Highlights
-
•
Guidelines are unclear which ICU patients should be tested for respiratory viruses.
-
•
Less than half of ICU patients with CAP or HAP are tested for viral pathogens.
-
•
In ICU patients who were tested, the prevalence of viral infections is 16–34%.
-
•
There is room for increasing routine testing of influenza virus in CAP and HAP.
Keywords: Respiratory tract infections, Virus diseases, Pneumonia, Routine diagnostic tests, Intensive care, Influenza
Abstract
Background
Clinical guidelines suggest testing for respiratory viruses during the influenza season, but are unclear which categories of patients on the intensive care unit (ICU) should be tested.
Objective
We described the clinical practice of diagnostic testing for respiratory virus infections in patients presenting to ICU with suspected community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).
Study design
Prospective observational study in consecutive CAP and HAP patients with an ICU stay of more than 24 h in two tertiary care hospitals in The Netherlands, from 2011 to December 2013. The proportion of patients receiving diagnostic testing with PCR for the presence of respiratory viruses in respiratory tract specimens was determined.
Results
In total, 1452 patients were included, of which 712 patients presented with CAP and 740 with HAP. In CAP, 282 of 712 (40%) were tested for respiratory viruses (190 of 417 (46%) during the influenza season). In HAP, 95 of 740 (13%) were tested (50 of 372 (13%) during the influenza season). Regardless of the season, virus diagnostic tests were ordered significantly more often in patients with comorbidities, and in those presenting with elevated CRP and leucopenia. In patients who were tested during the influenza season, the prevalence of influenza was 14% in patients with CAP and 10% in those with HAP. Influenza was absent during the summer in both groups.
Conclusions
Less than half of patients admitted to the ICU with suspected pneumonia were tested for the presence of viral pathogens, either in or outside the influenza season.
1. Background
Respiratory virus infections are important causes of community-acquired pneumonia (CAP) and respiratory failure both in children and adults [1]. Epidemiological studies show that the prevalence of viral respiratory tract infections can be as high as 41% in critically ill patients admitted to the intensive care unit (ICU) with a suspected CAP, and up to 34% in hospital-acquired pneumonia (HAP) [2], [3], [4], [5], [6]. Detection of such infections in critically ill patients may have important implications for infection control measures such as isolation and, in case of (suspected) influenza, rapid initiation of antiviral medication [7], [8]. These measures have an impact on ICU resource use, mandating clear assessment of patients at risk of a viral respiratory tract infection.
A recent large retrospective study indicated that influenza infections are underdiagnosed in the critically ill [9]. However, current international clinical guidelines on virus diagnostics are not clear about which patients should receive testing in the ICU setting. The Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) consensus guidelines, as well as the International Guidelines for Management of Severe Sepsis and the European Respiratory Society/European Society for Clinical Microbiology and Infectious Diseases guidelines, state that testing for at least influenza should be considered in adult patients admitted with suspected respiratory infection during local epidemics [10], [11], [12], but all are unclear if all patients admitted to the ICU with a suspected CAP should be tested for respiratory viruses. There are no recommendations for virus testing in patients admitted to the ICU due to HAP. The current practice of testing for the presence of viral pathogens in critically ill patients with a suspected CAP or HAP is unknown.
2. Objectives
The practice of diagnostic testing for viral respiratory infections was described in patients admitted to the ICU with clinical symptoms suggestive for CAP or HAP. Also, the prevalence of virus infections as detected during routine care was reported.
3. Study design
3.1. Study population
This study is part of a multi-center prospective cohort study, in which consecutive patients admitted to the mixed ICUs of two tertiary care hospitals in The Netherlands were enrolled between January 1st 2011 and December 31st 2013 (clinicaltrials.gov Identifier: NCT01905033). For this study patients with suspected CAP or HAP were included. Exclusion criteria were admissions with a length of ICU stay of < 24 h and transfers from another ICU. The Ethics Committees of both participating centers approved an opt-out method of consent (protocol number 10-056C).
3.2. Study definitions
A suspected respiratory tract infection at ICU admission was defined by empiric or targeted use of systemic antibiotics for a suspected CAP or HAP initiated by the attending physicians, between seven days prior to, and two days after ICU admission. The most likely source of each infection was determined by assessment of clinical data, radiological imaging and culture results as ordered by routine care, using strict diagnostic criteria. These criteria were based on CDC criteria as well as the International Sepsis Forum Consensus Conference definitions for CAP and HAP, which were adapted to the Dutch situation as described previously [13]. All observers were trained in these definitions before the start of the study, and an electronic algorithm was used that alarmed the researchers when there were inconsistencies with other recorded clinical variables.
Respiratory virus diagnostics were defined as in-house polymerase chain reaction (PCR) tests ordered as per discretion of attending physicians on samples from the respiratory tract, either simplex or multiplex [14], [15], for any of the following viruses: influenza virus A and B, respiratory syncytial virus, human metapneumovirus, parainfluenza virus 1–4, human rhinovirus, coronavirus, adenovirus, enterovirus, human bocavirus and parechovirus. Samples from the respiratory tract included nasopharyngeal swabs, throat swabs, bronchoalveolar lavage fluid, tracheobronchial aspirates, sputum and pleural puncture fluid.
3.3. Data analysis
The characteristics of patients who were tested were compared to patients not tested using non-parametric descriptive statistics. Numerical data was compared by Kruskal-Wallis tests, categorical data by Chi-square tests. Subgroup analyses were performed for the influenza season period and the period outside the season separately. Influenza season was defined between November 1st and April 30th. The prevalence of viral infections in those who were tested was reported. Treatment with oseltamivir was recorded, and the continuation, discontinuation or start of treatment was related to influenza test results. All analyses were performed using SAS 9.2 (Cary, NC, USA). P values less than 0.05 were considered to represent statistical differences.
4. Results
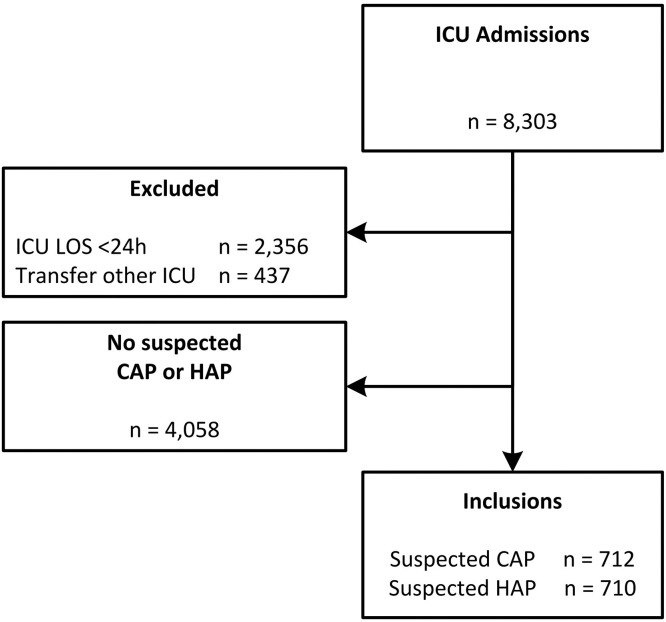
During the study period, a total of 8303 patients were admitted to the ICU, of whom 2356 were excluded because of an ICU stay of less than 24 h, 437 patients were transferred from another ICU, and 4058 did not have a suspected pneumonia. In total, 712 patients were included with a suspected CAP, and 740 patients with a suspected HAP (Fig. 1 ).
Fig. 1.
Flowchart of patient inclusion.
Abbreviations: CAP = community-acquired pneumonia; HAP = hospital-acquired pneumonia; ICU = intensive care unit; LOS = length of stay.
4.1. Proportion of patients tested for respiratory viruses
In the group of patients admitted to the ICU with a suspected CAP, 282 of 712 patients (40%) were tested for respiratory viruses; of patients admitted with a suspected HAP, 95 of 740 (13%) were tested (P = <0.001).
4.2. Characteristics of tested and non-tested patients with suspected CAP
Patients admitted with a suspected CAP who were tested, significantly more often had comorbidities (including chronic obstructive pulmonary disease (COPD), chronic renal insufficiency and immune deficiency) compared to those who were not tested (Table 1 ). Also, within 24 h of ICU admission, tested patients had a significantly lower acute physiology score (APS), while the Acute Physiology and Chronic Health Evaluation IV (APACHE IV) and Sequential Organ Failure Assessment (SOFA) score upon admission did not differ. Also, tested patients had higher C-reactive protein (CRP) levels, more often leucopenia, and more often acute renal failure compared to non-tested patients.
Table 1.
Baseline characteristics of patients admitted with a suspected CAP and HAP.
| Suspected CAP |
Suspected HAP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not tested (n = 430) | Tested (n = 282) | p value | Not tested (n = 645) | Tested (n = 95) | p value | |||||
| Age, median years (Q1, Q3) | 61.0 | (48.0, 71.0) | 62.0 | (48.0, 72.0) | 0.28 | 65.0 | (55.0, 73.0) | 59.0 | (50.0, 68.0) | <0.001 |
| Male, n (%) | 289 | (67%) | 153 | (54%) | <0.001 | 414 | (64%) | 62 | (65%) | 0.84 |
| BMI, median (Q1, Q3) | 24.8 | (22.5, 27.9) | 24.7 | (21.5, 29.0) | 0.73 | 24.8 | (22.2, 28.1) | 23.4 | (20.4, 27.5) | 0.007 |
| Medical admission, n (%) | 363 | (84%) | 279 | (99%) | <0.001 | 504 | (78%) | 89 | (94%) | <0.001 |
| Hospital days prior to ICU admission, median (Q1, Q3) | 0.0 | (0.0, 1.0) | 0.0 | (0.0, 1.0) | 0.88 | 8.0 | (4.0, 17.0) | 8.0 | (4.0, 18.0) | 0.70 |
| Comorbidities | ||||||||||
| COPD, n (%) | 62 | (14%) | 73 | (26%) | <0.001 | 101 | (16%) | 19 | (20%) | 0.28 |
| Congestive heart failure, n (%) | 24 | (6%) | 17 | (6%) | 0.80 | 43 | (7%) | 5 | (5%) | 0.60 |
| Diabetes mellitus, n (%) | 77 | (18%) | 59 | (21%) | 0.32 | 125 | (19%) | 17 | (18%) | 0.73 |
| Chronic renal insufficiency, n (%) | 41 | (10%) | 41 | (15%) | 0.041 | 60 | (9%) | 17 | (18%) | 0.010 |
| Malignancy, n (%) | 39 | (9%) | 24 | (9%) | 0.80 | 126 | (20%) | 15 | (16%) | 0.39 |
| Splenectomy, n (%) | 1 | (0%) | 0 | (0%) | 0.42 | 6 | (1%) | 0 | (0%) | 0.35 |
| Immune deficiency, n (%)a | 48 | (11%) | 84 | (30%) | <0.001 | 71 | (11%) | 42 | (44%) | <0.001 |
| APACHE IV Score, median (Q1, Q3) | 76.0 | (59.0, 104.0) | 75.5 | (59.0, 94.0) | 0.39 | 75.0 | (62.0, 93.0) | 87.0 | (70.0, 100.0) | <0.001 |
| Acute Physiology Score, median (Q1,Q3) | 64.0 | (47.5, 92.0) | 61.0 | (46.0, 77.0) | 0.023 | 63.0 | (50.0, 80.0) | 71.0 | (61.0, 84.0) | 0.005 |
| SOFA score on admission, median (Q1, Q3) | 7.0 | (5.0, 9.0) | 7.0 | (5.0, 9.0) | 0.50 | 7.0 | (5.0, 9.0) | 9.0 | (6.0, 10.0) | <0.001 |
| In the first 24 h of admission | ||||||||||
| Highest central body temperature, median °C (Q1,Q3) | 37.7 | (36.8, 38.5) | 37.8 | (37.0, 38.6) | 0.044 | 38.0 | (37.3, 38.7) | 38.0 | (37.4, 38.7) | 0.90 |
| First measured CRP, median mg/L (Q1, Q3) | 40.0 | (5.0, 154.0) | 135.0 | (42.0, 235.0) | <0.001 | 114.5 | (51.0, 213.0) | 160.0 | (92.0, 282.0) | 0.001 |
| Highest leucocytes, median cells·109/L (Q1, Q3) | 14.6 | (10.4, 19.0) | 11.7 | (7.9, 17.7) | <0.001 | 14.8 | (10.4, 19.9) | 12.5 | (2.1, 19.4) | 0.011 |
| Leucopenia, n (%)b | 43 | (10%) | 48 | (17%) | 0.006 | 44 | (7%) | 26 | (27%) | <0.001 |
| Use of vasoactive medication >1 h, n (%) | 262 | (61%) | 170 | (60%) | 0.86 | 400 | (62%) | 65 | (68%) | 0.23 |
| Acute renal failure, n (%) | 42 | (10%) | 42 | (15%) | 0.038 | 77 | (12%) | 16 | (17%) | 0.18 |
| Highest serum lactate, median mmol/L (Q1, Q3) | 2.8 | (1.7, 4.8) | 2.2 | (1.4, 3.2) | <0.001 | 1.9 | (1.3, 3.3) | 1.8 | (1.3, 2.9) | 0.74 |
Characteristics of patients tested for respiratory viruses were compared to patients who were not tested. Abbreviations: APACHE IV = Acute Physiology and Chronic Health Evaluation IV; BMI = body mass index; CAP = community-acquired pneumonia; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; HAP = hospital-acquired pneumonia; ICU = intensive care unit; SOFA = sequential organ failure assessment.
Immunodeficiency was defined as a history of solid organ or stem cell transplantation, infection with the human immunodeficiency virus, hematological malignancy, use of immunosuppressive medication (prednisone >0.1 mg/kg for >3 months, prednisone >75 mg/day for >1 week, or equivalent), chemotherapy/radiotherapy in the year before ICU admission, and any known humoral or cellular immune deficiency.
Leucopenia was defined as <4 × 109/L leucocytes.
4.3. Characteristics of tested and non-tested patients with suspected HAP
Patients admitted with a suspected HAP who were tested, more often had chronic renal insufficiency and immune deficiency compared to non-tested patients (Table 1). Tested patients also had significantly higher CRP levels, more often leucopenia, acute renal failure and higher severity of all illness scores compared to patients with a suspected HAP who were not tested.
4.4. Seasonal influence on viral diagnostic testing
In the influenza season, 190 of 417 (46%) patients admitted with a suspected CAP were tested for respiratory viruses; outside the season 92 of 295 (32%) were tested (P = <0.001). Of the patients admitted with a suspected HAP, 50 of 372 (13%) in and 45 of 368 (12%) outside the season were tested (P = 0.62). In CAP patients admitted within the influenza season, baseline characteristics were compared between tested and non-tested patients, showing similar differences in characteristics as in the total group of suspected CAP patients, with the exception of the APS, which did not differ between tested and non-tested patients (Supplementary Table S1).
4.5. Timing and results of diagnostic tests
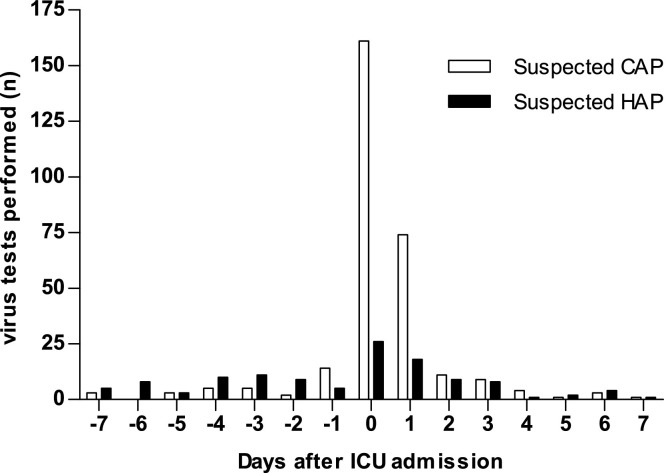
The prevalence of viral respiratory tract infections in tested patients differed between suspected CAP and HAP, and between patients admitted in and outside the influenza season (Table 2 ). In the influenza season, viruses were found in 65 of 190 (34%) of suspected CAP patients, and in 17 of 50 (34%) of suspected HAP patients. Outside the influenza season, 17 of 92 (19%) suspected CAP patients and 7 of 45 (16%) suspected HAP patients tested positive for at least 1 virus. In the influenza season, the most prevalent pathogen was influenza virus (26 of 190 (14%) in suspected CAP and 5 of 49 (10%) in suspected HAP). Outside the influenza season, influenza virus was not found. Ordering of virus tests was mostly performed on the day of ICU admission, in both suspected CAP and HAP cases (Fig. 2 ). In all patients that were tested for influenza virus, the results of the test changed antiviral prescription in 157/367 (43%) of patients: in 149 of 158 (94%) patients oseltamivir treatment was discontinued after a negative test, and in 8 of 9 (89%) patients without empirical antiviral treatment, oseltamivir was started after a positive test result.
Table 2.
Prevalence of viral respiratory tract infections as found by routine diagnostics.
| Virus | Suspected CAP in influenza seasona |
Suspected CAP outside influenza seasona |
Suspected HAP in influenza seasona |
Suspected HAP outside influenza seasona |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients tested (n) | Virus positive (n, %) | Patients tested (n) | Virus positive (n, %) | Patients tested (n) | Virus positive (n, %) | Patients tested (n) | Virus positive (n, %) | |||||
| adenovirus | 173 | 3 | (2%) | 88 | 1 | (1%) | 44 | 0 | (0%) | 39 | 1 | (3%) |
| bocavirus | 172 | 0 | (0%) | 88 | 0 | (0%) | 44 | 0 | (0%) | 39 | 0 | (0%) |
| coronavirus | 179 | 13 | (7%) | 89 | 1 | (1%) | 44 | 4 | (9%) | 39 | 0 | (0%) |
| enterovirus | 134 | 1 | (1%) | 73 | 0 | (0%) | 27 | 0 | (0%) | 24 | 0 | (0%) |
| human metapneumovirus | 173 | 9 | (5%) | 88 | 0 | (0%) | 44 | 1 | (2%) | 39 | 2 | (5%) |
| influenza virus | 190 | 26 | (14%) | 89 | 0 | (0%) | 49 | 5 | (10%) | 39 | 0 | (0%) |
| parechovirus | 134 | 0 | (0%) | 73 | 0 | (0%) | 27 | 0 | (0%) | 24 | 0 | (0%) |
| parainfluenza virus | 173 | 1 | (1%) | 88 | 4 | (5%) | 44 | 2 | (5%) | 39 | 3 | (8%) |
| rhinovirus | 179 | 13 | (7%) | 89 | 12 | (14%) | 44 | 3 | (7%) | 39 | 2 | (5%) |
| respiratory syncytial virus | 179 | 5 | (3%) | 89 | 2 | (2%) | 45 | 3 | (7%) | 39 | 0 | (0%) |
| total (any virus)b | 190 | 65 | (34%) | 92 | 17 | (19%) | 50 | 17 | (34%) | 45 | 7 | (16%) |
Abbreviations: CAP = community-acquired pneumonia; HAP = hospital-acquired pneumonia.
Influenza season was defined from November 1st to April 30th.
A patient with an infection with more than one virus counts as 1 virus-positive patient.
Fig. 2.
Timing of virus diagnostic tests, as performed by attending physicians.
Abbreviations: CAP = community-acquired pneumonia; HAP = hospital-acquired pneumonia; ICU = intensive care unit.
5. Discussion
Our study shows that 46% of patients admitted to the ICU with a suspected CAP during the influenza season was tested for the presence of viral pathogens, whereas 32% of CAP patients was tested outside the season. Patients admitted with a suspected HAP were tested in 13% and 12% in both seasons respectively. Regardless of the season, patients with comorbidities (including COPD and immune deficiency) and inflammation biomarkers (elevated CRP and leucopenia) were tested significantly more often. In patients who were tested, the prevalence of viral respiratory tract infections was similar in suspected CAP and HAP cases.
The results of this study show that less than half of patients admitted to the ICU with a suspected pneumonia were tested for viral infections. This may reflect a lack of awareness and clear clinical guidelines on virus diagnostics for ICU patients admitted with a suspected CAP or HAP. While international guidelines are unclear if all critically ill patients with suspected pneumonia for influenza during the winter season should be tested, the IDSA/ATS guidelines also state that “Patients with CAP should be investigated for specific pathogens that would significantly alter standard (empirical) management decisions, when the presence of such pathogens is suspected on the basis of clinical and epidemiologic clues” [10]. Although a positive virus test does not necessarily indicate virus-related or virus-induced critical illness [16], [17], detecting an influenza infection has consequences for antiviral treatment and quarantine measures [7], [8]. Consequently, some experts advise testing for influenza in all patients admitted with severe pulmonary infection during the influenza season [18], [19], [20]. Indeed, our study shows that the results of the influenza PCR changed the oseltamivir prescription in 43% of patients. Whether testing for non-influenza viruses should be routinely performed in patients with severe pneumonia remains unclear. The US Healthcare Infection Control Practices Advisory Committee recommends droplet isolation for infections with adenovirus and rhinovirus in adults [25], however the clinical significance of most non-influenza viruses in adult ICU patients in particular remains controversial [21], [22], [23], [24]. More research on the clinical burden of non-influenza viruses in patients with severe pneumonia is needed to determine if routine testing for other respiratory viruses is also warranted. Furthermore, more insight in the disease burden that comes with viral infection may limit unnecessary use of antibiotics.
Alternatively, low prevalence of testing may be due to difficulties in establishing a clinical suspicion of a viral respiratory tract infection in the critically ill. As testing in our study was more often performed in patients who had more comorbidities, higher CRP and leucopenia, physicians may believe that viral infection is characterized by these characteristics. However, symptoms of influenza-like-illness may be mild or absent in hospitalized patients [26], and there is yet no clinical algorithm to distinguish viral pneumonia from bacterial pneumonia [1]. Although the risk factors for more severe and complicated viral respiratory tract infections are well established, such as chronic lung- and heart disease and immunodeficiency, this does not imply that ICU patients with these characteristics are at a higher risk of having a viral infection on admittance [3]. Selective testing in the ICU setting may lead to underdiagnosis of viral infections. Indeed, a large retrospective study comparing the predicted amount of influenza-related ICU admissions to the reported admissions with an influenza diagnosis, suggested that over 90% of cases are either not diagnosed or not reported [9]. Small prospective observational studies show that 50–70% of detected influenza infections were unsuspected by the attending physicians [27], [28]. In addition, in the current study the majority of virus tests was ordered in the first 2 days of ICU admission. Interestingly however, there were also virus tests being ordered up to 7 days after ICU admission. While we do not know the reasons for ordering these tests, it may suggest that an infection with a respiratory virus may have been occasionally overlooked at ICU admission. Taken together, one could suggest that viral testing of ICU patients with a suspected respiratory tract infection should not depend on symptom severity.
Remarkably, the frequency of viral testing did not differ very much between in and outside the influenza season. Outside the influenza season, the prevalence of viral infection dropped but was still 19% in CAP and 16% in HAP cases. Whether this indicates that virus testing should also be performed outside the season cannot be concluded from our study, because only a selected group of patients were tested. Of note, influenza virus was absent outside the season.
HAP patients were tested significantly less often for viral infections than CAP patients. However, in the tested patients, the overall prevalence of viral infections was similar in CAP and HAP patients. Our data may suggest that HAP patients also have a considerable risk of having a viral infection, which is in accordance with retrospective studies on the prevalence of viral infections in the ICU that include HAP [2], [6].
The strength of the current study design is based on the fact that suspected cases of CAP and HAP were prospectively assessed by a trained team of research physicians according to validated definitions, and that the attending medical staff was not aware of our study aims, which otherwise could have interfered with the actual practice of performing viral diagnostic tests. An important limitation of the current study design is that the prevalence of viral infections was not systematically tested in all patients with a suspected respiratory infection, which hampers the estimation of the prevalence of viral respiratory tract infections.
In conclusion, our study shows that less than half of the ICU patients admitted with a suspected pneumonia, either in or outside the influenza season, are tested for the presence of viral pathogens and that the decision to test seems primarily to depend on patient comorbidities and inflammation biomarker profile. As guidelines clearly recommend to consider testing for influenza virus during local epidemics, and detecting influenza virus has consequences for treatment and isolation measures, there is room for increasing routine influenza virus testing in patients with suspected CAP and HAP admitted to the ICU during the influenza season.
Conflict of interest
None declared.
Funding
This work was supported by the Center for Translational Molecular Medicine (http://www.ctmm.nl), project MARS [grant number 04I-201].
Ethical approval
The local Ethics Committees of both participating centers approved the study with opt-out consent (protocol number 10-056C).
Acknowledgements
We thank the participating ICUs, physicians and nurses of the University Medical Center Utrecht (Utrecht, The Netherlands) and Academic Medical Center (Amsterdam, The Netherlands) for their help in data acquisition.
*The MARS Consortium includes the following persons: Friso M. de Beer, MD; Gerie J. Glas, MD; Roosmarijn T. M. van Hooijdonk, MD; Janneke Horn, MD, PhD; Mischa A. Huson, MD; Tom van der Poll, MD, PhD; Laura R. A. Schouten, MD; Marleen Straat, MD; Lonneke A. van Vught, MD; Maryse A. Wiewel, MD; Esther Witteveen, MD; Luuk Wieske, MD, PhD (Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands); Jos F. Frencken, MD; Peter Klein Klouwenberg MD, PharmD, PhD (University Medical Center Utrecht, Utrecht, The Netherlands)
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2016.08.295.
Contributor Information
Frank van Someren Gréve, Email: frankvsg@gmail.com.
David S.Y. Ong, Email: davidsyong@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi S.-H., Hong S.-B., Ko G.-B., Lee Y., Park H.J., Park S.-Y. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am. J. Respir. Crit. Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 3.Østby A.C., Gubbels S., Baake G., Nielsen L.P., Riedel C., Arpi M. Respiratory virology and microbiology in intensive care units: a prospective cohort study. APMIS. 2013;121:1097–1108. doi: 10.1111/apm.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiemken T., Peyrani P., Bryant K., Kelley R.R., Summersgill J., Arnold F. Incidence of respiratory viruses in patients with community-acquired pneumonia admitted to the intensive care unit: results from the Severe Influenza Pneumonia Surveillance (SIPS) project. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:705–710. doi: 10.1007/s10096-012-1802-8. [DOI] [PubMed] [Google Scholar]
- 5.Cillóniz C., Ewig S., Ferrer M., Polverino E., Gabarrús A., Puig de la Bellacasa J. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit. Care. 2011;15:R209. doi: 10.1186/cc10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong H.-L., Hong S.-B., Ko G.-B., Huh J.W., Sung H., Do K.-H. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One. 2014;9:e95865. doi: 10.1371/journal.pone.0095865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron E.J., Miller J.M., Weinstein M.P., Richter S.S., Gilligan P.H., Thomson R.B. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 Recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM) Clin. Infect. Dis. 2013;57:485–488. doi: 10.1093/cid/cit441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uyeki T.M. Preventing and controlling influenza with available interventions. N. Engl. J. Med. 2014;370 doi: 10.1056/NEJMp1400034. 140122140218007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz J.R., Neuzil K.M., Shay D.K., Rue T.C., Neradilek M.B., Zhou H. The burden of influenza-associated critical illness hospitalizations. Crit. Care Med. 2014;42:2325–2332. doi: 10.1097/CCM.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007;44(Suppl. 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 12.Woodhead M.A., Blasi F., Ewig S., Garau J., Huchon G., Ieven M. Guidelines for the management of adult lower respiratory tract infections–full version. Clin. Microbiol. Infect. 2011;6:E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein Klouwenberg P.M.C., Ong D.S.Y., Bos L.D.J., de Beer F.M., van Hooijdonk R.T.M., Huson M.A. Interobserver agreement of centers for disease control and Prevention criteria for classifying infections in critically ill patients. Crit. Care Med. 2013;41:2373–2378. doi: 10.1097/CCM.0b013e3182923712. [DOI] [PubMed] [Google Scholar]
- 14.Jansen R.R., Schinkel J., Koekkoek S., Pajkrt D., Beld M., de Jong M.D. Development and evaluation of a four-tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J. Clin. Virol. 2011;51:179–185. doi: 10.1016/j.jcv.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Pol A.C., van Loon A.M., Wolfs T.F.W., Jansen N.J.G., Nijhuis M., Breteler E.K. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real-time PCR in samples from patients with respiratory symptoms. J. Clin. Microbiol. 2007;45:2260–2262. doi: 10.1128/JCM.00848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daubin C., Parienti J.-J., Vincent S., Vabret A., du Cheyron D., Ramakers M. Epidemiology and clinical outcome of virus-positive respiratory samples in ventilated patients: a prospective cohort study. Crit. Care. 2006;10:R142. doi: 10.1186/cc5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luyt C.E. Virus diseases in ICU patients: a long time underestimated; but be aware of overestimation. Intensive Care Med. 2006;32:968–970. doi: 10.1007/s00134-006-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wunderink R.G., Waterer G.W. Community-acquired pneumonia. N. Engl. J. Med. 2014;370:543–551. doi: 10.1056/NEJMcp1214869. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz J.R., Neuzil K.M., Cooke C.R. The authors reply. Crit. Care Med. 2015;43:e118. doi: 10.1097/CCM.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen C., Kaku S., Tutera D., Kuschner W.G., Barr J. Viral respiratory infections of adults in the intensive care unit. J. Intensive Care Med. 2016;(31):427–441. doi: 10.1177/0885066615585944. [DOI] [PubMed] [Google Scholar]
- 21.Ong D.S.Y., Faber T.E., Klein Klouwenberg P.M.C., Cremer O.L., Christiaan Boerma E., Sietses M. Respiratory syncytial virus in critically ill adult patients with community-acquired respiratory failure: a prospective observational study. Clin Microbiol. Infect. 2013 doi: 10.1111/1469-0691.12503. Off Publ Eur Soc Clin Microbiol Infect Dis: (online only) [DOI] [PubMed] [Google Scholar]
- 22.Schnell D., Gits-Muselli M., Canet E., Lemiale V., Schlemmer B., Simon F. Burden of respiratory viruses in patients with acute respiratory failure. J. Med. Virol. 2013 doi: 10.1002/jmv.23760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luchsinger V., Ruiz M., Zunino E., Martínez M.A., Machado C., Piedra P.A. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax. 2013;68:1000–1006. doi: 10.1136/thoraxjnl-2013-203551. [DOI] [PubMed] [Google Scholar]
- 24.Miggins M., Hasan A., Hohmann S., Southwick F., Casella G., Schain D. The potential influence of common viral infections diagnosed during hospitalization among critically ill patients in the United States. PLoS One. 2011;6:e18890. doi: 10.1371/journal.pone.0018890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel J.D., Rhinehart E., Jackson M., Chiarello L. Health care infection control practices advisory committee: 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babcock H.M., Merz L.R., Fraser V.J. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect. Control Hosp. Epidemiol. 2006;27:266–270. doi: 10.1086/501539. [DOI] [PubMed] [Google Scholar]
- 27.Giannella M., Rodriguez-Sanchez B., Lopez Roa P., Catalan P., Munoz P., Garcia Viedma D. Should lower respiratory tract secretions from intensive care patients be systematically screened for influenza virus during the influenza season? Crit. Care. 2012;16:R104. doi: 10.1186/cc11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Someren Gréve F., Schultz M.J., de Jong M.D., Juffermans N.P. Influenza and other respiratory viruses are underdiagnosed in critically ill patients. Crit. Care Med. 2015;43:e117. doi: 10.1097/CCM.0000000000000849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.