Highlights
-
•
The influence of an influenza diagnosis on patient managed during ED visits is examined.
-
•
An influenza diagnosis in the ED is actionable, impacting empiric management in 61% of cases.
-
•
The clinical diagnosis of influenza, based on presenting symptoms, lacks sensitivity at 36%.
-
•
A 30 min result from collection to report could be achieved in the ED for 91.7%, of cases tested.
-
•
ED testing resulted in savings of $200.40/ED visit but is dependent on avoiding planned admissions.
Keywords: Emergency department, Influenza, RT-PCR, Liat, Rapid, Testing
Abstract
Background
Emergency Departments (ED) are challenged during influenza season by patients who present acutely during sporadic ED visits. ED management is largely empiric, often occurring without reliable diagnostics needed for targeted therapies, safe outpatient discharge, or hospital admissions.
Objective
To evaluate the impact of the influenza diagnosis on physician decision making during ED visits using the Cobas Liat® influenza A + B assay.
Study design
Prospective study assessing the impact of rapid (<30 min), reverse-transcriptase polymerase chain reaction (RT-PCR) influenza testing on physician decision making in the ED. Physician responses established pre-and post-diagnosis management courses which required confirmation via secondary documentation in the medical record. Changes in physician decision making were analyzed across four clinical touchpoints: (i) admission/discharge status, (ii) medical procedures, (iii) antiviral and antibiotic prescribing, and (iv) laboratory studies.
Results
An influenza diagnosis changed patient management courses, relative to empiric, pre-diagnosis plans, in in 61% of the cases resulting in cost savings of $49,420-to-$42,270 over 143 patients and 104 days during influenza season resulting in a cost savings of $200.40/ED visit. Evaluation over 2000 ED patient visits projects cost savings > $578,000 due to deferred admissions, and reduction in antiviral prescribing. Sensitivity of ED-based influenza testing using the Cobas Liat® assay was equivalent to centralized lab testing at 98.8% sensitivity and 98.5% specificity respectively.
Conclusion
Providing rapid, RT-PCR influenza testing to ED settings is actionable and used to guide patient care decisions. Understanding the cascade of events linked to the influenza diagnosis in the ED provides overall cost savings which offset the cost of providing ED-based testing.
1. Background
Seasonal influenza is a significant cause of morbidity and mortality contributing to >200,000 hospitalizations and up to 50,000 deaths annually in the United States alone [1]. Diagnostics are frequently challenged by circulating influenza strains and debates over the ideal test(s) for suspected influenza cases are long standing [2,3]. Balancing needs for rapid, sensitive, cost-effective testing is difficult. Reverse-transcriptase polymerase chain reaction (RT-PCR) is acknowledged as gold-standard testing for influenza because many enzyme immune-assays (EIA) lack sensitivity [2]. However, RT-PCR assays are not widely used among Emergency Departments (ED) and ancillary clinics. Recent surveys of 240 US hospitals revealed that 67% rely solely on EIA testing and that fewer than 26% of US hospitals surveyed reported the availability of molecular assays as options within hospitals [4,5].
EDs are particularly challenged during influenza season because they are frequently used as areas of primary medical care. ED visits are sporadic and patients can present acutely, with unknown or incomplete medical histories making establishing ongoing medical relationships and follow-up care difficult. Physician decision making in the ED is largely empiric, occurring without access to diagnostic testing supporting targeted therapy and safe outpatient discharge, or hospital admission for patients in whom their illness requires further care in a general hospital bed or in the ICU.
Molecular influenza testing for ED patients is an area of active investigation and many groups have compared new molecular platforms to traditional testing methods demonstrating increased sensitivity and improved antiviral utilization [6]. Newer PCR assays technologies contribute to overall reductions in length of inpatient stay and time to isolation [7], however, access to RT-PCR was still unable to reach nearly 50% of patients in time to direct admission decisions in the ED [8] because testing occurs in centralized laboratories. Thus, key questions surrounding the clinical utility and financial impact of rapid molecular testing for ED patients still exist.
Few studies have attempted to examine rapid molecular testing within the context of the ED visit, during patient history and physical (H&P exams). Studies examining clinical impact of molecular testing among ED patients limit their analysis to analytical sensitivity and specificity comparisons [9]. Additional studies targeting test utilization focus on antiviral prescribing alone [10], overlooking the cascade of management decisions accompanying an influenza diagnosis. Without studies designed to assess the value of ED-based testing within the time frame of the patient encounter, it remains difficult to assess whether RT-PCR testing performed in the ED provides additional value compared to testing performed in centralized hospital laboratories, typically after the ED visit.
Central to the concept of establishing clinical utility is the assumption that test results will be used to direct patient management during the ED visit and in time to influence physician decision making. Systematic analysis of this assumption is lacking. To address this unanswered question, we employed a novel prospective study design during a 24 h/7days/week evaluation during 104 days of the 2014–2015 influenza season. The primary aim was to evaluate the impact of a new rapid RT-PCR assay, the Roche Cobas Liat® (Liat®) assay, on physician decision making in the ED during the patient visit. Changes in physician decision making post influenza diagnosis were analyzed across four clinical touchpoints. Secondary aims targeted the cost-effectiveness of RT-PCR testing in the ED, which were determined based on hospital charges, physician documentation, international classification of disease coding (ICD) and national ED benchmarking data.
2. Materials and methods
2.1. Study population
The study occurred over 104 days (February to May) during the 2014–2015 influenza season at Hennepin County Medical Center (HCMC) ED ( > 109,000 patient visits annually). Influenza activity in the community was high, averaging 73.9 cases per 100,000 persons resulting in >4000 hospitalizations state-wide with over 2000 of those admissions occurring in metropolitan Minneapolis/St. Paul hospitals, including HCMC [11]. Influenza B viruses predominated from February 2015 to May 23, 2015. During the study period, the CDC’s adjusted vaccination efficacy (VE) against influenza was 23%. Reduced protection against influenza viruses for the 2014–2015 season occurred because > 80% of circulating influenza viruses analyzed by the CDC experienced antigenic “drift” in comparison to viruses covered in the vaccine [12]. Patients presenting to the ED during the study period with at least one sign compatible with influenza infection, including; fever (>= 37.8 °C), cough, sore throat, and muscle aches were eligible to enroll.
2.2. Study design
An investigative group comprising ED physicians, medical residents, students, volunteers, and laboratory staff was assembled to examine the impact of rapid influenza testing on clinical decision making in the emergency department (CLADE). The study was approved by the HCMC Ethics and Institutional Review Board. Changes in physician decision making represented the dependent variable. Patient management plans prior to the influenza diagnosis and prior to testing served as the control variable with which to assess the impact of a rapid, RT-PCR testing in the ED.
2.3. Documentation of management plans and response to the influenza diagnoses
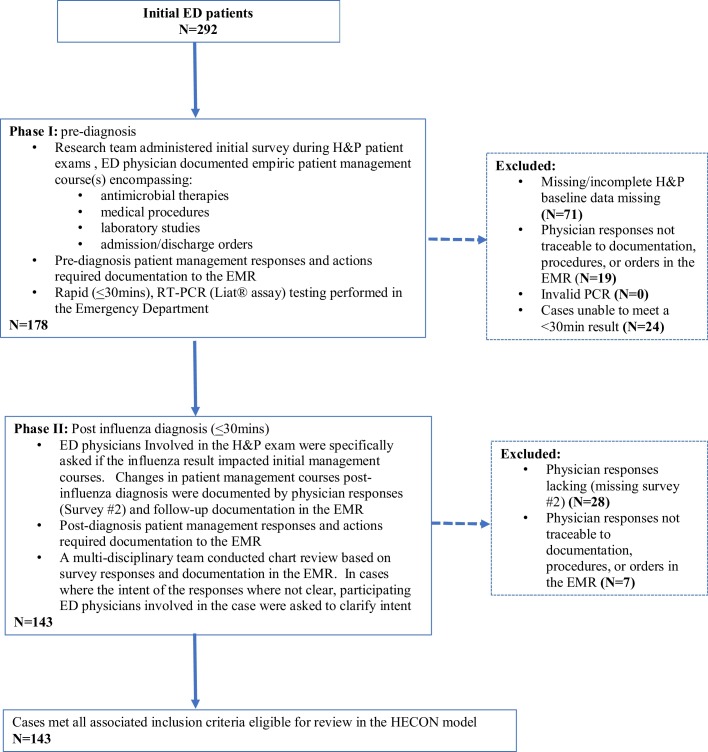
A two-stage prospective study design defining pre-and post-diagnosis management courses captured the relative impact of the influenza diagnosis (Fig. 1 ). Stage 1 defined empiric management courses for ED patients presenting with at least one symptom compatible with influenza. Study surveys, administered to ED physicians prior to influenza testing, as part of ED H&P exams, defined baseline ED management courses. Study surveys comprised a 5-page, 14-question questionnaire spanning four clinical touchpoints: (i) admission/discharge status, (ii) medical procedures, (iii) antiviral and antibiotic prescribing, and (iv) laboratory studies. ED physicians documented the clinical likelihood of influenza based on evaluation of presenting patient symptoms only. The clinical likelihood of influenza was assessed based on a physician’s assessment of high, moderate, or low likelihood on influenza. To minimize subjectivity often associated with survey responses, we required secondary documentation of responses related to medical orders, procedures, labs, and medications stated on the surveys to match documentation and orders reported to electronic medical record (EMR).
Fig. 1.
Study Design.
At the time of the study the Liat® assay had yet to receive Clinical Laboratory Improvement Amendments (CLIA) waiver as a waived test that could be run by non-laboratory personnel outside of the laboratory. Our hospital utilizes a satellite laboratory located in the ED but staffed by laboratory personnel who performed influenza testing on the Liat® assay in the ED. Upon receipt of the influenza RT-PCR test result, secondary study surveys (survey 2) were distributed to the same ED physicians involved in initial H&P exams and captured the impact of the influenza diagnosis and the RT-PCR test result on patient management courses. Survey 2 responses also required ED physicians to document changes in medical management in the EMR and traceability to survey responses in the EMR were required.
2.4. Influenza testing
A total of 292 unique influenza samples from symptomatic patients were collected via nasopharyngeal flocked swabs in 1 ml of universal viral transport media (UTM) (Becton Dickinson; Sparks MD, USA). Samples were tested on the Liat® influenza A/B assay which detects the presence of influenza A and influenza B viral RNA. All testing was performed as per product insert available for the assay. Briefly, nasopharyngeal samples collected in universal transport media were transferred using transfer pipettes to uncapped Liat® cartridges containing all PCR consumables, including extraction. Samples were filled to pre-set volumes established by the Liat® cartridge. Cartridges were re-sealed and placed into the Liat® instrument.
The sensitivity and specificity of Liat® testing was compared to the GenMark E-sensor® RVP assay (Carlsbad, California, USA) as our current gold-standard test result used at our hospital and performed in the centralized laboratory. All GenMark testing was performed in accordance with the manufactures instructions and RNA extraction was performed using the FDA-cleared application of the BioMerieux Easymag (Marcy-I’Etoile, France). Confirmation of positive or negative Liat® results were confirmed with a corresponding positive or negative result from the GenMark E-sensor®. For cases in which test results between the Liat® and the GenMark E-sensor assays differed, a third molecular assay, the Biofire RP assay (Salt Lake City, Utah, USA) was used on the same sample and served as the resolver between conflicting Liat® and GenMark E-sensor results. Biofire RP testing was performed as per manufacturer’s instructions.
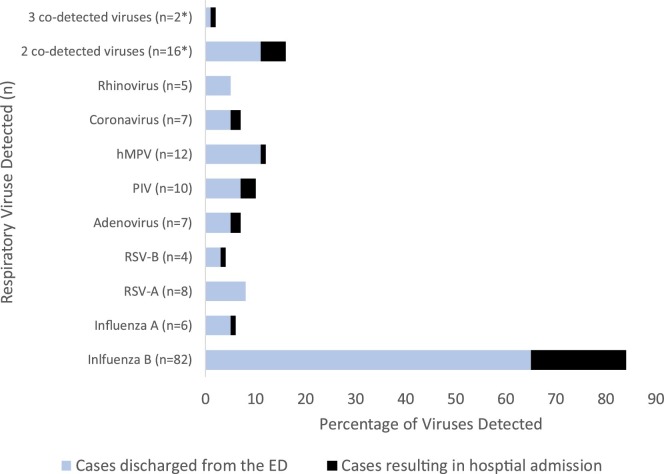
The recognition of other respiratory viruses in patients who present with influenza symptoms is an area of recent investigation which has been made possible by the introduction of a number of FDA cleared respiratory panel-based assays. The GenMark E-sensor assay used as the comparator test in our study detects 10 additional respiratory viruses other than influenza, thus we were able to report the distribution of other respiratory viruses seen in the 143 cases reviewed (Fig. 2 ).
Fig. 2.
Detection of Respiratory Viruses Among Patients Presenting to the Emergency Department.
Values represent percentage of cases for which a respiratory virus was detected. RSV = Respiratory Syncytial Virus. hMPV = human Metapneumonvirus. PIV = Parainfluenza Virus1-3. Influenza B virus was detected in 9 cases with an additional respiratory virus (2 co-detected viruses) and a single case in which three respiratory viruses were detected (3 co-detected viruses). Data represents n = 143 patients meeting all inclusion criteria but total 161 because of the number of cases in which more than one virus was detected but totals. Dark bars represent cases involving hospital admissions from the ED. Light bars depict cases discharged from the ED. Of the cases examined, 21% required admission to the hospital and influenza virus was detected in 68% (23/34) cases. A respiratory virus other than influenza accounted for the remaining 32% of hospital admissions. Of these cases, coronavirus, adenovirus, and PIV were detected in 6%, 6%, and 9% of admitted patients.
2.5. Exclusion criteria for follow up patient evaluation
2.5.1. Exclusion criteria #1
It is often difficult to define the consequence of test results on patient management because other medical conditions impact care. We limited evaluation to patients with a main diagnosis code of pneumonia and influenza (ICD-9 480–488), acute respiratory infections (ICD-9 460–466), or chronic obstructive pulmonary disease and allied conditions (ICD-9490–496). Patients in whom respiratory illness was not coded as the primary medical condition or patients who presented with multiple complexity codes were excluded from evaluation because the impact of the rapid influenza diagnosis could not be as clearly captured compared to patients who presented with isolated and defined respiratory illness.
2.5.2. Exclusion criteria 2
Physician survey responses required verification. We required ED to document survey responses and management plans in the EMR. Cases for which survey responses (primary study surveys; pre-diagnosis) could not be traced to corresponding medical order or medical notes were excluded.
2.5.3. Exclusion criteria #3
In consultation with ED staff, an expected influenza result of ≤30 min was established as a threshold for expected turn-around time needed to guide physician decision making within a typical ED visit. While 143 cases met all associated inclusion criteria and were used for evaluation we felt it was important to assess the turn-around time achieved with the Liat® assay in the ED with the total of 292 total cases tested by the Liat® assay.
2.5.4. Exclusion criteria 4
Cases for which survey responses (secondary study surveys; post-diagnosis) could not be traced to corresponding medical order or medical notes were excluded. In addition to verifying the accuracy of physician responses, secondary documentation was needed in order to assess costs associated with medical management resultant from the influenza diagnosis. In the US, medical coding, and hospital billing is dependent on physician documentation. Therefore, any changes in medical management stated by physicians required traceability to the EMR which defined ICD coding required for a HECON analysis. Failure to compete either phase 1 surveys (n = 71) or phase 2 surveys (n = 28), or the inability to verify physician responses/actions in the EMR (n = 34) were excluded (Fig. 1).
2.6. Health care economic analysis
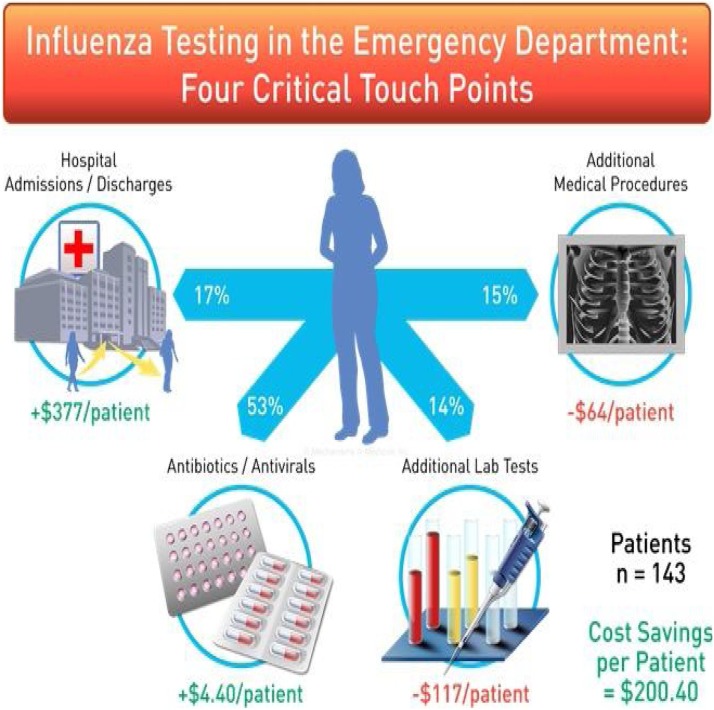
A HECON model was developed to describe the impact of rapid RT-PCR influenza testing in the ED. Management of suspected influenza patients were examined over four clinical touchpoints: (i) admission/discharge status, (ii) medical procedures, (iii) antiviral/antibiotic prescribing, and (iv) laboratory studies (Fig. 3 ). Costs associated with laboratory studies, anti-infective therapies, and medical procedures where taken directly from hospital billing. Costs associated with the admission from the ED to the hospital, for diagnosis of pneumonia and respiratory infection (without complexity coding), were taken from pre-established benchmark standards determined by the National Inpatient Sample databases including the Nationwide Emergency Department Sample and Healthcare Cost and Utilization Project [[13], [14]]. Statistical analysis was performed using binomial one sample test where the null hypothesis was Proportion = 0
Fig. 3.
Association of the Influenza Diagnosis on Patient Management Examined Across Four Touch Points.
Changes in patient management were determined for four cirtitcal touchpoints including changes (relative to initial pre-diagnosis management and H&P exam) admssion/discharge orders, antibiotics/antiviral perscribing, medical procedrues (ultrasound, chest xray, lumbar puncture, electrocardiogram, computerized tomography scan,) and laboratory studies (troponin, sputum culture, blood culture, procalicitonin, respiratory viral panel PCR, d-dimer, urine culture, urine analysis, CBC, c-reactive protein, BMP, Tuberculosis IGRA, legionella urinary antigen, liver enzyme testing). A total of 17%, 53%, 15%, and 17% initial hospital admission orders, anti-microbial prescriptions, medical procedures, and laboratory testing were impacted in response to the rapid influenza test result. Actual costs associated with changes in management reflect both incurred and deferred costs.
3. Results
3.1. Description of patient demographics and comparative test sensitivity
A total of 292 unique patient samples inclusive of both adult (80%) and pediatric (20%) cases were tested in the ED using the Liat® assay and this larger patient set was used to define the analytical test performance of the Liat® assay (Table 1 ) as well as to define the practicality of providing <30 min turn-around times in the ED using the largest patient data set available to us. All patients tested (n = 292) presented with symptoms compatible with the CDC ILI case definition and selection of patients were randomly distributed between February to May.
Table 1.
Sensitivity of the Cobas Liat® Influenza A/B assay compared to the Genmark RVP.
| Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) |
|---|---|---|---|
| N = 292 cases; 84 positive cases, 208 negative cases | |||
| 96.5% (90.03–99.27) | 98.06 (95.1–99.5) | 95.4% (88.5–98.7) | 98.75% (95.8–99.7) |
| Adjusted sensitivity/Specificity/PPV/NPV based on discordant analysis | |||
| 98.8% (93.5–99.9) | 98.5 (95.8–99.7) | 96.5%** (90.0–99.3) | 99.5%** (97.3–99) |
292 test results represent the total number of initial ED patients, regardless of exclusion criteria. *Based on Disease Prevalence of 29.2%. Testing compared to GenMark RVP assay performed in the centralized molecular laboratory.
Adjusted sensitivity based on results of discordant analysis which used Biofire testing and the confirmation of second molecular result needed to verify a positive influenza diagnosis.
**Based on adjusted 28.72% Disease prevalence.
Although 292 patient samples were tested with the Liat® assay, a total of n = 143 (49%) cases met all associated inclusion criteria required for further evaluation in the healthcare economic analysis (HECON) (Fig. 1). All n = 143 patients presented with at least one symptom (fever > = 37.8 °C, cough, sore throat, and muscle ache) consistent with influenza. Median patient age was 37.5 years (range 6 weeks to 86 years). Of these 143 patients, 31% met the CDC criteria for ILI, defined as temperature ≥37.8 °C and at least 1 of: cough or sore throat without another obvious cause, and 73% (104/143) were considered high risk for influenza infection according to established risk factors [[15], [16]].
Influenza-positivity rate was 29% (n = 84). Sensitivity and specificity of the Liat® was 96.5% (95% CI, 90.3-99.3%) and 98.06% (95% CI, 95.1-99.5%), respectively (Table 1). Results between the Liat® and GenMark assays different in 2.4% (n = 7) of the cases. Adjusted data resolved 3/7 (43%) cases in favor of the original Liat® influenza result. Adjusted sensitivities based on addition of a third resolver assay (Biofire RP) were reported at 98.8% (95% CI, 93.5-99.9%) and 98.5% (95% CI, 95.8-99.7%) (Table 1).
The ILI case definition reported a sensitivity of 34.1% (95% CI, 20.5-49.9) and a specificity of 50% (95% CI, 39.2-60.9%) respectively (Table 2 ). Clinical diagnosis, determined by ED physician assessment, reported a sensitivity and specificity of 36% (95% CI, 10.8-38.5%) and 85.6% (95% CI, 77.3-91%) respectively. Presence of fever carried a sensitivity and specificity of 25.7% (95% CI, 12.5-43.3) and 52.1% (95% CI, 42.7-61.5) respectively.
Table 2.
Sensitivity of the Clinical Assessment of Influenza in the ED.
| N = 44/143 (31%) |
N = 61/143 (43%) |
N = 99/143 |
N = 21/143 |
N = 23/143 |
|
|---|---|---|---|---|---|
| ILI case definition* | Fever as presenting symptom | Physician assessment! low probability | Physician assessment! moderate probability | Physician assessment high! probability of influenza | |
| Sensitivity% (95% CI) | 34.1 (20.5–49.9) | 25.7 (12.5–43.3) | 15.0 (5.7–29.8) | 62.5 (45.8–77.3) | 36% (10.8–38.5) |
| Specificity% (95% CI) | 50.0 (39.2–60.9) | 52.1 (42.7–61.5 | 85.6 (77.3–91.7) | 28.9 (20.4–38.6) | 85.58 (77.3–91.7) |
| False positive rate% | 61% | 75% | (31% false Negative) | 69% | 64% |
| PPV (95% CI) | 25.4 (15–38.4) | 13.85 (6.5–24.6) | 28.75 (11.3–52.2) | 25.3 (17.1–34.9) | 37.5 (18.8–59.4) |
| NPV (95% CI) | 60.3 (48.1–71.6) | 70.1 (59.4–79.5) | 72.4 (63.6–80.0) | 66.7 (51.1–80.0) | 74.2 (65.4–81.7) |
| Positive likelihood ratio (95% CI) | 2.71 (2.35–3.13) | 0.54 (0.3–0.97) | 1.04 (0.4–2.5) | 0.88 (0.67–1.2) | 1.56 (0.7–3.3) |
| Negative likelihood ratio (95% CI) | 1.42 (1.1–1.85) | 0.99 (0.9–1.2) | 1.30 (0.79–2.2) | 0.91 (0.8–1.1) |
Based on total number of ED patients meeting all inclusion criteria (N = 143).
95% confidence interval.
*The ILI case definition in use by the CDC is defined by: ILIcdc. Fever (at least 37.8 ° Celsius) At least 1 of: cough or sore throat. Without a known cause other than influenza
! Physician assessment of influenza was determined by directly querying the likelihood of influenza in presenting ED patients based on presenting symptoms alone and made prior to influenza testing. Clinically likelihood of influenza was recorded as direct responses from ED physicians based on their assessment of high, moderate, and low likelihood on influenza.
Among influenza negative cases, a respiratory virus other than influenza A or influenza B was detected in 43% (61/143) of cases. Two or more viruses in an individual patient were detected in 13% (18/143) of cases and in 12% of influenza positive cases. Of the cases examined, 23.7% (30/143) required admission to the hospital and influenza virus was detected in 68% (23/34) of these cases. A respiratory virus other than influenza accounted for the remaining 32% of hospital admissions. Of these cases, coronavirus, adenovirus, and parainfluenza viruses were detected in 6%, 6%, and 9% of admitted patients respectively.
3.2. The effect of influenza test results on clinical decision making in the ED
Influenza was detected in 61/143(43%) of the cases evaluated, however, changes in patient management, resultant from rapid influenza testing in the ED, involved 87/143(61%) (p = 0001) (Table 3 ). Changes in patient management were assessed relative to empiric management courses, established prior to influenza testing. Changes to patient management courses were 2.3 times more likely to occur among influenza negative cases compared to influenza positive cases, highlighting the importance of sensitive testing which can assure a high negative predictive value.
Table 3.
Impact of Rapid Influenza testing on clinical decision making in the ED for suspected Influenza Patients (n = 143).
| Clinical Touchpoint | Total cases impacted (%) | % reduction in utilization/change in discharge | % increase in utilization or admission |
|---|---|---|---|
| 61%* (87/143) | |||
| Antimicrobial! prescribing total | 58% (83/143) | 10% (14/143) 
|
15% (21/143) 
|
| Antibiotic prescribing | 33.5% (48/143) | 9% (13/143) 
|
33.6% (48/143) 
|
| Antiviral prescribing | 39.2% (56/143) | 24.5% (35/143) 
|
14.7% (21/143) 
|
| Medical Procedures/Imaging | 15.4% (22/143) | 2.1% (3/143) 
|
13.2% (19/143) 
|
| Laboratory studies | 14% (20/143) | 2.8% (4/143) 
|
11.1% (16/143) 
|
| Hospital Admission/Discharge | 18% (26/143) | 10.5% (15/143) 
|
7.7% (11/143) 
|
Based on total number of ED patients meeting all inclusion criteria (N = 143).
*P = .001; binomial one sample test where the null hypothesis was Proportion = 0.
! Antimicrobial prescribing refers to sum of antibiotic and antiviral prescribing.
Administration and discontinuation of both anti-viral and anti-microbials as a result of the influenza diagnosis were the most commonly documented change in management occurring in 58% (n = 83/143) (p = .001) of the cases which resulted in a decreased inappropriate/unsupported antibiotic and anti-viral and prescribing by 24.5% and 9% respectively. Inappropriate antibiotic prescribing was defined as use of an antibiotic for a confirmed influenza infection. Conversely, inappropriate anti-viral (oseltamivir) prescribing was defines as use of an antiviral in the setting of a negative influenza diagnosis. Negative influenza results increased laboratory testing and additional medical procedures in 14% (n = 20) and 15% (n = 22) of the cases respectively. Changes to admission and discharge status occurred in 18% (26/143) of the cases accounting for 30% (26/87) of all documented changes in patient management. A total of 15/143 patient (10.5%) avoided an initially planned hospital admission when provided with the rapid influenza result. Conversely, 11/143 (7.7%) patients for whom an initial hospital admission was not planned were subsequently admitted because of a positive influenza test result.
Whilst debates over the turn-around time needed to provide PCR testing for influenza in the ED exist, there is little debate regarding the intent of ED-based PCR testing. In order for testing to provide tangible benefits, results should be actionable where the key objective of testing should be to produce results quicker than could be routinely offered by contemporary or centralized testing or within the context of discrete patient visits. In consultation with ED staff, we defined a turn-around time for Liat® testing at <30 min. Among the total 292 cases tested, 91.7%, (268/292) received an influenza result in the ED within ≤30 min from the order entry. For 24 cases, a “rapid” result (≤30 min) could not be achieved. Among cases for which a < 30 min turn-around time could not be achieved the average turn-around time was 46-min (range 36–120-min). However, among these 24 cases, 45.8% (11/24) involved patient encounters in the ED stabilization room which require higher levels of care within the ED. These patients often have longer, more complicated ED management, thus it is understandable why many of the patients seen in ED stabilization rooms would experience delays in laboratory testing because testing becomes less of a priority compared to the resuscitation of the patient that occurs in ED stabilization rooms. Additional factors contributing to turn-around times >30 min could not be determined for the remaining 13 cases.
3.3. Financial implications of influenza testing in the ED among suspected influenza patients
Deferred costs associated with antiviral prescribing resulted in net cost savings of $1288 over 104 days for 143 unique patient encounters but increased antibiotic utilization by net $601. Costs associated with combined antiviral/antibiotic prescribing, accounted for net savings of $616 equating to an overall cost savings of $4.40/patient/ED visit. Follow up medical procedures accounted for an increase of $64/patient/ED visit. An increase in laboratory testing post influenza diagnosis was noted resulting in a net increase of $117/patient/ED visit. Changes to admission/discharge orders were associated with a net cost savings of $42,270 to $49,420 over a 104-day period resulting in cost savings of $377.00/patient/ED visit. Overall costs savings associated with access to RT-PCR testing in the ED resulted in $200.40/patient/ED visit. Projections over an extended influenza season (2000 ED visits) including estimated cost of providing testing resulted in net cost savings of $578,627 to $678,627 (Table 4 ).
Table 4.
Healthcare Economic Model based on 2000 ED visits.
| Patient involved | Costs Incurred ($USD) | Cost Avoidance ($USD) | Net ($USD) | Cost per ED visit ($USD) | |
|---|---|---|---|---|---|
| N = 143 (observed) | +$377/ED visit | ||||
| Anti-viral medication | −1932 | +3220 | +1288 | +4.40 | |
| Antibiotics | −2912 | −2311 | −601 | ||
| Laboratory Studies | −1872 | +468 | −1404 | −117.00 | |
| Medical Procedures/Imaging | −1216 | +192 | −1024 | −64.00 | |
| Admission Change (n = 11) | (new admission) | 155.573 | + 56,570 | ||
| Admission Change (n = 15) | (deferred admission) | 212,145 | |||
| Costs of RT-PCR testing* | 50 −100/test | −7150 to 14,300 | |||
| Overall Healthcare associated costs | + 49,420 to 42, 270 | ||||
| Totals | N = 2000 (projected) | ||||
| Anti-viral medication | 27,020 | 45,034 | +18,014 | ||
| Antibiotics | 13,706 | 5090 | −8615 | ||
| Laboratory Studies | 26,181 | 6545 | +19,635 | ||
| Medical Procedures/Imaging | 17,006 | 2685 | −14,320 | ||
| Admission Change (n = 153) | (new admission) | 2,163,879 | |||
| Admission Change (n = 209) | (deferred admission) | +2,967,062 | |||
| Cost of RT-PCR testing | $100,000–200,000 | −100,000 to 200,000 | |||
| Overall Healthcare associated costs | +578,627 to 678,627 | ||||
Costs associated with antiviral, antimicrobial, and laboratory studies were taken from direct hospital billing. The costs associated with ED admissions were based on established data sets available through HCUP Nationwide Emergency Department Sample (NEDS). Healthcare Cost and Utilization Project (HCUP). 2007, 2008, 2009. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nedsoverview.jsp. *Cost associated with RT-PCR testing is provided as an estimate, as institution-specific pricing will vary.
4. Discussion
Optimal patient management relies on recognition of appropriate clinical symptoms by a knowledgeable healthcare provider and the availability of reliable diagnostics with strong predictive values capable of allowing management decisions to be made with confidence.
We demonstrate that access to rapid (≤30 min), sensitive, RT-PCR testing for influenza impacted patient management in 61% of cases, demonstrating rapid influenza results in the ED are actionable and used by ED physicians to help guide patient management. Value propositions examining rapid RT-PCR testing demonstrate reductions in unnecessary antibiotic prescriptions [19] decreased time to diagnosis [6], and reduced length of stay within EDs [20]. Rogers and colleagues recently compared rapid multiplex PCR testing to traditional batch PCR testing among pediatric ED patients, further demonstrating the associations between rapid diagnosis antibiotic stewardship, reduced length of stay, and decreased time to diagnosis [8]. However, despite statistical significance with respect to time to diagnosis, testing was unavailable for 48% of pediatric ED patients in time to influence ED admission [8]. While such studies define the clinical value of rapid RT-PCR-based influenza however, they fall short of establishing clinical utility of ED testing because testing occurs in centralized laboratory locations after the ED visit. Our study is one of the first attempts to examine the role of providing the diagnosis during the ED visit and within the ED using rapid point of care molecular testing.
Our experience demonstrates that providing the diagnosis nearer to patient presentation continues to be an actionable event that impacts medical management for 61% of cases. The greatest impact on empiric medical management affected antimicrobial stewardship processes, highlighting the relationship between rapid diagnostics and appropriate antibiotic and antiviral prescribing previously described [[10], [5]]. As developments in small molecule and monoclonal therapies for influenza infection grow [[21], [22], [23], [24]], the importance of access to rapid, sensitive diagnostics capable of guiding antiviral prescribing and treatment decisions will become important, a finding supported by our experience. Requirements for diagnostic support of suspected influenza cases is particularly relevant given recent experience demonstrating that clinical recognition of influenza in presenting ED patients lacks sensitivity [[25], [15]]. Our experience confirms that of previous investigators who found that clinical sensitivity for influenza among ED patients was 36% [25].
Ancillary test ordering, including laboratory testing and medical procedures, increased post influenza diagnosis. This finding is in contrast to previous studies that concluded rapid testing can control ancillary testing [[26], [8], [27]]. These differences can be explained, in part, because we examined a wider range of laboratory and medical procedures compared to other studies. Additionally, studies demonstrating reductions in test ordering frequently focus only on positive influenza results. Given this bias, it is highly likely that the vast majority of influenza testing, which is often negative, results in greater utilization of laboratory testing because testing expands to support narrowing differential diagnoses.
Cost savings associated with a rapid RT-PCR testing in the ED was $200.40/patient visit, based largely on deferral of a handful of patients in whom the influenza diagnosis allowed for safe discharge from the ED. Expanded data over 2000 ED-patient visits reports cost savings of $ 578, 627 to $678,627, demonstrating diagnostic support is an important factor in supporting efficient patient care while helping to limit healthcare costs.
We conclude that our findings are an underestimation of the overall clinical impact of influenza testing observed in our study because of our study design. Many influenza series rely on pre-and post-testing cohorts which are subject to differences in patient acuity, seasonal influenza strains, and differences among physician behavior which collectively influence data sets. Patients who are tested for influenza during flu season often present with additional co-morbidities. In such patients, management decisions and length of hospital stay are influenced by medical issues independent of influenza status which are not accounted for in the vast majority of pre-and post-intervention studies. In order to determine the impact of the influenza diagnosis on patient management we felt it was important to minimize confounding variables which independently affect physician management decisions. While this approach allowed us to further target the impact of influenza testing, it also excluded a number of patients for whom the influenza diagnosis is important. Additionally, 66% (n = 31) of the patients in whom a ≤30 min test result could not be achieved spent time in the ED stabilization room. The acuity of patients who require stabilization in the ED is high and while these patients undoubtedly have additional medical conditions other than respiratory infection they also benefit from rapid access to their influenza diagnosis. Thus, while we limited evaluation to patients in whom influenza testing could be stringently analyzed, we acknowledge that some patients for whom an influenza diagnosis would be helpful were excluded as part of our study design. Limiting patient enrollment to define respiratory infection allowed us to target the effect of the diagnosis, and while this study group demonstrated positive clinical and financial impacts of the rapid influenza diagnosis it is likely an underestimation of the value of providing rapid ED-based RT-PCR testing.
The costs and infrastructure needed to support ED-based testing fall outside the scope of the current study aimed at defining the utilization of the influenza diagnosis in the ED. However, recent experience with the Liat® assay using various ED and clinic locations indicates that testing can be reliably performed by non-laboratory staff such as nurses, physicians, or allied-health professionals [28]. It should be noted that during our study, testing occurred in the ED environment but was performed by laboratory staff. While we acknowledge the utility of testing using non-laboratory personnel in the ED, the primary aims of our study were to: [1] provide influenza testing within the ED; [2] to deliver a result within the time frame of typical ED visits; and [3] to further describe the impact of the diagnosis on patient management. Any utility gained by using non-laboratory personnel to perform testing requires first demonstrating that providing testing is of clinical benefit. The infrastructure needed to support point-of care testing is complex, requiring staffing support, laboratory oversight (where available), physician space, and education, which are often poorly defined in many studies and were not addressed in our study. Many of these variables were in place prior to our study, which did not impact our costs associated with testing, although we acknowledge that the costs associated with ED-based testing may be different for others. Our findings demonstrate that providing the RT-PCR testing in the ED during the patient visit is an actionable result that influenced and changed physician decision making in 61% of the cases and can be a cost-effective approach. These conclusions lend support for additional studies that can systematically describe the operational insights needed for point-of care testing.
Reports describing the epidemiology of respiratory viruses other than influenza among ED patients [17] and clinical concerns over viruses other than influenza [18] continue to evolve and lend support for comprehensive panel-based approaches to respiratory virus testing. Whether syndromic-respiratory virus testing can be performed during ED visits in time to influence management is currently a debate, and many panel-based respiratory tests are often reserved or hospitalized or acutely ill patients because the utility of comprehensive respiratory virus testing has not been established among ED populations. Our findings support influenza infection as the primary virus detected in presenting patients and the primary virus associated with the majority to hospitalized patients from the ED.
A potential limitation of our study was the lack of circulating influenza A virus at the time of evaluation as only 4% of cases were positive for an influenza A virus. Recent studies indicates the LIAT assay is highly sensitive against both influenza A & B viruses [9]. The lack of influenza A present during the time of our study is a reality of the epidemiology of seasonal influenza infections. However, clinical experience demonstrates that both influenza A and B can cause similar illness, present with overlapping clinical presentations and typically respond similarly to oseltamivir therapy. Efforts to capture the impact of the influenza diagnosis in the ED are inclusive of “influenza infection,” thus we interpret our findings in the context of influenza despite the relatively low numbers of influenza A infection encountered during the time of our study.
Benefits of diagnostic testing are derived from identifying and treating true positives, weighed against the harm or failing to detect influenza in symptomatic patients. During the events of pandemic H1N1 outbreak, early treatment was associated with a decrease in mortality compared to no treatment (odds ratio 0.35; 95% confidence interval 0.18–0.71) [29] emphasizing the importance of diagnostic support for timely antiviral response.
As US healthcare struggles with rising costs and waste in healthcare expenditure, diagnostic testing is increasingly viewed as an important component of patient management, which can influence hospital costs. Expanding diagnostic testing to non-traditional testing locations such as EDs is an area of active interest but cost-benefit analyses are required. We conclude that applying rapid, accurate RT-PCR testing for influenza in the ED influenced patient management for 61% of cases studied resulting in cost savings of $49,420-to-$42,270 or $200.40/ED visit. Providing rapid, RT-PCR testing to ED settings should warrant further considerations.
Financial support and conflicts of interest
Partial funding for this study was provided by an unrestricted educational grant from Roche molecular to GTH and from the Minneapolis Medical Research (grant no. 7627). We thank Dr. Sara Love for providing critical comments and we thank the faculty and staff in the Department of Emergency Medicine at Hennepin County Medical Center.
Contributor Information
Glen T. Hansen, Email: Glen.Hansen@hcmed.org.
Johanna Moore, Email: Johanna.Moor@hcmed.org.
Emily Herding, Email: Emily.Herdin@hcmed.org.
Tami Gooch, Email: Tami.Gooc@hcmed.org.
Diane Hirigoyen, Email: Diane.Hirigoyen@hcmed.org.
Kevan Hanson, Email: Kevan.Hanso@hcmed.org.
Marcia Deike, Email: Marica.Dieke@hcmed.org.
References
- 1.CDC Estimates of deaths associated with seasonal Influenza–United states, 1976–2007. Morb. Mortal Wkly Rev. 2010;59(33):1057–1062. 2. [PubMed] [Google Scholar]
- 2.Dunn J.J., Ginocchio C.C. Can newly developed, rapid immunochromatographic antigen detection tests be reliably used for the laboratory diagnosis of influenza virus infections? J. Clin. Microbiol. 2015;53(6):1790–1796. doi: 10.1128/JCM.02739-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chartrand C., Pai M. How accurate are rapid influenza diagnostic tests? Expert Rev. Anti-Infect. Ther. 2012;10(6):615–617. doi: 10.1586/eri.12.49. [DOI] [PubMed] [Google Scholar]
- 4.Su S., Fry A.M., Kirley P.D., Aragon D., Yousey-Hindes K., Meek J., Openo K., Oni O., Sharangpani R., Morin C., Hollick G., Lung K., Laidler M., Lindegren M.L., Schaffner W., Atkinson A., Chaves S.S. Survey of influenza and other respiratory viruses diagnostic testing in US hospitals, 2012–2013. Influenza Other Respir. Viruses. 2016;10(2):86–92. doi: 10.1111/irv.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busson L., Mahadeb B., De Foor M., Vandenberg O.H.M. Contribution of a rapid influenza diagnostic test to manage hospitalized patients with suspected influenza. Diagn. Microbiol. Infect. Dis. 2017;87(3):238–242. doi: 10.1016/j.diagmicrobio.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Xu M., Qin X., Astion M.L., Rutledge J.C., Simpson J., Jerome K.R., Englund J.A., Zerr D.M., Migita R.T., Rich S., Childs J.C., Cent A., Del Beccaro M.A. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am. J. Clin. Pathol. 2013;139:118–123. doi: 10.1309/AJCPH7X3NLYZPHBW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rappo U., Schuetz A.N., Jenkins S.G., Calfee D.P., Walsh T.J., Wells M.T., Hollenberg J.P., Glesby M.J. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J. Clin. Microbiol. 2016;54(8):2096–2103. doi: 10.1128/JCM.00549-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers B.B., Shankar P., Jerris R.C., Kotzbauer D., Anderson E.J., Watson J.R., O’Brien L.A., Uwindatwa F., McNamara K., Bost J.E. Impact of a rapid respiratory panel test on patient outcomes. Arch. Pathol. Lab. Med. 2015;139(5):636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 9.Nolte F.S., Gauld L., Barrett S.B. Direct comparison of alere™ i and cobas® liat influenza a and B tests for rapid detection of influenza virus infection. J. Clin. Microbiol. 2016;(August 31) doi: 10.1128/JCM.01586-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson R.E., Stockmann C., Hersh A.L., Pavia A.T., Korgenksi K., Daly J.A., Couturier M.R., Ampofo K., Thorell E.A., Doby E.H., Robison J.A., Blaschke A.J. Economic analysis of rapid and sensitive polymerase chain reaction testing in the emergency department for influenza infections in children. Pediatr. Infect. Dis. J. 2015;34(6):577–582. doi: 10.1097/INF.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 11.http://www.health.state.mn.us/divs/idepc/diseases/flu/stats/2015summary.pdf.
- 12.https://www.cdc.gov/flu/pastseasons/1415season.htm.
- 13.HCUP Nationwide Emergency Departmet Sample (NEDS). Healthcare cost and utilization Project (HCUP). 2013, Www.hcup-us.ahrq.gov/nedsoverview.jsp.
- 14.Www.hcup-us.ahrq.gov/reports/statbriefs/sb168, https://www.hcup-us.ahrq.gov/reports/statbriefs/sb168-Hospital-Costs-United-States-2011.jsp, (2009).
- 15.Falsey A.R., Baran A., Walsh E. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir. Viruses. 2015;(Suppl. 1):23–29. doi: 10.1111/irv.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.cdc.gov/flu/professionals/diagnosis/rapidclin.htm.
- 17.Yu X., Lu R., Wang Z., Zhu N., Wang W., Julian D., Chris B., Lu J.T.W. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in beijing. PLoS One. 2012;7(2):e32174. doi: 10.1371/journal.pone.0032174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turgay C., Emine T., Ozlem K., Muhammet S.P., Haydar A.T. A rare cause of acute flaccid paralysis: human coronaviruses. J. Pediatr. Neurosci. 2015;10(3):280–281. doi: 10.4103/1817-1745.165716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozkaya E., Cambaz N., Coşkun Y., Mete F., Geyik M.S.N. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Pediatr. 2009;98(10):1589–1592. doi: 10.1111/j.1651-2227.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonner A.B., Monroe K.W., Talley L.I., Klasner A.E., Kimberlin D.W. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2) doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 21.Jordan P.C., Stevens S.K., Tam Y., Pemberton R.P., Chaudhuri S., Stoycheva A.D., Dyatkina N., Wang G., Symons J.A., Deval J.B.L. Activation pathway of a nucleoside analog inhibiting respiratory syncytial virus polymerase. ACS Chem. Biol. 2017;20(12):83–91. doi: 10.1021/acschembio.6b00788. [DOI] [PubMed] [Google Scholar]
- 22.Perron M., Stray K., Kinkade A., Theodore D., Lee G., Eisenberg E., Sangi M., Gilbert B.E., Jordan R., Piedra P.A., Toms G.L., Mackman R.C.T. GS-5806 inhibits a broad range of respiratory syncytial virus clinical isolates by blocking the virus-cell fusion process. Antimicrob. Agents Chemother. 2015;60(3):1264–1273. doi: 10.1128/AAC.01497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baranovich T., Jones J.C., Russier M., Vogel P., Szretter K.J., Sloan S.E., Seiler P., Trevejo J.M., Webby R.J., Govorkova E.A. The hemagglutinin stem-Binding monoclonal antibody VIS410 controls influenza virus-induced acute respiratory distress syndrome. Antimicrob. Agents Chemother. 2016;25(60):4. doi: 10.1128/AAC.02457-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paules C.I., Lakdawala S., McAuliffe J.M., Paskel M., Vogel L., Kallewaard N.L., Zhu Q.S.K. The hemagglutinin a stem antibody MEDI8852 prevents and controls disease and limits transmission of pandemic influenza viruses. J. Infect. Dis. 2017;216(3):356–365. doi: 10.1093/infdis/jix292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugas A.F., Valsamakis A., Atreya M.R., Thind K., Alarcon Manchego P., Faisal A., Gaydos C.A., Rothman R.E. Clinical diagnosis of influenza in the ED. Am. J. Emerg. Med. 2015;33(6):770–775. doi: 10.1016/j.ajem.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaschke A.J., Shapiro D.J., Pavia A.T., Byington C.L., Ampofo K., Stockmann C.H.A. A national study of the impact of rapid influenza testing on clinical care in the emergency department. J. Pediatr. Infect. Dis. Soc. 2014;3(2):112–118. doi: 10.1093/jpids/pit071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito S., Marchisio P., Morelli P., Crovari P.P.N. Effect of a rapid influenza diagnosis. Arch. Dis. Childhood. 2003;88:525–526. doi: 10.1136/adc.88.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson J., Schechter-Perkins E.M., Mitchell P., Mace S., Tian Y., Williams K., Luo R., Yen-Lieberman B. Multi-center evaluation of the cobas® Liat® Influenza A/B & RSV assay for rapid point of care diagnosis. J. Clin. Virol. 2017;95:5–9. doi: 10.1016/j.jcv.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Muthuri S.G., Myles P.R., Venkatesan S., Leonardi-Bee J., Nguyen-Van-Tam J.S. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J. Infect. Dis. 2013;15(207):553–563. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]