Abstract
Background
To explore the association between serum angiotensin-converting enzyme 2 (ACE2) levels and postoperative morbidity and mortality after major pulmonary resection in non-small cell lung cancer (NSCLC) patients.
Methods
Preoperative and postoperative serum ACE2 levels in 320 NSCLC patients who underwent major pulmonary resection were measured. The serum ACE2 levels on postoperative day 1 were divided into quartile categories.
Results
After adjustment for age, sex, body mass index, current smoking status, forced expiratory volume in 1 second, coronary heart disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and tumour clinical stages, the risk of developing postoperative morbidities was significantly higher in the lowest serum ACE2 level quartile than in the highest quartile (hazard ratio, 2.12; 95% CI, 1.57-6.23; p=0.008). NSCLC patients with a serum ACE2 level ≤3.21 ng/mL had significantly higher rates of pneumonia, pleural effusion, atrial fibrillation as well as higher in-hospital mortality after major pulmonary resection, compared with those with a serum ACE2 level >3.21ng/mL.
Conclusions
The serum ACE2 level one day post surgery is an independent risk factor for postoperative morbidities after major pulmonary resection in NSCLC patients. Thus, it could be used as a prognostic factor for postoperative morbidities after major pulmonary resection in NSCLC patients.
Keywords: Angiotensin-converting enzyme 2, Pulmonary resection, Non-small cell lung cancer, Morbidity, Mortality
Introduction
The major comorbidity in patients with non-small cell lung cancer (NSCLC) is of cardiovascular nature and is reported to be up to 23% [1]. The incidence of cardiovascular disease has been described to be a major risk factor for morbidity and mortality following surgery for NSCLC [1], [2], [3], [4], [5].
The renin-angiotensin system (RAS) plays a crucial role in cardiovascular regulation [6]. In the RAS, angiotensin-converting enzyme (ACE) metabolises angiotensin I (Ang I) to form angiotensin II (Ang II), which exerts direct trophic actions on cardiovascular cells [6]. Local Ang II production is of key importance in the pathophysiology of the RAS in cardiovascular system [7]. Recently, ACE2, a new member of the RAS, was found to function as a negative regulator of the Ang system by metabolising Ang II to a putatively protective peptide Ang-(1-7) with high efficiency [8], [9], [10]. ACE2 is expressed and active in most tissues. The highest expression of ACE2 is observed in the endothelium, the lungs, and the heart [11]. It has been widely accepted that ACE2 is critical in balancing the activity of the ACE-Ang II axis and thus plays a pivotal role in the body as an endogenous regulator of the RAS [10]. In the present study, we for the first time explored the association between serum ACE2 levels and postoperative morbidity and mortality after major pulmonary resection in NSCLC patients.
Materials and methods
Patients
From January 2008 to March 2013, 320 Han Chinese NSCLC patients who underwent major pulmonary resection were enrolled in this study. The inclusion criteria included: (1) Definitely diagnosed with NSCLC; (2) Underwent major pulmonary resection including lobectomy, bilobectomy, and pneumonectomy, but not segmentectomy and wedge resection; (3) Had not received any treatment for NSCLC before pulmonary resection. Patients with other concurrent malignancies, congenital heart diseases, or a previous cardiovascular surgical history were excluded. This study was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University. Before the start of the study, all participants provided written informed consent.
Data collection
Operations were performed under general anaesthesia through lateral thoracotomy completed with radical systematic lymphadenectomy. Staging was reevaluated according to the seventh edition of the TNM classification [12]. Histologic typing was carried out according to the World Health Organization histologic classification [13]. Morbidity was defined as any postoperative event, such as pneumonia, prolonged air leak with postoperative chest tube drainage >7 days, atrial fibrillation, or pleural effusion requiring renewed drainage. Operative mortality was defined as in-hospital mortality. Blood samples were drawn on preoperative day 3, preoperative day 1, postoperative day 1, and postoperative 3, respectively. All blood samples were subject to ELISA assays for serum ACE2 levels using an ACE2 (human) ELISA Kit (K4918-100) purchased from BioVision (Milpitas, CA, USA).
Statistical analysis
Serum ACE2 levels were divided into quartile categories: ≤3.21, 3.22-3.86, 3.87-4.52, and ≥4.53 ng/mL. The adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated using the Cox proportional hazard model. All continuous variable values were expressed as Mean ± SD. Comparisons of means between two groups was performed with student t tests. Categorical variables were expressed as n(%) and analysed with Chi-square tests or Fisher's exact tests where appropriate. All statistical analyses were performed with SAS 9.1.3. The statistical significance level of this study was set at a two-tailed α=0.05.
Results
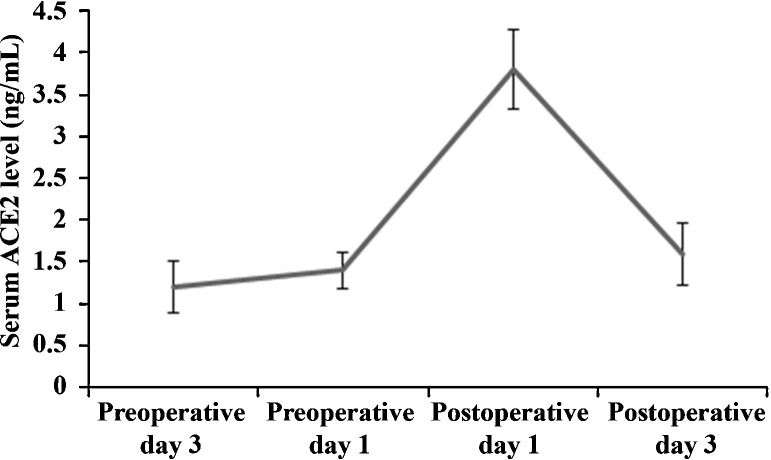
Serum ACE2 levels were measured in blood samples collected on preoperative day 3, preoperative day 1, postoperative day 1 and postoperative day 3. As shown in Fig. 1 , the serum ACE2 level in NSCLC patients was at baseline level preoperatively, peaked 24 hours after major pulmonary resection, and returned to baseline level three days after the surgery. We divided the serum ACE2 levels on postoperative day 1 into quartile categories: ≤3.21, 3.22-3.86, 3.87-4.42, and ≥4.43 ng/mL. As shown in Table 1 , there were no significant differences among the quartile categories in age, sex, body mass index (BMI), current smoking status, forced expiratory volume in 1 second (FEV1), and prevalence of coronary heart disease, hypertension, diabetes mellitus and chronic obstructive pulmonary disease (COPD). There were also no significant differences among the quartile categories in distribution of NSCLC histology and clinical stages (Table 2 ).
Figure 1.
Preoperative and postoperative serum angiotensin-converting enzyme 2 (ACE2) levels in non-small cell lung cancer patients undergoing major pulmonary resection.
Table 1.
General characteristics of study subjects by quartiles of serum angiotensin-converting enzyme 2 (ACE2) levels on postoperative day 1.
| Serum ACE2 levels (ng/mL) |
p | ||||
|---|---|---|---|---|---|
| ≤3.21 (n = 80) |
3.22-3.86 (n = 80) |
3.87-4.42 (n = 80) |
≥4.43 (n = 80) |
||
| Age (years) | 64.3±9.5 | 64.1 ± 8.8 | 63.6 ± 9.2 | 63.1 ± 10.2 | 0.33 |
| Age group (years) n(%) | |||||
| ≤50 | 8 (10.0) | 8 (10.0) | 7 (8.8) | 9 (11.3) | 0.98 |
| 51-60 | 15 (18.8) | 19 (23.8) | 21 (26.2) | 22 (27.5) | |
| 61-70 | 24 (30.0) | 21 (26.2) | 22 (27.5) | 20 (25.0) | |
| 71-80 | 33 (41.2) | 32 (40.0) | 30 (37.5) | 29 (36.2) | |
| Sex n(%) | |||||
| Male | 51 (63.8) | 49 (61.3) | 55 (68.8) | 50 (62.5) | 0.77 |
| Female | 29 (36.2) | 31 (38.7) | 25 (31.2) | 30 (37.5) | |
| Pulmonary resection n(%) | |||||
| Pneumonectomy | 10 (12.5) | 10 (12.5) | 7 (8.8) | 8 (10.0) | 0.80 |
| Lobectomy | 66 (82.5) | 64 (80.0) | 70 (87.5) | 65 (81.2) | |
| Bilobectomy | 4 (5.0) | 6 (7.5) | 3 (3.7) | 7 (8.8) | |
| FEV1 (%) | 77.5 ± 5.4 | 76.9 ± 5.9 | 76.4 ± 6.5 | 75.8 ± 6.9 | 0.20 |
| Coronary heart disease n(%) | 14 (17.5) | 11 (13.8) | 11 (13.8) | 10 (12.5) | 0.82 |
| Hypertension n(%) | 31 (38.8) | 28 (35.0) | 27 (33.8) | 23 (28.8) | 0.61 |
| Diabetes mellitus n(%) | 14 (17.5) | 13 (16.3) | 15 (18.8) | 11 (13.8) | 0.85 |
| COPD n(%) | 40 (50.0) | 35 (43.8) | 36 (45.0) | 31 (38.8) | 0.56 |
| Body mass index (kg/m2) | 17.5 ± 4.2 | 17.9 ± 5.0 | 18.3 ± 4.7 | 18.1 ± 2.9 | 0.35 |
| Current smoking | 32 (40.0) | 27 (33.8) | 36 (45.0) | 30 (37.5) | 0.52 |
Note: FEV1, forced expiratory volume in 1 second; COPD, chronic obstructive pulmonary disease.
Table 2.
Tumour characteristics of study subjects by quartiles of serum angiotensin-converting enzyme 2 (ACE2) levels on postoperative day 1.
| Serum ACE2 levels (ng/mL) |
p | ||||
|---|---|---|---|---|---|
| ≤3.21 (n = 80) |
3.22-3.86 (n = 80) |
3.87-4.42 (n = 80) |
≥4.43 (n = 80) |
||
| Histology n(%) | |||||
| Adenocarcinoma | 72 (90.0) | 73 (91.3) | 74 (92.5) | 68 (85.0) | 0.93 |
| Squamous cell carcinoma | 3 (3.8) | 2 (2.5) | 2 (2.5) | 4 (5.0) | |
| Adenosquamous cell carcinoma | 1 (1.2) | 1 (1.2) | 0 (0) | 1 (1.2) | |
| Neuroendocrine carcinoma | 4 (5.0) | 4 (5.0) | 4 (5.0) | 7 (8.8) | |
| Clinical stages n(%) | |||||
| IA | 25 (31.3) | 23 (28.8) | 22 (27.5) | 21 (26.3) | 1.00 |
| IB | 28 (35.0) | 27 (33.8) | 27 (33.8) | 25 (31.3) | |
| IIA | 10 (12.5) | 12 (15.0) | 12 (15.0) | 13 (16.2) | |
| IIB | 4 (5.0) | 4 (5.0) | 5 (6.2) | 6 (7.5) | |
| IIIA | 12 (15.0) | 13 (16.2) | 12 (15.0) | 13 (16.2) | |
| IIIB | 1 (1.2) | 1 (1.2) | 2 (2.5) | 2 (2.5) | |
As shown in Table 3 , postoperative morbidities increased with descending quartiles of serum ACE2 levels, and the risk was significantly higher in the first quartile than in the second, third, and fourth quartiles (Model 1). After adjustment for age, sex, BMI, current smoking status, FEV1, coronary heart disease, hypertension, diabetes mellitus, COPD, and tumour clinical stages (model 2), the risk of developing postoperative morbidities was significantly higher in the lowest serum ACE2 level quartile than in the highest quartile (hazard ration, 2.12; 95% CI, 1.57-6.23; p = 0.008).
Table 3.
Adjusted hazard ratios of postoperative morbidity by quartiles of serum angiotensin-converting enzyme 2 (ACE2) levels on postoperative day 1.
| Serum ACE2 levels (ng/mL) |
p for trend (across categories) | Continuous log scale* | p for trend (continuous) | ||||
|---|---|---|---|---|---|---|---|
| ≤3.21 (n = 80) |
3.22-3.86 (n = 80) |
3.87-4.42 (n = 80) |
≥ 4.43 (n = 80) |
||||
| Postoperative morbidity (%) | 38.8 | 20.0 | 18.8 | 11.3 | |||
| Model 1b HR (95% CI) |
4.2 (1.88-9.37) | 1.75 (0.74-4.13) | 1.62 (0.68-3.85) | 1 (Reference) | <0.001 | 2.27 (1.73-4.25) | <0.001 |
| Model 2b HR (95% CI) |
2.12 (1.57-6.23) | 1.29 (0.62-3.37) | 1.20 (0.40-3.11) | 1 (Reference) | 0.008 | 1.92 (1.51-2.48) | 0.005 |
Note: HR, hazard ratio. *HR per 1-SD decrease of log-transformed ACE2. aModel 1: adjusted for age, sex, and body mass index (BMI). bModel 2: adjusted for age, sex, BMI, forced expiratory volume in 1 second (FEV1), coronary heart disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, current smoking status, and tumour clinical stages.
We then examined the association between the lowest serum ACE2 level quartile (≤3.21 ng/mL) and the incidence of typical morbidities after major pulmonary resection, including pneumonia, prolonged air leak, pleural effusion, and atrial fibrillation. In the analyses, we combined the second, third, and fourth quartiles of serum ACE2 levels into one category (>3.21 ng/mL; n = 240) to compare with the first serum ACE2 level quartile (≤3.21 ng/mL; n = 80). As shown in Table 4 , NSCLC patients with a serum ACE2 level ≤3.21ng/mL had significantly higher rates of pneumonia, pleural effusion, atrial fibrillation as well as higher in-hospital mortality after major pulmonary resection, compared with those with a serum ACE2 level >3.21ng/mL. Further analyses by the method of pulmonary resection revealed that the serum ACE2 level did not have a significant effect on the rates of postoperative morbidities and in-hospital mortality in patients undergoing pneumonectomy (n = 35) (Table 5 ). However, in patients undergoing lobectomy/bilobectomy (n = 285), those with a serum ACE2 level ≤3.21ng/mL had significantly higher rates of pleural effusion and atrial fibrillation than those with a serum ACE2 level >3.21ng/mL (Table 6 ). According to the data in Table 5, Table 6, patients undergoing pneumonectomy had higher rates of postoperative morbidities and in-hospital mortality than those undergoing lobectomy/bilobectomy.
Table 4.
Association of serum angiotensin-converting enzyme 2 (ACE2) levels with postoperative morbidities and in-hospital mortality in total patients.
| Morbidity | Total (n = 320) |
Serum ACE2 levels (ng/mL) |
p | |
|---|---|---|---|---|
| ≤3.21 (n = 80) |
>3.21 (n = 240) |
|||
| Pneumonia n(%) | 16 (5) | 8 (10) | 8 (3.3) | 0.033 |
| Prolonged air leak n(%) | 18 (5.6) | 5 (6.3) | 13 (5.4) | 0.782 |
| Pleural effusion n(%) | 8 (2.5) | 5 (6.3) | 3 (1.3) | 0.026 |
| Atrial fibrillation n(%) | 29 (9.1) | 13 (16.3) | 16 (6.7) | 0.014 |
| In-hospital mortality n(%) | 9 (2.8) | 5 (6.3) | 4 (1.7) | 0.046 |
Table 5.
Association of serum angiotensin-converting enzyme 2 (ACE2) levels with postoperative morbidities and in-hospital mortality in patients undergoing pneumonectomy.
| Morbidity | Total (n = 35) |
Serum ACE2 levels (ng/mL) |
p | |
|---|---|---|---|---|
| ≤3.21 (n = 10) |
>3.21 (n = 25) |
|||
| Pneumonia n(%) | 7 (20.0) | 4 (40.0) | 3 (12.0) | 0.155 |
| Prolonged air leak n(%) | 3 (8.6) | 1 (10.0) | 2 (8.0) | 1.000 |
| Pleural effusion n(%) | 2 (5.8) | 1 (10.0) | 1 (4.0) | 0.496 |
| Atrial fibrillation n(%) | 4 (11.4) | 2 (20.0) | 2 (8.0) | 0.561 |
| In-hospital mortality n(%) | 2 (5.7) | 1 (10.0) | 1 (4.0) | 0.496 |
Table 6.
Association of serum angiotensin-converting enzyme 2 (ACE2) levels with postoperative morbidities and in-hospital mortality in patients undergoing lobectomy/bilobectomy.
| Morbidity | Total (n = 285) |
Serum ACE2 levels (ng/mL) |
p | |
|---|---|---|---|---|
| ≤3.21 (n = 70) |
>3.21 (n = 215) |
|||
| Pneumonia n(%) | 9 (3.2) | 4 (5.7) | 5 (2.3) | 0.230 |
| Prolonged air leak n(%) | 15 (5.3) | 4 (5.7) | 11 (5.1) | 0.767 |
| Pleural effusion n(%) | 6 (2.1) | 4 (5.7) | 2 (1.0) | 0.034 |
| Atrial fibrillation n(%) | 25 (8.8) | 11 (15.7) | 14 (6.5) | 0.027 |
| In-hospital mortality n(%) | 7 (2.5) | 3 (4.3) | 3 (1.4) | 0.160 |
Discussion
The prevalence of cardiovascular diseases in lung cancer patients is known to be twice as high as in the general population [1]. There have been numerous reports on the negative impact of cardiovascular comorbidity on postoperative outcome after pulmonary resection in patients with NSCLC [1], [2], [3], [4], [5], [14]. As an endogenous regulator of the RAS, ACE2 plays a crucial role in cardiovascular regulation [10]. In the present study, we provide the first evidence that the serum ACE2 level is associated with postoperative morbidities and mortality after major pulmonary resection in NSCLC patients.
ACE2 is highly expressed in the lungs as a membrane-bound protein in the vascular endothelium, alveolar epithelial cells, smooth muscle cells of the pulmonary vasculature, and bronchial epithelia [15]. Pulmonary ACE2 appears to have a role in regulating the balance of circulating Ang II/Ang 1-7 levels. Ang II induces pulmonary vasoconstriction in response to hypoxia, which is important in preventing shunting in patients with lung injury [16]. Locally increased Ang II production also triggers increasing vascular permeability facilitating pulmonary oedema [17]. A recent study suggests that ACE2 protects from severe acute lung failure and COPD, and possibly, pulmonary arterial hypertension [15]. ACE2 protein also appears to be the entry-point receptor for the severe acute respiratory syndrome (SARS) coronavirus [18], [19]. In addition, interplay between ACE and ACE2 reportedly plays a pivotal role in the development of tuberculous pleural effusions [20]. Thus, ACE2 appears directly involved in pulmonary vascular regulation as well as in pulmonary infection and pleural effusion. In agreement with the previous studies, we found in the present study that the serum ACE2 level in the lowest quartile was associated with significantly higher rates of pneumonia and pleural effusion after major pulmonary resection in NSCLC patients.
Pan et al. examined expression of ACE2 in the fibrillating atria of pigs and its involvement in fibrotic pathogenesis during atrial fibrillation [21]. Their study results suggest that down-regulation of ACE2 may be associated with pacing-induced sustained atrial fibrillation. In line with this report, we found in the present study that the serum ACE2 level in the lowest quartile was associated with significantly higher rates of atrial fibrillation after major pulmonary resection in NSCLC patients. The high rates of postoperative morbidities in patients with serum ACE2 level in the lowest quartile sufficiently explain the increased in-hospital mortality rate in the patients.
Pneumonectomy is a common type of pulmonary resection performed in 10%-30% of patients offered curative resection for primary lung cancer. However, despite improvements in operative techniques and postoperative care, pneumonectomy remains a high-risk procedure [22]. In agreement with this, patients undergoing pneumonectomy had higher rates of postoperative morbidities and in-hospital mortality than those undergoing lobectomy/bilobectomy in this study. We found that the serum ACE2 level in NSCLC patients was significantly elevated 24 hours after major pulmonary resection and returned to baseline level on day 3 postoperatively. Therefore, only the serum ACE2 level on postoperative day 1 was used for subsequent hazard ratio and association analyses. The high prevalence of cardiovascular diseases in lung cancer patients can be explained with common risk factors such as older age and tobacco consumption [23], [24]. Thus, the hazard ratio analysis was adjusted for age, current smoking status, and a variety of other confounding factors that may affect the incidence of postoperative morbidities and/or serum ACE2 levels, including sex, BMI, FEV1, coronary heart disease, hypertension, diabetes mellitus, COPD, and tumour clinical stages. After adjustment for the confounding factors, the risk of developing postoperative morbidities remained significantly higher in the lowest serum ACE2 level quartile than in the highest quartile on postoperative day 1, indicating that the serum ACE2 level one day post surgery is an independent risk factor for postoperative morbidities after major pulmonary resection in NSCLC patients. Thus, the serum ACE2 level one day post surgery could be used as a biomarker or prognostic factor for postoperative morbidities after major pulmonary resection in NSCLC patients. In addition, our findings also suggest that supplementing ACE2 could be a potential therapy for preventing or improving postoperative morbidities after major pulmonary resection. Given the important role of Ang II/ACE2 signalling in pulmonary vascular regulation, pulmonary infection and pleural effusion, and possibly atrial fibrillation [15], [16], [17], [18], [19], [20], [21], an increase of ACE2 after major pulmonary resection, albeit short-lived, may be a critical compensatory mechanism against initiation of early postoperative morbidities, which leads to reduced manifestation of postoperative morbidities and mortality. Further studies are needed to uncover the underlying mechanisms.
This study was a prospective study with multiple measurements of the serum ACE2 level, which enabled us to conduct the study in a more controlled manner and thus to generate reliable data. There are several limitations in this study: (1) We only assessed short-term outcomes in this study. Nevertheless, evaluation of long-term survival outcomes is underway with the same patient cohort. (2) This study suggests that the serum ACE2 level one day post surgery could be used as a prognostic factor for postoperative morbidities after major pulmonary resection in NSCLC patients, the clinical application value of which still needs to be validated in future studies with a larger patient population. (3) Although the total number of patients undergoing major pulmonary resection was well enough for achieving the study objectives, only a small number of patients underwent pneumonectomy. Thus, in stratified analyses by pneumonectomy and lobectomy, the relatively small sample size of patients undergoing pneumonectomy (n = 35) may not be adequate for achieving results with adequate statistical power. We did not find significant effects of the serum ACE2 level on postoperative morbidities and in-hospital mortality in patients undergoing pneumonectomy (n = 35), which was very likely due to an inadequate sample size.
Conclusion
The serum ACE2 level one day post surgery was an independent risk factor for postoperative morbidities after major pulmonary resection in NSCLC patients. Low serum ACE2 levels were associated with increased rates of postoperative morbidities as well as higher in-hospital mortality after major pulmonary resection in NSCLC patients. Thus, the serum ACE2 level one day post surgery resection could be used as a prognostic factor for postoperative morbidities after major pulmonary resection in NSCLC patients.
Conflict of Interest
None declared.
Acknowledgements
This work was supported by Hunan Provincial Natural Science Foundation (grants #08C2315 and #12C3821), P.R. China.
References
- 1.Janssen-Heijnen M.L., Schipper R.M., Razenberg P.P., Crommelin M.A., Coebergh J.W. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 2.Ambrogi V., Pompeo E., Elia S., Pistolese G.R., Mineo T.C. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:811–817. doi: 10.1016/s1010-7940(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 3.Pavia R., Spinelli F., Monaco M., Mondello B., Monaco F., Gaeta R. Lung cancer and cardiovascular diseases: occurrence, comorbidity and surgical timing. J Cardiovasc Surg. 2007;48:227–231. [PubMed] [Google Scholar]
- 4.Volpino P., Cangemi R., Fiori E., Cangemi B., De Cesare A., Corsi N. Risk of mortality from cardiovascular and respiratory causes in patients with chronic obstructive pulmonary disease submitted to follow-up after lung resection for non-small cell lung cancer. J Cardiovasc Surg. 2007;48:375–383. [PubMed] [Google Scholar]
- 5.Janssen-Heijnen M.L., Maas H.A., Houterman S., Lemmens V.E., Rutten H.J., Coebergh J.W. Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43:2179–2193. doi: 10.1016/j.ejca.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Domenighetti A.A., Wang Q., Egger M., Richards S.M., Pedrazzini T., Delbridge L.M. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- 7.Mazzolai L., Pedrazzini T., Nicoud F., Gabbiani G., Brunner H.R., Nussberger J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensives mice. Hypertension. 2000;35:985–991. doi: 10.1161/01.hyp.35.4.985. [DOI] [PubMed] [Google Scholar]
- 8.Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 9.Der Sarkissian S., Huentelman M.J., Stewart J., Katovich M.J., Raizada M.K. ACE2: a novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163–198. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Raizada M.K., Ferreira A.J. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 11.Tikellis C., Thomas M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rami-Porta R., Crowley J.J., Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 13.Travis W.D., Colby T.V., Corrin B. 3rd edition. Springer; Berlin: 1999. Histological typing of lung and pleural tumours. [Google Scholar]
- 14.Senbaklavaci O., Taspinar H., Hartert M., Vahl C.F. Impact of previous cardiovascular surgery on postoperative morbidity and mortality after major pulmonary resection for non-small cell lung cancer. Langenbecks Arch Surg. 2013 doi: 10.1007/s00423-013-1081-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Li Y., Zeng Y., Wu R., Ou J. Endothelin-1 Downregulates Angiotensin-Converting Enzyme-2 Expression in Human Bronchial Epithelial Cells. Pharmacology. 2013;91:297–304. doi: 10.1159/000350395. [DOI] [PubMed] [Google Scholar]
- 16.Kiely D.G., Cargill R.I., Wheeldon N.M., Coutie W.J., Lipworth B.J. Haemodynamic and endocrine effects of type 1 angiotensin II receptor blockade in patients with hypoxaemic cor pulmonale. Cardiovasc Res. 1997;33:201–208. doi: 10.1016/s0008-6363(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh W.Y., Kuan T.C., Cheng K.S., Liao Y.C., Chen M.Y., Lin P.H. ACE/ACE2 ratio and MMP-9 activity as potential biomarkers in tuberculous pleural effusions. Int J Biol Sci. 2012;8:1197–1205. doi: 10.7150/ijbs.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan C.H., Lin J.L., Lai L.P., Chen C.L., Stephen Huang S.K., Lin C.S. Downregulation of angiotensin converting enzyme II is associated with pacing-induced sustained atrial fibrillation. FEBS Lett. 2007;581:526–534. doi: 10.1016/j.febslet.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y., Zhou D., Peng W., Liu T., Chen H. Determinants of perioperative morbidity and mortality after pneumonectomy. Thorac Cardiovasc Surg. 2004;52:45–48. doi: 10.1055/s-2004-815801. [DOI] [PubMed] [Google Scholar]
- 23.Reicher-Reiss H., Jonas M., Goldbourt U., Boyko V., Modan B. Selectively increased risk of cancer in men with coronary heart disease. Am J Cardiol. 2001;87:459–462. doi: 10.1016/s0002-9149(00)01405-3. [DOI] [PubMed] [Google Scholar]
- 24.Licker M., de Perrot M., Hohn L., Tschopp J.M., Robert J., Frey J.G. Perioperative mortality and major cardio-pulmonary complications after lung surgery for non-small cell carcinoma. Eur J Cardiothorac Surg. 1999;15:314–319. doi: 10.1016/s1010-7940(99)00006-8. [DOI] [PubMed] [Google Scholar]