Highlights
-
•
A prospective cohort of young children were studied for RTIs.
-
•
Blood MxA protein levels were elevated with symptomatic virus infections.
-
•
MxA response was demonstrated for rhinoviruses in clinical setting.
-
•
Immunization with live virus vaccine had a modest effect on MxA levels.
Keywords: MxA protein, Respiratory virus infection, Rhinovirus, Rotavirus vaccine, Interferon, STEPS study
Abstract
Background
Type I interferon induced MxA response can differentiate viral from bacterial infections, but MxA responses in rhinovirus or asymptomatic virus infections are not known.
Objective
To study MxA protein levels in healthy state and during respiratory virus infection of young children in an observational prospective cohort.
Study design
Blood samples and nasal swabs were collected from 153 and 77 children with and without symptoms of respiratory infections, respectively. Blood MxA protein levels were measured by an enzyme immunoassay and PCR methods were used for the detection of respiratory viruses in nasal swabs.
Results
Respiratory viruses were detected in 81% of symptomatic children. They had higher blood MxA protein levels (median [interquartile range]) than asymptomatic virus-negative children (695 [345–1370] μg/L vs. 110 [55–170] μg/L; p < 0.001). Within asymptomatic children, no significant difference was observed in MxA responses between virus-positive and virus-negative groups. A cut-off level of 175 μg/L had 92% sensitivity and 77% specificity for a symptomatic respiratory virus infection. Rhinovirus, respiratory syncytial virus, parainfluenza virus, influenza virus, coronavirus, and human metapneumovirus infections were associated with elevated MxA responses. Asymptomatic virus-negative children vaccinated with a live virus vaccine had elevated MxA protein levels (240 [120–540] μg/L), but significantly lower than children with an acute respiratory infection, who had not received vaccinations (740 [350–1425] μg/L; p < 0.001).
Conclusion
Blood MxA protein levels are increased in young children with symptomatic respiratory virus infections, including rhinovirus infections. MxA is an informative general marker for the most common acute virus infections.
1. Background
Respiratory tract infections (RTIs) cause a high disease burden to children in developed countries and are a major cause of mortality in developing countries [1]. A surrogate marker for virus infections would be very useful to guide antimicrobial therapy decisions and discriminate virus infections from other inflammatory conditions.
Myxovirus resistance protein A (MxA) is an intracellular, cytoplasmic GTPase that has activity against a wide range of viruses [2], [3], [4], [5], [6], [7]. It is induced exclusively by type I and III interferons (IFNs) as a specific response to virus infections and its basal levels in healthy immunotolerant individuals are low [8], [9]. With a half-life in blood leukocytes of 2–4 days, the level remains elevated shortly after an acute infection [8], [10], [11].
Although several different viruses induce MxA expression, controversy concerning the MxA response to rhinovirus (RV) still exists [12], [13]. As RVs are the most frequent cause of RTIs in children and adults [14], [15], [16], determining the MxA response to RV infection would be critical for its potential use as a sensitive marker for a virus infection.
2. Objectives
Here, we compared blood MxA protein levels in young children with respiratory infections caused by RV or other respiratory viruses with asymptomatic virus-negative children. We also studied whether the MxA response to symptomatic respiratory virus infections can be differentiated from the responses to asymptomatic infections or immunizations.
3. Study design
3.1. Patients
This study was conducted within the prospective observational birth-cohort study Steps to the Healthy Development and Well-being of Children (STEPS) [17]. Of 1827 recruited children, 923 were followed intensively for RTIs during the first two years of life. From February 2009 through April 2011, blood samples for MxA and C-reactive protein (CRP) determinations and white blood cell counts (WBC) were collected from children 1 to 24 months of age who presented to the study clinic with symptoms of an acute RTI and fever or ill appearance. The existence and duration of symptoms (rhinorrhoea, cough, wheezing, fever, and other symptoms) and the findings of clinical examination were uniformly documented. Fever was defined as body temperature ≥38 °C per rectum or ≥37.5 °C from axilla or tympanic membrane. Families were encouraged to visit the study clinic when children experienced symptoms of RTI. Alternatively, trained parents collected nasal swabs from their children at home and mailed the specimens to the study clinic [18], [19].
Blood samples for MxA and nasal swabs for viruses were collected also from children at scheduled visits to the study clinic at the age of 2 and 13 months, and blood samples for MxA were collected from their asymptomatic parents. The possible presence of respiratory symptoms was documented at the scheduled visits to identify true asymptomatic children, as children often have mild respiratory symptoms. Those without any respiratory symptoms, and negative for respiratory viruses, served as a control group of uninfected, asymptomatic children. Any samples collected at different time points from the same child were analyzed as separate cases.
Childhood vaccination histories were collected from the electronic registries of regional well baby clinics. All vaccinations administered to the child during the 30 days preceding the MxA sample were included in the analysis.
3.2. MxA, CRP, and WBC measurements
MxA protein levels were determined by an enzyme immunoassay (EIA) from a capillary whole blood sample taken by fingertip prick (children visiting for RTI) or from a peripheral venous whole blood sample taken by antecubital venipuncture (scheduled visits). Blood samples were diluted 1:20 in hypotonic buffer and stored at −70 °C until EIA was performed as described in the supplementary data and in earlier studies [9], [20], [21], [22]. CRP and WBC were measured from capillary whole blood samples. A rapid CRP test was performed with Orion QuikRead 101 (Orion Diagnostica, Espoo, Finland) and rapid WBC count was done using HemoCue WBC (HemoCue, Ängelholm, Sweden) point-of-care analyser according to the manufacturer's instructions.
3.3. Virus detection
The nasal swabs were suspended in phosphate buffered saline and subjected to automated nucleic acid extraction by using NucliSense easyMag (BioMerieux, Boxtel, Netherlands) or MagnaPure 96 (Roche, Penzberg, Germany) instrument. Extracted RNA was reverse transcribed and the cDNA was amplified using real-time PCR for RV, enterovirus and respiratory syncytial virus (RSV) as described earlier [23] with the modification that primers (forward, 5′-TGA-ACA-GTT-TRA-CAT-TAC-CAA-GTG-A-3′; reverse 5′-CCA-CGA-TTT-TTR-TTG-GAT-GC-3′) and probe (5′-ROX-CCA-TAG-CAT-GAC-ACW-AT-BHQ-3′; A, locked nucleic acid analog) specific for RSV F gene were included in the same assay. Seeplex RV12 multiplex PCR assay (Seegene, Seoul, Korea) for RV, RSV-A, RSV-B, adenovirus (AdV), influenza (Flu) A and B viruses, parainfluenza virus (PIV) types 1, 2 and 3, human metapneumovirus (HMPV) and coronaviruses (CoV) 229E/NL63 and OC43/HKU1 was performed according to the manufacturer's instructions. A separate PCR test was used for detection of human bocavirus (HBoV) [24]. For RV and RSV, a positive result in either one of the tests was considered as a positive test result.
3.4. Statistical analyses
Mann–Whitney U test was used to compare blood MxA protein levels and χ 2 test or Fisher's exact test were used for categorical data. Kruskal–Wallis test was used for multiple comparisons followed by pairwise Mann–Whitney U test with Bonferroni correction. A decision threshold for MxA was calculated by receiver operating characteristic (ROC) analysis and correlations between blood MxA and viral loads or duration of symptoms were calculated by Spearman's correlation. P values <0.05 were considered statistically significant. Statistical analyses were performed by using IBM SPSS Statistics for Macintosh, Version 21.0.
4. Results
A total number of 242 blood samples were collected from 200 children. During study clinic visits for an acute RTI, blood samples for MxA, CRP and WBC determinations were collected from 132 children. Nine children were excluded from the study because of missing nasal swabs and 3 because of bacterial infections (2 children with a culture confirmed pyelonephritis and 1 with orbital cellulitis, with MxA and CRP levels of 50 μg/L and 63 mg/L; 90 μg/L and 112 mg/L; 80 μg/L and 104 mg/L, respectively), resulting in a group of 120 children visiting for RTI (Table 1 ). Blood MxA was also measured from 110 children at scheduled visits at the age of 2 (n = 67) and 13 months (n = 43). Of these children, 33 had symptoms of respiratory infection at the time of sample collection. In addition, blood MxA level was determined in 44 asymptomatic parents (age, 25–56 years; males, 41%). Clinical, laboratory, and virus findings of the study subjects are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the children and the etiology of virus infections detected by PCR.
| Children visiting for RTI | Children visiting at the age of 2 or 13 months (n = 110) |
||
|---|---|---|---|
| (n = 120) | RTI symptoms (n = 33) | No symptoms (n = 77) | |
| Age (years) | 1.08 ± 0.42 | 0.64 ± 0.46 | 0.55 ± 0.44 |
| Sex | |||
| Male | 70 (58%) | 22 (67%) | 42 (55%) |
| Symptoms | |||
| Respiratory symptoms | 115 (96%) | 33 (100%) | 0 |
| Fever | 107 (89%) | 0 | 0 |
| Diagnoses | |||
| Acute upper RTI | 94 (78%) | NA | NA |
| Acute otitis media | 26 (22%) | NA | NA |
| Wheezing illness | 17 (14%) | NA | NA |
| Laryngitis | 4 (3%) | NA | NA |
| Laboratory values | |||
| Blood MxA (μg/l) | 855 (383–1438) | 220 (75–660) | 110 (60–185) |
| White blood cell count (E9/l) | 9.37 ± 4.07 | ND | ND |
| C-reactive protein (mg/l) | 13.6 ± 19.8 | ND | ND |
| Virus detection | |||
| Virus-positive | 100 (83%) | 24 (72%) | 12 (16%)a |
| Rhinovirus | 31 (26%) | 15 (45%) | 4 (5%) |
| Respiratory syncytial virus | 15 (13%) | 1 (3%) | 2 (3%) |
| Coronavirus OC43/HKU1 | 8 (7%) | 1 (3%) | 2 (3%) |
| Parainfluenza virus type 3 | 7 (6%) | 0 | 1 (1%) |
| Human metapneumovirus | 6 (5%) | 0 | 0 |
| Coronavirus 229E/NL63 | 1 (1%) | 3 (9%) | 2 (3%) |
| Human bocavirus | 4 (3%) | 1 (3%) | 0 |
| Influenza A virus | 3 (3%) | 1 (3%) | 0 |
| Parainfluenzavirus type 1 | 2 (2%) | 0 | 0 |
| Influenza B virus | 1 (1%) | 0 | 0 |
| Adenovirus | 1 (1%) | 0 | 0 |
| Parainfluenzavirus type 2 | 0 | 0 | 1 (1%) |
| Co-infection | 21 (18%)b | 2 (6%)c | 0 |
RTI, respiratory tract infection; NA, not available; ND, not determined.
Diagnoses, white blood cell counts and C-reactive protein levels were available for children visiting for RTI and examined by a physician. Part of the children (n = 33) visiting at the age of 2 or 13 months had mild symptoms of RTI at the time of sample collection, not necessitating an evaluation by a physician.
Values are n (%), mean ± SD, and for MxA, median (IQR).
The overall rate of virus detection in all screened asymptomatic children (n = 163) was 7%.
Co-infections (no.): Respiratory syncytial virus with either rhinovirus (2), parainfluenza virus (3), bocavirus (3), human metapneumovirus (1), coronavirus (1), adenovirus (1), or coronavirus and bocavirus (1); rhinovirus with coronavirus (3), bocavirus (1), parainfluenza virus (1), enterovirus (1), metapneumovirus (1), or parainfluenzavirus and bocavirus (1); coronavirus with bocavirus (1).
Co-infections: Bocavirus with either respiratory syncytial virus (1), or rhinovirus (1).
ROC analysis was used to estimate the ability of MxA to differentiate children with a laboratory-confirmed, symptomatic respiratory virus infection from asymptomatic virus-negative children. The greatest sum of sensitivity (92%) and specificity (77%) was obtained with a cut-off level of 175 μg/L as the upper limit of normal range. A slighly lower specificity (75%) was obtained when the comparison group was asymptomatic children regardless of virus detection.
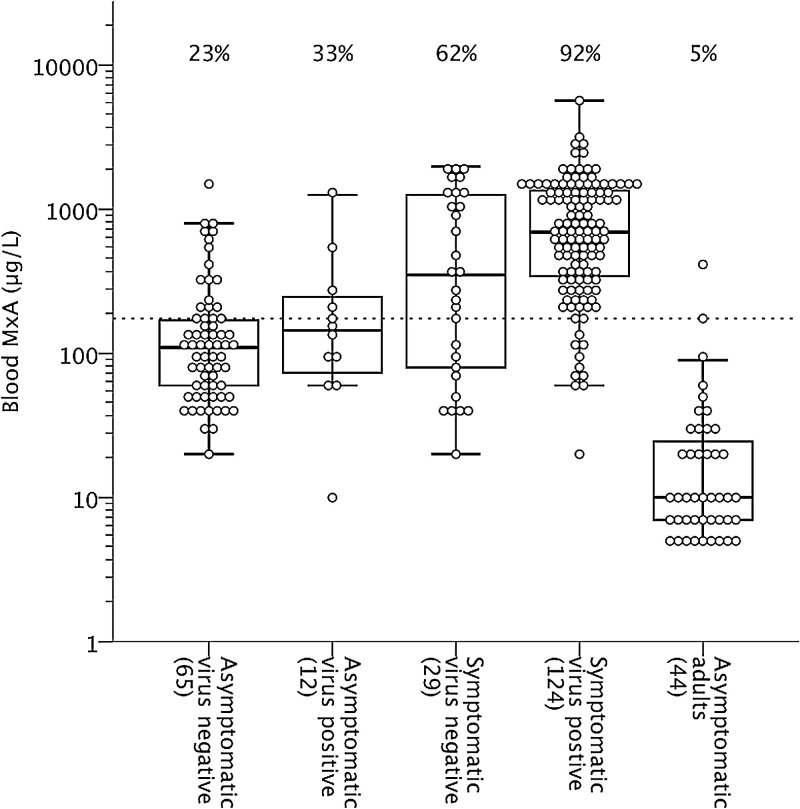
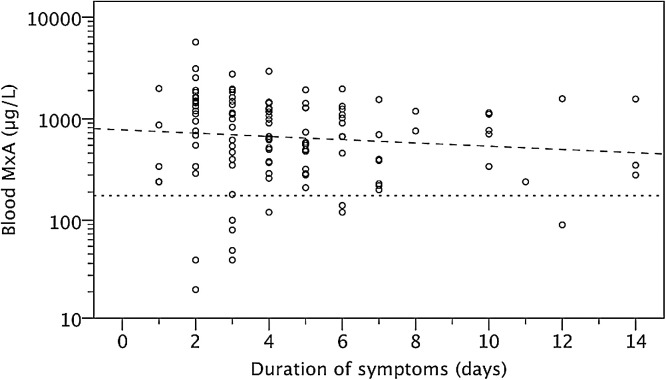
The blood MxA protein levels in children, according to the presence of respiratory symptoms and virus detection, and in asymptomatic adults, are shown in Fig. 1 . The basal level of blood MxA (median [interquartile range]) was significantly higher in asymptomatic children than in asymptomatic adults (110 [60–185] μg/L vs. 10 [10–30] μg/L; p < 0.001). MxA protein levels did not differ in asymptomatic children at 2 months compared to 13 months of age (120 [60–215] μg/L vs. 90 [60–165] μg/L; p 0.55). Children with a symptomatic, laboratory confirmed respiratory virus infection had higher MxA levels than asymptomatic virus-negative children (695 [340–1370] μg/L; vs. 110 [55–170] μg/L; p < 0.001). The levels were not significantly higher in asymptomatic virus-positive children (145 [70–270] μg/L; p 0.34) as compared to asymptomatic virus-negative children. Febrile infections were associated with higher MxA levels than respiratory infections without fever (930 [415–1440] μg/L vs. 240 [95–725] μg/L; p < 0.001). Wheezing illness or the presence of acute otitis media could not be differentiated by MxA levels from uncomplicated upper RTIs (data not shown). MxA levels had a weak negative correlation with the duration of symptoms preceding the sampling, but the assay sensitivity was not affected (Fig. 2 ).
Fig. 1.
Blood MxA protein levels in children according to virus detection and presence of symptoms, and in asymptomatic adults. Boxes show median and IQR, and whiskers show 10th and 90th percentiles. Values below the sensitivity limit of 10 μg/L are presented as 7 or 5 μg/L. Values above different groups indicate the ratios of values above the cut-off level of 175 μg/L.
Fig. 2.
Blood MxA protein levels in relation to the duration of fever or respiratory symptoms before MxA measurement. All symptomatic children with recorded duration of symptoms for 1–14 days and with (n = 103) or without (n = 21) detected virus are included. Spearman's rho −0.193; p 0.032; correlation line (– – –); 175 μg/L cut-off (- - -).
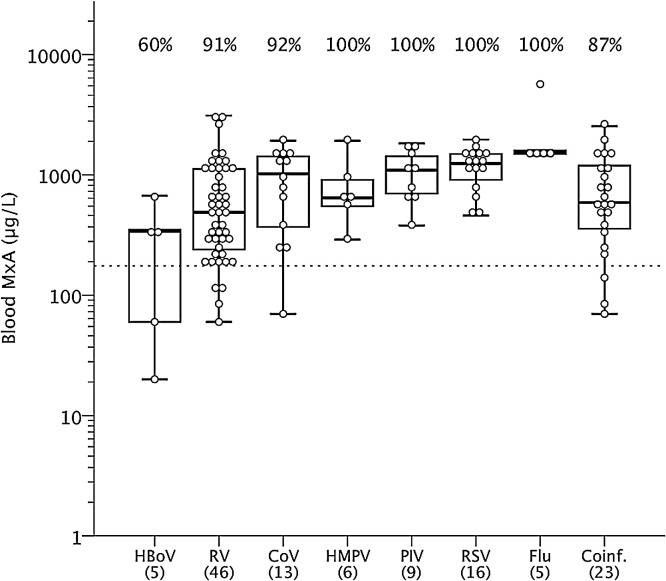
Symptomatic RV, RSV, PIV, Flu, CoV and HMPV infections were associated with elevated MxA levels, whereas MxA levels of HBoV infected children were not found to be different (p 1.00) from those of asymptomatic virus negative children (Fig. 3 ). The MxA levels in children positive for more than one virus were similar to those seen in children positive only for one virus (590 [320–1200] μg/L vs. 760 [345–1405] μg/L; p 0.48). There was no significant correlation between the RV or RSV RNA copy numbers and the blood MxA levels (RV log of copies vs. MxA, Spearman's rho −0.259, p 0.064 and RSV log of copies vs. MxA, Spearman's rho −0.112, p 0.56).
Fig. 3.
Blood MxA protein levels in symptomatic virus-positive children according to detected viruses. Boxes show median and IQR, and whiskers show 10th and 90th percentiles. Values above different groups indicate the ratios of values above the cut-off level of 175 μg/L. HBoV, human bocavirus; RV, rhinovirus; CoV, coronavirus; HMPV, human metapneumovirus; PIV, parainfluenza virus (types 1–3); RSV, respiratory syncytial virus; Flu, influenza virus (A or B). A single case with adenovirus is not presented.
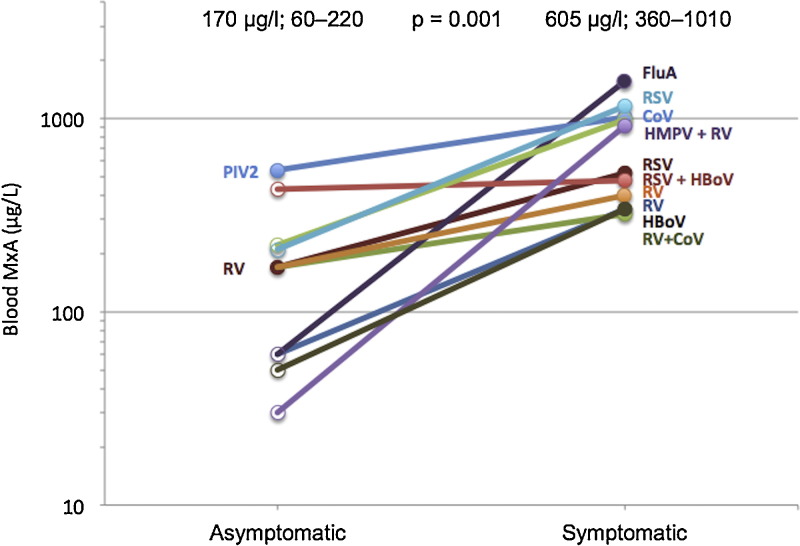
Samples were available from 11 children both during the healthy, asymptomatic state and later during a respiratory infection demonstrating individual MxA responses and significant differences between the states (Fig. 4 ).
Fig. 4.
Blood MxA protein levels and virus findings in the same individuals during the healthy state and during a respiratory infection. Median and IQR values are shown above the groups; p 0.001 by the Mann–Whitney U test. HBoV, human bocavirus; RV, rhinovirus; HMPV, human metapneumovirus; CoV, coronavirus; PIV2, parainfluenza virus type 2; RSV, respiratory syncytial virus; FluA, influenza A virus.
The information on vaccinations was available for 207 cases (90%). Of 59 asymptomatic virus-negative children, 19 were vaccinated with a live virus vaccine (18 with rotavirus vaccine and 1 with MMR- and VZV-vaccines, simultaneously), 8 with inactivated viral, bacterial or combination vaccines, and 32 were not vaccinated within a month preceding MxA sampling. Within the group of asymptomatic virus-negative children, the MxA levels were higher in children vaccinated with a live virus vaccine as compared to children without preceding vaccinations (240 [120–540] μg/L vs. 85 [50–130] μg/L; p 0.001), but the MxA level was not elevated in children vaccinated with inactivated vaccines (75 [50–135] μg/L; p 0.85). Children with an acute respiratory infection, who had not received vaccinations (n = 103), had significantly higher MxA levels (740 [350–1425] μg/L) than asymptomatic virus-negative children vaccinated with a live virus vaccine (p < 0.001).
5. Discussion
In this study, symptomatic infections caused by common respiratory viruses, including RV, were associated with elevated MxA levels in the blood. According to previous studies, MxA is induced during influenza-, parainfluenza-, RS-, and adenovirus infections in children, but the capability of RV to induce MxA protein expression has been controversial [12], [25], [26], [27]. In vitro and animal experiments show that RV infection is able to induce type I IFN response [28] and thus it would be expected that MxA or other IFN-inducible proteins would also be induced. However, an earlier flow cytometric analysis from isolated blood lymphocytes showed only a weak MxA response to RV [12]. The patients were adults and the severity of infection, or the presence of fever, were not specified. The discrepant results may be explained by the different clinical setup, different methodology used, and apparently a milder RV infection in adults [8], [12]. In the current study, MxA was measured from the whole blood lysates by EIA, which is less time-consuming and has the potential for a rapid diagnostic use.
By using 175 μg/L as the cut-off level, elevated MxA had 92% sensitivity and 77% specificity for a symptomatic respiratory virus infection. This suggests that MxA can be used as a marker of respiratory virus infection in children. Earlier, MxA has been shown to discriminate between viral and bacterial infection in febrile children, but the number of investigated respiratory viruses in these studies has been limited [11], [27]. In the present study, a systematic detection of 14 respiratory viruses by PCR was performed, including HMPV, CoV, and HBoV, which have not been assessed in similar studies earlier. The MxA response varied according to the causative virus. In RSV, PIV, Flu and HMPV the disease was associated with high MxA levels while in RV and CoV infections MxA levels remained at a lower but yet clearly higher levels as compared to samples obtained from patients suffering from asymptomatic or non-viral infections. Febrile infections were associated with higher MxA levels as compared to non-febrile infections reflecting a stronger systemic immune response. Our data indicate that in children, most of the common respiratory viruses are capable of inducing an efficient IFN response, which readily correlates with elevated expression of blood cell MxA protein.
Asymptomatic virus infections are common in children especially during the epidemic seasons with reported prevalences of up to 45% [29]. Blood cell MxA levels were not significantly elevated in asymptomatic virus-positive children as compared to asymptomatic virus-negative children in the present study. This provides further evidence for the discriminatory power of blood MxA protein for differentiation between viral and non-viral causes of febrile infections.
Viral co-infections are common in children as diagnosed by sensitive PCR assays, but it is controversial whether infections by multiple viruses cause more severe diseases as compared to infections caused by a single virus [30]. Furthermore, a higher viral load may be associated with the causative role of the virus in the illness or with more severe symptoms [31]. Co-infections or high viral loads in single virus infections were not associated with higher MxA levels in our study. However, more detailed studies on the immune response in these conditions, as well as on MxA response during viral-bacterial co-infections, are warranted.
Low detected numbers of some of the investigated viruses is a limitation for the generalization of our data. The presence of HBoV DNA in nasal secretions did not associate with an elevated MxA level, but there were only five cases with HBoV as the sole virus which may preclude definitive conclusions of whether HBoV is able or not able to induce type I IFN and MxA response. Although we analyzed 14 viruses with PCR, there are additional viruses, e.g. PIV type 4 that were not included in the assay and could explain symptoms in some children with negative PCR results. Still, the virus detection rate in symptomatic subjects was as high as 81%. In this out-of-hospital study, there were only few children with a sole bacterial infection, but it is of note that they had low MxA protein levels (50–90 μg/L).
Scheduled visits in the STEPS study included antecubital venipuncture to collect peripheral venous whole blood of which a sample was separated for the laboratory work of this study. When a child visited the clinic because of RTI, a capillary whole blood sample was taken by fingertip prick. Chieux and coworkers observed an excellent correlation but 24% lower MxA levels in capillary blood as compared to venous blood [26]. Here, MxA levels during a respiratory infection in capillary blood were significantly higher than during healthy state in venous blood. This indicates that the strong MxA response produced during a virus infection exceeds the minor difference caused by a different sampling type.
Recent immunization with a live virus vaccine, which in most of our cases was rotavirus vaccine, was associated with an increased MxA level. Inactivated vaccines had no such effect. This finding is in line with the study of Roers et al., who showed that MxA protein is induced in healthy volunteers after immunization with a live yellow fever virus vaccine [10]. This data suggests that recent vaccinations with live virus vaccines should be taken into consideration when interpreting the MxA results.
In conclusion, our study demonstrates that blood MxA levels are increased in young children with symptomatic respiratory virus infections, including RV infections. Detection of MxA protein in blood leucocytes should be reconsidered as a broad-spectrum diagnostic marker for respiratory virus infections in young children.
Funding
This work was supported by Academy of Finland (Grant No. 140251); The Pediatric Research Foundation (Finland); The National Graduate School of Clinical Investigation; the University of Turku; Abo Akademi University; and Turku University Hospital.
Competing interests
None declared.
Ethical approval
The Ethics Committee of the Hospital District of Southwest Finland approved the study. Parents of participating children gave their written, informed consent.
Acknowledgements
We thank Maria Kivivirta, Tiina Ylinen and Eila Pelkonen for their technical assistance, all families who participated to this study, the midwives for their help in recruiting the families, the whole STEPS Study team, and Robert M. Badeau, Ph.D. of the University of Turku Language Center, who performed the linguistic proofreading. Presented in part at the 15th Annual Meeting of the European Society for Clinical Virology, Madrid, Spain 2012, and at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 2013.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2014.11.018.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Wussow P., Jakschies D., Hochkeppel H.K., Fibich C., Penner L., Deicher H. The human intracellular Mx-homologous protein is specifically induced by type I interferons. Eur J Immunol. 1990;20:2015–2019. doi: 10.1002/eji.1830200920. [DOI] [PubMed] [Google Scholar]
- 3.Simon A., Fäh J., Haller O., Staeheli P. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J Virol. 1991;65:968–971. doi: 10.1128/jvi.65.2.968-971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronni T., Matikainen S., Lehtonen A., Palvimo J., Dellis J., Van Eylen F. The proximal interferon-stimulated response elements are essential for interferon responsiveness: a promoter analysis of the antiviral MxA gene. J Interferon Cytokine Res. 1998;18:773–781. doi: 10.1089/jir.1998.18.773. [DOI] [PubMed] [Google Scholar]
- 5.Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 6.Holzinger D., Jorns C., Stertz S., Boisson-Dupuis S., Thimme R., Weidmann M. Induction of MxA gene expression by influenza A virus requires type I or type III interferon signaling. J Virol. 2007;81:7776–7785. doi: 10.1128/JVI.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller O., Kochs G. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. J Interferon Cytokine Res. 2011;31:79–87. doi: 10.1089/jir.2010.0076. [DOI] [PubMed] [Google Scholar]
- 8.Ronni T., Melén K., Malygin A., Julkunen I. Control of IFN-inducible MxA gene expression in human cells. J Immunol. 1993;150:1715–1726. [PubMed] [Google Scholar]
- 9.Maria N.I., Brkic Z., Waris M., van Helden-Meeuwsen C.G., Heezen K., van de Merwe J.P. MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren's syndrome. Ann Rheum Dis. 2014;73:1052–1059. doi: 10.1136/annrheumdis-2012-202552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roers A., Hochkeppel H.K., Horisberger M.A., Hovanessian A., Haller O. MxA gene expression after live virus vaccination: a sensitive marker for endogenous type I interferon. J Infect Dis. 1994;169:807–813. doi: 10.1093/infdis/169.4.807. [DOI] [PubMed] [Google Scholar]
- 11.Halminen M., Ilonen J., Julkunen I., Ruuskanen O., Simell O., Mäkelä M.J. Expression of MxA protein in blood lymphocytes discriminates between viral and bacterial infections in febrile children. Pediatr Res. 1997;41:647–650. doi: 10.1203/00006450-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Mäkelä M.J., Halminen M., Ruuskanen O., Puhakka T., Pirhonen J., Julkunen I. Lack of induction by rhinoviruses of systemic type I interferon production or enhanced MxA protein expression during the common cold. Eur J Clin Microbiol Infect Dis. 1999;18:665–668. doi: 10.1007/s100960050370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvaggi C., Pierangeli A., Fabiani M., Spano L., Nicolai A., Papoff P. Interferon lambda 1-3 expression in infants hospitalized for RSV or HRV associated bronchiolitis. J Infect. 2014;68:467–477. doi: 10.1016/j.jinf.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller E.K., Lu X., Erdman D.D., Poehling K.A., Zhu Y., Griffin M.R. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay I.M., Lambert S.B., Faux C.E., Arden K.E., Nissen M.D., Sloots T.P. Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period. J Infect Dis. 2013;207:1433–1441. doi: 10.1093/infdis/jis476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagström H., Rautava P., Kaljonen A., Räihä H., Pihlaja P., Korpilahti P. Cohort profile: Steps to the healthy development and well-being of children (the STEPS Study) Int J Epidemiol. 2013;42:1273–1284. doi: 10.1093/ije/dys150. [DOI] [PubMed] [Google Scholar]
- 18.Peltola V., Waris M., Osterback R., Susi P., Ruuskanen O., Hyypia T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 19.Waris M., Osterback R., Lahti E., Vuorinen T., Ruuskanen O., Peltola V. Comparison of sampling methods for the detection of human rhinovirus RNA. J Clin Virol. 2013;58:200–204. doi: 10.1016/j.jcv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Vallittu A.M., Erälinna J.P., Ilonen J., Salmi A.A., Waris M. MxA protein assay for optimal monitoring of IFN-beta bioactivity in the treatment of MS patients. Acta Neurol Scand. 2008;118:12–17. doi: 10.1111/j.1600-0404.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 21.Bellingan G., Maksimow M., Howell D.C., Stotz M., Beale R., Beatty M. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. Lancet Respir Med. 2014;2:98–107. doi: 10.1016/S2213-2600(13)70259-5. [DOI] [PubMed] [Google Scholar]
- 22.Ruuskanen O., Waris M., Kainulainen L. Treatment of persistent rhinovirus infection with pegylated interferon alpha 2a and ribavirin in patients with hypogammaglobulinemia. Clin Infect Dis. 2014;58:1784–1786. doi: 10.1093/cid/ciu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osterback R., Tevaluoto T., Ylinen T., Peltola V., Susi P P., Hyypiä T. Simultaneous detection and differentiation of human rhino- and enteroviruses in clinical specimens by real-time PCR with locked nucleic Acid probes. J Clin Microbiol. 2013;51:3960–3967. doi: 10.1128/JCM.01646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskenvuo M., Möttönen M., Waris M., Allander T., Salmi T.T., Ruuskanen O. Human bocavirus in children with acute lymphoblastic leukemia. Eur J Pediatr. 2008;167:1011–1015. doi: 10.1007/s00431-007-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forster J., Schweizer M., Schumacher R.F., Kaufmehl K., Lob S. MxA protein in infants and children with respiratory tract infection. Acta Paediatr. 1996;85:163–167. doi: 10.1111/j.1651-2227.1996.tb13985.x. [DOI] [PubMed] [Google Scholar]
- 26.Chieux V., Hober D., Chehadeh W., Harvey J., Alm G., Cousin J. MxA protein in capillary blood of children with viral infections. J Med Virol. 1999;59:547–551. [PubMed] [Google Scholar]
- 27.Nakabayashi M., Adachi Y., Itazawa T., Okabe Y., Kanegane H., Kawamura M. MxA-based recognition of viral illness in febrile children by a whole blood assay. Pediatr Res. 2006;60:770–774. doi: 10.1203/01.pdr.0000246098.65888.5b. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett N.W., Slater L., Glanville N. Defining critical roles for NF-(B p65 and type I interferon in innate immunity to rhinovirus. EMBO Mol Med. 2012;4:1244–1260. doi: 10.1002/emmm.201201650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jartti T., Jartti L., Peltola V., Waris M., Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27:1103–1107. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 30.Chorazy M.L., Lebeck M.G., McCarthy T.A., Richter S.S., Torner J.C., Gray G.C. Polymicrobial acute respiratory infections in a hospital-based pediatric population. Pediatr Infect Dis J. 2013;32:460–466. doi: 10.1097/INF.0b013e31828683ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen R.R., Wieringa J., Koekkoek S.M., Visser C.E., Pajkrt D., Molenkamp R. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.