Highlights
-
•
3.71% of children with ARTI exhibited HAdV positive.
-
•
HAdV-2, HAdV-3 and HAdV-7 were the predominant types identified from ARTI children.
-
•
74.85% of HAdV were co-detected with other respiratory pathogens, most commonly HRV.
-
•
The co-detection rate of HAdV-C was significant higher than those of HAdV-B.
-
•
HAdV-7 positive children may not present more severe clinical outcome.
Abbreviations: ARTI, acute respiratory tract infection; HAdV, Human adenovirus; CAP, community-acquired pneumonia; NPA, nasopharyngeal aspirate; FluA, Influenza A; FluB, Influenza B; 09H1, Influenza A H1N1 pdm09; H3, Influenza H3N2; HPIV, Human parainfluenza virus; RSV, Respiratory syncytial virus; HMPV, Human metapneumovirus; HRV, Rhinovirus; HBoV, Human bocavirus; HCoV, Human coronavirus; Ch, Chlamydia; Mp, Mycoplasma pneumoniae
Keywords: Adenovirus, Acute respiratory tract infection, Molecular epidemiology, Clinical characterization, Children
Abstract
Background
Human adenovirus (HAdV) is a common pathogen in children that can cause acute respiratory tract infection (ARTI), but the molecular epidemiological and clinical information relating to HAdV among hospitalized children with ARTI are few reported in China.
Objectives
To evaluate the epidemiological, clinical, and molecular characteristics of HAdV infections among hospitalized children with ARTI in Hebei, Northern China from June 2017 to May 2018.
Study design
A 12-month longitudinal, retrospective study on HAdV, typed by nested polymerase chain reaction targeting the hexon gene’s hypervariable region (typing was merely performed by sequencing of the hexon neutralization epitope and thus genotypes could not be identified unequivocally), associated with ARTI was performed. The epidemiological and clinical data of different types of HAdV were analyzed using statistical product and service solutions (SPSS) 21.0 software.
Results
HAdV was detected in 330 (3.71%) of the 8906 specimens, with most (88.48%, 292/330) HAdV-positives cases detected among children < 3 years old. HAdV were detected throughout the year with a higher prevalence in spring. 11 types were identified, with HAdV-2 (33.33%, 110/330) as the predominant type, followed by HAdV-3 (21.21%, 70/330) and HAdV-7 (13.94%, 46/330). Of the 330 HAdV-positive specimens, 247 (74.85%) were co-detected with other respiratory pathogens, most commonly rhinovirus (HRV) (58.7%, 145/247). Additionally, patients with HAdV-7 positive had longer duration of fever than HAdV-2 or -3 positive patients.
Conclusions
During the study period, HAdV-2, HAdV-3 and HAdV-7 were the predominant types identified from children with ARTI in Hebei Province. Pediatric patients with HAdV-7 positive may not present more severe clinical outcome except a longer duration of fever.
1. Background
Human adenovirus (HAdV) is considered as an important causative agent of acute respiratory tract infection (ARTI) in children [1,2], and accounts for at least 5 to 10% of pediatric ARTI [3]. HAdV is a non-enveloped, double-stranded DNA virus belonging to the Mastadenovirus genus (Adenoviridae family). There are currently seven different HAdV species (A to G), including 103 HAdV types (HAdV types up to type number 51 were defined as serotypes and higher type numbers were defined as genotypes) (http://hadvwg.gmu.edu/). HAdV-1, 2, 3, 4, 5, 6, 7, 11, 14 and the re-emergent type HAdV-55 are known to cause ARTI [[4], [5], [6]]. Although HAdV is associated with mild to moderate disease in most cases, types 3 and 7 were increasingly reported [7,8] associated with severe, and even life-threatening infections and outbreaks. Therefore, investigating the epidemiological and clinical manifestations of different types of HAdV infections is helpful to the prevention and control of HAdV circulating.
2. Objectives
Previous studies have reported the adenoviral epidemiology in Beijing and Guangzhou, China [9,10]. However, information on HAdV among hospitalized children with ARTI is limited in Hebei, China. To perfect molecular epidemiological and clinical information of HAdV infections, we conducted a retrospective study to evaluate the epidemiological, clinical, and molecular characteristics of HAdV infections among hospitalized children with ARTI in Hebei, Northern China from June 2017 to May 2018.
3. Study design
3.1. Population and definitions
Hospitalized children (<18 years) with ARTI from Children’s Hospital of Hebei Province from May 2017 to April 2018, were retrospectively enrolled in this study. Children with signs and symptoms of respiratory tract infection (such as fever, coughing, nasal obstruction, sneeze), or lower respiratory infection signs (tachypnea, dyspnea, or wheezing/rales upon auscultation etc.) were defined as ARTI. Community-acquired pneumonia (CAP) was diagnosed by radiographic evidence (consolidation, other infiltrate or pleural effusion). Severe CAP was defined as an CAP that presented with one of the following clinical presentations: oxygen saturation<92%, cyanosis; respiratory rate >70 (infants) or 50 (older children) breaths/min; significant tachycardia for level of fever; prolonged central capillary refill time >2 s; difficulty in breathing; intermittent apnoea (infants), grunting; not feeding (infants); chronic conditions (eg, congenital heart disease, chronic lung disease of prematurity, chronic respiratory conditions leading to infection such as cystic fibrosis, bronchiectasis, immune deficiency). This study was approved by the Institutional Review Board of the Children’s Hospital of Hebei Province, affiliated to Hebei Medical University. Data records and collected clinical specimens were de-identified and completely anonymous so informed consent was waived.
3.2. Information collection and detection of pathogens
Demographic and clinical data were obtained from the hospital’s database. The nasopharyngeal aspirate (NPA) specimens collected from each patient were extracted total nucleic acids using EasyPure Viral DNA/RNA Kit (TransGen Biotech, Beijing, China) according to the manufacturer's instructions. Thirteen common respiratory pathogens and subtypes (Influenza A (Flu A), Influenza B (Flu B), Influenza A H1N1 pdm09 (09H1), influenza H3N2 (H3), human parainfluenza virus (HPIV), respiratory syncytial virus (RSV), HAdV, human metapneumovirus (HMPV), rhinovirus (HRV), human bocavirus (HBoV), human coronavirus (HCoV), Chlamydia (Ch) and Mycoplasma pneumoniae (Mp)) were detected by using a GeXP-based multiplex reverse transcription PCR assay [11]. HAdV-positive samples were molecularly typed by nested PCR amplification and sequencing that targeted hypervariable regions 1-6 of the hexon gene, as described previously [12].
Meanwhile, bronchial alveolar lavage fluids (if available), blood, and pleural effusion (if available) from HAdV-positive patients during the entire hospital admission were used to identify any bacteria or fungi.
3.3. Statistical analysis
Epidemiological and clinical data were analyzed using statistical product and service solutions (SPSS) 21.0 software. Categorical variables were evaluated by Chi-square test and Fisher’s exact test where appropriate. Continuous variables were compared by a Mann-Whitney test. All tests were two-tailed and the value of P < 0.05 was considered to represent a statistically significant difference.
4. Results
4.1. Characteristics of the children with ARTI
A total of 8906 hospitalized children with ARTI at the Children’s Hospital of Hebei Province, affiliated to Hebei Medical University were enrolled, and all children survived. Among those children, 5458 (61.28%) were males and 3448 (38.72%) were females (Table1 ). The age range was from 1 month to 18 years of age with a median age of 1.5 years. From them, 6955 (78.09%) patients were younger than 3 years old (Table 1).
Table 1.
HAdV-positive in children of different ages and gender with ARTI.
| Variable | total ARIT(N) | HAdV-positive (N (%)) | P |
|---|---|---|---|
| Gender | |||
| Male | 5458 | 209 (3.83) | 0.436 |
| Female | 3448 | 121 (3.51) | |
| Age (years) | |||
| ≤1 | 3787 | 110 (2.9) | <0.001 |
| >1 to ≤2 | 2054 | 139 (6.77) | |
| >2to≤3 | 1114 | 43 (3.86) | |
| >3 | 1951 | 38 (1.95) | |
| Total | 8906 | 330 (3.71) | |
| HAdV: Human adenovirus, ARTI: Acute respiratory tract infection. | |||
4.2. Epidemiology of HAdV
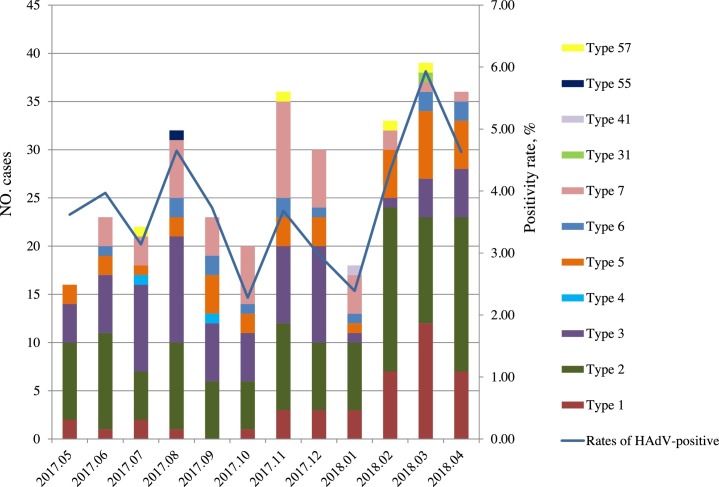
Among the 8906 children, 330 (3.71%) HAdV-positive cases were detected. And most (88.48%, 292/330) HAdV-positives cases detected among children < 3 years old. No significant differences were observed in males 209 (63.33%) and females 121 (36.67%) in the rate of HAdV-positive (P = 0.436). Children at age groups > 1 to ≤ 2 years had higher HAdV-positive rates than other age groups (P < 0.001) (Table 1). Low HAdV-positive rates were detected in October 2017 (2.28%) and January 2018 (2.39 %), while a peak positive rate occurred during March-April 2018 (Fig.1 ).
Fig. 1.
Seasonal distribution of HAdV and diffenert HAdV types in children with ARTI from May 2017 to April 2018. HAdV: Human adenovirus, ARTI: acute respiratory tract infection.
4.3. Typing of HAdV
We identified 11 HAdV types in 330 samples. HAdV-2 [33.33% (110/330)] was the most prevalent type, followed by HAdV-3 [21.52% (70/330)] and HAdV-7 [13.94% (46/330)] (Table 2 ). Beside, 2 cases with multiple HAdV types (HAdV-2 and HAdV-3) were identified. HAdV-1 and 2 positive cases were more detected during February to Apirl, 2018; HAdV-3 was more prevalent during July to August, 2017 and November to December, 2017; HAdV-5 was more frequent in March, 2018 and HAdV-7 in November 2017. In contrast, HAdV-4, 6, 31, 41, 55 and 57 were sporadic detected during the study period (Fig. 1).
Table 2.
Molecular types of HAdV in single-detection and co-detection groups.
| Total | Single-detection | Co-detection | P | ||
|---|---|---|---|---|---|
| HAdV -positive | 330 | 81 (24.55) | 247 (74.85) | - | |
| Gender, male | 209 (63.33) | 55 (67.9) | 153 (61.94) | 0.334 | |
| Age, median (IQR) (y) | 1.33 (1.17) | 1.75 (2) | 1.25 (1.17) | 0.032 | |
| Genotypes | |||||
| HAdV -A | 31 | 1 (0.3) | 1 (100) | - | |
| HAdV -B | 117 (35.45) | 41 (35.04) | 76 (64.96) | 0.001b | |
| 3 | 70 (21.21) | 22 (31.43) | 48 (68.57) | ||
| 7 | 46 (13.94) | 18 (39.13) | 28 (60.87) | ||
| 55 | 1 (0.3) | 1 (100) | - | ||
| HAdV -C | 207 (62.73) | 38 (18.36) | 169 (81.64) | ||
| 1 | 42 (12.73) | 10 (23.81) | 32 (76.19) | ||
| 2 | 110 (33.33) | 19 (17.276) | 91 (82.73) | ||
| 5 | 37 (11.21) | 7 (18.92) | 30 (81.08) | ||
| 6 | 14 (4.24) | 1 (7.14) | 13 (92.86) | ||
| 57 | 4 (1.21) | 1 (25) | 3 (75) | ||
| HAdV -E | 4 | 2 (0.61) | 1 (50) | 1 (50) | |
| HAdV -F | 41 | 1 (0.3) | - | 1 (100) | |
| HAdV -2&3a | 2 (0.61) | - | - | ||
Data are presented as No. (%) unless otherwise indicated. IQR: interquartile range. aHAdV -2&3: HAdV -2 co-infection with HAdV -3. b The mixed detection rate of HAdV -B was compared with those of HAdV-C.
4.4. Co-detected with other respiratory pathogens
Of the 330 HAdV-positive cases, 247 (74.85%) were co-detected with other respiratory pathogens including 163 (65.99%, 163/247) with one other pathogens, 74 (29.96%, 74/247) with two other pathogens, and 10 (4.05%, 10/247) with three other pathogens. The most frequently identified mixed infection was HRV (58.7%, 145/247), as shown in Table 3 . The median age of children with co-detection was 1.25 years and younger than children with single-detection (median age was 1.75 years, P = 0.032). Compared with HAdV-B positive cases, the mixed detection rate of HAdV-C was significantly higher (P = 0.001). Detailed co-detection distribution of different types was shown in Table 2.
Table 3.
Co-detection of HAdV with other respiratory pathogens.
| 2 pathogens (N = 163) |
3 pathogens (N = 74) |
4 pathogens (N = 10) |
|||
|---|---|---|---|---|---|
| Etiologic agents (HAdV+) | Proportion | Etiologic agents (HAdV+) | Proportion | Etiologic agents (HAdV+) | Proportion |
| HRV | 80 | HRV+HPIV | 23 | HRV + Mp+HPIV | 2 |
| RSV | 25 | HRV + RSV | 13 | HRV + Mp+HBoV | 2 |
| HPIV | 16 | HRV+HMPV | 7 | HRV + Mp+HMPV | 1 |
| HMPV | 13 | HRV+HCoV | 7 | HRV + Mp + FluB | 1 |
| HBoV | 8 | HRV + Mp | 3 | HRV+HPIV+HCoV | 1 |
| Mp | 8 | HRV+HBoV | 3 | HRV + RSV+09H1 | 1 |
| 09H1 | 6 | HPIV+HCoV | 3 | HRV+HCoV+H. influenzae | 1 |
| S. Pneumoniae | 3 | HCoV + RSV | 3 | HBoV + Mp + RSV | 1 |
| FluB | 2 | RSV+09H1 | 2 | ||
| HCoV | 1 | HPIV+HMPV | 2 | ||
| H3 | 1 | HPIV + RSV | 1 | ||
| HPIV+09H1 | 1 | ||||
| HBoV+HCoV | 1 | ||||
| HBoV+H. influenzae | 1 | ||||
| HMPV + FluB | 1 | ||||
| 09H1+FluB | 1 | ||||
| RSV + S. Pneumoniae | 1 | ||||
| H. influenzae + S. aureus | 1 | ||||
HAdV: Human adenovirus, HRV: Rhinovirus, RSV: Respiratory syncytial virus, HPIV: Human parainfluenza virus, HMPV: Human metapneumovirus, HBoV: Human bocavirus, Mp: Mycoplasma pneumoniae, 09H1: Influenza A H1N1 pdm09, S. pneumoniae: Streptococcus pneumoniae, FluB: Influenza B, HCoV: Human coronavirus, H3: Influenza H3N2, H. influenzae: Haemophilus influenza, S. aureus: Staphylococcus aureus.
4.5. Demographic and clinical data of different types of HAdV
After excluding 247 cases with co-detection, 2 cases with multiple types, 1 case diagnosed with bronchial foreign body and 1 case diagnosed with herpangina, demographic and clinical data of 79 cases were collected and shown in supplementary material S1. Fever, cough, and sore throat were the most common clinical features among different types. CAP was the most common diagnoses of the patients. 2 patients with HAdV-2 positive were admitted to PICU, 2 patients (1 with HAdV-1 positive and 1 with HAdV-3 positive) required respiratory support with mechanical ventilation, and 5 patients (2 with HAdV-2 positive, 1 with HAdV-3 positive and 2 with HAdV-7 positive) underwent immunoglobulin therapy. All HAdV-positive patients recovered fully from respiratory infections.
Because HAdV-2, HAdV-3 and HAdV-7 were the most predominant types among patients with HAdV positive, the age and gender distribution, clinical characteristics, and laboratory findings of patients with single HAdV-2, HAdV-3 and HAdV-7 positive were further compared to exclude the possible effect of other respiratory pathogens infection. The HAdV-7 positive patients had a longer median duration of fever, followed the HAdV-3 positive patients and the HAdV-2 positive patients were shortest (P = 0.006). Runny nose was observed in 33.33% of HAdV-2 positive patients, 22.73% of HAdV-3 positive patients and only 5.56% of HAdV-7 positive patients (P = 0.008). HAdV-3 and HAdV-7 positive patients were more likely to experience sore throat (P = 0.015) and had higher leukocyte count (P = 0.048) than HAdV-2 positive patients. Underlying respiratory diseases were more frequently observed with the HAdV-3 positive patients (27.27%) and the HAdV-7 positive patients (11.11%) compared to HAdV-2 positive patients (0%) (P = 0.044). No significant differences were found in the age and gender distribution, complications and clinical outcomes when compared among the three HAdV types.
5. Discussion
HAdV is a common pathogen associated with ARTI in hospitalized children. Among our studied cohort, 3.71% of paediatric patients with ARTI exhibited HAdV positive, which is lower than the finding (5.64%) in Beijing [9] during the same period. Such discrepant HAdV detection rates were easy to be explained by different regions. Previous studies from Beijing and Guangzhou [9,10] revealed that HAdV infections occurred throughout the year with the highest prevalence in the summer. However, a peak positive rate occurred during March-April in the present study, which is consistent with another study that has reported seasonal peak for HAdV infections in spring in Northern China [13]. We found that HAdV-positives cases detected predominantly (88.48%) in children under 3 years of age, with a peaking in children aged 1-2 years (P < 0.001), demonstrating that most children become infected by HAdV at an early age. A recent repeated cross sectional study [14] drew similar findings that the HAdV positive rate of children at the age of <3 years was 63.51% and the rate of HAdV infection peaking in children aged l-3 years in Northern China (P = 0.021).
HAdV -2, -3 and -7 are the most prevalent species in China [15,16], but the type distribution of HAdV varies for different regions and periods of studies. HAdV-7 was the most prevalent types from 2007 to 2012 in Beijing [17], HAdV-3 dominated from 2017 to 2018 in Beijing [9] and from 2012 to 2013 in Guangzhou [8]. While throughout the present study period, HAdV-2 was the most prevalent type, followed by HAdV-3 and -7.
HAdV-55 is a recombinant virus which has a genomic backbone of type 14 and a neutralization epitope of type 11, and once caused an outbreak of respiratory tract infection in Shanxi Province, China, in 2006 [18]. In the present study, a hexon sequence identical to type 55 was detected from a boy aged eight months. This boy diagnosed with CAP and presented with a ten-days of fever (Maximum temperature = 40 °C), cough and sore throat, but no PICU admission, mechanical ventilation and immunoglobulin therapy required during the hospitalization period. Although this hexon sequence is also almost identical to type 11 which is not closely related to respiratory infections, it was thought as type 55 according to clinical feature of this boy. This is the first report of mono-detection of HAdV-55 in children with ARTI in Hebei Province, though HAdV-55 infection has been reported in other provinces in China [17,19].
Another notable finding of this study was the identifying of HAdV-57 in 4 respiratory samples. HAdV-57 was first isolated from the feces of a healthy child as part of an acute flaccid paralysis surveillance program. The detection of HAdV-57 in respiratory samples collected from pediatric patients with ARTI was first reported by a study from 2007 to 2012 in Beijing [17]. However, all the three HAdV-57-infected cases were co-infected with other respiratory viruses in their study. In this present study, HAdV-57 alone was identified in a previously healthy 10-months girl who diagnosed with bronchitis requiring hospitalization. The girl not only presented with signs and symptoms of respiratory tract infection, including fever (Maximum temperature = 37.5 °C), cough, runny nose, nasal congestion, sore throat and dyspnea, but also accompanied by vomiting. Unfortunately, the further HAdV detection on her stool sample was not performed.
Similar to the report from Beijing (69.6%), Nouthern China [17], results here showed that 74.85% of HAdV were co-detected with one or more other respiratory tract pathogens and that coinfections were more frequently observed in younger children. Moreover, co-detection rates of different HAdV types varied, especially the co-detection rate of HAdV-C was significant higher than those of HAdV-B (P = 0.001). Nested PCR with a low limit of detection was used for typing of HAdV in the present study. Thus low level HAdV DNA shedding of latent HAdV infections could be detected, which may be explain the high rate of co-detection of HAdV-C with other pathogens.
In a recent study in Taiwan [15], clinical features of HAdV- 2, -3, and -7 mono-infections in children were investigated in an outbreak, and drawn a conclusion that childhood HAdV-2, HAdV-3 and HAdV-7 infections may exhibit different clinical manifestations and HAdV-7 caused more severe disease characteristics and outcomes. Similarly, through a comprehensive series of assays in vitro and in vivo as well as clinical correlates, a study in Chongqing [20] shown that HAdV-7 replicates more robustly than HAdV-3, and promotes an exacerbated cytokine response, causing a more severe airway inflammation. Our results revealed that patients with HAdV-7 positive experienced longer durations of fever than HAdV-2 or HAdV-3 positive patients, which was consistent with the results reported by Taiwan [15]. However, although patients with HAdV-7 positive had a higher rate of severe CAP and tended to require longer hospital stays in our study, no significant difference was found. Meanwhile, no patients with HAdV-7 positive needed PICU admission and mechanical ventilation in our study. These results may not support the conclusion from previous investigations [15,20] that HAdV-7 positive patients may have more severe clinical consequence.
One potential weakness of our data is that there was small sample size, since we have excluded the possible interference by any other co-infected respiratory pathogens. So, a larger sample size of case-control study is needed to illustrate the effectiveness of our results. Another limitation of this study is that typing of HAdV was merely performed by sequencing of the hexon neutralization epitope. However, some genotypes share their neutralization epitope with one of the (sero-)types. Thus we cannot exclude completely that the hexon sequence though as type 55 was truly type 11 in this study.
6. Conclusions
Our study reported the molecular epidemiology and clinical characterization of among the HAdV-positive pediatric hospitalized patients with ARTI in Hebei, Northern China in 2017-2018, providing reliable scientific basis for diagnosis, prevention and control for the future of HAdV infection in Hebei region.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Children’s Hospital of Hebei Province, affiliated to Hebei Medical University. Data records and collected clinical specimens were de-identified and completely anonymous so informed consent was waived.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declaration of Competing Interest
The authors declare that there are no competing interests.
Funding
This work was supported by grants from the Hebei Key Project Plan for Medical Science Research (20190834).
Authors’ contributions
MCZ, GXL and ZSF conceived the study. MCZ, YHG and FZQ performed the experiments. YHG, FZQ, LW and SY conducted the clinical work. MCZ, YHG and FZQ wrote this article, ZSF revised it. All the authors have read and approved the final version of this manuscript.
CRediT authorship contribution statement
Meng-chuan Zhao: Conceptualization, Methodology, Writing - original draft, Funding acquisition. Ying-hui Guo: Methodology, Investigation, Writing - original draft. Fang-zhou Qiu: Methodology, Investigation, Writing - original draft. Le Wang: Formal analysis, Resources. Shuo Yang: Investigation, Resources. Zhi-shan Feng: Conceptualization, Writing - review & editing, Funding acquisition. Gui-xia Li: Conceptualization, Writing - review & editing, Supervision.
Acknowledgements
Not applicable.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2019.104254.
Contributor Information
Meng-chuan Zhao, Email: zhaomengchuan1989@163.com.
Ying-hui Guo, Email: 18503292337@139.com.
Fang-zhou Qiu, Email: qiufangzhou91@sina.com.
Le Wang, Email: luka_wl@163.com.
Shuo Yang, Email: hbcheysy@163.com.
Zhi-shan Feng, Email: 1173791762@qq.com.
Gui-xia Li, Email: 13832179762@139.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Sun J., Xiao Y., Zhang M., Ao T., Lang S., Wang J. Serum inflammatory markers in patients with adenovirus respiratory infection. Med Sci Monit. 2018;24:3848–3855. doi: 10.12659/MSM.910692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wo Y., Lu Q.B., Huang D.D., Li X.K., Guo C.T., Wang H.Y. Epidemical features of HAdV-3 and HAdV-7 in pediatric pneumonia in Chongqing, China. Arch Virol. 2015;160:633–638. doi: 10.1007/s00705-014-2308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandkovsky U., Vargas L., Florescu D.F. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep. 2014;16:416. doi: 10.1007/s11908-014-0416-y. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y.C., Lu P.L., Lin K.H., Chu P.Y., Wang C.F., Lin J.H. Molecular epidemiology and phylogenetic analysis of human adenovirus caused an outbreak in Taiwan during 2011. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127377. DOI: 10. 1371/journal.pone.0127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang G., Yu D., Zhu Z., Zhao H., Wang P., Gray G.C. Outbreak of febrile respiratory illness associated with human adenovirus type 14p1 in Gansu Province, China. Influenza Other Respir Viruses. 2013;7:1048–1054. doi: 10.1111/irv.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Q.B., Tong Y.G., Wo Y., Wang H.Y., Liu E.M., Gray G.C. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009-2012. Influenza Other Respir Viruses. 2014;8:302–308. doi: 10.1111/irv.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai C.Y., Lee C.J., Lu C.Y., Lee P.I., Shao P.L., Wu E.T. Adenovirus serotype 3 and 7 infection with acute respiratoryfailure in children in Taiwan, 2010-2011. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J., Choi E.H., Lee H.J. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991-2007) J Med Virol. 2010;82:624–631. doi: 10.1002/jmv.21701. [DOI] [PubMed] [Google Scholar]
- 9.Yao L.H., Wang C., Wei T.L., Wang H., Ma F.L., Zheng L.S. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017-2018. Virol J. 2019;16(1):78. doi: 10.1186/s12985-019-1185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Liu F., Wang C., Zhao M., Deng L., Zhong J. Molecular Identification and Epidemiological Features of Human Adenoviruses Associated with Acute Respiratory Infections in Hospitalized Children in Southern China, 2012-2013. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao M.C., Li G.X., Zhang D., Zhou H.Y., Wang H., Yang S. Clinical evaluation of a new single-tube multiplex reverse transcription PCR assay for simultaneous detection of 11 respiratory viruses, Mycoplasma pneumoniae and Chlamydia in hospitalized children with acute respiratory infect-ions. Diagn Microbiol Infect Dis. 2017;88:115–119. doi: 10.1016/j.diagmicrobio.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X., Erdman D.D. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch Virol. 2006;151:1587–1602. doi: 10.1007/s00705-005-0722-7. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Zhou W., Zhao Y., Wang Y., Xie Z., Lou Y. Molecular typing and epidemiology profiles of human adenovirus infection among paediatric patients with severe acute respiratory infection in China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Y.L., Zhu Y., Xu B.P., Li C.C., Chen A.H., Deng L. Multicenter study of human adenovirus infection in pediatric community-acquired pneumonia in China. Zhonghua Er Ke Za Zhi. 2019;57:27–32. doi: 10.3760/cma.j.issn.0578-1310.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Lin M.R., Yang S.L., Gong Y.N., Kuo C.C., Chiu C.H., Chen C.J. Clinical and molecular features of adenovirus type 2, 3, and 7 infections in children in an outbreak in Taiwan, 2011. Clin Microbiol Infect. 2017;23:110–116. doi: 10.1016/j.cmi.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wo Y., Lu Q.B., Huang D.D., Li X.K., Guo C.T., Wang H.Y. Epidemical features of HAdV-3 and HAdV-7 in pediatric pneumonia in Chongqing, China. Arch Virol. 2015;160:633–638. doi: 10.1007/s00705-014-2308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C., Xiao Y., Zhang J., Ren L., Li J., Xie Z. Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007 to 2012. BMC Infect Dis. 2015;15:408. doi: 10.1186/s12879-015-1126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z., Zhang Y., Xu S., Yu P., Tian X., Wang L. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol. 2009;47:697–703. doi: 10.1128/JCM.01769-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Kong M., Su X., Zou M., Guo L., Dong X. An outbreak of acute respiratory disease in China caused by human adenovirus type B55 in a physical training facility. Int J Infect Dis. 2014;28:117–122. doi: 10.1016/j.ijid.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Y., Tang Z., Ye Z., Mo S., Tian X., Ni K. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect Dis. 2019;19:36. doi: 10.1186/s12879-018-3651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.