Highlights
-
•
We analyzed for saffold virus in myocardial specimens from humans with myocarditis.
-
•
One of 150 examined specimens was detected positive for saffold virus type 2.
-
•
Saffold virus was detected in three anatomical compartments.
-
•
Histological evidence of inflammation was found in two related organs.
-
•
Saffold virus is a possible cause of human myocarditis.
Keywords: Human myocarditis, Virology, Saffold virus, PCR, Forensic medicine
Abstract
Background
Saffold virus was described in 2007 as one of the first human viruses within the genus cardioviruses. Cardioviruses may cause severe infections of the myocardium in animals, and several studies have associated saffold virus with human disease. As a result, saffold virus has been isolated from different anatomical compartments, including the myocardium, but, until now, it has not been possible to demonstrate the accompanying histopathological signs of inflammation.
Objectives
The aim of the study was to examine if saffold virus is capable of causing invasive infection in the human myocardium.
Study design
Using real-time PCR, we retrospectively examined formalin-fixed paraffin embedded cardiac tissue specimens from 150 deceased individuals diagnosed with myocarditis at autopsy. The results were compared with histological findings.
Results and conclusions
Saffold virus was detected in the myocardium, lung tissue and blood of one child and was accompanied by histopathological inflammation in the heart and lungs, which was supportive of a viral infection. These findings suggest that cardioviruses may be associated with myocarditis in humans.
1. Background
Cardiovirus is a genus within the picornavirus family that has been known for several decades. It consists of two main species: Cardiovirus A-B. Cardiovirus A contains two serotypes of Encephalomyocarditis virus (EMCV), which are well known causes of myocarditis in animals. Human infections with EMCV were recently diagnosed using isolation and serology, but no human case of myocarditis due to EMCV has been identified [1]. The Cardiovirus B species includes Theiler’s murine encephalomyelitis virus, which is known to cause encephalitis and myocarditis [2], Thera virus, Vilyuisk human encephalomyelitis virus and the recent described human virus; Saffold virus (SAFV).
SAFV was discovered in 2007 when it was isolated from a stool sample from a child with fever of unknown origin [3]. The virus is distributed worldwide. SAFV has been isolated from respiratory specimens and stool samples from children with respiratory and gastrointestinal symptoms [4], [5] and, in a few studies, the virus has been detected in the cerebrospinal fluid and the myocardium [6], [7]. However, the detection of SAFV has not been associated with microscopic evidence of inflammation in the affected organs. No larger studies on the occurrence of SAFV in myocardial tissue specimens from humans with myocarditis have been reported.
2. Objectives
Using real-time PCR, we examined a cohort of 150 deceased individuals diagnosed with myocarditis to investigate if SAFV is capable of causing invasive infection in the human myocardium.
3. Study design
We retrospectively examined formalin-fixed paraffin embedded (FFPE) cardiac tissue specimens from 150 deceased individuals diagnosed with myocarditis at autopsy (106 men and 44 women; median age 34.7 years, age range 3 weeks to 77 years; 13 cases below the age of 5 years). The cases were selected from a database at The Institute of Forensic Medicine, Aarhus University, Denmark and represented all autopsy cases diagnosed with myocarditis in the period 1992–2010. Four myocardial tissue samples from each heart were examined: one from the anterior wall of the left ventricle; one from the posterior wall of the left ventricle, one from the interventricular septum and one from the right ventricle. Three consecutive sections fro each location were stained for Hematoxylin & Eosin, CD3 and CD68.
Total nucleic acids were extracted from 20 μm tissue sections using a previously described, customized protocol that utilizes the automated Maxwell 16 system (Promega) in combination with proteinase K digestion and incubation in a lysis buffer [8]. The presence of RNA after extraction was confirmed in 91% of the myocarditis cases by reverse-transcriptase PCR amplification of a 121 basepair fragment of the mRNA transcript from the human LONP1 gene as described previously [9]. All samples were tested for SAFV using a reverse-transcriptase real-time PCR assay and a genotyping was performed as previously described [7].
The laboratory workflow was performed according to usual guidelines where all procedures for the extraction and PCR were handled in different laboratories. Furthermore, all samples were processed separately from each other and from all other materials in order to reduce the risk of contamination. All assays included positive and negative control reactions.
4. Results
SAFV was detected at low concentrations upon repeated measurements (ct value = 39) in one of the 150 deceased individuals. This patient was a 2-year, 7-month-old previously healthy boy who died suddenly and unexpected after one day of slight fever. Using direct VP2 genotyping analysis, we determined the identified SAFV to be SAFV genotype 2. SAFV was also detectable in frozen blood (ct-value 30) and respiratory secretion (ct-value 22) while the cerebrospinal fluid was negative. As the present case was a forensic autopsy case routine examinations for other pathogens were performed prior to this study in order to establish the cause of death. Staphylococcus aureus, Haemophilus influenzae and non-hemolytic streptococci were isolated from lung tissue while enterovirus was detected in respiratory secretion. The results are shown in Table 1 .
Table 1.
The results of the routine examinations and PCR analysis for saffold virus—overview.
| Routine examinationsa | Examination of saffold virus | |
|---|---|---|
| Histological examination | ||
| Myocardium | Acute myocarditis | |
| Lung tissue | Acute interstitial pneumonia | |
| Otherb | Normal tissue | |
| Bacteriological examination | ||
| Lung tissue |
Staphylococcus aureus Non-hemolytic streptococci Haemophilus influenzae |
|
| Blood | Negative | |
| Cerebrospinal fluid | Negative | |
| Virological analysisc | ||
| Myocardium | Negative | Positive |
| Blood | Negative | Positive |
| Respiratory secretion | Enterovirus | Positive |
| Cerebrospinal fluid | Negative | Negative |
| Lung tissue (FFPE) | Negatived | Negative |
Examinations performed in relation to autopsy.
Liver, brain, kidney, prostate, bone marrow, pancreas, testicles, thyroid gland, thymus, spleen, epiglottis and peripheral muscle.
Routine virological analysis: adenovirus, coronavirus, enterovirus, parechovirus, influenzavirus, parainfluenza virus, parvovirus B19, human herpes simplex virus 1 + 2, varicella zoster virus and rhinovirus.
Only examined for enterovirus and saffold virus.
Histological examination revealed acute inflammation in the myocardium (Fig. 1, Fig. 2 ) and in the lung tissue (Fig. 3 ) while the other examined organs revealed no signs of inflammation or other diseases. Due to the inflammatory alterations and the detection of enterovirus in the respiratory secretion, analysis for SAFV and enteroviruses was also performed on FFPE lung tissue, which despite repeated trials was negative for both viruses.
Fig. 1.
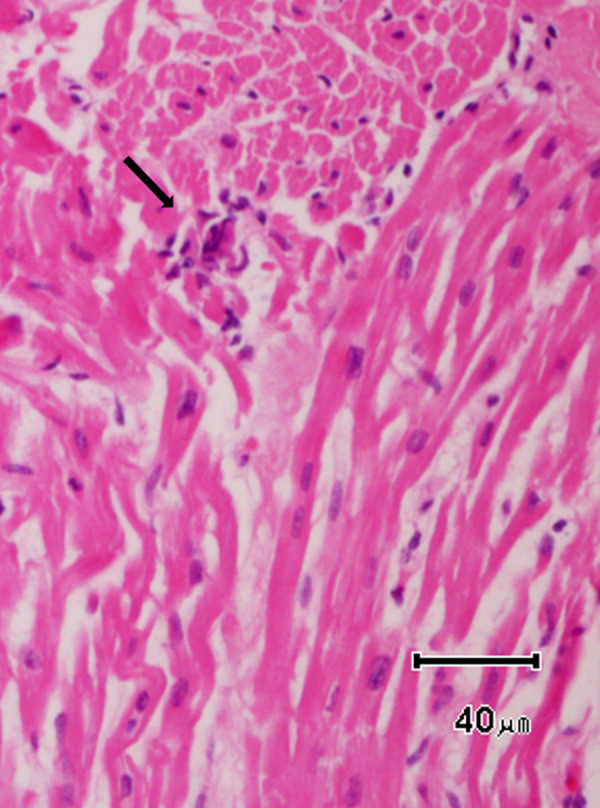
Acute lymphocytic myocarditis. Histological section of the myocardium illustrating a small lymphocytic focus with associated degeneration of the myocytes (arrow). Hematoxylin and Eosin × 200.
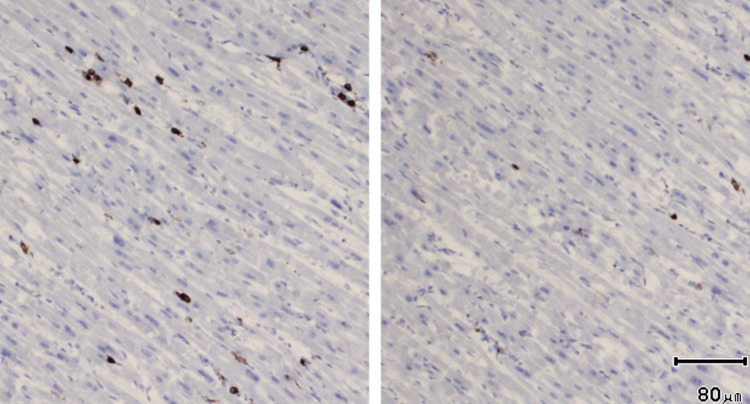
Fig. 2.
Acute lymphocytic myocarditis. Histological sections of the myocardium illustrating an area with an increased amount of CD45-positive lymphocytes (left picture) compared to normal myocardium (right picture). CD45 × 100.
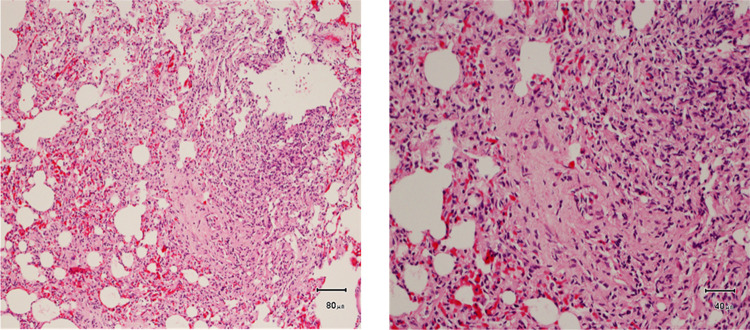
Fig. 3.
Interstitial pneumonia. Histological section of the lung illustrating lymphocytic infiltration into the interstitial tissue. Hematoxylin and Eosin × 100 (left) and ×200 (right).
5. Discussion
Since the discovery of SAFV many efforts have been made to clarify its possible pathogenicity in humans. In addition to the positive findings in respiratory specimens and stool samples, SAFV has been detected in the cerebrospinal fluid and the myocardium, indicating an invasive potential [6], [7]. In the first study, SAFV was found in the cerebrospinal fluid of two children ≤4 years of age, one of which died without preexisting symptoms. In this child, SAFV was also detected in a myocardial biopsy. In the second study, 10 myocardial biopsies were tested, and one was positive in a nested PCR assay, but not in real-time PCR. However, no histopathological alteration could be demonstrated in the myocardium of either of the two cases. In our study, we found SAFV in the respiratory secretion, blood and myocardial tissue of a child and histological evidence of inflammation in two related organs (heart and lungs). This combination is novel and may point to a role of SAFV in organ-related diseases.
The histological alterations in the myocardium and lung tissue primarily consisted of lymphocytes, which is suggestive of an infection of viral origin. The inflammatory changes in the myocardium were less pronounced than the changes in the lungs. Myocarditis is known to be a condition that causes both focal and diffuse inflammation, and even a small lesion may have a significant clinical impact [10]. The presence of viremia and the detection of SAFV in respiratory secretion and myocardium combined with the absence of other detectable pathogens in the myocardium, made SAFV plausible as a causative agent of the inflammatory changes. The low concentration of SAFV in the myocardium compared to blood and respiratory secretion may have been caused by the use of FFPE myocardial tissue as there is a known decreased sensitivity of virus detection in FFPE tissue. This may also be a likely explanation for the negative result of SAFV analysis on FFPE lung tissue. However, a higher concentration of viral RNA in the myocardium compared to lung tissue is also a possibility. The possibility of detecting RNA targets in postmortem tissue samples depends on several parameters including postmortem interval, fixation time, pH in fixation solution, and RNA extraction method [11]. In this study, we could detect a control RNA target in more than 90% of the study samples suggesting a reasonable RNA detection sensitivity and integrity in our FFPE material. Other studies have established that FFPE tissue material is usable for reverse-transcriptase PCR analysis of virus and messenger RNA, although the analytical sensitivity is reduced when compared to the use of matched frozen tissue material [12].
Three different types of bacteria were isolated from the lung tissue. Although S. aureus and H. influenzae may cause pneumonia, the histological picture is not in agreement with a bacterial etiology. Rather, the findings represent post-mortal growth of bacteria in the airways, and the presence of non-hemolytic streptococci supports this view.
A limitation of our study is that a verification of intracellular RNA e.g., by in situ hybridization (ISH) was not performed. A positive PCR result in the myocardium might represent the presence of blood in the tissue. However, the sensitivity of ISH is markedly lower than RT-PCR and a negative result of ISH in our case cannot rule out the presence of the virus. Future studies preferably using unfixed material should establish the presence of the virus inside the infected myocytes. Another limitation is the age of the study cohort varying from 3 weeks to 77 years, with the mean being 34.7 years. Thirteen cases were below the age of 5 years. In prior studies as well as in this study, SAFV has mainly been detected in different compartments of young children, indicating that infection with SAFV occurs early in life [10], [13], [14]. Determining the exact significance of SAFV for myocarditis would require a study including a large cohort of young children.
We found one SAFV-positive case of 150 deceased individuals. Despite a low prevalence that does not allow us to make a definite conclusion on the association of SAFV with myocarditis, we find the result remarkable because cardioviruses are well-known causes of myocarditis in animals [2]. In conjunction with prior studies it seems evident that SAFV plays a role in invasive human infections and our results suggest that SAFV may also be associated with myocarditis in humans.
Conflict of interests
The authors state that they have no conflict of interest.
Funding
This work was supported by the Beckett Foundation, Kong Christian den Tiendes Foundation, Kirsten Anthonius Foundation, Aarhus University and Aalborg University.
Ethical approval
The study was approved by the national ethics committee, Denmark, protocol no. 1209317.
Acknowledgements
The authors are grateful to Mette Skov Mikkelsen, Bente Wormstrup and Anette Funder for their excellent technical assistance.
Contributor Information
Trine Skov Nielsen, Email: tsn@retsmedicin.au.dk.
Alex Yde Nielsen, Email: alex.christian.yde.nielsen@regionh.dk.
Jytte Banner, Email: jytte.banner@sund.ku.
Jakob Hansen, Email: jah@forens.au.dk.
Ulrik Baandrup, Email: utb@rn.dk.
Lars Peter Nielsen, Email: larspn@gmail.com.
References
- 1.Oberste M.S., Gotuzzo E., Blair P., Nix W.A., Ksiazek T.G., Comer J.A. Human febrile illness caused by encephalomyocarditis virus infection, Peru. Emerg. Infect. Dis. 2009;15(April (4)):640–646. doi: 10.3201/eid1504.081428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez R.M., Rinehart J.E., Wollmann R., Roos R.P. Theiler’s murine encephalomyelitis virus-induced cardiac and skeletal muscle disease. J. Virol. 1996;70(December (12)):8926–8933. doi: 10.1128/jvi.70.12.8926-8933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones M.S., Lukashov V.V., Ganac R.D., Schnurr D.P. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J. Clin. Microbiol. 2007;45(July (7)):2144–2150. doi: 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abed Y., Boivin G. New saffold cardioviruses in 3 children, Canada. Emerg. Infect. Dis. 2008;14(May (5)):834–836. doi: 10.3201/eid1405.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blinkova O., Kapoor A., Victoria J., Jones M., Wolfe N., Naeem A. Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J. Virol. 2009;83(May (9)):4631–4641. doi: 10.1128/JVI.02085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drexler J.F., Baumgarte S., Eschbach-Bludau M., Simon A., Kemen C., Bode U. Human cardioviruses, meningitis, and sudden infant death syndrome in children. Emerg. Infect. Dis. 2011;17(December (12)):2313–2315. doi: 10.3201/eid1712.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen A.C., Bottiger B., Banner J., Hoffmann T., Nielsen L.P. Serious invasive Saffold virus infections in children, 2009. Emerg. Infect. Dis. 2012;18(January (1)):7–12. doi: 10.3201/eid1801.110725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loewe R. RNA-Isolation aus FFPE-Gewebe: Säulchen- und Bead-basierte Methoden. Laborwelt. 2011;12(05):38–39. [Google Scholar]
- 9.Nielsen T.S., Hansen J., Nielsen L.P., Baandrup U.T., Banner J. The presence of enterovirus, adenovirus, and parvovirus B19 in myocardial tissue samples from autopsies: an evaluation of their frequencies in deceased individuals with myocarditis and in non-inflamed control hearts. Forensic Sci. Med. Pathol. 2014;10(September (3)):344–350. doi: 10.1007/s12024-014-9570-7. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen T.S., Nyengaard J.R., Moller J., Banner J., Nielsen L.P., Baandrup U.T. Quantitative diagnosis of lymphocytic myocarditis in forensic medicine. Forensic Sci. Int. 2014;238(May):9–15. doi: 10.1016/j.forsciint.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Bass B.P., Engel K.B., Greytak S.R., Moore H.M. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch. Pathol. Lab. Med. 2014;138(November (11)):1520–1530. doi: 10.5858/arpa.2013-0691-RA. [DOI] [PubMed] [Google Scholar]
- 12.van der Linden A., Blokker B.M., Kap M., Weustink A.C., Robertus J.L., Riegman P.H. Post-mortem tissue biopsies obtained at minimally invasive autopsy: an RNA-quality analysis. PLoS One. 2014;9(12):e115675. doi: 10.1371/journal.pone.0115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu C.Y., Greninger A.L., Chen E.C., Haggerty T.D., Parsonnet J., Delwart E. Cultivation and serological characterization of a human Theiler’s-like cardiovirus associated with diarrheal disease. J. Virol. 2010;84(May (9)):4407–4414. doi: 10.1128/JVI.02536-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoll J., Erkens H.S., Lanke K., Verduyn L.F., Melchers W.J., Schoondermark van de Ven E. Saffold virus, a human Theiler’s-like cardiovirus, is ubiquitous and causes infection early in life. PLoS Pathog. 2009;5(May (5)):e1000416. doi: 10.1371/journal.ppat.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]