Highlights
-
•
The mobile suitcase laboratory is easy to use in low-resource settings.
-
•
The Diagnostics-in-a-Suitcase depends on using cold-chain-independent reagents.
-
•
The electricity was supplied from a motor vehicle battery or solar panel battery.
-
•
The H7N9 RT-RPA assays provided results within 10 min.
Keywords: Recombinase polymerase amplification assay, Diagnostics-in-a-Suitcase, Avian influenza A (H7N9)
Abstract
Background
In developing countries, equipment necessary for diagnosis is only available in few central laboratories, which are less accessible and of limited capacity to test large numbers of incoming samples. Moreover, the transport conditions of samples are inadequate, therefore leading to unreliable results.
Objectives
The development of a rapid, inexpensive, and simple test would allow mobile detection of viruses.
Study design
A suitcase laboratory “Diagnostics-in-a-Suitcase” (56 cm × 45.5 cm × 26.5 cm) containing all reagents and devices necessary for performing a reverse transcription recombinase polymerase amplification (RT-RPA) assay was developed. As an example, two RT-RPA assays were established for the detection of hemagglutinin (H) and neuraminidase (N) genes of the novel avian influenza (H7N9) virus.
Results
The sensitivities of the H7 and the N9 RT-RPA assays were 10 and 100 RNA molecules, respectively. The assays were performed at a single temperature (42 °C). The results were obtained within 2–7 min. The H7N9 RT-RPA assays did not show a cross-detection either of any other respiratory viruses affecting humans and/or birds or of the human or chicken genomes. All reagents were used, stored, and transported at ambient temperature, that is, cold chain independent. In addition, the Diagnostics-in-a-Suitcase was operated by a solar-powered battery.
Conclusions
The developed assay protocol and mobile setup performed well. Moreover, it can be easily implemented to perform diagnoses at airports, quarantine stations, or farms for rapid on-site viral nucleic acid detection.
1. Background
Reliable rapid diagnostic tools can generally help confirm infection in suspected cases during disease outbreaks, and therefore they can help improve hygiene management and prevent the further spread of the disease. The most extensively used tools are rapid antigen detection tests (RDTs) [1], [2]. RDTs are simple, fast (10–15 min), and suitable for point-of-care screening. Nevertheless, many reports stress the limited sensitivity and specificity of RDTs [2], [3], [4], [5], and RDT results are yet to be confirmed by real-time polymerase chain reaction (PCR).
Currently, real-time PCR is the standard of molecular diagnosis [6], [7], [8]. Samples are sent to regional or central laboratories for testing. Alternatively, a mobile real-time PCR system can be used at an outbreak site for the detection of pathogen nucleic acid. However, mobile real-time PCR devices are large, expensive, and complex. In addition, the test run time varies between 50 and 90 min [9].
For rapid wide-scale testing, robust easy-to-use molecular test systems that can be applied in the field provide a big advantage, as they allow rapid on-site nucleic acid detection at the point of need. Smart mobile assays should be able to discover silent or clinical relevant cases even in rural areas with low infrastructure.
Recombinase polymerase amplification (RPA) [10] represents an alternative technology to the real-time PCR. RPA is an isothermal DNA amplification and detection technology. This assay is rapid (2–15 min) and operated via a compact portable device (i.e., a tube scanner, 19 cm × 17.5 cm) [11], [12], [13], [14].
2. Objectives
In this study, reverse transcription RPA (RT-RPA) assays were developed for the detection of avian influenza A (H7N9) virus. Moreover, “Diagnostics-in-a-Suitcase” (DiaS) was established in order to facilitate its use at the site of an outbreak.
3. Study design
3.1. Viral nucleic acid and oligonucleotides
The Quality Control for Molecular Diagnostics, Glasgow, Scotland, UK; the Landesgesundheitsamt Niedersachsen, Hanover, Germany; and Robert Koch Institute, Berlin, Germany, provided the human viruses and viral nucleic acids (Table 1 ) used in this study. The avian respiratory viral nucleic acid panel (Table 1) was provided by the Friedrich-Loeffler-Institute, Greifswald-Insel Riems, Germany.
Table 1.
List of viral nucleic acids. H7N9 RT-RPA assays detected only H7 and N9 RNA but not other nucleic acids of viruses causing respiratory manifestations.
| Viral nucleic acid | H7 RT-RPA assay | N9 RT-RPA assay |
|---|---|---|
| Human respiratory viruses | ||
| Coronavirus 229E, NL63, and OC43 | − | − |
| SARS coronavirus | − | − |
| MERS coronavirus | − | − |
| Influenza A (H1N1 pdm09) | − | − |
| Influenza A (H3N2) | − | − |
| Influenza A (H5N1) | − | − |
| Influenza A (H1N1 H275Y) | − | − |
| Influenza B (Victoria) | − | − |
| Influenza B (Yamagata) | − | − |
| Parainfluenza virus 1–4 (patient isolate)* | − | − |
| Respiratory syncytial virus A and B | − | − |
| Human rhinovirus A 16 | − | − |
| Human rhinovirus A 90 | − | − |
| Human rhinovirus A 8 | − | − |
| Human rhinovirus B 5 | − | − |
| Human rhinovirus B 42 | − | − |
| Human rhinovirus C | − | − |
| Enterovirus 68 | − | − |
| Human metapneumovirus A1 and B2 | − | − |
| Adenovirus type 1, 4, and 34 | − | − |
| Avian respiratory viruses | ||
| A/Anhui/1/13 (H7N9) | + | + |
| A/Chicken/Germany/79 “Taucha” (H7N7) | + | − |
| A/Chicken/Brescia/19/02 (H7N7) | + | − |
| A/Chicken/Germany/R28/03 (H7N7) | + | − |
| A/Cygnus olor/Germany/R1377/07 (H5N1) | − | − |
| A/Chicken/Egypt/0833-NLQP/2008 (H5N1) | − | − |
| A/Chicken/Vietnam/P78/05 (H5N1) | − | − |
| Newcastle disease virus clone 30 | − | − |
| Infectious laryngotracheitis virus U76 | − | − |
| Infectious laryngotracheitis virus (A489) | − | − |
| Infectious bronchitis M41 | − | − |
Two RT-RPA assays were developed. As no program or strict rules for designing RPA primers and probe were available, three forward primers (FPs), three reverse primers (RPs), and one exo-probes (exo-Ps) were tested for each assay to select the combination producing the highest RT-RPA assay analytical sensitivity (Table 2 ). Oligonucleotides were synthesized by TIB MOLBIOL (Berlin, Germany).
Table 2.
Sequence of primers and exo-probes for H7N9 RT-RPA assays.
| Name | Sequence 5′–3′ | Amplicon size (nt) |
|---|---|---|
| H7-RPA-FP | CACAGCAAATACAGGGAAGAGGCAATGCAAAATAG | 121 |
| H7-RPA-RP | GAAGTATGAAACATGATGCCCCGAAGCT | |
| H7-RPA-P | CAAAGTATCACATCTT (BHQ1-dT)(THF)(FAM-dT)AGCCGCTGCTTAGTTTGACTGGGTCAATCTG-PH | |
| N9-RPA-FP | CAGAGGGAAACACTCAAACGGAACAATACAC | 129 |
| N9-RPA-RP | CTAGTACTTGACCACCCAATGCATTCCACCCTGC | |
| N9-RPA-P | CTGCTGTTGTACAC(BHQ1-dT)(THF)(FAM-dT)GGGCGGTGATGATAGTGGCCAGCTTATCAG-PH | |
H7-RPA-FP and H7-RPA-RP are forward and reverse RPA primers for the H7 RT-RPA assay; N9-RPA-FP and N9-RPA-RP are forward and reverse RPA primers for the N9 RT-RPA assay; H7-RPA-P and N9-RPA-P are exo-probes; BHQ1-dT, thymidine nucleotide carrying Black Hole Quencher-1; THF, tetrahydrofuran spacer; FAM-dT, thymidine nucleotide-carrying fluorophore; nt, nucleotides.
3.2. Diagnostics-in-a-Suitcase
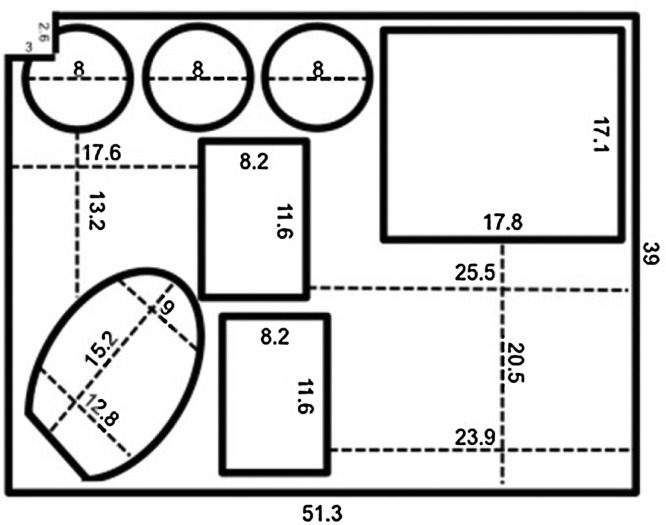
To simplify the use of H7N9 RT-RPA assays at the site of an outbreak, DiaS (Fig. 1 ) was created. DiaS contains all reagents and equipment necessary for performing H7N9 RT-RPA assays. A case of size 56 cm × 45.5 cm × 26.5 cm (ZARGES GmbH, Weilheim, Germany) is lined with foam at its base to act as a shock absorber during transport. A PVC layer is attached to the foam, into which indents are cut and sealed to render them watertight (Fig. 2 ). These indents house the equipment needed to perform the sample processing and amplification reactions: a tube scanner (Twista, TwistDx, Cambridge, UK), disinfectant wipes (Pursept®-A Xpress, Merz Consumer Hygiene GmbH, Luetjenburg, Germany), a waste container (Sarstedt, Nuembrecht, Germany), a vortex (lab-Dancer, IKA®, Staufen, Germany), a Sprout® Minicentrifuge (OMNILAB, Bremen, Germany), and two easily refillable Pipette Tip boxes (BRAND, Wertheim, Germany), in addition to a micro-tube rack, scissors, and a magnetic stand (Beckman Coulter, Krefeld, Germany). The lid of the case contains a box for gloves, an FFP3 mask, protection goggles, 1–10- and 10–100-μl automatic micropipettes (Eppendorf AG, Hamburg, Germany), marker and boxes for storage buffers, and RPA kits. A solar panel and a power pack (Yeti 150 set, GOALZERO, South Bluffdale, UT, USA) provide the energy needed.
Fig. 1.

Diagnostics-in-a-Suitcase. It contains all equipment and reagents for performing the RT-RPA assay.
Fig. 2.
Layout of the PVC layer of the Diagnostics-in-a-Suitcase. All measurements are in centimeters. The inside of the bold line represents the cutouts whereas the dotted lines represent the distance between two points.
3.3. Workflow in the DiaS
The workflow consisted of total viral nucleic acid extraction using magnetic beads (Dynabeads® SILANE viral NA, Life Technologies, Darmstadt, Germany) according to the manufacturer's instructions with few modifications. In summary, 200 μl of the sample was incubated with 50 μl of proteinase K and 300 μl of lysis buffer for 5 min at room temperature. Thereafter, 150 μl of isopropanol and 50 μl of Dynabeads were added and incubated for 10 min followed by two washing steps. Nucleic acid bound to the Dynabeads was left to dry for 5 min, followed by elution. The total time needed for the extraction was 30 min. For the RT-RPA assay, the ready-to-use TwistAmp™ RT exo (TwistDx, Cambridge, UK) was used as described [11]. Briefly, RPA primers (420 nM), the probe (120 nM), 45 μl of rehydration buffer containing 14 mM Mg acetate, and 5 μl of extracted RNA were added. The tube was closed and mixed well, and then placed in the tube scanner for 10 min at 42 °C. The total time for the RT-RPA test was 15 min. All pipetting steps were performed in the suitcase area. Surfaces outside of the suitcase were not used.
3.4. Analytical sensitivity and specificity of H7N9 RT-RPA assays
In vitro transcribed HA and NA RNAs of the H7N9 A/Anhui/1/2013 virus (accession numbers: EPI439507, EPI439509) were provided by the Friedrich-Loeffler-Institute, Greifswald-Insel Riems, Germany [15]. To determine the analytical sensitivity of RT-RPA assays, RNA molecular standard quantities ranging from 107 to 101 RNA molecules/μl were used. The RT-RPA assay was performed using the ready-to-use TwistAmp™ RT exo (TwistDx, Cambridge, UK) as described before [15]. Using PRISM (Graphpad Software Inc., San Diego, CA, USA), the threshold time was plotted against molecules detected and a semilog regression was calculated. To determine the limit of detection at 95% probability, a probit regression was performed using STATISTICA (StatSoft, Hamburg, Germany). The specificity of RT-RPA assays was determined by testing six H7N9-negative chicken samples and a reference human genome. In addition, the cross-reactivity was tested using the viral nucleic acids listed in Table 1. For comparison, H7N9 real-time RT-PCR was performed as previously described [16].
4. Results
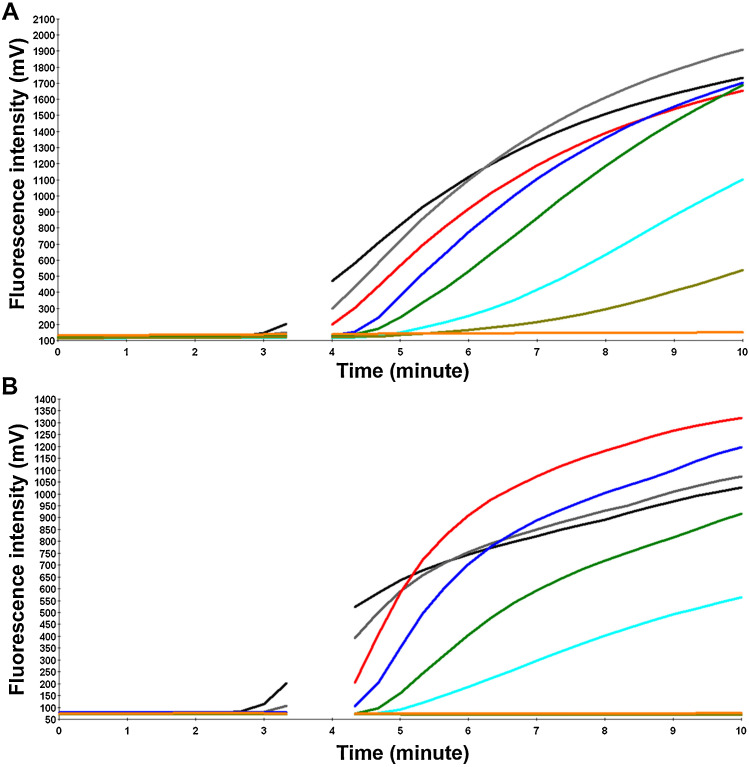
Two RT-RPA assays were designed for the detection of avian influenza A (H7N9) virus. Two molecular in vitro transcribed RNA standards (H7 and N9) were implemented to evaluate both RT-RPA assays. A dilution range of 107–101 molecules/μl of the RNA H7 and N9 molecular standards was used to select the RT-RPA primer and probe combination to produce the highest assay analytical sensitivity. RPA primers and exo-Ps listed in Table 2 yielded an analytical sensitivity between 10 and 100 RNA molecules for the H7 and N9 RT-RPA assays, respectively (Fig. 3 ).
Fig. 3.
Analytical sensitivity of H7N9 RT-RPA assays. (A) The H7 and (B) the N9 RT-RPA assays. Fluorescence development via real-time detection using a dilution range of 107–101 RNA molecules/μl of the H7 and N9 RNA molecular standards (graph generated by ESEquant tube scanner studio software). The sensitivities were 10 and 100 RNA molecules for the H7 (A) and the N9 (B) RT-RPA assays, respectively. The reverse transcription took place in the first minute. To increase the sensitivity, a mixing step was performed after 3 min (no fluorescence signal was detected). 107 represented by black line; 106, gray; 105, red; 104, blue; 103, green; 102, cyan; 101, dark khaki; and negative control, orange. (For interpretation of the references to color in this text, the reader is referred to the web version of the article.)
Using the data of eight RT-RPA runs of RNA molecular standard serial dilutions, a semilog regression (Fig. 4 ) and probit regression analysis (Fig. 5 ) were carried out. The run times for both assays were between 2 and 7 min (Fig. 4). The limits of detection in 95% of cases were calculated to be 14 (Fig. 5A) and 179 (Fig. 5B) RNA molecules detected for the H7 and the N9 RT-RPA assays, respectively. Both the H7 and N9 RT-RPA assays were specific. They detected neither viral nucleic acids of other respiratory viruses (Table 1) nor the human or the avian genome. Unfortunately, no clinical samples were available to test the clinical sensitivity of the assay.
Fig. 4.
Reproducibility of H7N9 RT-RPA assays. (A) H7 and (B) N9 RT-RPA assays. Semilogarithmic regression of the data collected from eight H7N9 RT-RPA test runs on the RNA molecular standard using Prism Software. Both assays yielded results between 2 and 7 min. In the H7 RT-RPA assay, 107–102 RNA molecules were detected in eight out of eight, and 10 in six of eight RT-RPA runs. In the N9 assay, 107–103 RNA molecules were detected in all RT-RPA runs, 102 in seven out of eight, and 10 in two out of eight.
Fig. 5.
Probit analysis of H7N9 RT-RPA assays. (A) H7 and (B) N9 RT-RPA assays. Probit regression analysis using STATISTICA software on the data set of the eight RT-RPA assay runs. The limit of detection at 95% probability (14 and 179 RNA molecules for the H7 and N9 RT-RPA assays, respectively) is represented by a triangle.
5. Discussion
The avian influenza A (H7N9) virus, which mainly infects birds, also occasionally infects humans [17]. As of 22 December 2013, 71 deaths of 166 laboratory-confirmed human cases were recorded by the World Health Organization (WHO) in restricted parts of China including Hong Kong. By 14 November 2014, the number of confirmed human cases increased to 458, 177 of which were fatalities. In addition, infected cases were reported in Taiwan and Malaysia [18], suggesting the spread of the virus to other countries as well. Thus, it is absolutely necessary to detect an H7N9 outbreak as early as possible to initiate appropriate control measures and prevent further spread among birds or transmission to humans.
Several real-time RT-PCRs were developed for sensitive detection of avian influenza (H7N9) virus [15], [16], [19]. They produced results in 50–90 min. To avoid contamination of reagents or the workplace with PCR amplicons, the preparation of the master mix, addition of the sample RNA to the mix, and the PCR reaction must be performed in separate biological safety cabinets or pipetting hoods. Moreover, real-time RT-PCR reagents must be stored in a freezer at −20 °C. Therefore, real-time RT-PCR is not easy to use at the point of need or in the field.
The detection of avian influenza (H7N9) virus was also performed by reverse transcription-loop-mediated isothermal amplification (RT-LAMP) [20], [21], [22], [23], [24]. RT-LAMP amplification was achieved at 60 °C in >30 min, and readout was done with the naked eye or SYBR Green-based detection. However, RT-LAMP needs six to 12 primers for the amplification of the H7 and N9 genes, which are not easy to design especially for highly variable RNA viruses. By contrast, the H7N9 RT-RPA assays developed in this study were very fast (2–7 min). They were operated at a single temperature (42 °C), and only four primers and two exo-Ps were needed for the amplification and detection of two genes (H7 and N9). Moreover, the analytical sensitivity and specificity of the RT-RPA assays were as good as that of the published real-time-RT-PCR [16].
To allow for the use of the RT-RPA assay at quarantine stations, ports, or the site of outbreak, the developed DiaS contains the complete equipment and reagents needed to perform on-site RPA-based diagnostic test. DiaS has the same size as a standard suitcase, which is easy to carry, transport, and ship. The DiaS currently costs around 5000 euro. The system can be used with a solar panel and/or power pack as a source of energy for easy use in low-resource settings. All RT-RPA reagents and oligonucleotides can be stored at ambient temperature for up to 3 months. RNA extraction is achieved by a magnetic-bead-based method, which reduces the risk of environmental contamination because no centrifugation step is needed. Six samples can be tested at the same time, in addition to negative and positive controls. Kits for up to 98 samples can be stored in the DiaS. The DiaS storage box can be refilled whenever needed. Results are displayed as positive or negative on the screen of the tube scanner without the need of a laptop. Overall, nine pipetting steps are needed. Further improvements to reduce the pipetting steps could be to include lyophilized RPA primers and probe into the RPA pellet, to employ a multiplex RT-RPA assay, and to apply simple non-purification lysis. In addition, generic influenza RT-RPA assays for detection of influenza type A and B as well as H5N1 are being developed, but they have not yet been included [25].
In conclusion, the developed DiaS represents a portable, rapid, and simple platform for the detection of avian influenza A (H7N9) virus at the point of need and in low-resource settings. DiaS depends on the RPA technique for identifying the RNA of the H7N9 virus, which can be optimized for the detection of the genome of other infectious agents as well.
Funding
The development of avian influenza A (H7N9) assays was funded by the Federal Ministry of Education and Research (BMBF), Germany (project name: RESCheck, grant no: FKN: 16SV5436). The funders had no role in design of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors have declared that no competing interests exist.
Ethical approval
This is non-applicable as no materials from humans or animals were used.
Acknowledgments
The authors thank Dr. Bernd Hoffmann, Friedrich-Loeffler-Institute, Greifswald-Insel Riems, Germany, for providing the H7 and N9 in vitro transcribed RNA, avian respiratory viruses, and H7N9 negative chicken samples; the Global Initiative on Sharing All Influenza Data (GISAID) for the avian influenza A (H7N9) sequence; and Quality Control for Molecular Diagnostics (QCMD), Glasgow, Scotland, UK, Landesgesundheitsamt Niedersachsen, Hanover, Germany, and Robert Koch Institute, Berlin, Germany, for human viruses and viral nucleic acid. We thank the University Medical Centre, Goettingen, Germany, for providing support and workspace until 30 September 2014. Mr. Manfred Lawendel helped us fix the PVC layer to the bottom of the case. We thank Doris Heidenreich for technical help, and Dr. Christiane Stahl-Hennig and Shereen Petersen for English proofreading.
References
- 1.Meseko C.A., Oladokun A.T., Ekong P.S., Fasina F.O., Shittu I.A., Sulaiman L.K. Rapid antigen detection in the diagnosis of highly pathogenic avian influenza (H5N1) virus in Nigeria. Diagn. Microbiol. Infect. Dis. 2010;68:163–165. doi: 10.1016/j.diagmicrobio.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Taylor J., McPhie K., Druce J., Birch C., Dwyer D.E. Evaluation of twenty rapid antigen tests for the detection of human influenza A H5N1, H3N2, H1N1, and B viruses. J. Med. Virol. 2009;81:1918–1922. doi: 10.1002/jmv.21604. [DOI] [PubMed] [Google Scholar]
- 3.Blacksell S.D. Commercial dengue rapid diagnostic tests for point-of-care application: recent evaluations and future needs? J. Biomed. Biotechnol. 2012;2012:151967. doi: 10.1155/2012/151967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blacksell S.D., Doust J.A., Newton P.N., Peacock S.J., Day N.P., Dondorp A.M. A systematic review and meta-analysis of the diagnostic accuracy of rapid immunochromatographic assays for the detection of dengue virus IgM antibodies during acute infection. Trans. R. Soc. Trop. Med. Hyg. 2006;100:775–784. doi: 10.1016/j.trstmh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Peeling R.W., Artsob H., Pelegrino J.L., Buchy P., Cardosa M.J., Devi S. Evaluation of diagnostic tests: dengue. Nat. Rev. Microbiol. 2010;8:S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 6.Chen W., He B., Li C., Zhang X., Wu W., Yin X. Real-time RT-PCR for H5N1 avian influenza A virus detection. J. Med. Microbiol. 2007;56:603–607. doi: 10.1099/jmm.0.47014-0. [DOI] [PubMed] [Google Scholar]
- 7.Sidoti F., Rizzo F., Costa C., Astegiano S., Curtoni A., Mandola M.L. Development of real time RT-PCR assays for detection of type A influenza virus and for subtyping of avian H5 and H7 hemagglutinin subtypes. Mol. Biotechnol. 2010;44:41–50. doi: 10.1007/s12033-009-9211-7. [DOI] [PubMed] [Google Scholar]
- 8.Spackman E., Senne D.A., Bulaga L.L., Myers T.J., Perdue M.L., Garber L.P. Development of real-time RT-PCR for the detection of avian influenza virus. Avian Dis. 2003;47:1079–1082. doi: 10.1637/0005-2086-47.s3.1079. [DOI] [PubMed] [Google Scholar]
- 9.Almassian D.R., Cockrell L.M., Nelson W.M. Portable nucleic acid thermocyclers. Chem. Soc. Rev. 2013;42:8769–8798. doi: 10.1039/c3cs60144g. [DOI] [PubMed] [Google Scholar]
- 10.Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS Biol. 2006;4:e204. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abd El Wahed A., El-Deeb A., El-Tholoth M., Abd El Kader H., Ahmed A., Hassan S. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS ONE. 2013;8:e71642. doi: 10.1371/journal.pone.0071642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abd El Wahed A., Patel P., Heidenreich D., Hufert F.T., Weidmann M. Reverse transcription recombinase polymerase amplification assay for the detection of Middle East respiratory syndrome coronavirus. PLoS Curr. 2013:5. doi: 10.1371/currents.outbreaks.62df1c7c75ffc96cd59034531e2e8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amer H.M., Abd El Wahed A., Shalaby M.A., Almajhdi F.N., Hufert F.T., Weidmann M. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. J. Virol. Methods. 2013;193:337–340. doi: 10.1016/j.jviromet.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euler M., Wang Y., Nentwich O., Piepenburg O., Hufert F.T., Weidmann M. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J. Clin. Virol. 2012;54:308–312. doi: 10.1016/j.jcv.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kalthoff D., Bogs J., Harder T., Grund C., Pohlmann A., Beer M. Nucleic acid-based detection of influenza A virus subtypes H7 and N9 with a special emphasis on the avian H7N9 virus. Euro Surveill. 2014;19(10) doi: 10.2807/1560-7917.es2014.19.10.20731. pii:20731. [DOI] [PubMed] [Google Scholar]
- 16.Corman V.M., Eickmann M., Landt O., Bleicker T., Brunink S., Eschbach-Bludau M. Specific detection by real-time reverse-transcription PCR assays of a novel avian influenza A(H7N9) strain associated with human spillover infections in China. Euro surveillance: bulletin Europeen sur les maladies transmissibles. Eur. Commun. Dis. Bull. 2013;18:20461. [PubMed] [Google Scholar]
- 17.Jernigan D.B., Cox N.J. H7N9: preparing for the unexpected in influenza. Ann. Rev. Med. 2015;66:361–371. doi: 10.1146/annurev-med-010714-112311. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Avian influenza A (H7N9) virus. http://wwwwhoint/influenza/human_animal_interface/influenza_h7n9/en/.
- 19.Fan J., Cui D., Lau S., Xie G., Guo X., Zheng S. Detection of a novel avian influenza A (H7N9) virus in humans by multiplex one-step real-time RT-PCR assay. BMC Infect. Dis. 2014;14:541. doi: 10.1186/1471-2334-14-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao H., Zhao Y., Wang Y., Xu X., Shi J., Zeng X. Development of a reverse transcription loop-mediated isothermal amplification method for the rapid detection of subtype H7N9 avian influenza virus. BioMed Res. Int. 2014;2014:525064. doi: 10.1155/2014/525064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Y., Wu B., Qi X., Zhao K., Guo X., Zhu Y. Rapid and sensitive detection of novel avian-origin influenza A (H7N9) virus by reverse transcription loop-mediated isothermal amplification combined with a lateral-flow device. PLoS ONE. 2013;8:e69941. doi: 10.1371/journal.pone.0069941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Nian Q.G., Li J., Hu Y., Li X.F., Zhang Y. Development of reverse-transcription loop-mediated isothermal amplification assay for rapid detection of novel avian influenza A (H7N9) virus. BMC Microbiol. 2014;14:271. doi: 10.1186/s12866-014-0271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakauchi M., Takayama I., Takahashi H., Tashiro M., Kageyama T. Development of a reverse transcription loop-mediated isothermal amplification assay for the rapid diagnosis of avian influenza A (H7N9) virus infection. J. Virol. Methods. 2014;204:101–104. doi: 10.1016/j.jviromet.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Feng Y., Hu D., Lv H., Zhu J., Cao M. Rapid and sensitive detection of H7N9 avian influenza virus by use of reverse transcription-loop-mediated isothermal amplification. J. Clin. Microbiol. 2013;51:3760–3764. doi: 10.1128/JCM.01907-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehnts K.L. Georg August University Goettingen; 2013. Development of a Panel of Recombinase Polymerase Amplification Assays for Detection of Respiratory Viruses (MD thesis) https://ediss.uni-goettingen.de/handle/11858/00-1735-0000-0001-BAD4-F?locale-attribute=en. [Google Scholar]