Highlights
-
•
Changing our respiratory panel assay decreased turn-around-time from 27.1 to 1.4 h.
-
•
The faster assay was associated with decreased empiric antibiotic use, less x-rays, and shorter LOS.
-
•
The faster assay improved optimal use of oseltamivir in influenza infected patients.
Keywords: Antimicrobial stewardship, Rapid diagnostics, Acute respiratory tract infections, Pediatric
Abstract
Background
Empiric antibiotic treatment is common among children with acute respiratory tract infections (ARTI), despite infections being predominately viral. The use of molecular respiratory panel assays has become increasingly common for medical care of patients with ARTIs.
Study design
This was a 6-year retrospective, single-centered study of pediatric inpatients who tested positive for an ARTI respiratory pathogen. We examined the relationship between clinical outcomes and whether the patient was tested using the Luminex Respiratory Viral Panel ([RVP]; in-use: Dec. 2009 – Jul. 2012) or Biofire Respiratory Pathogen Panel ([RP]; in-use Aug. 2012 – Jun. 2016). The prevalence and duration of pre-test empiric antibiotics, post-test oseltamivir administration to influenza patients, chest x-rays and length of stay between the two assays was compared.
Results
A total of 5142 patients (1264 RVP; 3878 RP) were included. The median laboratory turn-around-time for RP was significantly shorter than RVP (1.4 vs. 27.1 h, respectively; p < .001). Patients tested with RP were less likely to receive empiric antibiotics (OR: 0.45; p < .001; 95% CI: 0.39, 0.52) and had a shorter duration of empiric broad-spectrum antibiotics (6.4 h vs. 32.9 h; p < .001) compared to RVP patients. RP influenza patients had increased oseltamivir use post- test compared to RVP influenza patients (OR: 13.56; p < .001; 95% CI: 7.29, 25.20).
Conclusions
Rapid molecular testing positively impacts patient management of ARTIs. Adopting assays with a shorter turn-around-time improves decision making by decreasing empirical antibiotic use and duration, decreasing chest x-rays, increasing timely oseltamivir administration, and reducing length of stay.
1. Background
Acute respiratory tract infections (ARTI) —including the common cold, otitis media, pharyngitis, acute bronchiolitis, and pneumonia—are the most common diagnoses among patients seeking medical care in the US, and account for the majority of all antibiotic prescriptions [1,2]. Donnelly et al. estimated that each year there are 43 million emergency department (ED) visits for patients <5 years of age with a diagnosis of ARTI (rate: 354 per 1000 ED visits). [3]. Antimicrobials are not indicated for the common cold, bronchitis/bronchiolitis and the vast majority of pharyngitis cases, and specific criteria exists to target appropriate antibiotic use for otitis media, sinusitis and streptococcal pharyngitis. Yet, antibiotic prescribing is very common among children with ARTI, for bronchitis (71%), sinusitis (89%), and acute otitis media (86%) [4]. A study evaluating bronchiolitis management before and after the 2006 AAP guidelines found a significant reduction in RSV testing (p < .001) and decreased corticosteroids and bronchodilators use (p < .001) [5]. However, the trend in antibiotic use did not change (p = .07) in the post-AAP guideline period further highlighting the significant problem of overuse of antibiotics for ARTI.
While the use of stringent diagnostic criteria to confirm the diagnosis of ARTI is key in optimizing antibiotic prescribing, empiric treatment for ARTI remains common because viral symptoms are often clinically similar and difficult to distinguish from those caused by bacteria. Therefore, laboratory testing that provides accurate and prompt detection of pathogens associated with respiratory infections is important for proper patient care management. Molecular respiratory panel (MRP) assays are becoming increasingly popular due to their ability to detect multiple pathogens with high sensitivity and specificity and documented cost savings [[6], [7], [8]]. While research has demonstrated that MRPs may have a positive impact on patient outcomes such as decreasing empiric antibiotic exposures, length of hospital stay (LOS), and improving timely oseltamivir treatment for influenza patients [[9], [10], [11], [12], [13], [14]], there is a dearth of information on whether this clinical impact is conditional on the turn-around-time (TAT) of the MRP assay.
Both xTAG Respiratory Viral Panel (RVP) and Biofire Respiratory Panel (RP) are FDA-cleared MRP assays that can detect 12 and 20 respiratory pathogens, respectively. The objective of this study was to compare the impact of RVP and RP on clinical outcomes for patients that were admitted in our hospital from 2009 to 2016. We hypothesized that the rapid detection of respiratory pathogens by RP compared to RVP would be positively associated with changes in antibiotic treatment, initiation of oseltamivir and LOS on pediatric patients <18 years old.
2. Methods
2.1. Study sample
We performed a retrospective cohort study of pediatric patients who were admitted to our 354-bed free-standing children’s hospital between December 2009– June 2016 and who tested positive for at least one of the respiratory pathogens on either the RVP (Luminex Inc., Texas) or RP (Biofire LLC, Idaho) MRP assay. Data were abstracted from the electronic medical record for patients where the RVP or RP assay occurred either while admitted as an inpatient or during the ED encounter. For patients initially seen at the ED and subsequently admitted, all medical services were considered one clinical episode. Patients were excluded for all who were either 1) undergoing immune suppressive therapy; 2) were admitted to the NICU; 3) had a LOS greater than 7 days; or 4) RVP or RP assay was not ordered within the first 48 h of hospitalization. This study was reviewed and approved by the Children’s Mercy Hospital Institutional Review Board.
2.2. Molecular-multiplex assays
In our hospital the RVP assay was introduced in December 2009 and was replaced by the RP assay in August 2012. Patients’ respiratory samples were tested by either the RVP or RP based on test in use date. At our institution RVP tests were run in batch mode once daily, 7 days a week, whereas RP tests were run as the samples arrived (24/7) in the clinical lab.
2.3. Study outcomes
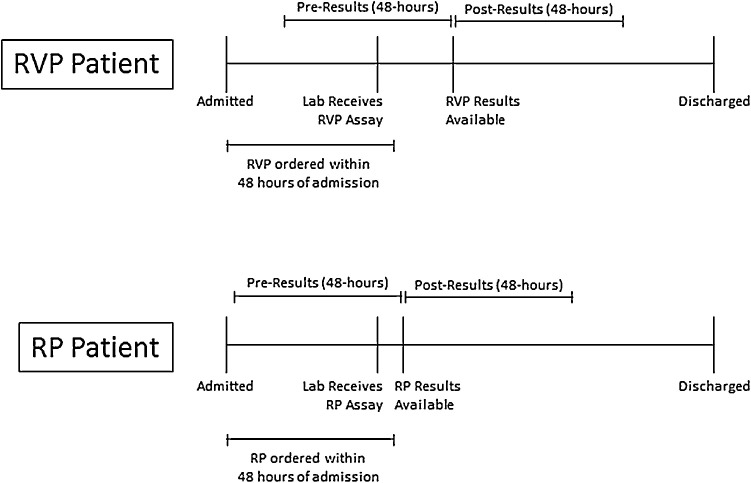
The primary outcome of this study was appropriate antimicrobial therapy. Binary treatment indicators were based on antimicrobial use during a 48-hour period both before and after the MRP assay results were reported in the laboratory information system (Fig. 1 ), including: 1) use of empiric systemic antibiotic treatment between time of admission and result availability and 2) administering oseltamivir to influenza positive patients during the 48 h following result availability. The prevalence of narrow- and broad-spectrum empiric antibiotic treatment was also calculated, including the duration of empiric therapy, which was defined as the time difference, in hours, between the first and last antibiotic administration. We considered penicillin, ampicillin and clindamycin as narrow-spectrum antibiotics; all other antibiotics were considered broad-spectrum. We also evaluated use of chest radiographs within the first 48 h of admission and LOS (in hours) as secondary outcomes. Lastly, we calculated the turn-around-time ([TAT]; i.e., time from when the clinical laboratory received the specimen to when the test was completed and results reported in the laboratory information system) for each specimen tested.
Fig. 1.
Timing of Antibiotic Treatment Categories Relative to MRP Assay.
2.4. Clinical predictors
We dichotomized our study time period into either RVP patients or RP patients. The patient’s age at the time of admission was categorized into either <90 days (infant sepsis work-up), 3–24 months (upper age range from 2015 AAP guideline on bronchiolitis management [15]) or 2 years and older. A mutually exclusive pathogen indicator was created based on the organism(s) detected in the RVP/RP assay (rhinovirus/enterovirus, influenza virus, respiratory syncytial virus (RSV), adenovirus, human metapneumovirus, parainfluenza, co-detection [2+ viruses], or bacterial pathogen). For example, a patient who was positive for RSV only was categorized as ‘RSV’ whereas a patient who tested positive for both RSV and rhinovirus/enterovirus was categorized as ‘co-detection’. Any patient with a bacterial pathogen, regardless of concurrent viral infection, was categorized as ‘bacterial pathogen’. In order to further depict clinical presentation and medical decision making, we included the location where the MRP assay was ordered (PICU, ED, medical/surgical, or other) and the calendar quarter.
2.5. Data analysis
The frequency distribution of our categorical outcomes was calculated for both RVP/RP time periods, with Pearson’s chi-square used to compare proportions. Hospital LOS and TAT were treated as continuous outcomes with the Mann-Whitney U test used to compare distributions. Multivariable logistic models were used for each of our categorical outcomes to examine the relationship with RVP/RP time period, after adjusting to medical service, patient age, pathogen, and seasonality. All analysis were completed using SAS (SAS 9.4; SAS Institute, Cary, NC).
3. Results
A total of 12,803 patients in whom either one of the MRP assays (Luminex RVP or Biofire RP) was obtained during the study time period were initially identified. We excluded 5129 (40.1%) patients who tested negative for any pathogens since the focus of the current study was to describe the impact of rapid detection of respiratory pathogens in management of hospitalized patients. We further excluded 349 (2.7%) who were admitted to either the NICU or hematology/oncology ward, 456 (3.6%) who did not have the RVP/RP ordered within the first 48 h of admission, 708 (5.5%) who had a LOS > 7 days, 1007 (7.9%) who were seen in the ED but were not admitted to the hospital, and 12 (.09%) who were ≥18 years old at the time of the admission. Our final study group included 5142 patients
A total of 1264 (24.6%) patients were tested with the RVP assay (Table 1 ). Those patients who were ≥2 years old represented the largest age group in our analysis (n = 2246; 43.7%). Nearly half of our patients tested positive for rhinovirus/enterovirus only whereas 110 (2.1%) patients were positive for ≥1 bacterial organisms.
Table 1.
Demographic and Clinical Characteristics of ARTI Patients with Respiratory Pathogen Detection.
| No. (%) of Tested (N = 5142) | ||
|---|---|---|
| MRP Assay | ||
| Luminex | 1264 (24.6%) | |
| Biofire | 3878 (75.4%) | |
| Age at Admission | ||
| <90 days | 819 (15.9%) | |
| 3-24 months | 2077 (40.4%) | |
| 2+ years | 2246 (43.7%) | |
| Organism | ||
| Any Bacterial Organism a | 110 (2.1%) | |
| Rhino/Entero | 2518 (49.0%) | |
| Influenza | 237 (4.6%) | |
| RSV | 706 (13.7%) | |
| Metapneumo | 344 (6.7%) | |
| Coronavirus | 134 (2.6%) | |
| Parainfluenza | 322 (6.3%) | |
| Adenovirus | 191 (3.7%) | |
| Viral Co-detection | 580 (11.3%) | |
| Location of MRP Order | ||
| PICU | 151 (2.9%) | |
| ED | 1439 (28.0%) | |
| Med/Surg | 3301 (64.2%) | |
| Other/Unknown | 251 (4.9%) | |
| Calendar Quarter of Order | ||
| Q1 | 1840 (35.8%) | |
| Q2 | 1193 (23.2%) | |
| Q3 | 1027 (20.0%) | |
| Q4 | 1082 (21.0%) | |
Includes M. pneumoniae, C. pneumoniae, andB. pertussis.
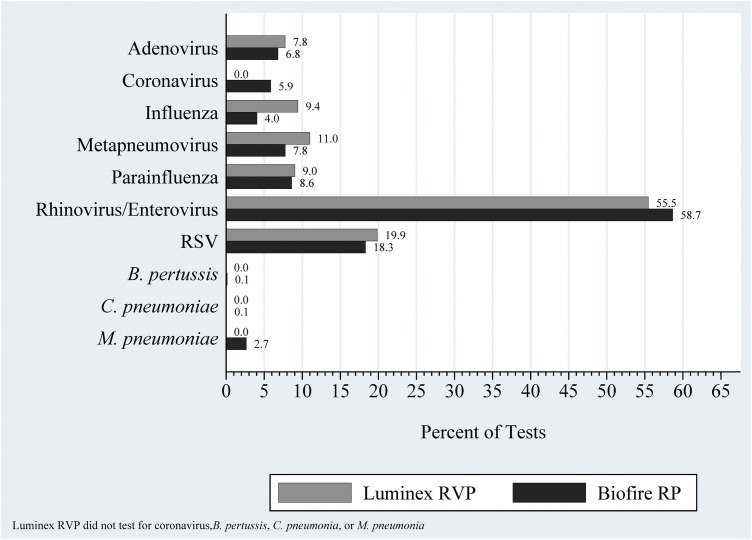
The frequency distribution of respiratory pathogens between the two RP/RVP time periods were comparable (Fig. 2 ), with the exception of influenza (RVP: 119 [9.4%] vs. RP: 157 [4.1%]; p < .001) and human metapneumovirus (RVP: 139 [11.0%] vs. RP: 301 [7.8%]; p < .001), which suggests reasonable stability in the epidemiology of respiratory pathogens during our study time period.
Fig. 2.
Distribution of Respiratory Pathogens, by Study Time Period.
Patients who were tested with RP were significantly less likely to receive any empiric antibiotic therapy prior to results from the MRP assay reported in the laboratory information system when compared to RVP patients (32.0% vs. 51.1%; p < .001 [Table 2 ]). After adjusting for patient age, organism, location, and seasonality, RP patients continued to be less likely to have empiric therapy (odds ratio [OR]: 0.45; 95% confidence interval [CI]: 0.39, 0.52). When considering just those patients who had >1 empiric antibiotic administration, RP patients had a significantly shorter median duration of broad-spectrum (6.4 h vs. 32.9 h; p < .001) and narrow-spectrum therapy (9.1 h vs. 31.4 h; p < .001) compared to RVP patients.
Table 2.
Comparison of Medication Administrations, Chest X-rays, Turn-around-Time, and LOS by MRP Assay Type.
| Unadjusted |
Adjusted b |
|||||
|---|---|---|---|---|---|---|
| Luminex | Biofire | p-value a | OR (95% CI) | p-value | ||
| Empiric antibiotics -- no. (%) | 646 (51.1%) | 1239 (32.0%) | <.001 | 0.45 (0.39, 0.52) | <.001 | |
| Empiric broad-spectrum | 447 (35.4%) | 829 (21.4%) | <.001 | 0.50 (0.43, 0.58) | <.001 | |
| Broad-spectrum duration (hours) c -- median [IQR] | 32.9 [22.9, 45.8] | 6.4 [1.9, 13.2] | <.001 | --- | --- | |
| Empiric narrow-spectrum -- no. (%) | 382 (30.2%) | 625 (16.1%) | <.001 | 0.44 (0.38, 0.52) | <.001 | |
| Narrow-spectrum duration (hours) c-- median [IQR] | 31.4 [18.1, 43.4] | 9.1 [6.1, 16.3] | <.001 | --- | --- | |
| Oseltamivir post-test d -- no. (%) | 23 (19.3%) | 124 (79.0%) | <.001 | 13.56 (7.29, 25.20) | <.001 | |
| Chest x-rays within 48 hours of admission | 803 (63.5%) | 2245 (57.9%) | <.001 | 0.71 (0.62, 0.82) | <.001 | |
| Length of stay (hours) -- median [IQR] | 54.3 [38.6, 89.0] | 49.0 [32.2, 78.9] | <.001 | --- | --- | |
| Turn-around-time (hours) | 27.1 [24.7, 50.3] | 1.4 [1.2, 1.7] | <.001 | --- | --- | |
Abbreviation: IQR, inter-quartile range; OR, odds ratio; CI, confidence interval.
Based on Fisher's Exact test for categorical and Kruskal-Wallis test for continuous.
Odds relative to Luminex after adjusting for patient age, organism, location, and calendar quarter.
Among patients with more than one empiric antibiotic administration.
Among patients positive for influenza.
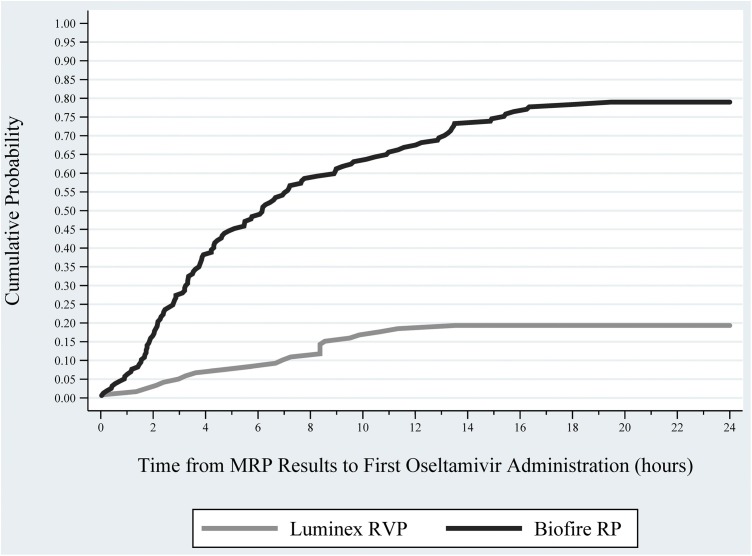
RP influenza patients were more likely to receive oseltamivir during the 48 h after result availability when compared to RVP influenza patients (79.0% vs. 19.3%; p < .001). After adjusting for patient age, order location, and calendar quarter, RP patients were more likely to receive post-MRP oseltamivir (OR: 13.56; 95% CI: 7.29, 25.20) when compared to RVP patients. Nearly 50% of RP influenza patients received oseltamivir within 6 h following the results (Fig. 3 ), whereas less than 10% of RVP patients received a dose during that same time period.
Fig. 3.
Probability of Oseltamivir Initiation Among Confirmed Influenza Patients, by Study Time Period.
The median LOS for RP patients (49.0 h) was significantly shorter when compared with RVP patients (54.3 h; p < .001). In addition, the median TAT for patients tested with the RP assay (1.4 h) was significantly shorter compared to the RVP (27.1 h; p < .001).
Empiric antibiotics were more likely for patients who had detection of a bacterial organism (67.3%), influenza (43.9%), RSV (45.8%), and parainfluenza (45.3%) relative to patients with rhinovirus/enterovirus (30.9%) [Table 3 ]. After adjusting for MRP assay, patient age, order location, and calendar quarter, an increased likelihood of empiric antibiotics was observed for patients with a bacterial organism (OR: 5.50; 95% CI: 3.63, 8.35) and parainfluenza (OR: 1.75; 95% CI: 1.37, 2.24).
Table 3.
Prevalence of Empiric Antibiotic Administration by Respiratory Organism.
| Unadjusted |
Adjusted a |
||||
|---|---|---|---|---|---|
| Received -- no. (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Any Bacterial Organism b | 74 (67.3%) | 4.59 (3.05, 6.89) | <.001 | 5.50 (3.63, 8.35) | <.001 |
| Rhino/Entero | 779 (30.9%) | -ref- | --- | -ref- | --- |
| Influenza | 104 (43.9%) | 1.75 (1.33, 2.29) | <.001 | 1.42 (1.07, 1.89) | 0.015 |
| RSV | 323 (45.8%) | 1.88 (1.59, 2.23) | <.001 | 1.62 (1.34, 1.97) | <.001 |
| Metapneumo | 154 (44.8%) | 1.81 (1.44, 2.27) | <.001 | 1.60 (1.25, 2.04) | <.001 |
| Parainfluenza | 146 (45.3%) | 1.85 (1.46, 2.34) | <.001 | 1.75 (1.37, 2.24) | <.001 |
| Adenovirus | 56 (29.3%) | 0.93 (0.67, 1.28) | 0.641 | 0.84 (0.60, 1.17) | 0.307 |
| Coronavirus | 43 (32.1%) | 1.05 (0.73, 1.53) | 0.779 | 1.08 (0.73, 1.60) | 0.688 |
| Viral Co-detection | 206 (35.5%) | 1.23 (1.02, 1.49) | 0.033 | 1.15 (0.94, 1.41) | 0.179 |
Abbreviation: OR, odds ratio; CI, confidence interval.
Odds after adjusting for MRP assay, patient age, nurse unit, and calendar quarter.
IncludesM. pneumoniae, C. pneumoniae, andB. pertussis.
4. Discussion
Utilizing MRP assays may increase the likelihood of optimal treatment of viral ARTI [[9], [10], [11], [12]]. However, since some of these studies have been restricted to adult patients [11], examined clinical impact for influenza patients only [12] or only included data from peak respiratory seasons [9,10] more studies are needed to provide a comprehensive assessment of the clinical impact for pediatric ARTI patients. Our study included 5142 pediatric patients with positive respiratory pathogens that were collected over 6 years. We observed that relying on rapid diagnostic testing for ARTI cases may have a direct impact for the patient management, including reducing unnecessary antibiotic and radiation exposure, and reducing LOS.
Despite ARTIs being predominately viral in nature, empiric antibiotic treatment for ARTI remains prevalent. Kronman et al. found that antibiotics were frequently prescribed for bronchitis, sinusitis, and acute otitis media patients. [4] Byington et al. examined patients who received a direct fluorescent assay for respiratory pathogens and found that only 12.8% of patients who also received bacterial cultures tested positive for a bacterial infections [16]. We propose that use of MRP assays significantly improves diagnostic yield and accurately identifies viral etiology in majority of patients (∼60%; internal data monitoring over past 5 years), providing clinicians added confidence in antibiotic treatment decisions. Our findings provide much needed data to federal programs and insurance agencies that question the value of MRP assays in management of ARTI patients [17]. We feel our study contributes to existing evidence [18] on how rapid molecular diagnostics may further optimize appropriate care and management of ARTI patients.
Our study found that patients tested with RP, which had a shorter TAT, were less likely to receive empiric antibiotics. We hypothesized that having a shorter TAT may influence the clinician’s decision making to delay empiric antibiotic therapy knowing that assay results would be available within a few hours. A study among patients who tested positive using a rapid influenza test found that when the clinician was aware of the results they were significantly less likely to prescribe antibiotics (7.3% vs. 24.5%) and more likely to give an antiviral prescription (18.8% vs. 6.7%) relative to when the clinician was unaware the patient was positive for influenza. [13] In our study, patients with RP test results available approximately 26 h faster than RVP were less likely to have empiric antibiotic therapy (32.0% vs. 51.1%) as well as a significantly shorter duration of empiric broad-spectrum (6.4 vs. 32.9 h) and narrow-spectrum therapy (9.1 vs. 31.4 h).
According to CDC guidelines, anti-influenza therapy is recommended for patients <2 years with signs and symptoms of flu-like illness. [19] In addition, multiple observational studies have demonstrated that receipt of oseltamivir significantly improves patient outcomes for patients with influenza [[20], [21], [22], [23], [24], [25]], although clinical improvement is more likely if oseltamivir is initiated shortly after symptom onset [22,[21], [22], [23], [24], [25]]. A prospective study of ICU influenza patients compared early treatment vs. late treatment oseltamivir administration and found that early treatment had shorter ICU LOS (18.4 vs. 22.7 days), shorter hospital LOS (27.2 vs. 34.0 days), and decreased likelihood of mortality (21.5% vs. 34.3%) [24]. In our study, nearly 80% of RP influenza patients received oseltamivir within 48 h of result availability, compared with 19.3% for RVP influenza patients. Utilization of tests with shorter TAT may ensure that optimal treatment for influenza infections is given.
Determination of cost effectiveness is important when hospitals decide on adopting rapid diagnostic testing. An increasing number of studies have concluded that rapid diagnostics that test for ARTI viruses provide a cost savings. [6,7] In our study, RP patients had decreased empirical antibiotic use, decreased likelihood of chest x-rays, and shorter LOS which could translate into decreased hospital cost. Further cost effectiveness research is needed that also considers the cost of the rapid test for all patients, not just those who test positive.
There were limitations with this study. We excluded patients seen in the either the NICU or hematology/oncology ward as well as patients who had a LOS > 7 days. However, we purposely excluded these patients in an attempt to have a sample of otherwise healthy children. Second, this was a single-center study at a freestanding pediatric hospital, therefore our results may not generalize to all centers that provide care for ARTI. Third, we did not examine concurrent bacterial infections, which could partly explain empiric antibiotic treatment. Since the prevalence of concurrent bacterial infection is unlikely to be influenced by the assay type (i.e., RVP/RP), we would argue the potential of this biasing our results is minimal. We observed less human metapneumovirus and influenza cases during the RP phase which may have influenced treatment strategies, especially oseltamivir use. Patients tested but never admitted were excluded, thus our inpatient sample may represent patients with more severe ARTI symptoms. Lastly, we did exclude patients who tested negative, thus our study does not provide a complete representation of ARTI patients but rather addresses the importance of rapid respiratory pathogen detection on management of hospitalized patients. We intend to conduct future research on MRP assays that includes both negative and positive patients.
This study demonstrates that adopting MRP assays with a shorter TAT can have a significant improvement for pediatric inpatients with viral ARTI, including decreased exposure to empirical antibiotics, decreased exposure to chest x-rays, increased optimization of timely oseltamivir administration, and shorter LOS. By providing respiratory pathogen results in a timely manner, rapid diagnostics have the ability to help guide clinicians on judicious antibiotic use for ARTI patients.
Funding
None.
Conflict of interest
None.
Author contributions statement
Brian Lee: Conception, Methodology, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Visualization. Ferdaus Hassan: Conception, Methodology, Writing – Original Draft, Writing – Review & Editing. Mary Anne Jackson: Conception, Writing – Review & Editing. Rangaraj Selvarangan: Conception, Methodology, Writing – Review & Editing, Visualization.
References
- 1.Grijalva C.G., Nuorti J.P., Griffin M.R. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCaig L.F., Besser R.E., Hughes J.M. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096–3102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly J.P., Baddley J.W., Wang H.E. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob. Agents Chemother. 2014;58(3):1451–1457. doi: 10.1128/AAC.02039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronman M.P., Zhou C., Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics. 2014;134(4):e956–65. doi: 10.1542/peds.2014-0605. [DOI] [PubMed] [Google Scholar]
- 5.Parikh K., Hall M., Teach S.J. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133(1):e1–7. doi: 10.1542/peds.2013-2005. [DOI] [PubMed] [Google Scholar]
- 6.Barenfanger J., Drake C., Leon N., Mueller T., Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 2000;38(8):2824–2828. doi: 10.1128/jcm.38.8.2824-2828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahony J.B., Blackhouse G., Babwah J. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J. Clin. Microbiol. 2009;47(9):2812–2817. doi: 10.1128/JCM.00556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babady N.E., Mead P., Stiles J. Comparison of the Luminex xTAG RVP Fast assay and the Idaho Technology FilmArray RP assay for detection of respiratory viruses in pediatric patients at a cancer hospital. J. Clin. Microbiol. 2012;50(7):2282–2288. doi: 10.1128/JCM.06186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wishaupt J.O., Russcher A., Smeets L.C., Versteegh F.G., Hartwig N.G. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128(5):e1113–20. doi: 10.1542/peds.2010-2779. [DOI] [PubMed] [Google Scholar]
- 10.Rogers B.B., Shankar P., Jerris R.C. Impact of a rapid respiratory panel test on patient outcomes. Arch. Pathol. Lab. Med. 2015;139(5):636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 11.Brittain-Long R., Westin J., Olofsson S., Lindh M., Andersson L.M. Access to a polymerase chain reaction assay method targeting 13 respiratory viruses can reduce antibiotics: a randomised, controlled trial. BMC Med. 2011;9:44. doi: 10.1186/1741-7015-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M., Qin X., Astion M.L. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am. J. Clin. Pathol. 2013;139(1):118–123. doi: 10.1309/AJCPH7X3NLYZPHBW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonner A.B., Monroe K.W., Talley L.I., Klasner A.E., Kimberlin D.W. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 14.Subramony A., Zachariah P., Krones A., Whittier S., Saiman L. Impact of multiplex polymerase chain reaction testing for respiratory pathogens on healthcare resource utilization for pediatric inpatients. J. Pediatr. 2016;173:196–201. doi: 10.1016/j.jpeds.2016.02.050. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralston S.L., Lieberthal A.S., Meissner H.C. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 16.Byington C.L., Castillo H., Gerber K. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children’s hospital. Arch. Pediatr. Adolesc. Med. 2002;156(12):1230–1234. doi: 10.1001/archpedi.156.12.1230. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services . 2018. PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Multiplex Nucleic Acid Amplified Tests for Respiratory Viral Panels (DL37713) Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=37712&ver=3&DocID=DL37713&SearchType=Advanced&bc=IAAAABAAAAAA&, (Accessed 28 March 2018) [Google Scholar]
- 18.Infectious Disease Society of America . Better Tests, Better Care. Available at: http://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocac/Current_Topics_and_Issues/Diagnostics/Better%20Tests%20Better%20Care.pdf, Accessed 4/5/2018.
- 19.Fiore A.E., Fry A., Shay D. Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- 20.Barr C.E., Schulman K., Iacuzio D., Bradley J.S. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza. Curr. Med. Res. Opin. 2007;23(3):523–531. doi: 10.1185/030079906x167499. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson K.G., Aoki F.Y., Osterhaus A.D. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355(9218):1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 22.Spagnuolo P.J., Zhang M., Xu Y. Effects of antiviral treatment on influenza-related complications over four influenza seasons: 2006–2010. Curr. Med. Res. Opin. 2016;32(8):1399–1407. doi: 10.1080/03007995.2016.1176016. [DOI] [PubMed] [Google Scholar]
- 23.Viasus D., Pano-Pardo J.R., Pachon J. Timing of oseltamivir administration and outcomes in hospitalized adults with pandemic 2009 influenza A(H1N1) virus infection. Chest. 2011;140(4):1025–1032. doi: 10.1378/chest.10-2792. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez A., Diaz E., Martin-Loeches I. Impact of early oseltamivir treatment on outcome in critically ill patients with 2009 pandemic influenza A. J. Antimicrob. Chemother. 2011;66(5):1140–1149. doi: 10.1093/jac/dkq511. [DOI] [PubMed] [Google Scholar]
- 25.Muthuri S.G., Venkatesan S., Myles P.R. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir. Med. 2014;2(5):395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]