Highlights
-
•
Systematic antimicrobials are recommended in community-acquired pneumonia of child.
-
•
A large panel of bacteria and viruses was detected in 85 children exhibiting CAP.
-
•
More than 60% of children with CAP exhibited an exclusive viral infection.
-
•
A co-infection with at least 2 viruses was observed in >40% of the children.
-
•
Data suggest that the use of antimicrobials in child's CAP should be revisited.
Keywords: Children, Community-acquired-pneumonia, PCR, Viral pneumonia, Epidemiology
Abstract
Background
The management of children with community-acquired pneumonia (CAP) is largely influenced by the development of new molecular diagnostic tests that allow the simultaneous detection of a wide range of pathogens.
Objectives
Evaluation of a diagnostic approach including multiplex PCR assays for revisiting the epidemiology and etiology of CAP in children at hospital.
Study design
Children of all ages consulting at the Emergency Department of the University hospital of Saint-Etienne, France, during the 2012–2013 winter period were included. In addition to bacterial cultures, the following pathogens were detected using biplex commercially-available rt-PCR tests: adenovirus, respiratory syncytial virus, human metapneumovirus, bocavirus, rhinovirus/enterovirus, coronavirus, influenza viruses A and B, parainfluenza viruses, Mycoplasma pneumoniae and Chlamydophila pneumonia.
Results
From 85 patients with CAP, at least one pathogen was identified in 81 cases (95.3%), including 4 bacterial exclusive infections (4.7%), 53 viral exclusive infections (62.4%) and 24 mixed infections (28.2%). Coinfection by at least two viruses was observed in 37 cases (43.5%). Mean age was higher in the case of documented bacterial infection (P < 0.05). In the subgroup of viral exclusive infection, the mean age of severe cases was 2.0 years vs 3.8 years in mild and moderate cases (P < 0.05).
Conclusions
These findings highlight the huge proportion of CAP of viral origin, the high number of co-infection by multiple viruses and the low number of bacterial CAP, notably in children under 5 years, and address the need to re-evaluate the indications of empiric antimicrobial treatment in this age group.
1. Background
Community-acquired pneumonia (CAP) is the leading cause of death in children under five years of age in the world [1]. In developed countries, the systematic prescription of antimicrobial drugs to patients with CAP has led to a dramatic reduction in mortality linked to this pathology [2], [3]. However, a bacterial origin of CAP has not been documented in a large proportion of cases despite extensive aetiological investigations. The current recommendations [4], [5], [6] encourage pediatricians to prescribe a probabilistic antimicrobial treatment, even when no bacterial infection is documented, which results in prolonged use of antibiotics and in the possible selection of resistant strains within the endogenous flora [7].
Until the beginning of the current century, the absence of documented bacterial infection was attributed to the difficulty in obtaining deep respiratory specimens that are not contaminated by bacteria from the local flora [8] together with the lower sensitivity of blood cultures in proving bacterial sepsis [9]. At this time, most of the epidemiological data from pediatric CAP relied on traditional bacteriological cultures. With the occurrence of new diagnostic tools and notably of multiplex PCR assays able to simultaneously detect a large panel of viruses and atypical bacteria, it now appears that a large proportion of CAP could be related to viral infection [10], [11], [12]. Many studies have evaluated these new tools but most of them were limited to subgroups of children notably to the young [13], [14], to hospitalized children [11], [13], [14], [15], [16], [17], or for selected pathogens [10], [12], [18], [19].
2. Objective
The aim of the present study was to document the presence of a large variety of pathogens in respiratory specimens from children attending the Pediatric Emergency Department of the University hospital of Saint-Etienne, France, during a six-month period and presenting a CAP based on clinical and radiological evidence. The microbiological diagnostic approach combined bacterial cultures and biplex commercially available rt-PCR tests detecting a wide range of respiratory pathogens.
3. Study design
3.1. Clinical data
A single center epidemiological observational study was conducted over a six-month period (November 2012 to April 2013) on children aging from one month to 16.5 years and presenting with CAP at the Pediatric Emergency Department of the University hospital of Saint-Etienne, France. The study was submitted for approval to the local Ethics Committee. After oral information was given together with a form explaining the content of the research, a consent form was signed by a parent or legal tutor before inclusion of each patient.
A CAP case was defined [6] as a subject with fever of at least 38.5 °C, an age-corrected polypnea [20] and a chest radiograph showing images of acute pneumonia confirmed by a second examiner (a pediatric radiologist for ambulatory children or a pediatrician for hospitalized patients). A few subjects were excluded after this second reading, notably in the case of associated bronchiolitis.
The data collected at inclusion comprised the demographic characteristics of the child, their vaccine status, the smoking habits of parents, the date of the beginning of the current respiratory episode and the drugs, including antimicrobials, that they received during this period. According to the current guidelines [4], [5], [6], a pneumonia was defined as severe for this study if the patient presented at least one of the following criteria: respiratory rate above 70 per minute in infants less than 1 year of age and above 50 per minute for older children, a tachycardia adjusted to age, a capillary refill time >3 s and an oxygen saturation <92%.
A control visit was systematically carried out at days 2 and 5 either by phone call for ambulatory-treated children or after a physical examination in the service of hospitalization for children admitted to hospital.
3.2. Biological investigations
A number of biological parameters were recorded systematically, including C reactive protein (CRP), procalcitonin (PCT), white blood cell count and natremia.
Nasopharyngeal secretions obtained by sputum induction [21], [22] were sampled at entry for all the participants. The following tests were performed at inclusion: standard detection of conventional bacteria by culture, detection of five different viruses (respiratory syncytial virus (RSV), influenza viruses A and B, parainfluenza viruses, metapneumovirus and adenovirus) by indirect immunofluorescence (IFI) assay and detection of atypical bacteria by PCR as previously described [23].
In parallel, blood cultures and pneumococcal antigenuria were tested if prescribed by the clinician, notably in the case of hospitalization.
In addition to the test listed above that were performed at the time of hospital attendance, a rtPCR assay was performed at the end of the study on an aliquot fraction kept frozen at −80 °C for the whole panel of nasopharyngeal aspirates, as previously described [24]. Briefly, 200 μl of aspirate was extracted on NUCLISENS® easyMAG™ (bioMérieux, Marcy l’Etoile, France). The Respiratory Multi Well System (MWS) r-gene™ (Chla/Myco pneumo, Influenza A/B, RSV/hMPV, AD/hBoV, HCoV/HPIV, and Rhino&EV/CC) from bioMérieux was used for molecular testing. It consists of a series of biplex assays detecting either a couple of pathogens or a single pathogen and a cell control (CC) attesting for the presence of cellular nucleic acids within the specimen. The following pathogens were tested: Mycoplasma pneumoniae and Chlamydophila pneumoniae, influenza viruses A and B, RSV and human metapneumovirus (hMPV), adenovirus (ADV) and bocavirus (BoV), parainfluenza viruses and coronaviruses, rhinovirus/enterovirus (hREV) and a cell control.
3.3. Statistical comparison of bacterial and non-bacterial cases
An univaried analysis was performed to compare the cases documented as probably related to a bacterial infection (threshold of 107 CFU/ml for conventional cultures [25], [26] or the presence of atypical bacterium by PCR in nasopharyngeal aspirates), and the others. Comparisons adjusted for age, severity of pneumonia and mono/multiple infection were also performed. The chi-square test or the Fisher exact test was used to compare qualitative variables whereas the Student t test was used for quantitative variables. A P value of 0.05 was considered as statistically significant.
A multivariate analysis of factors independently associated with detection of bacterial was secondarily performed; the parameters included in the logistic regression model were those with P < 0.10 by univaried analysis.
4. Results
4.1. Clinical characteristics of included cases
Over the six-month period of the study, 95 children thought to have CAP were included; 10 of them were excluded secondarily, comprising 7 cases with non-CAP infection, 2 cases without nasopharyngeal aspirate and one case of CAP whose inclusion was not consented by the child's family. With reference to the total number of cases of CAP recorded over the same period in the Pediatric Emergency Department (n = 97), the representation rate was of 87.6% (85/97).
The demographic and clinical characteristics of the 85 included cases together with the mode of management (ambulatory or hospital) and the probabilistic antimicrobial treatment are listed in Table 1 .
Table 1.
Demographic and clinical characteristics of the 85 patients included in the study.
| Characteristics (unit) | Numerical data |
|---|---|
| Median age (years) | 2.8 |
| Interquartile range (years) | 1.5–5.7 |
| Categories by age | |
| Infants (<2 years old) – No. (%) | 30 (35.3) |
| Preschool children (2 – 5 years old) – No. (%) | 32 (37.6) |
| Children (5 – 12 years old) – No. (%) | 19 (22.3) |
| Teenagers (12 – 18 years old) – No. (%) | 4 (4.7) |
| Gender – No. females (%) | 39 (46) |
| Vaccinal status | |
| Up-to-date pneumococcal vaccine – No. (%) | 61 (71.7) |
| Up-to-date Haemophilus influenzae B vaccine – No. (%) | 68 (80) |
| Medical history | |
| Previous hospitalization – No. (%) | 17 (20) |
| Pulmonary disease – No. (%) | 26 (30.5) |
| - Bronchiolitis – No. (%) | 9 (10.5) |
| - Asthma – No. (%) | 9 (10.5) |
| - Pneumonia – No. (%) | 4 (4.7) |
| Parental smoking habits | |
| No smoking parent – No. (%) | 44 (51.7) |
| At least one smoking parent – No. (%) | 28(32.8) |
| Medication before emergency's consultation | |
| None – No. (%) | 16 (18.8) |
| Acetaminophen – No. (%) | 58 (68.2) |
| Non-steroidal anti-inflammatory drug – No. (%) | 15 (17.6) |
| Antimicrobial treatment– No. (%) | 18 (21.2) |
| Average duration of symptoms before emergency consultation (days) | 4 |
| Radiological localization of lesions | |
| Right upper lobe – No. (%) | 12 (14.1) |
| Right lower lobe – No. (%) | 21 (24.7) |
| Right middle lobe – No. (%) | 13 (15.3) |
| Left upper lobe – No. (%) | 8 (9.4) |
| Left lower lobe – No. (%) | 24 (28.2) |
| Interstitial syndromea – No. (%) | 13 (15.3) |
| At least one criterion of severe pneumonia at entry | 26 (30.6) |
| Hospitalization at entry – No. (%) | 36 (42.4) |
| Antimicrobial treatment | |
| Amoxicillin – No. (%) | 50 (58.8) |
| Co-amoxiclav – No. (%) | 18 (21.2) |
| Erythromycin – No. (%) | 8 (9.4) |
| Amoxicillin + erythromycin – No. (%) | 4 (4.7) |
| None – No. (%) | 4 (4.7) |
| Others – No. (%) | 1 (1.2) |
Interstitial syndrome was associated with lobar lesions in 6 cases and was the only radiological sign in 7 cases.
Apyrexia was observed in 85.9 and 98.8% of cases at day 2 and 5, respectively. From the 35 children hospitalized at entry, 33 (94.3%) and 5 (14.3%) were still hospitalized at day 2 and 5, respectively. Only one child needed intensive care within the Pediatric Intensive Care Unit. The antimicrobial treatment was modified in only three cases. Neither fatal cases nor immediate sequelae were observed during the study. Twenty-six cases (30.6%) were classified as severe CAP.
The general biological characteristics of the included cases were as follows: the median count of leukocytes was 14.7 109 per liter (interquartile range 10.84–20.08), with 66.5% of neutrophils; the CRP median rate was of 64.5 mg/L (interquartile range: 19–163), the PCT median rate was of 0.87 μg/L (interquartile range: 0.17–5.14); an hyponatremia (<135 mEq/L) was observed in 23 cases (27%).
4.2. Microbiological results
By using IFI prospectively, only 26 of the 85 nasopharyngeal aspirates (30.6%) were found positive, including 17 RSV, 9 ADV and 4 influenzavirus B; all of these infections were confirmed by the retrospective rt-PCR assay. The pneumococcal antigenuria, available in 60 cases, was positive in 13 patients, 3 of them exhibiting a threshold of 107 CFU/ml by conventional culture. In the three other pneumococcal infections, no antigenuria was available. Blood culture, performed in 38 cases (44%), was found positive for Streptococcus pneumoniae in only one case.
By combining bacterial culture and retrospective rt-PCR assays, at least one pathogen was identified in 81 cases (95.3%). The number of detected pathogens was of 0, 1, 2, 3, 4 and 5 in 4 (4.7%), 30 (35.3%), 34 (40.0%), 13 (15.3%), 3 (3.5%) and 1 (1.2%) specimens, respectively (Table 2 ). From the 85 cases of CAP, 4 bacterial exclusive infections were observed (4.7%), 24 infections with at least one bacterium and one virus (28.2%) and 53 viral exclusive infections (62.4%). Coinfection by at least two viruses was observed in 37 cases (43.5%). Table 3 displays the distribution of the identified pathogens and specifies the various associations, the most common being ADV/RSV (10 cases), Haemophilus influenzae/ADV(8 cases) and hREV/BoV (8 cases).
Table 2.
Distribution of the 85 cases of CAP according to the number of detected bacteria and viruses.
| Number of bacteria | Number of viruses |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | |
| 0 | 4 | 28 | 22 | 2 | 1 |
| 1 | 2 | 10 | 10 | 2 | – |
| 2 | 2 | 1 | 1 | – | – |
Table 3.
Detailed presentation of cases of community-acquired pneumonia exhibiting an infection with at least 2 pathogens. Gray boxes indicate the total number of positive cases for each pathogen. White boxes corresponds to the cases with co-infection.
| Infectious agents | ADV | ||||||||||||||
| Adenovirus (ADV) | 32 | RSV | |||||||||||||
| Respiratory syncytial virus (RSV) | 10 | 28 | BoV | ||||||||||||
| Bocavirus (BoV) | 4 | 5 | 18 | Hi | |||||||||||
| Haemophilus influenza (Hi) | 8 | 6 | 0 | 11 | IVB | ||||||||||
| Influenzavirus (IVB) | 3 | 1 | 1 | 1 | 11 | hMPV | |||||||||
| Human metapneumovirus (hMPV) | 5 | 1 | 1 | 2 | 0 | 11 | HREV | ||||||||
| Human rhinovirus or enterovirus (HREV) | 2 | 3 | 8 | 1 | 0 | 1 | 11 | Mc | |||||||
| Moraxella catarrhalis (Mc) | 2 | 1 | 4 | 0 | 2 | 0 | 2 | 9 | Spn | ||||||
| Streptococcus pneumonia (Spn) | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | PIV | |||||
| Parainfluenza virus (PIV) | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | IVA | ||||
| Influenzavirus A (IVA) | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 | Mp | |||
| Mycoplasma pneumonia (Mp) | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | Spy | ||
| Streptococcus pyogenes (Spy) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | Mn | |
| Moraxella nonliquefaciens (Mn) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | hCoV |
| Coronavirus (CoV) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total of coinfections (%) | 26 (81.2) | 19 (67.9) | 18 (100) | 11 (100) | 6 (54.5) | 10 (90.9) | 6 (54.5) | 9 (100) | 5 (83.3) | 4 (80) | 4 (80) | 3 (75) | 1 (100) | 1 (100) | 1 (100) |
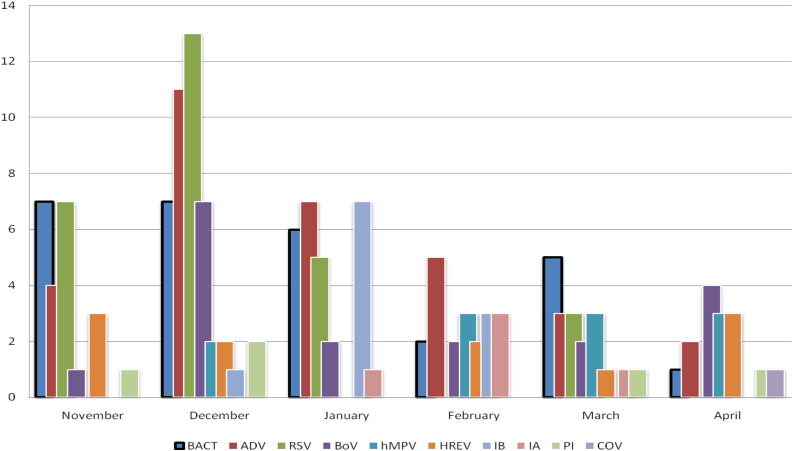
The monthly distribution of microbiological infections is depicted in Fig. 1 .
Fig. 1.
Monthly distribution of microbiological infection. BACT: bacteria. ADV: adenovirus; RSV: respiratory syncytial virus; BoV: bocavirus; hMPV: human metapneumovirus; HREV: human rhinovirus and enterovirus; IB: influenzavirus B; IA: influenzavirus A; PI: parainfluenza virus; COV: coronavirus.
4.3. Statistical analysis
As shown in Table 4 , none of the variables tested was statistically correlated to the presence of a bacterial pathogen by univaried analysis, with the exception of age that was higher in the case of documented bacterial infection (mean age of 5.45 vs 3.49 years; P < 0.05 by Student t test) and the presence of abdominal pain at clinical examination at entry (P = 0.02). Concerning biological parameters, no correlation was observed between bacterial and non-bacterial cases for the most of them, notably for CRP and PCT, with the exception of the white blood cell count that was higher in case of viral infection. Severity was statistically associated neither to the bacterial or non bacterial etiology of CAP, nor to a younger age, except in the subgroup of viral exclusive infection (n = 53), in which the mean age of severe cases was 2.0 years vs 3.8 years in mild and moderate cases (P < 0.05 by Student t test). Coinfection was not associated to a younger age or a more severe disease, even if the number of detected pathogens tended to be related to the severity of CAP (2.1 infected agents in severe cases vs 1.7 in non-severe cases, P = 0.09 by Student t test).
Table 4.
Data from individuals with versus without bacterial infection.
| Characteristics (unit) | Bacterial infection (n = 28) | Non bacterial infection (n = 57) | pa |
|---|---|---|---|
| Mean age (years) | 5.45 | 3.49 | 0.0210 |
| Gender (No. females) | 13 | 26 | 1 |
| Vaccinal status (No.) | |||
| Up-to-date pneumococcal vaccine | 17 | 44 | 0.3744 |
| Up-to-date Haemophilus influenzae B vaccine | 24 | 44 | 1 |
| Medical history (No.) | |||
| Previous hospitalization | 7 | 10 | 0.5647 |
| Pulmonary disease | 7 | 19 | 0.4662 |
| Parental smoking habits (No.) | |||
| At least one smoking parent | 8 | 20 | 0.6105 |
| Medication before emergency's consultation (No.) | |||
| None | 6 | 11 | 1 |
| Acetaminophen | 19 | 41 | 0.6176 |
| Non-steroidal anti-inflammatory drug | 5 | 10 | 1 |
| Corticosteroids | 3 | 2 | 0.3526 |
| Average duration of symptoms before emergency consultation (days) | 4.3 | 3.9 | 0.4829 |
| At least one criterion of severe pneumonia at entry (No.) | 10 | 16 | 0.6170 |
| Hospitalization at entry | 9 | 26 | 0.2466 |
| Still hospitalized at day 2 | 10b | 23 | 0.8137 |
| Clinical examination at entry (No.) | |||
| Asthenia | 21 | 38 | 0.4662 |
| Chest pain | 4 | 7 | 1 |
| Abdominal pain | 11 | 9 | 0.0279 |
| Cough | 22 | 45 | 1 |
| Wheezing | 5 | 9 | 1 |
| Crackles | 11 | 11 | 0.0656 |
| Biology at entry | |||
| White blood cells (G/L) | 13.45 | 17.67 | 0.0181 |
| Polynuclears neutrophils (%) | 66.7 | 62.3 | 0.3607 |
| Platelets (G/L) | 293 | 350 | 0.0513 |
| CRP (mg/L) | 105 | 111 | 0.8366 |
| Procalcitonin (μg/L) | 6.3 | 4.9 | 0.5783 |
| Natremia (mEq/L) | 135.2 | 136.3 | 0.0809 |
Fisher's exact test for categorical data and Student t-test for continuous values.
By comparaison to the precedent line, a patient was hospitalized after coming back home.
By multivariate analysis, none of the tested variables was independently associated with bacterial infection.
5. Discussion
The demographic characteristics of the included patients were coherent with those of the literature [6] in terms of age (median age of 2.8 years; interquartile range: 1.5–5.7 years) and sex ratio (1.18 to the benefit of males). Those patients with CAP were shown to exhibit different risk factors as illustrated by a lower vaccination coverage as compared to that of the French population, an elevated rate of previous hospitalization (17/85, 20.0%), a history of frequent respiratory disease (26/85; 30.6%) and a high level of smoking habits in parents (Table 1).
Approximately 4 children out of 5 reached hospital without having consulted another physician; most of them had already received an antipyretic treatment, mainly acetaminophen but also non-steroidal anti-inflammatory drugs (NSAID), despite the fact that the use of NSAID may be harmful [27].
At entry, 95.3% (81/85) of children had received an antimicrobial treatment. In most cases, the first choice for antimicrobial drug was amoxicillin, as currently recommended [4], [5], [6].
Despite the limited size of the present study and its restriction to a single center, its originality lies in the diversity of the included population in terms of age and mode of management (ambulatory and hospitalization), together with the detection of a large range of pathogens including viruses and bacteria.
From a microbiological point of view, it is first useful to justify the definition of what level of detection constitutes a causative agent in children with CAP included in this study. Concerning bacterial loads, the threshold of 107 CFU/ml was retained as recommended by European experts when induced sputum specimen are used [25]. It helps the discarding of pneumococcal colonization with prevalence of up to 57–65% in children of less than 5 years old [28], [29] from true infection. By contrast, “the asymptomatic carrier state of viruses is rather uncommon for most respiratory viruses [12], [30], apart from the post active phase of respiratory virosis” [25], which justifies to consider the detection of viral genome in respiratory specimens as a marker of probable viral infection.
In the more exhaustive studies performed earlier, the percentage of elucidated cases from a microbiological point of view ranged from 72% to 86% [6], [11], [30] whereas it was of 95.3% in the present study detecting a wide spectrum of infectious agents with respiratory tropism. As reported earlier [30], viral infections were shown to be predominant in children under 5 years of age and only 32% of all CAP were found to be of bacterial origin.
Eighteen children received antimicrobial therapy before emergency consultation, which could be considered as a source of false-negative culture. However, all of them were always symptomatic at entry, which implies that a bacterium, if present, had a significant opportunity to be recovered by culture.
An interesting finding of this study is the large proportion of viral coinfection (43.5%), much higher than that previously reported [10], [12], [30], notably for bocavirus, metapneumovirus and adenovirus that were detected in association with at least one other virus in more than 80% of the CAP cases involving these agents (Table 2). It has been suggested that infection by several viruses could enhance the severity of CAP [12], [30], [31], [32]. In the present study, a trend was observed in the association between the mean number of infectious agents and the severity of CAP as defined above (P = 0.09); a larger effective size would have been needed to determine a statistically significant difference.
In terms of clinical evolution, neither death nor major complications were reported in this study, despite rates of 30.6% for severe pneumonia and 42.4% for hospitalization. There was no statistical difference in the severity of CAP between viral and bacterial infections. Almost all of the children received antibiotics, which was unnecessary in a large proportion of cases (62.4%) for which only viruses were detected. This finding raises the question of the systematic use of antimicrobials to treat childhood CAP, which is still recommended in different guidelines [4], [5], [6].
The present findings, together with those of others, allowed the identification of a subpopulation of children less than 5 years of age with mild or moderate symptoms for which a viral etiology of CAP is highly probable. This situation represents half of the cases recorded in this study: 62 children (72.9%) were less than five-years old, and 40 of them presented a mild or moderate CAP, which corresponds to 47.1% of all cases. The use of a rapid molecular test detecting a large set of viral and bacterial pathogens within 2 or 3 hours, such as that described in [33], would allow an improvement in the management of the antimicrobial treatment. In the case of positive result, it would be recommended to avoid the use of amoxicillin as a first-intent therapy (or to prescribe erythromycin in the case of detection of an agent of atypical pneumonia) and to reconsider the use of antimicrobial treatment 24–48 h later according to the clinical evolution and to the results of bacterial cultures. Conversely, the negativity of the rapid test would lead to the empiric prescription of amoxicillin, as currently recommended [4], [5], [6]. It is obvious that this attitude would be dedicated to CAP with mild or moderate symptoms and that CAP with severe presentation at entry should include a systematic probabilistic antimicrobial therapy, whatever the results of PCR assay. The present results are indicative that this strategy could dramatically reduce the proportion of unnecessary antimicrobial treatments in mild or moderate child CAP. Wider studies are needed to prospectively evaluate the benefits of this approach in terms of patient recovery, prevention of antibiotic resistance and medical economy.
Funding
The reagents used for the retrospective analysis were funded by a grant from the Medical Association of Pediatricians of the University Hospital of Saint-Etienne for research and studies (ADERPS), France.
Competing interests
The authors declare that they have no conflict of interest regarding the object of this study.
Ethical approval
The study was submitted for approval to the local Ethics Committee.
Acknowledgements
The authors are indebted to the patients’ families who gave their consent for participating to this study. Philip Lawrence is warmly acknowledged for his careful checking of English language. The reagents used for the retrospective analysis were funded by a grant from the Medical Association of Paediatrician of the University-Hospital of Saint-Etienne for research and studies (ADERPS).
References
- 1.Bryce J., Boschi-Pinto C., Shibuya K., Black R.E. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Black R.E., Cousens S., Johnson H.L., Lawn J.E., Rudan I., Bassani D.G. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Rudan I., Boschi-Pinto C., Biloglav Z., Mulholland K., Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley J.S., Byington C.L., Shah S.S., Alverson B., Carter E.R., Harrison C. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 doi: 10.1093/cid/cir531. cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uehara S., Sunakawa K., Eguchi H., Ouchi K., Okada K., Kurosaki T. Japanese guidelines for the management of respiratory infectious diseases in children 2007 with focus on pneumonia. Pediatr Int. 2011;53:264–276. doi: 10.1111/j.1442-200x.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- 6.Harris M., Clark J., Coote N., Fletcher P., Harnden A., McKean M. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl. 2):1–23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 7.Esposito S., Cohen R., Domingo J.D., Pecurariu O.F., Greenberg D., Heininger U. Antibiotic therapy for pediatric community-acquired pneumonia: do we know when, what and for how long to treat? Pediatr Infect Dis J. 2012;31:e78–e85. doi: 10.1097/INF.0b013e318255dc5b. [DOI] [PubMed] [Google Scholar]
- 8.Murdoch D.R., O’Brien K.L., Scott J.A.G., Karron R.A., Bhat N., Driscoll A.J. Breathing new life into pneumonia diagnostics. J Clin Microbiol. 2009;47:3405–3408. doi: 10.1128/JCM.01685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements H., Stephenson T.J. Blood culture is poor method of confirming pneumococcus as cause of childhood pneumonia. Br Med J. 1996;313:757. doi: 10.1136/bmj.313.7059.757b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf D.G., Greenberg D., Shemer-Avni Y., Givon-Lavi N., Bar-Ziv J., Dagan R. Association of human metapneumovirus with radiologically diagnosed community-acquired alveolar pneumonia in young children. J Pediatr. 2010;156:115–120. doi: 10.1016/j.jpeds.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamano-Hasegawa K., Morozumi M., Nakayama E., Chiba N., Murayama S.Y., Takayanagi R. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14:424–432. doi: 10.1007/s10156-008-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cilla G., Oñate E., Perez-Yarza E.G., Montes M., Vicente D., Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol. 2008;80:1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsolia M.N., Psarras S., Bossios A., Audi H., Paldanius M., Gourgiotis D. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39:681–686. doi: 10.1086/422996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cevey-Macherel M., Galetto-Lacour A., Gervaix A., Siegrist C.-A., Bille J., Bescher-Ninet B. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr. 2009;168:1429–1436. doi: 10.1007/s00431-009-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond P., Clark J., Wheeler J., Galloway A., Freeman R., Cant A. Community acquired pneumonia – a prospective UK study. Arch Dis Child. 2000;83:408–412. doi: 10.1136/adc.83.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michelow I.C., Olsen K., Lozano J., Rollins N.K., Duffy L.B., Ziegler T. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 17.Lin P.-Y., Lin T.-Y., Huang Y.-C., Tsao K.-C., Huang Y.-L. Human metapneumovirus and community-acquired pneumonia in children. Chang Gung Med J. 2005;28:683–688. [PubMed] [Google Scholar]
- 18.Bellau-Pujol S., Vabret A., Legrand L., Dina J., Gouarin S., Petitjean-Lecherbonnier J. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126:53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Principi N., Esposito S., Blasi F., Allegra L., Mowgli study group Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;32:1281–1289. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 20.Palafox M., Guiscafré H., Reyes H., Munoz O., Martínez H. Diagnostic value of tachypnoea in pneumonia defined radiologically. Arch Dis Child. 2000;82:41–45. doi: 10.1136/adc.82.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert S.B., Whiley D.M., O’Neill N.T., Andrews E.C., Canavan F.M., Bletchly C. Comparing nose-throat swabs and nasopharyngeal aspirates collected from children with symptoms for respiratory virus identification using real-time polymerase chain reaction. Pediatrics. 2008;122:e615–e620. doi: 10.1542/peds.2008-0691. [DOI] [PubMed] [Google Scholar]
- 22.Lahti E., Peltola V., Waris M., Virkki R., Rantakokko-Jalava K., Jalava J. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64:252–257. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 23.Ginevra C., Barranger C., Ros A., Mory O., Stephan J.-L., Freymuth F. Development and evaluation of Chlamylege, a new commercial test allowing simultaneous detection and identification of Legionella, Chlamydophila pneumoniae, and Mycoplasma pneumoniae in clinical respiratory specimens by multiplex PCR. J Clin Microbiol. 2005;43:3247–3254. doi: 10.1128/JCM.43.7.3247-3254.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillet S., Lardeux M., Dina J., Grattard F., Verhoeven P., Le Goff J. Comparative evaluation of six commercialized multiplex PCR kits for the diagnosis of respiratory infections. PLoS ONE. 2013;8:e72174. doi: 10.1371/journal.pone.0072174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freymuth F., Ieven M., Wallet F. Lower respiratory tract infections. In: Cornaglia G, Courcol R, Herrmann J.L., Kahlmeter G, Peigue Lafeuille H, Vila J, SFM, ESCMID, editors. European manual of clinical microbiology. 1st ed. Epernay; France: 2012. pp. 153–161. [Google Scholar]
- 26.Murdoch D.R., O’Brien K.L., Driscoll A.J., Karron R.A., Bhat N., Pneumonia Methods Working Group Laboratory methods for determining pneumonia etiology in children. Clin Infect Dis. 2012;54:S146–S152. doi: 10.1093/cid/cir1073. [DOI] [PubMed] [Google Scholar]
- 27.François P., Desrumaux A., Cans C., Pin I., Pavese P., Labarère J. Prevalence and risk factors of suppurative complications in children with pneumonia. Acta Pædiatr. 2010;99:861–866. doi: 10.1111/j.1651-2227.2010.01734.x. [DOI] [PubMed] [Google Scholar]
- 28.Millar E.V., Watt J.P., Bronsdon M.A., Dallas J., Reid R., Santosham M. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47:989–996. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 29.Abdullahi O., Nyiro J., Lewa P., Slack M., Scott J.A.G. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi District, Kenya. Pediatr Infect Dis J. 2008;27:59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esposito S., Daleno C., Prunotto G., Scala A., Tagliabue C., Borzani I. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses. 2013;7:18–26. doi: 10.1111/j.1750-2659.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fry A.M., Lu X., Chittaganpitch M., Peret T., Fischer J., Dowell S.F. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito S., Principi N. Unsolved problems in the approach to pediatric community-acquired pneumonia. Curr Opin Infect Dis. 2012;25:286–291. doi: 10.1097/QCO.0b013e328352b60c. [DOI] [PubMed] [Google Scholar]
- 33.Pierce V.M., Elkan M., Leet M., McGowan K.L., Hodinka R.L. Comparison of the Idaho Technology FilmArray system to real-time PCR for detection of respiratory pathogens in children. J Clin Microbiol. 2012;50:364–371. doi: 10.1128/JCM.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]