Summary
Echinacea is one of the most commonly used herbal products, but controversy exists about its benefit in the prevention and treatment of the common cold. Thus, we did a meta-analysis evaluating the effect of echinacea on the incidence and duration of the common cold. 14 unique studies were included in the meta-analysis. Incidence of the common cold was reported as an odds ratio (OR) with 95% CI, and duration of the common cold was reported as the weighted mean difference (WMD) with 95% CI. Weighted averages and mean differences were calculated by a random-effects model (DerSimonian-Laird methodology). Heterogeneity was assessed by the Q statistic and review of L'Abbé plots, and publication bias was assessed through the Egger weighted regression statistic and visual inspection of funnel plots. Echinacea decreased the odds of developing the common cold by 58% (OR 0·42; 95% CI 0·25–0·71; Q statistic p<0·001) and the duration of a cold by 1·4 days (WMD −1·44, −2·24 to −0·64; p=0·01). Similarly, significant reductions were maintained in subgroup analyses limited to Echinaguard/Echinacin use, concomitant supplement use, method of cold exposure, Jadad scores less than 3, or use of a fixed-effects model. Published evidence supports echinacea's benefit in decreasing the incidence and duration of the common cold.
Introduction
According to the National Institute of Allergy and Infectious Diseases, the US population has 1 billion colds annually. Adults have between two and four colds per year, whereas children have between six and ten colds.1 Although rhinovirus and coronavirus are the most common viruses precipitating cold symptoms, approximately 200 other viruses are also known to cause the common cold. In the USA, about 40% of lost work time and 30% of time lost from school are attributed to symptoms caused by the common cold.2 The common cold is also associated with a large financial burden on society, with about US$1·5 billion spent annually for physicians' visits and another $2 billion spent on non-prescription cough and cold treatments.3
In 2002, approximately 20% of the adult US population used nutraceuticals (herbal products, functional foods, animal based supplements). Echinacea, a collection of nine related plant species indigenous to North America, was the most common nutraceutical used and was consumed by over 40·3% of these people.4, 5 Echinacea angustifolia, Echinacea pallida, and Echinacea purpurea (figure 1 ) are the most common species recognised for their medicinal value.6 The mechanism of action underlying the proposed immunostimulatory effects of echinacea remains unclear. Some evidence suggests that upregulation of tumour necrosis factor-α mRNA, which is stimulated by agonistic activity of the cannabinoid receptor (CB2) by alkamides present in echinacea, has a role.7
Figure 1.
Echinacea purpurea flower
© 2007 Maxine Adcock/Science Photo Library
The German Commission E, WHO, and the Canadian Natural Health Products Directorate have advocated echinacea use for the common cold.8, 9, 10, 11, 12 However, there is controversy about the efficacy of echinacea for the prevention or treatment of the common cold with some studies showing benefit and others showing a null effect. Meta-analysis can be useful in situations such as this, since it can show what the preponderance of evidence in the published work suggests. A past systematic review by Melchart and colleagues13 concluded that echinacea preparations from the aerial part of the plant were effective for the treatment of colds but the evidence for the prevention of a cold was lacking.13 It is important to note, however, that this review excluded studies using an experimental rhinovirus infection and echinacea preparations with supplements, and it did not include a more recent study by Turner and colleagues.14 We therefore did a meta-analysis evaluating the effect of echinacea on the incidence and duration of the common cold in randomised placebo-controlled studies.
Methods
Search strategy and selection criteria
A literature search using the terms “Echinacea” and “Purple coneflower” (limited to human beings and clinical trials) was done by three independent reviewers (SAS, CMW, and CIC) using Medline (1966 to April, 2006), CINAHL (Cumulative Index to Nursing and Allied Health Literature; 1982 to April, 2005), Web of Science (1994 to April, 2006), the Cochrane Database of Systematic Reviews (October to December, 2005). Hand searches of references in the echinacea monograph of the Natural Medicines Comprehensive Database and in relevant primary and review articles were also done.
Trials were included for analysis if they met the following inclusion criteria: randomised placebo-controlled trials evaluating echinacea-containing products in the prevention and/or treatment of the common cold with adequately reported data on either cold incidence or duration.
In cases where a study evaluated the effects of different echinacea species or formulations compared with placebo, when possible the data from the echinacea arms were pooled and compared with the placebo arm.14, 15 When data were reported separately for bacterial and viral infections, only the latter was extracted for inclusion in the analysis.13, 16
Validity assessment
The following methodological features, most relevant to the control of bias, were assessed: randomisation, random allocation concealment, masking of treatment allocation, blinding, and withdrawals. Jadad scores were calculated to aid in the identification of reports with overall weaker study methodologies.17 All studies were reviewed and evaluated by three independent reviewers (SAS, CMW, and CIC) with disagreement resolved by consensus.
Data abstraction
All data were independently abstracted by three investigators (SAS, CMW, and CIC) through the use of a standardised data abstraction tool. The following information was sought from each article: author identification, year of publication, geographical location of the study, study funding source, type of study design (prospective or retrospective, randomised or observational, presence and type of control, blinded or open-label), study population, sample size, duration of patient follow-up, echinacea product used (specific species, dose, preparation type, and branded or unbranded), presence or absence of concomitantly administered supplement, mode of virus exposure (natural or inoculation), and definition for incidence or duration of cold (when reported).
Statistical analysis
Incidence of the common cold was treated as a dichotomous variable and reported as an odds ratio with its 95% CI using a DerSimonian and Laird random-effects model. Calculation of odds ratios can be problematic when there is an absence of events in one of the comparator groups. For these studies, a nominal value (0·5 colds) was added in each 2×2 cell to enable calculation of an odds ratio.18, 19
Risk difference was also calculated to aid in the assessment of not only statistical but clinical significance as well. Duration of illness was treated as a continuous variable and the weighted mean difference (WMD) was calculated as the difference between the mean days of the common cold in the echinacea and control groups. Again, a DerSimonian and Laird random-effects model was used in calculating the weighted mean difference and its 95% CI. Only one study20 provided 95% CIs for continuous data, for this study the standard deviation of the mean was calculated from the 95% CI using standard methods.19 Statistical heterogeneity was addressed using the Q statistic (p<0·1 considered significant). Heterogeneity was also assessed through visual inspection of L'Abbé plots. All statistical analyses were done using StatsDirect Version 2.4.6 (StatsDirect Ltd, Cheshire, UK).
Numerous subgroup analyses to assess sources of clinical heterogeneity were done. The concomitant administration of additional nutraceuticals with echinacea could potentially result in synergistic, additive, or inhibitory interactions. Therefore, echinacea's efficacy was assessed both in the presence and absence of other nutraceuticals. Because of the lack of regulation of herbal products, concern has arisen regarding the content of active ingredients contained in various products. A study that evaluated echinacea preparations available in a retail setting showed that six (10%) of 59 preparations contained no measurable echinacea.21 Furthermore, only nine (43%) of 21 standardised preparations met the quality standards as described on the label.21 Five studies in this meta-analysis included an E purpurea product extracted in 22% alcohol (Echinaguard and Echinacin, Madaus AG, Cologne, Germany).22, 23, 24, 25, 26, 27 As such, an analysis of benefit using these two products was done in a subgroup. Finally, studies included in this meta-analysis examined patients who were exposed to (or contracted) a cold either naturally13, 15, 16, 23, 24, 25, 26, 27, 28, 29, 30 or through investigator inoculation.14, 22, 31 Since the effect this might have had on the efficacy of echinacea was not known, separate analyses were done to evaluate studies using natural and investigator-inoculated virus.
Studies of poorer methodological quality, such as unblinded or open-labelled trials might exhibit exaggerated treatment effects. Excluding them might result in increased internal validity but could reduce external validity of the analysis. Additionally, the selection of a random versus fixed-effect model in meta-analyses is controversial. The use of a random-effect model in the calculation of confidence intervals results in wider intervals and thus a more conservative estimate of treatment effect compared with a fixed-effect model. To reconcile these issues, sensitivity analysis was done, in which the meta-analysis was reanalysed excluding studies of weaker methodology (Jadad score less than 3) and using a fixed-effects model (Mantel-Haenszel methodology).
Egger weighted regression statistics and a visual inspection of funnel plots were used to assess for the presence of publication bias.
Results
Trials included
Study identification, inclusion, and exclusion are shown in figure 2 . Our initial search strategy yielded 738 citations. Of these, 665 were excluded manually and electronically by limiting our search to human beings and clinical trials. The remaining 73 abstracts were reviewed of which 50 were excluded for evaluating echinacea for outcomes other than cold incidence or duration. Therefore, 23 abstracts remained and underwent full-text article review. Eight of the 23 studies did not report data on either primary endpoint of the analysis (incidence or duration), and one of the 23 used an active control. Therefore, 14 unique studies14, 15, 16, 20, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32 were therefore included in this meta-analysis, encompassing 1356 study participants for incidence and 1630 participants for duration.
Figure 2.
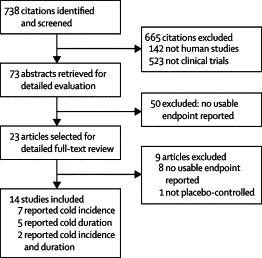
Study identification, inclusion, and exclusion
Table 1 shows characteristics of the included studies. Seven studies evaluated monotherapy with E purpurea,22, 23, 24, 25, 27, 30, 32 one study evaluated E angustifolia,14 one evaluated E pallida,13, 16 one study did not specify which specific species of echinacea was studied,31 and four studies evaluated a combination of different Echinacea species.15, 20, 28, 29 Two studies evaluated echinacea's effect in children.28, 32 Five studies used either Echinaguard or Echinacin products made by one company, Madaus AG.22, 23, 24, 25, 27 Virus exposure using rhinovirus inoculation was done in three studies,14, 22, 31 and four studies evaluated the effect of echinacea along with a supplement.20, 28, 29, 30
Table 1.
Characteristics of included studies
| Patient population | Echinacea species | Use of Echinaguard or Echinacin | Concomitant supplement | Dose | Virus exposure | Duration | Jadad score | |
|---|---|---|---|---|---|---|---|---|
| Turner et al (2005)14 | Healthy volunteers | E angustifolia | No | No | Three times a day equivalent to 900 mg/day | Inoculation with rhinovirus 39 | 7 days pre and 5 days post-inoculation | 4 |
| Cohen et al (2004)28 | Healthy volunteers, children | E purpurea/E angustifolia | No | Vitamin C, propolis | 5 mL twice a day for ages 1–3 years, 7·5 mL twice a day for ages 4–5 years. Increase to four times a day during episode flare only | Natural | 12 weeks | 5 |
| Sperber et al (2004)22 | Healthy volunteers | E purpurea | Echinaguard | No | 2.5 mL three times a day | Inoculation with rhinovirus 39 | 7 days pre and 5 days post-inoculation | 4 |
| Taylor et al (2003)32 | Active cold, children | E purpurea | No | No | 3·75 mL twice a day for ages 2–5 years and 5 mL twice a day for ages 6–11 years | Natural | 10 days | 5 |
| Barrett et al (2002)20 | Active cold | E purpurea/E angustifolia | No | Thyme, peppermint, citric acid | 6 g on day 1 and 3 g on subsequent days | Natural | 10 days | 5 |
| Schulten et al (2001)23 | Healthy volunteers | E purpurea | Echinacin | No | 5 mL twice a day | Natural | At first sign of cold for 10 days | 5 |
| Turner et al (2000)31 | Healthy volunteers | Not specified | No | No | 300 mg three times a day | Inoculation with rhinovirus 23 | 14 days pre and 5 days post-inoculation | 1 |
| Lindenmuth and Lindenmuth (2000)29 | Active cold | E purpurea/E angustifolia | No | Lemongrass leaf, spearmint | Five to six bags per day titrated down to one bag on day 5 | Natural | 12 weeks | 3 |
| Grimm and Muller (1999)24 | Healthy volunteers | E purpurea | Echinacin | No | 4 mL twice a day | Natural | 8 weeks | 5 |
| Berg (1998)25 | Healthy volunteers | E purpurea | Echinacin | No | 8 mL/day | Natural | 28 days | 1 |
| Melchart et al (1998)15 | Healthy volunteers | E purpurea/E angustifolia | No | No | 50 drops twice a day for 12 weeks | Natural | 12 weeks | 5 |
| Hoheisel et al (1997)27 | Healthy volunteers | E purpurea | Echinaguard | No | 20 drops every 2 h in water on day 1 followed by three times a day for 9 days | Natural | At first sign of cold for 10 days | 5 |
| Scaglione and Lund (1995)30 | Active cold | E purpurea | No | Vitamin C, rosemary leaf, eucalyptus, fennel seed | Four tablets daily equivalent to 100 mg/day | Natural | For duration of the cold | 2 |
| Braunig and Knick (1993)16 | Active cold | E pallida | No | No | 90 drops equivalent to 900 mg/day | Natural | 8–10 days | 3 |
Meta-analyses outcomes
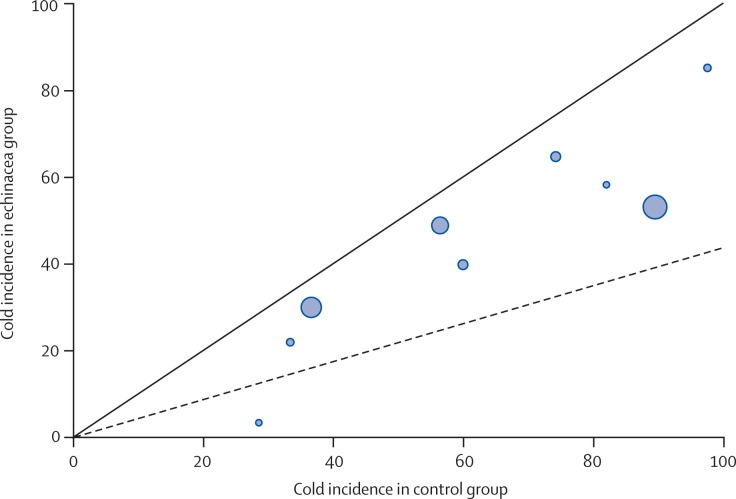
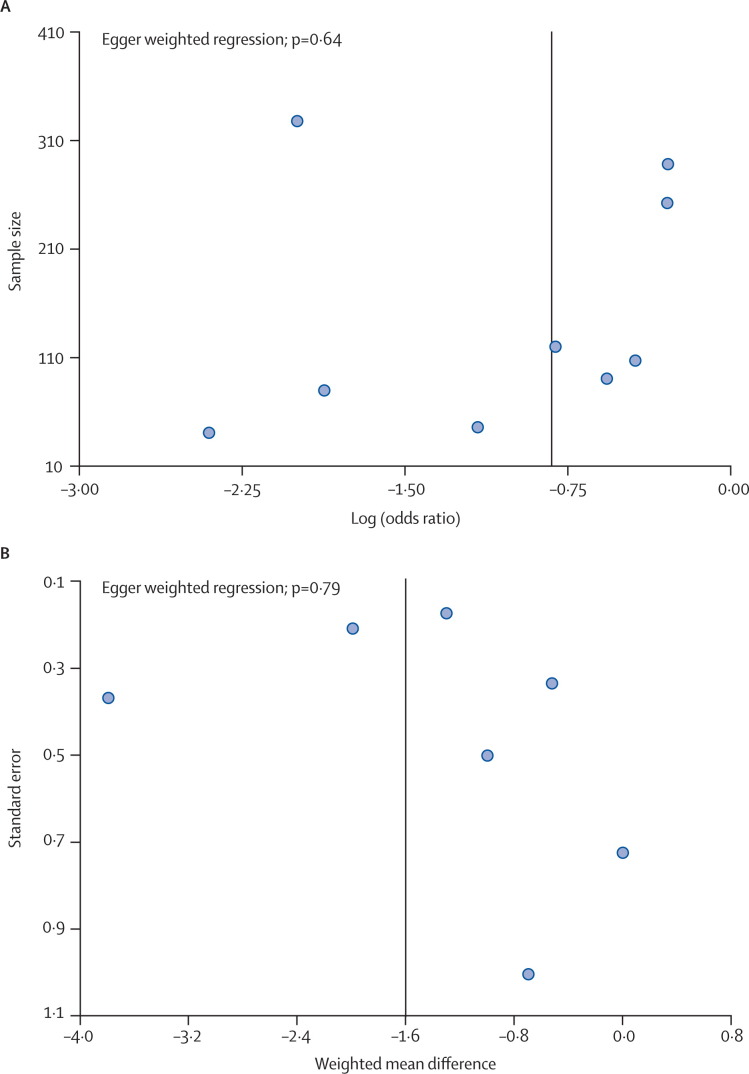
A summary of individual study data on the incidence and duration of colds is provided in table 2 . Meta-analysis showed that echinacea decreased the odds of a patient contracting a cold by 58% (odds ratio [OR] 0·42, 95% CI 0·25–0·71; Q statistic p<0·001), corresponding with a risk difference of −0·17 (−0·25 to −0·08; number needed to treat 6). Echinacea was also found to decrease the duration of cold by 1·4 days (WMD −1·44, −2·24 to −0·64; p=0·01), as shown in figure 3 and figure 4 . The Q statistic showed significant heterogeneity in both the incidence and duration analyses. However, review of the L'Abbé plot for incidence showed that included studies generally agreed on echinacea's positive effect, but not the magnitude of the benefit (figure 5 ). Some degree of asymmetry was noted upon review of the funnel plots for both the incidence and duration analyses, resulting in our inability to rule out the presence of publication bias in our analyses (figure 6 ). However, when publication bias was assessed using the Egger weighted regression statistic, no significant publication bias was detected for either the incidence or duration analyses (p=0·64 and p=0·79, respectively).
Table 2.
Individual study characteristics
| Analyses included in study | Incidence in echinacea group* | Incidence in control group* | Number of patients with cold in echinacea group | Number of patients with cold in control group | Mean duration in echinacea group (SD) | Mean duration in control group (SD) | |
|---|---|---|---|---|---|---|---|
| Turner et al (2005)14 | Incidence of cold | 73/149 | 58/103 | NA | NA | NA | NA |
| Cohen et al (2004)28 | Incidence of cold, duration of cold | 85/160 | 150/168 | 138† | 308† | 1·60 (1·90) | 2·90 (1·60) |
| Sperber et al (2004)22 | Incidence of cold | 14/24 | 18/22 | NA | NA | NA | NA |
| Taylor et al (2003)32 | Duration of cold | NA | NA | 337† | 370† | 9·00 (9·37) | 9.00 (9·81) |
| Barrett et al (2002)20 | Duration of cold | NA | NA | 69 | 73 | 6·27‡ | 5·75‡ |
| Schulten et al (2001)23 | Incidence of cold | 35/41 | 38/39 | NA | NA | NA | NA |
| Turner et al (2000)31 | Incidence of cold | 11/50 | 14/42 | NA | NA | NA | NA |
| Lindenmuth and | Duration of cold | NA | NA | 48 | 47 | 2·34 (1·08) | 4·33 (0·93) |
| Lindenmuth (2000)29 | |||||||
| Grimm and Muller (1999)24 | Incidence of cold | 35/54 | 40/54 | NA | NA | NA | NA |
| Berg (1998)25 | Incidence of cold | 0/14 | 7/26 | NA | NA | NA | NA |
| Melchart et al (1998)15 | Incidence of cold, duration of cold | 60/199 | 33/90 | 60 | 33 | 8·00 (5·10) | 8·7 (3·60) |
| Hoheisel et al (1997)27 | Incidence of cold | 24/60 | 36/60 | NA | NA | NA | NA |
| Scaglione and Lund (1995)30 | Duration of cold | NA | NA | 16 | 16 | 3·37 (1·25) | 4·37 (1·57) |
| Braunig and Knick (1993)16 | Duration of cold | NA | NA | 70 | 45 | 9·10 (1·8) | 12·9 (2·1) |
NA=not applicable.
Data shown as number of events/total population.
Reported data is number of cold episodes, not number of patients with cold.
Reported data as difference of −0·52 days, 95% CI −1·09 to −0·22.
Figure 3.
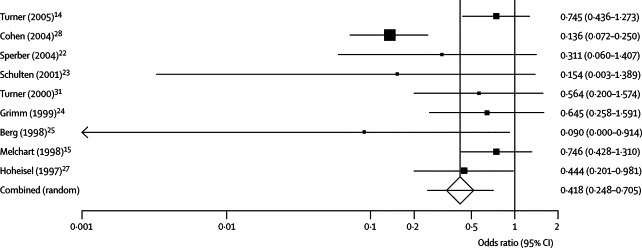
The effect of echinacea on incidence of common cold
The squares represent individual studies and the size of the square represents the weight given to each study in the meta-analysis. Error bars represent 95% CIs. The diamond represents the combined result. The solid vertical line extending upwards from 1·0 is the null value.
Figure 4.
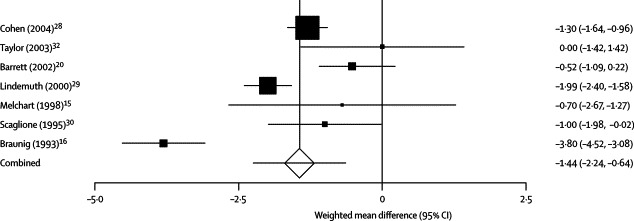
The effect of echinacea on duration of common cold
The squares represent individual studies and the size of the square represents the weight given to each study in the meta-analysis. Error bars represent 95% CIs. The diamond represents the combined result. The solid vertical line extending upwards from 0 is the null value.
Figure 5.
L'Abbé plot for incidence of common cold
Each dot represents an individual study. Symbol size represents sample size.
Figure 6.
Funnel plots of common cold incidence and duration
(A) Incidence of cold. (B) Duration of cold. Vertical line represents the combined effect observed in the analysis.
Subgroup and sensitivity analysis
Table 3 depicts the results of subgroup and sensitivity analyses. Regardless of whether echinacea was administered in the presence or absence of other supplements or nutraceuticals, substantial reductions in the incidence of the common cold were seen. Whereas the subgroup of those receiving echinacea with a supplement showed a significant effect on shorting the duration of cold in its own right (p<0·0001), the subgroup receiving echinacea without a supplement showed only a trend towards benefit (p=0·27).
Table 3.
Results of subgroup and sensitivity analysis
|
Incidence of cold |
Duration of cold |
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of studies | Echinacea group* | Control group* | Odds ratio (95% CI) random effects | Number of studies | Number of participants, echinacea | Number of participants, control | Weighted mean difference (95% CI) random effects | |
| All studies | 9 | 337/751 (45%) | 394/604 (65%) | 0.42 (0·25 to 0·71) | 7 | 738 | 892 | −1·44 (−2·24 to −0·64) |
| Fixed-effects model | 9 | 337/751 (45%) | 394/604 (65%) | 0.44 (0·34 to 0·56) | 7 | 738 | 892 | −1·59 (−2·25 to −0·94) |
| Excluding studies with Jadad score less than 3 | 7 | 326/687 (47%) | 373/536 (70%) | 0·42 (0·23 to 0·76) | 6 | 722 | 876 | −1·51 (−2·40 to −0·61) |
| Excluding Cohen et al (2004)28 | 8 | 252/591 (43%) | 244/436 (56%) | 0·61 (0·46 to 0·81) | 6 | 600 | 584 | −1·43 (−2·53 to −0·33) |
| Studies evaluating echinacea without a supplement | 8 | 252/591 (43%) | 244/436 (56%) | 0·61 (0·46 to 0·81) | 3 | 467 | 448 | −1·57 (−4·34 to 1·19) |
| Studies evaluating echinacea with a supplement | 1 | 85/160 (53%) | 150/168 (89%) | 0·14 (0·07 to 0·25) | 4 | 271 | 444 | −1.25 (−1·87 to −0·65) |
| Studies using Echinaguard/Echinacin | 5 | 108/193 (56%) | 139/201 (69%) | 0·44 (0·27 to 0·71) | 0 | 0 | 0 | NA |
| Natural virus exposure only | 6 | 239/514 (46%) | 304/437 (70%) | 0·35 (0·16 to 0·74) | 7 | 738 | 892 | −1.44 (−2·24 to −0·64) |
| Rhinovirus exposure only | 3 | 98/223 (44%) | 90/167 (54%) | 0·65 (0·42 to 0·99) | 0 | 0 | 0 | NA |
NA=not applicable.
Data shown as number events/total population (%).
In the analysis limited to five studies evaluating Echinaguard or Echinacin products,22, 23, 24, 25, 27 similar significant reductions in patients' odds compared with the overall analysis were observed (p=0·0009). A reduction in the odds of contracting a cold was observed when virus exposure occurred naturally or was investigator inoculated. A decrease in duration of cold was also maintained when only natural virus exposure studies were evaluated.13, 15, 16, 20, 28, 29, 30, 32
For the endpoint of cold duration, no studies used Echinaguard/Echinacin or evaluated investigational rhinovirus inoculation; thus these analyses could not be undertaken. Finally, neither the use of a fixed-effects model instead of a random-effects model nor the exclusion of studies with a Jadad score less than 3 had any effect on overall study conclusions.
Discussion
More than 800 products containing echinacea are available, which come in tablet, extract, fresh juice, tincture, and tea formulations.33 There are three commonly used species of echinacea, differing parts of the plant can be used in different products (flower, stem, root), and the same plant species may contain differing levels of constituent molecules in different parts of the year or geographical location. Although concentration variances exist, all three species of echinacea contain water-soluble polysaccharides, a lipophilic fraction (alkamides, polyacetylenes), caffeoyl conjugates (echinacoside, chicoric acid, caffeic acid) and flavonoids.34, 35 It is yet to be determined if it is one, a few, or the combined effect of many constituents (mainly alkamides, chicoric acid, and polysaccharides) that induce immunostimulation. Despite all of these factors that can influence the efficacy of echinacea and the different doses that can be used, the results of our meta-analysis show that echinacea reduces the incidence as well as the duration of the common cold.
Since the Echinaguard or Echinacin products both contain the fresh pressed juice of E purpurea in 22% alcohol extract, were manufactured by the same company, and were evaluated in five different studies,22, 23, 24, 25, 26, 27 we analysed the benefits of these products separately and found a reduction in the incidence of cold by 56%. This might be important since the variability in the echinacea product evaluated between these trials would be minimised.
Our meta-analysis had only one cold incidence study28 that used echinacea with other supplements (vitamin C and propolis). This study found an 86% reduction in the incidence of the common cold. As such, we cannot determine if the combination of echinacea with other nutraceuticals yields better results than echinacea alone. Several experimental studies have shown that vitamin C might have effects on the immune system.36 Propolis, a natural resinous product collected by honeybees from various plant sources, has also been used in the prevention of respiratory infections.37 For cold duration as the outcome, four studies20, 28, 29, 30 used echinacea combined with additional supplements (vitamin C, propolis, lemongrass leaf, spearmint, peppermint, thyme, citric acid, rosemary leaf, eucalyptus, and fennel seed) and yielded a 1·3-day shorter duration of cold than placebo. Echinacea used alone, although showing a similar benefit, did not show a significantly shorter duration of cold than placebo (p=0·27), suggesting that this sub-group analysis was underpowered. Comparing the results on duration of cold in the overall analysis to the subgroup analyses suggests that the benefit is caused by echinacea rather than the other supplements.
We evaluated the method of viral exposure on the outcome of cold induction. If echinacea was given prophylactically in an attempt to reduce the incidence of natural cold induction, the incidence was reduced by 65% versus placebo. When echinacea was given as prophylaxis against cold induction caused by direct rhinovirus inoculation, the incidence was only reduced by 35%. One postulation for the possible reduced benefits with direct inoculation is that echinacea works better on preventing the common cold caused by viruses other than rhinovirus. With over 200 viruses capable of causing the common cold, echinacea could have modest effect against rhinovirus but marked effects against other viruses. Of the direct rhinovirus inoculation trials, the most touted is the study by Turner and colleagues14 published in 2005. The authors compared patients given E angustifolia equivalent to 900 mg/day with placebo and showed that echinacea did not have “clinically significant effects on infection with a rhinovirus or on the clinical illness that results from it”. The German Commission E has approved E purpurea at a recommended dose of 900 mg but has not approved E angustifolia.8 The 1999 WHO monograph recommends E angustifolia at a dose of 3 g, a dose more than three times the dose used by Turner and colleagues.38 As such, the dose used in this trial may have been too low to be fully effective.
The previous meta-analysis done by Melchart and colleagues13 and updated in November, 2005,39 included 16 trials encompassing 22 analyses and showed a benefit of echinacea for the treatment but not prevention of a common cold.13, 39 By comparison, our meta-analysis included 14 trials encompassing 16 analyses. Although our results are in agreement with the previous meta-analysis, our results suggest an additional benefit of echinacea for use in the prevention, as well as the treatment, of a cold. The meta-analysis by Linde and colleagues39 assessed a cold severity endpoint, included two unpublished evaluations, and excluded studies that used experimental rhinovirus inoculation or that combined echinacea with other nutraceutical ingredients. In our analysis we chose not to evaluate cold severity because of concerns about the potential heterogeneity of the methods used for cold severity assessment in the studies. We included studies evaluating echinacea with other nutraceuticals in our analysis, as well as studies evaluating direct rhinovirus inoculation, which have the highest internal validity since the virus, the degree of exposure, and the exact time of exposure are all known. We decided to address the effect of these potential confounders through the use of subgroup and sensitivity analyses rather than through exclusion, which provides more information from which to make a determination of the efficacy of echinacea. Furthermore, we included one study that was published after Melchart and colleagues updated their analysis39 and excluded unpublished studies because data in such studies have not undergone rigorous peer review.
There are several limitations to this meta-analysis that must be addressed. First, the studies by Barrett20 and Turner14 and their colleagues used alfalfa and a mixture of alcohol beverages, respectively, as their placebo arm. Since alfalfa and alcoholic concoctions may have immunostimulatory benefits, their use in the placebo arm is controversial.40 Although we agree that this may be a potential confounder in our analyses, it should be noted that if these agents do in fact have beneficial properties that reduce the incidence and/or duration of the common cold, then this would result in an underestimation of echinacea's benefit. Second, although the Egger statistic shows absence of publication bias, our funnel plot shows asymmetry, suggesting that the potential for publication bias cannot be eliminated. Publication bias arises when trials with negative outcomes have a lower propensity to be published. Third, heterogeneity was present in our meta-analysis; however, the L'Abbé plot shows that the heterogeneity is a result of studies' disagreement in the magnitude, but not the direction, of echinacea's benefit. Furthermore, after doing various subgroup analyses to assess the effect of clinical heterogeneity, echinacea maintained significant effects on the reduction of cold incidence and duration. Finally, this analysis focuses on the efficacy but not the safety of echinacea. Although adverse events with echinacea are not commonly reported, gastrointestinal upset and rash have been reported.6 Much more work needs to be done to elucidate the safety of prolonged therapy since its effect on the rate-corrected QT interval, blood pressure, and other safety parameters is not well known. Of note, echinacea is a human cytochrome P450 3A4 enzyme inhibitor so the potential for drug interactions also needs to be assessed.41
Conclusion
An analysis of the current evidence in the literature suggests that echinacea has a benefit in decreasing the incidence and duration of the common cold; however, large-scale randomised prospective studies controlling for variables such as species, quality of preparation and dose of echinacea, method of cold induction, and objectivity of study endpoints evaluated are needed before echinacea for the prevention or treatment of the common cold can become standard practice.
Search strategy and selection criteria
These are described in detail in the Methods section on page 473.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.National Institute of Allergy and Infectious Diseases The common cold, health matters fact sheets. http://www3.niaid.nih.gov/healthscience/healthtopics/colds/overview.htm (accessed May 30, 2007).
- 2.Kirkpatrick GL. The common cold. Prim Care. 1996;23:657–675. doi: 10.1016/S0095-4543(05)70355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giles JT, Palat CT, 3rd, Chien SH, Chang ZG, Kennedy DT. Evaluation of echinacea for treatment of the common cold. Pharmacotherapy. 2000;20:690–697. doi: 10.1592/phco.20.7.690.35173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; number 343. National Center for Health Statistics; Hyattsville, Maryland: 2004. [PubMed] [Google Scholar]
- 5.Caruso TJ, Gwaltney JM., Jr. Treatment of the common cold with echinacea: a structured review. Clin Infect Dis. 2005;40:807–810. doi: 10.1086/428061. [DOI] [PubMed] [Google Scholar]
- 6.Huntley AL, Coon JT, Ernst E. The safety of herbal medicinal products derived from Echinacea species. Drug Saf. 2005;28:387–400. doi: 10.2165/00002018-200528050-00003. [DOI] [PubMed] [Google Scholar]
- 7.Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Med. 2005;71:701–705. doi: 10.1055/s-2005-871290. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal M, Busse WR, Goldberg A, Hall T, Riggins CW, Rister RS, editors. The complete German Commission E monographs: therapeutic guide to herbal medicines. American Botanical Council; Austin, TX: 1998. [Google Scholar]
- 9.WHO . WHO monographs on selected medicinal plants. World Health Organization; Geneva: 1999. Echinacea; pp. 125–144. [Google Scholar]
- 10.Health Canada Echinacea angustifolia: natural health products directorate. Draft January, 2004. http://hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/monograph/mono_echinacea_e.html (accessed May 30, 2007).
- 11.Health Canada Echinacea purpurea: natural health products directorate. Draft January 19, 2004. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodnatur/mono_echinacea_purpurea_e.pdf (accessed May 18, 2007).
- 12.Health Canada Echinacea pallida: natural health products directorate. Draft January 19, 2004. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodnatur/mono_echinacea_pallida_e.pdf (accessed May 18, 2007).
- 13.Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2000;2 doi: 10.1002/14651858.CD000530. CD000530. [DOI] [PubMed] [Google Scholar]
- 14.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 15.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extract for the prevention of upper respiratory tract infections: A double-blind, placebo-controlled randomized trial. Arch Fam Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 16.Braunig B, Knick E. Therapeutic experiences with Echinacea pallida in influenzal infections. Naturheilpraxis mit Naturmedizin. 1993;1:72–75. (in German). [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 4.2.6 [updated September 2006] John Wiley & Sons Ltd; Chichester, UK: 2006. [Google Scholar]
- 20.Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D'Alessio D. Treatment of the common cold with unrefined echinacea. A randomized, double-blind, placebo controlled trial. Ann Intern Med. 2002;137:939–946. doi: 10.7326/0003-4819-137-12-200212170-00006. [DOI] [PubMed] [Google Scholar]
- 21.Gilroy CM, Steiner JF, Byers T, Shapiro H, Georgian W. Echinacea and truth in labeling. Arch Intern Med. 2003;163:699–704. doi: 10.1001/archinte.163.6.699. [DOI] [PubMed] [Google Scholar]
- 22.Sperber SJ, Shah LP, Gilbert RD, Ritchey TW, Monto A. Echinacea purpurea for prevention of experimental rhinovirus colds. Clin Infect Dis. 2004;38:1367–1371. doi: 10.1086/386324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulten B, Bulitta M, Brigitta B, Koster U, Schafer M. Efficacy of Echinacea purpurea in patients with a common cold: a placebo-controlled, randomised, double-blind clinical trial. Arzneimittelforschung. 2001;51:563–568. doi: 10.1055/s-0031-1300080. [DOI] [PubMed] [Google Scholar]
- 24.Grimm W, Muller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106:138–143. doi: 10.1016/s0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 25.Berg A, Northoff H, Konig D. Influence of echinacin (EC31) treatment on the exercise-induced immune response in athletes. J Clin Res. 1998;1:367–380. [Google Scholar]
- 26.Blumenthal M, Hall T, Goldberg A, editors. The ABC clinical guide to herbs. American Botanical Council; Austin, TX: 2003. [Google Scholar]
- 27.Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schafer M. Echinaguard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res. 1997;9:261–269. [Google Scholar]
- 28.Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med. 2004;158:217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 29.Lindenmuth GF, Lindenmuth EB. The efficacy of echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double-blind placebo-controlled study. J Altern Complement Med. 2000;6:327–334. doi: 10.1089/10755530050120691. [DOI] [PubMed] [Google Scholar]
- 30.Scaglione F, Lund B. Efficacy in the treatment of the common cold of a preparation containing an echinacea extract. Int J Immunother. 1995;11:163–166. [Google Scholar]
- 31.Turner RB, Riker DK, Gangemi JD. Ineffectiveness of echinacea for prevention of experimental rhinovirus colds. Antimicrob Agents Chemother. 2000;44:1708–1709. doi: 10.1128/aac.44.6.1708-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor JA, Weber W, Standish L, Quinn H, Goesling J, McGann M, Calabrese C. Efficacy and safety of echinacea in treating upper respiratory tract infections in children: a randomized controlled trial. JAMA. 2003;290:2824–2830. doi: 10.1001/jama.290.21.2824. [DOI] [PubMed] [Google Scholar]
- 33.Islam J, Carter R. Use of echinacea in upper respiratory tract infection. South Med J. 2005;98:311–318. doi: 10.1097/01.SMJ.0000154783.93532.78. [DOI] [PubMed] [Google Scholar]
- 34.Gruenwald J, editor. Physicians' desk reference for herbal medicines. 3rd edn. Thomson PDR; Montvale, NJ: 2004. p. 267. [Google Scholar]
- 35.Dennehy C, Tsourounis C. Herbs and nutritional supplements. In: Koda-Kimble MA, Young LY, Kradjan WA, Guglielmo BJ, Alldredge BK, Corelli RL, editors. Applied therapeutics: the clinical use of drugs. 8th edn. Lippincott Williams and Wilkins; Baltimore, MD: 2005. pp. 3–15. [Google Scholar]
- 36.Hemila H. Vitamin C supplementation and respiratory infections: a systematic review. Mil Med. 2004;169:920–925. doi: 10.7205/milmed.169.11.920. [DOI] [PubMed] [Google Scholar]
- 37.Burdock GA. Review of the biological properties and toxicity of bee propolis. Food Chem Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 38.Blumenthal M, Farnsworth NR. Echinacea angustifolia in rhinovirus infections. N Engl J Med. 2005;353:1971–1972. doi: 10.1056/NEJM200511033531818. [DOI] [PubMed] [Google Scholar]
- 39.Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2006;1 doi: 10.1002/14651858.CD000530.pub2. CD000530. [DOI] [PubMed] [Google Scholar]
- 40.Millea PJ. Echinacea for the common cold. Ann Intern Med. 2003;139:7. doi: 10.7326/0003-4819-139-7-200310070-00020. [DOI] [PubMed] [Google Scholar]
- 41.Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]