Abstract
Background
Rhinoviruses (RVs) may cause pneumonia, but the characteristics of RV-associated pneumonia have not been adequately evaluated.
Objective
We aimed to compare characteristics, complications, and outcomes between severe RV- and influenza virus (IFV)-associated pneumonia in adults.
Study design
We used prospective cohort data of adult patients with severe pneumonia who had been admitted to the medical intensive care unit of a tertiary care hospital over a 4-year period. The clinical features and outcomes of 27 patients with RV-positive bronchoscopic bronchoalveolar lavage (BAL) fluid were compared to those of 51 pneumonia patients with IFV-positive BAL fluid or IFV-positive nasopharyngeal specimens.
Results
Of 356 patients who underwent bronchoscopic BAL and respiratory virus polymerase chain reaction (PCR), RV was the most commonly identified virus (8.1%) from BAL fluid. Patients with RV-associated pneumonia were more likely to be immunocompromised than patients with IFV-associated pneumonia (81.5% vs. 33.3%, p < 0.001). Bacterial coinfection tended to be less common in the RV group (18.5% vs. 37.3%, p = 0.09). Although septic shock was less common in the RV group (29.6% vs. 54.9%, p = 0.03), other clinical manifestations, laboratory findings, and radiologic patterns were similar between the groups. The 28-day mortality of patients with severe RV- and IFV-associated pneumonia was similarly high (29.6% vs. 35.3% respectively, p = 0.61).
Conclusions
Severe RV-associated pneumonia patients were more likely to be immunocompromised and less likely to present septic shock. Overall clinical features were similar and mortalities of both groups were comparably high. Studies of larger cohorts encompassing mild to moderate pneumonia patients are needed.
Abbreviations: RV, rhinovirus; IFV, influenza virus; BAL, bronchoalveolar lavage; PCR, polymerase chain reaction; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; APACHE, Acute Physiological and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment
Keywords: Rhinovirus, Pneumonia, Influenza
1. Background
RV is a non-enveloped, non-segmented single-strand RNA virus of the genus Enterovirus and the family Picornaviridae [1]. In adults, RVs are a predominant etiology of upper respiratory tract infections, also known as ‘common colds’, and are frequently associated with exacerbation of asthma [2] and COPD [3]. RVs’ ability to reach and infect the lower respiratory tract epithelium has been demonstrated recently [4], [5], [6], [7], and the link between RV and pneumonia has gained recent attention [8], [9], [10], [11], [12]. However, the role of RV in the pathogenesis of pneumonia remains controversial.
Histopathological examination of lung tissue specimens, including in situ hybridization of RV RNA, would be helpful to establish the role of RVs in pneumonia development. However, such examination is rarely possible in clinical practice. A prior report suggests that RVs infect a small subset of lower airway epithelial cells [7] and activate marked inflammatory responses, rather than directly invade lower airways in the lower respiratory tract epithelium [13], [14], [15]. Therefore, RV detection using bronchoalveolar lavage (BAL) fluid specimens from severe pneumonia patients may be an indirect but meaningful indicator for the pathogenic role of RV. However, clinical data using BAL specimens from unselected adult patients with RV-associated pneumonia are scarce [10], [16], [17], with some studies investigating small subsets of a retrospective series of respiratory virus infection [16], [17]. The majority of prior studies using BAL specimens were confined to small groups of hematopoietic stem cell transplant patients [12], [18], [19], [20], which limits our understanding of RV-associated pneumonia.
IFV is the major cause of severe viral pneumonia in adults during the epidemic season, and clinical features, complications, and outcomes of IFV-associated pneumonia have been well documented. Therefore, comparing RV- and IFV-associated pneumonia may help clinicians understand the clinical characteristics of RV-associated pneumonia.
2. Objectives
The aim of this study was to investigate the clinical and laboratory characteristics, complications, and outcomes of severe RV-associated pneumonia identified by bronchoscopic BAL in adults and to compare them with IFV-associated pneumonia.
3. Study design
3.1. Study design, setting, patient population, and data collection
This study was part of our ongoing prospective observational study regarding severe pneumonia in critically ill adult patients [21], [22], [23]. Since March 2010, all patients ≥16 years of age who were clinically suspected of having severe pneumonia were admitted to a 28-bed medical ICU of the Asan Medical Center, a 2700-bed tertiary care hospital in Seoul, Republic of Korea. Patients were prospectively identified and monitored until the time of discharge or death. The collected data included demographic characteristics, underlying diseases or conditions, symptoms and signs, laboratory test results, radiologic findings, treatment, complications, ICU and hospital lengths of stay, and mortalities. This study was approved by the Asan Medical Center Institutional Review Board. Due to the observational nature of the study, a waiver of the informed consent was allowed.
3.2. Patient selection
The current study included severe pneumonia patients with RV- or IFV-positive respiratory specimens admitted to the medical ICU between March 2010 and February 2014. For the RV group, only BAL fluid RV polymerase chain reaction (PCR)-positive cases were included. The IFV group included both BAL fluid and nasopharyngeal specimen IFV PCR-positive cases. Cases of mixed RV and IFV infection were excluded from the analysis.
3.3. Definitions
Pneumonia was defined and categorized as previously described [24], [25], [26]. Severe pneumonia was defined as the need for mechanical ventilation or septic shock at the time of ICU admission [25]. Immunocompromised state was defined as previously described [27].
3.4. Bronchoscopic BAL procedure and microbiological evaluation
BAL procedure and microbiological evaluation of the BAL fluid were performed following a previously described standardized protocol [21], [22], [23]. Respiratory virus PCR was performed by multiplex reverse-transcription PCR assay using the Seeplex RV 15 ACE Detection kit (Seegene Inc., Seoul, Korea). This kit simultaneously detects all RV species including RV groups A, B, and C; IFV A and B; adenovirus, parainfluenza virus types 1, 2, 3, and 4; respiratory syncytial virus types A and B; human metapneumovirus; enterovirus; human coronaviruses OC43/HKU1; human coronaviruses 229E/NL63; and bocavirus. The primers were targeted to the 5′-noncoding region and designed to distinguish between RV and enterovirus.
3.5. Statistical analysis
Demographic, clinical, and laboratory parameters were compared between RV patients and IFV patients. Categorical variables were compared by chi-square or Fisher's exact test, and continuous variables were compared by Student's t-test or Mann–Whitney U test. P values of <0.05 were considered statistically significant. All analyses were performed using SPSS version 21.0 (SPSS, Chicago, IL).
4. Results
4.1. Study population
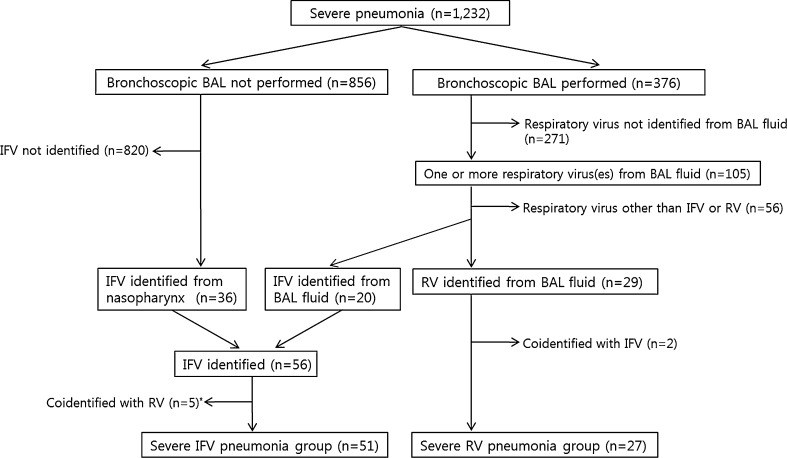
Fig. 1 shows the patient selection process and the reasons for exclusion. During the 48 months of the study period, a total of 1232 patients were admitted to the medical ICU with a diagnosis of severe pneumonia (260 with community-acquired pneumonia [CAP], 475 with healthcare-associated pneumonia [HCAP], and 497 with hospital-acquired pneumonia [HAP]). Of these patients, 376 underwent bronchoscopic BAL. Multiplex respiratory virus PCR was performed on the BAL fluid in 356 cases (94.7%). Among them, one or more viruses were identified in the BAL fluid of 105 patients (29.5%, 105/356). RV was the most common virus identified (8.1%, 29/356), followed by parainfluenza virus (6.7%, 24/356), respiratory syncytial virus (6.5%, 25/356), IFV (5.6%, 20/356), human coronavirus (2.2%, 8/356), human metapneumovirus (2.0%, 7/356), and adenovirus (1.1%, 4/356). In addition to 20 IFV-positive cases identified by BAL fluid, 36 IFV-positive cases with severe pneumonia were identified by nasopharyngeal specimen. After excluding the patients who were PCR-positive for both RV and IFV, we included 27 severe RV-associated pneumonia patients and 51 severe IFV-associated pneumonia patients (45 IFV A and 9 IFV B) in our analysis.
Fig. 1.
Patients selection process. BAL = bronchoalveolar lavage, IFV = influenza virus, RV = rhinovirus. *includes three RV samples from nasopharyngeal specimens.
4.2. Demographics, underlying diseases/conditions, and category of pneumonia
The demographic, underlying diseases/conditions, and category of pneumonia are summarized in Table 1 . The proportion of male patients was higher in the RV group than in the IFV group (77.8% vs. 54.9%, p = 0.047). The median ages of the RV group patients and the IFV group patients were 66.0 years and 62.0 years, respectively. Structural lung disease, hematologic malignancy, and diabetes mellitus were the most common underlying diseases in both groups. Of these, hematologic malignancy was significantly more common in the RV group (40.7% vs. 13.7%, p = 0.007). A greater proportion of RV patients were immunocompromised (81.5% vs. 33.3%, p < 0.001) and current smokers (14.8% vs. 1.9%, p = 0.04). CAP was significantly more common in the IFV group (11.1% vs. 37.3%, p = 0.02), and HCAP was more common in the RV group (55.6% vs. 31.4%, p = 0.04).
Table 1.
Demographics, underlying diseases/conditions, and categories of pneumonia.
| Total (n = 78) |
Rhinovirus (n = 27) |
Influenza virus (n = 51) |
p-Value | |
|---|---|---|---|---|
| Male sex | 49 (62.8) | 21 (77.8) | 28 (54.9) | 0.047 |
| Age, median (interquartile range) | 65.0 (53.8–71.0) | 66.0 (57.0–70.0) | 62.0 (53.0–73.0) | 0.77 |
| Underlying disease or conditiona | ||||
| Structural lung disease | 22 (28.2) | 7 (25.9) | 15 (29.4) | 0.75 |
| Chronic obstructive lung disease | 10 (12.8) | 3 (11.1) | 7 (13.7) | 1.00 |
| Interstitial lung disease | 7 (9.0) | 4 (14.8) | 3 (5.9) | 0.23 |
| Bronchiectasis | 4 (5.1) | 1 (3.7) | 3 (5.9) | 1.00 |
| Destroyed lung due to tuberculosis | 1 (1.3) | 0 | 1 (2.0) | 1.00 |
| Pneumoconiosis | 1 (1.3) | 0 | 1 (2.0) | 1.00 |
| Hematologic malignancy | 18 (23.1) | 11 (40.7) | 7 (13.7) | 0.007 |
| Diabetes mellitus | 16 (20.5) | 7 (25.9) | 9 (17.6) | 0.39 |
| Solid cancer | 10 (12.8) | 3 (11.1) | 7 (13.7) | 1.00 |
| End-stage renal disease | 2 (2.6) | 0 | 2 (3.9) | 0.54 |
| Congestive heart failure | 4 (5.1) | 0 | 4 (7.8) | 0.29 |
| Liver cirrhosis | 1 (1.3) | 1 (3.7) | 0 | 0.35 |
| Chronic renal failure | 3 (3.8) | 0 | 3 (5.9) | 0.55 |
| s/p cerebraovascular attack | 1 (1.3) | 0 | 1 (2.0) | 1.00 |
| Solid organ transplantation | 3 (3.8) | 2 (7.4) | 1 (2.0) | 0.27 |
| Hematopoietic stem cell transplantation | 9 (11.5) | 4 (14.8) | 5 (9.8) | 0.71 |
| Immunocompromised stateb | 39 (50.0) | 22 (81.5) | 17 (33.3) | < 0.001 |
| Receipt of immunosuppressant | 21 (26.9) | 14 (51.9) | 7 (13.7) | < 0.001 |
| Recent chemotherapy | 17 (21.8) | 9 (33.3) | 8 (15.7) | 0.07 |
| Active smoker | 5 (6.2) | 4 (14.8) | 1 (1.9) | 0.04 |
| Ex-smoker | 28 (35.9) | 11 (40.7) | 17 (33.3) | 0.52 |
| Recent surgery (within 1 month) | 3 (3.8) | 2 (7.4) | 1 (2.0) | 0.27 |
| Neutropeniac | 8 (10.3) | 5 (18.5) | 3 (5.9) | 0.12 |
| Category of pneumonia | ||||
| Community-acquired pneumonia | 22 (28.2) | 3 (11.1) | 19 (37.3) | 0.02 |
| Healthcare-associated pneumonia | 31 (39.7) | 15 (55.6) | 16 (31.4) | 0.04 |
| Hospital-acquired pneumonia | 25 (32.1) | 9 (33.3) | 16 (31.4) | 0.86 |
Data are presented as No. (%) unless otherwise stated.
Some patients had one or more underlying diseases or conditions.
Defined as one of the following conditions: (i) daily receipt of immunosuppressants, including corticosteroids, (ii) human immunodeficiency virus infection, (iii) solid organ or hematopoietic stem cell transplant recipients, (iv) receipt of chemotherapy for underlying malignancy during the previous 6 months, and (v) underlying immune deficiency disorder.
Absolute neutrophil count < 500/mm3.
The characteristics of five RV patients who were not immunocompromised are summarized in Table 2 . Of these patients, two had underlying COPD. Another two patients were 70 years old and 81 years old, respectively, and neither had remarkable underlying disease, history of hospitalization, or identifiable co-pathogens. The last patient had underlying diabetes mellitus and had undergone recent esophageal surgery. RV was co-identified with respiratory syncytial virus A and Acinetobacter baumannii in this patient.
Table 2.
Characteristics of five non-immunocompromised patients with severe rhinovirus-associated pneumonia.
| Patient No. | Year/ month |
Age /sex |
Underlying disease or condition | Category of pneumonia | Nasopharyngeal rhinovirus PCR | Co-pathogen | CT findings | Complication | Outcome (cause of death) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010/ April |
73/ male |
COPD | CAP | Not done | None | Bilateral mulifocal pathy consolidation and ground-glass opacities with interlobular septal thickening | Right ventricular failure | Died at hospital day 9 (pulmonary embolism) |
| 2 | 2010/ June |
84/ male |
COPD, bronchiectasis | HAP | Negative | None | Both lower lung consolidation | Atrial fibrillation, hypoxic brain damage | Alive |
| 3 | 2010/ September |
70/ male |
None | CAP | Positive | None | Both lower lung consolidation | None | Alive |
| 4 | 2010/ October |
81/ male |
None | CAP | Positive | None | Right mid-lobe and lower lobe consolidation, left hydropneumothorax | Acinetobacter baumannii ventilator-associated pneumonia | Died at hospital day 81 (ventilator-associated pneumonia) |
| 5 | 2012/ January |
64/ male |
Diabetes mellitus, fishbone-associated esophageal abscess → s/p surgical drainage | HAP | Negative | A. baumannii + respiratory syncytial virus A | Bilateral multifocal ground-glass opacity | Methicillin-resistant Staphylococcus aureus endocarditis | Alive |
ARDS, acute respiratory distress syndrome; CAP, community-acquired pneumonia; COPD, chronic obstructive lung disease; CT, computed tomography; HAP, hospital-acquired pneumonia.
4.3. Co-pathogens identified
Table 3 shows the co-pathogens identified with RV and IFV. Twelve of the RV patients (44.4%) and 26 (51.0%) of the IFV patients had one or more other respiratory pathogens identified from either BAL fluid or other specimens. Viral coinfection tended to be more common in the RV group (29.6% vs. 11.8%, p = 0.07) and bacterial coinfection tended to be more common in the IFV group (18.5% vs. 39.3%, p = 0.09). Cytomegalovirus (n = 4) and respiratory syncytial virus (n = 4) were the most common viral co-pathogens for the RV and IFV groups, respectively. Staphylococcus aureus was the most frequent bacterial co-pathogen (n = 7), followed by A. baumannii (n = 5) and Streptococcus pneumoniae (n = 5). S. aureus was found in the IFV group only (0% vs. 13.7%, p = 0.09) and P. jirovecii was found in the RV group only (14.8% vs. 0%, p = 0.01).
Table 3.
Identity of co-pathogens in patients with severe human rhinovirus- and influenza virus-associated pneumonia.
| Co-pathogensa | Total (n = 78) |
Rhinovirus (n = 27) |
Influenza virus (n = 51) |
p-Value |
|---|---|---|---|---|
| Any | 38 (48.7) | 12 (44.4) | 26 (51.0) | 0.58 |
| Other virus | 14 (17.9) | 8 (29.6) | 6 (11.8) | 0.07 |
| Respiratory syncytial virus | 6 | 2b | 4 | |
| Cytomegalovirus | 4 | 4 | 0 | |
| Human coronavirusc | 4 | 1b | 3 | |
| Parainfluenza virus | 1 | 1d | 0 | |
| Bacteria | 24 (30.8) | 5 (18.5) | 19 (37.3) | 0.09 |
| S. aureus | 7 | 0 | 7 | 0.09 |
| A. baumannii | 5 | 2 | 3e | |
| S. pneumoniae | 5 | 2 | 3 | |
| P. aeruginosa | 2 | 0 | 2e | |
| K. pneumoniae | 2 | 0 | 2 | |
| S. pyogenes | 1 | 0 | 1 | |
| C. striatum | 1 | 0 | 1 | |
| E. cloacae | 1 | 1 | 0 | |
| C. freundii | 1 | 0 | 1 | |
| Non-tuberculous mycobacteria, unspecified | 1 | 0 | 1 | |
| Aspergillus species | 6 (7.7) | 2 (7.4) | 4 (7.8) | 1.00 |
| Pneumocystis jirovecii | 4 (5.1) | 4 (14.8) | 0 | 0.01 |
Include pathogens identified from BAL fluid specimen or other specimens including nasopharyngeal specimen, sputum, endotracheal aspirate, and blood culture. Categories of coinfection were not mutually exclusive. Some cases were associated with two or more category of pathogens.
Respiratory syncytial virus A and human coronavirus 229E/NL63 were identified together.
Include two human coronavirus OC43/HKU-1 and two human coronavirus 229E/NL63.
PIV-1 and PIV-3 were co-identified.
In one patient, P. aeruginosa and A. baumannii were co-identified.
4.4. Clinical manifestations, laboratory findings, radiological findings, and seasonality
Clinical, laboratory, and radiologic characteristics are compared in Table 4 . Dyspnea, sputum, and fever were the most common symptoms in both groups. Cough tended to be less common in the RV group (66.7% vs. 84.3%, p = 0.07). Septic shock at the time of ICU admission was significantly more common in the IFV group (29.6% vs. 54.9%, p = 0.03). However, the rate of mechanical ventilation and severity index, measured by APACHE II score and SOFA score, did not differ between the two groups. Bacterial coinfection was significantly associated with septic shock (with bacterial coinfection, 66.7% [16/24] vs. without bacterial coinfection, 37.0% [20/54], p = 0.02). When the analysis was confined to patients without coinfection, the incidence of septic shock at ICU admission was similar between the two groups (RV group, 40.0% [6/15] vs. IFV group, 44.4% [11/25], p = 0.80).
Table 4.
Clinical, laboratory, and radiologic chacracteristics of patients with severe human rhinovirus-associated pneumonia and severe influenza virus-associated pneumonia.
| Total (n = 78) |
Rhinovirus (n = 27) |
Influenza virus (n = 51) |
p-Value | |
|---|---|---|---|---|
| Dyspnea | 73 (93.6) | 27 (100) | 46 (90.2) | 0.16 |
| Sputum | 63 (80.8) | 21 (77.8) | 42 (82.4) | 0.63 |
| Fever > 38 °C | 62 (79.5) | 19 (70.4) | 43 (84.3) | 0.15 |
| Cough | 61 (78.2) | 18 (66.7) | 43 (84.3) | 0.07 |
| Altered mentality | 18 (23.1) | 6 (22.2) | 12 (23.5) | 0.90 |
| Diarrhea | 5 (6.4) | 3 (11.1) | 2 (3.9) | 0.33 |
| Septic shock at ICU admission | 36 (46.2) | 8 (29.6) | 28 (54.9) | 0.03 |
| Mechanical ventilation | 75 (96.2) | 26 (96.3) | 49 (96.1) | 1.00 |
| APACHE II score (mean ± SD) | 24.4 ± 6.4 | 25.6 ± 6.9 | 23.7 ± 6.0 | 0.23 |
| SOFA score (median, IQR) | 10.0 (7.0–12.5) | 10.0 (7.3–12.0) | 10.0 (6.0–13.0) | 0.82 |
| Laboratory findings (median, IQR) | ||||
| White blood cells/mm3 | 9500 (4400–15,150) | 9400 (5100–15,800) | 9600 (4100–15,000) | 0.85 |
| Platelets, 103/mm3 | 146 (82–212) | 154 (83–219) | 136 (77–206) | 0.63 |
| C-reactive protein, mg/dl | 13.4 (6.1–20.8) | 13.4 (6.9–19.6) | 13.4 (5.7–23.2) | 0.99 |
| Procalcitonin, ng/ml | 1.5 (0.2–10.0) | 1.9 (0.2–24.4) | 1.1 (0.2–5.4) | 0.40 |
| BAL fluid cellular analysis (median, IQR) | ||||
| Total WBC count, cell/μL | 280 (135–1330) | 210 (100–930) | 500 (180–1790) | 0.17 |
| Neutrophils, % | 77.0 (39.3–88.8) | 76.0 (39.5–87.5) | 80.0 (38.0–89.0) | 0.95 |
| Lymphocytes, % | 6.0 (2.0–15.8) | 6.0 (2.5–14.0) | 6.0 (2.0–21.0) | 0.89 |
| Macrophages, % | 15.5 (6.3–41.0) | 17.0 (6.0–39.5) | 14.0 (6.0–45.0) | 0.92 |
| Radiologic findings | ||||
| Bilateral involvement | 74 (94.9) | 25 (92.6) | 49 (96.1) | 0.61 |
| Diffuse involvement | 46 (59.0) | 16 (59.3) | 30 (58.8) | 0.97 |
| Dominant pattern | 0.71 | |||
| Peribronchial pattern | 36 (46.2) | 12 (44.4) | 24 (47.1) | |
| Interstitial pattern | 21 (26.9) | 10 (37.0) | 11 (21.6) | |
| Lobar pattern | 19 (24.4) | 5 (18.5) | 14 (27.5) | |
| Other | 2 (2.6) | 0 | 2 (3.9) | |
| Ground-glass opacity | 31 (39.7) | 14 (51.9) | 17 (33.3) | 0.11 |
| Pleural effusion | 15 (19.2) | 8 (29.6) | 7 (13.7) | 0.09 |
| Nodule | 11 (14.1) | 3 (11.1) | 8 (15.7) | 0.74 |
Data are presented as No. (%) unless otherwise stated.
APACHE, acute physiology and chronic health evaluation; BAL, bronchoalveolar lavage; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation; SOFA, sequential organ failure assessment; WBC, white blood cell count.
Peripheral white blood cell counts (WBC), platelet counts, serum C-reactive protein levels, and procalcitonin levels were not significantly different between the two groups. Median total WBC counts and differential counts in the BAL fluid were not significantly different between the groups.
The radiologic patterns of pneumonia were mostly bilateral and often showed diffuse involvement in both groups. Differences in radiologic patterns between the two groups were not significant. Ground-glass opacity (51.9% vs. 33.3%, p = 0.11) and pleural effusion (29.6% vs. 13.7%, p = 0.09) tended to be more common in the RV group.
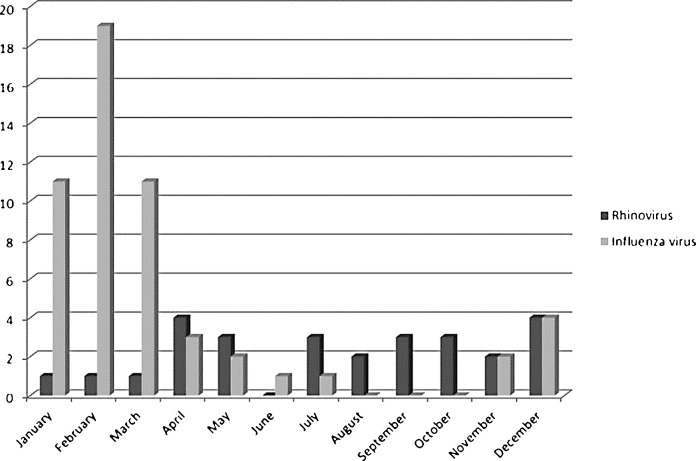
Fig. 2 shows the monthly distribution of RV and IFV cases over 48 months. RV-associated pneumonia occurred sporadically throughout the year whereas IFV-associated pneumonia occurred predominately from December to March.
Fig. 2.
Monthly distribution of severe pneumonia associated with rhinovirus and influenza virus.
4.5. Outcomes
Table 5 summarizes the comparisons of outcomes between the two groups. Median length of ICU stay and the proportion of patients complicated by ventilator-associated pneumonia were similar between the two groups. The 28-day mortality, ICU mortality, and in-hospital mortality were not significantly different between groups. The presence of bacterial coinfection was not associated with increased in-hospital mortality in either the RV group (bacterial coinfection, 60.0% vs. no bacterial coinfection, 54.5%, p = 1.00) or the IFV group (bacterial coinfection, 47.4% vs. no bacterial coinfection, 53.1%, p = 0.69).
Table 5.
Outcomes in patients with severe human rhinovirus- and influenza virus-associated pneumonia.
| Total (n = 78) |
Rhinovirus (n = 27) |
Influenza virus (n = 51) |
p-Value | |
|---|---|---|---|---|
| ICU stay, day (median, IQR) | 13.0 (6.0–27.0) | 13.0 (8.0–30.0) | 13.0 (5.0–27.0) | 0.55 |
| Complicated by ventilator-associated pneumonia | 18 (23.1) | 5 (18.5) | 13 (25.5) | 0.49 |
| Mortality | ||||
| 7-day mortality | 7 (9.0) | 2 (7.4) | 5 (9.8) | 1.00 |
| 14-day mortality | 16 (20.5) | 6 (22.2) | 10 (19.6) | 0.79 |
| 21-day mortality | 21(26.9) | 6 (22.2) | 15 (29.4) | 0.50 |
| 28-day mortality | 26 (33.3) | 8 (29.6) | 18 (35.3) | 0.61 |
| 60-day mortality | 38 (48.7) | 14 (51.9) | 24 (47.1) | 0.69 |
| ICU mortality | 35 (44.9) | 13 (48.1) | 22 (43.1) | 0.67 |
| In-hospital mortality | 41 (52.6) | 15 (55.6) | 26 (51.0) | 0.70 |
ICU, intensive care unit; IQR, interquartile range.
5. Discussion
This study compared the clinical characteristics and outcomes of severe RV-associated pneumonia with those of severe IFV-associated pneumonia. RV-associated pneumonia was commonly associated with an immunocompromised state although about 20% of the RV patients were not immunocompromised. Additionally, the RV-associated patients tended to have more viral coinfections and fewer bacterial coinfections. Despite fewer cases of septic shock at the time of ICU admission in RV-associated pneumonia, clinical and laboratory features and outcomes did not differ between the groups.
A limited number of adult RV-associated pneumonia cases identified by bronchoscopic BAL have been reported previously [10], [12], [16], [17], [18], [19], [20]. The main characteristics of these cases are summarized in supplementary Table 1. All of the prior reports included small number of cases, most of which, with the exception of a report from a tertiary care hospital in U.S.A [10], occurred in severely immunocompromised patients. This group retrospectively reviewed clinical features of 20 patients (including three children) who had RV cultured from their BAL over a 10-year period. Twelve (60.0%) needed mechanical ventilation. In-hospital mortality was relatively low (25.0%), which may be due to the lower frequency of severe immunocompromised conditions, such as hematopoietic stem cell transplant, in their patients. Notably, our study included five non-immunocompromised patients, two of which had underlying COPD and one who had undergone recent surgery. Another two elderly patients had no underlying illness or identifiable co-pathogen, suggesting that RV may be the sole cause of severe pneumonia in non-immunocompromised elderly patients.
Bacterial coinfection is a well-known complication of severe or fatal IFV infection, with reported rates of 18–55% [28]. The two most common bacterial co-pathogens, S. aureus and S. pneumoniae, are common colonizers of the nasopharynx [29], [30], [31]. In the present study, bacterial coinfection tended to be less frequent in the RV group than in the IFV group, and S. aureus was exclusively identified in the IFV group. Bacterial coinfection in influenza infection is likely caused by IFV propagation along the respiratory tree, together with mucociliary clearance impairment and IFV-induced respiratory tract epithelial cell damage [28]. By contrast, RVs involve only a small proportion of epithelial cells in the upper [32] and lower airway epithelium [7]. Therefore, the lower frequency of bacterial coinfection and septic shock may be due to the reduced extent of epithelial damage with RV infection. Since our study used different inclusion criteria for the two groups and included only patients with severe pneumonia admitted to medical ICU, the difference in the bacterial coinfection rate between the groups may have been biased. The proportion of individuals admitted to the ICU because of secondary bacterial pneumonia rather than viral pneumonia may be higher in the IFV group. Bacterial coinfection is a key cause of death in 20th century influenza pandemics [29], [30], and bacterial coinfection was also associated with an increased risk of death in 2009 pandemic influenza A/H1N1 (pH1N1) [31], [33]. In our results, however, bacterial coinfection was not associated with poorer outcomes in either IFV group or RV group, although the small numbers in our group may limit the generalizability of our findings and warrant further investigation.
Although cough tended to occur more frequently in the IFV group, other respiratory symptoms, laboratory findings, and radiographic findings were indistinguishable between the two groups. Recently, a U.S. group compared the characteristics between 2009 pH1N1 and other respiratory viruses in patients hospitalized with respiratory illness [34] and found that the presence of fever, cough, and gastrointestinal symptoms increased the likelihood of pH1N1 almost 10-fold in the pediatric population. Our study was limited to severe pneumonia patients admitted to the ICU, and more than two-thirds of both groups had respiratory symptoms. Therefore, a symptom-based approach is likely not reliable in differentiating clinical diagnoses among adult patients with severe pneumonia. The seasonal distribution of each respiratory virus and the temporal occurrence of respiratory viral illness in the community may help predict viral pathogens. Nevertheless, PCR tests for respiratory viruses are reliable and should be included for etiological diagnosis in patients with severe pneumonia.
Although septic shock at the time of initial presentation was less common in the RV group than in the IFV group, it was significantly associated with bacterial coinfection. Therefore, the presence of bacterial coinfections may be responsible for more severe manifestations of pneumonia at the time of ICU admission. Nevertheless, mortalities of patients were similarly high in both groups, likely reflecting the more frequent presence of immunocompromised conditions in the RV group. Taken together, our data suggest that host factor is an important contributor to the development of severe RV-associated pneumonia and affects the outcome.
Our study has several limitations. First, protected specimen brushing was not used during the study period, meaning BAL fluid may have been contaminated by upper airway secretions during the bronchoscopic procedure. Second, even in BAL fluid RV PCR-positive cases, RVs might be present as non-pathogenic colonizers. Third, because we did not perform a molecular study for serogrouping and serotyping, the impact of RV species and strains could not be evaluated. Finally, the RV group only included PCR-positive cases from BAL fluid, whereas the IFV group also included PCR-positive cases from nasopharyngeal specimens, indicating a possibility for biases associated with these different inclusion criteria.
In conclusion, severe RV-associated pneumonia patients were more likely to be immunocompromised and less likely to have bacterial coinfection and septic shock compared with patients who had severe IFV-associated pneumonia. However, both groups of pneumonia showed similar clinical and laboratory features and were associated with comparably high mortalities. Since our current study used different inclusion criteria for the two groups and included only severe pneumonia patients admitted to an ICU, further investigations are warranted.
Funding
This study was supported by grant 2012-389 from the Asan Institute of Life Sciences, Seoul, Republic of Korea.
Competing interests
The authors declare that there are no conflicts of interest to disclose.
Ethical approval
The study was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (2010-0079).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2014.11.010.
Appendix A. Supplementary data
References
- 1.Jacobs S.E., Lamson D.M., St George K., Walsh T.J. Human rhinoviruses. Clin Microbiol Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minor T.E., Dick E.C., Baker J.W., Ouellette J.J., Cohen M., Reed C.E. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976;113:149–153. doi: 10.1164/arrd.1976.113.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg S.B., Allen M., Wilson J., Atmar R.L. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 4.Gern J.E., Galagan D.M., Jarjour N.N., Dick E.C., Busse W.W. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos N.G., Sanderson G., Hunter J., Johnston S.L. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58:100–104. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos N.G., Bates P.J., Bardin P.G., Papi A., Leir S.H., Fraenkel D.J. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 7.Mosser A.G., Brockman-Schneider R., Amineva S., Burchell L., Sedgwick J.B., Busse W.W. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson K.G., Kent J., Hammersley V., Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119–1123. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Sahly H.M., Atmar R.L., Glezen W.P., Greenberg S.B. Spectrum of clinical illness in hospitalized patients with common cold virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malcolm E., Arruda E., Hayden F.G., Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol: the Off Publ Pan Am Soc Clin Virol. 2001;21:9–16. doi: 10.1016/s1386-6532(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 11.Falsey A.R., Walsh E.E., Hayden F.G. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ison M.G., Hayden F.G., Kaiser L., Corey L., Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36:1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bardin P.G., Fraenkel D.J., Sanderson G., Lampe F., Holgate S.T. Lower airways inflammatory response during rhinovirus colds. Int Arch Allergy Immunol. 1995;107:127–129. doi: 10.1159/000236951. [DOI] [PubMed] [Google Scholar]
- 14.Johnston S.L. Natural and experimental rhinovirus infections of the lower respiratory tract. Am J Respir Crit Care Med. 1995;152:S46–S52. doi: 10.1164/ajrccm/152.4_Pt_2.S46. [DOI] [PubMed] [Google Scholar]
- 15.Fraenkel D.J., Bardin P.G., Sanderson G., Lampe F., Johnston S.L., Holgate S.T. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 16.Connolly M.G., Jr., Baughman R.P., Dohn M.N., Linnemann C.C., Jr. Recovery of viruses other than cytomegalovirus from bronchoalveolar lavage fluid. Chest. 1994;105:1775–1781. doi: 10.1378/chest.105.6.1775. [DOI] [PubMed] [Google Scholar]
- 17.Garbino J., Gerbase M.W., Wunderli W., Kolarova L., Nicod L.P., Rochat T. Respiratory viruses and severe lower respiratory tract complications in hospitalized patients. Chest. 2004;125:1033–1039. doi: 10.1378/chest.125.3.1033. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S., Champlin R., Couch R., Englund J., Raad I., Malik S. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 19.Gutman J.A., Peck A.J., Kuypers J., Boeckh M. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant. 2007;40:809–811. doi: 10.1038/sj.bmt.1705827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowden R.A. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. discussion 42-3. [DOI] [PubMed] [Google Scholar]
- 21.Choi S.H., Hong S.B., Ko G.B., Lee Y., Park H.J., Park S.Y. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 22.Hong H.L., Hong S.B., Ko G.B., Huh J.W., Sung H., Do K.H. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One. 2014;9:e95865. doi: 10.1371/journal.pone.0095865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi S.H., Hong S.B., Hong H.L., Kim S.H., Huh J.W., Sung H. Usefulness of cellular analysis of bronchoalveolar lavage fluid for predicting the etiology of pneumonia in critically ill patients. PLoS One. 2014;9:e97346. doi: 10.1371/journal.pone.0097346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carratala J., Mykietiuk A., Fernandez-Sabe N., Suarez C., Dorca J., Verdaguer R. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 25.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 27.Micek S.T., Kollef K.E., Reichley R.M., Roubinian N., Kollef M.H. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chertow D.S., Memoli M.J. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 29.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzmann S.W., Adler J.L., Sullivan R.J., Jr., Marine W.M. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969. Arch Intern Med. 1971;127:1037–1041. [PubMed] [Google Scholar]
- 31.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R., 3rd Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arruda E., Boyle T.R., Winther B., Pevear D.C., Gwaltney J.M., Jr., Hayden F.G. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 33.Palacios G., Hornig M., Cisterna D., Savji N., Bussetti A.V., Kapoor V. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan P.A., Mermel L.A., Andrea S.B., McCulloh R., Mills J.P., Echenique I. Distinguishing characteristics between pandemic 2009-2010 influenza A (H1N1) and other viruses in patients hospitalized with respiratory illness. PLoS One. 2011;6:e24734. doi: 10.1371/journal.pone.0024734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.