection with a rhinovirus or other respiratory virus and wheezing and oxygen treatment.
| Outcome of interest | Factors | Wheezing |
Oxgen |
||||
|---|---|---|---|---|---|---|---|
| p-values | OR | 95% CI | p-values | OR | 95% CI | ||
| PIV3 | RV coinfectionb | 1.0000 | 1.25 | 0.10–15.11 | 0.1196 | 8.00 | 0.96–66.95 |
| other virus coinfectionc | 0.4065 | 0.44 | 0.08–2.55 | 0.4065 | 2.29 | 0.39–13.33 | |
| RV coinfection vs. other virus coinfection | 0.6000 | 2.86 | 0.24–33.90 | 0.3348 | 3.50 | 0.43–28.45 | |
| RSV | RV coinfection | 1.0000 | 0.86 | 0.17–4.28 | 0.1186 | 5.14 | 0.71–37.15 |
| other virus coinfection | 0.6936 | 0.74 | 0.14–3.78 | 0.0946 | 6.00 | 0.81–44.35 | |
| RV coinfection vs. other virus coinfection | 1.0000 | 1.17 | 0.17–8.09 | 1.0000 | 0.86 | 0.12–5.94 | |
| hMPV | RV coinfection | 0.6746 | 0.64 | 0.12–3.32 | 0.5934 | 2.07 | 0.32–13.25 |
| other virus coinfection | 1.0000 | 1.44 | 0.25–8.22 | 0.3262 | 3.10 | 0.58–16.59 | |
| RV coinfection vs. other virus coinfection | 0.6084 | 0.44 | 0.05–3.98 | 1.0000 | 0.67 | 0.08–5.88 | |
| BoV | RV coinfection | 1.0000 | 1.19 | 0.19–7.46 | 1.0000 | 1.20 | 0.19–7.77 |
| other virus coinfection | 0.4334 | 2.29 | 0.50–10.50 | 0.6905 | 0.63 | 0.13–2.99 | |
| RV coinfection vs. other virus coinfection | 0.6460 | 0.52 | 0.09–2.99 | 0.6460 | 1.92 | 0.33–11.03 | |
| AdV | RV coinfection | 0.1038 | 3.57 | 0.81–15.71 | 0.0114a | 5.97 | 1.52–23.43 |
| other virus coinfection | 0.1991 | 2.68 | 0.68–10.53 | 0.4947 | 1.75 | 0.44–6.98 | |
| RV coinfection vs. other virus coinfection | 1.0000 | 1.33 | 0.25–7.01 | 0.1031 | 3.40 | 0.88–13.19 | |
| RVA vs. RVBd | 0.6258 | 0.58 | 0.07–4.43 | 1.0000 | 0.45 | 0.02–9.02 | |
| RVA vs. RVC | 0.6633 | 1.28 | 0.54–3.05 | 0.4626 | 1.49 | 0.56–3.95 | |
| RVB vs. RVC | 0.5868 | 2.23 | 0.28–17.61 | 0.5588 | 3.29 | 0.16–65.92 | |
p < 0.05. No other significant between-group differences.
single infection vs. dual infection with a rhinovirus (RV).
single infection vs. dual infection with respiratory viruses other than rhinoviruses.
comparison of clinical severity of single infections by rhinoviral genogroups.
5. Discussion
In this study, separate real-time PCR assays were used to detect 12 RNA viruses and two DNA viruses, and real-time reverse transcription (RT) PCR was used to detect influenza viruses A and B. Of the 512 samples analyzed, 424 were positive 1 virus or more. The overall viral detection rate was 83%, which was much higher than in similar past reports [5], [6], [7], [18]. The reason may be that our method had many detection targets. Furthermore, the higher detection rates among young children likely correspond to the higher incidence of viral respiratory tract infections in children, although other factors such as pre-existing immunity might also have played a role [4]. Since the specimens from children aged 1 year or younger accounted for 68% of those studied, it is considered that the rate of viral detection and the rate of concurrent infections became higher than in past reference data. The largest number of viruses detected in one sample was 5 in the nasal aspirate from a 9-month-old girl. When comparing the number of viruses detected per specimen, it was found that the specimens from younger patients tended to include more than one virus (data not shown).
Seasonal distribution was seen for each virus. The epidemic peak of each virus was about the same as in another report from Japan [19]. Seasonal influenza virus type A migrates globally between epidemics and is reintroduced every winter season in temperate climates [20], although the underlying cause of the seasonality of the other respiratory viruses remains unknown. It has been suggested that rhinovirus infections could reduce subsequent RS virus and influenza virus type A infections by inducing an interferon response, thereby creating an undesirable environment for these viruses [21], [22]. In this study, the peaks for the various viral epidemics did not coincide. Thus, it is thought that some kind of interference by viruses may influence epidemics of respiratory viruses.
In this study, human rhinoviruses were the most common viruses. Rhinoviruses are thought to be mainly associated with the common cold, causing mild respiratory symptoms [23]. These viruses are classified into three species and divided into more than 160 serotypes or genotypes. Thus they are among the mostly commonly detected viruses in respiratory specimens of children [10]. However, recent reports suggest that rhinovirus infections may induce and/or exacerbate asthma and be responsible for lower respiratory tract infections with severe symptoms [24], [25].
Based on the sequence data, rhinovirus C was detected mainly in the winter, whereas rhinovirus A was detected all year round, with a high proportion of positive samples in June (44% of the samples). Although rhinovirus B was detected, its seasonality was not clear. However, it became clear that there was a difference in the epidemic seasons of rhinoviruses A and C. Furthermore, the detected rhinoviruses consisted of 32 genotypes of group A, 5 genotypes of group B and 21 genotypes of group C, suggesting that multiple genotypes were brought into the area and that epidemics of some of them might occur at the same time (Supplementary Table 1). Rhinovirus A consists of 80 serotypes and B consists of 32 types, including genotypes, and there are now 55 rhinovirus C genotypes proposed [17]. It is not clear whether the genotypes of the rhinoviruses detected in this study cause severe illness.
We also compared the clinical symptoms of single infections and dual infections by rhinoviruses and other respiratory viruses of the children infected by one of the five most commonly detected respiratory viruses. The results revealed that there were no significant differences in the number of days of hospitalization, the necessity for oxygen inhalation or the existence of wheezing between the children with single infections and those with dual infections. In former reports that evaluated the impacts of rhinoviruses on lower respiratory infections, there were only marginal differences between the different rhinovirus groups and between single rhinovirus infection and rhinovirus coinfection [26], [27]. Though there was no significant difference in the number of hospitalization days of patients with single infections by rhinoviruses or other respiratory viruses, our data suggested the importance of rhinoviruses as a potential cause of pediatric pneumonia. Recently, our group evaluated the prevalence of rhinovirus infections among asymptomatic children [10]. Rhinoviruses were often detected in their throats at a time without any symptoms. Since rhinoviruses do not exist in the upper respiratory tract for a long time even if a child does not show symptoms, these were “active” asymptomatic infections rather than persistent infections.
In conclusion, rhinoviruses are causative agents of various conditions ranging from asymptomatic infection to lower respiratory tract infection and pneumonia. Rhinovirus coinfection with other respiratory viruses is not responsible for more severe symptoms, so it is hypothesized that the factors related to disease severity are mainly the underlying conditions of the children.
Funding
This work was supported by Grant-in-Aid for Scientific Research (C) Grant number from the Japan Society for the Promotion of Science.
Competing interest
None declared.
Ethical approval
This study was approved by the Osaka Prefectural Institute of Public Health ethical committee (No. 1302-05-01).
Acknowledgements
The authors would like to thank Maki Otsuka for helpful technical assistance in amplification and Kim Barrymore for editing the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2015.10.001.
Contributor Information
Saeko Morikawa, Email: morikawa@iph.pref.osaka.jp.
Urara Kohdera, Email: kohdera@nakano-kodomo.or.jp.
Taisuke Hosaka, Email: thosaka@pd6.so-net.ne.jp.
Kousuke Ishii, Email: kousuke.22.ishii@gmail.com.
Shohei Akagawa, Email: shohei@mbk.nifty.com.
Satoshi Hiroi, Email: hiroi@iph.pref.osaka.jp.
Tetsuo Kase, Email: kasetetsuo@iph.pref.osaka.jp.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Tregoning J.S., Schwarze J. Respiratory viral infection in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabello C., Manjarrez M.E., Olvera R., Villalba J., Valle L., Paramo I. Frequency of viruses associated with acute respiratory infections in children younger than five years of age at a locality of Mexico City. Mem. Inst. Oswaldo Cruz. 2006;101:21–24. doi: 10.1590/s0074-02762006000100005. [DOI] [PubMed] [Google Scholar]
- 3.Piippo-Savolainen E., Korppi M. Wheezy babies—wheezy adults? Review on long-term outcome until adulthood after early childhood wheezing. Acta Pediatr. 2008;97:5–11. doi: 10.1111/j.1651-2227.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 4.Brittain-Long R., Anderson L.M., Olofsson S., Lindh M., Westin J. Seasonal variations of 15 respiratory agents illustrated by the application of a multiplex polymerase chain reaction assay. Scand. J. Infect. Dis. 2012;44:9–17. doi: 10.3109/00365548.2011.598876. [DOI] [PubMed] [Google Scholar]
- 5.Wishaupt J.O., Russcher A., Smeets L.C., Versteegh F.G.A., Hartwig N.G. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113–e1120. doi: 10.1542/peds.2010-2779. [DOI] [PubMed] [Google Scholar]
- 6.Al-Ayed M.S., Asaad A.M., Qureshi M.A., Ameen M.S. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse-transcriptase polymerase chain reaction. Saudi Med. J. 2014;35:1348–1353. [PMC free article] [PubMed] [Google Scholar]
- 7.Weigl J.A., Puppe W., Meyer C.U., Berner R., Forster J., Schumitt H.J., Zepp F. Ten years’ experience with year-round active surveillance of up to 19 respiratory pathogens in children. Eur. J. Pediatr. 2007;166:957–966. doi: 10.1007/s00431-007-0496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepiller Q., Barth H., Lefebvre F., Herbrecht R., Lutz P., Kessler R., Fafi-Kremer S., Stoll-Keller F. High incidence but low burden of coronaviruses and preferential associations between respiratory viruses. J. Clin. Microbiol. 2013;51:3039–3046. doi: 10.1128/JCM.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prill M.M., Iwane M.K., Edwards K.M., Williams J.V., Weinberg G.A., Staat M.A. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr. Infect. Dis. J. 2012;31:235–240. doi: 10.1097/INF.0b013e31823e07fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morikawa S., Hiroi S., Kase T. Detection of respiratory viruses in gargle specimens of healthy children. J. Clin. Virol. 2015;64:59–63. doi: 10.1016/j.jcv.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiko H., Shimada Y., Yonaha M., Hashimoto O., Hayashi A., Sakae K., Takeda N. Molecular diagnosis of human enteroviruses by phylogeny-based classification by use of the VP4 sequence. J. Infect. Dis. 2002;185:744–754. doi: 10.1086/339298. [DOI] [PubMed] [Google Scholar]
- 12.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 13.Mclntyre C.L., Knowles N.J., Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 2013;94:1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henquell C., Mirand A., Deusebis A.L., Regagnon C., Archimbaud C., Chambon M. Prospective genotyping of human rhinoviruses in children and adults during the winter of 2009–2010. J. Clin. Virol. 2012;53:280–284. doi: 10.1016/j.jcv.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Kaida A., Kubo H., Takakura K., Togawa M., Shiomi M., Koudera U., Iritani N. Molecular epidemiology of human rhinovirus C in patients with acute respiratory tract infections in Osaka City, Japan. Jpn. J. Infect. Dis. 2011;64:488–492. [PubMed] [Google Scholar]
- 16.Simmonds P., Mclntyre C., Savolainen-kopra C., Tapparel C., Mackay I.M., Hovi T. Proposals for the classification of human rhinovirus species C into genotypically assigned types. J. Gen. Virol. 2010;91:2409–2419. doi: 10.1099/vir.0.023994-0. [DOI] [PubMed] [Google Scholar]
- 17.Picornaviridae study group. http://www.picornaviridae.com (accessed 13.07.15.).
- 18.Kuypers J., Wright N., Ferrenberg J., Huang M.-L., Cent A., Corey L., Morrow R. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J. Clin. Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada H., Ogura A., Hotta C., Wakui T., Ogawa T., Terai M. Epidemiological study of respiratory viruses detected in patients under two years old who required admission because of lower respiratory disease. Kansenshogaku Zassi. 2014;88:423–429. doi: 10.11150/kansenshogakuzasshi.88.423. [DOI] [PubMed] [Google Scholar]
- 20.Nelson M.I., Simonsen L., Viboud C., Miller M.A., Holmes E.C. Phylogenetic analysis reveals the global migration of seasonal influenza A viruses. PloS Pathog. 2007;3:e131. doi: 10.1371/journal.ppat.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greer R.M., McErlean P., Arden K.E., Faux C.E., Nitsche A., Lambert S.B. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J. Clin. Virol. 2009;45:10–15. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linde A., Rotzen-Ostlund M., zweygberg-Wirgart B., Rubinova S., Brytting M. Does viral interference affect spread of influenza? Euro Surveill. 2009;14:pii19354. [PubMed] [Google Scholar]
- 23.Ruohola A., Waris M., Allander T., Ziegler T., Heikkinen T., Ruuskanen O. Viral etiology of common cold in children. Finland Emerg. Infect. Dis. 2009;15:344–346. doi: 10.3201/eid1502.081468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E., Printz M.C. Wheezing rhinovirus illness in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gern J.E. The ABCs of rhinoviruses, wheezing and asthma. J. Virol. 2010;84:7418–7426. doi: 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito S., Daleno C., Tagliabue C., Scala A., Tenconi R., Borzani I., Fossali E., Pelucci C., Piralla A., Principi N. Impact of rhinoviruses on pediatric community-acquired pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1637–1645. doi: 10.1007/s10096-011-1487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paula N.T., Carneiro B.M., Yokosawa J., Freitas G.R.O., Olivaira T.F.M., Costa L.F., Silveira H.L., Queiroz D.A.O. Human rhinovirus in the lower respiratory tract infections of young children and the possible involvement of a secondary respiratory viral agent. Mem. Inst. Oswaldo Cruz. 2011;106:316–321. doi: 10.1590/s0074-02762011000300010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.