Abstract
Oligonucleotide-based therapeutics have been hailed as ‘the next great wave of the biotechnology revolution’ starting with antisense oligonucleotides (ASOs) nearly 20 years ago to RNA interference (RNAi) currently. Is RNAi just the latest research tool or does it have real potential as a therapeutic drug modality? As a research tool, it is evident that RNAi has revolutionized the biological sciences by allowing selective silencing of messenger RNA (mRNA) expression. With the advent of the postgenomic era, RNAi offers a therapeutic platform on which to identify potential picomolar active drug candidates to any target, including those that are conventionally undruggable. In this review, we will discuss the progress made in developing RNAi therapeutics for the treatment of respiratory diseases.
Introduction
RNA interference (RNAi) represents a natural endogenous mechanism that cells utilize to regulate RNA expression (for review, see [1, 2, 3]). It is possible to harness this pathway, through multiple means including micro-RNA (miRNA), vector-based short hairpin RNA (shRNA), and synthetic small interfering RNA (siRNA). Gene silencing can be induced by siRNA through a sequence-specific cleavage of perfectly complementary messenger RNA (mRNA), whereas miRNAs mediate translational repression and transcript degradation for imperfectly complementary targets (Figure 1 ). The ability of siRNA to potently, but reversibly, silence genes in vivo makes it particularly well suited as a therapeutic. In addition, because they enter the RNAi pathway later, siRNAs are less likely to interfere with gene regulation by endogenous miRNA [4, 5]. Synthetic siRNAs also have other potential advantages, as drug-like properties of siRNA can often be improved through introduction of chemical modifications, and manufacturing processes are usually amenable to scaled-up production. As a result, siRNAs are the class of RNAi therapeutic that are in most advanced preclinical and clinical studies.
Figure 1.
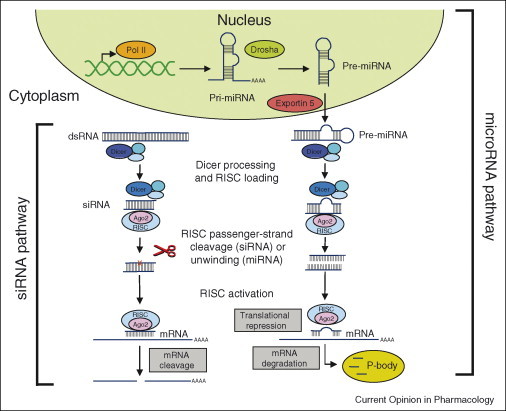
RNA interference in mammalian cells. RNA interference (RNAi) pathways involve either small interfering RNA (siRNA) or micro-RNA (miRNA). The siRNA pathway involves cleavage of long double-stranded RNA (dsRNA) by the Dicer enzyme complex to yield siRNA. These siRNA are then loaded into Argonaute 2 (AGO2) and the RNAi-induced silencing complex (RISC). If the loaded RNA duplex has perfect sequence complementarity, AGO2 cleaves the passenger (sense) strand so that active RISC containing the guide (antisense) strand is produced. The active RISC with guide strand recognizes target sites on mRNA and AGO2 then performs site-specific cleavage of the mRNA. RNAi therapeutics developed to harness the siRNA pathway usually involves delivery of synthetic siRNA into the cell cytoplasm. By contrast, the miRNA pathway begins with endogenously encoded primary micro-RNA transcripts (pri-miRNA) that are transcribed by polymerase II (Pol II). These are then processed by the Drosha enzyme complex to form precursor micro-RNA (pre-miRNA), and exported to the cell cytoplasm by exportin 5. The precursor miRNA is bound in the cytoplasm by the Dicer complex which processes it further for loading into the AGO2–RISC complex. When the RNA duplex loaded into RISC has imperfect sequence complementarity, the passenger (sense) strand is unwound leaving a mature miRNA bound to active RISC. The RISC–AGO2-bound miRNA then recognizes target sites (usually in the 3′-UTR) in the mRNA, leading to translation repression. Binding of miRNA to target mRNA may also lead in certain cases to mRNA degradation in processing (P)-bodies. Modified with permission from Nature Reviews Drug Discovery (3).
Identification of potentially potent lead siRNA candidates usually begins with bioinformatic design and siRNA synthesis, followed by in vitro characterization of siRNA for activity and specificity, which ultimately results in the selection of siRNA drug candidates (usually with subnanomolar or picomolar activity) (Figure 2 ). Having identified potentially optimized siRNA with drug-like properties, the major hurdle of effective delivery to the target cell remains. This typically involves investigation into in vivo efficacy and safety, along with additional studies examining pharmacokinetics, biodistribution, and cellular uptake of the formulated siRNA (Figure 2). Multiple approaches for the delivery of siRNA have been published ranging from the relative simplicity of direct administration of saline-formulated siRNA, to liposome-based and polymer-based nanoparticles approaches, to siRNA conjugation and complexation approaches [6]. With the formulation and delivery approaches employed today, silencing can be achieved in several tissues and cell types, most notably through direct delivery of siRNA to the central nervous system and lung epithelial cells, and through systemic delivery of siRNA to hepatocytes and tumor cells [3, 7]. Although multiple routes of administration using siRNA have been published, ranging from direct injection into target tissues to systemic administration, use of siRNA for the treatment of respiratory diseases has tended to focus on direct intratracheal or intranasal delivery of siRNA to the lungs (Table 1 ). In addition, while several different formulations have been investigated for the delivery of siRNA to the lungs, most reported successes have used simple saline formulations (Table 1).
Figure 2.
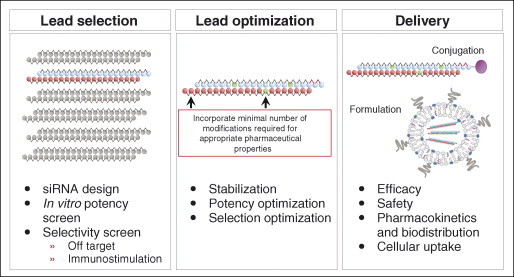
Developing an RNAi therapeutic. Several steps are typically required in the identification of an RNAi therapeutic. Selection of lead siRNA candidates usually begins with bioinformatic design, extends through empiric testing of potential siRNA lead candidates in a variety of in vitro assays for those with an appropriate selectivity and immunostimulatory profile. Lead optimization then proceeds with the introduction of stabilizing chemical modifications as required, along with additional in vitro testing for selectivity and potency. The final step involves the identification of an effective and safe delivery formulation. Multiple delivery strategies exist including siRNA conjugation or siRNA encapsulation into nanoparticles, such as liposomes. Beyond the assessment of efficacy and safety, other in vivo parameters such as pharmacokinetics, biodistribution and cellular uptake are also usually investigated. Success typically leads to further research and development activities including formulation optimization and manufacturing, analytic method development, and comprehensive safety/pharmacology analyses in multiple species.
Table 1.
In vivo efficacy using siRNA in pulmonary models
| Target | Formulation | Route | Model | Reference |
|---|---|---|---|---|
| RSV-P, PIV-P | Saline or lipoplex | Intranasal | RSV infection; PIV infection | [8••] |
| SARS | D5W or surfactant | Intranasal | SARS infection | [9•] |
| Influenza A — NP, PA | PEI | Intravenous | Influenza virus infection | [10] |
| Saline/oligofectamine | Intravenous hydrodynamic/intranasal | Influenza virus infection | [12] | |
| HO-1 | Saline | Intranasal | Hyperoxic acute lung injury | [15•] |
| KC, MIP-2, Fas | Saline | Intranasal | Septic acute lung injury | [17, 18] |
| Angiopoietin 2 | Saline | Intranasal | Hyperoxic acute lung injury | [16•] |
| DDR1 | Saline | Intranasal | Bleomycin-induced fibrosis | [31] |
| GFP | Chitosan NP | Intranasal | GFP transgenic | [24•] |
| Caveolin | Liposomes | Intravenous | Vascular permeability | [29] |
| IL-13 | jetPEI | Intravenous | Airway hypersensitivity | [30] |
Reports of positive in vivo efficacy in lung are listed along with formulation, route of administration and animal model utilized. All animal models were conducted in the mouse except for the SARS infection study which was performed in nonhuman primates. D5W, 5% dextrose; NP, nanoparticles; PEI, polyethylenimine; RSV, respiratory syncytial virus; PIV, parainfluenza virus; SARS, severe acute respiratory distress syndrome; GFP, green fluorescent protein.
Direct instillation of siRNA into the lung offers several important benefits. Delivery of siRNA directly to the lung has the advantage that, as for any drug, the dose of siRNA required for efficacy is substantially lower when administered near the target cell type. In addition, direct delivery might also reduce any theoretical undesired systemic side effects. Lastly and most importantly in the context of treating respiratory disease, instillation of siRNA (for instance by intranasal or intratracheal administration) allows direct access to lung epithelial cells, important cell types in a variety of pulmonary disorders. Among the myriad of lung pathologies in which epithelial cells are thought to play an important role are diseases such as cystic fibrosis, chronic obstructive pulmonary disease, asthma, and pulmonary fibrosis. In addition, multiple viruses infect the lung epithelium, including respiratory syncytial virus (RSV), parainfluenza virus (PIV), influenza, rhinoviruses, and severe acute respiratory syndrome (SARS) corona virus (SCV). As a consequence many viral and endogenous gene targets, especially those pertaining to lung epithelial cells, have been silenced using siRNA.
Pulmonary viral targets
Multiple airborne diseases infect lung or use it as an entry site for systemic infection. SiRNA can be rapidly designed to target the causal agent of viral infections — the virus itself. Not surprisingly, siRNA therapeutics have been designed targeting many different respiratory viruses, including RSV [8••], PIV [8••], SARS-SCV [9•], and influenza [10, 11, 12] (Table 1). In an important study Barik and colleagues demonstrated in mice that a single intranasal administration of 70 μg of siRNA targeting either the RSV or PIV P protein (an essential subunit of the viral RNA polymerase) reduced viral titers for both RSV and PIV by more than three orders of magnitude when administered before infection either unformulated or in the form of a lipoplex with Transit-TKO [8••]. Moreover, therapeutic application of the RSV siRNA up to three days post-infection reduced viral titers by more than two orders of magnitude. Disease pathology associated with RSV infection was also significantly alleviated by RSV siRNA treatment with no observable side effects. Importantly, the specificity of this antiviral effect was clearly demonstrated as the RSV-directed and PIV-directed siRNA only affected replication of the intended virus. The authors also showed that a combination of a RSV-directed and PIV-directed siRNA could be administered and was effective even in the case of viral coinfection [8••]. Using a similar approach, intranasally administered siRNA, targeting the SARS corona virus, formulated in 5% glucose substantially inhibited viral replication and improved lung pathology in a nonhuman primate model of viral infection [9•]. In summary, these results underscore the ability of direct lung instillation of saline-formulated virus-specific siRNA to impact viral replication and improve disease pathology in species ranging from rodents to nonhuman primates.
Another virus that has been published to be susceptible to siRNA-mediated inhibition is influenza. In two separate but related studies, it was shown that the same siRNA molecules directed against the nucleoprotein (NP) and acid polymerase (PA) influenza genes could protect mice against lethal influenza infection, including H5N1 virus [10, 12]. In one study, polyethylenimine (PEI) complexes of siRNA were injected intravenously and shown to reduce viral titers by about 10-fold [10]. PEI-complexed siRNA reduced viral load even when administered postinfection. In the second study, a hydrodynamic injection of siRNA followed by intranasal administration of oligofectamine/siRNA complexes reduced viral titers by at least 10-fold and conferred 100% survival to lethally infected mice [12]. Limited attempts to detect nonspecific activation of the immune system by siRNA were reported as negative [10]. Given the potent immunostimulatory properties of the flu siRNA utilized in these studies (and relative lack thereof in the control GFP siRNA used), the protection seen in the flu infection model may be mediated in large part by siRNA stimulation of innate immunity. The ability of certain unmodified siRNAs to elicit the production of proinflammatory mediators, including TNF-α and interferons, highlights the care that must be taken when examining in vivo endpoints which are sensitive to the release of such cytokines. Fortunately, several studies have shown that the introduction of 2′ base modifications can abolish double-stranded RNA-induced cytokine production [13, 14].
Endogenous lung targets
In addition to targeting viral genomes, successful siRNA-mediated silencing of multiple endogenous lung targets has been reported. Many endogenous lung targets have been silenced in the context of acute lung injury (ALI) models (Table 1). In a mouse experimental ischemia-reperfusion injury model intranasal administration of a saline-formulated heme oxygenase 1 (HO1)-specific siRNA-attenuated disease [15•]. Although ischemia-reperfusion-induced HO1 expression in multiple organs, including in epithelial cells in the lung, intranasally administered siRNA only silenced gene expression in the lung. In another study, hyperoxia-induced lung injury in mice was notably ameliorated by the intranasal administration of saline-formulated chemically modified angiopoietin 2-specific siRNA [16•]. Injury as well as consequential inflammation, mortality, cell death and vascular permeability were all dramatically reduced to levels equivalent to those seen in angiopoietin 2-deficient mice. Lastly, several other studies utilizing hemorrhage-induced and septic challenge models of ALI, have demonstrated that intratracheal administration of saline-formulated siRNA-targeting keratinocyte-derived chemokine [17], macrophage inflammatory protein 2 [17], and Fas [18] resulted in target mRNA silencing in the lung and an amelioration in disease pathology.
In summary, these reports suggest that under certain conditions, the direct administration of saline-formulated siRNA is able to specifically silence both viral and endogenous gene targets expressed in lung epithelial cells. Further studies are required to fully understand the mechanism of siRNA uptake in the lung, the contribution of lung injury to siRNA uptake, the nature of the epithelial cells being targeted, and whether other lung cell types might also be targeted.
Lung delivery approaches
Despite some success in delivering saline-based siRNA formulations directly to the lung in rodents and nonhuman primates, further improvements are desirable. Formulations could be designed to improve cellular uptake and cytoplasmic localization of siRNA, to broaden the cell types to which siRNA can be delivered, and to improve the pharmacokinetic and lung distribution profile of siRNA. While a large body of literature and experience in gene therapy to the lung supports the feasibility of this approach [19, 20], airway-directed oligonucleotide delivery is not simple. The lung has evolved both physical and immunologic barriers that can hinder the effectiveness of delivery formulations (for review, see [21, 22]). To date, several different formulations have been tested in the context of direct lung instillation of siRNA with mixed results (Table 1).
The cationic lipid Genzyme Lipid (GL) 67 is a well-established vehicle for in vivo lung gene transfer that has been utilized in human clinical trials. Griesenbach et al. [23] assessed the in vivo efficiency of GL67-mediated uptake of siRNAs into airway epithelium in mice. Intranasally delivered GL67-formulated siRNA localized mostly to alveolar macrophages and functional silencing in airway epithelial cells was marginal as siRNAs specific to beta-galactosidase reduced mRNA levels in the airway epithelium of K18-lacZ transgenic mice by one-third with no decrease in protein levels detected [23]. Another established approach for direct lung delivery of nucleic acids involves chitosan nanoparticles. Chitosan is a well-tolerated natural biodegradable polymer that forms cationic complexes with nucleic acids. Chitosan–siRNA duplexes, ranging in size from 40 to 600 nm, were shown to reduce by 40% EGFP expression in bronchiole epithelial cells of transgenic mice after the intranasal administration of chitosan/EGFP siRNA formulations [24•]. A chitosan-based nanoparticle was also used to directly deliver a shRNA targeting the RSV NS1 gene and resulted in significant antiviral activity [25]. More recent work has identified degree of deacetylation and higher chitosan molecular weight as important parameters for efficient siRNA-mediated knockdown [26]. Further formulation optimization is probably required before chitosan can be considered a robust formulation for siRNA lung delivery. A third approach involves the use of cationic cell penetrating peptides (CPPs) such as Tat to mediate siRNA delivery across the cellular membrane. Unfortunately, despite previous studies showing efficacy of siRNA/CPPs in vitro, conjugation of siRNA to Tat-(48–60) and penetratin failed to result in siRNA-mediated knockdown of p38 MAPK mRNA in mouse lung in vivo [27]. Lastly, influenza virus envelopes or virosomes have been published to deliver siRNA to the lung [28]. The use of virosomes can be especially beneficial for antiviral siRNA delivery in the lung, because virosomes are expected to be taken up by the cells infected with the virus. However, progress in virosome research is hampered by the difficulty in manufacturing viral envelopes.
Although most effort on new siRNA formulations has focused on improving direct lung delivery, several approaches have used systemic administration to target siRNA to the lungs. Cationic liposomes have been utilized to deliver caveolin-1 siRNA, leading to a >90% reduction of caveolin-1 in mouse lung endothelia and resulting in the expected physiological effects [29]. Lastly, Lively et al. recently demonstrated that PEI-complexed siRNA-directed against IL-13 reduced airway resistance in the allergen-induced airway hypersensitivity mouse model when delivered intravenously [30].
Clinical trials
To date, half a dozen human clinical trials are ongoing using RNAi, including one for the treatment of RSV infection. ALN-RSV01, an siRNA targeting the viral nucleocapsid (N) gene, has completed phase I intranasal and inhalation studies in healthy adults and was found to be generally well tolerated (Alnylam Pharmaceuticals [Cambridge, MA] press release). ALN-RSV01 has been evaluated in a phase II intranasal adult experimental infection study with top-line results just reported, showing ALN-RSV01 was safe and well tolerated, and demonstrated statistically significant antiviral activity (Alnylam Pharmaceuticals press release).
Conclusions
In the development of siRNA therapeutics several major challenges remain to be overcome, of which delivery is paramount. Although some success has been achieved with direct instillation of saline-formulated siRNA, further progress is required. Formulations are needed to improve cellular uptake and cytoplasmic localization of siRNA, to broaden the lung cell types to which siRNA can be delivered, and to improve the pharmacokinetic and lung distribution profile of siRNA. The rapid preclinical progress, and encouraging initial clinical results, suggests that siRNA therapeutics may have a useful role in the treatment of respiratory disease. Ultimately, the verdict on the utility of RNAi as a broad therapeutic drug class remains to be rendered, and will rest on human clinical trial data.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
The authors disclose a competing financial interest in Alnylam Pharmaceuticals.
Contributor Information
Antonin de Fougerolles, Email: tdefougerolles@alnylam.com.
Tatiana Novobrantseva, Email: tnovobrantseva@alnylam.com.
References
- 1.Aagaard L., Rossi J.J. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bumcrot D., Manoharan M., Koteliansky V., Sah D.W. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Fougerolles A., Vornlocher H.P., Maraganore J., Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm D., Streetz K.L., Jopling C.L., Storm T.A., Pandey K., Davis C.R., Marion P., Salazar F., Kay M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 5.John M., Constien R., Akinc A., Goldberg M., Moon Y.A., Spranger M., Hadwiger P., Soutschek J., Vornlocher H.P., Manoharan M. Effective RNAi-mediated gene silencing without interruption of the endogenous microRNA pathway. Nature. 2007;449:745–747. doi: 10.1038/nature06179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novobrantseva TNA A., Borodovsky A., de Fougerolles A. Delivering silence: advancements in developing siRNA therapeutics. Curr Opin Drug Discov Devel. 2008;11:217–224. [PubMed] [Google Scholar]
- 7.de Fougerolles A. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 8••.Bitko V., Musiyenko A., Shulyayeva O., Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]; More than three orders of magnitude viral load reduction by intranasally administered saline-formulated siRNA in doses as low as 70 g per animal.
- 9•.Li B.J., Tang Q., Cheng D., Qin C., Xie F.Y., Wei Q., Xu J., Liu Y., Zheng B.J., Woodle M.C. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]; Antiviral activity of direct intranasal instillation of saline-formulated siRNA in the lung of nonhuman primates is demonstrated in this study.
- 10.Ge Q., Filip L., Bai A., Nguyen T., Eisen H.N., Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci U S A. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas M., Lu J.J., Ge Q., Zhang C., Chen J., Klibanov A.M. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci U S A. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V., Guenthner-Biller M., Bourquin C., Ablasser A., Schlee M., Uematsu S., Noronha A., Manoharan M., Akira S., de Fougerolles A. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 14.Morrissey D.V., Lockridge J.A., Shaw L., Blanchard K., Jensen K., Breen W., Hartsough K., Machemer L., Radka S., Jadhav V. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 15•.Zhang X., Shan P., Jiang D., Noble P.W., Abraham N.G., Kappas A., Lee P.J. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]; Report demonstrating endogenous target silencing in the lung through intranasal delivery of saline-formulated HO-1 siRNA.
- 16•.Bhandari V., Choo-Wing R., Lee C.G., Zhu Z., Nedrelow J.H., Chupp G.L., Zhang X., Matthay M.A., Ware L.B., Homer R.J. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chemically modified siRNA targeting Ang2 and delivered intranasally is shown to effectively reduce lung pathology and mouse morbidity.
- 17.Lomas-Neira J.L., Chung C.S., Wesche D.E., Perl M., Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 18.Perl M., Chung C.S., Lomas-Neira J., Rachel T.M., Biffl W.L., Cioffi W.G., Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White A.F., Ponnazhagan S. Airway epithelium directed gene therapy for cystic fibrosis. Med Chem. 2006;2:499–503. doi: 10.2174/157340606778250180. [DOI] [PubMed] [Google Scholar]
- 20.Ziady A.G., Davis P.B. Current prospects for gene therapy of cystic fibrosis. Curr Opin Pharmacol. 2006;6:515–521. doi: 10.1016/j.coph.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Birchall J. Pulmonary delivery of nucleic acids. Expert Opin Drug Deliv. 2007;4:575–578. doi: 10.1517/17425247.4.6.575. [DOI] [PubMed] [Google Scholar]
- 22.Weiss D.J. Delivery of DNA to lung airway epithelium. Methods Mol Biol. 2004;246:53–68. doi: 10.1385/1-59259-650-9:53. [DOI] [PubMed] [Google Scholar]
- 23.Griesenbach U., Kitson C., Escudero Garcia S., Farley R., Singh C., Somerton L., Painter H., Smith R.L., Gill D.R., Hyde S.C. Cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir Res. 2006;7:26. doi: 10.1186/1465-9921-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Howard K.A., Rahbek U.L., Liu X., Damgaard C.K., Glud S.Z., Andersen M.O., Hovgaard M.B., Schmitz A., Nyengaard J.R., Besenbacher F. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]; The authors introduce chitosan-based siRNA nanoparticles and demonstrate in vivo RNA interference in bronchiole epithelial cells.
- 25.Zhang W., Yang H., Kong X., Mohapatra S., San Juan-Vergara H., Hellermann G., Behera S., Singam R., Lockey R.F., Mohapatra S.S. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- 26.Liu X., Howard K.A., Dong M., Andersen M.O., Rahbek U.L., Johnsen M.G., Hansen O.C., Besenbacher F., Kjems J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28:1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Moschos S.A., Williams A.E., Lindsay M.A. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochem Soc Trans. 2007;35:807–810. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 28.de Jonge J., Holtrop M., Wilschut J., Huckriede A. Reconstituted influenza virus envelopes as an efficient carrier system for cellular delivery of small-interfering RNAs. Gene Ther. 2006;13:400–411. doi: 10.1038/sj.gt.3302673. [DOI] [PubMed] [Google Scholar]
- 29.Miyawaki-Shimizu K., Predescu D., Shimizu J., Broman M., Predescu S., Malik A.B. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 30.Lively T.N., Kossen K., Balhorn A., Koya T., Zinnen S., Takeda K., Lucas J.J., Polisky B., Richards I.M., Gelfand E.W. Effect of chemically modified IL-13 short interfering RNA on development of airway hyperresponsiveness in mice. J Allergy Clin Immunol. 2008;121:88–94. doi: 10.1016/j.jaci.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuyama W., Watanabe M., Shirahama Y., Hirano R., Mitsuyama H., Higashimoto I., Osame M., Arimura K. Suppression of discoidin domain receptor 1 by RNA interference attenuates lung inflammation. J Immunol. 2006;176:1928–1936. doi: 10.4049/jimmunol.176.3.1928. [DOI] [PubMed] [Google Scholar]


