Highlights
-
•
Respiratory viruses were detected in 32% of Hospital Acquired Pneumonia.
-
•
Virus/bacteria co-infection was associated with higher length of stay and mortality.
-
•
We distinguished acute acquired viral infection and long-term viral carriage.
Keywords: Respiratory virus, Nosocomial, Pneumonia, Hospital-acquired pneumonia, Intensive care unit
Abstract
Background
Data on the frequency and role of respiratory viruses (RVs) in hospital-acquired pneumonia (HAP) are still scarce.
Objectives
We assessed the proportion of RVs and their impact on the outcome of hospital-acquired pneumonia (HAP) in the intensive care unit (ICU).
Study design
Cases of HAP were retrospectively selected among patients who underwent screening for RVs by multiplex PCR (mPCR) in the ICU of a French tertiary care hospital from May 2014 to April 2016. ICU length of stay and in-hospital mortality were compared between four groups defined according to the identified pathogens: virus only (V), virus/bacteria (V/B), bacteria only (B) and no pathogen (Neg). When available, previous mPCR was retrieved in order to assess possible chronic viral carriage.
Results
Overall, 95/999 (10%) ICU patients who underwent mPCR had HAP (V(17,18%), V/B(13,14%), B(60,63%), Neg(5,5%)). Median age was 61 years and 45 (47%) were immunocompromised. Influenza (27%) and rhinovirus (27%) were the most common RVs. V/B group had higher mortality rate than B and V groups (62% vs. 40% and 35%, p = 0.3) and a significantly longer length of stay (31 days (18–48)) than V group (5 days (3–11), p = 0.0002)) and B group (14.5 days (5.5–25.5), p = 0.007)). Among the 15 patients with available mPCR tests before viral HAP, seven were negative and eight were positive corresponding to long-term carriage of community-acquired viruses.
Discussion
RVs were detected in 32% of HAP patients who underwent mPCR. Two situations were encountered: (i) acute acquired viral infection; (ii) long-term viral carriage (mostly rhinovirus) especially in immunocompromised patients complicated by a virus/bacteria coinfection. The latter was associated with a longer length of stay and a trend toward a higher mortality.
1. Background
Respiratory viruses (RVs) are known to constitute a large burden in community-acquired respiratory infections [1], [2], [3], [4]. With regards to nosocomial infections, RVs (excluding herpes simplex virus (HSV) and cytomegalovirus (CMV)) have traditionally been paid little attention, except in hematopoietic stem cell or solid organ transplant recipients [5], [6]. Although hospital-acquired pneumonia (HAP) is the second most common nosocomial infection in the developed countries and is associated with high mortality and morbidity [7], [8], it has always been seen to be driven by bacteria, with no role of RVs [9]. However, in a recent South Korean study 22.5% of cases of HAP were related to viral infections, and 59.5% to bacterial infections. This finding, showing a non-negligible role of viruses, can be explained by recent improvements in detection methods and needs to be explored further. Indeed, improvements in the sensitivity of detection techniques such as multiplex polymerase chain reaction (mPCR) have greatly enhanced the ability to detect RVs [10], [11]. However, data on the frequency and role of RVs in HAPs and especially their outcome are still scarce. Apart from influenza and respiratory syncytial virus (RSV), other viruses are not usually investigated because of weak evidence of their pathogenicity, lack of available treatment, and the high cost of mPCR tests. Furthermore, the role of viral-bacterial coinfection is still unclear in pneumonia and all of the few investigations using mPCR were conducted on community-acquired pneumonia presenting viral-bacterial coinfection and yielded divergent data on pathogenicity and prognosis [2], [12], [13], [14].
2. Objectives
We retrospectively reviewed all cases of HAP who underwent screening for RVs in patients admitted to or in an intensive care unit (ICU) between 2014 and 2016 in order to estimate the proportion of HAP associated with RVs, bacteria or viral-bacterial coinfection and to compare their impact on prognosis.
3. Study design
3.1. Study population and data collection
The study was conducted at the medical ICU of the Bichat-Claude Bernard Hospital, a teaching tertiary referral hospital in Paris, France. All medical records of patients who underwent mPCR assay for RVs during an ICU stay from May 1, 2014 to April 31, 2016 were retrospectively reviewed. Patients with community-acquired pneumonia or with a diagnosis other than pneumonia were excluded. Only adult patients diagnosed with HAP who underwent screening for RVs at the time of suspected pneumonia were included in the study. When multiple episodes of HAP occurred in the same patient, only the first episode was taken into consideration. Patients included were classified in one of the following four groups according to the microorganisms identified from specimens collected within 72 h after the diagnosis of pneumonia: virus only, bacteria only, virus/bacteria and no pathogens. Of note, the use of mPCR for respiratory virus screening in the ICU of the Bichat-Claude Bernard Hospital is not systematic and is left at physician’s discretion. Its use that started in 2013 and was at first limited to immunocompromised patients has progressively expanded over the years. We compared characteristics of those who did not undergo screening for RVs to those who underwent and were included in the study. For this we identified the total number of HAP that occurred during the study period in the ICU using the primary diagnosis coded according to the Tenth Revision of the International Classification of Diseases (ICD10).
Clinical, laboratory data including microbiological tests, and radiological findings at diagnosis of HAP data were retrieved from the patients’ medical charts. Length of ICU stay after diagnosis of pneumonia and in-hospital mortality were collected and compared between groups. In order to differentiate hospital-acquired viruses from long-term viral shedding in patients with at least one virus found, all available mPCR tests performed during the same hospital stay before HAP diagnosis were retrieved.
All definitions are detailed in Supplemental data 1.
3.2. Laboratory data
All patients enrolled in this study underwent bronchoscopy with bronchoalveolar lavage (BAL) or endotracheal aspirate (ETA). Other microbiological evaluations were performed at the physician’s discretion. These evaluations may have included one or more sets of blood culture (gram stain and culture), culture and histological examination for the diagnosis of fungus, PCR for diagnosis of cytomegalovirus (CMV) or herpes simplex virus (HSV) in ETA or BAL fluid and screening for RVs on nasopharyngeal swabs or in ETA or BAL fluid.
RVs were tested for by mPCR assay, using the AnyplexTM II RV16 Detection kit (Seegene® Inc., Seoul, Korea) [11] and allowing the detection of influenza A and B viruses, adenovirus, parainfluenza virus (types 1, 2, 3, and 4), RSV types (A and B), picornavirus (rhinoviruses, enteroviruses), human metapneumovirus, human coronaviruses (229E, NL63, OC43 and HKU1), and bocavirus.
Microorganisms identified from specimens collected within 72 h after the diagnosis of pneumonia were considered as pathogens.
3.3. Statistical analysis
Patient characteristics and outcome are reported as numbers and percentages for categorical variables and as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables. Data from the no pathogen group are not displayed. In-hospital and 28-day mortality and median ICU length of stay after HAP diagnosis in patients discharged alive were considered as outcome of interest and compared between the virus only, bacteria only and virus/bacteria groups. The Kruskal-Wallis test and Fisher’s exact test were used, as appropriate, for univariate analysis. There were no missing data. P values less than 0.05 were considered statistically significant. All statistical analyses were conducted using STATA software (V12, ©1996–2014 StataCorp, College Station, Texas, USA).
4. Results
4.1. Patient characteristics
Among the 999 ICU patients who underwent an mPCR assay to test for RVs during the study period, 95 were considered to have HAP and were included in the study. During the same period, HAP were admitted or occurred in the ICU in 143 patients. Thus, screening for RVs was performed in 66% (95/143) of HAP patients. The characteristics of the 48 HAP patients not tested for RVs were similar to those of the 95 patients described in this study, except for immune status. Patients tested for RVs were more likely to be immunocompromised (47% vs. 19%, p = 0.001).
The patients had a median age of 61 (IQR, 52–69) years and 71 (75%) were male. Forty-five patients (47%) were considered as immunocompromised. Sixty-four patients (67%) had at least one chronic underlying disease (most frequently diabetes mellitus (37%) and structural lung disease (28%)). The median length of hospital stay prior to HAP diagnosis was 17 (9–36) days. The median SAPS II score at admission was 52 [34–61].
Baseline characteristics are listed in Table 1 .
Table 1.
Demographics, clinical characteristics and outcomes of patients with hospital-acquired pneumonia according to pathogens identified.
| Total (n = 95) | Virus only (n = 17) | Bacteria only (n = 60) | Virus/Bacteria Coinfection (n = 13) | p-value | |
|---|---|---|---|---|---|
| Age, years (median [IQR]) | 61 [52–69] | 61 [50–66] | 61.5 [54.5–69] | 58 [53–65] | 0.68 |
| Male, n (%) | 71 (75) | 13 (76) | 44 (73) | 11 (85) | 0.76 |
| Active smoking, n (%) | 30 (32) | 7 (41) | 16 (27) | 5 (38) | 0.39 |
| Underlying conditions, n (%) | 64 (67) | 13 (76) | 40 (67) | 11 (85) | 0.40 |
| Structural Lung Disease | 27 (28) | 6 (35) | 1 (23) | 5 (38) | 0.38 |
| Chronic Heart Failure | 16 (17) | 3 (18) | 11 (19) | 1 (8) | 0.83 |
| End-Stage Renal Failure | 5 (5) | 2 (12) | 3 (5) | 0 | 0.37 |
| Liver cirrhosis | 3 (3) | 0 | 3 (5) | 0 | 1.0 |
| Diabetes mellitus | 36 (37) | 8 (47) | 22 (37) | 4 (31) | 0.62 |
| Immunocompromised State, n(%) | 45 (47) | 10 (59) | 22 (37) | 9 (69) | 0.05 |
| Solid Transplant Recipient | 25 (26) | 6 (35) | 16 (27) | 2 (15) | 0.49 |
| Bone Marrow Transplant | 0 | 0 | 0 | 0 | – |
| Solid Cancer | 4 (4) | 1 (6) | 2 (3) | 0 | 1.0 |
| Malignant Blood Disease | 5 (5) | 1 (6) | 1 (2) | 1 (8) | 0.26 |
| Immunosuppressive treatment | 35 (36) | 8 (47) | 18 (30) | 5 (38) | 0.38 |
| HIV (Uncontrolled) | 4 (4) | 0 | 3 (5) | 1 (8) | 0.58 |
| Autoimmune disease | 6 (6) | 1 (6) | 1 (2) | 3 (23) | 0.01 |
| Hospital stay prior to HAP diagnosis, median [IQR], days | 17 [9–36] | 26 [9–46] | 16 [9–37] | 17 [9–20] | 0.72 |
| Ward of occurrence, n (%) | 0.08 | ||||
| Outside Intensive Care Unit, n (%) | 32 (34) | 9 (53) | 17 (28) | 2 (15) | – |
| Intensive Care Unit, n(%) | 63 (66) | 8 (47) | 43 (72) | 11 (85) | – |
| VAP, n (%) | 60 (95) | 6 (75) | 42 (98) | 11 (100) | 0.06 |
| NV-ICUAP, n (%) | 3 (5) | 2 (25) | 1 (2) | 0 | – |
| SAPS II Score at admission, median [IQR] | 52 [34–61] | 44 [30–57] | 55 [35–63] | 47 [37–63] | 0.35 |
| ICU length of stay after HAP diagnosis in patients alive at release, median [IQR], days | 14 [4–26] | 5 [3–11] | 14.5 [5.5–25.5] | 31 [18–48] | 0.0002 |
| In-hospital mortality, n (%) | 38 (40) | 6 (35) | 24 (40) | 8 (62) | 0.30 |
| Mortality at Day 28, n (%) | 40 (42) | 7 (41) | 25 (42) | 8 (62) | 0.45 |
HAP: hospital-acquired pneumonia; IQR: interquartile range; VAP: ventilator-associated pneumonia; NV-ICUAP: non-ventilated intensive care unit-acquired pneumonia; SAPS: simplified acute physiology score; ICU: intensive care unit.
4.2. Microbiological results
At least one pathogen was identified in 90 out of 95 (95%) patients. Bacterial infection was observed in 73 patients (77%) and 60 (63%) had bacterial infections solely. Respiratory viral infection was found in 30 (32%) patients and 17 (17/95, 18%) had viral infections solely. Viral/bacterial co-infection concerned 13 (14%) cases. Among the 73 patients with bacterial infections identified in respiratory specimens, 7 (9%) had concomitant positive blood cultures. HSV was found in 7 (7%) cases and fungal infection in 3 (3%) cases (Pneumocystis jirovecii n = 1, Aspergillus species n = 2). RVs were more likely to be found in immunocompromised patients (19/45, 42% vs. 11/50, 22%, p = 0.04). Distribution of all pathogens identified is shown in Table 2 .
Table 2.
Distribution of identified pathogens among patients with hospital-acquired pneumonia.
| Pathogens | N (%) |
|---|---|
| Bacteria (n = 90) | – |
| Non-fermenting Gram-negative bacilli | 37 (39) |
| Pseudomonas aeruginosa | 29 (31) |
| Stenotrophomas maltophilia | 6 (6) |
| Acinetobacter baumanii | 2 (2) |
| Enterobacteria | 34 (36) |
| Enterobacter species | 12 (13) |
| Escherichia coli | 8 (8) |
| Klebsiella species | 7 (7) |
| Hafnia alveii | 4 (4) |
| Proteus mirabilis | 1 (1) |
| Citrobacter koserii | 1 (1) |
| Serratia marcescens | 1 (1) |
| MSSAa | 10 (11) |
| Others | 9 (9) |
| Enterococcus species | 4 (4) |
| Haemophilius influenza | 3 (3) |
| Branhamella catarrhalis | 2 (2) |
| Respiratory Viruses (n = 30) | – |
| Influenza | 8 (8) |
| Rhinovirus | 8 (8) |
| Respiratory Syncytial Virus (RSV) | 5 (5) |
| Parainfluenza | 4 (4) |
| Bocavirus | 2 (2) |
| Adenovirus | 1 (1) |
| Coronavirus | 1 (1) |
| Metapneumovirus | 1 (1) |
| Others (n = 10) | – |
| Herpes Simplex Virus | 7 (7) |
| Aspergillus species | 2 (2) |
| Pneumocystis jirovecii | 1 (1) |
Methicillin-sensitive Staphylococcus aureus.
4.2.1. Bacterial pathogens
A total of 90 bacteria were identified in 73 patients. Two and three concomitant bacteria were identified for 13 and 1 patients, respectively. Non-fermenting Gram-negative bacilli, Enterobacteriaceae and MSSA (methicillin-sensitive Staphylococcus aureus) were found in 37 (39%), 34 (36%) and 10 (11%) cases, respectively.
4.2.2. Respiratory viral pathogens
Overall, 30 RVs were identified in 30 patients (30/95, 32%). None of these patients were infected with more than one type of virus. All viruses were detected from BAL fluid specimens or the ETA. Among the 30 patients, 22 underwent solely bronchoscopy with BAL, 6 underwent both bronchoscopy with BAL and ETA and two only ETA. Eleven patients also had a nasopharyngeal swab.
Patients had a median age of 58 years (IQR 52–66), 73% had underlying conditions and 63% were immunocompromised. The median length of hospital stay prior to pneumonia was 17 days (IQR 9–36).
Influenza virus and Rhinovirus were the two most commonly identified viruses (n = 8 for each virus, 27% (8/30)). Other viruses were RSV (n = 5, 17%), parainfluenza virus (n = 4, 13%), bocavirus (n = 2, 7%), human coronavirus (n = 1, 3%), human metapneumovirus (n = 1, 3%), and adenovirus (n = 1, 3%). Among the 13 patients with respiratory virus-bacterial coinfection, three and two of them also presented HSV and fungus, respectively. Patients with coinfection were significantly more likely to have autoimmune disease than patients with solely bacteria (23% vs. 2%, p = 0.02).
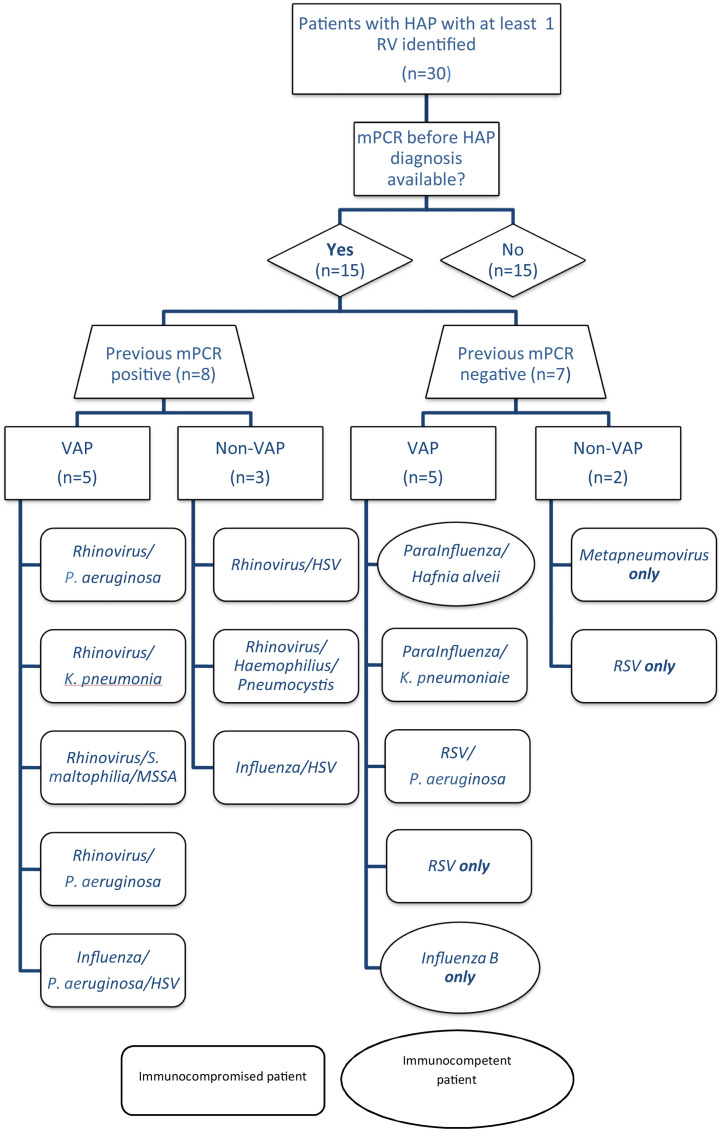
Fifteen of the 30 patients with HAP (50%) and at least one RV identified had a mPCR prior to the HAP episode that was performed during the same hospital stay (Fig. 1 ). The prior mPCR was negative in seven patients (7/15, 47%) who underwent it on average 15 (Standard Deviation (SD) 6.4) days before HAP diagnosis. These patients were considered to have acquired RVs in the hospital. Their characteristics and outcomes are shown in Supplementary Table S1. Five of the cases occurred in ICU ventilated patients and two in non-ventilated patients from a long-term care facility. Identified viruses were RSV (n = 3), parainfluenza (n = 2), metapneumovirus (n = 1) and influenza (n = 1) and 4/7 cases were virus only infection. No grouped cases among these seven patients were identified.
Fig. 1.
Distribution of respiratory viruses in patients with mPCR testing before HAP diagnosis.
RV: respiratory virus, mPCR: multiplex polymerase chain reaction; VAP: ventilator-acquired pneumonia; MSSA: methicillin-sensitive Staphylococcus aureus.
On the other hand, eight patients (8/15, 53%) had a prior positive mPCR on average 11 (SD 5.0) days before HAP diagnosis, with the same virus identified at the time of HAP diagnosis. These patients, all immunocompromised, were considered to be long-term carriers, infected in the community. Identified viruses were rhinovirus (n = 6) and influenza (n = 2) and all eight cases were coinfection HAP (viral-bacterial n = 4, viral-HSV-bacterial n = 1, viral-bacterial-Aspergillus n = 1 and viral-HSV n = 2), suggesting a potential role of some RVs in the occurrence of bacterial VAP in immunocompromised patients.
4.3. Outcomes between groups (Table 1)
The in-hospital mortality rate and 28-day mortality rate of all the included patients were 40% (38/95) and 42% (40/95), respectively, with a median (IQR) ICU length of stay after HAP diagnosis of 14 days (4–26) in patients alive at discharge. Mortality rates were higher in the coinfected (virus/bacteria) group than in both bacteria only and virus only groups but the difference did not reach statistical significance (62% vs. 40% and 35%, p = 0.3 for in-hospital mortality; 62% vs. 42% and 41%, p = 0.45 for 28-day mortality).
Among patients alive at hospital discharge, the virus/bacteria coinfection group had a significantly longer length of stay (31 days (18–48)) than both the virus only group (5 days (3–11), p = 0.0002)) and the bacteria only group (14.5 days (5.5–25.5), p = 0.007)).
5. Discussion
In this retrospective study conducted over two years in one teaching tertiary referral hospital in France, RVs were identified in 32% of 95 HAP patients admitted to or in the ICU who underwent respiratory mPCR testing. Influenza virus (27%), rhinovirus (27%) and RSV (17%) were the most commonly identified viruses. Patients concerned by viral HAP were more likely to be immunocompromised than those with bacterial HAP. Patients with virus/bacteria coinfection had a higher mortality, although this did not reach statistical significance, and a longer length of stay in the ICU than both the bacteria only and virus only groups.
Our results regarding the proportion and the nature of RVs found are consistent with those presented by Hong et al. in their single-center study of 262 cases of severe HAP in Seoul, South Korea over a two-year period. In that study, 119 (45%) were immunocompromised, 251 (96%) patients were ventilated and RVs were identified in 22.5% of cases with a predominance of RSV, parainfluenza virus and rhinovirus [15]. However, unlike Hong et al., the virus/bacteria group in our study presented a longer ICU length of stay and a trend to higher mortality rates than both the virus only and bacteria only groups. In the Hong et al. study, contrary to our study in which all patients underwent BAL fluid or ETA arguing in favor of their active role, only 41% (107/262) of patients had BAL performed and RVs were diagnosed on BAL fluid testing in 63% (37/59 patients).
In community-acquired pneumonia, previous studies have reported the clinical interaction between influenza and S. pneumoniae or S. aureus as a major contributor to influenza mortality [2], [16], [17], [18], [19]. It is becoming clear that similar interactions may occur with other RVs, including RSV, human metapneumovirus, and possibly rhinovirus or parainfluenza virus [20], [21], [22], [23], [24]. However, clinical evidence from prospective studies on the role of coinfection in severity is somewhat conflicting [2], [14], [25], [26]. In our study it should be underlined that all eight HAP cases with long-term shedding of community-acquired viruses involved immunocompromised patients with coinfection with other pathogens. Shedding of rhinoviruses for more than 4 weeks is not uncommon even in immunocompetent individuals [27] and especially in patients with immunodeficiency in whom chronic rhinovirus carriage for as long as 4–12 months has been documented [28]. The significance of rhinovirus detection in respiratory specimens from pneumonia patients is a subject of some debate. Some findings have demonstrated that rhinovirus infection alters the respiratory microbiome and may precipitate secondary bacterial infections in patients with COPD [29], [30].
We found a high rate (50%) of proven nosocomial HAP in patients who underwent a prior RVs screening during their hospital stay. Virus distribution, viral-bacterial coinfection rate, and patient prognosis differed greatly from long-term virus shedding cases. Our findings support the possible nosocomial transmission of various RVs and not only influenza and RSV in debilitated patients who have prolonged hospitalizations [31], [32], and highlight the need for infection control measurements targeted at health care personnel and family members to control further hospital transmission.
Several limitations of our work should, however, be acknowledged. First, this study was single-center and retrospective. Second, during the study period, mPCR testing for RVs was not systematic in the ICU, but was left instead to the clinician’s discretion, which may have led to selection bias and to an underestimation of the total number of cases of HAP in admitted patients to the ICU or occurring in the ICU. We tried to overcome this issue by estimating the total number of HAP during the study period and comparing characteristics of patients with HAP who underwent RVs screening and those who did not. The group of patients who underwent RVs screening were more frequently immunocompromised. Third, the 48-h delay traditionally used for the definition of nosocomial cases is derived from data concerning bacteria. This delay may not be appropriate for RVs for which incubation and shedding data are still partial in these populations and can differ from one virus to another. Most authors agree to a median delay for symptoms of 2–7 days for all RVs [33]. We used previous RV tests done before HAP diagnosis when available to try to overcome this issue. Finally, our study design prevented assessment of the pathogenic role of RVs. However, the fact that all RVs were obtained from BAL fluid or ETA argues in favor of their active role.
In conclusion, in our setting, RVs were tested for in two-thirds of patients (mostly immunocompromised) admitted with HAP or with HAP that occurred in the ICU, and RVs were identified in 32% of them, of which 43% were respiratory virus/bacterial coinfection. The latter seems to be associated with poorer outcomes compared with bacteria only or virus only pneumonia. RVs found in HAP could be divided in two categories: (i) community-acquired viruses with chronic shedding (i.e. mainly rhinovirus) in immunocompromised patients, associated with other microorganisms at the time of HAP, and (ii) hospital-acquired viruses (RSV, parainfluenza, influenza and human metapneumovirus) in debilitated patients with prolonged hospitalization. Our retrospective data did not demonstrate a causal relationship between viral shedding and outcome, but emphasize the need for further systematic prospective studies to assess the temporality and consequences of both viral infections and viral/bacterial coinfection.
Funding
The current work received no fund.
Competing interest
The authors declare no commercial or other association that might pose a conflict of interest.
Ethical approval
The need for informed consent was waived in view of the retrospective observational nature of the study with no intervention performed. This study was conducted in agreement with French regulations on privacy and data collection and treatment and was approved by the Advisory Committee on Information Processing for Research (CCTIRS, authorization 16.788), the French Data Protection Authority (CNIL, declaration 2003604) and the local ethics committee.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2017.04.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Lieberman D., Shimoni A., Shemer-Avni Y., Keren-Naos A., Shtainberg R., Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138:811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings L.C., Anderson T.P., Beynon K.A., Chua A., Laing R.T.R., Werno A.M. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 3.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das D., Le Floch H., Houhou N., Epelboin L., Hausfater P., Khalil A. Viruses detected by systematic multiplex polymerase chain reaction in adults with suspected community-acquired pneumonia attending emergency departments in France. Clin. Microbiol. Infect. 2015;21:608. doi: 10.1016/j.cmi.2015.02.014. e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigt S.S., Gregson A.L., Deng J.C., Lynch J.P., Belperio J.A. Respiratory viral infections in hematopoietic stem cell and solid organ transplant recipients. Semin. Respir. Crit. Care Med. 2011;32:471–493. doi: 10.1055/s-0031-1283286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renaud C., Campbell A.P. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr. Opin. Infect. Dis. 2011;24:333–343. doi: 10.1097/QCO.0b013e3283480440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards M.J.M., Edwards J.R.M., Culver D.H., Gaynes R.P. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Kollef M.H. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit. Care Med. 2004;32:1396–1405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- 9.Daubin C., Vincent S., Vabret A., du Cheyron D., Parienti J.-J., Ramakers M. Nosocomial viral ventilator-associated pneumonia in the intensive care unit: a prospective cohort study. Intensive Care Med. 2005;31:1116–1122. doi: 10.1007/s00134-005-2706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolte F.S., Marshall D.J., Rasberry C., Schievelbein S., Banks G.G., Storch G.A. MultiCode-PLx system for multiplexed detection of seventeen respiratory viruses. J. Clin. Microbiol. 2007;45:2779–2786. doi: 10.1128/JCM.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.-K., Oh S.-H., Yun K.A., Sung H., Kim M.-N. Comparison of Anyplex II RV16 with the xTAG respiratory viral panel and Seeplex RV15 for detection of respiratory viruses. J. Clin. Microbiol. 2013;51:1137–1141. doi: 10.1128/JCM.02958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchsinger V., Ruiz M., Zunino E., Martinez M.A., Machado C., Piedra P.A. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax. 2013;68:1000–1006. doi: 10.1136/thoraxjnl-2013-203551. [DOI] [PubMed] [Google Scholar]
- 13.McCullers J.A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voiriot G., Visseaux B., Cohen J., Nguyen L.B.L., Neuville M., Morbieu C. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit. Care. 2016;20:375. doi: 10.1186/s13054-016-1517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong H.-L., Hong S.-B., Ko G.-B., Huh J.W., Sung H., Do K.-H. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One. 2014;9:e95865. doi: 10.1371/journal.pone.0095865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seki M., Kosai K., Yanagihara K., Higashiyama Y., Kurihara S., Izumikawa K. Disease severity in patients with simultaneous influenza and bacterial pneumonia. Intern. Med. 2007;46:953–958. doi: 10.2169/internalmedicine.46.6364. [DOI] [PubMed] [Google Scholar]
- 17.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R. Critical illness from 2009 pandemic influenza A (H1N1) virus and bacterial co-infection in the United States. Crit. Care Med. 2012;40:1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estenssoro E., Ríos F.G., Apezteguía C., Reina R., Neira J., Ceraso D.H. Pandemic 2009 influenza A in Argentina. Am. J. Respir. Crit. Care Med. 2010;182:41–48. doi: 10.1164/201001-0037OC. [DOI] [PubMed] [Google Scholar]
- 20.Ampofo K., Bender J., Sheng X., Korgenski K., Daly J., Pavia A.T. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008;122:229–237. doi: 10.1542/peds.2007-3192. [DOI] [PubMed] [Google Scholar]
- 21.Talbot T.R., Poehling K.A., Hartert T.V., Arbogast P.G., Halasa N.B., Edwards K.M. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am. J. Med. 2005;118:285–291. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Ishizuka S., Yamaya M., Suzuki T., Takahashi H., Ida S., Sasaki T. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J. Infect. Dis. 2003;188:1928–1939. doi: 10.1086/379833. [DOI] [PubMed] [Google Scholar]
- 23.Smith C.M., Sandrini S., Datta S., Freestone P., Shafeeq S., Radhakrishnan P. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. Am. J. Respir. Crit. Care Med. 2014;190:196–207. doi: 10.1164/rccm.201311-2110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolter N., Tempia S., Cohen C., Madhi S.A., Venter M., Moyes J. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J. Infect. Dis. 2014;210:1649–1657. doi: 10.1093/infdis/jiu326. [DOI] [PubMed] [Google Scholar]
- 25.Pavia A.T. What is the role of respiratory viruses in community acquired pneumonia; what is the best therapy for influenza and other viral causes of CAP? Infect. Dis. Clin. North Am. 2013;27:157–175. doi: 10.1016/j.idc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diederen B.M.W., Eerden M.M.V.D., Vlaspolder F., Boersma W.G., Kluytmans J.A.J.W., Peeters M.F. Detection of respiratory viruses and Legionella spp. by real-time polymerase chain reaction in patients with community acquired pneumonia. Scand. J. Infect. Dis. 2009;41:45–50. doi: 10.1080/00365540802448799. [DOI] [PubMed] [Google Scholar]
- 27.Zlateva K.T., de Vries J.J.C., Coenjaerts F.E.J., van Loon A.M., Verheij T., Little P. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur. Respir. J. 2014;44:169–177. doi: 10.1183/09031936.00172113. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser L., Aubert J.-D., Pache J.-C., Deffernez C., Rochat T., Garbino J. Chronic rhinoviral infection in lung transplant recipients. Am. J. Respir. Crit. Care Med. 2006;174:1392–1399. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 29.Molyneaux P.L., Mallia P., Cox M.J., Footitt J., Willis-Owen S.A.G., Homola D. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188:1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S.-H., Huh J.W., Hong S.-B., Lee J.Y., Kim S.-H., Sung H. Clinical characteristics and outcomes of severe rhinovirus-associated pneumonia identified by bronchoscopic bronchoalveolar lavage in adults: comparison with severe influenza virus-associated pneumonia. J. Clin. Virol. 2015;62:41–47. doi: 10.1016/j.jcv.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boivin G., Serres G.D., Hamelin M.-E., Côté S., Argouin M., Tremblay G. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin. Infect. Dis. 2007;44:1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 32.Aitken C., Jeffries D.J. Nosocomial spread of viral disease. Clin. Microbiol. Rev. 2001;14:528–546. doi: 10.1128/CMR.14.3.528-546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect. Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.