Summary
The oceans are a unique resource that provide a diverse array of natural products, primarily from invertebrates such as sponges, tunicates, bryozoans, and molluscs, and from marine bacteria and cyanobacteria. As infectious diseases evolve and develop resistance to existing pharmaceuticals, the marine environment provides novel leads against fungal, parasitic, bacterial, and viral diseases. Many marine natural products have successfully advanced to the late stages of clinical trials, including dolastatin 10, ecteinascidin-743, kahalalide F, and aplidine, and a growing number of candidates have been selected as promising leads for extended preclinical assessment. Although many marine-product clinical trials are for cancer chemotherapy, drug resistance, emerging infectious diseases, and the threat of bioterrorism have all contributed to the interest in assessing natural ocean products in the treatment of infectious organisms. In this review, we focus on the pharmacologically tested marine leads that have shown in-vivo efficacy or potent in-vitro activity against infectious and parasitic diseases.
Although the diversity of life in the terrestrial environment is extraordinary, the greatest biodiversity is in the world's oceans, with 34 of the 36 phyla of life represented. The oceans cover more than 70% of the earth's surface and contains more than 300 000 described species of plants and animals.1, 2 Macroscopic plants and animals have adapted to all regions of the oceans, including polar, temperate, and tropical areas. The diversity in species is extraordinarily rich on coral reefs, where there are around 1000 species per m2 in some areas, and the Indo-Pacific Ocean has the world's greatest tropical marine biodiversity.2 The marine environment represents a treasure of useful products awaiting discovery for the treatment of infectious diseases (figure 1 ). Ecological pressures, including competition for space, the fouling of the surface, predation, and successfully reproducing have led to the evolution of unique secondary metabolites with various biological activities.3 The importance that these secondary metabolites play in the control of infectious and parasitic organisms was for many years largely overlooked.
Figure 1.
Acanthostrongylophora sp sponge.
In the past 30–40 years, marine plants and animals have been the focus of a worldwide effort to define the natural products of the marine environment. A small number of marine plants, animals, and microbes have already yielded more than 12 000 novel chemicals, with hundreds of new compounds still being discovered every year. These discovery efforts have yielded several bioactive metabolites that have been successfully developed by the pharmaceutical industry.4, 5 Several clinically useful drugs, investigational drug candidates, and pharmacological tools have already resulted from these marine-product discovery programmes.4, 5 One of the marine natural products that is currently under clinical investigation as a potential new anticancer agents is the marine alkaloid ecteinascidin-743, isolated from the tunicate Ecteinascidia turbinate.6 Ecteinascidin is a broad-spectrum antitumour agent and is among the most advanced compounds with clinical applications.6
In this review, we focus on the pharmacologically tested marine leads that have shown in-vivo efficacy or potent in-vitro activity against infectious and parasitic diseases, including malaria, toxoplasmosis, trypanosomiasis, and viral, bacterial, and fungal infections. Our objective was to highlight the most promising compounds that have the greatest potential to lead to clinically useful treatments. There was not room to review treatments for specific infectious diseases, but there are several helpful reviews for antiparasitic drugs,7 antinematodal drugs,8 antituberculosis agents,9 antiviral leads,10 and antifungal agents.11 Furthermore, there are some excellent general and extensive reviews focusing on marine natural products, such as those by Capon,12 Newman and colleagues,13 and Faulkner.14
Antifungal activity
The antifungal screening of marine samples has led to the characterisation of many unprecedented natural products in regard to antifungal activity and chemical structures. The frequency of invasive fungal infection has risen substantially with the increasing numbers of immunocompromised patients, such as those infected with HIV, receiving cancer chemotherapy, immunosuppressive therapy, or treatment with broad-spectrum antibiotics.11 Evidence is mounting that fungi display highly specific adaptations in the marine environment that include the production of unique secondary metabolites.11 The fact that marine organisms contain secondary metabolites different from their terrestrial counterparts in structure and biological activity has led to the hypothesis that marine organisms may contain efficient antifungal compounds with different modes of action and selective antifungal activity compared with human cells.11
Jasplakinolide is the first example of a cyclodepsipeptide isolated from a sponge (figure 1) and was identified from a Jaspis sp collected in Fiji.15 Jasplakinolide, also named jaspamide, is a 19-membered macrocyclic depsipeptide with selective in-vitro antimicrobial activity and a minimum inhibitory concentration (MIC) of 25 μg/mL against Candida albicans. The in-vivo topical activity of a 2% solution of jasplakinolide against a candida vaginal infection in mice is similar to that of miconazole nitrate.15
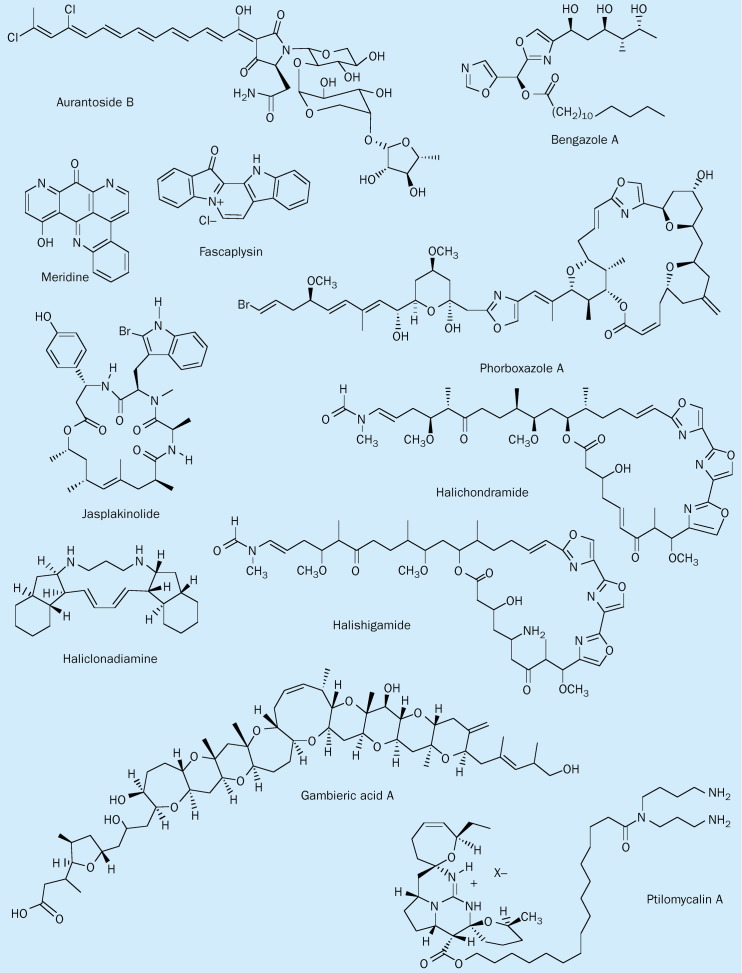
Gambieric acids are extremely potent antifungal metabolites isolated from a strain of the epiphytic marine dinoflagellate Gambierdiscus toxicus. These metabolites are the first antifungal representatives of the brevetoxin-type (fused polyether rings) structures, consisting of nine contiguous ether rings and one isolated tetrahydrofuran (figure 2 ). Gambieric acid A inhibits the growth of Aspergillus niger at a concentration of 10 ng/disk. The potency of gambieric acids exceeds that of amphotericin B by 2000-fold.16 Toxic effects in mice or cultured mammalian cells are moderate for a dose of 1 mg/kg given by intraperitoneal injection. Additional marine natural products with antifungal activity are listed in table 1 .
Figure 2.
Chemical structure of antifungal marine natural products.
Table 1.
Antifungal marine natural products
| Compound | Type* | Source | Activity MIC | Cytotoxicity | Reference |
|---|---|---|---|---|---|
| Aurantoside B | Polyketide | Siliquariaspongia japonica sponge | Aspergillus fumigatus 0·63 μg/mL | IC50 > μg/mL(P-388 murine leukaemia cells) | 17 |
| C albicans 0·16 μg/mL | |||||
| Phorboxazole A | Macrolide | Phorbas sp sponge | Calbicans 0·1 μg/disk | Cytostatic | 18 |
| Saccharomyces carlsbergensis 0·1 μg/disk | GI50 <0·8 × 10−3 μg/mL | ||||
| Halishigamide A | Macrolide | Halichondria sp sponge | Trichophyton mentagrophytes 0·1 μg/Ml | L1210 IC50 0·0036 μg/mL | 19 |
| Highly toxic to mice at 1·4 mg/kg | 20 | ||||
| Fascaplysin | Bis (indole) alkaloid | Fascaplysinopsis sp sponge | S cerevisiae 0·1 μg/disk (20 mm zone) C albicans at 1 μg/disk (11 mm zone) | L1210 IC50 0·2 μg/mL | 21 |
| Meridine | Polycyclic alkaloid | Corticium sp sponge | C albicans 0·2 μg/mL Cryptococcus neoformans 0·8 μg/mL | Not measured | 22 |
| Bengazole A | Oxazole-containing fatty-acid ester | Jaspis sp sponge | C albicans 0·5 μg/disk | Not measured | 23 |
| Ptilomycalin A | Polycyclic guanidine alkaloid | Ptilocaulis spiculifer sponge | C albicans 0·8 μg/mL, HSV 0·2 μg/mL | P388 IC50 0·1 μg/mL | 24 |
| Haliclonadiamine Alkaloid | Haliclona sp | C albicans 1 μg/disk | Not measured | 25 |
GI50=50% growth inhibition; HSV=herpes simplex virus; KB=nasopharyngeal cancer.
Structures illustrated in figure 2.
Most antifungal compounds from marine origin are cytotoxic. Consequently, they have not generally been considered promising antifungal agents for clinical applications. We have reviewed marine natural products that show potent antifungal activity, but evidence of cytotoxicity was not available for all.11 In many cases, the assessment of whether antifungal activity outweighs cytotoxic effects will be required, followed by rational modifications to improve the therapeutic index for these molecules.
Antituberculosis activity
The search for new antituberculosis agents has become increasingly important with the emergence of multidrug-resistant strains of Mycobacterium tuberculosis. Despite the small number of investigators looking at marine products as potential leads against M tuberculosis, the research in this area has been productive (figure 3 ).
Figure 3.
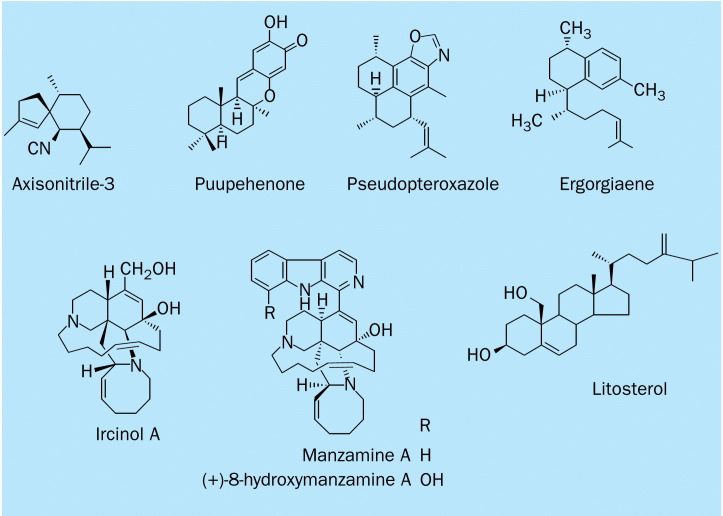
Chemical structure of antimycobacterial marine natural products.
The alkaloid (+)-8-hydroxymanzamine A is characterised by a complex heterocyclic ring system attached to a -carboline moiety. (+)-8-hydroxymanzamine A was first isolated from a sponge Pachypellina sp and later from an undescribed Petrosiidae genus.26 This alkaloid exhibits potent inhibitory activity against M tuberculosis H37Rv, with an MIC of 0·91 μg/mL.27 The likely biogenetic precursor ircinol A does not have the -carboline moiety but still has an MIC of 1·93 μg/mL. Ircinol A represents a useful candidate for assessment in vivo against M tuberculosis, since it shows low cytotoxicity and structural complexity compared with the other manzamine-type alkaloids.27 In addition, manzamine A inhibits M tuberculosis H37Rv, with an MIC of 1·56 μg/mL.27
Axisonitrile-3 is a cyanosesquiterpene isolated from the sponge Acanthella klethra and shows potent inhibitory activity against M tuberculosis, with an MIC of 2·0 μg/mL.28 Pseudopteroxazole, a benzoxazole diterpene alkaloid isolated from the West Indian gorgonian Pseudopterogorgia elisabethae, induces 97% growth inhibition for M tuberculosis H37Rv at a concentration of 12·5 μg/mL without substantial toxic effects.29 Ergorgiaene, a serrulatane-based diterpene (also known as biflorane) was isolated from the hexane extract of the same West Indian gorgonian, and induced 96% growth inhibition for M tuberculosis H37Rv at a concentration of 12·5μg/mL.30
Litosterol is a C-19 hydroxysteroid isolated from an Okinawan soft coral Litophyton viridis. It inhibited 90% of the growth of M tuberculosis with an MIC of 3·13 μg/mL. The poor solubility of litosterol in the aqueous tissue culture media obscured assessment of cytotoxic effects.9, 31 Puupehenone induced 99% inhibition of M tuberculosis H37Rv growth, with an MIC of 12·5 μg/mL and a 50% inhibitory concentration (IC}50 of 2·0 μg/mL. The puupehenones are shikimate-sesquiterpene derived metabolites isolated from sponges of the order Verongida and Dictyoceratida, collected from the Hawaiian Islands.9, 32
Anthelmintic activity
Anthelmintics are drugs used to rid host organisms of helminth parasites. Parasitism by nematodes (unsegmented worms that constitute the phylum Nematoda) represents a major issue in the commercial livestock industry, and contributes substantially to malnutrition and disease in human beings. Particularly difficult to eradicate is Ascaris lumbricoides, the large gut worm, which causes malnutrition and obstructive bowel disease, and the soil-transmitted blood-sucking hookworms Ancyclostoma duodenale and Necator americanus, which lead to severe blood loss and iron-deficient anaemia, decreased food intake, impaired digestion, malabsorption, and poor growth rate.33 Despite the availability of excellent commercial anthelmintics, growing resistance to key structural classes (benzimidazoles and macrolides) necessitates the search for new bioactive agents.33
Jasplakinolide represents a potent antiparasitic as well as antifungal agent. It exhibited an in-vitro 50% effective dose of less than 1 μg/mL against the nematode Nippostrongylus braziliensis.15 A dihydroxytetrahydrofuran from the south Australian marine brown alga Notheia anomala exhibits potent and selective nematocidal activity against
Haemonchus contortus and Trichostrongylus colubriformis, with a lethal dose in 50% respectively.34 This tetrahydrofuran inhibits the development of eggs for the infective free-living third stage of these two species. This degree of nematocidal activity is similar to that of the commercially available nematocides levamisole and closantel. Unfortunately, attempts to translate this in-vitro nematocidal activity to in vivo proved unsuccessful, but is not surprising given the hydrophobic properties of this drug.34 However, understanding of the in-vitro mode of action of these compounds may yet contribute to the discovery of new and improved anthelmintics.
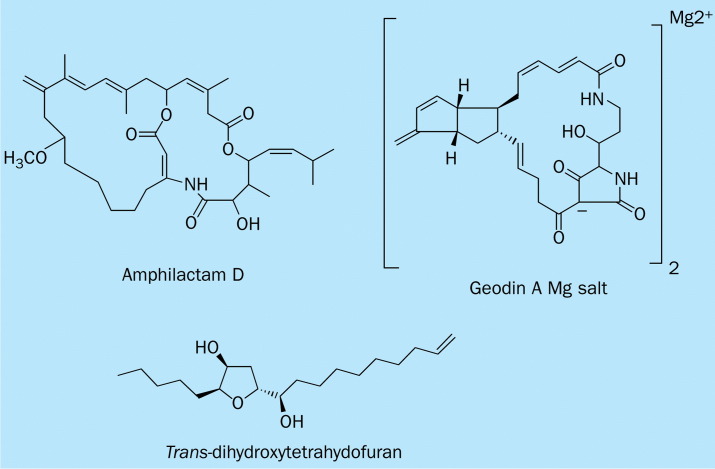
The amphilactams, isolated from a sponge of Amphimedon sp collected in the Great Australian Bight, feature an unusual carbon skeleton and an enamino lactone or lactam moiety (figure 4 ). Amphilactam D has an in-vitro nematocidal activity against the free-living stages of the parasitic nematode H contortus μg/mL. This substance inhibits larval development at the L1 stage, but has little or no activity against nematode eggs. This degree of in-vitro activity is similar to that of existing commercial anthelmintics, such as levamisole and closantel, and is thought to merit in-vivo assessment.35
Figure 4.
Chemical structure of anthelmintic marine natural products.
A sponge, Geodia sp, collected from southern Australia has yielded a potent nematocidal agent, geodin A magnesium salt, which is a macrocyclic polyketide lactam tetramic acid showing an LD99 value of 1·0μg/mL.36
Antiprotozoal activity
Diseases caused by protozoan parasites lead to high rates of mortality and morbidity worldwide. We have focused on the marine-derived natural products showing promising efficacy against the protozoal infections caused by Leishmania sp, Trypanosoma sp, Toxoplasma sp, and Plasmodium sp (figure 5 ).
Figure 5.
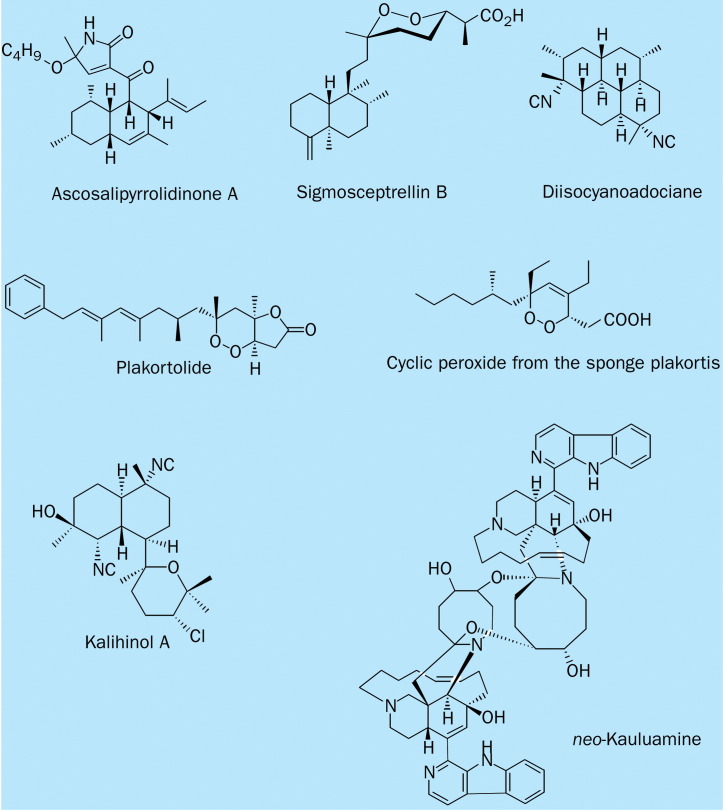
Chemical structure of antiprotozoal marine natural products.
Leishmaniasis is a disease caused by an obligate intracellular parasite of the genus Leishmania. Types of disease range from self-healing ulcers (cutaneous leishmaniasis) to progressive nasopharyngeal infections (mucocutaneous leishmaniasis) to disseminating visceral leishmaniasis, which is generally fatal if left untreated.37 In Mediterranean countries adult visceral leishmaniasis is recognised as an AIDS-related opportunistic disease, largely due to reactivation of latent infections by immunosuppression. The most common drugs for the treatment of leishmaniasis contain pentavalent antimonials, such as sodium stibogluconate and meglumine antimonite, that have cardiotoxic effects at the recommended doses and consequently signify the urgent need for alternative treatments.38
Sponges of the genus Plakortis are well known for their production of cyclic peroxides. Two peroxides produced by the Palauan sponge Plakortis aff angulospiculatus are active against Leishmania mexicana. The most active cyclic peroxide LD50 0·29 μg/mL) causes lysis of the cell membrane after 24h at a concentration of 1·0 μg/mL, and a striking decrease in motility after 30 min. This peroxide is, however, less effective than ketoconazole LD500·06 μg/mL).39
Toxoplasmosis can be transmitted to human beings from cats infected with the parasite Toxoplasma gondii. The severity ranges from a self-limiting adenopathy to fatal encephalitis. This infection is particularly dangerous for pregnant women and immuno-compromised individuals; congenital infection of infants is fatal in most cases.40 New drugs or drug combinations that can eradicate the tissue cysts that cause relapses are urgently needed, particularly for patients intolerant to folate inhibitors (pyrimethamine, trimethoprim), which frequently cause undesirable side-effects. The remaining group of drugs used for the treatment of toxoplasmosis include the macrolides, which act only against the tachyzoite, leaving the cysts unaffected.41
The alkaloid manzamine A displays 70% inhibition of the T gondii parasite, at 0·054 μg/mL concentration, without cell toxic effects. The activity increases notably at concentrations of 0·54 μg/mL and 5·40 μg/mL, even though it is accompanied by an increase in the cytotoxic effects for the host cells, and was therefore selected for in-vivo assessment. A daily intraperitoneal dose of 8 mg/kg of manzamine Afor 8 consecutive days, started on day 1 after infection, prolonged the survival of Swiss Webster mice to 20 days, compared with 16 days for untreated controls. Exploration of new manzamines, structure-activity relation studies, and studies of optimum dose will be important to improve the in-vivo efficacy of the manzamines against T gondii.42 Sigmosceptrellin-B isolated from the Red Sea sponge Diacarnus erythraeanus, exhibits potent in-vitro activity against T gondii at a concentration of 0·039 μg/mL without severe toxic effects. Sigmosceptrellin-B is active (84–99% inhibition) against the parasite in human diploid fibroblast cells.43 A cyclic peroxylactone, plakortide, was isolated from the Jamaican sponge Plakinastrella onkodes and has potent in-vitro activity against T gondi with an IC50 of 0·023 μg/mL and no toxic effects for the host cells up to a concentration 0·12 μg/mL.44
The flagellated protozoa Trypanosoma cruzi and Trypanosoma brucei are, respectively, the causal agents of South American trypanosomiasis (Chagas disease) and human African trypanosomiasis (sleeping sickness). The drugs used to treat these diseases, including pentamidine and suramin, require parenteral administration and are effective only against the early haemolymphatic stage of the disease. The arsenical drug melarsoprol is available for late-stage central nervous system infection, but it causes reactive encephalopathy and is difficult to administer. The nitroheterocyclic drug benznidazole, used to treat acute stages of Chagas disease, is not effective against the chronic phases of the disease and is poorly tolerated, which clearly indicates the need for new drugs with structures and mechanisms of action different from those that are presently used.45
From the green alga Ulva sp the endophytic and obligate marine fungus Ascochyta salicorniae was isolated and cultivated on a preparative scale, producing a structurally unusual tetramic acid metabolite ascosalipyrrolidinone A, which shows activity against T cruzi with an MIC of 1·1 μg/mL, whereas the control (benznidazole) has an MIC of 30·0 μg/mL. The main limitation for further development of this compound is its cytotoxic effects at 3·7 μg/mL tested against rat skeletal muscle myoblast cells.46
Malaria is a particularly serious disease in sub-Sahran Africa,47 but it is also a serious public-health issue in certain regions of southeast Asia and South America. Most malaria cases and deaths are caused by the parasite Plasmodium falciparum.47 Since removal of the vector of transmission (the anopheles mosquito) is almost impossible, new antimalarial agents providing novel mechanisms of action will always be needed to combat resistance to drugs such as chloroquine, mefloquine, quinine, and sulfadoxine-pyrimethamine.
Manzamine A exhibits potent in-vitro activity against P falciparum (D6 clone), with an MIC of 0·0045 μg/mL, compared with control-drug (chloroquine and artemisinin) MICs of 0·0155 μg/mL and 0·010 μg/mL, respectively.48 Manzamine A inhibits the growth of the rodent malaria parasite Plasmodium berghei in vivo, with more than 90% of the asexual erythrocytic stages of P berghei inhibited after one intraperitoneal injection of 50 or 100 mol/kg manzamine A into infected mice. This treatment prolongs the survival of highly parasitaemic mice to more than 10 days compared with 2–3 days among untreated controls, 2 days among mice treated with artemisinin, and 6 days among mice treated with chloroquine; it has a 40% recovery rate 60 days after one injection. Oral administration of an oil suspension of manzamine A also substantially reduces parasitaemia. Interestingly, the manzamine A hydroxyl derivative (−)-8-hydroxymanzamine A, as well as the dimer neo-kauluamine, extend the lives of P berghei-infected mice far more than the two most important human therapeutic antimalarial drugs when administered as one intraperitoneal dose of 100 mol/kg.
Manzamine A and selected derivatives have a rapid onset of action because of high bioavailability and sustained antiparasitic activity with no apparent toxic effects.48 Manzamine A has a similar therapeutic index to chloroquine, whose toxic dose at 500 mol/kg is ten times higher than the dose required (50 mol/kg) to clear the parasite if administered three times with 2-day intervals.48 The effectiveness of manzamine A and selected analogues against malaria makes them one of the most promising anti-infective leads to be discovered from the oceans, and understanding the structure-activity relation of this unique class of alkaloids is certain to lead to more effective and safer manzamine-related antimalarial drug leads.
The previously mentioned antifungal macrolide halichondramide also has notable activity against P falciparumμg/mL, (D6 clone), with an IC50 of 0·002 μg/ml, which is approaching the value for the most active clinically used compounds (mefloquine 0·0003 μg/mL). Although halichondramide has a much lower selectivity index than currently used drugs, it represents a potential new class of compounds in the development of alternative chemotherapy for the treatment of malaria after further structure-activity relation investigations.49
An example of a marine secondary metabolite containing the isonitrile group and eliciting significant antimalarial activity is di-isocyanoadociane, a tetracyclic diterpene with an isocycloamphilectane skeleton. Di-isocyanoadociane has been isolated from the sponge Cymbastela hooperi (axinellidae, halichondrida) and displayed antiplasmodial potency, with IC50 values of 0·005μg/mL, and selectivity that rivals the in-vitro results obtained from clinically used antimalarial drugs (chloroquine and artemisinin).50 The kalihinane diterpenoids exhibit several types of biological activities, of which the antimalarial activity of kalihinol A is the most noteworthy. Kalihinol A was isolated from an Okinawan marine sponge, Acanthella sp, and it possesses notable in-vitro antimalarial activity (EC500 0·0005 μg/mL) and selectivity index (SI 317)similar to those for mefloquine.51
Antibacterial activity
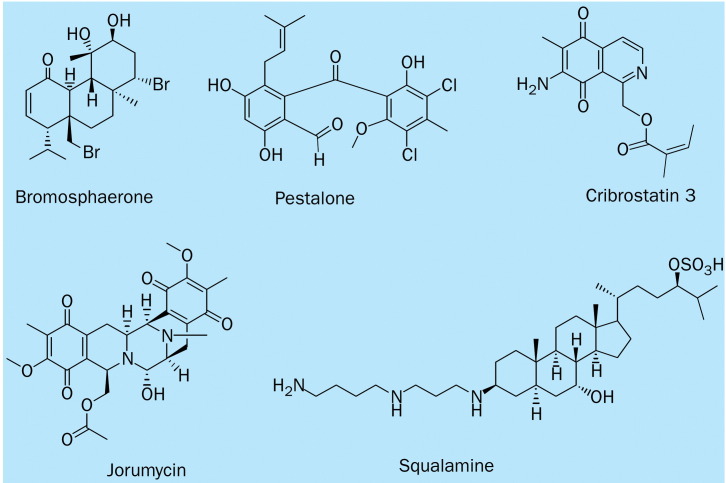
The development of resistance to current antibacterials continues to be a serious difficulty in the treatment of infectious diseases, and therefore the discovery and development of new antibiotics has become a high priority in biomedical research. In the continuing effort by the marine natural products community, many antibacterial agents have been identified; we have focused only on those that seem to possess the greatest potential (figure 6 ).
Figure 6.
Chemical structure of antibacterial marine natural products.
Squalamine is the first aminosterol isolated from the dogfish shark Squalus acanthias (squalidae). It has potent antimicrobial activity, with an MIC of 1·0 μg/mL against Staphylococcus aureus, and antiangiogenic and antitumour properties. It is currently being tested in clinical trials for the treatment of advanced non-small-cell lung cancer.52
Cribrostatins were isolated from a blue sponge Cribrochalina sp, and showed potent antineoplastic and antimicrobial activities. Cribrostatin 3 has potent inhibitory activity against Neisseria gonorrheae, with an MIC of 0·09 μg/mL. Cribrostatin 3 is also active against penicillin-resistant N gonorrheae (clinical isolate), with an MIC of 0·39 μg/mL.53
Sphaerococcus coronopifolius, a cosmopolitan red alga collected along the Atlantic coast of Morocco, contains the potent antibacterial diterpene, bromosphaerone. It shows antibacterial activity against S aureus, with an MIC of 0·047 μg/mL.54
A marine fungus isolated from the surface of the brown alga Rosenvingea sp, of the genus Pestalotia, collected in the Bahamas, was cocultured with a unicellular marine bacterium to yield pestalone. Pestalone has potent antibiotic activity against meticillin-resistant S aureus, with an MIC of 0·037 μg/mL, and vancomycin-resistant Enterococcus faecium, with an MIC of 0·078 μg/mL. These results represent an important achievement in showing that mixed fermentation can induce the biosynthesis of novel antibiotics, suggesting that this method may have use for drug discovery in the future. In addition, the potency of this agent toward drug-resistant pathogens suggests that pestalone should be assessed in more advanced animal models against infectious diseases.55
Jorumycin is a dimeric isoquinoline alkaloid that was isolated from the mantle and mucus of the Pacific nudibranch Jorunna funebris. It inhibits the growth of various Gram-positive bacteria—eg, Bacillus subtilis, S aureus—at a concentration of 0·050 μg/mL, with an inhibition zone of 16 mm. Despite this high potency, there are several limitations for further investigation, which include its cytotoxic effects at an IC50 of 0·012 μg/mL and the difficulty in obtaining a pure stable preparation.56
Antiviral activities
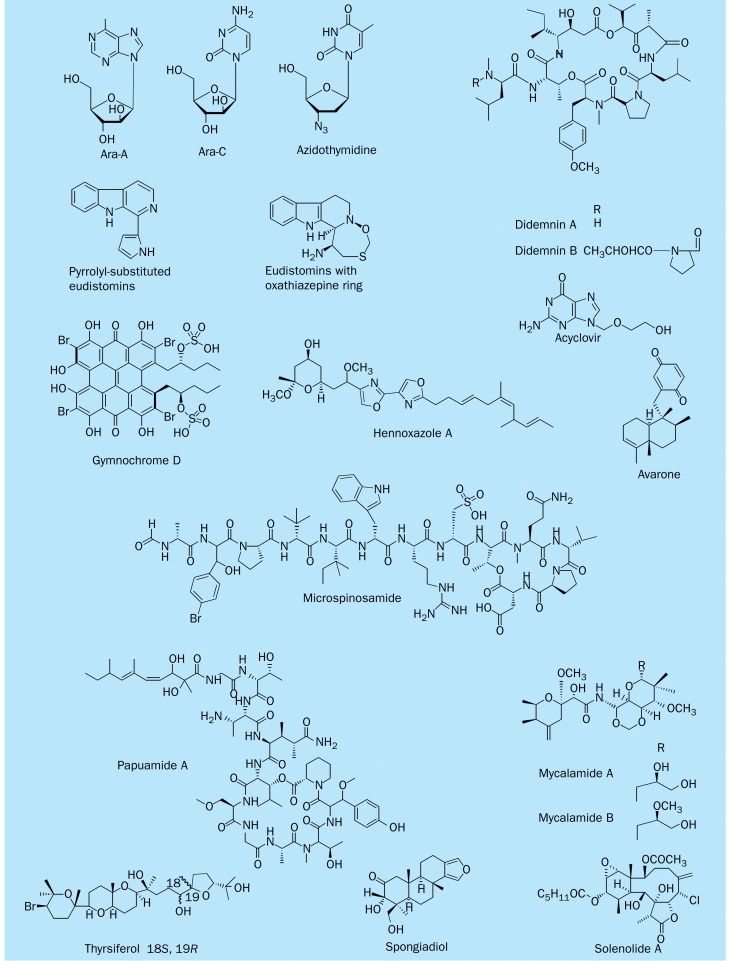
Viruses have remained resistant to treatment or prophylaxis longer than any other infectious organism. The search for viral chemotherapeutic agents from marine sources has yielded several promising therapeutic leads reported to display notable antiviral activity (figure 7 ).
Figure 7.
Chemical structure of antiviral marine natural products
Perhaps the most important antiviral lead of marine origin reported thus far is the nucleoside ara-A. Ara-A is a semisynthetic compound based on the arabinosyl nucleosides isolated from the sponge Cryptotethia crypta.57 Once it was realised that biological systems would recognise the nucleoside base after modifications of the sugar moiety, chemists began to substitute the typical pentoses with acyclic entities or with substituted sugars, leading to the drug azidothymidine (zidovudine). Ara-A (vidarabine), ara-C (1–D-arabinosylcytosine; cytarabine), acyclovir, and azidothymidine are in clinical use and are all examples of products of semisynthetic modifications of the arabinosyl nucleosides.58
The didemnins are a family of closely related cyclic depsipeptides obtained from Trididemnum solidum, a Caribbean tunicate, or sea squirt, of the family Didemnidae.59 In addition to their antitumour activity, they exhibit significant in-vitro and in-vivo antiviral properties. In 1982, Canonico and colleagues60 reported that didemnin B has in-vitro inhibitory effects against the viruses causing Rift Valley fever LD50 0·04 μg/mL), Venezuelan equine encephalomyelitisLD50 0·08 μg/mL), and yellow fever (LD50 0·08 μg/mL). In-vivo didemnin B at a dose of 0·25 mg/kg daily in mice infected with Rift Valley fever virus gave a 90% survival rate. Despite its important antiviral activity, didemnin B is cytotoxic and inhibits cellular DNA, RNA, and protein synthesis at concentrations close to those at which viral growth was inhibited, and hence it has low antiviral selectivity and therapeutic index. The didemnins might, however, be modifiable or useful in combination with other antiviral agents to combat such difficult diseases.61 Didemnin B interferes with mitogenic signal transmission such as inhibiting kinases, phosphatases, and elongation factors. Since 1986, didemnin B has been subjected to phase 1 and 2 clinical trials for cancer by different groups, and the consensus is that with the dose schedules used, the drugs provided little efficacy and substantial toxic effects.61
Eudistomins represent a large family of-carboline alkaloids that have been isolated from shallow-water tunicates from the genus Eudistoma. There are four types of eudistomins, including: unsubstituted, pyrrolyl-substituted, pyrrolinyl-substituted, and tetrahydro–carbolines containing a uniquely condensed oxathiazepine ring system. The in-vitro antiviral potency of the eudistomins against herpes simplex viruses 1 and 2 ranges from 5 ng/disk to 500 ng/disk and follows a trend in which the oxathiazepino-tetrahydro–carbolines show greater potency than 1-pyrrolinyl-substituted, which in turn have better antiviral activity than the 1-pyrrolyl-substituted compounds.62
Perry and colleagues63 reported isolating and investigating the structure of mycalamide A from a New Zealand sponge Mycale sp. They found that a material consisting of 2% mycalamide A was effective against A59 coronavirus in vivo in mice at 0·2 g/kg daily, with 100% survival after 14 days. When pure mycalamide A has been obtained, it inhibited herpes simplex virus 1 and poliovirus type 1 at 0·005 g/disk.63 When mycalamide B was isolated, it showed greater antiviral activity and cytotoxicity than mycalamide A. In-vitro antiviral testing showed a minimum dose of inhibition of 0·001–0·002 g/disk for mycalamide B. Mycalamide A and B are protein synthesis inhibitors.64 Further antiviral marine natural products are presented in table 2 .
Table 2.
Antiviral marine natural products
| Compound | Type* | Source | Activity | Reference |
|---|---|---|---|---|
| Papuamides A | Cyclic depsipeptides | Theonella mirabilis and T swinhoei sponges | HIV-1RF IC50 0·004μ g/mL | 65 |
| Avarone | Sesquiterpene hydroquinone | Dysidea avara sponge | HIV-1 0·1–1·0 μg/mL | 66 |
| Gymnochrome D | Brominated phenanthroperylenequinone igments | Fossil crinoid Gymnocrinus richeri | Dengue virus at doses <1·0 g/mL | 67 |
| Microspinosamide | Cyclic depsipeptide | Sidonops microspinosa sponge | HIV-1 EC50 0·2μg/mL | 68 |
| Solenolide A | Diterpene lactone | Gorgonian of the genus Solenopodium | Rhinovirus IC50 0·39μg/mL, poliovirus III, herpesvirus, and Ann Arbor and Maryland viruses | 69 |
| Hennoxazole A | Oxazole-containing alkaloid | Polyfibrospongia sp sponge | HSV1 IC50 0·6 μg/mL | 70 |
| Thyrsiferol | Triterpene | Red alga Laurencia venusta | VSV and HSV1 0·1–0·5 μg/disk | 71 |
| Spongiadiol | Tetracyclic furanoditerpene | Deep-water Spongia sp | HSV1 0·5 μg/disk | 72 |
HSV1=herpes simplex virus 1; VSV=vesicular stomatitis virus.
Structures illustrated in figure 2.
The way forward
The unprecedented diversity of marine natural products combined with an improved global awareness about the need for new anti-infective treatments is certain to result in an increased effort to move the product of the marine environment into clinical applications. Although marine-product leads have historically been confronted with many obstacles, including a sustainable supply—ie, the compounds may account for less than 10−6% of the wet weight73—there have been substantial advances, which suggest that sustainable sourcing will be achievable. We have learned from terrestrial drug leads that continuous and exhaustive harvesting from nature is not reliable and puts the respective species under risk of extinction. Consequently, the large-scale production of metabolites from marine natural products for clinical applications is a real challenge and alternative strategies for environmentally sound and economically feasible supplies are clearly needed.
Traditionally, among the first options to be explored for the supply of marine-derived small molecules is chemical synthesis. Unfortunately, the structural complexity of marine molecules, which suggests novel mechanisms of action and high selectivity, has also resulted in few economically feasible strategies for total chemical synthesis. One successful example of the synthetic production of a marine-product drug in unlimited quantities is the conus toxin ziconotide, because of its peptide nature.74 A second but also fairly labour-intensive strategy is to do studies of the biological roles of marine natural product pharmacophores, with a clearly defined structural moiety, and attempt to define whether the critical pharmacophore via synthesis, chemical degradation, modification, or a combination of these, can result in more practical drugs based on a marine prototype.
The aquaculture of the source organisms, including sponges, tunicates, and bryozoans, with the aim of securing a steady supply of drug product, has progressed notably in cancer applications, although in most cases the biomass currently generated is still far short of those required should a marine-based drug finally enter the market.75 Furthermore, the cultivation of invertebrates in their natural environment is subject to several uncertainties, such as destruction by storms or disease. An intriguing strategy has been to identify the true producers of bioactive compounds to find out whether or not they are of microbial origin, based on the fact that most if not all of the marine invertebrates harbour large communities of microorganisms, including bacteria, cyanobacteria, and fungi within their tissues.76 Many studies have successfully provided data to support the involvement of microorganisms in the biosynthesis of natural products isolated from invertebrates. The major breakthrough in the characterisation of the microbial communities associated with sponges involve the application of molecular methods such as fluorescence in-situ hybridisation, with the use of group-specific 16 S rRNA-targeted oligonucleotide probes. This approach has led to the identification of the bacterial communities associated with many marine invertebrates.77
If bacteria or other associated microorganisms do produce these compounds of interest, the careful design of special media for culture would be useful for large-scale fermentation. Currently, only an estimated 5% or less of the bacteria seen in marine samples by microscopic methods can be cultivated under standard conditions.78 As a result, molecular approaches offer particularly promising alternatives through the transfer of biosynthetic gene clusters to a vector suitable for large-scale fermentation, thereby avoiding the difficulties in culturing symbiotic bacteria.
Clearly, the world's oceans will play an important part in the future control of the global infectious-disease burden. Although substantial progress has been made in identifying novel drug leads from the ocean's resources, great efforts are still needed to advance to clinical applications.
Search strategy and selection criteria
We identified data for this review by searches of SciFinder Scholar (http://cas.org/SCIFINDER/SCHOLAR/index.html), Marine Lit, and references from relevant articles. Search terms were “antifungal marine”, “antimycobacterial marine”, “antibiotic marine”, “antiviral marine”, “antibacterial marine”, “anthelmintic marine”, and “marine natural products”. We reviewed English-language and French-language papers.
Acknowledgments
Acknowledgments
The preparation of this review was supported by NIH grants R01AI 36596 and KO2AI01502 from the National Institute of Allergy and Infectious Diseases, and the Egyptian government (predoctoral fellowship for MD). We thank Charles Stanley for his assistance in the preparation of this review.
Conflict of interest
None declared.
References
- 1.Jimeno JM. A clinical armamentarium of marine-derived anti-cancer compounds. Anticancer Drugs. 2002;13(suppl 1):S15–S19. [PubMed] [Google Scholar]
- 2.Pomponi SA. The bioprocess-technological potential of the sea. J Biotechnol. 1999;70:5–13. [Google Scholar]
- 3.Ireland CM, Copp BR, Foster MP, McDonald LA, Radisky DC, Swersey JC. Bioactive compounds from the sea. In: Martin RE, Carter EP, Davis LM, editors. Marine and freshwater products handbook. Technomic Publishing; Lanceaster. PA: 2000. pp. 641–661. [Google Scholar]
- 4.Konig GM, Wright AD, Sticher O, Angerhofer CK, Pezzuto JM. Biological activities of selected marine natural products. Planta Med. 1994;60:532–537. doi: 10.1055/s-2006-959565. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner DJ. Marine natural products. Nat Prod Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- 6.Cvetkovic RS, Figgitt DP, Plosker GL. ET-743. Drugs. 2002;62:1185–1192. doi: 10.2165/00003495-200262080-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kayser O, Kiderlen AF, Croft SL. Natural products as potential antiparasitic drugs. Stud Nat Prod Chem. 2002;26:779–848. bioactive natural products part G. [Google Scholar]
- 8.Ghisalberti EL. Secondary metabolites with antinematodal activity. Stud Nat Prod Chem. 2002;26:425–506. bioactive natural products part G. [Google Scholar]
- 9.El Sayed KA, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT. Marine natural products as antituberculosis agents. Tetrahedron. 2000;56:949–953. [Google Scholar]
- 10.El Sayed KA. Natural products as antiviral agents. Stud Nat Prod Chem 2000; 24 (bioactive natural products part E): 473–572.
- 11.Li H-Y, Matsunaga S, Fusetani N. Antifungal metabolites from marine sponges. Curr Org Chem. 1998;2:649–682. [Google Scholar]
- 12.Capon RJ. Marine bioprospecting: trawling for treasure and pleasure. Eur J Org Chem. 2001:633–645. [Google Scholar]
- 13.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 15.Crews P, Manes LV, Boehler M. Jasplakinolide a cyclodepsipeptide from the marine sponge Jaspis sp. Tetrahedron Lett. 1986;27:2797–2800. [Google Scholar]
- 16.Nagai H, Murata M, Torigoe K, Satake M, Yasumoto T. Gambieric acids: new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus. J Org Chem. 1992;57:5448–5453. [Google Scholar]
- 17.Sata NU, Matsunaga S, Fusetani N. Aurantosides D E and F: new antifungal tetramic acid glycosides from the marine sponge Siliquariaspongia japonica. J Nat Prod. 1999;62:969–971. doi: 10.1021/np9900021. [DOI] [PubMed] [Google Scholar]
- 18.Searle PA, Molinski TF. Phorboxazoles A and B: potent cytostatic macrolides from marine sponge Phorbas sp. J Am Chem Soc. 1995;117:8126–8131. [Google Scholar]
- 19.Kobayashi J, Tsuda M, Fuse H, Sasaki T, Mikami Y. Halishigamides A-D new cytotoxic oxazole-containing metabolites from Okinawan sponge Halichondria sp. J Nat Prod. 1997;60:150–154. [Google Scholar]
- 20.Kernan MR, Molinski TF, Faulkner DJ. Macrocyclic antifungal metabolites from the Spanish dancer nudibranch Hexabranchus sanguineus and sponges of the genus Halichondria. J Org Chem. 1988;53:5014–5020. [Google Scholar]
- 21.Roll DM, Ireland CM, Lu HSM, Clardy J. Fascaplysin: an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. J Org Chem. 1988;53:3276–3278. [Google Scholar]
- 22.McCarthy PJ, Pitts TP, Gunawardana GP, Kelly-Borges M, Pomponi SA. Antifungal activity of meridine a natural product from the marine sponge Corticium sp. J Nat Prod. 1992;55:1664–1668. doi: 10.1021/np50089a016. [DOI] [PubMed] [Google Scholar]
- 23.Searle PA, Richter RK, Molinski TF. Bengazoles C-G from the sponge Jaspis sp synthesis of the side chain and determination of absolute configuration. J Org Chem. 1996;61:4073–4079. doi: 10.1021/jo952261a. [DOI] [PubMed] [Google Scholar]
- 24.Kashman Y, Hirsh S, McConnell OJ, Ohtani I, Kusumi T, Kakisawa H. Ptilomycalin A: a novel polycyclic guanidine alkaloid of marine origin. J Am Chem Soc. 1989;111:8925–8926. [Google Scholar]
- 25.Fahy E, Molinski TF, Harper MK. Haliclonadiamine: an antimicrobial alkaloid from the sponge Haliclona sp. Tetrahedron Lett. 1988;29:3427–3428. [Google Scholar]
- 26.Ichiba T, Corgiat JM, Scheuer PJ, Kelly-Borges M. 8-Hydroxymanzamine A a-carboline alkaloid from a sponge Pachypellina sp. J Nat Prod. 1994;57:168–170. doi: 10.1021/np50103a027. [DOI] [PubMed] [Google Scholar]
- 27.Yousaf M, El Sayed KA, Rao KV. 1234-oxamanzamines novel biocatalytic and natural products from manzamine producing Indo-Pacific sponges. Tetrahedron. 2002;58:7397–7402. [Google Scholar]
- 28.Konig GM, Wright AD, Franzblau SG. Assessment of antimycobacterial activity of a series of mainly marine derived natural products. Planta Med. 2000;66:337–342. doi: 10.1055/s-2000-8534. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez AD, Ramirez C, Rodriguez GE., II Novel antimycobacterial benzoxazole alkaloids from the West Indian sea whip Pseudopterogorgia elisabethae. Org Lett. 1999;1:527–530. doi: 10.1021/ol9907116. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez AD, Ramirez C. Serrulatane diterpenes with antimycobacterial activity isolated from the West Indian sea whip Pseudopterogorgia elisabethae. J Nat Prod. 2001;64:100–102. doi: 10.1021/np000196g. [DOI] [PubMed] [Google Scholar]
- 31.Iguchi K, Saitoh S, Yamada Y. Novel 19-oxygenated sterols from the Okinawan soft coral Litophyton viridis. Chem Pharm Bull. 1989;37:2553–2554. [Google Scholar]
- 32.Nasu SS, Yeung BKS, Hamann MT, Scheuer PJ, Kelly-Borges M, Goins KD. Puupehenone-related metabolites from two Hawaiian sponges Hyrtios spp. J Org Chem. 1995;60:7290–7292. [Google Scholar]
- 33.Crompton DWT, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 34.Capon RJ, Barrow RA, Rochfort S. Marine nematodes: tetrahydrofurans from a Southern Australian brown alga Notheia anomala. Tetrahedron. 1998;54:2227–2242. [Google Scholar]
- 35.Ovenden SPB, Capon RJ. Amphilactams A-D: novel nematocides from Southern Australian marine sponges of the genus Amphimedon. J Org Chem. 1999;64:1140–1144. [Google Scholar]
- 36.Capon RJ, Skene C, Lacey E, Gill JH, Wadsworth D, Friedel T. Geodin A magnesium salt: a novel nematocide from a Southern Australian marine sponge. GeodiaJ Nat Prod. 1999;62:1256–1259. doi: 10.1021/np990144v. [DOI] [PubMed] [Google Scholar]
- 37.Berman J. Chemotherapy of leishmaniasis: recent advances in the treatment of visceral disease. Curr Opin Infect Dis. 1998;11:707–710. [PubMed] [Google Scholar]
- 38.Alvar J, Canavate C, Guiterrez-Solar B. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Compagnone R, Pina IC, Rangel HR. Antileishmanial cyclic peroxides from the Palauan sponge Plakortis aff Angulospiculatus. Tetrahedron. 1998;54:3057–3068. [Google Scholar]
- 40.Tuazon CU. Toxoplasmosis in AIDS patients. J AntimicrobChemother. 1989;23(suppl A):77–82. doi: 10.1093/jac/23.suppl_a.77. [DOI] [PubMed] [Google Scholar]
- 41.Derouin F. Anti-toxoplasmosis drugs. Curr Opin Invest Drugs. 2001;2:1368–1374. [PubMed] [Google Scholar]
- 42.El Sayed KA, Kelly M, Kara UAK. New manzamine alkaloids with potent activity against infectious diseases. J Am Chem Soc. 2001;123:1804–1808. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- 43.El Sayed KA, Hamann MT, Hashish NE, Shier WT, Kelly M, Khan AA. Antimalarial antiviral and antitoxoplasmosis norsesterterpene peroxide acids from the Red Sea sponge Diacarnus erythraeanus. J Nat Prod. 2001;64:522–524. doi: 10.1021/np000529+. [DOI] [PubMed] [Google Scholar]
- 44.Perry TL, Dickerson A, Khan AA. New peroxylactones from the Jamaican sponge Plakinastrella onkodes with inhibitory activity against the AIDS opportunistic parasitic infection Toxoplasma gondii. Tetrahedron. 2001;57:1483–1487. [Google Scholar]
- 45.Barrett MP, Mottram JC, Coombs GH. Recent advances in identifying and validating drug targets in trypanosomes and leishmanias. Trends Microbiol. 1999;7:82–88. doi: 10.1016/s0966-842x(98)01433-4. [DOI] [PubMed] [Google Scholar]
- 46.Osterhage C, Kaminsky R, Konig GM, Wright AD. Ascosalipyrrolidinone A: an antimicrobial alkaloid from the obligate marine fungus Ascochyta salicorniae. J Org Chem. 2000;65:6412–6417. doi: 10.1021/jo000307g. [DOI] [PubMed] [Google Scholar]
- 47.Mishra SK, Satpathy SK, Mohanty S. Survey of malaria treatment and deaths. Bull World Health Organ. 1999;77:1020. [PMC free article] [PubMed] [Google Scholar]
- 48.Ang KKH, Holmes MJ, Higa T, Hamann MT, Kara UAK. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob Agents Chemother. 2000;44:1645–1649. doi: 10.1128/aac.44.6.1645-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Sayed KA, Dunbar DC, Goins DK. The marine environment: a resource for prototype antimalarial agents. J Nat Toxins. 1996;5:261–285. [Google Scholar]
- 50.Konig GM, Wright AD, Angerhofer CK. Novel potent antimalarial diterpene isocyanates isothiocyanates and isonitriles from the tropical marine sponge Cymbastela hooperi. J Org Chem. 1996;61:3259–3267. [Google Scholar]
- 51.Miyaoka H, Shimomura M, Kimura H, Yamada Y. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge Acanthella sp. Tetrahedron. 1998;54:13467–13474. [Google Scholar]
- 52.Rao MN, Shinnar AE, Noecker LA. Aminosterols from the dogfish shark Squalus acanthias. J Nat Prod. 2000;63:631–635. doi: 10.1021/np990514f. [DOI] [PubMed] [Google Scholar]
- 53.Pettit GR, Knight JC, Collins JC, Herald DL, Young VG. Antineoplastic agents 430 Isolation and structure of cribrostatins 3 4 and 5 from the Republic of Maldives Cribrochalina sp. J Nat Prod. 2000;63:793–798. doi: 10.1021/np990618q. [DOI] [PubMed] [Google Scholar]
- 54.Etahiri S, Bultel-Ponce V, Caux C, Guyot M. New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J Nat Prod. 2001;64:1024–1027. doi: 10.1021/np0002684. [DOI] [PubMed] [Google Scholar]
- 55.Cueto M, Jensen PR, Kauffman C, Fenical W, Lobkovsky E, Clardy J. Pestalone a new antibiotic produced by a marine fungus in response to bacterial challenge. J Nat Prod. 2001;64:1444–1446. doi: 10.1021/np0102713. [DOI] [PubMed] [Google Scholar]
- 56.Fontana A, Cavaliere P, Wahidulla S, Naik CG, Cimino G. A new antitumor isoquinoline alkaloid from the marine nudibranch Jorunna funebris. Tetrahedron. 2000;56:7305–7308. [Google Scholar]
- 57.Bergmann W, Feeney RJ. Contributions to the study of marine products: the nucleosides of sponges. J Org Chem. 1951;16:981–987. [Google Scholar]
- 58.De Clerq E. New anti-HIV agents and targets. Med Res Rev. 2002;22:531–565. doi: 10.1002/med.10021. [DOI] [PubMed] [Google Scholar]
- 59.Rinehart KL, Gloer JB, Cook JC, Mizsak SA, Scahill TA. Structures of the didemnins antiviral and cytotoxic depsipeptides from a Caribbean tunicate. J Am Chem Soc. 1981;103:1857–1859. [Google Scholar]
- 60.Canonico PG, Pannier WL, Huggins JW, Rinehart KL. Inhibition of RNA viruses in vitro and in Rift Valley fever-infected mice by didemnins A and B. Antimicrob AgentsChemother. 1982;22:696–697. doi: 10.1128/aac.22.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vera MD, Joullie MM. Natural products as probes of cell biology: 20 years of didemnin research. Med Res Rev. 2002;22:102–145. doi: 10.1002/med.10003. [DOI] [PubMed] [Google Scholar]
- 62.Rinehart KL, Kobayashi J, Harbour GC, Hughes RG, Jr, Mizsak SA, Scahill TA. Eudistomins C E K and L potent antiviral compounds containing a novel oxathiazepine ring from the Caribbean tunicate Eudistoma olivaceum. J Am ChemSoc. 1984;106:1524–1526. [Google Scholar]
- 63.Perry NB, Blunt JW, Munro MHG, Pannell LK. Mycalamide A an antiviral compound from a New Zealand sponge of the genus Mycale. J Am Chem Soc. 1988;110:4850–4851. [Google Scholar]
- 64.Perry NB, Blunt JW, Munro MHG, Thompson AM. Antiviral and antitumor agents from a New Zealand sponge Mycale sp 2: structures and solution conformations of mycalamides A and B. J Org Chem. 1990;55:223–227. [Google Scholar]
- 65.Ford PW, Gustafson KR, McKee TC, Shigematzu N. Papuamides A-D HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc. 1999;121:5899–5909. [Google Scholar]
- 66.Sarin PS, Sun D, Thornton A, Muller WE. Inhibition of replication of the etiologic agent of acquired immune deficiency syndrome (human T-lymphotropic retrovirus/ lymphadenopathy-associated virus) by avarol and avarone. J Natl Cancer Inst. 1987;78:663–666. [PubMed] [Google Scholar]
- 67.Laille M, Gerald F. Debitus C: in vitro antiviral activity on dengue virus of marine natural products. Cell Mol Life Sci. 1998;54:167–170. doi: 10.1007/s000180050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rashid MA, Gustafson KR, Cartner LK, Shigematsu N, Pannell LK, Boyd MR. Microspinosamide a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J Nat Prod. 2001;64:117–121. doi: 10.1021/np0002379. [DOI] [PubMed] [Google Scholar]
- 69.Groweiss A, Look SA, Fenical W. Solenolides new antiinflammatory and antiviral diterpenoids from a marine octocoral of the genus Solenopodium. J Org Chem. 1988;53:2401–2406. [Google Scholar]
- 70.Ichiba T, Yoshida WY, Scheuer PJ, Higa T, Gravalos DG. Hennoxazoles bioactive bisoxazoles from a marine sponge. J Am ChemSoc. 1991;113:3173–3174. [Google Scholar]
- 71.Sakemi S, Higa T, Jefford CW, Bernardinelli G. Venustatriol a new anti-viral triterpenes tetracyclic ether from Laurencia venusta. Tetrahedron Lett. 1986;27:4287–4290. [Google Scholar]
- 72.Komoto S, McConnell OJ, Cross SS. Antitumor and antiviral furanoditerpenoids from a marine sponge. Chem Abstr. 1987;111:50424x. [Google Scholar]
- 73.Proksch P, Edrada RA, Ebel R. Drugs from the seas: current status and microbiological implications. Appl Microbiol Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- 74.Olivera BM. -Conotoxin MVIIA: from marine snail venom to analgesic drug. In: Fusetani N, editor. Drugs from the sea. Karger; Basel: 2000. pp. 74–85. [Google Scholar]
- 75.Mendola D. Aquacultural production of bryostatin 1 and ecteinascidin 743. In: Fusetani N, editor. Drugs from the sea. Karger; Basel: 2000. pp. 120–133. [Google Scholar]
- 76.Vacelet J, Donadey C. Electron microscope study of the association between some sponges and bacteria. J Exp Mar Ecol. 1977;30:301–314. [Google Scholar]
- 77.Friedrich AB, Merkert H, Fendert T, Hacker J, Proksch P, Hentschel U. Microbial diversity in the marine sponge Aplysina cavernicola analyzed by fluorescence in situ hybridization(FISH) Mar Biol. 1999;134:461–470. [Google Scholar]
- 78.Fenical W. Chemical studies of marine bacteria: developing a new resource. Chem Rev. 1993;93:1673–1683. [Google Scholar]