Abstract
Cryptosporidiosis is a significant diarrhoeal disease in both people and animals across the world and is caused by several species of the protozoan parasite Cryptosporidium. Recent research has highlighted the longer-term consequences of the disease for malnourished children, involving growth stunting and cognitive deficits, and significant growth and production losses for livestock. There are no vaccines currently available to prevent the disease and few treatment options in either humans or animals, which has been a significant limiting factor in disease control to date. A One Health approach to tackle zoonotic cryptosporidiosis looking at new advances in veterinary, public, and environmental health research may offer several advantages and new options to help control the disease.
Keywords: cryptosporidiosis, One Health, veterinary, public, environment
Highlights
Development of new vaccines for livestock and people to reduce disease and shedding of Cryptosporidium oocysts is a key One Health goal.
An integrated genotyping approach to detect and differentiate Cryptosporidium parasites in both veterinary and public health will inform source tracking, epidemiology, and surveillance.
The application of relevant clinical in vivo models and novel in vitro systems will progress understanding of host–pathogen interactions and enable efficacy testing of new therapeutics and vaccines.
New methods to treat Cryptosporidium-contaminated livestock and human waste will reduce oocyst contamination of the environment and help protect water catchments.
Knowledge exchange and education are vital to encourage a One Health approach to tackle cryptosporidiosis.
Cryptosporidium and One Health
The protozoan parasite Cryptosporidium is a significant cause of diarrhoeal disease in humans and animals globally [1]. There are currently over 40 recognised species of Cryptosporidium [2], with several of these species being found in humans. Those species most frequently detected are Cryptosporidium hominis and Cryptosporidium parvum, and other less frequently detected species include Cryptosporidium meleagridis, Cryptosporidium felis, Cryptosporidium canis, Cryptosporidium ubiquitum, Cryptosporidium cuniculus, Cryptosporidium muris, and Cryptosporidium viatorum. Infection and disease associated with Cryptosporidium parasites has significant health, welfare, and economic impacts (Box 1 ). Cryptosporidium spp. are highly successful parasites due to their large host range, high oocyst (see Glossary) output from infected individuals, water-borne transmission route, and low infectious dose. Recent modelling studies have shown a significant risk of infection from as low as one oocyst [3].
Box 1. Impact of Cryptosporidiosis.
Cryptosporidiosis is a significant diarrhoeal disease in both people and animals worldwide. Young children and people with compromised immune systems are very vulnerable to disease and the importance of Cryptosporidium in human health was first recognised during the AIDs epidemic in the 1980s [75]. From 2007 to 2017 the global prevalence of Cryptosporidium in HIV/AIDS patients was 10.9% [76]. A large-scale epidemiology study involving 22 500 children in sub-Saharan Africa and south east Asia found that Cryptosporidium was a major cause of severe diarrhoea in very young children and was the only gastrointestinal pathogen presenting a significant risk of death [15]. Cryptosporidium is also recognised as an important food-borne pathogen [8], being responsible for more than 8 million cases of food-borne illness annually [77].
Burden of disease studies, focussed on acute illness, estimate 4.2 million disability adjusted life years lost in children under 5 years [64], with longer term sequelae, including growth faltering and cognitive defects. Disease impact studies in high-income countries found longer term impacts following acute infection involving persistent abdominal pain, myalgia/arthralgia and fatigue [78], irritable bowel syndrome [79], and a recent study has shown a strong association between Cryptosporidium infection and human colon cancer [80].
Cryptosporidiosis is a major diarrhoeal disease in neonatal calves and other animals. In the cattle industry, production losses associated with the disease include death of the calf, costs incurred in the diagnosis, treatment and supportive therapies employed, and the extra costs of feed and husbandry for the animals to reach market weight [81]. Cattle infected with Cryptosporidium and monitored from birth to 210 days showed a correlation with infection and a lower live weight gain along with poorer production performance [82]. Long-term production impacts on growth and carcase weights following acute cryptosporidiosis in lambs was also reported on Australian sheep farms [83].
Outbreaks of cryptosporidiosis associated with contaminated water supplies can result in significant economic and health impacts. The large waterborne outbreak in Wisconsin affected 403 000 people and was estimated to cost USD 96.2 million [84]. In Sweden it was estimated that 50 000 sick leave days were attributed following a waterborne cryptosporidiosis outbreak where the attack rate was 45% of 60 000 residents [85]. Detection of Cryptosporidium oocysts in public water supplies often results in condemnation of supplies, public notices to boil water, and provision of bottled water. In a recent waterborne outbreak in Ireland a boil water notice was put on for 158 days, affecting over 120 432 people and costing around EUR 19 million [86].
Alt-text: Box 1
Infection occurs through oral ingestion of the oocyst stage of the parasite from contaminated faeces, food, drink, and pasture (for grazing animals), and following ingestion, the sporozoites are released from the oocysts and invade and undergo asexual development in the epithelial cells of the gastrointestinal tract of the host. This is followed by a sexual phase of development resulting in the production of potentially genetically diverse oocysts that are shed fully infective in the faeces. Oocysts may also ‘hatch’ before they are shed from the host, causing re-infection and exponential increases in parasite burden, leading to chronic infection, particularly in immunocompromised hosts [4]. Cryptosporidium parasites have a remarkable capacity to reproduce in the host. The rapid multiplication of the parasite in the gut cells causes tissue damage and destruction of the intestinal epithelial cells with stunting of the villi, reducing the absorptive surface of the gut, leading to malnutrition, dehydration, and diarrhoea [5].
During acute infection, and for a week or so following clinical recovery, the infected host will shed very large numbers of infectious oocysts in faeces [6]. Work with neonatal calves has shown that one calf can shed around 100 million infectious oocysts in faeces, which is a massive source of environmental contamination and poses an infection risk for other vulnerable hosts [7]. Cryptosporidium oocysts are microscopic (most species are 4–6 μm in diameter) and have a tough waxy wall composed of lipids and glycoproteins, which enables the parasite to survive a wide range of conditions, including temperatures from –22°C to 60°C. This wall protects the parasite against many common disinfectants, which makes Cryptosporidium hard to control on the farm, in drinking water, swimming pools, and in fresh produce [8,9].
Not all Cryptosporidium spp. in livestock are of public health significance, but the species of most zoonotic importance for human infection is C. parvum [1], which will be the focus for this review. Livestock, in particular young calves, are very vulnerable to cryptosporidiosis and a recent modelling study estimated the global load of Cryptosporidium parasites in livestock manure to be in the region of 3.2 × 1023 oocysts per year, with cattle being the predominant source [10]. Therefore, improving our understanding of environmental transmission routes of zoonotic Cryptosporidium and oocyst survival is important in assessing and mitigating against disease risk and is essential for a One Health approach to tackle human and animal cryptosporidiosis. Currently, there are no effective vaccines to prevent cryptosporidiosis in humans or in livestock and there are few safe and effective therapeutic options available. Cryptosporidiosis is a disease that is well placed for a collaborative interdisciplinary approach to tackle it effectively and previous authors have advocated the benefits of adopting a One Health approach [11]. This review will discuss progress made in this area, with greater emphasis on the benefits and opportunities from tackling the disease in livestock, and look at recent advances in scientific technologies that may lead to much needed prevention and control strategies.
Disease, Transmission, and Diagnostics
Human infection is usually of the small bowel via the faecal–oral route, although there is growing evidence for respiratory involvement in some populations [12]. Gastrointestinal symptoms, including profuse watery diarrhoea, abdominal pain, and nausea, typically start between 2 and 10 days after ingestion of oocysts and arise from malabsorption and inflammation [13]. Some populations are especially vulnerable to Cryptosporidium, and the parasite was included in the World Health Organization’s Neglected Diseases Initiative in 2004 [14]. This was in recognition of the links to poverty, the disproportionate impact on young, malnourished children with subsequent growth and development impairment [15,16], and the severe impact on immunocompromised people. Long-term health consequences are also now recognised in people (Box 1).
Disease in Livestock
Globally, cryptosporidiosis affects a large number of animal species, including the main livestock animals such as: cattle, sheep, goats, pigs, rabbits, horses, donkeys, water buffalo, camels, and poultry [17] and zoonotic Cryptosporidium spp. have also been reported in wildlife species, including rabbits [18], deer [19], wild mammals [20], and fish [21].
Infection with C. parvum is one of the most important causes of diarrhoeal disease in neonatal (<6 weeks old) calves in many countries worldwide [22]. The parasite is the most frequently diagnosed cause of neonatal calf diarrhoea in the UKi and C. parvum also causes significant disease in lambs and goats [23]. Sheep and goat production is an important agricultural industry in Europe, particularly in less favoured areas for agriculture, where they are an important source of meat and dairy products. It is estimated that 98% of the goat population is in developing countries, mainly in Africa and Asia, where they are an important source of meat, milk, fibre, and fertilizer and help to improve the livelihoods of the world’s poorest communities [24]. Children often play an important role in tending grazing livestock in many countries and, due to their close proximity to the animals, are very likely to come into contact with Cryptosporidium parasites. Recent data from China has also shown the presence of C. parvum in yaks, which may pose a public health risk [25].
The parasite causes disease in both dairy and beef herds and the prevalence of infection can be high in young calves, particularly in intensively reared management systems, with various clinical outcomes being reported, ranging from death of the calf to growth retardation and some animals with no obvious clinical signs [26]. The disease can also cause long-term production impacts in livestock (Box 1). The minimal infectious dose for neonatal calves is very low, with 17 oocysts causing infection and a correlation of a dose-dependent relationship between oocyst intake and clinical signs [27].
Clinical signs in acutely infected calves include yellow watery faeces, reluctance to feed, dehydration, and in severe cases can result in death [22]. The gut takes a few weeks to recover from infection and regain its ability to effectively absorb nutrients [5]. Oocyst shedding in the faeces of infected calves can be observed from day 4 postinfection, lasting for a further 10 days to 2 weeks and shedding can occur after diarrhoea has ceased. Therefore, asymptomatic animals may also pose a risk of infection to other animals and to people. This is particularly pertinent in managing risk of zoonotic transmission of Cryptosporidium at open farms and petting zoos [28].
Transmission Pathways for Zoonotic Cryptosporidium Oocysts
Figure 1 (Key Figure) illustrates some of the main sources and transmission pathways for zoonotic Cryptosporidium parasites and further information about transmission sources to people are shown in Box 2 . Environmental contamination with Cryptosporidium oocysts is widespread globally and it has been suggested that Cryptosporidium poses the biggest pathogen threat to the water industry [29]. Of 199 documented outbreaks of human parasitic protozoal disease due to water contamination in the period 2004–2010, Cryptosporidium spp. accounted for the majority of outbreaks [30]. How the oocysts reach water courses is not clear, as fate and transfer of oocysts from host faecal material through the environment to water courses is not well understood. Most scientists and water industry experts consider this mainly to be due to overland flow, particularly in periods of high rainfall or around intense rainfall events [31]. The effect of climate change (which is predicted to increase winter precipitation and summer extreme precipitation events) on pathogen loading to surface waters was recently explored, but predicted to have limited effect on the risk to public health, using models for catchment pathogen loads [32]. As livestock pastures frequently surround reservoirs ultimately destined for human drinking water, this can cause problems for water providers.
Figure 1.
Key Figure. Main Zoonotic Sources and Transmission Pathways of Cryptosporidium Parasites.
Zoonotic Cryptosporidium parasites are transmitted from livestock and wildlife through long-lived oocysts in their faeces, which can contaminate the environment, water, and food, producing a source of infection to people.
Box 2. Transmission of Cryptosporidium.
People may become infected directly by ingestion of infective oocysts through the faecal–oral route. This may be occupational [87], through contact with infected animals [88], or indirectly through the consumption of contaminated drinking water [89] or food [8]. Proportional source attribution data are sparse for Cryptosporidium. One study in Canada attributed source to sporadic cryptosporidiosis cases from exposure data and found that water was the most commonly reported likely source of infection (48% of cases), followed by contact with livestock (21%), person-to-person contact (15%), food-borne transmission (8%), and contact with pets (8%) [90]. More recent expert elicitation generally concurs with this order [91].
General social and economic zoonotic risk factors have also been identified for human cryptosporidiosis. C. parvum was more common in areas with lower human population densities, where there are a higher ratio of the number of farms to human inhabitants, or a higher ratio of the number of private water supplies to human inhabitants and in areas with high ruminant livestock density [92]. In England and Wales, C. parvum was linked to living in an area with a high estimate of Cryptosporidium applied to land from manure [93].
Additionally, through the identification of the infecting species and subtypes, the variable transmission and epidemiology of cryptosporidiosis becomes clearer and interventions can be directly applied, whether these are broadly hygiene and sanitation measures on farms, in homes, and institutions, or in potable or recreational water and the food chain.
Although most C. parvum subtypes are zoonotic, a subspecies that is human-adapted has been proposed as C. parvum anthroponosum [94]. Geographical analysis indicates that the human-adapted species (C. hominis, some C. parvum subtypes) are relatively more prevalent in resource-poor countries, whereas zoonotic C. parvum dominates in North America, Europe, Australia, and parts of the Middle East [95].
Transmission varies seasonally; in most European countries cryptosporidiosis cases are mainly reported in late summer and autumn (August–October) [35], with some countries also having a smaller peak in spring. There is good evidence from outbreaks and molecular analytical epidemiology that the spring peak is mostly attributable to C. parvum and may be related to lambing and calving, while the summer/autumn peak is mainly C. hominis and is associated with swimming pools and foreign travel [96].
Alt-text: Box 2
Diagnostics and Epidemiology
The true incidence of human cryptosporidiosis is not known, although the population at most risk is clearly children under the age of five and the immunocompromised. Diagnosis requires laboratory confirmation, usually through testing a stool sample [33]. Stool samples will not necessarily be tested for Cryptosporidium unless the patient falls into a selected group (e.g., young children; HIV-AIDS; recent foreign travel) or a request is made. Traditionally, stained microscopy is used to observe oocysts, and infection rates among (presumably immunocompetent) diarrhoea patients of 6.1% and 2.1% in developing and developed areas, respectively, have been reported, contrasting with 24% and 14% among HIV positive diarrhoea patients [34]. Differences in the sensitivity, specificity, and the positive predictive value of the diagnostic tests can impact burden of disease estimates; in the Global Enteric Multicentre Study, an improved ELISA was used and revealed the significant contribution of Cryptosporidium to childhood morbidity and mortality [16].
Differences in access to health care, sample submission, and diagnostic practice contribute to the ascertainment of cryptosporidiosis and, along with variation in reporting requirements, make interpretation of surveillance data within and between countries difficult [35]. In 2016, there were 3.8 confirmed cases per 100 000 population reported in the EU/EEA by 21/32 countries [36]. One study in the UK that measured under-ascertainment found that for every case reported to national surveillance there were an estimated 8.2 undiagnosed cases in the community [37].
Diagnosis of Cryptosporidium infection in livestock is traditionally done by the direct examination of faecal samples using microscopy and modified Ziehl-Neelsen or auramine phenol staining. Immune chromatography lateral flow assays are used to give a rapid diagnostic and are widely used for point-of-care testing on farms. Molecular assays are essential to give further information on parasite species and genotype [38]. Different Cryptosporidium species can be detected using multiplex PCR assays [39] or by direct sequencing but this is not done as a routine diagnostic test. Molecular-based diagnostics are widely used in commercial labs, often using real time PCR (that is helpful to detect low concentrations of oocysts) and are increasingly used in human diagnostics.
Diagnostic epidemiology studies on a whole catchment scale are essential when considering risk-based water safety assessments [40]. Such studies are scarce but have shown links between livestock and wildlife C. parvum infection with water contamination. Human infection through contamination of the public water supply has resulted in outbreaks and hospitalisation of consumers. In one study in Scotland, where this had occurred, further investigation showed that livestock and wild red and roe deer were contributing to catchment parasite loading and water contamination [19]. Another catchment study in Cumbria, UK, concluded that the distribution of Cryptosporidium species in surface waters, livestock, and wildlife were also linked [40]. Gene sequencing and PCR-restriction fragment length polymorphism analysis showed the presence of the same C. parvum strain in water and faecal samples collected from neonatal calves on dairy farms in Parana, Brazil, highlighting the risk of zoonotic strains contaminating water supplies used for human and animal consumption [41]. A recent study in Australia showed the presence of zoonotic Cryptosporidium spp. in livestock and wild mammals in water catchments [20]. In addition, C. parvum has been detected in edible marine fish in European waters, indicating the extent of environmental contamination with this zoonotic pathogen [21].
Application of Genotyping Tools
Assignment to species level is useful in determining zoonotic potential of the parasite, but to determine transmission dynamics and source of infection, more discriminatory power is required [42]. Genotyping within C. parvum has mainly been based on a single locus through sequencing of a polymorphic region of the Gp60 gene, which has a putative role in virulence. Whilst this is a useful library typing tool, it does not provide adequate differentiation for local or regional epidemiological questions, such as outbreak investigations. A literature review of multilocus genotyping schemes for C. parvum showed that these are usually based on single nucleotide polymorphisms or on micro/minisatellite regions [43]. In multilocus fragment typing (MLFT), repeat units within the genome (micro/minisatellites) are amplified and either sequenced or analysed for size, reflecting the length polymorphisms due to variable numbers of repeat motifs, which forms the basis for genotyping. Alleles at different loci, or markers, are combined to give a multilocus genotype.
Robinson and Chalmers [43] favour fragment sizing for surveillance and outbreak investigations, due to the potential to provide rapid, cost-effective results that are discriminatory enough to address source attribution. Work in bovine-derived C. parvum assessed an MLFT typing tool and demonstrated good discriminatory ability compared with Gp60 sequencing alone [44]. It would be desirable for a One Health approach to develop an integrated genotyping approach that could be used by both veterinary and public health researchers, as advocated in a recent EU Cost actionii. The markers that MLFT is based on are single copy genes and as such this approach has limitations for environmental samples where there is likely to be a low quantity of C. parvum DNA present. This presents a problem for whole-catchment approaches, where MLFT could be a useful tool in determining C. parvum transmission dynamics and innovative application of amplification technologies could be used to overcome such barriers.
Interventions and New Opportunities to Tackle Cryptosporidiosis
There are currently few options to help prevent and treat cryptosporidiosis in humans with no vaccines and only one FDA approved drug, nitazoxanide, available. Nitazoxanide has efficacy in immunocompetent people but is not effective in severely immunocompromised individuals [45]. Education about routes of transmission and hand washing are practical preventative environmental public health measures to help reduce disease incidence [46].
Disease Prevention and Control in Livestock
Due to the lack of vaccines for cryptosporidiosis in livestock, prevention and control of disease currently involves supporting the resilience of calves in the first few weeks of life. This will include making sure they receive adequate quantities of good quality colostrum in the first few hours of life [47] and housing in clean, dry, and warm pens with raised feeding and water troughs to minimise exposure to Cryptosporidium parasites in the environment. Deep and clean straw bedding will also help minimise contact with contaminated faeces and regular cleaning out of calf pens along with steam cleaning, as oocysts are inactivated at temperatures above 60°C [48]. Disinfection with recommended products can help to reduce build-up of contaminated faeces on the farm, where disinfectants containing hydrogen peroxide are the most effective [22]. A recent study has also suggested that disinfection of calf pens with hydrated lime may help to reduce onset and severity of cryptosporidiosis in calves [49]. It is advisable to house calves in similar age groups, as the younger calves are more susceptible to disease and may be vulnerable to infection if they are moved into an environment contaminated by older calves.
Calves affected by cryptosporidiosis should be housed separately until at least 1 week after diarrhoea stops, as the animal may still be shedding oocysts. Calves can be given supportive rehydration therapy and kept warm, clean, and dry. There are currently two licenced treatment products for use in calves; one is halofuginone lactate [50], which is used as a preventative treatment and is given within 24 hours of the onset of clinical signs and continued for 7 days. The drug acts to reduce shedding of the parasite and to reduce the severity and duration of diarrhoea, although it can be toxic in dehydrated animals so it needs to be used appropriately. Paromomycin [51] is recently available in UK under veterinary prescription and can be applied for 7 days, following a diagnosis of Cryptosporidium infection, and has been shown to reduce oocyst shedding and diarrhoea, although there is also a risk of toxicity with this drug.
Treatment of Manure to Reduce Viability of Cryptosporidium
A recent study used spatially explicit process-based modelling to estimate the global Cryptosporidium loads in livestock manure and estimated this to be in the region of 3.2 × 1023 oocysts per year, with cattle being the predominant source [10]. As manure is applied to land, oocysts can be transported via run-off into surface waters and may be a source of infection to both animals and people. Asia has the highest oocyst load from livestock manure, followed by Africa, South America, and Europe [10].
The effective management of manure and slurry on farms to reduce viability of Cryptosporidium oocysts will have an impact on disease risk in the wider environment and in particular for water catchments [52]. Such on-farm practices include the proper composting of manure, as heat (>60°C) will inactivate the oocysts; slurry storage, as ammonia and low pH will help to inactivate oocysts; and treatment with mesophilic and thermophilic anaerobic digestion to significantly reduce oocyst viability [10]. Fencing of livestock away from streams and water courses and use of vegetated and riparian buffer strips can help to slow down the transfer of Cryptosporidium oocysts from livestock faecal matter into water courses [52].
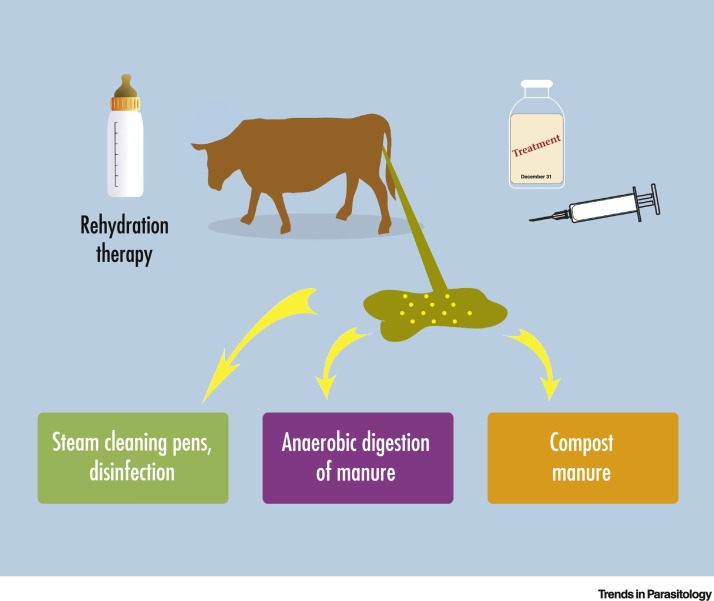
Applying measures to prevent and control cryptosporidiosis in livestock will have significant benefits for livestock health and welfare and will increase the efficiency of production, bringing economic benefits to livestock farmers in many regions of the world. In addition, applying methods on farm to minimise the environmental contamination with faeces containing infective Cryptosporidium oocysts will also help to minimise risk to other animals and to people through protection of the environment and water catchments (Figure 2 ).
Figure 2.
Interventions to Reduce the Impact of Cryptosporidiosis in Cattle and Environmental Contamination.
Supportive and therapeutic treatments can reduce disease and shedding of oocysts in neonatal calves. Cleaning farm buildings and treating infected manure can reduce contamination of the environment.
Advances in Drug Discovery
There has been considerable recent investment in Cryptosporidium drug discovery and there are now several classes of compounds of lead, late lead, and preclinical status. These include bumped kinase inhibitors that target Cryptosporidium calcium-dependant protein kinase 1 (CDPK1) and are effective in several in vivo models of cryptosporidiosis, including gnotobiotic piglets and calf models, which showed a reduction in oocyst shedding and clinical signs [53,54]. Compounds that target tRNA synthetases, enzymes that charge tRNAs with their cognate amino acid for use in protein synthesis, have also proven effective against Cryptosporidium [55]. Several groups carried out screens of compounds with activity on related parasites and identified new anti-cryptosporidial compounds [56]. These compounds progressed from in vitro screens, to small animal models, and to neonatal calf studies. Because C. parvum infects both humans and cattle, new medicines for treating human patients may also be effective for controlling livestock cryptosporidiosis.
A major question in drug discovery is a requirement for gastrointestinal versus systemic exposure. Because Cryptosporidium are usually located in the brush border epithelium of the gastrointestinal tract, systemic exposure may not be required to cure the infection [57]. Current drug efforts are working to better understand the PK/PD distribution required to cure cryptosporidiosis. Modelling this for both human and cattle will be important in developing effective treatments.
Vaccines for Livestock?
There are currently no vaccines available to prevent cryptosporidiosis in farm livestock and as the disease mainly occurs in very young calves it might be difficult to generate protective immunity to the parasite quickly enough through active vaccination. An alternative strategy such as that currently used to vaccinate dams against other pathogens causing diarrhoeal disease (e.g., rotavirus, coronavirus, and Escherichia coli) may also work for Cryptosporidium by generating specific immunity in the dam, which is passed to the calf through the colostrum, thus helping the calf to resist the disease during these crucial early weeks of life [58]. Promising results using a passive immunity approach have been achieved through immunisation of dams with recombinant P23 and CP15 proteins to generate hyperimmune colostrum containing specific antibodies and other immune factors that can help protect neonatal calves against disease caused by Cryptosporidium infection [59].
The protective immune mechanisms in calves against Cryptosporidium parasites are not well understood and are likely to involve both humoral and cell-mediated immune responses [22,58]. Antibody responses against several dominant Cryptosporidium antigens, such as gp15, cp15, and cp23 that are thought to play a role in parasite attachment and invasion of host cells, may be important as vaccine candidates [60].
Both animals and humans develop protective immunity after exposure to and recovery from Cryptosporidium infections. Understanding what drives immunity will aid development of much needed vaccines. Recent work by Sateriale et al. [61] developed a new mouse model to dissect immunological responses to Cryptosporidium. They isolated a mouse-specific Cryptosporidium tyzzeri strain that infects the small intestine of immunologically competent mice, closely mimicking human infection. This model is genetically tractable for both the host and parasite, and promises to provide mechanistic understanding of protective immunological responses that can drive rational vaccine design.
An effective vaccine that would minimise disease in livestock and reduce shedding of oocysts in faeces would have a significant impact on livestock health and welfare and also would reduce the contamination of the farm environment and wider catchment areas with infective oocysts [58].
Protecting Water Catchments from Contaminated Livestock Faeces
A large outbreak of cryptosporidiosis that occurred in Scotland in 2000 was associated with drinking water supplied from Loch Katrine and the catchment area surrounding the loch had a large sheep and cattle population. Livestock were removed from the area by the water authorities as a precaution and a rapid gravity filtration system was introduced, which successfully reduced the Cryptosporidium oocysts from the finished water [62]. An important example of health authorities working together with farmers to help protect water catchments is one of the largest water supply systems in the USA, providing over 9 million people in the New York City area with 4.5 billion litres daily from a single source of unfiltered water. Farmers in the Catskills area had intensified livestock production, leading to increased pollution from faeces and manure getting into the water shed [63]. The New York City authorities worked with the farmers to set up a whole farm planning system to customise pollution control on each farm to maximise effectiveness and minimise costiii. By continuing to work effectively with the farmers, the New York City authorities were able to protect the environment in the water catchment area and avoid the multi-billion dollar cost of filtering the water supply, illustrating very well how a managed environment will produce good quality water.
A One Health Approach
Adopting a One Health approach offers several opportunities to help tackle cryptosporidiosis, in particular, disease caused by C. parvum and other zoonotic species that affect both animals and people. The impact of the disease is far reaching and recent studies have highlighted the long-term consequences of clinical disease in both humans and livestock animals (Box 1). The true burden of disease in both public [64] and veterinary health is unknown, as many incidences of infection and disease go undiagnosed or unreported. With livestock farming moving towards increasingly efficient and sustainable production to minimise impact on the environment, there is much to be gained from tackling cryptosporidiosis. As livestock are also a major source of infectious and long-lived oocysts that can infect other animals, wildlife, and people [22], measures to reduce oocyst shedding and methods of storing and treating farm manure [10] to prevent oocyst contamination of the wider environment are important mitigation strategies.
New Diagnostic Targets
Rapid point-of-care detection is needed to enable quick and effective intervention and treatment. In addition, an integrated genotyping approach, which could be used by both veterinary and public health researchers [44], would help in disease surveillance identifying sources of infection and routes of transmission, examine evolving epidemiology patterns, and aid in the analysis of parasite virulence. Sensitive detection and genotyping methods are required when looking at environmental samples that may contain very few oocysts and a test to confirm oocyst viability would be highly desirable for analysis of infection risk. A recent EU research project involving research organisations and industrial partners across Europe looked at emerging technologies for early detection of waterborne pathogens, including Cryptosporidium, to enable improved water safety and public health (Aquavalens, 2017iv ). Collaboration and cooperation between countries on surveillance strategies and techniques would be of great benefit to enable sharing of information and disease epidemiology data.
Protective Immunity and Prospects for Vaccination
Developing vaccines for use in livestock to prevent disease and reduce oocyst shedding would be a key priority for a One Health approach. This intervention would not only benefit animal health and welfare, it would also have additional benefits to reduce parasite contamination of the environment through reduced oocyst shedding and would therefore also benefit public health [58]. The most feasible approach would be to target cattle where the parasite is highly prevalent in the first instance, as they are the most significant contributors to contaminated manure globally [10].
There are currently no human vaccines available and this would be a very desirable target, particularly for high-risk groups such as young children in developing countries and immuno-comprised individuals, where infection with the parasite can have very severe clinical consequences. The feasibility of a vaccination approach has been supported by studies showing the development of a degree of resistance to the parasite following repeated exposure [65]. In addition, sero-epidemiology studies in Scotland looked at the prevalence of antibodies against Cryptosporidium sporozoite antigens in populations before and after the introduction of enhanced filtration to remove Cryptosporidium oocysts for the water supply and found a significant reduction in seroprevalence, indicating a potential reduction in immune resilience to the parasite [66]. Several promising candidate antigens have been identified, along with knowledge of likely protective immune responses and different vaccine delivery strategies to target mucosal immune responses, recently reviewed [60]. Of interest is the level of cross-protective immunity between C. hominis and C. parvum, as this will be an important consideration for vaccine design. A serological study was conducted in children in Bangladesh and found that although most children were infected with C. hominis, they also had cross-reactive antibodies to the C. parvum antigen Cp23 [67]. A further study looked at cross-protective immunity between the two species using an in vivo gnotobiotic pig model and showed than prior infection with C. hominis protected against a further C. hominis challenge and gave partial protection against a C. parvum challenge [68], showing that there is a degree of cross-immunity between the two Cryptosporidium species.
The gnotobiotic piglet model shows similar clinical signs of diarrhoea as seen in human infection following challenge with C. hominis and is therefore a relevant clinical model with which to test out new therapies and vaccines [69]. Neonatal cattle [27] and sheep [70] are also relevant clinical models for C. parvum in particular, to improve understanding of host–parasite interactions, to test out novel therapeutic agents, and to provide sources of Cryptosporidium oocysts for research and diagnostic purposes.
In Vitro Systems and Genetic Manipulation
Recently, there has been some exciting progress on the in vitro culture of Cryptosporidium parasites using hollow-fibre technology [71] and the demonstration of the complete life cycle of C. parvum when cultured in human and mouse intestinal organoids [72,73]. Heo et al. microinjected Cryptosporidium into the lumen of three-dimensional human-derived organoids [72], while Wilke et al. infected monolayers of mouse-derived organoids cultured at an air–liquid interface in transwells [73]. Both culture systems support long-term growth (>20 days) and produce viable, infectious oocysts. Additionally, Wilke et al. generated transgenic parasites in vitro in organoid co-culture and used these parasites to perform a genetic cross [73]. These advances will help to reduce the numbers of animals used in research and for production of oocysts.
Important advances have also been made in the genetic engineering of Cryptosporidium parasites [74]. Use of CRISPR/Cas9 in Cryptosporidium has enabled the generation of reporter strains to advance drug discovery. Manipulation of genes of interest allows us to interrogate the function of genes directly in the parasite for the first time. Further tool development, including protein tagging and conditional knockdown, will improve our understanding of the parasite biology and host pathogen interactions [74]. These new technologies are very exciting, as both host and parasite are accessible and tractable for fundamental research, and provide more relevant models for drug and vaccine discovery. Similarly, other hosts and species of Cryptosporidium may be amenable to culture and laboratory exploration in the future. This would be especially impactful for C. hominis, which lacks an in vitro system and relies on a resource intensive gnotobiotic piglet animal model. Along with increasing awareness and understanding of the impact of cryptosporidiosis in people and animals, there is a renewed impetus to tackle cryptosporidiosis. Understanding key transmission routes and main sources of infection can also help to develop mitigating strategies to prevent direct infection and to protect against indirect transmission through water, food, and the environment.
Concluding Remarks
Cryptosporidium really lends itself to a One Health approach and with the recent technological advances and increased awareness of the long-term impacts for people, animals, and our natural environment, the opportunity exists to work collaboratively across the different sectors to tackle this global disease (see Outstanding Questions). A key target would be the development of a vaccine that could be used to help prevent disease and oocyst shedding in neonatal calves, as this would not only improve the health and welfare of the animals, it would also reduce environmental contamination as these animals are a main reservoir of C. parvum oocysts. Due to the very young age of the calves when they first encounter the parasite, a vaccination approach involving passive transfer of immunity in colostrum is most likely to be successful. Applying an integrated genotyping approach that may be used in both veterinary and public health would aid in source tracking and surveillance and inform epidemiology studies. Treatment of livestock and human faecal waste to reduce viability of Cryptosporidium oocysts would help to minimise contamination of the environment with infectious parasites and protect human and animal health.
Outstanding Questions.
Would a vaccine relying on passive transfer of immunity be effective in neonatal calves to reduce incidence of cryptosporidiosis and shedding of oocysts into the environment?
Would an integrated genotyping approach in veterinary and public health help with source tracking, epidemiology, and surveillance?
What treatments would be effective to reduce viability of Cryptosporidium oocysts in manure?
Will some of the new anti-cryptosporidial drug compounds identified be effective in both people and livestock?
Will the application of CRISPR/Cas9 in Cryptosporidium enable the manipulation of genes of interest to facilitate the direct interrogation of the function of specific genes?
Alt-text: Outstanding Questions
There have been some very exciting new advances in research into Cryptosporidium parasites, involving the development and application of relevant clinical in vivo models of disease, new in vitro systems, and the ability to conduct genetic manipulation studies. These will greatly advance our knowledge of host–parasite interactions and help facilitate the testing of new therapeutic compounds and vaccine targets. By combining our knowledge and expertise from the veterinary, public, and environmental health areas, along with the new advances in biology, genetics, and host–pathogen interactions, we have an exciting opportunity to tackle cryptosporidiosis using a One Health approach.
Acknowledgements
We would like to thank Hazel Simm at Moredun Research Institute for graphic design. E.A.I. and B.W. were funded by the Scottish Government Rural and Environment Science and Analytical Services and M.C.P. was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society.
Glossary
- Calcium-dependant protein kinase 1 (CDPK1)
the calcium-dependant protein kinase family are expanded in apicomplexan parasites and are plant-like and do not occur in animal cells, which makes them interesting targets for drug development.
- CRISPR/Cas9
a technology that enables genome editing by removing, adding, or altering sections of the DNA sequence.
- Disability adjusted life years
a measure of overall disease burden, expressed as the number of years lost to ill health, disability, or early death.
- Finished water
water that has passed through a water treatment plant.
- Gnotobiotic
an animal derived by aseptic caesarean section and reared in isolator conditions.
- Gp60
a 60-kDa glycoprotein gene of Cryptosporidium used as a target for molecular typing studies.
- Hyperimmune colostrum
produced by immunisation of cows during pregnancy, resulting in high levels of specific antibodies in the colostrum, the first milk produced after birth.
- Mesophilic
an organism that grows best in moderate temperature (20°C–45°C).
- Multilocus fragment typing (MLFT)
multilocus fragment typing uses tandem repeat units within the genome, often called micro- or minisatellites, which are amplified by PCR and the amplicons visualised by gel electrophoresis.
- Oocyst
environmentally resistant transmissive life cycle stage of Cryptosporidium spp.
- Organoids
self-organised three-dimensional tissue culture organ models derived from stem cells that show realistic micro-anatomy.
- PK/PD
pharmacokinetics (PK) describes the drug concentration in body fluids and pharmacodynamics (PD) describes the observed effect of the drug.
- Potable
water that is safe to drink or to use in food preparation.
- Rapid gravity filtration system
type of filter used in water purification.
- Thermophilic
an organism that grows best in high temperatures (41–122°C).
- Zoonotic
pertaining to a zoonosis, a disease that can be transmitted from animals to people.
Resources
iwww.gov.uk/government/collections/animal-disease-surveillance-reportsiiwww.euro.fbp.orgiiihttps://hdl.handle.net/10919/66907ivhttp://aquavalens.org/home1References
- 1.Chalmers R.M. Cryptosporidium. In: Percival S.L., editor. Microbiology of Waterborne Diseases. 2nd edn. Academic Press; 2014. pp. 287–326. [Google Scholar]
- 2.Feng Y. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Messner M.J., Berger P. Cryptosporidium infection risk: results of a new dose-response modelling risk. Risk Anal. 2016;36:1969–1982. doi: 10.1111/risa.12541. [DOI] [PubMed] [Google Scholar]
- 4.Hunter P.R., Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002;15:145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein P. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet. Parasitol. 2008;152:53–59. doi: 10.1016/j.vetpar.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxby D. Shedding of oocysts by immunocompetent individuals with cryptosporidiosis. Epidemiol. Infect. 1985;95:703–709. doi: 10.1017/s0022172400060812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nydam D.V. Number of Cryptosporidium parvum oocysts or Giardia spp cysts shed by dairy calves after natural infection. Am. J. Vet. Res. 2001;62:1612–1615. doi: 10.2460/ajvr.2001.62.1612. [DOI] [PubMed] [Google Scholar]
- 8.Koutsoumanis K. Pubic health risks associated with food-borne parasites. EFSA J. 2018;16:5495. doi: 10.2903/j.efsa.2018.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushkin G.G. Evidence for structural role for acid fast lipids in oocyst walls of Cryptosporidium, Toxoplasma and Eimeria. mBio. 2013;4 doi: 10.1128/mBio.00387-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeulen L.C. Global Cryptosporidium loads from livestock manure. Environ. Sci. Technol. 2017;51:8663–8671. doi: 10.1021/acs.est.7b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan U. Cryptosporidium in humans and animals – a One Health approach to prophylaxis. Parasite Immunol. 2016;38:535–547. doi: 10.1111/pim.12350. [DOI] [PubMed] [Google Scholar]
- 12.Sponseller J.K. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clin. Microbiol. Rev. 2014;27:575–586. doi: 10.1128/CMR.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farthing M.J.G. Clinical aspects of human cryptosporidiosis. In: Petry F., editor. Cryptosporidiosis and Microsporidiosis. Karger; 2000. pp. 50–74. [Google Scholar]
- 14.Savioli L. Giardia and Cryptosporidium join the “neglected diseases initiative”. Trends Parasitol. 2006;22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kotloff K.L. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study GEMS): a prospective, case control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 16.Platts-Mills J.A. Pathogen-specific burdens of community diarrhea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob. Health. 2015;9:564–575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson L.J. Cryptosporidiosis in farmed animals. In: Caccio S., Widmer G., editors. Cryptosporidium: Parasite and Disease. Springer; 2014. pp. 149–235. [Google Scholar]
- 18.Puleston R.L. The first recorded outbreak of cryptosporidiosis due to Cryptosporidium cuniculus (formerly rabbit genotype), following a water quality incident. J. Water Health. 2014;12:41–50. doi: 10.2166/wh.2013.097. [DOI] [PubMed] [Google Scholar]
- 19.Wells E.A. Prevalence, species identification and genotyping Cryptosporidium from livestock and deer in a catchment in the Cairngorms with a history of a contaminated public water supply. Parasit. Vectors. 2015;8:66. doi: 10.1186/s13071-015-0684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zahedi A. Cryptosporidium species and subtypes in animals inhabiting drinking water catchments in three states across Australia. Water Res. 2018;134:327–340. doi: 10.1016/j.watres.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Certad G. Prevalence, molecular identification and risk factors for Cryptosporidium infection in edible marine fish: a survey across sea areas surrounding France. Front. Microbiol. 2019;10:1037. doi: 10.3389/fmicb.2019.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson S. Bovine cryptosporidiosis: impact, host-parasite interaction and control strategies. Vet. Res. 2017;48:42. doi: 10.1186/s13567-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright S.E., Coop R.L. Cryptosporidiosis and coccidiosis. In: Aitken I.D., editor. Diseases of Sheep. 4th edn. Blackwell; 2007. pp. 179–185. [Google Scholar]
- 24.Peacock C. Goats-a pathway out of poverty. Small Rum. Res. 2005;60:179–186. [Google Scholar]
- 25.Meng Q. Molecular characterisation of Cryptosporidium spp and Giardia duodenalis from yaks in the central western region of China. BMC Microbiol. 2015;15:108. doi: 10.1186/s12866-015-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brook E. Prevalence and risk factors for Cryptosporidium spp infection in young calves. Vet. Parasitol. 2008;152:46–52. doi: 10.1016/j.vetpar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Zambrinski J.A. Cryptosporidium parvum: determination of ID50 and the dose-response relationship in experimentally challenged dairy calves. Vet. Parasitol. 2013;197:104–112. doi: 10.1016/j.vetpar.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gormley F.J. Zoonotic cryptosporidiosis from petting farms, England and Wales, 1992-2009. Emerg. Infect. Dis. 2011;17:151–152. doi: 10.3201/eid1701.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmers R.M. Waterborne cryptosporidiosis outbreaks. Ann. Ist. Super. Sanita. 2012;48:429–446. doi: 10.4415/ANN_12_04_10. [DOI] [PubMed] [Google Scholar]
- 30.Karanis P. Cryptosporidium: waterborne and foodborne transmission and worldwide outbreaks. In: Kallel A., editor. Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions. Springer; 2018. pp. 41–44. [Google Scholar]
- 31.Brankston G. Assessing the impact of environmental exposures and Cryptosporidium infection in cattle on human incidence of cryptosporidiosis in Southwestern Ontario, Canada. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterk A. Effect of climate change on runoff of Campylobacter and Cryptosporidium from land to surface water. Water Res. 2016;95:90–102. doi: 10.1016/j.watres.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Caccio S.M., Pozio E. Advances in the epidemiology, diagnosis and treatment of cryptosporidiosis. Exp. Rev. Anti Infect. Ther. 2006;4:429–443. doi: 10.1586/14787210.4.3.429. [DOI] [PubMed] [Google Scholar]
- 34.Adal K.A. Cryptosporidium and related species. In: Blaser M.J., editor. Infections of the Gastrointestinal Tract. Raven Press; 1995. pp. 1107–1128. [Google Scholar]
- 35.Caccio S.M., Chalmers R.M. Human cryptosporidiosis in Europe. Clin. Microbiol. Infect. 2016;22:471–480. doi: 10.1016/j.cmi.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 36.European Centre for Disease Prevention and Control . ECDC. Annual Epidemiological Report for 2016. ECDC; 2016. Cryptosporidiosis. [Google Scholar]
- 37.Tam C.C. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalmers R.M., Katzer F. Looking for Cryptosporidium: the application of advances in detection and diagnosis. Trends Parasitol. 2013;29:237–251. doi: 10.1016/j.pt.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson S. A multiplex PCR test to identify four common cattle-adapted Cryptosporidium species. Parasitol. Open. 2016;2:1–9. [Google Scholar]
- 40.Robinson G. A whole water catchment approach to investigating the origin and distribution of Cryptosporidium species. J. Appl. Microbiol. 2011;111:717–730. doi: 10.1111/j.1365-2672.2011.05068.x. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos Toledo R. Cryptosporidium spp. and Giardia spp. in feces and water and the associated exposure factors on dairy farms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chalmers R.M. Detection of Cryptosporidium species and sources of contamination with Cryptosporidium hominis during a waterborne outbreak in North West Wales. J. Water Health. 2010;8:311–325. doi: 10.2166/wh.2009.185. [DOI] [PubMed] [Google Scholar]
- 43.Robinson G., Chalmers R.M. Assessment of polymorphic genetic markers for multilocus typing of Cryptosporidium parvum and Cryptosporidium hominis. Exp. Parasitol. 2012;132:200–215. doi: 10.1016/j.exppara.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Hotchkiss E.J. Development of a framework for genotyping bovine derived Cryptosporidium parvum, using a multilocus fragment typing tool. Parasit. Vectors. 2015;8:500. doi: 10.1186/s13071-015-1107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparks H. Treatment of Cryptosporidium: what we know, gaps and the way forward. Curr. Trop. Med. Rep. 2015;2:181–187. doi: 10.1007/s40475-015-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalmers R.M. An evaluation of health protection practices for the investigation and management of Cryptosporidium in England and Wales. J. Public Health. 2018;40:114–120. doi: 10.1093/pubmed/fdw143. [DOI] [PubMed] [Google Scholar]
- 47.Meganck V. Advances in prevention and therapy of neonatal dairy calf diarrhoea: a systematical review with emphasis on colostrum management and fluid therapy. Acta Vet. Scand. 2014;56:75. doi: 10.1186/s13028-014-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson L.J. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 1992;58:3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjorkman C. Disinfection with hydrated lime may help manage cryptosporidiosis in calves. Vet. Parasitol. 2018;264:58–63. doi: 10.1016/j.vetpar.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trotz-Williams I.A. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves. Vet. Rec. 2011;168:509. doi: 10.1136/vr.d1492. [DOI] [PubMed] [Google Scholar]
- 51.Grinberg A. Controlling the onset of natural cryptosporidiosis in calves with paromomycin sulphate. Vet. Rec. 2002;151:606–608. doi: 10.1136/vr.151.20.606. [DOI] [PubMed] [Google Scholar]
- 52.Kay D. Effectiveness of best management practices for attenuating the transport of livestock derived pathogens within catchments. In: Dufour A., Bartram J., editors. Animal Waste Water Quality and Human Health. IWA Publishing; 2012. pp. 195–255. [Google Scholar]
- 53.Lendner M. A novel CDPK1 inhibitor--a potential treatment for cryptosporidiosis in calves? Parasit. Res. 2015;114:335–336. doi: 10.1007/s00436-014-4228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S. Therapeutic efficacy of bumped kinase inhibitor 1369 in a pig model of acute diarrhoea caused by Cryptosporidium hominis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00147-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckner F.S. Optimisation of methionyl tRNA synthetase inhibitors for treatment of Cryptosporidium infection. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02061-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lunde C.S. Identification of a potent benzoxaborale drug candidate for treating cryptosporidiosis. Nat. Comm. 2019;10:2816. doi: 10.1038/s41467-019-10687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold S.L.M. Necessity of bumped kinase inhibitor gastrointestinal exposure in treating Cryptosporidium infection. J. Infect. Dis. 2017;216:55–63. doi: 10.1093/infdis/jix247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Innes E.A. Developing vaccines to control protozoan parasites in ruminants: dead or alive? Vet. Parasitol. 2011;180:155–163. doi: 10.1016/j.vetpar.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 59.Jenkins M.C. Present and future control of cryptosporidiosis in humans and animals. Exp. Rev. Vaccines. 2004;3:669–671. doi: 10.1586/14760584.3.6.669. [DOI] [PubMed] [Google Scholar]
- 60.Mead J.R. Prospects for immunotherapy and vaccines against Cryptosporidium. Hum. Vaccine Immunother. 2014;10:1505–1513. doi: 10.4161/hv.28485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sateriale A. A genetically tractable, natural mouse model of cryptosporidiosis offers insights into host protective immunity. Cell Host Microbe. 2019;26:135–146. doi: 10.1016/j.chom.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollock K.G.J. Reduction in cryptosporidiosis associated with introduction of enhanced filtration of drinking water at Loch Katrine, Scotland. Epidemiol. Infect. 2014;142:56–62. doi: 10.1017/S0950268813000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Starkey S.R. Cryptosporidium and dairy cattle in the Catskill Delaware watershed: a quantitative risk assessment. Risk Anal. 2007;27:1469–1485. doi: 10.1111/j.1539-6924.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 64.Khalil I.A. Morbidity, mortality and long term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analysis study. Lancet Glob. Health. 2018;6:758–768. doi: 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okhuysen P.C. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 1998;66:441–443. doi: 10.1128/iai.66.2.441-443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramsey C.M. Effects of drinking water filtration on Cryptosporidium ser-epidemiology, Scotland. Emerg. Infect. Dis. 2014;20:70–77. doi: 10.3201/eid2001.120386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borad A.J. Systematic antibody responses to the immunodominant p23 antigen and p23 polymorphisms in children with cryptosporidiosis in Bangladesh. Am. J. Trop. Med. Hyg. 2012;86:214–222. doi: 10.4269/ajtmh.2012.11-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheuran A. Infection with Cryptosporidium hominis provides incomplete protection of the host against Cryptosporidium parvum. J. Infect. Dis. 2012;205:1019–1023. doi: 10.1093/infdis/jir874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S. The piglet acute diarrhoea model for evaluating efficacy of treatment and control of cryptosporidiosis. Hum. Vaccin. Immunother. 2019;15:1445–1452. doi: 10.1080/21645515.2018.1498436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blewett D.A. Infective dose size studies on Cryptosporidium parvum using gnotobiotic lambs. Water Sci. Technol. 1993;27:61–64. [Google Scholar]
- 71.Morada M. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int. J. Parasitol. 2016;46:21–29. doi: 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Heo I. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018;3:814–823. doi: 10.1038/s41564-018-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilke G. A stem cell derived platform enables complete Cryptosporidium development in vitro and genetic tractability. Cell Host Microbe. 2019;26:123–134. doi: 10.1016/j.chom.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawlowic M.C. Generating and maintaining transgenic Cryptosporidium parvum parasites. Curr. Protoc. Microbiol. 2017;46:20B.2.1–20B.2.32. doi: 10.1002/cpmc.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R.J. Widespread occurrence of Cryptosporidium infections in patients with HIV/AIDS: epidemiology, clinical feature, diagnosis and therapy. Acta Trop. 2018;187:257–263. doi: 10.1016/j.actatropica.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 76.Mekonnen Y. A review article on cryptosporidiosist. Acta Parasit. Glob. 2016;7:94–104. [Google Scholar]
- 77.Ryan U. Foodborne cryptosporidiosis. Int. J. Parasitol. 2018;48:1–12. doi: 10.1016/j.ijpara.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Insulander M. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol. Infect. 2012;141:1009–1020. doi: 10.1017/S0950268812001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stiff R.E. Long term health effects after resolution of acute Cryptosporidium parvum infection: a 1 year follow-up of outbreak associated cases. J. Med. Microbiol. 2017;66:1607–1611. doi: 10.1099/jmm.0.000609. [DOI] [PubMed] [Google Scholar]
- 80.Osman M. High association of Cryptosporidium spp. infection with colon adenocarcinoma in Lebanese patients. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olsen M.E. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 2004;20:185–191. doi: 10.1016/j.pt.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 82.Bueno da Silva A. Occurrence of Cryptosporidium spp. and its association with ponderal development and diarrhoea episodes in nellore mixed breed cattle. Acta Vet. Bras. 2019;13:24–29. [Google Scholar]
- 83.Jacobsen C. Greater intensity and frequency of Cryptosporidium and Giardia oocyst shedding beyond the neonatal period is associated with reduction in growth, carcase weight and dressing efficiency in sheep. Vet. Parasitol. 2016;228:42–51. doi: 10.1016/j.vetpar.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Corso P.S. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 2003;9:426–431. doi: 10.3201/eid0904.020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ridderstedt F. Sick leave due to diarrhoea caused by contamination of drinking water supply with Cryptosporidium hominis in Sweden: a retrospective study. J. Water Health. 2018;16:704–710. doi: 10.2166/wh.2017.311. [DOI] [PubMed] [Google Scholar]
- 86.Chyzheuskaya A. Economic assessment of waterborne outbreak of Cryptosporidium. Emerg. Infect. Dis. 2017;23:1650–1656. doi: 10.3201/eid2310.152037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nic Lochlainn L.M. Risk factors for sporadic cryptosporidiosis in the Netherlands: analysis of a 3 year population based case-control study coupled with genotyping, 2013-2016. J. Infect. Dis. 2019;219:1121–1129. doi: 10.1093/infdis/jiy634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunter P.R., Thompson R.C.A. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 2005;35:1181–1190. doi: 10.1016/j.ijpara.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Betancourt W.Q., Rose J.B. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 2004;126:219–234. doi: 10.1016/j.vetpar.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 90.Majowicz S.E. Descriptive analysis of endemic cryptosporidiosis cases reported in Ontario, 1996-1997. Can. J. Public Health. 2001;92:62–66. doi: 10.1007/BF03404847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hald T. World Health Organisation estimates for the relative contributions of food to the burden of disease due to foodborne hazards: a structured expert elicitation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pollock K.G.T. Spatial and temporal epidemiology of sporadic human cryptosporidiosis in Scotland. Zoonoses Public Health. 2010;57:487–492. doi: 10.1111/j.1863-2378.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 93.Lake I.R. Case-control study of environmental and social factors influencing cryptosporidiosis. Eur. J. Epidemiol. 2007;22:805–811. doi: 10.1007/s10654-007-9179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nadar J.L. Evolutionary genomics of anthroponosis in Cryptosporidium. Nat. Microbiol. 2019;4:826–836. doi: 10.1038/s41564-019-0377-x. [DOI] [PubMed] [Google Scholar]
- 95.King P. Anthroponotic transmission of Cryptosporidium parvum predominates in countries with poorer sanitation: a systematic review and meta-analysis. Parasit. Vectors. 2019;12:16. doi: 10.1186/s13071-018-3263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chalmers R.M. Long term Cryptosporidium typing reveals the aetiology and species specific epidemiology of human cryptosporidiosis in England and Wales 2000-2003. Euro. Surveill. 2009;14:19086. doi: 10.2807/ese.14.02.19086-en. [DOI] [PubMed] [Google Scholar]