Highlights
-
•
6% of acute respiratory infections were linked to human metapneumovirus.
-
•
High heterogeneity was observed.
-
•
HMPV should be taken into account as a possible etiology in hospitalized ARI.
Keywords: Human metapneumovirus, Meta-analysis, Prevalence, Acute respiratory infection, Respiratory virus
Abstract
This meta-analysis aimed to estimate the prevalence of human metapneumovirus (hMPV) infections in patients hospitalized for acute respiratory infection (ARI) and to study factors associated with this prevalence. Medline and ScienceDirect databases were searched for prospective observational studies that screened hospitalized patients with ARI for hMPV by RT-PCR, with data available at December 27, 2014. The risk of bias was assessed regarding participation rate, definition of ARI, description of diagnostic technique, method of inclusion identical for all subjects, standardized and identical sampling method for all subjects, analysis performed according to the relevant subgroups, and presentation of data sources. Random-effect meta-analysis with arcsine transformation and meta-regressions was used. In the 75 articles included, the prevalence of hMPV among hospitalized ARI was 6.24% (95% CI 5.25–7.30). An effect of the duration of the inclusion period was observed (p = 0.0114), with a higher prevalence of hMPV in studies conducted during periods of 7–11 months (10.56%, 95% CI 5.97–16.27) or complete years (7.55%, 95% CI 5.90–9.38) than in periods of 6 months or less (5.36%, 95% CI 4.29–6.54). A significant increase in the incidence with increasing distance from the equator was observed (p = 0.0384). hMPV should be taken into account as a possible etiology in hospitalized ARI.
1. Introduction
Acute respiratory tract infections (ARI) are a major cause of morbidity and mortality in a world in which viral etiologies predominate [1], [2]. Despite improved diagnostic techniques, the pathogen remained unidentified in a significant proportion of cases, which led to the search for new viruses [3]. Among these, human metapneumovirus (hMPV) was first isolated by genetic analysis of nasopharyngeal samples from 28 hospitalized children. In these children, this virus, which belongs to the Paramyxoviridae family, was associated with signs of airway infection: cough, fever, runny nose, wheezing and dyspnea [4]. hMPV is an enveloped virus with a single negative-stranded RNA genome of ∼13 kb [5]. As with other pneumoviruses, eight transcription units have been described. Some genes (F for the fusion protein, N for the nucleocapsid, M for the matrix and L for the polymerase) allow the characterization of the virus by reverse transcriptase polymerase chain reaction (RT- PCR) [6]. More recently, immunofluorescence [7], [8] and multiplex real-time PCR techniques have been developed and virological diagnosis is more feasible in clinical practice.
Though hMPV infection was identified in 2001, many of its features remain to be determined. Among the many research topics, the question of the prevalence of hMPV infections among ARI has been raised by many observational studies on various types of inpatient populations. To our knowledge, no synthesis of the available data concerning the role of hMPV in patients hospitalized with ARI has been done.
The purpose of this meta-analysis was to estimate the prevalence of hMPV infections in patients hospitalized for ARI and to study factors associated with this prevalence.
2. Methods
2.1. Data sources
Medline and ScienceDirect databases were searched for studies published from the inception of each database until December 27, 2014, without restriction on language. The following keywords were used: (1) in Pubmed: (metapneumovirus [mesh] OR metapneumovirus[title/abstract]) AND (hospital[title/abstract] OR hospitalized[title/abstract]) NOT (“Disease Outbreaks”[Mesh] OR “Outbreak”[ti]) NOT(“Retrospective Studies”[Mesh] OR retrospective[title/abstract]); and (2) in ScienceDirect: TITLE-ABSTR-KEY(metapneumovirus) AND (TITLE-ABSTR-KEY(hospital) OR TITLE-ABSTR-KEY(hospitalized)) AND NOT TITLE(outbreak) AND NOT TITLE-ABSTR-KEY(retrospective). The titles and abstracts of potentially relevant studies were scanned. When the studies seemed to meet the eligibility criteria; or when the information was insufficient to exclude them; the full articles were read.
Articles dealing with literature reviews or meta-analyses were scanned for additional studies.
2.2. Inclusion and exclusion criteria
Only prospective observational studies dealing with hospitalized patients with a clinical diagnosis of ARI were included. All types of ARI (upper, lower, pneumonia, severe pneumonia, bronchiolitis…) were included. Only studies including fever alone in the inclusion criteria were excluded. The search for hMPV had to be conducted systematically or by sampling of the population in the presence of defined inclusion criteria (respiratory signs) and by PCR (which is considered the gold standard) performed on respiratory samples.
Studies dealing with patients discharged after emergency admission, or which concerned a particular population (patients with cancer, cystic fibrosis, premature newborns, patients already hospitalized, patients screened for hMPV only if other respiratory viruses were negative, outbreak investigation), or with insufficiently detailed data to conduct a meta-analysis were excluded.
2.3. Data extraction and quality assessment
The data were extracted by two independent readers using a standardized grid and included: number of viruses screened, number of patients screened, number of patients infected with hMPV, viral and bacterial co-infections, inclusion periods, hospital location (country, city, continent, latitude, longitude), the gene used for the PCR, inclusion and exclusion criteria (patients’ ages, definition of infection, severity), and the sampling method.
The study quality was evaluated by two independent readers using a standard form based on: (1) participation rate: more than 75% response (agree to participate) or analysis to show whether respondents and non-respondents were similar for the socio-demographic characteristics; (2) ARI correctly defined; (3) method of inclusion identical for all subjects; (4) description of diagnostic technique; (5) same type of sample collected for all patients (nasopharyngeal aspirate, nasal or throat swab…); (6) standardized method for sample collection (quantity of aspirate or of liquid used for the nasal wash…); (7) analysis performed according to relevant subgroups (by age classes, by center, or by symptomatology, for example); (8) presentation of data sources (counts are presented, not only percentages) [9], [10], [11], [12], [13]. For each item, one point was awarded if the criterion was met. This resulted in a maximum score of 8. If the risk of non-response bias was uncertain, half a point was attributed.
2.4. Statistical analyses
The prevalence of hMPV infections was calculated in a meta-analysis of the proportion of patients with a positive PCR for hMPV divided by the number of patients admitted for ARI. An arcsine transformation was used to stabilize the variation of proportions [14]. A random effect model was used according to DerSimonian-Laird’s method [15]. Sensitivity analyses were conducted by removing the lower quality studies: in the first step, we removed studies for which we were not sure that the sample was systematically taken in the presence of the inclusion criteria. In the second step, the remaining studies with a quality score lower than six were excluded. Funnel plots and a rank test with calculation of Kendall’s tau were used to highlight a publication bias [16].
Univariate meta-regressions were used to test for an effect of the publication year, the median year of inclusion, the continent, the hemisphere, the latitude, the absolute latitude (to reflect the distance from the equator), the longitude, the inclusion or not of chronic diseases (cardiac, pulmonary, immunodepression…), the inclusion of the first episode only, the sampling method (nasal wash, nasal swab…), the inclusion criteria (wheezing, ARI, pneumonia or other), the age category, and the inclusion period (complete year(s), less than 6 months corresponding to an epidemic period, more than 1 year with different numbers of months according to the year, and other: between 7 and 11 months).
Sub-group analyses were performed: (1) keeping only pediatric populations, (2) by inclusion criteria, and (3) for variables significant in multivariate analysis.
A P value of less than 0.20 in univariate analyses led to inclusion of the variable in multivariate models. For categorical variables, the global P value was considered for the inclusion in multivariate models. A P value of less than 0.05 was considered significant in multivariate analyses. Only variables that were significant in the multivariate model were kept in the multivariate final model. Analyses were performed with R [17] (“meta” [18] and “metafor” [19] packages).
3. Results
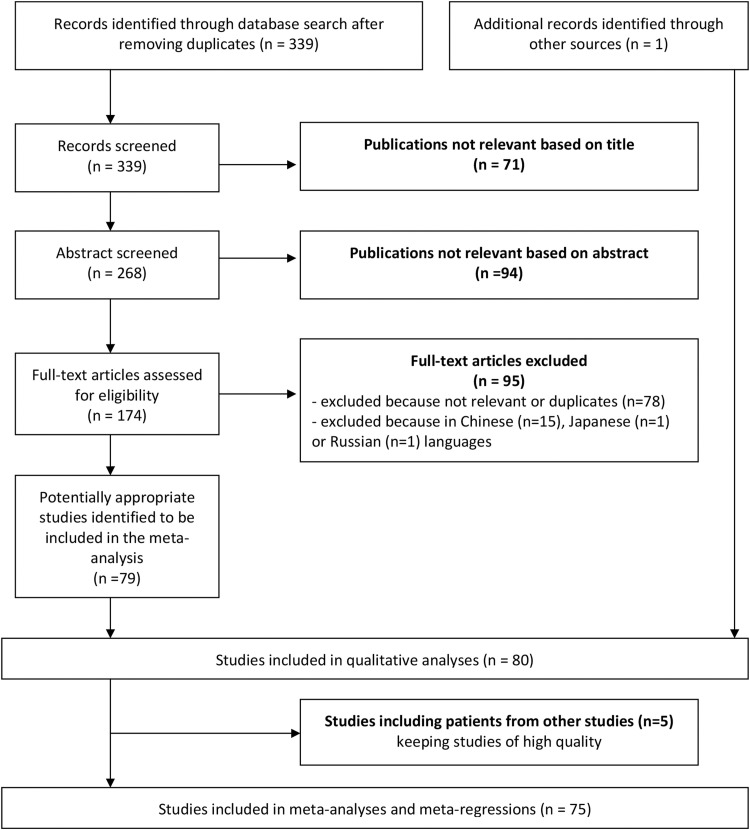
A total of 339 articles were obtained through the bibliographic search. Among these, 79 studies were retained (Fig. 1 ) [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98]. A recent meta-analysis of four respiratory viruses (not including hMPV) in acute lower respiratory infections in hospitalized children under five years of age was found [99]. One study with a search for hMPV was not retrieved with our bibliographic search strategy but was recovered from the manual search of this meta-analysis [100].
Fig. 1.
Flow chart showing the study selection process.
3.1. Description of included studies
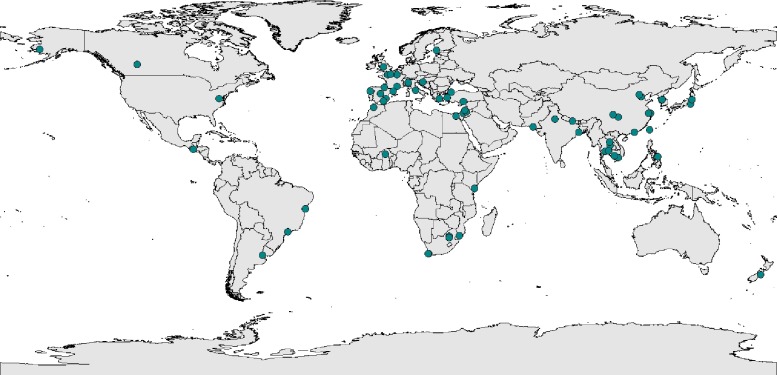
Most studies were conducted in Asia (36 studies) (Table 1 , Fig. 2 , Supplementary Table 1). Among the others, 23 were conducted in Europe, 11 in Africa, 5 in South America, 4 in North America, and 1 in Oceania.
Table 1.
Description of context and methods of included studies.
| Variable | Number (n = 80) | Proportion (%) |
|---|---|---|
| Continent | ||
| Africa | 11 | 13.75 |
| North America | 4 | 5.00 |
| South America | 5 | 6.25 |
| Asia (+ Oceania: 1 study) | 37 | 46.25 |
| Europe | 23 | 28.75 |
| Period of inclusion | ||
| Epidemic with 6 months or less | 13 | 16.25 |
| More than 6 months | 8 | 10.00 |
| Complete year(s) | 25 | 31.25 |
| Period varying according to the year | 34 | 42.50 |
| Sample method | ||
| Nasopharyngeal aspirate | 45 | 56.25 |
| Nasal or throat swab | 17 | 21.25 |
| Nasal wash | 9 | 11.25 |
| Bronchoalveolar lavage or several methods | 9 | 11.25 |
| Inclusion criteria (type of acute respiratory infection) | ||
| All acute respiratory infections | 31 | 38.75 |
| Bronchiolitis/expiratory wheezing | 16 | 20.00 |
| Bronchiolitis: inclusion if first episode only (n = 16) | 7 | 43.75 |
| Pneumonia | 13 | 16.25 |
| Low respiratory infections | 20 | 25.00 |
| Inclusion of severe forms only | 11 | 13.75 |
| Age | ||
| 0–3 years | 29 | 36.25 |
| 0–18 years | 45 | 58.25 |
| All ages | 4 | 5.00 |
| Adults only | 2 | 2.50 |
| Exclusion of chronic diseases | 18 | 22.50 |
Fig. 2.
Spatial distribution of the studies of human metapneumovirus among acute respiratory infections.
The size of the sample varied from 11 patients [85] to 28,369 [40], with a median of 320 (IQR 151.25–752.5). The inclusion period ranged from 3 months [61] to 5 years [40]. In most cases, the analyses were performed on nasopharyngeal or a nasal aspirates (45 studies). The N gene was most frequently used for the PCR primers. In most cases, the inclusion criterion was an ARI (including 31 studies with lower or upper respiratory tract infection and 20 studies with lower respiratory tract infection). Most studies included only pediatric populations (75 studies). Four studies included children and adults and two studies included adults only. Twenty-five studies were conducted in complete years, 34 over several years with varying inclusion periods depending on the year, 13 in one or several years but only during epidemic periods and 8 in one or several years during inclusion periods of seven to 11 months. Eleven studies included severe forms only, with various criteria for the definition of severity. The median quality score was 6.5 (IQR 5.5–7). The quality of studies is summarized in Supplementary Table 2, and details are shown in Supplementary Table 3.
Co-infections with other viruses were frequently observed (Supplementary Table 4).
3.2. Prevalence of hMPV among hospitalized ARI
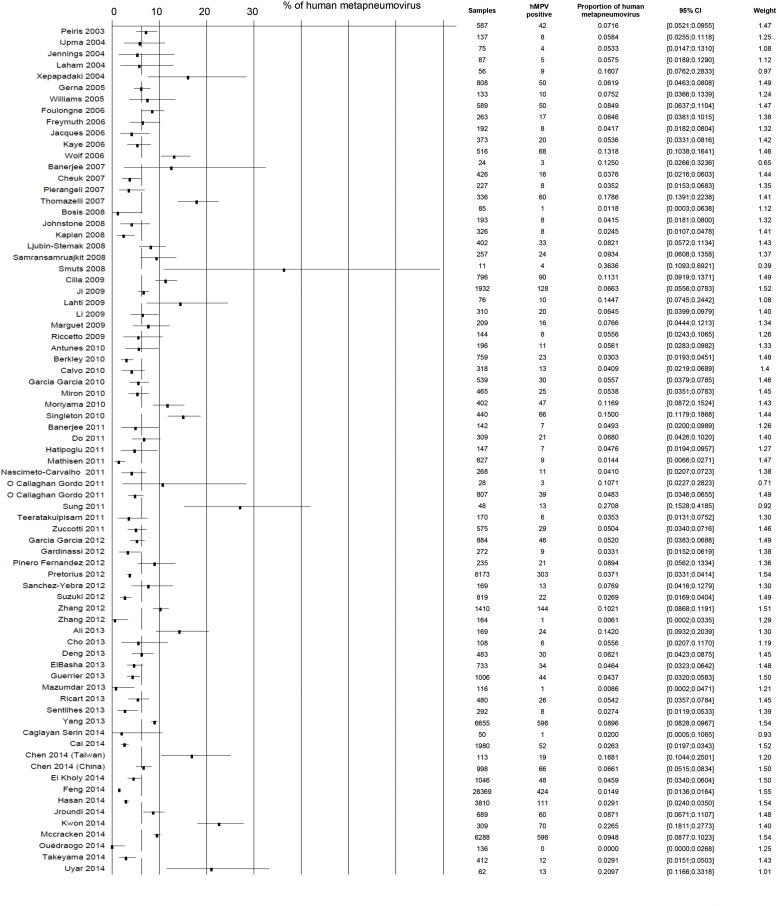
The prevalence of hMPV among patients hospitalized with ARI varied from 0% (95% CI 0.0–2.7) [74] to 36.4% (95% CI 10.9–69.2) [85] (Supplementary Table 4). The estimated prevalence of hMPV among hospitalized ARI was 6.39 (95% CI 5.45–7.39). The heterogeneity was very high (I2 = 96.8%, 95% CI 96.4–97.1). Several studies were conducted in populations which could have been included in part in other studies [25], [28], [29], [35], [43], [44], [45], [46], [73], [78], [80], [90]. In choosing studies to keep in the quantitative analyses, we privileged the higher quality studies. This led to 75 studies, which included 82,240 patients (Fig. 3 ), with a pooled prevalence of hMPV among ARI of 6.24 (95% CI 5.25–7.30). The heterogeneity remained high (I2 = 96.6%, 95% CI 96.2–97.0).
Fig. 3.
Forest plot of studies included in meta-analyses of prevalence of human metapneumovirus among hospitalized acute respiratory infections (n = 75). The dashed line represents the pooled prevalence (6.24%).
Sensitivity analyses led to consistent results. The prevalence of hMPV was 6.14% (95% CI 5.03–7.35) after the removal of 10 studies in which systematic inclusion was not sure and 6.26% (95% CI 5.29–7.31) after the removal of 6 other studies with a score of less than 6.
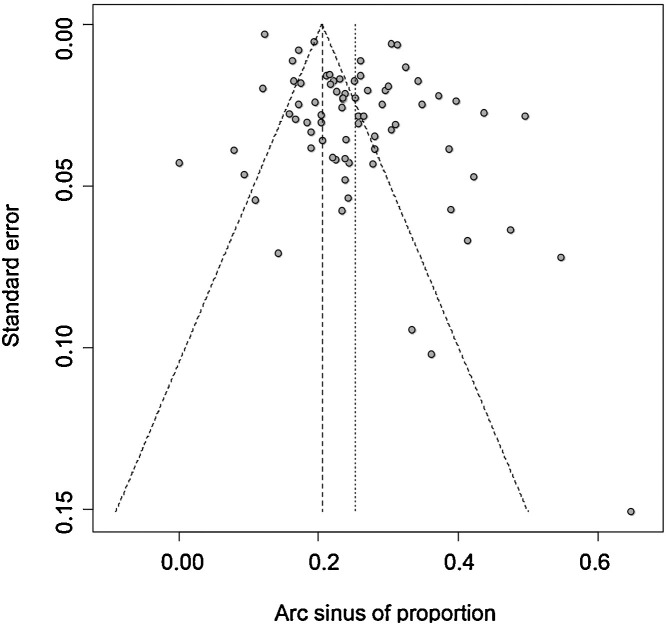
We found a higher prevalence of hMPV in small studies than in large studies (Fig. 4 ). The rank test confirmed the asymmetry of the funnel plot (p < 0.001).
Fig. 4.
Funnel plot of the meta-analysis of the prevalence of human metapneumovirus among hospitalized acute respiratory infections.
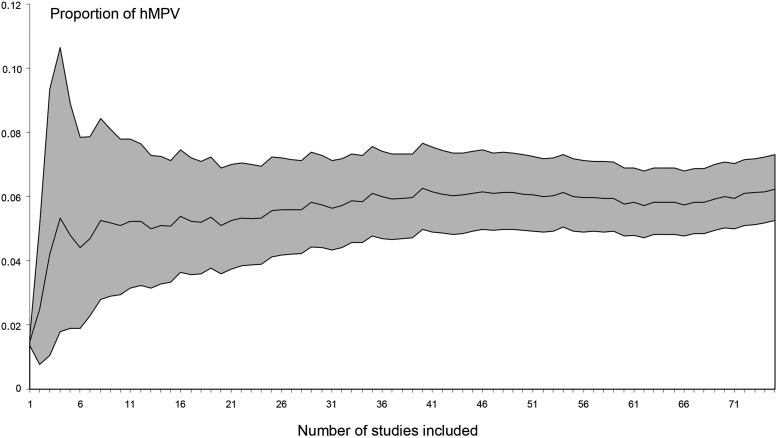
The cumulative meta-analysis, which included studies from the largest to the smallest, showed stabilization of the prevalence near to the 35th largest study (which included 336 patients) with a prevalence at around 6% (Fig. 5 ).
Fig. 5.
Cumulative meta-analyses of the prevalence of human metapneumovirus (hMPV) among hospitalized acute respiratory infections from the largest to smallest studies, with 95% confidence intervals.
3.3. Factors associated with the prevalence of hMPV infections
Univariate meta-regressions showed a higher prevalence when the inclusion period was 7–11 months per year (coefficient 0.096, P = 0.023) or complete years (coefficient 0.047, P = 0.100) than with epidemic periods of 6 months or less (Table 2 ). This variable was globally associated with the prevalence of hMPV infections (P = 0.014). Other tested variables were not statistically associated with the prevalence of hMPV infections.
Table 2.
Univariate and multivariate meta-regressions of the prevalence of human metapneumovirus infections in hospitalized acute respiratory infections (n = 75).
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Variable | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value |
| Publication year | −0.0045 (−0.0112; 0.0021) | 0.182 | ||
| Continent (reference: Africa)a | ||||
| North America | 0.0817 (−0.0241; 0.1874) | 0.130 | ||
| South America | 0.0642 (−0.0443; 0.1727) | 0.246 | ||
| Asia (+ Oceania: 1 study) | 0.0283 (−0.0372; 0.0938) | 0.397 | ||
| Europe | 0.0387 (−0.0321; 0.1095) | 0.284 | ||
| Latitude | 0.0002 (−0.0007; 0.0012) | 0.610 | ||
| Absolute latitude (reference: equator) | 0.0015 (−0.0003; 0.0033) | 0.102 | 0.0017 (0.0001–0.0033) | 0.038 |
| Longitude | −0.0001 (−0.0004; 0.0002) | 0.414 | ||
| Period of inclusion (reference: epidemic with 6 months or less)b | ||||
| More than 6 months | 0.0957 (0.0132; 0.1782) | 0.023 | 0.1071 (0.0239; 0.1903) | 0.012 |
| Complete year(s) | 0.0474 (−0.0091; 0.1040) | 0.100 | 0.0638 (0.0052; 0.1224) | 0.033 |
| Period varying according to the year | −0.0027 (−0.0561; 0.0508) | 0.921 | 0.0154 (−0.0407; 0.0715) | 0.592 |
| Sample method (reference: nasopharyngeal aspirate)a | ||||
| Nasal or throat swab | −0.0140 (−0.0590; 0.0311) | 0.543 | ||
| Nasal wash | 0.0263 (−0.0322; 0.0847) | 0.378 | ||
| Bronchiolo-alveolar washing or several methods | 0.0084 (−0.0542; 0.0711) | 0.792 | ||
| Inclusion criteria (reference: all acute respiratory infections)a | ||||
| Bronchiolitis/expiratory wheezing | 0.0199 (−0.0342; 0.0739) | 0.471 | ||
| Pneumonia | −0.0074 (−0.0644; 0.0496) | 0.799 | ||
| Low respiratory infections | 0.0337 (−0.0117; 0.0852) | 0.198 | ||
| Inclusion if first episode only | −0.0206 (−0.0875; 0.0464) | 0.547 | ||
| Inclusion of severe forms only | −0.0465 (−0.1073; 0.0143) | 0.134 | ||
| Age: 4 categories (reference 0–3 years)a | ||||
| 0-18 years | −0.0102 (−0.0534; 0.0331) | 0.646 | ||
| All ages | −0.0631 (−0.1510; 0.0249) | 0.160 | ||
| Adults | −0.0428 (−0.1683; 0.0827) | 0.504 | ||
| Age (adults or all ages versus children) | −0.0504 (−0.1202; 0.0194) | 0.157 | ||
| Exclusion of chronic diseases | −0.0102 (−0.0621; 0.0417) | 0.699 | ||
| Quality score | −0.0010 (−0.0222; 0.0203) | 0.928 | ||
| Number of samples | −4.3.106 (−8.9.106; 0.3.106) | 0.067 | ||
Global P value <0.200.
Global P value = 0.014 and 0.011 for univariate and multivariate analyses, respectively.
In multivariate analyses, the positive association with the absolute latitude became significant. The association with the inclusion period was still highlighted with a global P value for the likelihood ratio test of 0.0114. We found no associations with disease severity, age of inclusion, the number of samples included or the year. Further adjustments for age at inclusion did not significantly change the results (not shown).
3.4. Subgroup meta-analyses
In a subgroup analysis for pediatric patients (69 studies), the pooled prevalence was 6.40% (95% CI 5.51–7.35). Other subgroup analyses are shown in Table 3 .
Table 3.
Subgroup analyses of the prevalence of human metapneumovirus infections in hospitalized acute respiratory infections (n = 75).
| Subgroup | Number of studies | Pooled proportion | 95% CI |
|---|---|---|---|
| Inclusion criteria | |||
| Acute respiratory infection | 27 | 5.70 | 4.49–7.04 |
| Low acute respiratory infection | 18 | 7.44 | 4.99–10.32 |
| Bronchiolitis/expiratory wheezing | 17 | 6.45 | 4.82–8.30 |
| Pneumonia | 13 | 5.27 | 3.74–7.05 |
| Period of inclusion | |||
| 6 months or less in a year | 13 | 5.36 | 4.29–6.54 |
| 7 to 11 months in a year | 7 | 10.56 | 5.97–16.27 |
| Complete years | 23 | 7.55 | 5.90–9.38 |
| More than one year with different periods according to the year | 32 | 5.12 | 3.95–6.44 |
4. Discussion
In our meta-analysis of 75 studies with globally good methodological quality, the estimated prevalence of hMPV infections among hospitalized ARI was 6.24% (95% CI 5.25–7.30), with high heterogeneity. Sensitivity analyses gave similar results. We observed an effect of the period of inclusion with a significantly higher prevalence of hMPV infections in studies conducted during periods of 7–11 months or complete years than in periods of 6 months or less, and a significant increase in incidence with increasing distance from the equator.
In a recent meta-analysis that included 21 studies, Wang et al. found a similar prevalence of hMPV in childhood community-acquired pneumonia (6.1, 95% CI 4.1–8.1) [101]. Concerning other viruses, influenza, parainfluenza and adenovirus were found in proportions similar to that for hMPV: 6.3, 7.8 and 6.0% of cases, respectively. Respiratory syncytial virus, rhinovirus and bocavirus were more frequently observed (17.5%, 18.9% and 12.7%, respectively). Coronavirus was less frequent (3.9%). Luksic et al. provided a meta-analysis of the viral etiology of hospitalized acute lower respiratory infections in children under five years [99]. The prevalence of influenza viruses, parainfluenza viruses, and adenoviruses was 3.0% (95% CI 2.2–4.0), 2·7 (95% CI 1.9–3.7), and 5.8% (95% CI 3.4%–9.1%), respectively. The prevalence of coronavirus could not be estimated. Other viruses were not studied. Nair et al. reported 22% of respiratory syncytial virus in acute low respiratory infections in children under five years [102]. In the studies included in our meta-analysis, co-infections with other respiratory viruses were frequently observed. The prevalence of ARI due to hMPV only would have been lower, which would have been the case for other respiratory viruses, but this prevalence could not be estimated due to the diversity of the other viruses screened for.
Only studies using PCR to detect hMPV in patients hospitalized with an ARI, excluding fever without any respiratory symptoms, were included to limit the heterogeneity. Nevertheless, a high degree of heterogeneity was observed (I2 = 96.8%, 95% CI 96.4-97.1). This could be due to the diversity of study characteristics. Although meta-regressions took several study characteristics into account (sampling method, type of period, inclusion criteria, continent, year…), these variables explained only a very small part of the heterogeneity encountered. This could be due to the heterogeneousness of the studies’ characteristics persisting in each category. For example, inclusion criteria were classified in four categories (all ARI, low ARI, bronchiolitis or expiratory wheezing, and pneumonia), but “all ARI” or “low ARI” could be defined differently (or not defined in 19/45 cases) according to the study. For the above reasons, subgroup analysis according to the definition of ARI could not be performed. Furthermore, the difference in prevalences observed between studies could be due to the strains varying according to the year and the location, leading to epidemics of varying magnitude. Indeed, like influenza virus epidemics, hMPV epidemics have different magnitudes depending on the year, as shown in several studies conducted over several years [30], [40], [68], [84], [92], [103]. No linear effect of the year was observed. Non-linear effects were not tested because we hypothesized that the variations according to the year would be different from one country to another. Similar heterogeneity was found in other meta-analyses of respiratory viruses [99], [101], [102].
The prevalence of hMPV was higher in studies conducted during inclusion periods of 7–11 months or in complete year(s) than in studies conducted in periods of 6 months or less. These periods of 6 months or less probably corresponded to the epidemic period for ARI or influenza. However, they may not have corresponded to the epidemic period for hMPV. In several studies conducted in Europe or Asia during complete years, the epidemic peak occurred in March or April in Asia and February or March in Europe [32], [34], [40], [43], [49], [75], [98]. However, several other studies included patients between September-October and March-April, and these could have underestimated the prevalence of hMPV. In other studies, in particular in South and Central America (two studies in Brazil [73], [90], one study in Argentina [61] and one study in Guatemala [68]), hMPV infections occurred throughout the year, which was not the case for other respiratory viruses. Once again, studies conducted in epidemic periods of respiratory infections could have underestimated the prevalence of hMPV in ARI.
The prevalence of hMPV infections increased with distance from the equator. This could be due to the higher temperature and the seasonality of the climate, which is less pronounced near to the equator.
One of the strengths of this study is that it is the first meta-analysis about the prevalence of hMPV infections in hospitalized patients with ARI, and it included all ARI criteria in populations of all ages. We chose to include only hospitalized patients who were screened for hMPV using PCR, in prospective studies that included all patients (or random sampling of patients) in the presence of inclusion criteria to limit the heterogeneity and to improve the quality of data. A total of 80, mostly high-quality, studies were included in the qualitative analyses and 75 studies (which accounted for 82,240 patients) were included in the meta-analyses and meta-regressions. Retrospective studies were excluded to avoid the inclusion of studies in which hMPV was not systematically tested and to avoid other bias inherent to retrospective studies.
This study had some limitations. Firstly, a high degree of heterogeneity was observed and most of this heterogeneity could not be explained by the variables tested. All the characteristics of the study could not be taken into account because the information was not available. However, as mentioned above this heterogeneity probably reflects the reality of the prevalence of hMPV in ARI across countries and according to the year. Thus, the estimation of the worldwide prevalence of hMPV in ARI does not reflect the prevalence in every country in every year. Nonetheless, the observed heterogeneity is a result of interest. Secondly, a publication bias could be suspected from the funnel plot, the rank test, and the prevalence of hMPV, which decreased with the number of samples in univariate analysis. However, the cumulative meta-analysis from the largest to the smallest study showed stabilization of the prevalence at around 6% near the 35th largest study, and did not show a large increase with the smaller studies. Moreover, the asymmetry is probably due to the very high heterogeneity [104]. This is why we did not use methods such as trim-and-fill methods [105]. Thirdly, although the global quality of included studies was good, the definition of acute respiratory infection was not clear in many studies. Fourthly, the literature search strategy could be questionable and may have been restrictive, but 75 studies were included. Finally, despite the inclusion of all ages in our inclusion criteria, most of studies were conducted in pediatric populations. This study therefore mainly reflects the prevalence of hMPV in children.
In conclusion, hMPV should be taken into account as a possible etiology in hospitalized ARI. The prevalence of hMPV among ARI was 6.24% (95% CI 5.25–7.30). The heterogeneity was high (I2 = 96.6%, 95% CI 96.2–97.0) but the prevalence was stable over time. We observed a significantly higher prevalence of hMPV in studies conducted during periods of 7–11 months or complete years than in periods of 6 months or less and a significant increase in the incidence with increasing distance from the equator.
Conflicts of interests
The authors declare that there is no conflicts of interests.
Funding
None.
Acknowledgements
We thank Philip Bastable of Dijon University Hospital for his editorial assistance and Laetitia Leuci, Sarah Nouar, Rémi Sore, and Alicia Taha, medical students of Dijon University Hospital, for their help in extracting data and data entry.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2016.05.015.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.van Gageldonk-Lafeber A.B., Heijnen M.-L.A., Bartelds A.I.M., Peters M.F., van der Plas S.M., Wilbrink B. A case-Control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin. Infect. Dis. 2005;41:490–497. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The United Nations Children’s Fund (UNICEF)/World Health Organization (WHO). Pneumonia—The forgotten killer of children. 2006. Available at http://www.who.int/maternal_child_adolescent/documents/9280640489/en/ (last assessed 18.05.16).
- 3.Makela M., Puhakka T., Ruuskanen O. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Hoogen B.G., de Jong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Hoogen B.G., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–132. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 6.Maertzdorf J., Wang C.K., Brown J.B. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J. Clin. Microbiol. 2004;42:981–986. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerna G., Sarasini A., Percivalle E. Prospective study of human metapneumovirus infection: diagnosis, typing and virus quantification in nasopharyngeal secretions from pediatric patients. J. Clin. Virol. 2007;40:236–240. doi: 10.1016/j.jcv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Manoha C., Bour J.B., Pitoiset C., Darniot M., Aho S., Pothier P. Rapid and sensitive detection of metapneumovirus in clinical specimens by indirect fluorescence assay using a monoclonal antibody. J. Med. Virol. 2008;80:154–158. doi: 10.1002/jmv.21038. [DOI] [PubMed] [Google Scholar]
- 9.Al-Jader L.N., Newcombe R.G., Hayes S., Murray A., Layzell J., Harper P.S. Developing a quality scoring system for epidemiological surveys of genetic disorders. Clin. Genet. 2002;62:230–234. doi: 10.1034/j.1399-0004.2002.620308.x. [DOI] [PubMed] [Google Scholar]
- 10.Boyle M. Guidelines for evaluating prevalence studies. Evid. Based Mental Health. 1998;1:37–39. [Google Scholar]
- 11.Hoy D., Brooks P., Woolf A. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Shamliyan T., Kane R.L., Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J. Clin. Epidemiol. 2012;63:1061–1070. doi: 10.1016/j.jclinepi.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Shamliyan T.A., Kane R.L., Ansari M.T. Development quality criteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: pilot study of new checklists. J. Clin. Epidemiol. 2011;64:637–657. doi: 10.1016/j.jclinepi.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Rucker G., Schwarzer G., Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat. Med. 2008;27:746–763. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Begg C., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation For Statistical Computing, Vienna, Austria; 2014. Available at http://www.R-project.org/ (last assessed on 18.05.16).
- 18.Schwarzer Meta: meta-analysis with R. [R package]. Version 3. 6–0. 2014.
- 19.Viechtbauer W. Metafor: meta-analysis package for R. R package version 1. 9-3. J. Stat. Softw. 2014;36:1–48. [Google Scholar]
- 20.Ali A., Khowaja A.R., Bashir M.Z., Aziz F., Mustafa S., Zaidi A. Role of human metapneumovirus, influenza A virus and respiratory syncytial virus in causing WHO-defined severe pneumonia in children in a developing country. PLoS One. 2013;8:e74756. doi: 10.1371/journal.pone.0074756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antunes H., Rodrigues H., Silva N. Etiology of bronchiolitis in a hospitalized pediatric population: prospective multicenter study. J. Clin. Virol. 2010;48:134. doi: 10.1016/j.jcv.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee S., Bharaj P., Sullender W., Kabra S.K., Broor S. Human metapneumovirus infections among children with acute respiratory infections seen in a large referral hospital in India. J. Clin. Virol. 2007;38:70. doi: 10.1016/j.jcv.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S., Sullender W.M., Choudekar A. Detection and genetic diversity of human metapneumovirus in hospitalized children with acute respiratory infections in India. J. Med. Virol. 2011;83:1799–1810. doi: 10.1002/jmv.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkley J.A., Munywoki P., Ngama M. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosis S., Esposito S., Niesters H.G. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin. Microbiol. Infect. 2008;14:677–684. doi: 10.1111/j.1469-0691.2008.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caglayan Serin D., Pullukcu H., Cicek C., Sipahi O.R., Tasbakan S., Atalay S. Bacterial and viral etiology in hospitalized community acquired pneumonia with molecular methods and clinical evaluation. J. Infect. Dev. Ctries. 2014;8:510–518. doi: 10.3855/jidc.3560. [DOI] [PubMed] [Google Scholar]
- 27.Cai X.Y., Wang Q., Lin G.Y. Respiratory virus infections among children in South China. J. Med. Virol. 2014;86:1249–1255. doi: 10.1002/jmv.23931. [DOI] [PubMed] [Google Scholar]
- 28.Calvo C., Pozo F., Garcia-Garcia M.L. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99:883–887. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canducci F., Debiaggi M., Sampaolo M. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol. 2008;80:716–723. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.-W., Huang Y.-C., Ho T.-H., Huang C.-G., Tsao K.-C., Lin T.-Y. Viral etiology of bronchiolitis among pediatric inpatients in northern Taiwan with emphasis on newly identified respiratory viruses. J. Microbiol. Immunol. Infect. 2014;47:116. doi: 10.1016/j.jmii.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z.-R., Ji W., Wang Y.-Q. Etiology of acute bronchiolitis and the relationship with meteorological conditions in hospitalized infants in China. J. Formos. Med. Assoc. 2014;113:463–469. doi: 10.1016/j.jfma.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheuk D.K., Tang I.W., Chan K.H., Woo P.C., Peiris M.J., Chiu S.S. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr. Infect. Dis. J. 2007;26:995–1000. doi: 10.1097/INF.0b013e3181586b63. [DOI] [PubMed] [Google Scholar]
- 33.Cho H.J., Shim S.Y., Son D.W., Sun Y.H., Tchah H., Jeon I.S. Respiratory viruses in neonates hospitalized with acute lower respiratory tract infections. Pediatr. Int. 2013;55:49–53. doi: 10.1111/j.1442-200X.2012.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cilla G., Onate E., Perez-Yarza E.G., Montes M., Vicente D., Perez-Trallero E. Hospitalization rates for human metapneumovirus infection among 0- to 3-year-olds in Gipuzkoa (Basque Country), Spain. Epidemiol. Infect. 2009;137:66–72. doi: 10.1017/S0950268808000666. [DOI] [PubMed] [Google Scholar]
- 35.Cohen C., Walaza S., Moyes J. Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009–2012. Pediatr. Infect. Dis. J. 2015;34:66–72. doi: 10.1097/INF.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng J., Ma Z., Huang W. Respiratory virus multiplex RT-PCR assay sensitivities and influence factors in hospitalized children with lower respiratory tract infections. Virol Sin. 2013;28:97–102. doi: 10.1007/s12250-013-3312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Do A.H., van Doorn H.R., Nghiem M.N. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS One. 2011;6:e18176. doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Kholy A.A., Mostafa N.A., Ali A.A. Risk factors of prolonged hospital stay in children with viral severe acute respiratory infections. J. Infect. Dev. Ctries. 2014;8:1285–1293. doi: 10.3855/jidc.4682. [DOI] [PubMed] [Google Scholar]
- 39.ElBasha N., El Rifai N., Draz I., El Kholy A. Contribution of viruses to severe pneumonia in children. Gaz. Egypt. Paediatr. Assoc. 2013;61:73. [Google Scholar]
- 40.Feng L., Li Z., Zhao S. Viral etiologies of hospitalized acute lower respiratory infection patients in China, 2009–2013. PLoS One. 2014;9:e99419. doi: 10.1371/journal.pone.0099419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foulongne V., Guyon G., Rodiere M., Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr. Infect. Dis. J. 2006;25:354–359. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 42.Freymuth F., Vabret A., Cuvillon-Nimal D. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J. Med. Virol. 2006;78:1498–1504. doi: 10.1002/jmv.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Garcia M.L., Calvo C., Falcon A. Role of emerging respiratory viruses in children with severe acute wheezing. Pediatr. Pulmonol. 2010;45:585–591. doi: 10.1002/ppul.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Garcia M.L., Calvo C., Perez-Brena P., De Cea J.M., Acosta B., Casas I. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in Spain. Pediatr. Pulmonol. 2006;41:863–871. doi: 10.1002/ppul.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Garcia M.L., Calvo C., Pozo F., Villadangos P.A., Perez-Brena P., Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr. Infect. Dis. J. 2012;31:808–813. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 46.García-García M.L., Calvo Rey C., Pozo Sánchez F. Infecciones por bocavirus humano en niños españoles: características clínicas y epidemiológicas de un virus respiratorio emergente. An Pediatria. 2007;67:212. doi: 10.1016/S1695-4033(07)70609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardinassi L.G., Marques Simas P.V., Salomao J.B. Seasonality of viral respiratory infections in southeast of Brazil: the influence of temperature and air humidity. Braz. J. Microbiol. 2012;43:98–108. doi: 10.1590/S1517-838220120001000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerna G., Campanini G., Rovida F. Changing circulation rate of human metapneumovirus strains and types among hospitalized pediatric patients during three consecutive winter-spring seasons. Brief report. Arch. Virol. 2005;150:2365–2375. doi: 10.1007/s00705-005-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerrier G., Goyet S., Chheng E.T. Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr. Infect. Dis. J. 2013;32:e8–13. doi: 10.1097/INF.0b013e31826fd40d. [DOI] [PubMed] [Google Scholar]
- 50.Hasan R., Rhodes J., Thamthitiwat S. Incidence and etiology of acute lower respiratory tract infections in hospitalized children younger than 5 years in rural Thailand. Pediatr. Infect. Dis. J. 2014;33:e45–52. doi: 10.1097/INF.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatipoglu N., Somer A., Badur S. Viral etiology in hospitalized children with acute lower respiratory tract infection. Turk. J. Pediatr. 2011;53:508–516. [PubMed] [Google Scholar]
- 52.Ijpma F., Beekhuis D., Cotton M.F. Human metapneumovirus infection in hospital referred South African children. J. Med. Virol. 2004;73:486–493. doi: 10.1002/jmv.20116. [DOI] [PubMed] [Google Scholar]
- 53.Jrm Jacques, Bouscambert-Duchamp M., Hln Moret. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J. Clin. Virol. 2006;35:463. doi: 10.1016/j.jcv.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Jennings L.C., Anderson T.P., Werno A.M., Beynon K.A., Murdoch D.R. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr. Infect. Dis. J. 2004;23:1003–1007. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- 55.Ji W., Wang Y., Chen Z., Shao X., Ji Z., Xu J. Human metapneumovirus in children with acute respiratory tract infections in Suzhou, China 2005–2006. Scand. J. Infect. Dis. 2009;41:735–744. doi: 10.1080/00365540903148264. [DOI] [PubMed] [Google Scholar]
- 56.Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Human metapneumovirus pneumonia in adults: results of a prospective study. Clin. Infect. Dis. 2008;46:571–574. doi: 10.1086/526776. [DOI] [PubMed] [Google Scholar]
- 57.Jroundi I., Mahraoui C., Benmessaoud R. Risk factors for a poor outcome among children admitted with clinically severe pneumonia to a university hospital in Rabat, Morocco. Int. J. Infect. Dis. 2014;28:164–170. doi: 10.1016/j.ijid.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan N.M., Dove W., Abd-Eldayem S.A., Abu-Zeid A.F., Shamoon H.E., Hart C.A. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J. Med. Virol. 2008;80:168–174. doi: 10.1002/jmv.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaye M., Skidmore S., Osman H., Weinbren M., Warren R. Surveillance of respiratory virus infections in adult hospital admissions using rapid methods. Epidemiol. Infect. 2006;134:792–798. doi: 10.1017/S0950268805005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon J.M., Shim J.W., Kim D.S., Jung H.L., Park M.S., Shim J.Y. Prevalence of respiratory viral infection in children hospitalized for acute lower respiratory tract diseases, and association of rhinovirus and influenza virus with asthma exacerbations. Korean J. Pediatr. 2014;57:29–34. doi: 10.3345/kjp.2014.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laham F.R., Israele V., Casellas J.M. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 2004;189:2047–2056. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- 62.Lahti E., Peltola V., Waris M. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64:252–257. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 63.Li X.Y., Chen J.Y., Kong M. Prevalence of human metapneumovirus in hospitalized children with respiratory tract infections in Tianjin, China. Arch. Virol. 2009;154:1831–1836. doi: 10.1007/s00705-009-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ljubin-Sternak S., Santak M., Cepin-Bogovic J. Detection of genetic lineages of human metapneumovirus in Croatia during the winter season 2005/2006. J. Med. Virol. 2008;80:1282–1287. doi: 10.1002/jmv.21196. [DOI] [PubMed] [Google Scholar]
- 65.Marguet C., Lubrano M., Gueudin M. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One. 2009;4:e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathisen M., Basnet S., Sharma A. RNA viruses in young Nepalese children hospitalized with severe pneumonia. Pediatr. Infect. Dis. J. 2011;30:1032–1036. doi: 10.1097/INF.0b013e31822f845f. [DOI] [PubMed] [Google Scholar]
- 67.Mazumdar J., Chawla-Sarkar M., Rajendran K. Burden of respiratory tract infections among paediatric in and out-patient units during 2010–11. Eur. Rev. Med. Pharmacol. Sci. 2013;17:802–808. [PubMed] [Google Scholar]
- 68.McCracken J.P., Arvelo W., Ortiz J. Comparative epidemiology of human metapneumovirus- and respiratory syncytial virus-associated hospitalizations in Guatemala. Influenza Other Respir. Viruses. 2014;8:414–421. doi: 10.1111/irv.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miron D., Srugo I., Kra-Oz Z. Sole pathogen in acute bronchiolitis: is there a role for other organisms apart from respiratory syncytial virus? Pediatr. Infect. Dis. J. 2010;29:e7–e10. doi: 10.1097/INF.0b013e3181c2a212. [DOI] [PubMed] [Google Scholar]
- 70.Moriyama Y., Hamada H., Okada M. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur. J. Pediatr. 2010;169:1087–1092. doi: 10.1007/s00431-010-1183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nascimento-Carvalho C.M., Cardoso M.R., Ruuskanen O., Lappalainen M. Sole infection by human metapneumovirus among children with radiographically diagnosed community-acquired pneumonia in a tropical region. Influenza Other Respir. Viruses. 2011;5:285–287. doi: 10.1111/j.1750-2659.2011.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Callaghan-Gordo C., Bassat Q., Morais L. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr. Infect. Dis. J. 2011;30:39–44. doi: 10.1097/INF.0b013e3181f232fe. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira D.B., Durigon E.L., Carvalho A.C. Epidemiology and genetic variability of human metapneumovirus during a 4-year-long study in Southeastern Brazil. J. Med. Virol. 2009;81:915–921. doi: 10.1002/jmv.21436. [DOI] [PubMed] [Google Scholar]
- 74.Ouedraogo S., Traore B., Nene Bi Z.A. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso) PLoS One. 2014;9:e110435. doi: 10.1371/journal.pone.0110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peiris J.S., Tang W.H., Chan K.H. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg. Infect. Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pierangeli A., Gentile M., Di Marco P. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J. Med. Virol. 2007;79:463–468. doi: 10.1002/jmv.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinero Fernandez J.A., Alfayate Miguelez S., Menasalvas Ruiz A., Salvador Garca C., Moreno Docon A., Sanchez-Solis de Querol M. Epidemiology, clinical features and medical interventions in children hospitalized for bronchiolitis. An. Pediatr. 2012;77:391. doi: 10.1016/j.anpedi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pretorius M.A., Madhi S.A., Cohen C. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness–South Africa, 2009–2010. J. Infect. Dis. 2012;206(Suppl. 1):S159–65. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- 79.Ricart S., Garcia-Garcia J.J., Anton A. Analysis of human metapneumovirus and human bocavirus viral load. Pediatr. Infect. Dis. J. 2013;32:1032–1034. doi: 10.1097/INF.0b013e3182932f4f. [DOI] [PubMed] [Google Scholar]
- 80.Riccetto A.G., Silva L.H., Spilki F.R., Morcillo A.M., Arns C.W., Baracat E.C. Genotypes and clinical data of respiratory syncytial virus and metapneumovirus in brazilian infants: a new perspective. Braz. J. Infect. Dis. 2009;13:35–39. doi: 10.1590/s1413-86702009000100008. [DOI] [PubMed] [Google Scholar]
- 81.Samransamruajkit R., Hiranrat T., Chieochansin T. Prevalence, clinical presentations and complications among hospitalized children with influenza pneumonia. Jpn. J. Infect. Dis. 2008;61:446–449. [PubMed] [Google Scholar]
- 82.Sanchez-Yebra W., Avila-Carrillo J.A., Gimenez-Sanchez F. Viral agents causing lower respiratory tract infections in hospitalized children: evaluation of the Speed-Oligo(R) RSV assay for the detection of respiratory syncytial virus. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:243–250. doi: 10.1007/s10096-011-1300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sentilhes A.C., Choumlivong K., Celhay O. Respiratory virus infections in hospitalized children and adults in Lao PDR. Influenza Other Respir. Viruses. 2013;7:1070–1078. doi: 10.1111/irv.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singleton R.J., Bulkow L.R., Miernyk K. Viral respiratory infections in hospitalized and community control children in Alaska. J. Med. Virol. 2010;82:1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smuts H., Workman L., Zar H.J. Role of human metapneumovirus, human coronavirus NL63 and human bocavirus in infants and young children with acute wheezing. J. Med. Virol. 2008;80:906–912. doi: 10.1002/jmv.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung C.C., Chi H., Chiu N.C. Viral etiology of acute lower respiratory tract infections in hospitalized young children in Northern Taiwan. J. Microbiol. Immunol. Infect. 2011;44:184–190. doi: 10.1016/j.jmii.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki A., Lupisan S., Furuse Y. Respiratory viruses from hospitalized children with severe pneumonia in the Philippines. BMC Infect. Dis. 2012;12:267. doi: 10.1186/1471-2334-12-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeyama A., Hashimoto K., Sato M. Clinical and epidemiologic factors related to subsequent wheezing after virus-induced lower respiratory tract infections in hospitalized pediatric patients younger than 3 years. Eur. J. Pediatr. 2014;173:959–966. doi: 10.1007/s00431-014-2277-7. [DOI] [PubMed] [Google Scholar]
- 89.Teeratakulpisarn J., Ekalaksananan T., Pientong C., Limwattananon C. Human metapneumovirus and respiratory syncytial virus detection in young children with acute bronchiolitis. Asian Pac. J. Allergy Immunol. 2011;25:139–145. [PubMed] [Google Scholar]
- 90.Thomazelli L.M., Vieira S., Leal A.L. Surveillance of eight respiratory viruses in clinical samples of pediatric patients in southeast Brazil. J. Pediatr. (Rio. J.) 2007;83:422–428. doi: 10.2223/JPED.1694. [DOI] [PubMed] [Google Scholar]
- 91.Uyar M., Kuyucu N., Tezcan S., Aslan G., Tasdelen B. Determination of the frequency of human bocavirus and other respiratory viruses among 0–2 years age group children diagnosed as acute bronchiolitis. Mikrobiyol. Bul. 2014;48:242–258. doi: 10.5578/mb.7575. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y., Chen Z., Yan Y.D. Seasonal distribution and epidemiological characteristics of human metapneumovirus infections in pediatric inpatients in Southeast China. Arch. Virol. 2013;158:417–424. doi: 10.1007/s00705-012-1492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams J.V., Tollefson S.J., Heymann P.W., Carper H.T., Patrie J., Crowe J.E. Human metapneumovirus infection in children hospitalized for wheezing. J. Allergy Clin. Immunol. 2005;115:1311–1312. doi: 10.1016/j.jaci.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolf D.G., Greenberg D., Kalkstein D. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr. Infect. Dis. J. 2006;25:320–324. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 95.Xepapadaki P., Psarras S., Bossios A. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J. Clin. Virol. 2004;30:267. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang C., Du L.N., Zhang Z.Y. Detection and genetic diversity of human metapneumovirus in hospitalized children with acute respiratory infections in Southwest China. J. Clin. Microbiol. 2012;50:2714–2719. doi: 10.1128/JCM.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang G., Hu Y., Wang H., Zhang L., Bao Y., Zhou X. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PLoS One. 2012;7:e44568. doi: 10.1371/journal.pone.0044568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuccotti G., Dilillo D., Zappa A. Epidemiological and clinical features of respiratory viral infections in hospitalized children during the circulation of influenza virus A(H1N1) 2009. Influenza Other Respir. Viruses. 2011;5:e528–34. doi: 10.1111/j.1750-2659.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luksic I., Kearns P.K., Scott F., Rudan I., Campbell H., Nair H. Viral etiology of hospitalized acute lower respiratory infections in children under 5 years of age—a systematic review and meta-analysis. Croat. Med. J. 2013;54:122–134. doi: 10.3325/cmj.2013.54.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Callaghan-Gordo C., Diez-Padrisa N., Abacassamo F. Viral acute respiratory infections among infants visited in a rural hospital of southern Mozambique. Trop. Med. Int. Health. 2011;16:1054–1060. doi: 10.1111/j.1365-3156.2011.02811.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang M., Cai F., Wu X., Wu T., Su X., Shi Y. Incidence of viral infection detected by PCR and real-time PCR in childhood community-acquired pneumonia: a meta-analysis. Respirology. 2015;20:405–412. doi: 10.1111/resp.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nair H., Nokes D.J., Gessner B.D. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pitoiset C., Darniot M., Huet F., Aho S.L., Pothier P., Manoha C. Human metapneumovirus genotypes and severity of disease in young children (n = 100) during a 7-year study in Dijon hospital, France. J. Med. Virol. 2010;82:1782–1789. doi: 10.1002/jmv.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mavridis D., Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid. Based Ment. Health. 2014;17:30. doi: 10.1136/eb-2013-101699. [DOI] [PubMed] [Google Scholar]
- 105.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.