Abstract
Among the 11 members of the secreted phospholipase A2 (sPLA2) family, group IID, IIE, IIF and III sPLA2s (sPLA2-IID, -IIE, -IIF and -III, respectively) are “new” isoforms in the history of sPLA2 research. Relative to the better characterized sPLA2s (sPLA2-IB, -IIA, -V and -X), the enzymatic properties, distributions, and functions of these “new” sPLA2s have remained obscure until recently. Our current studies using knockout and transgenic mice for a nearly full set of sPLA2s, in combination with comprehensive lipidomics, have revealed unique and distinct roles of these “new” sPLA2s in specific biological events. Thus, sPLA2-IID is involved in immune suppression, sPLA2-IIE in metabolic regulation and hair follicle homeostasis, sPLA2-IIF in epidermal hyperplasia, and sPLA2-III in male reproduction, anaphylaxis, colonic diseases, and possibly atherosclerosis. In this article, we overview current understanding of the properties and functions of these sPLA2s and their underlying lipid pathways in vivo.
Keywords: Secreted phospholipase A2, Lipidomics, Knockout mice, Transgenic mice, Lipid-metabolic pathways, Pathophysiological function
1. Introduction
As already described in other reviews in this special issue, the secreted PLA2 (sPLA2) family contains 10 catalytically active isoforms (IB, IIA, IIC, IID, IIE, IIF, III, V, X and XIIA) and one inactive isoform (XIIB) in mammals [[1], [2], [3], [4], [5], [6]]. Individual sPLA2s exhibit unique tissue and cellular distributions and substrate selectivity, suggesting their distinct biological roles. Historically, sPLA2-IB and -IIA were purified and cloned in the 1980s, and sPLA2-IIC and -V were identified by genomic sequencing of the locus close to the sPLA2-IIA (PLA2G2A) gene in 1994 [[7], [8], [9], [10], [11]]. Soon afterwards, in the period of “sPLA2 hunting” research from 1997 to the early 2000s, sPLA2-IID, -IIE, -IIF, -III and -X as well as two sPLA2-XII isoforms were identified by EST database searches (“new” sPLA2s) by Lambeau's (a guest editor of this special issue) group, as well as by others [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. There is another sPLA2-related protein called otoconin-95, an inner ear structural protein that contains two catalytically inactive sPLA2-like domains [22].
Among the “new” sPLA2s, sPLA2-IID, -IIE and -IIF are classified as conventional sPLA2s (group I/II/V/X), which are closely related, low-molecular-weight enzymes with a highly conserved Ca2+-binding loop and a His/Asp catalytic dyad as well as conserved disulfide bonds. More specifically, they are members of the group II subfamily of sPLA2s, to which sPLA2-IIA and –IIC, as well as sPLA2-V, belong. The genes for these 6 group II subfamily sPLA2s are clustered at the same chromosomal locus (chromosomes 1 and 4 in human and mouse, respectively), suggesting that they originated from a common ancestral gene [18]. In contrast, sPLA2-III is an atypical sPLA2 showing closer similarity to bee venom group III sPLA2 than to the other mammalian sPLA2s [19]. Evolutionally, the group II subfamily sPLA2s exist only in vertebrates, while sPLA2s in the group III branch are present in vertebrates and insects but not in nematodes. Although currently known sPLA2 inhibitors can inhibit conventional sPLA2s to various degrees, no agent that specifically inhibits sPLA2-III or sPLA2-XIIA, another class of atypical sPLA2, has yet become available.
Although the properties and functions of sPLA2-IB, -IIA, -V and -X (see other reviews in this special issue) have been described in numerous studies, those of other sPLA2s have remained poorly understood for more than a decade. Recently, a series of studies using knockout and transgenic mice for nearly a full set of sPLA2s, together with comprehensive lipidomics approaches to identify their cognate substrates (phospholipids) and products (fatty acids, lysophospholipids or their metabolites), have clarified their distinct biological roles in vivo. Although genes for the group II subfamily sPLA2s are clustered in the same chromosome locus [18], the phenotypes observed in knockout mice for individual sPLA2s are distinct (see below), implying that they do not have compensatory functions. In this review, we provide an overview of current knowledge on the properties and functions of sPLA2-IID, -IIE, -IIF and -III. The roles of these sPLA2s and their underlying lipid-metabolic pathways are summarized in Table 1 .
Table 1.
Biological functions of sPLA2-IID, -IIE, -IIF and -III as revealed by studies using knockout (KO) and transgenic (TG) mice.
| Nomenclature | General names | Distributions | Lipid mobilizations | Phenotypes in KO mice | Phenotypes in TG mice | References |
|---|---|---|---|---|---|---|
| PLA2G2D | sPLA2-IID | Lymphoid DCs, M2 macrophages | Preferential production of ω3 PUFA-derived pro-resolving lipid mediators | Exacerbation of CHS and psoriasis | Reduction of CHS and psoriasis | [25,31] |
| Increased anti-tumor immunity, Protection from skin cancer | Decreased anti-tumor immunity | [31] | ||||
| Production of PGD2 | Increased anti-viral immunity, Protection from SARS-induced pneumonia | N.D. | [46] | |||
| PLA2G2E | sPLA2-IIE | Adipocytes | Hydrolysis of minor lipoprotein phospholipids (e.g. PE and PS) | Protection from diet-induced obesity and hyperlipidemia | N.D. | [62] |
| Unknown | Age-related fat accumulation and reduced lipolysis | N.D. | [66] | |||
| Hair follicles | Release of various unsaturated fatty acids and LPE species | Modest abnormalities in hair follicles | N.D. | [55] | ||
| PLA2G2F | sPLA2-IIF | Suprabasal keratinocytes | Production of lysoplasmalogen | Protection from psoriasis, CHS and skin cancer | Spontaneous development of psoriasis-like epidermal hyperplasia and alopecia, Exacerbation of skin cancer | [45] |
| PLA2G3 | sPLA2-III | Epididymal epithelial cells | Sperm membrane phospholipid remodeling | Male hypofertility | N.D. | [93] |
| Mast cells | Production of microenvironmental PGD2 | Impaired mast cell maturation and anaphylaxis | Increased mast cell maturation and anaphylaxis | [34] | ||
| Colonic epithelial cells | Production of LPA and LPI | Protection from colonic cancer and colitis | N.D. | [92] | ||
| Aortaa | Generation of LPC-rich small-dense LDL | N.D. | Exacerbation of atherosclerosis | [116] | ||
| Skina | Production of PGE2 | N.D. | Spontaneous skin inflammation | [124] |
N.D.; not done.
Results from TG overexpression.
2. sPLA2-IID
2.1. General aspects
sPLA2-IID, which is more similar to sPLA2-IIA (48% homology) than to other sPLA2s, was identified by three groups; Lambeau's group [14] and Hanasaki's group [13] independently identified the mouse and human enzymes by EST database searches, and another group isolated sPLA2-IID (called SPLASH) by subtraction cloning of splenic cDNA from wild-type (WT) and lymphotoxin-deficient mice, which have profound defects in the splenic microarchitecture [15]. Similar to sPLA2-IIA, sPLA2-IID is a basic protein (pI ~ 8.7) made up of 125 amino acids with 14 cysteines at exactly conserved positions. Likely because of its cationic nature, sPLA2-IID binds to heparin in vitro or heparan sulfate on the cell surface when overexpressed in cultured cells [23].
Initial studies revealed that recombinant sPLA2-IID had rather lower enzymatic activity than sPLA2-IIA in an assay using phospholipid vesicles with oleic acid (OA) or linoleic acid (LA) at the sn-2 position [14] and that transfection of sPLA2-IID into cultured cells (e.g. HEK293 cells) was able to augment IL-1β-induced arachidonic acid (AA) release and prostaglandin E2 (PGE2) generation, albeit at a lower potency than sPLA2-IIA [23,24]. However, these properties need to be interpreted with caution, since PLA2 enzyme assays employing artificial phospholipid vesicles comprising only one or a few phospholipid species, or cell-based studies in which sPLA2s are artificially overexpressed, or in which excess sPLA2s are added at super-physiological levels, do not necessarily reflect their true functional aspects in vivo. To comprehensively understand the pathophysiological functions of sPLA2s, it is important to consider as to when, where and to what degree any given sPLA2 is expressed, which phospholipid species in a given membrane component serve as the target substrates, which lipid metabolites are generated, and how these lipid metabolites modulate biological responses in relevant tissue microenvironments. It has become obvious that the functions of sPLA2s are not limited to the regulation of AA metabolism, which used to be the classical view in PLA2 research, but are also associated with mobilization of various fatty acids and lysophospholipids in specific tissue contexts, as described below.
sPLA2-IID is expressed mainly in dendritic cells (DCs) and macrophages, particularly CD4+CD11b+CD11c+MHC class IIlo DCs and M2-like macrophages, in secondary lymphoid organs such as the spleen and lymph nodes (LNs) of mice and humans [25]. As opposed to sPLA2-IIA (a so-called “inflammatory sPLA2”), whose expression is upregulated in various tissues in response to pro-inflammatory cytokines and lipopolysaccharide (LPS) [26], sPLA2-IID is downregulated in antigen-activated MHC class IIhi DCs [25] or LPS-stimulated tissues and macrophages [16,27]. A new lipidomics-based PLA2 enzyme assay using a natural phospholipid mixture extracted from a relevant tissue (lymphoid tissues in the case of sPLA2-IID) as a substrate (“natural membrane assay” [28]) has revealed that sPLA2-IID preferentially hydrolyzes phosphatidylethanolamine (PE) species with sn-2 polyunsaturated fatty acids (PUFAs), including ω6 AA and more efficiently ω3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), rather than those with OA and LA [25]. This enzymatic preference of sPLA2-IID for PE species with ω3 PUFAs as substrates, together with its distribution in lymphoid immune cells and downregulation by pro-inflammatory stimuli, suggests that sPLA2-IID has a role in resolution, rather than promotion, of the adaptive immune response.
2.2. Suppression of adaptive immune responses
The possibility that sPLA2-IID might have an immunosuppressive function was first demonstrated in the research field of T cell biology. Von Allmen et al. found that, among the various T cell subsets, sPLA2-IID is selectively expressed in Treg cells and has the capacity to promote Treg differentiation and function [29], although a subsequent study has shown that sPLA2-IID is expressed much more abundantly in antigen-presenting cells (DCs and macrophages) than in Treg cells [25]. Interestingly, an sPLA2-IID-Fc fusion protein inhibits the proliferation of CD4+ and CD8+ effector T cells in vitro and suppresses Th17-dependent diseases such as colitis and multiple sclerosis when administered to mice. However, it remained uncertain whether systemically administered artificial sPLA2-IID-Fc fusion protein indeed mirrored the intrinsic function of endogenous sPLA2-IID, and even if so, how this enzyme exerts its immunoregulatory functions. Although the sPLA2-IID-Fc fusion protein might act on the sPLA2 receptor (PLA2R1), sPLA2-IID has poor binding affinity for PLA2R1 [30] (overall binding properties of various sPLA2s to PLA2R1 are detailed in the other review in this special issue). Beyond this, the possibility that sPLA2-IID could transmit some signals through binding to an unknown receptor cannot be ruled out, and the distinction between an effect due to enzymatic activity versus a receptor-operated mechanism can only be addressed by using a catalytic mutant of sPLA2-IID, including knock-in mice such as the sPLA2-IID mutant “H48Q”. Nonetheless, the immunosuppressive function of sPLA2-IID has been established by a series of recent studies using sPLA2-IID-deficient (Pla2g2d −/−) and -transgenic (Pla2g2d-TG) mice, as described below.
In a model of Th1-dependent contact hypersensitivity (CHS), application of the hapten antigen dinitrofluorobenzene (DNFB) to abdominal skin (sensitization) followed by a second application of the same antigen to ear skin (elicitation) induces ear swelling. In the elicitation phase of CHS, the resolution, but not propagation, of inflammation in the skin and LNs is delayed in Pla2g2d −/− mice [25]. In this state, expression levels of the signature Th1 cytokines IFN-γ and IL-12 are highly elevated in the draining LNs, whereas those of the Treg markers FOXP3 and IL-10 are unaffected, by sPLA2-IID deficiency. Even in the late stage of the sensitization phase, IFN-γ expression in the LNs is substantially elevated in Pla2g2d −/− mice [31]. Moreover, DCs isolated from Pla2g2d −/− mice are hyper-activated even in the absence of stimulation, with increased secretion of IFN-γ and elevated surface expression of MHC class II [25]. In contrast, acute skin inflammation as evaluated by irritant dermatitis is not affected by sPLA2-IID deficiency [31]. These results suggest that the lack of sPLA2-IID augments DC-mediated Th1 immunity, rather than influencing Treg cell function and neutrophil-mediated acute inflammation.
Psoriasis is a common chronic skin diseases in western countries, characterized by epidermal hyperplasia (acanthosis), scaling, and erythematous plaque formation due to aberrant proliferation, differentiation and activation of keratinocytes as well as activation of Th17-type immunity [32]. In a model of imiquimod (IMQ)-induced psoriasis, Pla2g2d −/− mice display more severe epidermal hyperplasia than do Pla2g2d +/+ mice, with increased IL-17A+ or IL-22+ T cells in the affected skin and regional LNs [31]. Furthermore, DCs from Pla2g2d −/− mice produce greater amounts of IL-6 and IL-23, which play pivotal roles in Th17 immunity [33], than those from WT mice. Conversely, Pla2g2d-TG mice display milder inflammation in the CHS and psoriasis models [31]. Thus, sPLA2-IID suppresses the Th1- and Th17-dependent adaptive immune responses in CHS and psoriasis, respectively. This concept appears to corroborate the ability of a sPLA2-IID-Fc fusion protein to suppress colitis and multiple sclerosis, which are typical Th17-dependent disease models [29].
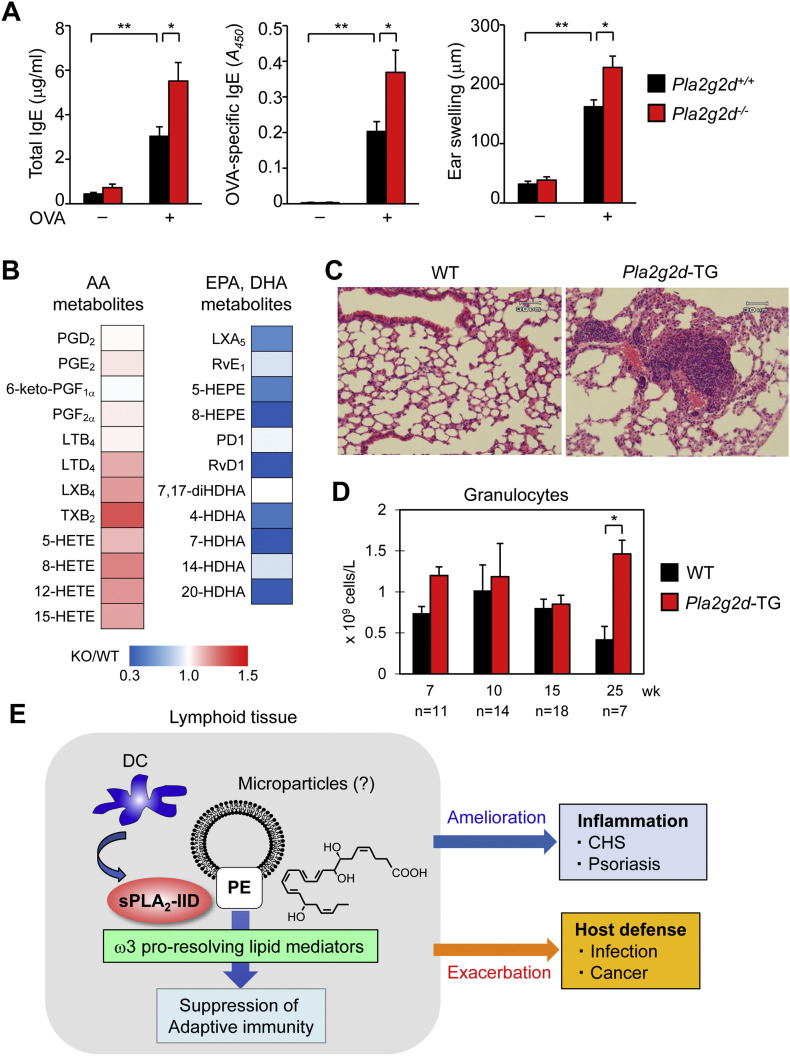
To address whether or not sPLA2-IID would also affect Th2 immunity, we sensitized Pla2g2d −/− and littermate Pla2g2d +/+ mice with ovalbumin (OVA) intraperitoneally and challenged them with the same antigen into the ears. In this model of Th2-dependent active cutaneous anaphylaxis, the serum levels of total and OVA-specific IgE, as well as ear swelling, were significantly increased in OVA-challenged Pla2g2d −/− mice relative to Pla2g2d +/+ mice (Fig. 1A). Since passive cutaneous anaphylaxis is not affected by the absence of sPLA2-IID [34], it is likely that the increased active anaphylactic response in Pla2g2d −/− mice is due to an increased level of OVA-specific IgE (an indication of the increased Th2 response) and thereby hyper-activation of mast cells sensitized by this IgE, rather than being attributable to some intrinsic alterations in mast cells or vascular endothelial cells. Thus, not only Th1/Th17 immunity, but also the Th2-dependent immune response is suppressed by sPLA2-IID.
Fig. 1.
Properties and functions of sPLA2-IID. (A) Increased Th2 response in Pla2g2d−/− mice. Pla2g2d+/+ and Pla2g2d−/− mice (male, 8 weeks old) were immunized intraperitoneally on days 0, 7, and 14 with 10 μg of chicken OVA (Sigma-Aldrich) in 100 μl of saline mixed with 200 μl of alum (Alu Gel S, which contained 2% Al(OH)3; Serva). Seven days after the last immunization, the left and right ears of the mice were injected intradermally with 30 μg of OVA. Ear swelling was measured at 30 min after OVA challenge. Total and OVA-specific IgE levels in sera were measured by ELISA (Bethyl Laboratories). OVA-induced IgE levels and ear edema were elevated in Pla2g2d−/− mice relative to Pla2g2d+/+ mice (n = 5–7, mean ± SEM, *p < 0.05, **p < 0.01). (B) Lipidomic heat map profiling of ω6 AA- and ω3 EPA/DHA-derived lipid mediators in lymph nodes of Pla2g2d−/− (KO) mice relative to Pla2g2d+/+ (WT) mice. EPA/DHA-derived lipid mediators were decreased in KO mice [25,31]. The elevation of several AA metabolites in KO mice might have been due to increased lymph node inflammation. HETE, hydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; LX, lipoxin; PD, protectin; Rv, resolvin. (C) Lung histology of WT and Pla2g2d-TG mice (male, 34 weeks old). The lungs of Pla2g2d-TG mice had more pronounced leukocyte infiltration than those of WT mice. (D) Aged (25 weeks old), but not young (7–15 weeks old), Pla2g2d-TG mice had more circulating granulocytes than did age-matched WT mice (mean ± SEM, *p < 0.05). The results in (C, D) suggest that the increased immunosuppressive tone in the TG mice results in more opportunistic infection, and thereby airway inflammation. (E) A schematic diagram of sPLA2-IID action. In lymphoid tissues, sPLA2-IID is preferentially expressed in DCs and hydrolyzes PE in microparticles to provide ω3 EPA/DHA-derived pro-resolving lipid mediators (the structure of RvD1 is shown), which dampen adaptive immunity. As such, sPLA2-IID ameliorates Th1/Th17-dependent inflammation in CHS and psoriasis and perturbs host defense against infection and cancer.
Lipidomics analyses of regional LNs and spleen, where sPLA2-IID is abundantly expressed, have revealed that the steady-state levels of ω3 PUFAs are markedly reduced in Pla2g2d −/− mice relative to Pla2g2d +/+ mice [25,31]. Moreover, the levels of ω3 PUFA metabolites, such as DHA-derived resolvin D1 (RvD1), are markedly lower in Pla2g2d −/− LNs than in Pla2g2d +/+ LNs (Fig. 1B). Conversely, the levels of ω3 PUFA metabolites are elevated in the LNs of Pla2g2d-TG mice relative to WT mice [31]. In contrast, the LN levels of eicosanoids (prostanoids and leukotrienes) are barely altered or even elevated by sPLA2-IID deletion, implying that these AA metabolites are largely derived from a sPLA2-IID-independent AA pool, possibly through the action of cytosolic PLA2 (cPLA2α) or other PLA2 subtype(s). Together with the substrate selectivity described above, it is likely that sPLA2-IID preferentially and constitutively hydrolyzes PUFA-containing PE species in LN membranes, probably those in microparticles (as in the case of sPLA2-IIA) [35,36], to mobilize ω3 PUFA-derived anti-inflammatory lipid mediators, which can put a brake on DC-committed adaptive immunity. It has been reported that leukocyte-derived microparticles spatiotemporally generated in inflammatory exudates during resolution contain esterified phospholipid precursors of anti-inflammatory lipid mediators [37]. We therefore speculate that similar microparticles constitutively generated in lymphoid tissues might be a target of sPLA2-IID, although this hypothesis needs further elucidation. A growing body of evidence indicates that ω3 PUFA-derived resolvins including RvD1 have the capacity to suppress acquired immunity by attenuating migration and activation of DCs, antigen presentation to T cells, and IgE class switching in B cells [31,[38], [39], [40], [41]]. Furthermore, consistent with the view that some ω3 PUFA metabolites such as maresins facilitate anti-inflammatory M2 polarization of macrophages [42,43], the splenic ratio of M2/M1 macrophages is decreased in Pla2g2d −/− mice relative to WT mice [31].
Collectively, sPLA2-IID is a “resolving sPLA2” that preferentially mobilizes ω3 PUFA metabolites in lymphoid organs, thereby ameliorating aggravated adaptive immunity. It should be noted that sPLA2-IID is not the only ω3 PUFA-releasing sPLA2 in vivo. Indeed, ω3 PUFAs are released by sPLA2-X in the colon and spermatozoa [44] and by sPLA2-IIF in the skin [45], where each of these sPLA2s has tissue-specific roles (see below).
2.3. Suppression of anti-viral and anti-tumor immunity
Although sPLA2-IID prevents exaggerated Th1/Th17 immunity in CHS and psoriasis, this beneficial immunosuppressive property is conversely disadvantageous in some circumstances such as host defense against infection and cancer. Indeed, sPLA2-IID prevents anti-viral and anti-tumor Th1 immunity, eventually exacerbating viral infection and tumor development toward worse outcomes.
sPLA2-IID expression in lung DCs increases with age in response to chronic exposure to oxidative stress. In aged (10–13 months old) mice, sPLA2-IID contributes to exacerbation, rather than amelioration, of pneumonia caused by infection with SARS coronavirus or influenza virus [46]. In this situation, sPLA2-IID is coupled with pulmonary mobilization of prostaglandin D2 (PGD2), an anti-inflammatory AA metabolite in this context that blocks DC migration and thereby Th1-driven anti-viral responses through its receptor, DP1. Accordingly, Pla2g2d −/− mice show increased migration of lung DCs to LNs, leading to augmented anti-viral T cell responses, which are protective against infection-induced lung inflammation and death. Although not tested in that study, it is conceivable that the steady-state reduction of ω3 PUFA metabolites in the LNs of Pla2g2d −/− mice (see above) may also contribute to the increased anti-viral immunity in this setting. Consistent with this notion, aged (>6 months old) Pla2g2d-TG mice show more profound leukocyte infiltration in the lung (Fig. 1C) and more granulocytes in the circulation (Fig. 1D) than age-matched WT mice, suggesting that the increased immunosuppressive tone by overexpression of sPLA2-IID results in more opportunistic infection and thereby lung inflammation.
Likewise, sPLA2-IID accelerates, rather than prevents, the development of skin tumors, likely because this enzyme attenuates anti-tumor Th1 immunity. In a model of chemical carcinogenesis, Pla2g2d −/− mice are highly protected against the development of skin cancer, accompanied by an increase of cytotoxic CD8α+IFN-γ+ T cells and M1-like macrophages, as well as a decrease of tumor-promoting M2-like macrophages [31]. Conversely, transgenic overexpression of sPLA2-IID shifts the immune balance toward suppression of the anti-tumor immunity. Reportedly, ectopic administration of ω3 PUFA metabolites [31,47,48] or systemic overproduction of these lipids in mice transgenic for Fat-1 (an ω3 PUFA synthase in Caenorhabditis elegans) [49] confers protective effects against infection-based inflammation or cancer xenograft by facilitating phagocytotic clearance of detrimental materials by neutrophils and macrophages. Apart from this systemic effect of ω3 PUFAs, the spatiotemporal supply of ω3 PUFAs by sPLA2-IID in local lymphoid niches may have a distinct impact on adaptive immunity by suppressing the functions of DCs and T cells.
Taken together, the immunosuppressive functions of sPLA2-IID provide favorable or unfavorable outcomes in distinct disease settings, protecting against inflammation and exacerbating infection and cancer (Fig. 1E). This points to the potential prophylactic or therapeutic use of an agent that would specifically stabilize or inhibit this enzyme according to disease context. In particular, specific inhibition of sPLA2-IID in patients with severe respiratory infection or those with certain types of cancer would be a potentially attractive therapeutic intervention for restoration of immunological functions, a concept reminiscent of the “immune checkpoint” therapy.
2.4. Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. This life-threatening disease not only creates problems resulting from airflow obstruction, but also has a major impact on cardiac function and air exchange, thereby resulting in systemic manifestations [50,51]. The presence of chronic and systemic inflammatory responses has an important influence on patient survival, because unexplained weight loss due to muscle wasting and adipose tissue depletion, a characteristic feature of advanced COPD, can be linked to systemic inflammation.
Interestingly, G80S polymorphism in the human sPLA2-IID (PLA2G2D) gene is associated with body weight loss in patients with COPD [52]. COPD patients carrying sPLA2-IID(Ser80) lose a significant amount of body weight in comparison with those carrying sPLA2-IID(Gly80). Although this mutation does not affect the in vitro enzymatic activity of sPLA2-IID, it enhances the expression of IL-6 and IL-8 in A549 cells (a human pulmonary epithelial cell line) [53]. A molecular model of human sPLA2-IID has revealed substantial differences between the native and mutant forms in terms of channel opening and the surface area for interfacial binding contact [54]. Given the immunosuppressive property of sPLA2-IID described above, the Ser80 mutant form might have weaker ability than the Gly80 native form to suppress inflammation. It is also possible that the body weight loss could be related to the anti-inflammatory function of sPLA2-IID in metabolically active tissues (e.g. adipose tissue), which will be described elsewhere.
3. sPLA2-IIE
3.1. General aspects
Mouse and human sPLA2-IIEs were identified by Lambeau's group [17] and Hanasaki's group [16] from the EST databases. sPLA2-IIE consists of 123 amino acids and is most similar to sPLA2-IIA with respect to the number and positions of cysteine residues as well as overall identity (51% homology). Similar to other group II subfamily sPLA2s (except for sPLA2-IIF; see below), sPLA2-IIE is a basic protein (pI ~ 8.1) and shows weak affinity for heparin. Within the limitations of the overexpression strategy using cultured cells, the ability of sPLA2-IIE to elicit AA release is weaker than that of sPLA2-IID [23,24].
Valentin et al. have reported that the enzymatic activity of sPLA2-IIE is much weaker than that of other sPLA2s [17], whereas Suzuki et al. have shown that the activity of sPLA2-IIE is comparable to that of sPLA2-IIA, hydrolyzing PE and to a lesser extent phosphatidylcholine (PC) with no fatty acid selectivity [16]. To reconcile the inconsistency between those two studies, we reevaluated the enzymatic activity of sPLA2-IIE using a lipidomics-based natural membrane assay with a phospholipid mixture extracted from mouse skin, a tissue where sPLA2-IIE is expressed abundantly (see below), as a substrate. It was found that sPLA2-IIE is as active as other sPLA2s if the phospholipid concentration is sufficiently high, whereas its activity is very weak at a low substrate concentration that allows other sPLA2s to remain fully active [55]. This suggests that the apparent Km of sPLA2-IIE toward this skin-extracted phospholipid mixture is higher than that of other sPLA2s. In the presence of a sufficiently high concentration of phospholipids, sPLA2-IIE is capable of releasing various unsaturated fatty acids including OA, LA, AA and DHA as well as lysphosphatidylethanolamine (LPE) in preference to lysophosphatidylcholine (LPC), a pattern similar to the results reported by Suzuki et al. [16]. This substrate selectivity is further supported by the crystal structure of human sPLA2-IIE, which shows overall similarity to that of human sPLA2-IIA, yet with substantial differences in terms of basic residue clusters at the interfacial site and C-terminal region [56].
In some inbred mouse strains such as C57BL/6, A/J, C58/J, P/J, 129/Sv and B10.RIII, sPLA2-IIA is entirely absent due to a frameshift mutation in its gene, whereas the gene is functional, but its expression is largely restricted to the intestine, in inbred strains such as BALB/c, C3H, NZB and DBA and outbred strains such as OF1 [57]. Instead, sPLA2-IIE expression is markedly induced in several mouse tissues upon LPS challenge [16]. Serum amyloid A, a pro-inflammatory mediator of lethal systemic inflammatory diseases, induces sPLA2-IIE expression in mouse macrophages [58]. In contrast, sPLA2-IIE expression is barely detectable in most human tissues, leading to the hypothesis that the functions of sPLA2-IIA in humans might be compensated by sPLA2-IIE in mice [4]. Nevertheless, a few studies have reported that sPLA2-IIE is expressed in human cells [59,60] and that polymorphism in the human sPLA2-IIE (PLA2G2E) gene is associated with ulcerative colitis [61], suggesting that sPLA2-IIE may be functional in humans in certain situations. Recent studies using sPLA2-IIE-deficient (Pla2g2e −/−) mice have revealed the novel roles of this sPLA2 in metabolic regulation and hair follicle homeostasis, as described below.
3.2. Metabolic regulation
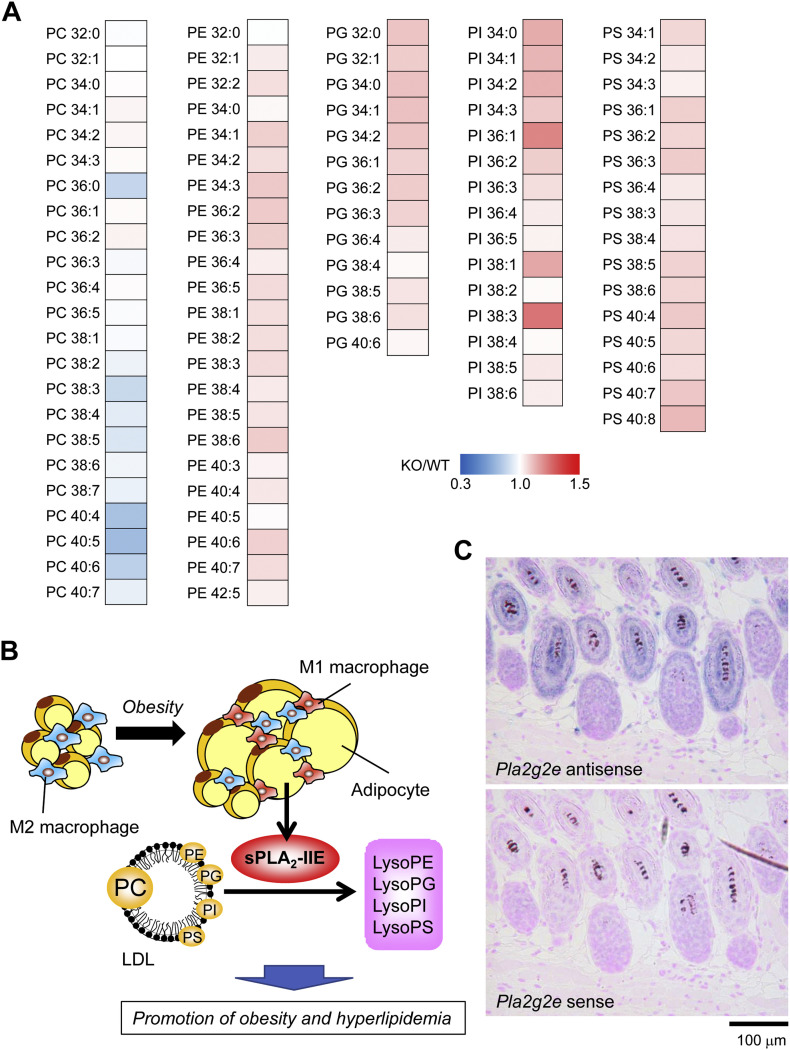
sPLA2-IIE is highly induced in hypertrophic white adipocytes in mice fed a high-fat diet or in genetically obese ob/ob mice [62]. An adipogenic stimulus is sufficient for the induction of sPLA2-IIE in 3T3-L1 adipocytes. In a model of diet-induced obesity, Pla2g2e −/− mice are modestly protected from obesity, hepatic steatosis and hyperlipidemia [62]. Lipidomics analysis of plasma lipoproteins obtained from Pla2g2e −/− mice in comparison with Pla2g2e +/+ mice has revealed that various molecular species of PE, phosphatidylserine (PS), phosphatidylinositol (PI) and phosphatidylglycerol (PG), but not those of PC, are all elevated in the null mice (Fig. 2A), suggesting that sPLA2-IIE preferentially hydrolyzes these minor lipoprotein phospholipids with no apparent fatty acid selectivity in vivo [62]. As such, sPLA2-IIE, a “metabolic sPLA2”, alters the lipid composition of lipoproteins, thereby moderately affecting lipid accumulation in adipose tissue and liver (Fig. 2B). However, the mechanism whereby the sPLA2-IIE-driven hydrolysis of minor lipoprotein phospholipids is linked to metabolic regulation still remains obscure. Since an increase of negative charges in lipoproteins renders the particles smaller [63], an increase of anionic phospholipids (e.g. PS) in lipoproteins resulting from sPLA2-IIE deficiency might afford such an effect. Alternatively, certain lysophospholipid species produced by sPLA2-IIE might have some metabolic effects, a possibility that awaits future studies. In fact, lysophosphatidylserine (LysoPS) and lysophsophatidylinositol (LPI) act on their cognate receptors that can affect inflammation and metabolism [64,65]. Importantly, the metabolic action of sPLA2-IIE contrasts with that of sPLA2-V, another diet-inducible “metabolic sPLA2” that hydrolyzes PC in low-density lipoprotein (LDL) to preferentially release OA and LA, thereby protecting against obesity, insulin resistance, fatty liver, and adipose tissue inflammation [62].
Fig. 2.
Properties and functions of sPLA2-IIE. (A) Lipidomic heat map profiling of phospholipids in LDL of Pla2g2e−/− (KO) mice relative to Pla2g2e+/+ (WT) mice fed a high-fat diet for 18 weeks. Most PE, PG, PI and PS molecular species were elevated in LDL of KO mice, suggesting that sPLA2-IIE acts on these minor lipoprotein phospholipids with no apparent fatty acid selectivity [62]. (B) A schematic diagram of the sPLA2-IIE-driven lipid pathway in lipoprotein metabolism during obesity. In obesity, sPLA2-IIE is induced in adipocytes, hydrolyzes minor lipoprotein phospholipids, and promotes obesity and hyperlipidemia. The roles of lysophospholipids released by sPLA2-IIE are unknown. (C) In situ hybridization of sPLA2-IIE in mouse skin. Intense Pla2g2e signal are localized to hair follicles [55].
On the other hand, another study has revealed that Pla2g2e −/− mice accumulate more epididymal fat than do Pla2g2e +/+ mice as they age [66]. During ex vivo adipogenesis, knockout or knockdown of sPLA2-IIE increases triglycerides in adipocytes, whereas its overexpression or exogenous addition facilitates lipolysis with increased release of glycerol. Although the reason for the discrepancy between these two studies is unclear, it might be attributable to the difference of experimental conditions (diet-induced versus age-associated obesity, high-fat versus chow diet, or female versus male) in the different animal facilities. One possible explanation is that sPLA2-IIE might have some additional effects on brown or beige fat, and thereby lipolysis and thermogenesis, a hypothesis that remains to be tested.
3.3. Hair follicle homeostasis
Hair follicles in the skin undergo repeated cycles of growth (anagen), regression (catagen) and rest (telogen) during life [67]. Perturbed skin lipid metabolism variably and often severely affects hair cycling, thereby causing hair loss or alopecia [68,69]. sPLA2-IIE is a “hair follicular sPLA2” that is expressed abundantly in hair follicles during the anagen period, being distributed in companion cells of the outer root sheath and cuticular cells of the inner root sheath [55] (Fig. 2C). Pla2g2e −/− mice exhibit mild skin abnormalities with perturbation of hair follicle ultrastructure and modest changes in the steady-state expression of a subset of skin genes. Lipidomics analysis has revealed that sPLA2-IIE mobilizes various unsaturated fatty acids and LPE species (both acyl and plasmalogen forms) in mouse skin. This substrate selectivity fits well with the in vitro enzymatic property of sPLA2-IIE (see above). However, it remains unclear which lipid metabolites mobilized by sPLA2-IIE are important for hair follicle homeostasis.
4. sPLA2-IIF
4.1. General aspects
Mouse and human sPLA2-IIFs were identified by Lambeau and his colleagues [17,18]. sPLA2-IIF consists of 148 amino acids harboring all of the structural features of group II subfamily sPLA2s, but has several unique characteristics. First, sPLA2-IIF is an acidic protein (pI ~ 5.8), in contrast to the other group II subfamily sPLA2s, which are basic. Second, although sPLA2s are active under neutral to mildly basic conditions in general, sPLA2-IIF retains its full enzymatic activity even at mildly acidic pH. This property may be related to the distribution of the enzyme in the epidermis (see below), where a mildly acidic environment is important for proper keratinocyte differentiation and function [70]. Third, sPLA2-IIF has a uniquely long C-terminal extension that is proline-rich and contains a single cysteine. The presence of this odd cysteine raises the possibility that sPLA2-IIF might occur as a covalent dimer (like several venom sPLA2s), although this has not been experimentally confirmed. Fourth, sPLA2-IIF is more hydrophobic than other sPLA2s. Probably as a result of this high hydrophobicity, sPLA2-IIF has a unique ability to penetrate and disrupt lipid monolayers and bilayers in vitro and to rapidly enter HEK293 cells in an endocytosis-independent manner to form unusual aggregates [71]. Within the limitations of the overexpression strategy, sPLA2-IIF can increase AA release with a potency comparable to sPLA2-III and superior to sPLA2-IIA when transfected into HEK293 cells or human fibroblasts (the rank order is X > V > III = IIF > IIA > IID > IIE) [24,72]. In the natural membrane assay using a phospholipid mixture extracted from mouse skin, sPLA2-IIF preferentially hydrolyzes PE, particularly plasmalogen-type PE (P-PE), to yield lysoplasmalogen (plasmalogen-type LPE; P-LPE) as well as DHA in preference to AA at a physiologically relevant concentration [45]. Of note, although high concentrations of sPLA2s often cleave all substrates non-selectively in vitro, as we have observed in skin lipid hydrolysis by recombinant sPLA2-IIF [45,55], the use of low concentrations of sPLA2s could reproduce the in vivo substrate selectivity in the natural membrane assay.
The epidermis is a highly organized stratified epithelium having four distinctive layers comprising the innermost stratum basale, the stratum spinosum, the stratum granulosum, and the outermost stratum corneum (SC) [73]. sPLA2-IIF is abundantly expressed in the suprabasal (spinous to SC) layers of the epidermis [45]. In cultured keratinocytes, sPLA2-IIF is markedly increased during cell differentiation in parallel with the induction of keratinocyte differentiation and activation markers such as KRT1, S100A9 and IL-36α, and robustly upregulated following stimulation with the Th17 cytokines IL-22 and IL-17A [45]. Moreover, sPLA2-IIF is highly expressed in the hyperplasic epidermis of patients with psoriasis [45]. These findings indicate that sPLA2-IIF may be associated with epidermal homeostasis and diseases, particularly with the pathology of psoriasis in which Th17 immunity plays a crucial role. Indeed, studies using sPLA2-IIF-deficient (Pla2g2f −/−) and transgenic (Pla2g2f-TG) mice have revealed the unique role of sPLA2-IIF, an “epidermal sPLA2”, in epidermal hyperplasic diseases including psoriasis and skin cancer, as described below.
4.2. Psoriasis
Perturbation of epidermal lipids variably and often profoundly affects skin homeostasis and barrier function, leading to skin disorders such as ichthyosis, psoriasis, atopic dermatitis and cancer [[74], [75], [76]]. Prior to the discovery of epidermal expression of sPLA2-IIF, it was recognized that several sPLA2s are expressed in mouse and human skins [[77], [78], [79], [80]]. Interestingly, transgenic overexpression of human sPLA2-IIA (PLA2G2A-TG) or sPLA2-X (PLA2G10-TG) in mice led to epidermal hyperplasia and alopecia [[81], [82], [83]], although endogenous expression of these two sPLA2s has been scarcely detected in mouse skin [45]. Later, it was shown that global or skin-specific Pla2g2f-TG mice spontaneously develop psoriasis-like epidermal hyperplasia and alopecia, with increased expression of a panel of psoriasis markers including S100A9 and IL-36α. Therefore, the skin phenotypes observed in PLA2G2A-TG or PLA2G10-TG mice may indicate that sPLA2-IIA or -X mimic the intrinsic actions of sPLA2-IIF when artificially overexpressed in the skin or that endogenous sPLA2-IIF is upregulated in the hyperplasic epidermis of these TG mice.
Pla2g2f −/− mice exhibit only mild skin abnormalities under the basal state, characterized by a fragile stratum corneum with modest perturbation of skin barrier function and acidity [45]. These phenotypes are evident in the abdominal, but not dorsal, skin of adult, but not newborn, Pla2g2f −/− mice, suggesting that sPLA2-IIF contributes to SC stability against environmental stresses, such as friction against the floor or prolonged exposure to skin microbiota, rather than to the central program of epidermal differentiation. After tape-stripping of the corneum, Pla2g2f −/− mice display delayed recovery from the skin barrier damage [80], suggesting that sPLA2-IIF accelerates epidermal repair. The impact of sPLA2-IIF ablation is more dramatic in primary keratinocytes, where the cells fail to be differentiated and undergo proper activation when sPLA2-IIF is genetically or pharmacologically inactivated [45]. The more profound effects of sPLA2-IIF deletion on keratinocytes in vitro than in vivo suggest that some mechanisms compensating for the lack of sPLA2-IIF might exist in vivo.
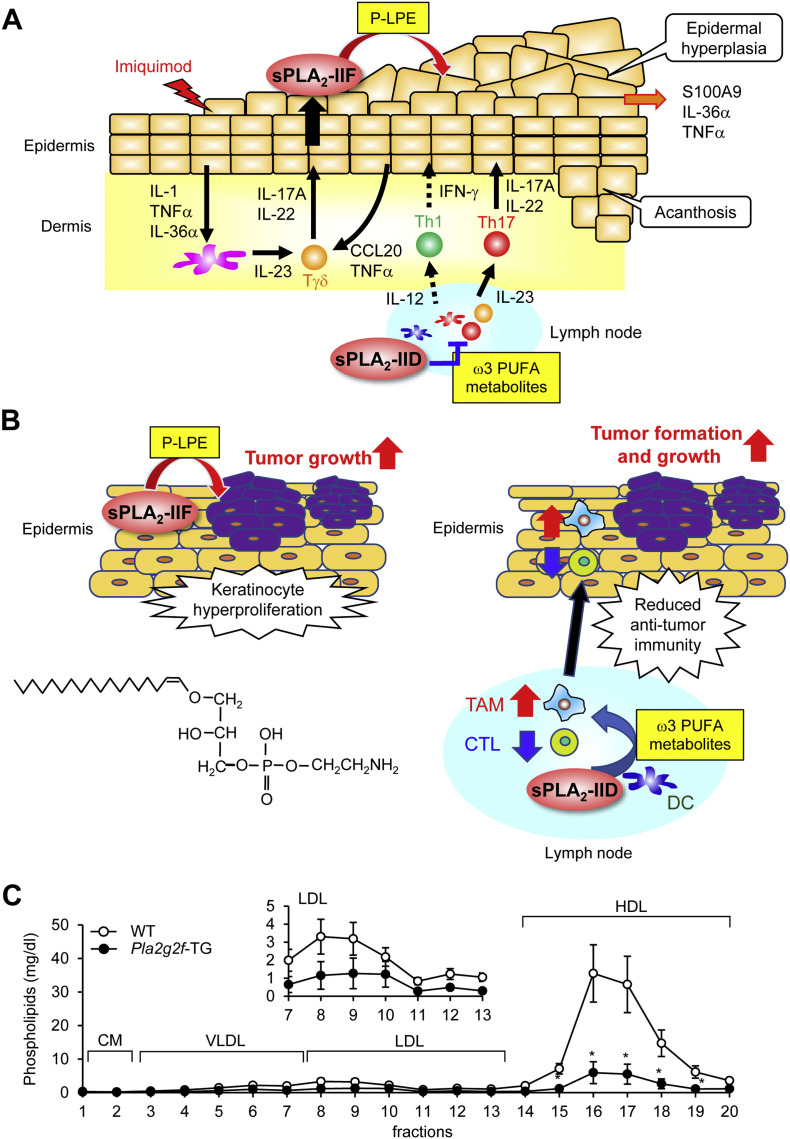
Strikingly, under pathological conditions, Pla2g2f −/− mice are protected from epidermal hyperplasia in models of Th17-dependent psoriasis and Th1-dependent CHS [45]. In primary keratinocytes from Pla2g2f −/− mice, IL-22- or IL-17A-induced expression of several psoriasis markers is markedly impaired. Mechanistically, sPLA2-IIF hydrolyzes P-PE secreted from keratinocytes to yield P-LPE, a unique lysophospholipid that facilitates the differentiation and activation of keratinocytes, leading to the propagation of skin inflammation. Indeed, the levels of P-LPE in the skin are correlated well with the expression levels of sPLA2-IIF in multiple skin disease models, and topical application of P-LPE to Pla2g2f −/− skin in vivo or supplementation of Pla2g2f −/− keratinocytes with P-LPE ex vivo restores the psoriasis-related phenotypes. Thus, in the pathology of psoriasis, sPLA2-IIF plays an exacerbating role by promoting aberrant proliferation and activation of keratinocytes through production of P-LPE in the suprabasal epidermis, whereas sPLA2-IID plays a resolving role by reducing the harmful Th17 immune response through production of ω3 PUFA-derived pro-resolving mediators in lymphoid tissues (see above) (Fig. 3A).
Fig. 3.
Properties and functions of sPLA2-IIF. (A, B) Distinct roles of sPLA2-IIF and sPLA2-IID in psoriasis and skin cancer [31,45]. (A) Following a psoriatic stimulus (imiquimod), sPLA2-IIF is induced in epidermal keratinocytes by Th17 cytokines derived from Tγδ and Th17 cells and hydrolyzes plasmalogen to give rise to lysoplasmalogen (P-LPE), which in turn promotes epidermal hyperplasia and inflammation. In contrast, sPLA2-IID blocks Th17 immunity in lymph nodes through production of ω3 PUFA metabolites, thereby putting a brake on psoriasis. (B) P-LPE produced by epidermal sPLA2-IIF promotes hypergrowth of skin cancer, without affecting its incidence. In contrast, ω3 PUFA metabolites produced by sPLA2-IID in lymph nodes decrease IFN-γ+CD8+ cytotoxic T cells (CTLs) and increase M2-like tumor-associated macrophages (TAMs), leading to reduced anti-tumor immunity. As such, sPLA2-IID facilitates tumor formation and growth. The structure of P-LPE is shown. (C) Lipoprotein profiles in Pla2g2f-TG and WT mice. The levels of phospholipids in HDL and LDL were markedly lower in Pla2g2f-TG mice than in WT mice (n = 4, mean ± SEM, *p < 0.05), suggesting that sPLA2-IIF, when overexpressed systematically, has the capacity to hydrolyze lipoprotein phospholipids in the circulation. LDL fractions are magnified in Inset. VLDL, very-low-density lipoprotein; HDL, high-density lipoprotein; CM, chylomicron.
4.3. Skin cancer
Skin-specific Pla2g2a-TG mice are sensitive to chemical carcinogenesis [82], even though endogenous sPLA2-IIA is not expressed in mouse skin. Likewise, Pla2g2f-TG mice are highly susceptible to the skin carcinogenesis model, with an apparent propensity to develop larger tumors than WT mice [45], implying again that the overexpressed sPLA2-IIA in Pla2g2a-TG mice mimics the action of sPLA2-IIF. Importantly, Pla2g2f −/− mice on a BALB/c background are markedly protected from the development of skin tumors, accompanied by lower production of P-LPE and unaltered production of canonical AA metabolites [45]. Among the sPLA2 knockout mouse strains tested so far, only Pla2g2d −/− and Pla2g2f −/− mice are protected against skin cancer through distinct mechanisms; sPLA2-IID deficiency increases anti-tumor immunity and thereby blocks tumor development (see above), whereas sPLA2-IIF deficiency ameliorates keratinocyte hyperproliferation (Fig. 3B) [31,45].
Taken together, the findings so far suggest that sPLA2-IIF promotes epidermal hyperplasic diseases including psoriasis and skin cancer and that P-LPE, a primary sPLA2-IIF product, represents a particular biomarker and bioactive lipid that reflects the expression and function of sPLA2-IIF. Given that sPLA2-IIF is expressed in the epidermis rather specifically and that Pla2g2f −/− mice display more profound skin phenotypes under pathological conditions than under physiological conditions, blocking or neutralizing this particular sPLA2 may be a novel approach for specific treatment of psoriasis, skin cancer, or other conditions characterized by epidermal hyperplasia. It remains to be clarified whether sPLA2-IIF-driven P-LPE would act on keratinocytes through a specific receptor or through other mechanism(s), and whether DHA, another sPLA2-IIF-driven product, would be metabolized to certain products that would affect skin homeostasis. The latter possibility seems plausible, since DHA and its metabolites have been shown to have the capacity to facilitate skin wound healing, suppress psoriasis, and prevent neoplastic transformation of keratinocytes [[84], [85], [86]].
4.4. Other potential functions
Recombinant sPLA2-IIF has a potent capacity to prevent malaria infection in vitro [87]. The anti-malaria property of sPLA2s is dependent on their ability to release PUFAs relative to other fatty acids from lipoproteins, sPLA2-IIF being the most PUFA-selective sPLA2. Indeed, lipoprotein phospholipids are potently hydrolyzed, with marked PUFA preference, when treated with sPLA2-IIF in vitro [87,88] or in Pla2g2f-TG mice in vivo (Fig. 3C). Beyond this ability to confer anti-malaria immunity, lipoprotein hydrolysis by sPLA2s would be expected to have some influence on systemic metabolism, as has been demonstrated for the “metabolic sPLA2s” sPLA2-IIE and -V (see above) [62]. In this context, it would be important to determine whether endogenous sPLA2-IIF has an opportunity to encounter plasma lipoproteins under certain in vivo conditions, and if so, which cells or tissues would secrete sPLA2-IIF in this context and how sPLA2-IIF-directed lipoprotein hydrolysis would affect immunity or metabolism. Although sPLA2-IIF is also substantially expressed in the intestinal epithelium [45], its deficiency does not significantly alter the sensitivity to colitis in an animal model [44].
5. sPLA2-III
5.1. General aspects
Human sPLA2-III was originally identified by Lambeau and his colleagues in 2000 [19]. sPLA2-III is an atypical sPLA2 whose structure is rather distinct from conventional group I/II/V/X sPLA2s except for the conserved catalytic site and the Ca2+-binding motif. Human sPLA2-III has 490 amino acids made up of a central sPLA2 domain (141 residues) with a typical group III feature that is flanked by unique N- and C-terminal domains (130 and 219 residues, respectively), and its gene maps to chromosome 22q. The central sPLA2 domain is similar to bee venom sPLA2 and possesses all of the features of group III sPLA2s including 10 cysteines. Unlike sPLA2-IB and -X, in which the N-terminal propeptide interferes with catalytic activity, the presence of the N- and C-terminal domains does not profoundly affect the activity of sPLA2-III [19,89]. Molecular modeling of the sPLA2 domain has revealed that sPLA2-III has unique structural features in comparison with conventional sPLA2s, such as a decrease in the volume of the substrate-binding hydrophobic channel [90].
When overexpressed in HEK293 cells or primary fibroblasts, sPLA2-III elicits AA release with a potency comparable to that of sPLA2-IIF and superior to that of sPLA2-IIA [24,89]. The N- and C-terminal domains are removed to give rise to a mature, sPLA2 domain-only form [91]. Overexpressed sPLA2-III in mammalian or insect cells is often N-glycosylated at two positions, which affect the secretion of the enzyme [91], although the N-glycosylation of endogenous sPLA2-III has not yet been confirmed in vivo. Evaluation of the enzymatic property using a lipidomics-based natural membrane assay with a mixture of colon-extracted phospholipids as the substrate has demonstrated that sPLA2-III hydrolyzes all phospholipid subclasses including PC, PE, PS, PI and PG (i.e. with no apparent polar head group specificity), tending to prefer the sn-2 position of PUFAs [92]. In mice, sPLA2-III is distributed in several tissues, showing the highest expression in the colon, skin, and male reproductive organs [92,93]. Indeed, mice null for sPLA2-III (Pla2g3 −/−) and those with transgenic overexpression of human sPLA2-III (PLA2G3-TG) display several remarkable phenotypes in these tissues, as described below. Importantly, these studies have revealed that the same sPLA2 may work in different tissues by different mechanisms for different biological effects.
5.2. Male reproduction
After the complex process of testicular germ cell differentiation, spermatozoa exit the seminiferous tubules of the testis into the epididymis. During the epididymal transit of spermatozoa, PC in the sperm membrane undergoes a dramatic shift in its sn-2 acyl groups from OA and AA to DHA and docosapentaenoic acid (DPA), and the increased proportion of DPA/DHA consequently contributes to increased sperm membrane fluidity, and thereby flagellar motility and oocyte fertilization [[94], [95], [96], [97]]. The percentage of DHA relative to total fatty acids is correlated with the normal morphology and fertility of sperm cells [98]. Male hypofertility in Pla2g3 −/− mice highlights a critical role of sPLA2-III in this epididymal sperm maturation process [93]. In fact, when mutant males are mated with WT females, the litter sizes are reduced in a genotype-related manner, with only 2–3 pups per litter after breeding of Pla2g3 −/− males with Pla2g3 +/+ females.
sPLA2-III is expressed in epididymal epithelial cells as well as testicular Sertoli cells [93]. In the epididymis, sPLA2-III is secreted from the epithelium into the lumen and acts on immature sperm cells passing through the duct in a paracrine manner to regulate phospholipid remodeling. Strikingly, sperm membrane phospholipid remodeling in the epididymis, but not testicular spermatogenesis, is severely compromised in Pla2g3 −/− mice [93]. Accordingly, Pla2g3 −/− spermatozoa, with a low proportion of DPA/DHA, have aberrant acrosomes and an abnormal axoneme configuration in flagella, resulting in reduced motility and fertility. Epididymal sPLA2-III may participate in deacylation of OA and AA from sperm phospholipids, followed by reacylation with DHA and DPA by a certain lysophospholipid acyltransferase (possibly LPAAT3 [99]) leading to an increase of DPA/DHA-containing PC in mature sperm cells. In the Pla2g3 −/− epididymis, impairment of the deacylation step may eventually perturb the subsequent reacylation with DPA/DHA, culminating in the asthenozoospermia phenotype.
In addition to Pla2g3 −/− mice, sPLA2-X-deficient (Pla2g10 −/−) mice also display sperm abnormality. In Pla2g10 −/− mice, spermatogenesis and epididymal sperm maturation occur normally, but subsequent sperm activation including the acrosome reaction is impaired, thus affecting fertilization [44,100,101]. sPLA2-X is secreted from the sperm acrosome and selectively hydrolyzes DPA/DHA-bearing PC species in sperm membranes to release DPA, DHA and LPC, among which DPA and to a lesser extent LPC can restore the fertilization capacity of Pla2g10 −/− sperm [44]. Thus, sPLA2-III promotes epididymal sperm maturation, allowing enrichment of DPA/DHA-containing PC species in sperm membranes, while sPLA2-X acts on these DPA/DHA-rich phospholipids to liberate DPA and LPC for successful fertilization, thus underscoring elegant cooperation of these two “reproductive sPLA2s” in the process of male reproduction. From a clinical standpoint, sPLA2-III and -X are potential targets for the development of male contraceptive agents or as potential diagnostic markers of male sterility.
5.3. Anaphylaxis
It has been well established that cPLA2α is essential for the production of PGD2 and leukotrienes by mast cells, a key effector cell population in allergy [102,103]. Beyond this, the hypothesis that sPLA2-III might participate in mast cell activation and allergy stemmed primarily from the fact that this enzyme is the sole mammalian homolog of bee venom sPLA2, which is a potent mast cell activator and anaphylaxis inducer [19]. Indeed, like bee venom sPLA2, exogenous human sPLA2-III elicits mast cell activation when injected into mouse skin [34]. Endogenous sPLA2-III is expressed in mouse and human mast cells, where it is stored in secretory granules and released upon cell activation. Detailed analysis of Pla2g3 −/− mice has revealed that sPLA2-III not merely acts as a mast cell activator, but also functions essentially as a regulator of mast cell maturation.
Microenvironmental alterations of mast cell phenotypes through intercellular communication with fibroblasts affect susceptibility to allergy [104,105]. However, the mechanisms underlying the maturation of mast cells toward an allergy-sensitive phenotype remain incompletely understood. Mast cell-dependent passive and active anaphylactic responses are markedly attenuated in Pla2g3 −/− mice and conversely augmented in PLA2G3-TG mice in a cell-autonomous manner [34]. Skin mast cells in Pla2g3 −/− mice are morphologically and functionally immature, with markedly lower histamine and protease contents in secretory granules, expression of mast cell maturation markers, and cell surface expression of FcεRI. Moreover, bone marrow-derived mast cells (a relatively immature mast cell population) prepared from Pla2g3 −/− mice exhibit impaired fibroblast-driven maturation and thereby IgE-dependent and even -independent activation in ex vivo culture. Importantly, similar mast cell abnormalities are also evident in mice lacking lipocalin-type PGD2 synthase (L-PGDS) or those lacking the PGD2 receptor DP1 [34]. Indeed, genetic or pharmacological inactivation of DP1 in mast cells or of L-PGDS in fibroblasts phenocopies that of sPLA2-III in mast cells toward defective mast cell maturation and anaphylaxis.
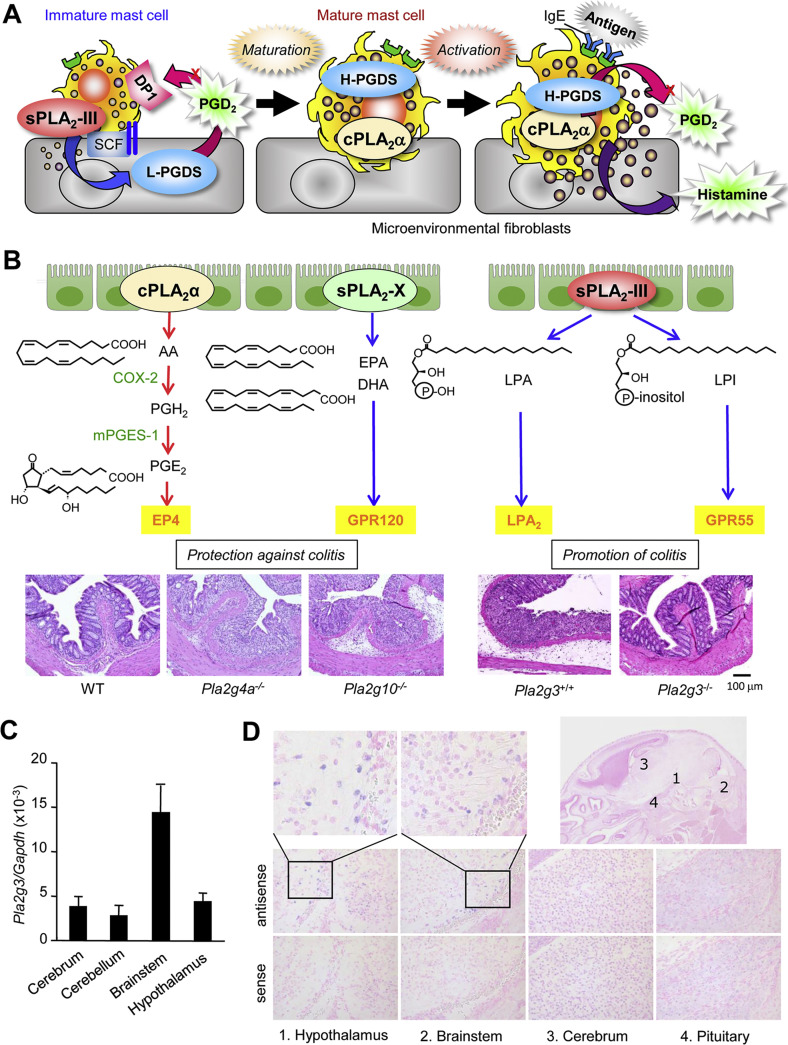
Collectively, sPLA2-III secreted from immature mast cells is functionally coupled with fibroblastic L-PGDS to provide a microenvironmental pool of PGD2, which in turn acts on DP1 on mast cells to promote their appropriate maturation (Fig. 4A). This PGD2-dependent paracrine circuit involving sPLA2-III, L-PGDS and DP1 explains a missing link required for fibroblast-driven maturation of mast cells [106]. Accordingly, a new agent that specifically inhibits this unique sPLA2 may be useful for the treatment of patients with mast cell-associated allergic and other diseases. It should be noted, however, that the mast cell defects observed in mice lacking sPLA2-III tend to be more severe than those observed in mice lacking L-PGDS or DP1 [34], suggesting that the full maturation of mast cells may require an additional sPLA2-III-driven lipid signal(s).
Fig. 4.
Properties and functions of sPLA2-III. (A) The role of sPLA2-III in mast cell maturation and allergy [34]. sPLA2-III is released from immature mast cells and coupled with fibroblastic L-PGDS to produce a microenvironmental pool of PGD2, which in turn acts on DP1 to promote mast cell maturation. Mature mast cells, which express cPLA2α and hematopoietic PGD2 synthase (H-PGDS) abundantly, release a distinct pool of PGD2 as well as histamine following activation by IgE and antigen, leading to allergic responses. Disturbance of the paracrine sPLA2-III-L-PGDS-DP1 circuit hampers the maturation and thereby activation of mast cells, resulting in impairment of allergic responses. (B) The roles of distinct PLA2s in the colon [44,92]. cPLA2α releases a pool of AA that is converted to PGH2 by cyclooxygenase-2 (COX-2) and then to PGE2 by microsomal PGE2 synthase (mPGES-1). This cPLA2α-driven PGE2 confers protection from colitis through its receptor EP4 [143]. sPLA2-X releases ω3 EPA/DHA, which blocks harmful Th17 responses in colitis through the PUFA receptor GPR120. In contrast, sPLA2-III supplies lysophospholipids such as LPA and LPI, which promote colitis and colorectal cancer probably through their receptors LPA2 and GPR55, respectively. Representative images of the colons of WT, Pla2g4a−/− and Pla2g10−/− mice treated with 1% DSS and those of Pla2g3+/+ and Pla2g3−/− mice treated with 1.5% DSS are shown. (C) Real-time PCR of Pla2g3 mRNA in the brain of 8-week-old C57BL/6 mice (n = 4, mean ± SEM). These results agree with a previous report [125]. (D) In situ hybridization of Pla2g3 mRNA in the brain of newborn C57BL/6 mice. Signals for Pla2g3 mRNA (blue) are located in several neurons within the hypothalamus and brainstem.
5.4. Colonic inflammation and cancer
Colorectal cancer is a frequent form of malignancy and a major cause of death in the Western hemisphere. Sporadic colon cancers exhibit some aspects of inflammation, and the pathogenesis of some types of colon cancer is associated with inflammatory bowel disease [107]. Several lines of evidence suggest a potential link between sPLA2-III and the development of colon cancer. Implantation of sPLA2-III-transfected colon cancer cells into nude mice leads to increased growth of tumor xenografts [91]. sPLA2-III has been proposed as a candidate biomarker for human colon cancer [108]. Higher expression of sPLA2-III in human colorectal cancer is positively correlated with a higher rate of lymph node metastasis and shorter survival [109]. Moreover, polymorphisms in the human sPLA2-III gene (PLA2G3) are significantly associated with a higher risk of colorectal cancer [110]. Importantly, a recent study has shown that sPLA2-III is expressed in colonic epithelial cells and that its genetic deletion protects against colon cancer and colitis [92].
Pla2g3 −/− mice are resistant to three distinct models of colon cancer, including those induced by azoxymethane (a model of carcinogen-induced cancer), by azoxymethane plus chronic treatment with dextran sulfate sodium (DSS) (a model of colitis-induced cancer), and by an APC mutation (Apc Min/+; a model of familial adenomatous polyposis) [92]. Furthermore, Pla2g3 −/− mice are less susceptible to DSS-induced acute colitis, with lower expression of pro-inflammatory and pathogenic Th17 cytokines and higher expression of epithelial barrier genes, than are Pla2g3 +/+ mice [92], implying that the amelioration of colonic inflammation by sPLA2-III ablation underlies the protection against colon cancer (Fig. 4B). Lipidomics analysis has revealed that the Pla2g3 −/− colon displays significant reduction of LPA and LPI species [92], which promote colon inflammation or cancer through their receptors LPA2 and GPR55, respectively [111,112]. Production of these lysophospholipids by sPLA2-III is evident in DSS-treated, but not in steady-state, colon, suggesting that sPLA2-III acts on labile or damaged epithelial membranes in this disease setting. The colonic action of sPLA2-III appears to be distinct from those of cPLA2α and sPLA2-X, which mobilize colon-protective PGE2 and ω3 PUFAs, respectively, in the colon and thereby protect against colitis [44] (Fig. 4B). Overall, these results establish a role for sPLA2-III in the aggravation of colonic inflammation and cancer, expand our understanding of the divergent roles of multiple PLA2 enzymes in the colon, and point to sPLA2-III as a novel druggable target for colorectal diseases.
5.5. Atherosclerosis
Clinically, an elevated plasma level of sPLA2-IIA is an independent risk factor for cardiovascular disease [113]. It has been proposed that sPLA2-mediated hydrolysis of lipoprotein phospholipids gives rise to a type of pro-atherogenic, small-dense LDL with an increased net negative charge, LPC content and aggregation propensity, as well as modified HDL whose anti-atherogenic function is decreased [114]. Indeed, LDL treated with several sPLA2s such as sPLA2-III, -V and -X facilitates the formation of lipid-laden foam cells from macrophages, a hallmark feature of atherosclerosis, in vitro [115,116]. On the basis of these backgrounds, the roles of conventional group I/II/V/X sPLA2s in atherosclerosis have been investigated using their transgenic or knockout mice in several studies, although the results have been controversial [[117], [118], [119], [120], [121]]. Although varespladib, a pan-sPLA2 inhibitor that broadly inhibits conventional group I/II/V/X sPLA2s, prevented the development of atherosclerosis in animal studies [122], a phase III clinical trial using this compound failed to demonstrate its therapeutic efficacy in patients with cardiovascular disease [123]. This is likely because any advantageous effect of the inhibition of pro-atherogenic sPLA2s would be cancelled out by the detrimental effect of the inhibition of anti-atherogenic sPLA2s.
PLA2G3-TG mice crossed with ApoE −/− mice, followed by supplementation with an atherogenic diet, develop more advanced atherosclerotic lesions than ApoE −/− mice, accompanied by marked increases in pro-atherogenic LPC-rich small-dense LDL and the pro-thrombotic AA metabolite thromboxane (TX) A2 [116]. PLA2G3-TG mice also develop systemic inflammation with increased age [124], suggesting that the elevated systemic inflammatory state may have an additional impact on promotion of atherosclerosis in these mice. Given that sPLA2-III is insensitive to pan-sPLA2 inhibitors, a new agent that targets this atypical sPLA2 might be useful for treatment of atherosclerosis. Nonetheless, although the analysis of PLA2G3-TG mice has revealed the pro-atherogenic potential of sPLA2-III, the definitive role of endogenous sPLA2-III in atherosclerosis awaits further clarification using Pla2g3 −/− mice.
5.6. Other potential roles
sPLA2-III is expressed in the central nervous system, where it is distributed in the brainstem, hypothalamus, spinal cord, and cerebral neocortex (Fig. 4C, D) [125]. The localization of sPLA2-III in dendrites or dendritic spines as well as postsynaptic structures in rat spinal cord suggests a potential role of this enzyme in neurotransmission or synaptic plasticity. In culture, sPLA2-III can promote neuronal outgrowth and survival [126]. In humans, PLA2G3 polymorphisms are associated with Alzheimer's disease [127]. The potential roles of sPLA2-III in neuronal function and diseases need to be evaluated using Pla2g3 −/− mice in future studies.
Interestingly, functional genomic screening has identified sPLA2-III as a negative regulator of ciliogenesis [128] The primary cilium is a microtubule-based organelle that projects from the cell surface and acts as an antenna to sense extracellular cues and regulate diverse signaling pathways [129,130]. Defective cilium formation is associated with many pathologic states, including classical ciliopathies, obesity and cancer [131,132]. Using a Pla2g3 knockdown strategy, it has been proposed that the production of lysophospholipids by sPLA2-III, whose expression is controlled by the transcription factor SREBP-1c, disturbs endosomal recycling and vesicular trafficking toward normal ciliogenesis [133]. Therefore, the functions of sPLA2-III in inflammation, cancer, and sperm flagellar motility (see above) might rely, at least in part, on the regulation of ciliogenesis by this enzyme.
6. Other poorly characterized sPLA2s
sPLA2-IIC has the structural features of group II sPLA2s, but possesses an extra sequence in the middle region, thus having 16 cysteines (i.e. 8 disulfides) [134]. Although sPLA2-IIC is expressed abundantly in meiotic cells in rodent testis [135], it is a pseudogene in humans [134]. Therefore, analysis of Pla2g2c −/− mice has not been performed. A cell biological study using Pla2g2c knockdown has shown that sPLA2-IIC is up-regulated in hepatitis B-infected hepatocytes to produce LPE, which is then presented to CD1d on natural killer T cells, leading to propagation of an anti-virus immune response [136].
The atypical group XII subfamily contains two isoforms, sPLA2-XIIA and -XIIB. The in vivo functions of sPLA2-XIIA are largely obscure, since studies using Pla2g12a −/− mice have not yet been reported. sPLA2-XIIA kills Gram-negative bacteria such as Helicobacter pylori even more efficiently than sPLA2-IIA, a “bactericidal sPLA2”, in vitro [137,138]. Forcible overexpression of sPLA2-XIIA in Xenopus laevis embryos facilitates olfactory sensory neurogenesis [139]. sPLA2-XIIA is present in axon terminals and dendrites in rat brain, and injection of its antisense oligonucleotide into the prefrontal cortex perturbs working memory and attention [140]. sPLA2-XIIB, preferentially expressed in the liver, is catalytically inactive due to the replacement of the catalytic histidine by a leucine residue [141]. Mice lacking sPLA2-XIIB (Pla2g12b −/−) display steatohepatitis due to impaired hepatic secretion of very-low-density lipoprotein through an unknown, probably non-catalytic, mechanism [142].
7. Concluding remarks
Studies during the last decade have revealed the pathophysiological functions of various sPLA2s, among which sPLA2-IID, -IIE, -IIF and -III are highlighted in this review. It is now clear that individual sPLA2s play unique and tissue-specific roles by acting on extracellular phospholipids, which include adjacent cell membranes, non-cellular lipid components, and foreign phospholipids such as those in microbes and food. The diversity of target phospholipids and products may explain why the sPLA2 family contains multiple isoforms. However, as most of our knowledge on sPLA2 functions has been obtained from mouse (mostly C57BL/6) studies, it is important to translate these studies to humans with caution. Although current data obtained from the knockout studies have suggested that individual sPLA2s are functionally non-redundant in most cases, the possibility that some of the functions could be compensated if sPLA2-IIA is normally expressed cannot be fully ruled out. Further analyses in this research field and their integration for therapeutic applications will benefit from advanced lipidomics that can monitor the sPLA2-associated lipid metabolism occurring within specific tissue niches in more detail. Hopefully, the next decade will yield a comprehensive map of sPLA2-driven lipid networks, allowing the development and therapeutic application of a new class of sPLA2 inhibitors.
Transparency document
Transparency document
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP15H05905, JP16H02613 (to M.M.), JP16K15122 (to Y.T.) and JP16H01372 (to K.Y.), and AMED-CREST JP18gm0710006 (to M.M.) and PRIME JP18gm5910012 (to K.Y.) from the Japan Agency for Medical Research and Development.
The authors have no conflicting financial interests.
Footnotes
This article is part of a Special Issue entitled Novel functions of phospholipase A2 Guest Editors: Makoto Murakami and Gerard Lambeau.
The Transparency document associated with this article can be found, in online version.
Contributor Information
Makoto Murakami, Email: makmurak@m.u-tokyo.ac.jp.
Kei Yamamoto, Email: kei@tokushima-u.ac.jp.
References
- 1.Lambeau G., Gelb M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 2.Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M., Sato H., Miki Y., Yamamoto K., Taketomi Y. A new era of secreted phospholipase A2. J. Lipid Res. 2015;56:1248–1261. doi: 10.1194/jlr.R058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami M., Yamamoto K., Miki Y., Murase R., Sato H., Taketomi Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv. Immunol. 2016;132:91–134. doi: 10.1016/bs.ai.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami M. Lipoquality control by phospholipase A2 enzymes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:677–702. doi: 10.2183/pjab.93.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seilhamer J.J., Randall T.L., Yamanaka M., Johnson L.K. Pancreatic phospholipase A2: isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 1986;5:519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- 8.Kramer R.M., Hession C., Johansen B., Hayes G., McGray P., Chow E.P., Tizard R., Pepinsky R.B. Structure and properties of a human non-pancreatic phospholipase A2. J. Biol. Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- 9.Seilhamer J.J., Pruzanski W., Vadas P., Plant S., Miller J.A., Kloss J., Johnson L.K. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J. Biol. Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- 10.Chen J., Engle S.J., Seilhamer J.J., Tischfield J.A. Cloning and recombinant expression of a novel human low molecular weight Ca2+-dependent phospholipase A2. J. Biol. Chem. 1994;269:2365–2368. [PubMed] [Google Scholar]
- 11.Chen J., Engle S.J., Seilhamer J.J., Tischfield J.A. Cloning and characterization of novel rat and mouse low molecular weight Ca2+-dependent phospholipase A2s containing 16 cysteines. J. Biol. Chem. 1994;269:23018–23024. [PubMed] [Google Scholar]
- 12.Cupillard L., Koumanov K., Mattei M.G., Lazdunski M., Lambeau G. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J. Biol. Chem. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaki J., Suzuki N., Higashino K., Yokota Y., Ono T., Kawamoto K., Fujii N., Arita H., Hanasaki K. Cloning and characterization of novel mouse and human secretory phospholipase A2s. J. Biol. Chem. 1999;274:24973–24979. doi: 10.1074/jbc.274.35.24973. [DOI] [PubMed] [Google Scholar]
- 14.Valentin E., Koduri R.S., Scimeca J.C., Carle G., Gelb M.H., Lazdunski M., Lambeau G. Cloning and recombinant expression of a novel mouse-secreted phospholipase A2. J. Biol. Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 15.Shakhov A.N., Rubtsov A.V., Lyakhov I.G., Tumanov A.V., Nedospasov S.A. SPLASH (PLA2IID), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes Immun. 2000;1:191–199. doi: 10.1038/sj.gene.6363659. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki N., Ishizaki J., Yokota Y., Higashino K., Ono T., Ikeda M., Fujii N., Kawamoto K., Hanasaki K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A2s. J. Biol. Chem. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- 17.Valentin E., Ghomashchi F., Gelb M.H., Lazdunski M., Lambeau G. On the diversity of secreted phospholipases A2. Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 1999;274:31195–31202. doi: 10.1074/jbc.274.44.31195. [DOI] [PubMed] [Google Scholar]
- 18.Valentin E., Singer A.G., Ghomashchi F., Lazdunski M., Gelb M.H., Lambeau G. Cloning and recombinant expression of human group IIF-secreted phospholipase A2. Biochem. Biophys. Res. Commun. 2000;279:223–228. doi: 10.1006/bbrc.2000.3908. [DOI] [PubMed] [Google Scholar]
- 19.Valentin E., Ghomashchi F., Gelb M.H., Lazdunski M., Lambeau G. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 2000;275:7492–7496. doi: 10.1074/jbc.275.11.7492. [DOI] [PubMed] [Google Scholar]
- 20.Gelb M.H., Valentin E., Ghomashchi F., Lazdunski M., Lambeau G. Cloning and recombinant expression of a structurally novel human secreted phospholipase A2. J. Biol. Chem. 2000;275:39823–39826. doi: 10.1074/jbc.C000671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho I.C., Arm J.P., Bingham C.O., 3rd, Choi A., Austen K.F., Glimcher L.H. A novel group of phospholipase A2s preferentially expressed in type 2 helper T cells. J. Biol. Chem. 2001;276:18321–18326. doi: 10.1074/jbc.M008837200. [DOI] [PubMed] [Google Scholar]
- 22.Verpy E., Leibovici M., Petit C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc. Natl. Acad. Sci. U. S. A. 1999;96:529–534. doi: 10.1073/pnas.96.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami M., Koduri R.S., Enomoto A., Shimbara S., Seki M., Yoshihara K., Singer A., Valentin E., Ghomashchi F., Lambeau G., Gelb M.H., Kudo I. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 2001;276:10083–10096. doi: 10.1074/jbc.M007877200. [DOI] [PubMed] [Google Scholar]
- 24.Masuda S., Murakami M., Mitsuishi M., Komiyama K., Ishikawa Y., Ishii T., Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem. J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miki Y., Yamamoto K., Taketomi Y., Sato H., Shimo K., Kobayashi T., Ishikawa Y., Ishii T., Nakanishi H., Ikeda K., Taguchi R., Kabashima K., Arita M., Arai H., Lambeau G., Bollinger J.M., Hara S., Gelb M.H., Murakami M. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013;210:1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruzanski W., Vadas P. Phospholipase A2—a mediator between proximal and distal effectors of inflammation. Immunol. Today. 1991;12:143–146. doi: 10.1016/S0167-5699(05)80042-8. [DOI] [PubMed] [Google Scholar]
- 27.Lindbom J., Ljungman A.G., Tagesson C. Interferon γ-induced gene expression of the novel secretory phospholipase A2 type IID in human monocyte-derived macrophages is inhibited by lipopolysaccharide. Inflammation. 2005;29:108–117. doi: 10.1007/s10753-006-9007-x. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K., Miki Y., Sato H., Murase R., Taketomi Y., Murakami M. Secreted phospholipase A2 specificity on natural membrane phospholipids. Methods Enzymol. 2017;583:101–117. doi: 10.1016/bs.mie.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 29.von Allmen C.E., Schmitz N., Bauer M., Hinton H.J., Kurrer M.O., Buser R.B., Gwerder M., Muntwiler S., Sparwasser T., Beerli R.R., Bachmann M.F. Secretory phospholipase A2-IID is an effector molecule of CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11673–11678. doi: 10.1073/pnas.0812569106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouault M., Le Calvez C., Boilard E., Surrel F., Singer A., Ghomashchi F., Bezzine S., Scarzello S., Bollinger J., Gelb M.H., Lambeau G. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 2007;46:1647–1662. doi: 10.1021/bi062119b. [DOI] [PubMed] [Google Scholar]
- 31.Miki Y., Kidoguchi Y., Sato M., Taketomi Y., Taya C., Muramatsu K., Gelb M.H., Yamamoto K., Murakami M. Dual roles of group IID phospholipase A2 in inflammation and cancer. J. Biol. Chem. 2016;291:15588–15601. doi: 10.1074/jbc.M116.734624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowes M.A., Suarez-Farinas M., Krueger J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 34.Taketomi Y., Ueno N., Kojima T., Sato H., Murase R., Yamamoto K., Tanaka S., Sakanaka M., Nakamura M., Nishito Y., Kawana M., Kambe N., Ikeda K., Taguchi R., Nakamizo S., Kabashima K., Gelb M.H., Arita M., Yokomizo T., Nakamura M., Watanabe K., Hirai H., Nakamura M., Okayama Y., Ra C., Aritake K., Urade Y., Morimoto K., Sugimoto Y., Shimizu T., Narumiya S., Hara S., Murakami M. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 2013;14:554–563. doi: 10.1038/ni.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boudreau L.H., Duchez A.C., Cloutier N., Soulet D., Martin N., Bollinger J., Pare A., Rousseau M., Naika G.S., Levesque T., Laflamme C., Marcoux G., Lambeau G., Farndale R.W., Pouliot M., Hamzeh-Cognasse H., Cognasse F., Garraud O., Nigrovic P.A., Guderley H., Lacroix S., Thibault L., Semple J.W., Gelb M.H., Boilard E. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duchez A.C., Boudreau L.H., Naika G.S., Bollinger J., Belleannee C., Cloutier N., Laffont B., Mendoza-Villarroel R.E., Levesque T., Rollet-Labelle E., Rousseau M., Allaeys I., Tremblay J.J., Poubelle P.E., Lambeau G., Pouliot M., Provost P., Soulet D., Gelb M.H., Boilard E. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3564–E3573. doi: 10.1073/pnas.1507905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norling L.V., Spite M., Yang R., Flower R.J., Perretti M., Serhan C.N. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawada Y., Honda T., Hanakawa S., Nakamizo S., Murata T., Ueharaguchi-Tanada Y., Ono S., Amano W., Nakajima S., Egawa G., Tanizaki H., Otsuka A., Kitoh A., Dainichi T., Ogawa N., Kobayashi Y., Yokomizo T., Arita M., Nakamura M., Miyachi Y., Kabashima K. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015;212:1921–1930. doi: 10.1084/jem.20150381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiurchiu V., Leuti A., Dalli J., Jacobsson A., Battistini L., Maccarrone M., Serhan C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramon S., Gao F., Serhan C.N., Phipps R.P. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 2012;189:1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim N., Ramon S., Thatcher T.H., Woeller C.F., Sime P.J., Phipps R.P. Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur. J. Immunol. 2016;46:81–91. doi: 10.1002/eji.201545673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titos E., Rius B., Gonzalez-Periz A., Lopez-Vicario C., Moran-Salvador E., Martinez-Clemente M., Arroyo V., Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 43.Dalli J., Zhu M., Vlasenko N.A., Deng B., Haeggstrom J.Z., Petasis N.A., Serhan C.N. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murase R., Sato H., Yamamoto K., Ushida A., Nishito Y., Ikeda K., Kobayashi T., Yamamoto T., Taketomi Y., Murakami M. Group X secreted phospholipase A2 releases ω3 polyunsaturated fatty acids, suppresses colitis, and promotes sperm fertility. J. Biol. Chem. 2016;291:6895–6911. doi: 10.1074/jbc.M116.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto K., Miki Y., Sato M., Taketomi Y., Nishito Y., Taya C., Muramatsu K., Ikeda K., Nakanishi H., Taguchi R., Kambe N., Kabashima K., Lambeau G., Gelb M.H., Murakami M. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 2015;212:1901–1919. doi: 10.1084/jem.20141904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vijay R., Hua X., Meyerholz D.K., Miki Y., Yamamoto K., Gelb M., Murakami M., Perlman S. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. J. Exp. Med. 2015;212:1851–1868. doi: 10.1084/jem.20150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiang N., Fredman G., Backhed F., Oh F., Vickery T., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia Q., Lupton J.R., Smith R., Weeks B.R., Callaway E., Davidson L.A., Kim W., Fan Y.Y., Yang P., Newman R.A., Kang J.X., McMurray D.N., Chapkin R.S. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oudijk E.J., Lammers J.W., Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2003;46:5s–13s. doi: 10.1183/09031936.03.00004603a. [DOI] [PubMed] [Google Scholar]
- 51.Barnes P.J., Celli B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 52.Takabatake N., Sata M., Inoue S., Shibata Y., Abe S., Wada T., Machiya J., Ji G., Matsuura T., Takeishi Y., Muramatsu M., Kubota I. A novel polymorphism in secretory phospholipase A2-IID is associated with body weight loss in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;172:1097–1104. doi: 10.1164/rccm.200503-319OC. [DOI] [PubMed] [Google Scholar]
- 53.Igarashi A., Shibata Y., Yamauchi K., Osaka D., Takabatake N., Abe S., Inoue S., Kimura T., Yamaguchi Y., Ishizaki J., Hanasaki K., Kubota I. Gly80Ser polymorphism of phospholipase A2-IID is associated with cytokine inducibility in A549 cells. Respiration. 2009;78:312–321. doi: 10.1159/000213243. [DOI] [PubMed] [Google Scholar]
- 54.Khan M.I., Gupta A.K., Kumar D.R., Kumar M., Ethayathulla A.S., Hariprasad G. Molecular modeling of Gly80 and Ser80 variants of human group IID phospholipase A2 and their receptor complexes: potential basis for weight loss in chronic obstructive pulmonary disease. J. Mol. Model. 2016;22:232. doi: 10.1007/s00894-016-3095-9. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto K., Miki Y., Sato H., Nishito Y., Gelb M.H., Taketomi Y., Murakami M. Expression and function of group IIE phospholipase A2 in mouse skin. J. Biol. Chem. 2016;291:15602–15613. doi: 10.1074/jbc.M116.734657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou S., Xu T., Xu J., Qu L., Xu Y., Chen L., Liu J. Structural basis for functional selectivity and ligand recognition revealed by crystal structures of human secreted phospholipase A2 group IIE. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacPhee M., Chepenik K.P., Liddell R.A., Nelson K.K., Siracusa L.D., Buchberg A.M. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 58.Zhu S., Wang Y., Chen W., Li W., Wang A., Wong S., Bao G., Li J., Yang H., Tracey K.J., D'Angelo J., Wang H. High-density lipoprotein (HDL) counter-regulates serum amyloid A (SAA)-induced sPLA2-IIE and sPLA2-V expression in macrophages. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Triggiani M., Giannattasio G., Calabrese C., Loffredo S., Granata F., Fiorello A., Santini M., Gelb M.H., Marone G. Lung mast cells are a source of secreted phospholipases A2. J. Allergy Clin. Immunol. 2009;558–565(565):e551–e553. doi: 10.1016/j.jaci.2009.04.035. (124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki K., Takahashi S., Watanabe K., Fujioka D., Nakamura T., Obata J.E., Kawabata K., Katoh R., Matsumoto M., Kugiyama K. The expression of groups IIE and V phospholipase A2 is associated with an increased expression of osteogenic molecules in human calcified aortic valves. J. Atheroscler. Thromb. 2014;21:1308–1325. doi: 10.5551/jat.24273. [DOI] [PubMed] [Google Scholar]
- 61.Silverberg M.S., Cho J.H., Rioux J.D., McGovern D.P., Wu J., Annese V., Achkar J.P., Goyette P., Scott R., Xu W., Barmada M.M., Klei L., Daly M.J., Abraham C., Bayless T.M., Bossa F., Griffiths A.M., Ippoliti A.F., Lahaie R.G., Latiano A., Pare P., Proctor D.D., Regueiro M.D., Steinhart A.H., Targan S.R., Schumm L.P., Kistner E.O., Lee A.T., Gregersen P.K., Rotter J.I., Brant S.R., Taylor K.D., Roeder K., Duerr R.H. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat. Genet. 2009;41:216–220. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato H., Taketomi Y., Ushida A., Isogai Y., Kojima T., Hirabayashi T., Miki Y., Yamamoto K., Nishito Y., Kobayashi T., Ikeda K., Taguchi R., Hara S., Ida S., Miyamoto Y., Watanabe M., Baba H., Miyata K., Oike Y., Gelb M.H., Murakami M. The adipocyte-inducible secreted phospholipases PLA2G5 and PLA2G2E play distinct roles in obesity. Cell Metab. 2014;20:119–132. doi: 10.1016/j.cmet.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hidaka A., Inoue K., Kutsukake S., Adachi M., Kakuta Y., Kojo S. Decrease in the particle size of low-density lipoprotein (LDL) by oxidation. Bioorg. Med. Chem. Lett. 2005;15:2781–2785. doi: 10.1016/j.bmcl.2005.03.117. [DOI] [PubMed] [Google Scholar]
- 64.Barnes M.J., Li C.M., Xu Y., An J., Huang Y., Cyster J.G. The lysophosphatidylserine receptor GPR174 constrains regulatory T cell development and function. J. Exp. Med. 2015;212:1011–1020. doi: 10.1084/jem.20141827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno-Navarrete J.M., Catalan V., Whyte L., Diaz-Arteaga A., Vazquez-Martinez R., Rotellar F., Guzman R., Gomez-Ambrosi J., Pulido M.R., Russell W.R., Imbernon M., Ross R.A., Malagon M.M., Dieguez C., Fernandez-Real J.M., Fruhbeck G., Nogueiras R. The l-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhi H., Qu L., Wu F., Chen L., Tao J. Group IIE secretory phospholipase A2 regulates lipolysis in adipocytes. Obesity (Silver Spring) 2015;23:760–768. doi: 10.1002/oby.21015. [DOI] [PubMed] [Google Scholar]
- 67.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kazantseva A., Goltsov A., Zinchenko R., Grigorenko A.P., Abrukova A.V., Moliaka Y.K., Kirillov A.G., Guo Z., Lyle S., Ginter E.K., Rogaev E.I. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science. 2006;314:982–985. doi: 10.1126/science.1133276. [DOI] [PubMed] [Google Scholar]
- 69.Inoue A., Arima N., Ishiguro J., Prestwich G.D., Arai H., Aoki J. LPA-producing enzyme PA-PLA1α regulates hair follicle development by modulating EGFR signalling. EMBO J. 2011;30:4248–4260. doi: 10.1038/emboj.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feingold K.R., Elias P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta. 2014;1841:280–294. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Wijewickrama G.T., Albanese A., Kim Y.J., Oh Y.S., Murray P.S., Takayanagi R., Tobe T., Masuda S., Murakami M., Kudo I., Ucker D.S., Murray D., Cho W. Unique membrane interaction mode of group IIF phospholipase A2. J. Biol. Chem. 2006;281:32741–32754. doi: 10.1074/jbc.M606311200. [DOI] [PubMed] [Google Scholar]
- 72.Murakami M., Yoshihara K., Shimbara S., Lambeau G., Gelb M.H., Singer A.G., Sawada M., Inagaki N., Nagai H., Ishihara M., Ishikawa Y., Ishii T., Kudo I. Cellular arachidonate-releasing function and inflammation-associated expression of group IIF secretory phospholipase A2. J. Biol. Chem. 2002;277:19145–19155. doi: 10.1074/jbc.M112385200. [DOI] [PubMed] [Google Scholar]
- 73.Elias P.M., Williams M.L., Holleran W.M., Jiang Y.J., Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J. Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jobard F., Lefevre C., Karaduman A., Blanchet-Bardon C., Emre S., Weissenbach J., Ozguc M., Lathrop M., Prud'homme J.F., Fischer J. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum. Mol. Genet. 2002;11:107–113. doi: 10.1093/hmg/11.1.107. [DOI] [PubMed] [Google Scholar]
- 75.Vasireddy V., Uchida Y., Salem N., Jr., Kim S.Y., Mandal M.N., Reddy G.B., Bodepudi R., Alderson N.L., Brown J.C., Hama H., Dlugosz A., Elias P.M., Holleran W.M., Ayyagari R. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirabayashi T., Anjo T., Kaneko A., Senoo Y., Shibata A., Takama H., Yokoyama K., Nishito Y., Ono T., Taya C., Muramatsu K., Fukami K., Munoz-Garcia A., Brash A.R., Ikeda K., Arita M., Akiyama M., Murakami M. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017;8 doi: 10.1038/ncomms14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li-Stiles B., Lo H.H., Fischer S.M. Identification and characterization of several forms of phospholipase A2 in mouse epidermal keratinocytes. J. Lipid Res. 1998;39:569–582. [PubMed] [Google Scholar]
- 78.Gurrieri S., Furstenberger G., Schadow A., Haas U., Singer A.G., Ghomashchi F., Pfeilschifter J., Lambeau G., Gelb M.H., Kaszkin M. Differentiation-dependent regulation of secreted phospholipases A2 in murine epidermis. J. Invest. Dermatol. 2003;121:156–164. doi: 10.1046/j.1523-1747.2003.12315.x. [DOI] [PubMed] [Google Scholar]
- 79.Haas U., Podda M., Behne M., Gurrieri S., Alonso A., Furstenberger G., Pfeilschifter J., Lambeau G., Gelb M.H., Kaszkin M. Characterization and differentiation-dependent regulation of secreted phospholipases A in human keratinocytes and in healthy and psoriatic human skin. J. Invest. Dermatol. 2005;124:204–211. doi: 10.1111/j.0022-202X.2004.23513.x. [DOI] [PubMed] [Google Scholar]
- 80.Ilic D., Bollinger J.M., Gelb M., Mauro T.M. sPLA2 and the epidermal barrier. Biochim. Biophys. Acta. 2014;1841:416–421. doi: 10.1016/j.bbalip.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grass D.S., Felkner R.H., Chiang M.Y., Wallace R.E., Nevalainen T.J., Bennett C.F., Swanson M.E. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J. Clin. Invest. 1996;97:2233–2241. doi: 10.1172/JCI118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulherkar R., Kirtane B.M., Ramchandani A., Mansukhani N.P., Kannan S., Naresh K.N. Expression of enhancing factor/phospholipase A2 in skin results in abnormal epidermis and increased sensitivity to chemical carcinogenesis. Oncogene. 2003;22:1936–1944. doi: 10.1038/sj.onc.1206229. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto K., Taketomi Y., Isogai Y., Miki Y., Sato H., Masuda S., Nishito Y., Morioka K., Ishimoto Y., Suzuki N., Yokota Y., Hanasaki K., Ishikawa Y., Ishii T., Kobayashi T., Fukami K., Ikeda K., Nakanishi H., Taguchi R., Murakami M. Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin. J. Biol. Chem. 2011;286:11616–11631. doi: 10.1074/jbc.M110.206714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nikolakopoulou Z., Nteliopoulos G., Michael-Titus A.T., Parkinson E.K. Omega-3 polyunsaturated fatty acids selectively inhibit growth in neoplastic oral keratinocytes by differentially activating ERK1/2. Carcinogenesis. 2013;34:2716–2725. doi: 10.1093/carcin/bgt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arantes E.L., Dragano N., Ramalho A., Vitorino D., de-Souza G.F., Lima M.H., Velloso L.A., Araujo E.P. Topical docosahexaenoic acid (DHA) accelerates skin wound healing in rats and activates GPR120. Biol. Res. Nurs. 2016;18:411–419. doi: 10.1177/1099800415621617. [DOI] [PubMed] [Google Scholar]
- 86.Xu J., Duan X., Hu F., Poorun D., Liu X., Wang X., Zhang S., Gan L., He M., Zhu K., Ming Z., Chen H. Resolvin D1 attenuates imiquimod-induced mice Psoriasiform dermatitis through MAPKs and NF-κB pathways. J. Dermatol. Sci. 2018;89:127–135. doi: 10.1016/j.jdermsci.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Guillaume C., Payre C., Jemel I., Jeammet L., Bezzine S., Naika G.S., Bollinger J., Grellier P., Gelb M.H., Schrevel J., Lambeau G., Deregnaucourt C. In vitro anti-Plasmodium falciparum properties of the full set of human secreted phospholipases A2. Infect. Immun. 2015;83:2453–2465. doi: 10.1128/IAI.02474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamamoto K., Isogai Y., Sato H., Taketomi Y., Murakami M. Secreted phospholipase A2, lipoprotein hydrolysis, and atherosclerosis: integration with lipidomics. Anal. Bioanal. Chem. 2011;400:1829–1842. doi: 10.1007/s00216-011-4864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murakami M., Masuda S., Shimbara S., Bezzine S., Lazdunski M., Lambeau G., Gelb M.H., Matsukura S., Kokubu F., Adachi M., Kudo I. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (groups III and XII) J. Biol. Chem. 2003;278:10657–10667. doi: 10.1074/jbc.M211325200. [DOI] [PubMed] [Google Scholar]
- 90.Hariprasad G., Kumar M., Kaur P., Singh T.P., Kumar R.P. Human group III PLA2 as a drug target: structural analysis and inhibitor binding studies. Int. J. Biol. Macromol. 2010;47:496–501. doi: 10.1016/j.ijbiomac.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Murakami M., Masuda S., Shimbara S., Ishikawa Y., Ishii T., Kudo I. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A2. J. Biol. Chem. 2005;280:24987–24998. doi: 10.1074/jbc.M502088200. [DOI] [PubMed] [Google Scholar]
- 92.Murase R., Taketomi Y., Miki Y., Nishito Y., Saito M., Fukami K., Yamamoto K., Murakami M. Group III phospholipase A2 promotes colitis and colorectal cancer. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-12434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato H., Taketomi Y., Isogai Y., Miki Y., Yamamoto K., Masuda S., Hosono T., Arata S., Ishikawa Y., Ishii T., Kobayashi T., Nakanishi H., Ikeda K., Taguchi R., Hara S., Kudo I., Murakami M. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 2010;120:1400–1414. doi: 10.1172/JCI40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lenzi A., Picardo M., Gandini L., Dondero F. Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update. 1996;2:246–256. doi: 10.1093/humupd/2.3.246. [DOI] [PubMed] [Google Scholar]
- 95.Lenzi A., Gandini L., Maresca V., Rago R., Sgro P., Dondero F., Picardo M. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 2000;6:226–231. doi: 10.1093/molehr/6.3.226. [DOI] [PubMed] [Google Scholar]
- 96.Furimsky A., Vuong N., Xu H., Kumarathasan P., Xu M., Weerachatyanukul W., Bou Khalil M., Kates M., Tanphaichitr N. Percoll gradient-centrifuged capacitated mouse sperm have increased fertilizing ability and higher contents of sulfogalactosylglycerolipid and docosahexaenoic acid-containing phosphatidylcholine compared to washed capacitated mouse sperm. Biol. Reprod. 2005;72:574–583. doi: 10.1095/biolreprod.104.036095. [DOI] [PubMed] [Google Scholar]
- 97.Rejraji H., Sion B., Prensier G., Carreras M., Motta C., Frenoux J.M., Vericel E., Grizard G., Vernet P., Drevet J.R. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol. Reprod. 2006;74:1104–1113. doi: 10.1095/biolreprod.105.049304. [DOI] [PubMed] [Google Scholar]
- 98.Aksoy Y., Aksoy H., Altinkaynak K., Aydin H.R., Ozkan A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fat. Acids. 2006;75:75–79. doi: 10.1016/j.plefa.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Iizuka-Hishikawa Y., Hishikawa D., Sasaki J., Takubo K., Goto M., Nagata K., Nakanishi H., Shindou H., Okamura T., Ito C., Toshimori K., Sasaki T., Shimizu T. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 2017;292:12065–12076. doi: 10.1074/jbc.M117.791277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Escoffier J., Jemel I., Tanemoto A., Taketomi Y., Payre C., Coatrieux C., Sato H., Yamamoto K., Masuda S., Pernet-Gallay K., Pierre V., Hara S., Murakami M., De Waard M., Lambeau G., Arnoult C. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 2010;120:1415–1428. doi: 10.1172/JCI40494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato H., Isogai Y., Masuda S., Taketomi Y., Miki Y., Kamei D., Hara S., Kobayashi T., Ishikawa Y., Ishii T., Ikeda K., Taguchi R., Ishimoto Y., Suzuki N., Yokota Y., Hanasaki K., Suzuki-Yamamoto T., Yamamoto K., Murakami M. Physiological roles of group X-secreted phospholipase A2 in reproduction, gastrointestinal phospholipid digestion, and neuronal function. J. Biol. Chem. 2011;286:11632–11648. doi: 10.1074/jbc.M110.206755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murakami M., Austen K.F., Arm J.P. The immediate phase of c-kit ligand stimulation of mouse bone marrow-derived mast cells elicits rapid leukotriene C4 generation through posttranslational activation of cytosolic phospholipase A2 and 5-lipoxygenase. J. Exp. Med. 1995;182:197–206. doi: 10.1084/jem.182.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ueno N., Taketomi Y., Yamamoto K., Hirabayashi T., Kamei D., Kita Y., Shimizu T., Shinzawa K., Tsujimoto Y., Ikeda K., Taguchi R., Murakami M. Analysis of two major intracellular phospholipases A2 (PLA2) in mast cells reveals crucial contribution of cytosolic PLA2α, not Ca2+-independent PLA2β, to lipid mobilization in proximal mast cells and distal fibroblasts. J. Biol. Chem. 2011;286:37249–37263. doi: 10.1074/jbc.M111.290312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rivera J., Fierro N.A., Olivera A., Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gurish M.F., Austen K.F. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 106.Starkl P., Marichal T., Galli S.J. PLA2G3 promotes mast cell maturation and function. Nat. Immunol. 2013;14:527–529. doi: 10.1038/ni.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 108.Mounier C.M., Wendum D., Greenspan E., Flejou J.F., Rosenberg D.W., Lambeau G. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br. J. Cancer. 2008;98:587–595. doi: 10.1038/sj.bjc.6604184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kazama S., Kitayama J., Hiyoshi M., Taketomi Y., Murakami M., Nishikawa T., Tanaka T., Tanaka J., Kiyomatsu T., Kawai K., Hata K., Yamaguchi H., Nozawa H., Ishihara S., Sunami E., Watanabe T. Phospholipase A2 group III and group X have opposing associations with prognosis in colorectal cancer. Anticancer Res. 2015;35:2983–2990. [PubMed] [Google Scholar]
- 110.Hoeft B., Linseisen J., Beckmann L., Muller-Decker K., Canzian F., Husing A., Kaaks R., Vogel U., Jakobsen M.U., Overvad K., Hansen R.D., Knuppel S., Boeing H., Trichopoulou A., Koumantaki Y., Trichopoulos D., Berrino F., Palli D., Panico S., Tumino R., Bueno-de-Mesquita H.B., van Duijnhoven F.J., van Gils C.H., Peeters P.H., Dumeaux V., Lund E., Huerta Castano J.M., Munoz X., Rodriguez L., Barricarte A., Manjer J., Jirstrom K., Van Guelpen B., Hallmans G., Spencer E.A., Crowe F.L., Khaw K.T., Wareham N., Morois S., Boutron-Ruault M.C., Clavel-Chapelon F., Chajes V., Jenab M., Boffetta P., Vineis P., Mouw T., Norat T., Riboli E., Nieters A. Polymorphisms in fatty-acid-metabolism-related genes are associated with colorectal cancer risk. Carcinogenesis. 2010;31:466–472. doi: 10.1093/carcin/bgp325. [DOI] [PubMed] [Google Scholar]
- 111.Lin S., Wang D., Iyer S., Ghaleb A.M., Shim H., Yang V.W., Chun J., Yun C.C. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stancic A., Jandl K., Hasenohrl C., Reichmann F., Marsche G., Schuligoi R., Heinemann A., Storr M., Schicho R. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 2015;27:1432–1445. doi: 10.1111/nmo.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kugiyama K., Ota Y., Takazoe K., Moriyama Y., Kawano H., Miyao Y., Sakamoto T., Soejima H., Ogawa H., Doi H., Sugiyama S., Yasue H. Circulating levels of secretory type II phospholipase A2 predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–1284. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- 114.Ishimoto Y., Yamada K., Yamamoto S., Ono T., Notoya M., Hanasaki K. Group V and X secretory phospholipase A2s-induced modification of high-density lipoprotein linked to the reduction of its antiatherogenic functions. Biochim. Biophys. Acta. 2003;1642:129–138. doi: 10.1016/s0167-4889(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 115.Hanasaki K., Yamada K., Yamamoto S., Ishimoto Y., Saiga A., Ono T., Ikeda M., Notoya M., Kamitani S., Arita H. Potent modification of low density lipoprotein by group X secretory phospholipase A2 is linked to macrophage foam cell formation. J. Biol. Chem. 2002;277:29116–29124. doi: 10.1074/jbc.M202867200. [DOI] [PubMed] [Google Scholar]
- 116.Sato H., Kato R., Isogai Y., Saka G., Ohtsuki M., Taketomi Y., Yamamoto K., Tsutsumi K., Yamada J., Masuda S., Ishikawa Y., Ishii T., Kobayashi T., Ikeda K., Taguchi R., Hatakeyama S., Hara S., Kudo I., Itabe H., Murakami M. Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J. Biol. Chem. 2008;283:33483–33497. doi: 10.1074/jbc.M804628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivandic B., Castellani L.W., Wang X.P., Qiao J.H., Mehrabian M., Navab M., Fogelman A.M., Grass D.S., Swanson M.E., de Beer M.C., de Beer F., Lusis A.J. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler. Thromb. Vasc. Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 118.Bostrom M.A., Boyanovsky B.B., Jordan C.T., Wadsworth M.P., Taatjes D.J., de Beer F.C., Webb N.R. Group v secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:600–606. doi: 10.1161/01.ATV.0000257133.60884.44. [DOI] [PubMed] [Google Scholar]
- 119.Boyanovsky B., Zack M., Forrest K., Webb N.R. The capacity of group V sPLA2 to increase atherogenicity of ApoE−/− and LDLR−/− mouse LDL in vitro predicts its atherogenic role in vivo. Arterioscler. Thromb. Vasc. Biol. 2009;29:532–538. doi: 10.1161/ATVBAHA.108.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zack M., Boyanovsky B.B., Shridas P., Bailey W., Forrest K., Howatt D.A., Gelb M.H., de Beer F.C., Daugherty A., Webb N.R. Group X secretory phospholipase A2 augments angiotensin II-induced inflammatory responses and abdominal aortic aneurysm formation in apoE-deficient mice. Atherosclerosis. 2011;214:58–64. doi: 10.1016/j.atherosclerosis.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ait-Oufella H., Herbin O., Lahoute C., Coatrieux C., Loyer X., Joffre J., Laurans L., Ramkhelawon B., Blanc-Brude O., Karabina S., Girard C.A., Payre C., Yamamoto K., Binder C.J., Murakami M., Tedgui A., Lambeau G., Mallat Z. Group X secreted phospholipase A2 limits the development of atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2013;33:466–473. doi: 10.1161/ATVBAHA.112.300309. [DOI] [PubMed] [Google Scholar]
- 122.Rosenson R.S., Hislop C., McConnell D., Elliott M., Stasiv Y., Wang N., Waters D.D., Investigators P. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 2009;373:649–658. doi: 10.1016/S0140-6736(09)60403-7. [DOI] [PubMed] [Google Scholar]
- 123.Nicholls S.J., Kastelein J.J., Schwartz G.G., Bash D., Rosenson R.S., Cavender M.A., Brennan D.M., Koenig W., Jukema J.W., Nambi V., Wright R.S., Menon V., Lincoff A.M., Nissen S.E., Investigators V.-. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 124.Sato H., Taketomi Y., Isogai Y., Masuda S., Kobayashi T., Yamamoto K., Murakami M. Group III secreted phospholipase A2 transgenic mice spontaneously develop inflammation. Biochem. J. 2009;421:17–27. doi: 10.1042/BJ20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang H., Siddiqi N.J., Alhomida A.S., Ong W.Y. Expression and localization of sPLA2-III in the rat CNS. Neurochem. Res. 2013;38:753–760. doi: 10.1007/s11064-013-0974-7. [DOI] [PubMed] [Google Scholar]
- 126.Masuda S., Yamamoto K., Hirabayashi T., Ishikawa Y., Ishii T., Kudo I., Murakami M. Human group III secreted phospholipase A2 promotes neuronal outgrowth and survival. Biochem. J. 2008;409:429–438. doi: 10.1042/BJ20070844. [DOI] [PubMed] [Google Scholar]
- 127.Martinez-Garcia A., Sastre I., Recuero M., Aldudo J., Vilella E., Mateo I., Sanchez-Juan P., Vargas T., Carro E., Bermejo-Pareja F., Rodriguez-Rodriguez E., Combarros O., Rosich-Estrago M., Frank A., Valdivieso F., Bullido M.J. PLA2G3, a gene involved in oxidative stress induced death, is associated with Alzheimer's disease. J. Alzheimers Dis. 2010;22:1181–1187. doi: 10.3233/JAD-2010-101348. [DOI] [PubMed] [Google Scholar]
- 128.Kim J., Lee J.E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J.G. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Christensen S.T., Pedersen L.B., Schneider L., Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. (Copenhagen, Denmark) [DOI] [PubMed] [Google Scholar]
- 130.Berbari N.F., O'Connor A.K., Haycraft C.J., Yoder B.K. The primary cilium as a complex signaling center. Curr. Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fliegauf M., Benzing T., Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 132.Sen Gupta P., Prodromou N.V., Chapple J.P. Can faulty antennae increase adiposity? The link between cilia proteins and obesity. J. Endocrinol. 2009;203:327–336. doi: 10.1677/JOE-09-0116. [DOI] [PubMed] [Google Scholar]
- 133.Gijs H.L., Willemarck N., Vanderhoydonc F., Khan N.A., Dehairs J., Derua R., Waelkens E., Taketomi Y., Murakami M., Agostinis P., Annaert W., Swinnen J.V. Primary cilium suppression by SREBP1c involves distortion of vesicular trafficking by PLA2G3. Mol. Biol. Cell. 2015;26:2321–2332. doi: 10.1091/mbc.E14-10-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tischfield J.A., Xia Y.R., Shih D.M., Klisak I., Chen J., Engle S.J., Siakotos A.N., Winstead M.V., Seilhamer J.J., Allamand V., Gyapay G., Lusis A.J. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 1996;32:328–333. doi: 10.1006/geno.1996.0126. [DOI] [PubMed] [Google Scholar]
- 135.Chen J., Shao C., Lazar V., Srivastava C.H., Lee W.H., Tischfield J.A. Localization of group IIc low molecular weight phospholipase A2 mRNA to meiotic cells in the mouse. J. Cell. Biochem. 1997;64:369–375. doi: 10.1002/(sici)1097-4644(19970301)64:3<369::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 136.Zeissig S., Murata K., Sweet L., Publicover J., Hu Z., Kaser A., Bosse E., Iqbal J., Hussain M.M., Balschun K., Rocken C., Arlt A., Gunther R., Hampe J., Schreiber S., Baron J.L., Moody D.B., Liang T.J., Blumberg R.S. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat. Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koduri R.S., Gronroos J.O., Laine V.J., Le Calvez C., Lambeau G., Nevalainen T.J., Gelb M.H. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 138.Huhtinen H.T., Gronroos J.O., Gronroos J.M., Uksila J., Gelb M.H., Nevalainen T.J., Laine V.J. Antibacterial effects of human group IIA and group XIIA phospholipase A2 against Helicobacter pylori in vitro. APMIS. 2006;114:127–130. doi: 10.1111/j.1600-0463.2006.apm_330.x. [DOI] [PubMed] [Google Scholar]
- 139.Munoz-Sanjuan I., Brivanlou A.H. Induction of ectopic olfactory structures and bone morphogenetic protein inhibition by Rossy, a group XII secreted phospholipase A2. Mol. Cell. Biol. 2005;25:3608–3619. doi: 10.1128/MCB.25.9.3608-3619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ee S.M., Lo Y.L., Shui G., Wenk M.R., Shin E.J., Kim H.C., Ong W.Y. Distribution of secretory phospholipase A2 XIIA in the brain and its role in lipid metabolism and cognition. Mol. Neurobiol. 2014;50:60–75. doi: 10.1007/s12035-014-8635-7. [DOI] [PubMed] [Google Scholar]
- 141.Rouault M., Bollinger J.G., Lazdunski M., Gelb M.H., Lambeau G. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry. 2003;42:11494–11503. doi: 10.1021/bi0349930. [DOI] [PubMed] [Google Scholar]
- 142.Guan M., Qu L., Tan W., Chen L., Wong C.W. Hepatocyte nuclear factor-4α regulates liver triglyceride metabolism in part through secreted phospholipase A2 GXIIB. Hepatology. 2011;53:458–466. doi: 10.1002/hep.24066. [DOI] [PubMed] [Google Scholar]
- 143.Kabashima K., Saji T., Murata T., Nagamachi M., Matsuoka T., Segi E., Tsuboi K., Sugimoto Y., Kobayashi T., Miyachi Y., Ichikawa A., Narumiya S. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document