Summary
Globalisation and its effect on human development has rendered an environment that is conducive for the rapid international spread of severe acute respiratory syndrome (SARS), and other new infectious diseases yet to emerge. After the unprecedented multi-country outbreak of avian influenza with human cases in the winter of 2003–2004, an influenza pandemic is a current threat. A critical review of problems and solutions encountered during the 2003–2004 SARS epidemics will serve as the basis for considering national preparedness steps that can be taken to facilitate the early detection of avian influenza, and a rapid response to an influenza pandemic should it occur.
A severe form of acute respiratory syndrome (SARS) associated with a novel coronavirus infection, is a bona fide newly emerged infectious disease that first surfaced in November 2002 in southern China.1, 2, 3, 4, 5, 6, 7 The virus is transmitted by respiratory droplets, close contact, or through contaminated environmental surfaces and fomites;8, 9, 10 on rare and special circumstances, aerosolised human excreta may also contribute to the transmission.11 The disease that spread to 29 countries in five continents over a few weeks has shown its potential to have a pandemic health impact in the absence of precautionary control measures.12 However, after the explosive outbreaks in several countries, transmission was shown to be containable using a variety of public-health measures, including vigorous isolation of all infected patients and quarantine of all close contacts.
The outcome of this multi-country outbreak clearly showed that SARS transmission can be effectively controlled, but only after all the affected countries simultaneously implemented multiple control measures, some of which were probably more cost-effective than others. Although it is not possible to compare these public-health measures through stringent control led trials, review of the temporal events and epidemic characteristics, in conjunction with the vast amount of information collected about the SARS coronavirus and its infection, has allowed the development of a plan of action in the event of a SARS recurrence.
The potential sources of SARS resurgence include: the natural animal reservoir, laboratory spillage, and an undetected low level of human infection that might be perpetuated under suitable circumstances related to seasonal variation or human behaviours. In the winter of 2003–2004, the four SARS cases reported in Guangdong could not all be traced to a clear source, which shows that the SARS coronavirus has not disappeared from nature altogether. In addition, laboratory-acquired SARS is a constant threat. Therefore, continued vigilance is necessary to minimise the potentially adverse public-health impact of any new SARS cases. The preparedness plans formulated in Taiwan, a SARS-affected country, is reviewed in this article, and should be of general interest to the international community.
Brief history of the SARS epidemic in Taiwan
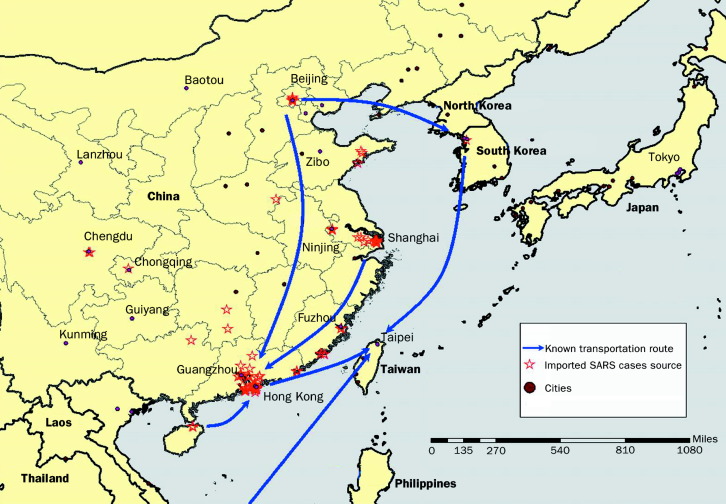
The SARS epidemic in Taiwan was marked by three distinct phases: the initial importation, a series of explosive nosocomial outbreaks, and the final containment phase. Between February 25, 2003, when the first SARS patient returned to Taiwan from China, and April 21, 2003, when a large-scale nosocomial infection was recognised, 30 probable and 50 suspected SARS cases were reported. All were visitors who had returned from various parts of China via Hong Kong (figure ). During this phase, all reported SARS patients regardless of whether they were considered to be suspected or probable (according to the criteria recommended by WHO) cases, were all hospitalised in negative-pressure isolation rooms, which had been established mainly for patients with tuberculosis. By the end of April, the major SARS epidemics in Hong Kong, Singapore, and Canada had plateaued, and the medical community in Taiwan became complacent since each imported SARS case was quickly identified, and secondary transmission was successfully blocked. Consequently, an unidentified SARS patient who had no travel history abroad, was not diagnosed promptly and that, coupled with a lapse in hospital infection-control practices, triggered a large-scale nosocomial SARS transmission in a municipal hospital in Taipei; this was followed by a series of nosocomial infections in seven hospitals due to the interhospital movement of unrecognised SARS patients. This chain of transmission from one hospital to another was subsequently confirmed by a genome analysis of the viruses, which verified the epidemiological link between outbreaks in all seven hospitals.13 In the end, 80% of the confirmed 346 SARS patients in Taiwan were acquired in a hospital, and 30% (n=102) of them were health-care workers. The scenario of nosocomial infection affecting both patient and health-care workers was similar in other countries,14 and highlights the potential threat that noscocomial infections have in debilitating an entire health-care system.
Figure 1.
Map of China and Taiwan with stars representing cities from where the 80 imported SARS cases (30 probable and 50 suspected) in Taiwan originated during the initial phase of importation; nearly all cases travelled via Hong Kong.
Points to consider
Strategic planning for the potential recurrence of SARS focuses on the two stages of SARS transmission: firstly, case containment through early diagnosis of the initial sporadic SARS patient and; secondly, accommodating the surge potential for SARS transmission—ie, a rapid increase of the number of affected patients in a short time. Chains of SARS transmission as shown so far, can be effectively blocked with early case detection and isolation of SARS patients, which in turn can be enhanced by strengthening border controls, laboratory diagnostic capacity, and the alert, response, and surveillance systems. SARS transmission in health-care settings often indiscriminately affects both health-care providers and patients, and can be explosive when involving unrecognised SARS patients.9, 14, 15 Continued vigilance and the availability of isolation wards are key to preventing a wide-scale outbreak. Considering the yearly influenza season might coincide with the return of SARS, formulating a meticulous strategy for patient triage is necessary to accommodate all SARS and SARS-like flu patients who require medical care in isolation.
Preventing SARS transmission
Border control
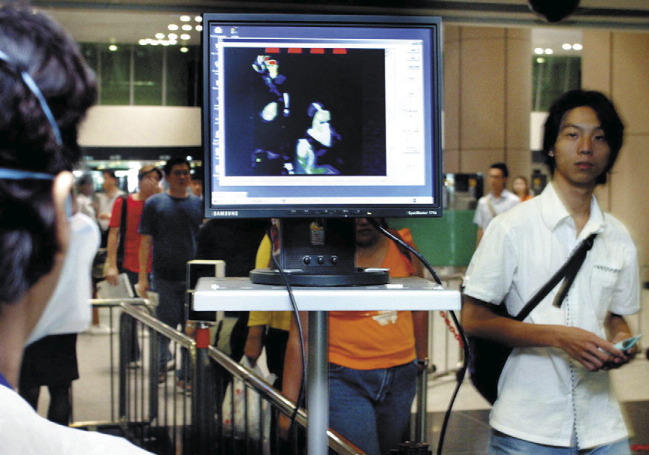
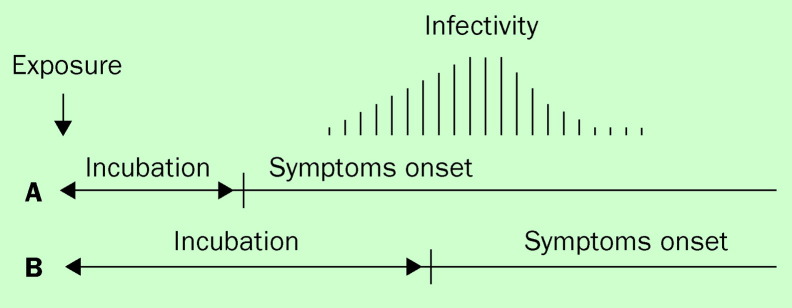
An infrared body temperature screening device that can accurately measure body temperature when inbound and outbound travellers walk through at their usual pace (figure 2 ) was established at the two international airports in Taiwan. During the SARS outbreak, passengers with fevers were prohibited from boarding the aeroplane. A hospital near each airport was designated to house, diagnose, and treat any passengers found with fever at the airport. Interestingly, in the summer through fall of 2003, 15 cases of imported dengue and/or malaria were identified at the airports. In part, it is believed that such a fever screening system would serve to deter SARS patients from international travel, and could reduce the international spread of SARS. However, due to the difference in the timing of infectivity in relation to the time of symptom onset (figure 3 ), how well this screening device can prevent international spread of infections other than SARS remains to be evaluated.
Figure 2.
A typical infrared body-temperature screening device used in airports and at the entrance of office buildings in Taipei. Passengers walk through at a usual pace. The faces of passengers whose body temperatures are higher than 38°C show up as a red image on the screen.
Figure 3.
According to the timing of a patient's infectivity in relation to the timing of symptoms onset, infectious diseases are broadly divided into two categories: (A) depicts infections, such as SARS and smallpox, where patients develop apparent symptoms before the infectivity begins, and (B) depicts infections, such as chickenpox, measles, and possibly influenza, where patients are infectious before they develop apparent symptoms. Quarantine and isolation are far more effective in category A than category B.
Strengthening laboratory diagnoses
Several nucleic acid-based diagnostic methods for SARS have been established at Taiwan's Center for Disease Control central laboratory and 12 contracted laboratories located in medical centres throughout the island.16 Confirmation of a SARS diagnosis by at least two laboratories is recommended, thus specimens of all suspected SARS patients are simultaneously sent to the CDC central laboratory and a designated contracted laboratory. The ability to confirm a SARS case within 12 h of hospital admission was shown in the most recent laboratory-acquired SARS case in December 2003.
To keep a rapid and smooth chain of flow of clinical specimens from fever patients to the CDC central laboratory beginning on August 19, 2003, 50 patients each week were selected from fever clinics to be tested for a variety of respiratory pathogens, including the SARS coronavirus, chlamydia, mycoplasma, dengue, and the Japanese encephalitis virus with the rapid antigen test. On October 15, 2003, in addition to the rapid antigen test, virus isolation and typing were carried out on the 50 weekly specimens. Beginning on November 17, 2003, the sampling was increased to 50 specimens per day. This practice continued until the influenza season ended in March 2004.
With the unexpected emergence of the H5N1 avian influenza in people during the winter of 2004, the preparedness plan in Taiwan for SARS has produced the additional benefit of consolidating the preparedness plans for possible influenza pandemics. Through the already existing viral laboratory network, methods for detecting H5 influenza serotype among patients were quickly established in all the reference laboratories, and have become one of the routine diagnostic items for severe respiratory infection. The intensified efforts to identify aetiological agents for respiratory diseases have put these laboratories in a well-prepared state to detect the avian influenza virus when it occurs in human beings. In fact, early detection of any avian influenza virus in human beings is believed to be the key to influenza pandemic prevention and has been the main focus of concern for clinical laboratories in Taiwan.
Strengthening alert, reporting, and the surveillance system
In general, fever patients are evaluated in designated fever clinics where laboratory specimens are collected and aetiological agents are identified with rapid tests as described above. The reporting of SARS patients was added to the preexisting web-based reporting system along with all other reportable infectious diseases, and can be accessed by all regional hospitals and medical centres. In addition, a special telephone line has been installed for reporting any unusual infectious diseases, imported or otherwise, or in any unusual clusters by the general public or any practising physicians. Several pre-existing surveillance systems are undergoing evaluation and revision, including emergency room-based syndromic surveillance that would allow analysis for targeted signs and symptoms related to infectious diseases—eg, fever, cough, respiratory distress, etc.
Rapid response and surge capacity: SARS and influenza A
The clinical presentation of SARS and influenza patients have considerable overlapping non-specific features. The seasonal peaks of influenza in Taiwan usually begin during week 47–48 every year and last for approximately 3 months.17 The first case of SARS in China occurred in mid-November 2002, and the subsequent SARS outbreak lasted throughout the winter. Therefore, it is possible that if the occurrence of SARS follows a certain seasonal propensity similar to other respiratory diseases,18, 19 it would coincide with the winter influenza season. The pre-existing influenza immunisation programme to cover individuals over 65 years of age was expanded in the winter of 2003–2004 to all healthcare workers and all public-health personnel. Moreover, intensified public education aiming to increase the vaccine coverage rate was part of the post-SARS public-education programme.
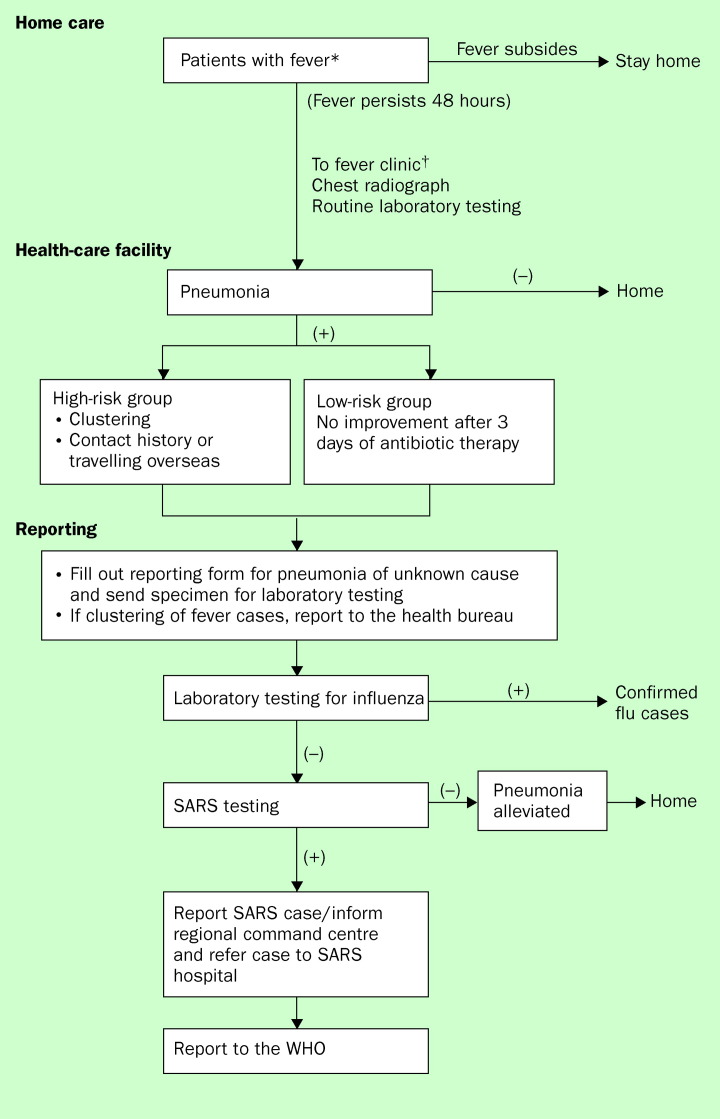
Considering the limited capacity of Taiwan's medical facilities to accommodate all SARS patients as well as the large number of SARS-like influenza patients, we developed an algorithm to triage all fever patients with respiratory illnesses (figure 4 ). This scheme can be implemented with the establishment of the fever hotline, fever-screening clinics in most healthcare facilities, and dedicated SARS hospitals—ie, high containment isolation hospital wards in a broad sense17, 20
Figure 4.
Algorithm to triage fever patients during a period when there are no known SARS transmissions occurring in the world. Elderly patients with other co-morbidity factors should seek health care early. †All those otherwise healthy individuals with fever should take antipyretics, stay home, and monitor their fever for 2 days before attending a physician.
If a fever occurs in a previously healthy individual, it is recommended that he or she stays home and calls the “177” fever hotline for consultation. Daily fever screening of all school children up to the ninth grade, a policy initiated during the SARS epidemic, has also been continued at all schools, and children with a body temperature of 38°C or more are kept in the school infirmary or sent home. Fever patients staying at home should be in isolation, and should wear a surgical mask whenever in contact with other family members. Those patients whose fever persists are evaluated in fever clinics where patients are routinely grouped and triaged according to their indications and case histories. During periods without any known SARS transmissions in the world, SARS will be considered in the differential diagnosis for laboratory testing only in the event of having pneumonia, or in the case of fever patients who cluster in time and space, or in the case of patients who do not respond to antibiotic therapy for 3 days. These measures are implemented to avoid testing a large number of clinical samples using the currently available nucleic acid-based reverse transcriptase-PCR method, because the small percentage of false-positives associated with this method would involve unnecessary public-health measures that would incur a heavy workload on hospital personnel, and possibly social panic.
Moreover, clinicians are advised to consider influenza A infection as one of the differential diagnoses when evaluating a SARS patient. Young children, elderly patients, and patients with a high risk for severe influenza infection should be evaluated early if a fever develops, and given antiviral drugs early when deemed necessary. In the winter of 2003–2004, the Taiwan CDC for the first time distributed an antiviral drug, oseltamivir, through public-health channels to health-care facilities for aggressive prophylactic therapy. The goal is to reduce the demand for isolation hospital wards if a SARS resurgence occurs. In addition, all influenza A viruses isolated from potential SARS patients are sent to the Taiwan CDC for subtyping. The interesting correlation of a low influenza season during the past winter in Taiwan, and the stringent preventive measures described above is currently being critically evaluated by the Taiwan CDC.
Other measures of sustainable development in the public-health system
Public-health law and policies
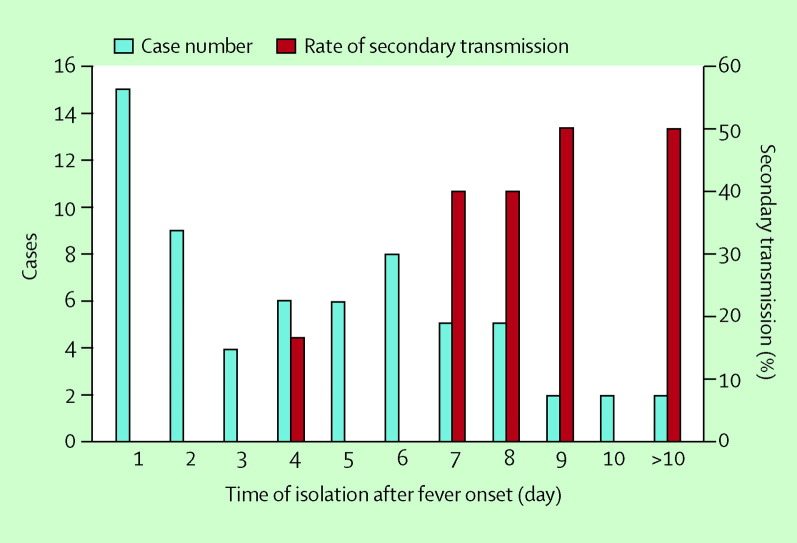
The SARS epidemic reactivated disease-control measures, such as the quarantine of contacts and compulsory isolation of SARS patients. The public-health code was revised in March 2003 to place the implementation and enforcement of these control measures on a legal ground. By the end of the SARS outbreak, 211 945 people, 0·92% of the total population in Taiwan, who either had contact with a SARS patient or had returned from a SARS-affected area, were quarantined for 10–14 days.21 Only 133 (0·06%) of the quarantined were later reported as probable or suspected SARS cases. Furthermore, among the limited number of confirmed SARS patients who acquired SARS infection outside of the hospital settings in Taiwan, SARS transmission to family members did not occur until day 3 of the onset of the fever (figure 5 ). After a review of the local and international epidemiological data that showed the infectivity of SARS patients began only after the onset of fever (figure 3), the quarantine policy was ultimately replaced by implementing stringent fever monitoring of the high-risk group and fever surveillance at the ports of entry.17, 22
Figure 5.
Non-hospital acquired SARS patients by time of isolation after onset of fever, and the rate of their secondary transmission to social and household contacts.
Further revision of the public-health code has been undertaken to accommodate the complex issues concerning the protection of rights and freedom of individuals in the context of the overall well-being of the public. In SARS patients, the onset of symptoms precedes the onset of infectiousness (figure 3), which supports the effectiveness of quarantine and fever surveillance. In the case of influenza A infection, however, the infectivity may precede the onset of illness. Thus, in the event of pandemic influenza, quarantine would not be an effective control measure. Rather, the preparation for the influenza pandemic is now focusing on stockpiling of effective antiviral drugs as well as the strengthening of the influenza vaccination programme. Taking into account the ongoing activity of H5N1 avian influenza outbreaks among human beings and birds in the neighbouring southeast Asian countries, strategies for regional alliance and a rapid delivery of international aid including antiviral drugs, to neighbouring affected countries for the purpose of containing transmission are being formulated.
Health-care epidemiology
More than 80% of all reported probable SARS cases acquired the SARS infection in hospitals, and health-care workers constitute a third of all these cases. This shows how the complex network of modern medical facilities with a mix of acute and chronically ill patients, inpatients, and outpatients is extremely vulnerable to the rapid and efficient transmission of infectious diseases. The ultimate SARS control would not have been possible if the hospital transmission of SARS was not blocked. After the SARS outbreak, in addition to heightening surveillance, monitoring, and education about hospital infection control, each health-care facility is required to instigate a health surveillance system for health-care workers so that any clustering of fever cases can be detected and investigated. The Taiwan CDC collects data passively on the health surveillance of workers from each health-care facility, and the reporting can be made active if indicated in the event of a resurgence of SARS. Chronic-care facilities and nursing homes are monitored via the same fever surveillance system.
Biosafety issues
In August and December 2003, two SARS cases were reported in Singapore and Taiwan in two research virologists working on the SARS coronavirus.23 The Taiwan CDC invited international and local experts to evaluate and discuss biosafety issues regarding practice, training, and regulation. National policy on monitoring and regulation of biosafety, as well as biosafety level III and IV practice standards is being formalised. A third incident of laboratory-acquired SARS cases that initiated transmission occurred in Beijing, and further stressed the importance of providing guidelines for biosafety standards and maintaining public-health vigilance.
A case in focus
In January and February 2004, four community-acquired SARS patients were reported from Guangdong,24 the southern Chinese province where SARS patients were first identified in the fall of 2002.1 Although no secondary transmission occurred, the source of these cases has not yet been completely identified, other than one case wherein the patient might have had an occupational exposure to infected palm civets in a restaurant. The SARS coronavirus isolated from the first of these four patients revealed a high homology with the SARS-like virus that had been isolated from the animals.25 The obscure source of the SARS coronavirus infection for these SARS patients suggests the possible persistence of SARS coronavirus in animal reservoirs in southern China. In January 2004, the Chinese authority banned all trading of palm civets in the market in Guangdong (NS Zhong, Guangzhou Institute of Respiratory Disease, China, personal communication). Whether the lack of further SARS cases is a result of the ban on palm civets remains to be seen. Whether palm civets are the authentic natural host of SARS coronavirus is still under debate; nevertheless, reintroduction of the SARS coronavirus from animal to human beings surely can occur again under certain conditions, which are as yet to be defined.
The close geographic and commercial links between Taiwan and China are reflected in a daily average of 10 000–20 000 visitors entering Taiwan from China. Taiwan is therefore confronted with the possible importation of SARS cases on a daily basis. As a result, the level for SARS alert was upgraded on January 1, 2004 to level A, which indicates the presence of confirmed SARS cases in other countries.26 Taiwan CDC kicked off several prevention measures, including intensifying fever surveillance of all travellers arriving from China, and expediting laboratory diagnosis of SARS coronavirus among testing for patients who recently had arrived from China and Hong Kong. In addition, stowaways from China, if caught in Taiwan are quarantined for 10 days before being sent home. Moreover, all employers are asked to provide assistance in conducting fever surveillance of their employees who travel to and from China or Hong Kong. The SARS alert was downgraded on February 25, 2004 when it was certain that no secondary transmission had occurred in Guangdong.
Looking to the future
The public-health system in Taiwan, as well as in many other countries, has geared up to minimise the adverse health impact of a possible influenza pandemic. It should be reiterated that prevention measures will have to be adjusted to account for the similarities and differences between SARS and influenza. Both SARS and pandemic influenza are likely to be zoonotic in nature, and establishing the capability for early detection in initial human cases is the key to preventing large-scale human transmission. While both diseases carry a considerable surge potential in terms of the number of patients and health-care workers potentially affected, antiviral drugs that can be used for prophylaxis are available to fight influenza. However, the demand for the antiviral may be high and will require countries to stock adequate supplies in advance. If transmission begins in human beings at any focal point, the speed at which influenza spreads will depend on how early it is detected, and how fast the international community can mobilise and deliver assistance, including providing antiviral drugs for prophylactic use. Therefore, in addition to a national preparedness plan, Taiwan scientists and government officials are also actively seeking international collaborations with neighbouring countries in Asia, since Taiwan is, hitherto, usually barred from activities coordinated by WHO.
Acknowledgments
Acknowledgments
The authors thank Weiju Chen and Ellie Chang for their assistance in preparing this manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Zhong NS, Zeng GQ. Our strategies for fighting severe acute respiratory syndrome(SARS) Am J Respir Crit Care Med. 2003;168:7–9. doi: 10.1164/rccm.200305-707OE. [DOI] [PubMed] [Google Scholar]
- 2.Zhong NS, Zheng BJ, Li YM. Epidemiology and cause of severe acute respiratorysyndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken T, Fouchier RA, Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouchier RA, Kuiken T, Schutten M. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 7.Peiris JS, Lai ST, Poon LL. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) WHO/CDS/CSR/GAR/2003·11. WHO; Geneva: 2003. http://www.who.int/csr/sars/en/WHOconsensus.pdf (accessed January 30, 2004). [Google Scholar]
- 9.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 10.Varia M, Wilson S, Sarwal S. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285–292. [PMC free article] [PubMed] [Google Scholar]
- 11.Health, Welfare & Food Bureau SARS Bulletin (18 April 2003) Hong Kong Special Administrative Region. Department of Health; 2003. http://www.info.gov.hk/dh/diseases/ap/eng/bulletin0418.htm (accessed January 30, 2004). [Google Scholar]
- 12.Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS) WHO; Geneva: 2003. http://www.who.int/csr/sars/country/table2003_09_23/en/ (accessed January 30, 2004). [Google Scholar]
- 13.Yeh SH, Wang HY, Tsai CY. Characterization of severe acute respiratory syndrome coronavirus genomes in Taiwan: Molecular epidemiology and genome evolution. Proc Natl Acad Sci U S A. 2004;101:2542–2547. doi: 10.1073/pnas.0307904100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow KY, Lee CE, Ling ML, Heng DM, Yap SG. Outbreak of severe acute respiratory syndrome in a tertiary hospital in Singapore, linked to an index patient with atypical presentation: epidemiological study. BMJ. 2004;328:195. doi: 10.1136/bmj.37939.465729.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmerman JM, Chu D, Chang H. Implications of unrecognized severe acute respiratory syndrome. Nurse Pract. 2003;28:21–31. doi: 10.1097/00006205-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Wu H-S, Chiu S-C, Tseng T-C. Serologic and molecular biologic methods for SARS-associated coronavirus infection, Taiwan. J Emerg Infect Dis. 2004;10:304–310. doi: 10.3201/eid1002.030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Disease Control, DOH, Executive Yuan, Taiwan Prevention and control of SARS in Taiwan. 2004/3/1. http://www.cdc.gov.tw (accessed September 15, 2004).
- 18.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowell SF, Ho M-S. Seasonality of infectious diseases and SARS-what we know can hurt us. Lancet Infect Dis4: 704–08. [DOI] [PMC free article] [PubMed]
- 20.Center for Disease Control, DOH, Executive Yuan, Taiwan Memoir of severe acute respiratory syndrome control in Taiwan. 2004/3/1. http://www.cdc.gov.tw/sarsen/ (accessed September 15, 2004).
- 21.Anon Use of quarantine to prevent transmission of severe acute respiratory syndrome—Taiwan, 2003. MMWR. 2003;52:680–683. (accessed September 15, 2004). [PubMed] [Google Scholar]
- 22.Lipsitch M, Cohen T, Cooper B. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Center for Disease Control DOH, Executive Yuan, Taiwan CDC Health Advisory: severe acute respiratory syndrome (SARS) in Taiwan. 2004/2/1. http://www.cdc.gov/ncidod/sars/taiwan17dec2003.htm (accessed September 15, 2004).
- 24.WHO Announcement of suspected severe acute respiratory syndrome (SARS) case, southern China-update. Wkly Epidemiol Rec. 2004 Jan 16;79:14–16. [PubMed] [Google Scholar]
- 25.Chinese SMEC Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 26.Center for Disease Control, DOH, Executive Yuan, Taiwan SARS website. http://www.cdc.gov.tw/sars/default.asp (accessed September 15, 2004).