Highlights
-
•
The number of cases per month were highest from November to May.
-
•
A respiratory-based primary diagnosis was the most common.
-
•
Requirements included ventilation, inotropes, renal replacement and ECMO.
-
•
Mean (SD) mortality risk was 4.8 (6.4)%, with a median (range) of 2.7 (0.2–39.1)%.
-
•
The total cost of PICU admissions was estimated to be £2,256,823 - £3,997,823.
Keywords: Human metapneumovirus, Intensive care, PICU, Cost
Abstract
Background
Human metapneumovirus (HMPV) is a pneumovirus known to cause respiratory disease in children. It was identified as a pathogen in 2001 and its healthcare burden and associated costs are not fully understood.
Objectives
This study aimed to assess the clinical characteristics of children with HMPV infection admitted to paediatric intensive care units (PICUs) across the United Kingdom (UK) over a nine-year period and to estimate the associated costs of care.
Study design
Data were collected from the UK paediatric intensive care audit network (PICANet) and costs calculated using the National Health Service (NHS) reference costing scheme.
Results
There were 114 admissions in which HMPV was detected. The number of admissions associated with a code of HMPV rose steadily over the study period (three in 2006 to 28 in 2014) and showed significant seasonal variability, with the peak season being from November to May. Children required varying levels of intensive care support from minimal to complex support including invasive ventilation, inotropes, renal replacement therapy and extracorporeal membrane oxygenation (ECMO). HMPV was associated with five deaths during the study period. The associated costs of PICU admissions were estimated to be between £2,256,823 and £3,997,823 over the study period, with estimated annual costs rising over the study period due to increasing HMPV admissions.
Conclusions
HMPV is associated with a significant healthcare burden and associated cost of care in PICUs in the UK.
1. Background
Human metapneumovirus (HMPV) is a pneumovirus that was first identified in the Netherlands in 2001 [1]. It is a ubiquitous virus, with most children being infected by 5 years of age, but continuing to experience re-infections throughout life [1]. Studies have shown that HMPV is identified in 3.9%–8.5% of children with clinical symptoms of upper respiratory tract infections (URTI) or lower respiratory tract infections (LRTI) who are hospitalised [[2], [3], [4], [5]]. The attributable fraction of HMPV (proportion of children who have HMPV detected where HMPV is considered to be the cause of the illness) to infected children with respiratory symptoms has been calculated to be between 73–90%, similar to that of RSV and influenza [6,7]. Annual rates of hospitalisation in children under 5 years of age associated with HMPV are approximately 1 in 1000 in the United States (US) [8]. This is a similar burden to influenza (1 in 1000) but lower than RSV (3 in 1000) [8]. Estimates of the prevalence of HMPV within the community setting are more variable and are influenced by the cohort being sampled, values range from 1.3% to 20% of those with symptoms of URTI/LRTI [[9], [10], [11]]. A study of 3257 children in the US has estimated the annual outpatient burden of HMPV to be 55 clinic visits and 13 emergency department visits per 1000 children [8].
HMPV shows seasonal variation, with the majority of infections being detected in January to April in the northern hemisphere [8]. It can cause a range of clinical syndromes from a mild URTI to severe LRTI requiring intensive care [1]. A study examining children presenting to an emergency department in Milan, Italy found that children with HMPV infection presented with mild URTIs, pharyngitis, acute otitis media, croup, acute wheeze, acute bronchiolitis and pneumonia [12]. A large study of children under 5 years of age admitted to hospital with respiratory illness or fever in the US showed that children with HMPV infections were more likely to need supplemental oxygen than their HMPV-negative counterparts (53% versus 36%) and had a longer average stay in the paediatric intensive care unit (PICU) (4.5 days versus 2 days) [8]. A study comparing the characteristics of children admitted to PICU with HMPV and RSV found that children with HMPV were more likely to present with pneumonia or pneumonitis than those with RSV (29% vs. 16%), but that both groups required similar levels of invasive and non-invasive ventilation [13]. There is currently no specific treatment or vaccine for HMPV infection [14].
Given the ubiquitous nature of HMPV, its potential clinical severity and the high burden of disease, it is becoming increasingly important to establish the impact of HMPV on paediatric populations.
2. Objectives
In this study we aimed to assess the clinical characteristics of children with HMPV infection admitted to PICUs in the United Kingdom (UK) over a nine-year period. A secondary aim was to estimate the associated cost of care.
3. Study design
Data were extracted retrospectively from the UK Paediatric Intensive Care Audit Network (PICANet) database for all children with a HMPV diagnostic code between 1st January 2006 and 31st December 2014. PICANet is a UK national database that includes clinical and laboratory data on almost all children admitted to a PICU in the UK, with an estimated case ascertainment of 99.4% in 2017 [15].
The data collected includes basic demographic information, timing of admission, length of PICU stay, primary diagnosis, microbiology results, intensive care support required, paediatric index of mortality 2 (PIM2) score (a severity scoring system used to help predict the outcome of patients admitted to PICU) and mortality.
Although detection of HMPV was recorded as a diagnostic code in all of the included children, data was not available on how the organism was detected (i.e. whether collected from the upper or lower respiratory tract or the diagnostic test used). In addition, the presence of HMPV may not have been the primary reason for admission to PICU and may have been an incidental finding.
Healthcare costs were calculated (UK£ for 2017) using the NHS reference costing scheme [16]. The healthcare costs are presented as mean data in order to preserve total costs. Critical care in the UK is divided into basic, intermediate and advanced and within advanced critical care there are a further 5 sub-levels. PICANet does not currently collect data on children in basic and intermediate critical care and thus all patients included in this study received at least advanced critical care level 1. Documentation from the Royal College of Paediatrics and Child Health (RCPCH) on paediatric critical care has outlined the 5 sub-levels of advanced critical care [17]. However, full details of all the care children received during their stay in PICU was not available through PICANet (e.g. the volume of fluid boluses they received), thus we could only differentiate admissions needing level 5 care (requiring ECMO) from those requiring level 1–4 care. We, therefore, calculated mean (minimum, maximum) costs for admissions requiring level 1–4 care and those requiring level 5 care.
3.1. Statistical analysis
Proportions were compared using the Chi squared or Fisher’s exact test. Continuous variables were tested for normality using the Shapiro-Wilk test and data were analysed using either the independent t-test or the Mann–Whitney U test as appropriate. Statistical analysis was carried out with Microsoft Excel and IBM SPSS Statistics (Version 24, New York, USA).
Ethical approval was not required for this study.
4. Results
4.1. Demographics and duration of stay
There were 114 admissions involving 103 patients during the study period. Over the study period, PICAnet estimated the total number of admissions to UK PICUs to be 144,800 [18]. This would mean that those admissions associated with HMPV contribute to approximately 0.08% of total PICU admissions or 0.8 per 1000 PICU admissions. 95 patients had one admission, five patients had two admissions, and three patients had three admissions. 56 patients were male (49.1%). The mean (standard deviation, SD) age at admission was 2.8 (3.7) years and median (range) age 12 months (<1 month–15.4 years). Mean (SD) length of stay was 9.2 (11.4) days with a median (range) of 5.8 (0.4–100) days.
4.2. Seasonal variation
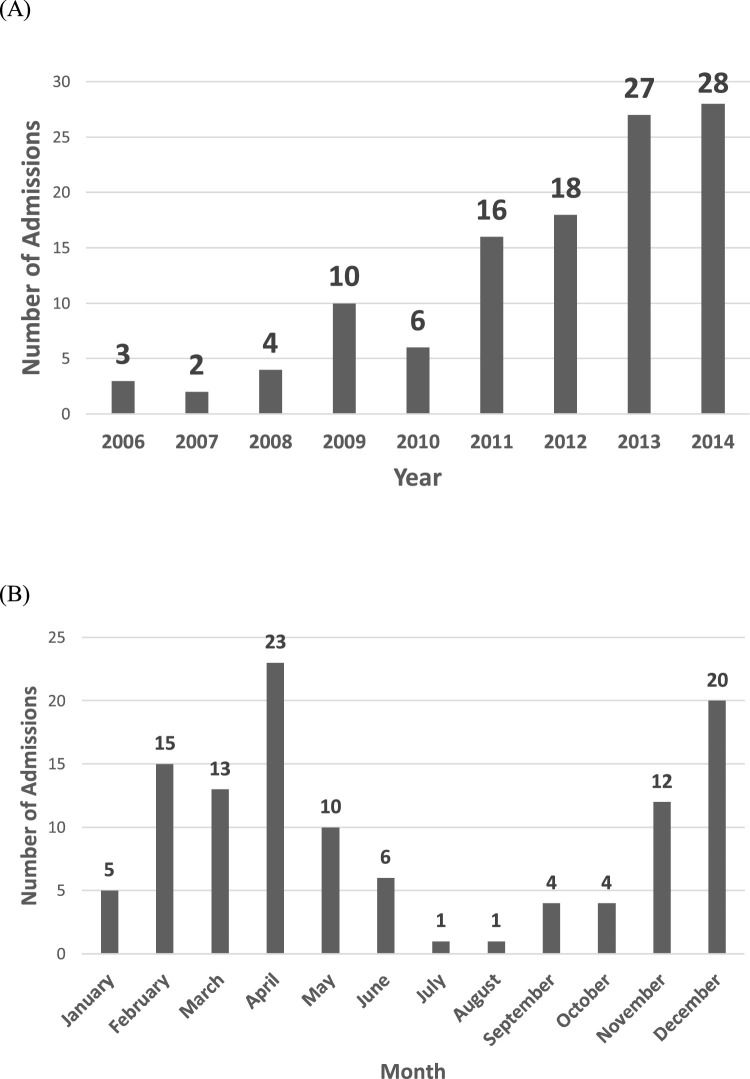
The number of patients with a code of HMPV increased gradually from three in 2006 to 28 in 2014 (Fig. 1 A). The number of cases per month were highest from November to May with 91% of all cases occurring between these months (Fig. 1B). The peak number of cases was in April which saw 23 (20.2%) cases over the study period. Only five (4%) cases were identified in January despite there being higher numbers of cases in the months either side.
Fig. 1.
(A) Number of admissions per year 2006–2014 and (B) Number of admissions per month.
4.3. Co-detection of other organisms
104 patients (91.2%) had HMPV alone detected, with the remainder of patients having another organism also detected including adenovirus (3.5%), rhinovirus (2.6%), RSV (0.9%), coronavirus (0.9%), parainfluenza virus (0.9%) and Group A Streptococcus (0.9%).
4.4. Primary diagnosis for PICU admission
There were 26 different diagnoses recorded as the primary diagnosis for PICU admission with a respiratory-based diagnosis the most common (84.2% of admissions) (Table 1 ).
Table 1.
Primary recorded diagnoses of children with HMPV detected during their admission. Data presented as n (%).
| Primary diagnosis | Number of admissions (%) |
|---|---|
| Infection of lower respiratory tract/pneumonia | 41 (35.9%) |
| Acute viral bronchiolitis | 29 (25.4%) |
| Acute respiratory failure | 11 (9.6%) |
| Sepsis | 4 (3.5%) |
| Laryngomalacia/Tracheobronchomalacia | 3 (2.6%) |
| Laryngotracheobronchitis | 3 (2.6%) |
| Recurrent apnoea | 2 (1.7%) |
| Respiratory distress | 2 (1.7%) |
| Neuroblastoma | 2 (1.7%) |
| Acute obliterating bronchiolitis | 1 (0.9%) |
| Adenovirus infection | 1 (0.9%) |
| Cardiac arrest with unsuccessful resuscitation | 1 (0.9%) |
| Encephalitis | 1 (0.9%) |
| Gastro-oesophageal reflux disease | 1 (0.9%) |
| Enzyme deficiency | 1 (0.9%) |
| Human metapneumovirus nucleic acid detection | 1 (0.9%) |
| Urea cycle defect | 1 (0.9%) |
| Meningitis | 1 (0.9%) |
| Meningococcal septicaemia | 1 (0.9%) |
| Pleural Effusion | 1 (0.9%) |
| Pleural Empyema | 1 (0.9%) |
| Portal hypertension | 1 (0.9%) |
| Status Epilepticus | 1 (0.9%) |
| Tetralogy of Fallot | 1 (0.9%) |
| Total anomalous pulmonary venous drainage | 1 (0.9%) |
| Unspecified encephalopathy | 1 (0.9%) |
4.5. PICU requirements
Children had a variety of intensive care requirements including invasive and non-invasive ventilation, inotropic support, renal replacement therapy and extracorporeal membrane oxygenation (ECMO). Many children required more than one type of support, either sequentially or simultaneously; ten children (8.8%) required none of the above interventions, 62 (54.4%) required one intervention, 33 (28.9%) required two interventions and nine (7.9%) required three interventions (Table 2 ).
Table 2.
Combinations of intensive care support required. Data presented as n (%) or mean (SD).
| Number (%) of children | Non-invasive ventilation | Invasive ventilation | Inotropic support | Renal replacement therapy | ECMO | Mean (SD) duration of therapy (Days) | Mean (SD) duration of therapy (Days) | Mean (SD) length of PICU stay (days) | Mean (SD) length of PICU stay (days) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) of children* | – | 34 (29.8%) | 90 (78.9%) | 26 (22.8%) | 3 (2.6%) | 2 (1.7%) | – | – | – | – |
| Mean (SD) duration of therapy (days) | – | 4.0 (3.6) | 9.7 (12.1) | 4.1 (3.8) | 7.7 (4.2) | 13.0 (5.0) | – | – | – | – |
| Number of interventions | ||||||||||
| 0 | 10 (8.8%) | X | X | X | X | X | N/A | N/A | 2.9 (2.4) | 2.9 (2.4) |
| 1 | 14 (12.2%) | ✓ | X | X | X | X | 5.1 (3.9) | 7.9 (12.7) | 5.6 (3.8) | 8.2 (12.6) |
| 1 | 48 (42.1%) | X | ✓ | X | X | X | 8.6 (14.2) | 8.9 (14.0) | ||
| 2 | 17 (14.9%) | X | ✓ | ✓ | X | X | 11.1 (10.8) | 10.4 (9.4) | 11.0 (10.7) | 11.9 (10.7) |
| 2 | 15 (13.2%) | ✓ | ✓ | X | X | X | 9.7 (7.8) | 13.1 (10.7) | ||
| 2 | 1 (0.9%) | X | ✓ | X | X | ✓ | 9 (0) | 7.6 (0) | ||
| 3 | 5 (4.4%) | ✓ | ✓ | ✓ | X | X | 10.8 (5.2) | 12.4 (6.6) | 12.7 (6.5) | 13.9 (6.9) |
| 3 | 3 (2.6%) | X | ✓ | ✓ | ✓ | X | 10.7 (4.2) | 12.2 (5.4) | ||
| 3 | 1 (0.9%) | X | ✓ | ✓ | X | ✓ | 26 (0) | 24.7 (0) |
Key: ✓=support was used, X = support was not used, N/A = not applicable.
The percentages add up to more than 100% as some children received more than one type of support.
4.6. Paediatric index of mortality 2 (PIM2) score and mortality
PIM2 scores, which give a value for mortality risk based on events within the first hour of admission to PICU, were collected for each admission. Mean (SD) mortality risk was 4.8 (6.4)%, with a median (range) of 2.7 (0.2–39.1)%. Five children died; the mean (SD) predicted mortality rate of those children was 14.3 (8.7)% compared with a predicted mortality among those who survived to discharged of 4.3 (5.9)% (P = 0.02). All those who died required invasive ventilatory support and four required inotropic support. The primary recorded diagnoses of those who died were: acute bronchiolitis, acute obliterating bronchiolitis, lower respiratory tract infection, sepsis and cardiac arrest with unsuccessful resuscitation.
4.7. Healthcare costs associated with HMPV
Costs of care were estimated using the NHS reference costing scheme; advanced critical care level 1 cost £1895/night, level 2 cost £2390/night, level 3 cost £2077/night, level 4 cost £3942/night and level 5 cost £5867/night [16]. The mean (minimum, maximum) costs for each admission requiring level 1–4 care (n = 112) were £23,442 (£17,287, £35,960) and the mean costs for each admission requiring level 5 care (n = 2) were £94,928.
The total cost of PICU admissions over the study period was estimated to be between £2,256,823 and £3,997,823, giving an annual estimated minimum cost of £282,102 and maximum cost of £499,728 over the study period. However, as the number of HMPV cases increased significantly over the study period, the costs for 2006 were estimated to only be between £59,390 (minimum cost) and £105,206 (maximum cost) but by 2014 they had increased to be between £554,307 (minimum cost) and £981,921 (maximum cost).
5. Discussion
HMPV was detected with increasing frequency amongst the UK PICU population between 2006 and 2014. This may have been due to increasing incidence of the virus, however, it is more likely to be attributable to increased awareness of HMPV as a potential pathogen and therefore increased testing specifically for it as well as an increase in the use of multiplex polymerase chain reaction (PCR) testing which detects multiple pathogens from a single sample. Other possible explanations include that six new PICU’s (of a total of 34) began adding data from their units to PICAnet during the course of this study, or that there was an overall 15% increase in PICU admissions in the UK over the study period [18].
The ubiquitous nature of HMPV is demonstrated by the equal gender spread and wide age range of affected patients. It is also clear that HMPV has a seasonal variation in its incidence with a ‘peak season’ occurring between November and May, with the maximal incidence occurring in April. Similar seasonal patterns have been shown by other studies in the northern hemisphere, with the season being noted to range from November through to May, with peaks occurring between February to April [8,[19], [20], [21], [22]]. Interestingly we found few cases in January despite there being more cases in the months either side. This may be due to the fact January is peak season for RSV and influenza and testing may have predominantly been focussed on these pathogens, especially in the early years of our study.
We investigated the types of support required by children whilst admitted to PICU; 104 (91.2%) required respiratory support and 27 (23.7%) required inotropes, renal replacement therapy and/or ECMO. Of the two (1.8%) patients requiring ECMO, one had a primary diagnosis of acute viral bronchiolitis and the other viral pneumonia. Both patients had HMPV coded as the sole infective organism. Mortality in the study group was 4.4% (five children). Of those, four had HMPV only identified, with one also having rhinovirus detected. All five children required invasive respiratory support and four required inotropes. None of the children who died received renal replacement therapy or ECMO. Three had respiratory-related primary diagnoses including acute bronchiolitis and lower respiratory tract infection implying HMPV was the primary cause of death in these children. We are unaware of previous studies systematically measuring HMPV mortality rates. These data demonstrate HMPV can cause severe respiratory disease requiring the highest levels of intensive care support and even death.
HMPV places a significant financial burden on the NHS, potentially costing up to £1 million per year in 2014. This is only inclusive of children admitted to PICU and thus the true costs of all healthcare episodes associated with HMPV will be much higher. A study in the US has estimated a mean healthcare cost annually of US$277 million for all hospitalisations associated with HMPV [22].
HMPV (0.8 per 1000 PICU admissions) appears to account for a considerably smaller burden of PICU services when compared to RSV and influenza. A similar study using PICAnet data looking at PICU admissions related to influenza between October 2003 and March 2017 showed an average of 64 PICU admissions per year. Assuming an average of 16,000 PICU admissions per year this would account for approximately 4 per 1000 PICU admissions [23]. Similar data for UK PICU admissions related to RSV are not available, however, one study has investigated all cause bronchiolitis admissions to UK PICUs. It is known that approximately 80% of bronchiolitis admissions are caused by RSV. If considering PICU admissions for bronchiolitis in infants under 1 year alone then those attributable to RSV within this cohort would be approximately 45 per 1000 PICU admissions [24]. The true burden of RSV admissions, including older children, will be far higher. If there is more routine and frequent testing for HMPV, as there is for RSV and influenza, the burden of HMPV may be continue to increase.
Our study has several strengths and some limitations. We have described the burden of HMPV infection in PICUs at a national level over a nine-year period and calculated the associated healthcare costs. In line with other similar studies using coding data a significant limitation of our study is the fact that some cases of HMPV may have been incidental findings in children admitted to PICU for other reasons and some cases may have been incorrectly coded, thus leading to either an over- or underestimation of the true number of cases. This places some restrictions on conclusions that can be drawn about HMPV as a causative organism of the clinical characteristics observed. In addition to this, 8.8% of children had another infective pathogen coded in addition to HMPV during their stay. However, 91.2% of patients only had HMPV coded during their PICU stay and 84.2% of patients had a respiratory-based primary diagnosis suggesting HMPV was the reason for PICU admission for many children.
This data serves to show that HMPV can cause severe infection in children of all ages, although primarily young children. HMPV should be considered a significant pathogenic virus alongside RSV and influenza, potentially causing significant morbidity and even mortality among children. It may result in a cost to the NHS of up to £1 million per year in the PICU setting alone. Further efforts should be made to develop treatments and prophylactic agents to help clinicians prevent and manage children with HMPV infections.
Ethical approval
Not required.
Competing interests
None declared.
Funding
None.
Acknowledgements
Paediatric Intensive Care Audit Network Annual Report 2017 (published November 2017): Universities of Leeds and Leicester ©2017 The University of Leeds, University of Leicester and the Healthcare Quality Improvement Partnership.
References
- 1.Van Den Hoogen B.G., De Jong J.C., Groen J., Kuiken T., De Groot R., Fouchier R.A.M. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7(June (6)):719–724. doi: 10.1038/89098. http://www.nature.com/articles/nm0601_719 [Internet] [cited 2018 Aug 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullins J.A., Erdman D.D., Weinberg G.A., Edwards K., Hall C.B., Walker F.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 2004;10(April (4)):700–705. doi: 10.3201/eid1004.030555. http://www.ncbi.nlm.nih.gov/pubmed/15200863 [Internet] [cited 2018 Sep 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin G., De Serres G., Côté S., Gilca R., Abed Y., Rochette L. Human metapneumovirus infections in hospitalized children. Emerg. Infect. Dis. 2003;9(June (6)):634–640. doi: 10.3201/eid0906.030017. http://www.ncbi.nlm.nih.gov/pubmed/12781001 [Internet] [cited 2018 Sep 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAdam A.J., Hasenbein M.E., Feldman H.A., Cole S.E., Offermann J.T., Riley A.M. Human metapneumovirus in children tested at a tertiary‐care hospital. J. Infect. Dis. 2004;190(July (1)):20–26. doi: 10.1086/421120. https://academic.oup.com/jid/article-lookup/doi/10.1086/421120 [Internet] [cited 2018 Sep 19] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Foulongne V., Guyon G., Rodière M., Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr. Infect. Dis. J. 2006;25(April (4)):354–359. doi: 10.1097/01.inf.0000207480.55201.f6. https://insights.ovid.com/crossref?an=00006454-200604000-00015 [Internet] [cited 2018 Sep 19] Available from: [DOI] [PubMed] [Google Scholar]
- 6.Zar H.J., Barnett W., Stadler A., Gardner-Lubbe S., Myer L., Nicol M.P. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir. Med. 2016;4(June (6)):463–472. doi: 10.1016/S2213-2600(16)00096-5. https://www.sciencedirect.com/science/article/pii/S2213260016000965 [Internet] [cited 2018 Aug 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta–analysis. J. Glob. Health. 2015;5(June (1)) doi: 10.7189/jogh.05.010408. http://www.ncbi.nlm.nih.gov/pubmed/26445672 010408. [Internet] [cited 2018 Aug 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards K.M., Zhu Y., Griffin M.R., Weinberg G.A., Hall C.B., Szilagyi P.G. Burden of human metapneumovirus infection in young children. N. Engl. J. Med. 2013;368(Feberuary (7)):633–643. doi: 10.1056/NEJMoa1204630. [Internet] [cited 2018 Aug 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams J.V., Harris P.A., Tollefson S.J., Halburnt-Rush L.L., Pingsterhaus J.M., Edwards K.M. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 2004;350(January (5)):443–450. doi: 10.1056/NEJMoa025472. [Internet] [cited 2018 Sep 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz H.P., Clancy R.R., Druce J.D., Birch C.J., Mackay I.M., Carlin J.B. Accuracy of a home throat culture program: a study of parent participation in health care. Pediatrics. 1974;53(May (5)):687–691. http://www.ncbi.nlm.nih.gov/pubmed/4826724 [Internet] [cited 2018 Sep 19] Available from: [PubMed] [Google Scholar]
- 11.Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg. Infect. Dis. 2002;8(September (9)):897–901. doi: 10.3201/eid0809.020084. http://www.ncbi.nlm.nih.gov/pubmed/12194763 [Internet] [cited 2018 Sep 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Principi N., Bosis S., Esposito S. Human metapneumovirus in paediatric patients. Clin. Microbiol. Infect. 2006;12(April (4)):301–308. doi: 10.1111/j.1469-0691.2005.01325.x. https://www.sciencedirect.com/science/article/pii/S1198743X14615962#bib16 [Internet] [cited 2018 Aug 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paget S.P., Andresen D.N., Kesson A.M., Egan J.R. Comparison of human metapneumovirus and respiratory syncytial virus in children admitted to a paediatric intensive care unit. J. Paediatr. Child Health. 2011;47(October (10)):737–741. doi: 10.1111/j.1440-1754.2011.02043.x. [Internet] [cited 2018 Sep 19] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Panda S., Mohakud N.K., Pena L., Kumar S. Human metapneumovirus: review of an important respiratory pathogen [Internet] Int. J. Infect. Dis. 2014;25:45–52. doi: 10.1016/j.ijid.2014.03.1394. https://www.sciencedirect.com/science/article/pii/S120197121401488X Elsevier [cited 2018 Aug 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltbower R., Kapetanstrataki M., Norman L., Butler S., Hiley V., Draper E. 2015. Summary Report Paediatric Intensive Care Audit Network Annual Report 2018 Organisation Key.www.picanet.org.uk [Internet] [cited 2018 Nov 12]. Available from: [Google Scholar]
- 16.NHS Improvement . 2017. Reference Costs.https://improvement.nhs.uk/resources/reference-costs/ [Google Scholar]
- 17.RCPCH . 2014. High Dependency Care for Children - Time to Move on; pp. 1–50.https://www.rcpch.ac.uk/sites/default/files/2018-07/high_dependency_care_for_children_-_time_to_move_on.pdf [cited 2018 Aug 29] Available from: [Google Scholar]
- 18.Parslow R., Mcshane P., Fleming T., Fleming S., Norman L., Batchelor J. 2014. National Paediatric Intensive Care “Decade of Data” Report 2014.https://www.picanet.org.uk/wp-content/uploads/sites/25/2018/05/PICANet_A_Decade_of_Data_2014_Annual_Report_Summary.pdf [Internet] [cited 2018 Nov 12]. Available from: [Google Scholar]
- 19.Horton K.C., Dueger E.L., Kandeel A., Abdallat M., El-Kholy A., Al-Awaidy S. Viral etiology, seasonality and severity of hospitalized patients with severe acute respiratory infections in the Eastern Mediterranean Region, 2007–2014. Schanzer DL, editor. PLoS One. 2017;12(July (7)) doi: 10.1371/journal.pone.0180954. http://dx.plos.org/10.1371/journal.pone.0180954 e0180954. [Internet] [cited 2018 Sep 2]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes A.K., Fowlkes A.L., Schneider E., Mutuc J.D., Armstrong G.L., Gerber S.I. Human metapneumovirus circulation in the United States, 2008 to 2014. Pediatrics. 2016;137(April (5)) doi: 10.1542/peds.2015-2927. http://www.ncbi.nlm.nih.gov/pubmed/27244790 e20152927. [Internet] [cited 2018 Sep 2]Available from: [DOI] [PubMed] [Google Scholar]
- 21.Rafiefard F., Yun Z.B., Örvell C. Epidemiologic characteristics and seasonal distribution of human metapneumovirus infections in five epidemic seasons in Stockholm, Sweden, 2002-2006. J. Med. Virol. 2008;80(September (9)):1631–1638. doi: 10.1002/jmv.21242. [Internet] [cited 2018 Sep 2]Available from: [DOI] [PubMed] [Google Scholar]
- 22.Davis C.R., Stockmann C., Pavia A.T., Byington C.L., Blaschke A.J., Hersh A.L. Incidence, morbidity, and costs of human metapneumovirus infection in hospitalized children. J. Pediatric Infect. Dis. Soc. 2016;5(September (3)):303–311. doi: 10.1093/jpids/piv027. https://academic.oup.com/jpids/article-lookup/doi/10.1093/jpids/piv027 [Internet] [cited 2018 Sep 2] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardelid P., Kapetanstrataki M., Norman L., Fleming S.J., Lister P., Gilbert R. Impact of the introduction of a universal childhood influenza vaccination programme on influenza-related admissions to paediatric intensive care units in England. BMJ Open Respir. Res. 2018;5(June (1)) doi: 10.1136/bmjresp-2018-000297. e000297. [Internet] [cited 2018 Nov 21]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green C.A., Yeates D., Goldacre A., Sande C., Parslow R.C., McShane P. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch. Dis. Child. 2016;101(Feberuary (2)):140–146. doi: 10.1136/archdischild-2015-308723. http://www.ncbi.nlm.nih.gov/pubmed/26342094 [Internet] [cited 2018 Nov 21]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]