Highlights
-
•
Evaluation of customized TaqMan® array card, targeting 34 pathogens simultaneously.
-
•
TAC assay identified virus in significantly more samples.
-
•
Even after exclusion of viruses that could not be detected by conventional testing.
-
•
Immunocompromised patient population with overall positivity rate of 52.4%.
-
•
Viral and total pathogen co-infection rate of 5.6% and 11.8%, respectively.
Keywords: Respiratory virus infections, Multi-pathogen molecular assay, Conventional methods, Immunocompromised patients
Abstract
Background
Respiratory viral infections can cause significant morbidity and mortality in immunocompromised patients. Conventional tests routinely available at most institutions are limited by the number of detectable pathogens, by a poor sensitivity and/or a long turnaround time.
Objectives
To compare the performance of routine conventional testing with direct fluorescent antibody assays and viral culture to a customized TaqMan® array card (TAC) real-time PCR method, targeting 24 viruses, 8 bacteria and 2 fungi simultaneously.
Study design
We collected 143 respiratory samples from 120 symptomatic immunocompromised patients. Samples for which conventional and TAC results were discordant underwent further verification testing.
Results
The TAC assay identified viral pathogens in more samples than did conventional testing (77/143 versus 27/143; McNemar P < 0.0001), even when TAC results for viruses that could not be detected by conventional testing were excluded from analysis (59/143 versus 26/143; P < 0.0001). In addition, the TAC assay identified 18 samples with non-viral pathogens. Verification testing confirmed positive TAC results for 50 out of 55 samples for which conventional testing was negative. Two out of three samples with a positive conventional test but negative TAC result were confirmed positive. A viral and a total pathogen co-infection rate of 5.6% and 11.8% were found, respectively.
Conclusions
The customized TAC assay resulted in a significantly increased identification of respiratory viruses. This study provides a practical real-life assessment of the performance of the TAC assay in a population for whom rapid and accurate diagnosis of viral and atypical pathogens is crucial for appropriate clinical management.
1. Background
Acute respiratory infections account for an estimated 75% of all acute morbidities in developed countries, and most of them (approximately 80%) have a viral etiology [1]. Viruses usually associated with self-limited upper respiratory tract illness in immunocompetent hosts, can evoke lower respiratory tract infections leading to bronchiolitis, pneumonitis/pneumonia or acute respiratory distress syndrome (ARDS) associated to a significant morbidity and mortality in immunocompromised hosts [2], [3], [4], [5], [6].
Until recently, the primary diagnostic tools for respiratory viruses included direct fluorescent antibody (DFA) assays, enzyme immunoassays, and viral culture. While DFA assays and enzyme immunoassays have a rapid turnaround time, sensitivity is limited, especially in adults. For cultivable viruses, viral culture is clearly more sensitive but it requires both several days of incubation, experienced lab technicians, and it is labor intensive. Molecular testing has greatly improved the laboratory's ability to diagnose viral respiratory tract infections [1]. Multiplex PCR coupled with fluidic microarrays using microbeads or DNA chips (oligonucleotides spotted onto a slide or chips) represents the latest diagnostic approach for the clinical laboratory. Many commercial as well as in-house developed assays exist nowadays which offer a syndromic based approach for the detection of respiratory pathogens. This study focusses on the TaqMan® Array Card (TAC) (Thermo Fisher Scientific, Life Technologies, Carlsbad, CA), a microfluidic technology based on singleplex, reverse transcription real-time PCR in a 384-well microfluidic card. These cards are already commercialized for multiple gene expression and micro- RNA expression analyses and have been extensively used in cancer research [7], [8], [9]. For infectious diseases diagnostics, only in-house developed custom-made TAC assays exist to date [10], [11], [12], [13], [14].
2. Objectives
The goals of the present study are firstly to characterize the performance of a customized respiratory TAC assay (Patent number PCT/GB2012/053076; premarket version Cambridge-Brugge) on bronchoalveolar lavage (BAL) and nose-throat swab (NTS) samples in immunocompromised patients in comparison to standard conventional testing for the detection of respiratory viruses. Secondly, we aim to gain insight in the role of respiratory viruses, atypical bacteria and opportunistic fungi as etiologic agents of upper and lower respiratory tract infections in immunocompromised patients.
3. Study design
3.1. Patients and samples
The study population included 120 adult immunocompromised patients with symptoms of an upper or lower respiratory tract infection that underwent testing for respiratory pathogens at the University Hospital Erasme between December 2014 and April 2015. Immunocompromised status was defined as presence of a disease and/or treatment known to impair the immune system, such as solid organ transplant (SOT) under immunosuppressive therapy, solid or hematological malignancy under chemotherapy, or other underlying disease needing long-term corticosteroids therapy or immunosuppressive therapy. Patients infected with HIV with a CD4 count <200/mm3 were also included. Electronic medical records were reviewed retrospectively for clinical details using a structured case report form. After conventional testing, the samples were aliquoted and stored at −80 °C until study testing.
3.2. Conventional testing
The following DFA respiratory virus tests were performed on all included BAL and NTS samples: influenza A (IA) and B (IB) viruses, adenovirus, respiratory syncytial virus (RSV) (Argene, Verniolle, France), parainfluenza viruses (PIV) 1–3 (Argene, Verniolle, France and Light Diagnostics, Millipore, USA) and human metapneumovirus (hMPV) (Light Diagnostics, Millipore, USA). For all samples, rapid viral culture (Shell vial LLC-MK2) for adenovirus, PIV1-3, RSV and influenza A/B was performed and additionally conventional viral culture on two supplementary cell lines (A549 and MRC-5) was realized for BAL samples.
3.3. Nucleic acid extraction
Nucleic acids were extracted from 800 μL input sample on the QiaSymphony (Qiagen, Valencia, CA) using the DSP viral pathogen midi kit in combination with the “Pathogen/Complex 800_V5_DSP” generic DNA-RNA extraction protocol and eluted in 130 μL. A dilution of Phocine Distemper Virus (PDV) was added during extraction as internal extraction and inhibition control.
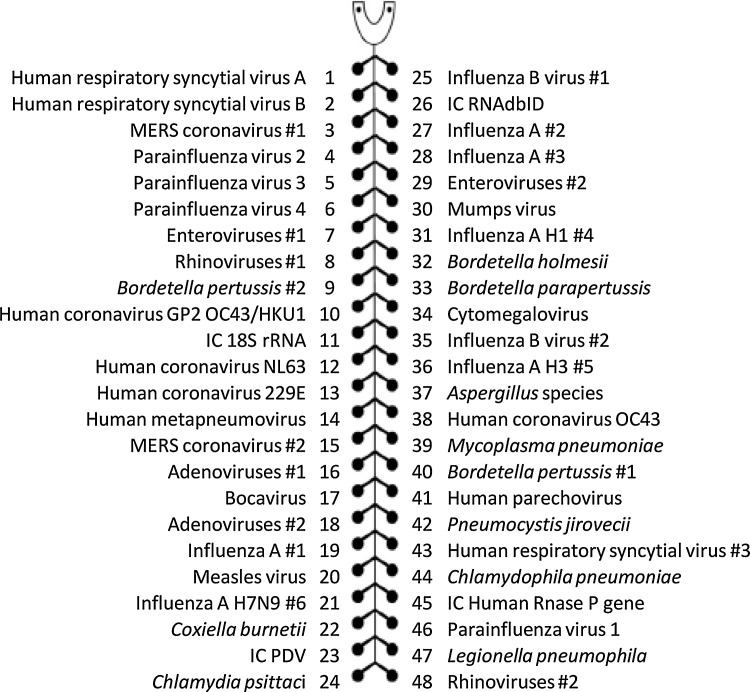
3.4. TAC testing
Patient samples were retrospectively tested for respiratory pathogens with a customized TAC respiratory panel which included testing for pathogens showed in Fig. 1 . For each patient sample, 78 μL of nucleic acid extract and 26 μL of TaqMan Fast Virus 1-step mastermix (Thermo Fisher Scientific, Life Technologies, Carlsbad, CA) were mixed and added to the TAC sample port. Reverse transcriptase real-time PCR was performed on the QuantStudio 7 (Thermo Fisher Scientific, Life Technologies, Carlsbad, CA) using following amplification protocol: 50 °C for 5 min, 95 °C for 20 s, and 40 cycles of 95 °C for 1 s followed by 60 °C for 20 s. The system reported a cycle threshold for each positive PCR assay. Whenever a clear exponential amplification curve was registered, even for one gene target, the result was considered positive for the specific pathogen. Based on the internal QC (PDV) data a%CV of 9.5% was registered, indicating a highly reproducible method.
Fig. 1.
TaqMan array card layout for one sample with composition of the respiratory pathogens included. These microfluidic cards contain 384 individual wells separated into eight loading ports with 48 wells each. For each sample, one internal positive control (IC) with Phocine Distemper Virus (PDV) and three human DNA/RNA controls are included. For several pathogens, more than one genetic target is included (indicated with #).
3.5. Verification PCR testing
Discordant conventional respiratory virus testing and TAC assay results were verified using real-time PCR assays. Verification testing was also performed for all non-viral pathogens detected by the TAC assay. The same nucleic acid extract was used for TAC and verification testing. Assay description, executive laboratory, target(s) and reference for each verification test are shown in Table S1 in the supplemental material.
3.6. Resolution of concordant and discordant results
Samples were considered to be positive for a specific respiratory pathogen if results from conventional testing were positive and matched the TAC results. Samples were considered to be negative if the conventional testing and TAC testing both yielded negative results. In cases where the conventional testing result and the TAC result were discordant, the result of the confirmatory testing was regarded as the final result.
3.7. Statistical analysis
Conventional testing and the TAC assay were compared using the exact two-sided McNemar's test. The calculated sensitivity, specificity, cPPV and cNPV and their corresponding exact 95% binomial confidence intervals were calculated separately for conventional testing and TAC testing. All statistical analyses were performed using the MedCalc software (Mariakerke, Belgium).
4. Results
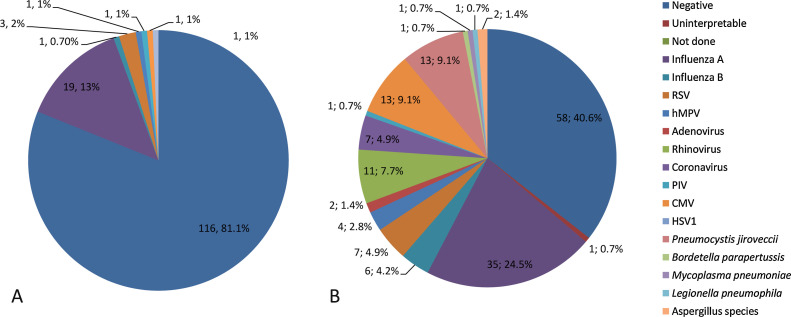
One hundred-forty three samples were obtained from 120 immunocompromised patients who underwent respiratory pathogen testing for symptoms of upper or lower respiratory tract infection. Patient and sample characteristics are shown in Table 1, Table 2 , respectively. Conventional testing identified 27 samples (18.9%) with one pathogen each among 143 samples. The TAC assay identified 77 samples with one or more viral respiratory pathogens (53.8%). Detailed results are shown in Fig. 2 and in Table 3 . Almost one fifth of all included samples were positive for IA virus. The positivity rate was higher for NTS samples (63.9%) than for BAL samples (45.7%), but the difference was not statistically significant. For NTS samples only, the viral distribution at genus level was as follows: IA (27.8%), CMV (8.3%), rhinovirus (6.5%), RSV and coronavirus (5.5%) the most frequently encountered, followed by IB and hMPV (3.7%). Adenovirus and parainfluenza viruses were detected in only a limited number of samples (≤3.0%). For BAL samples, less IA (14.3%), RSV and coronavirus (2.8%) were found but more CMV (11.4%), rhinovirus (8.6%) and IB (5.7%). Human MPV, adenovirus and PIV were not detected in BAL samples. In addition to the viral pathogens, the TAC assay identified 13 samples with P. jirovecii, 2 samples with Aspergillus species and 1 sample each with B. parapertussis, M. pneumoniae and L. pneumophila. When viral and non-viral pathogens were considered, more than one pathogen was detected in 17 samples (11.9%). Table 4 shows the combinations of these pathogens and Ct-values identified by TAC. Those most frequently involved in co-infections were CMV (n = 8), P. jirovecii (n = 7), rhinovirus (n = 5) and influenza A virus (n = 5).
Table 1.
Baseline characteristics of the 120 patients from whom respiratory samples were collected.
| Characteristic | Value |
|---|---|
| Age, median (range), Yrs. | 58.5 (22, 94) |
| Gender, male, No. (%) | 64 (53.3) |
| Underlying condition, No. (%) | |
|
59 (49.2) |
|
26 (21.7) |
|
21 (17.5) |
|
12 (10.0) |
|
2 (1.7) |
| Type of solid organ transplant, No. (%) | |
|
23 (19.2) |
|
19 (15.8) |
|
7 (5.8) |
|
7 (5.8) |
|
3 (2.5) |
| * Lung + kidney, lung + heart, kidney + liver | |
Table 2.
Type of sample and clinical indications of the 143 respiratory samples.
| No. (%) of samples (a) | |
|---|---|
| Type of sample | |
| Nose-throat swab | 108 (75) |
| Bronchoalveolar lavage | 35 (25) |
| Clinical indication for test | |
| upper respiratory tract infection | 29 (24) |
| lower respiratory tract infection | 93 (76) |
-
•20 patients with NTS and BAL for the same clinical indication.
-
•One patient with one NTS during first respiratory episode, 1 month later NTS and BAL during second respiratory episode.
-
•One patient with NTS and BAL for different respiratory episodes.
Fig. 2.
Results for conventional testing with DFA and viral culture (A) and TAC testing (B), including the numbers of pathogens and percentages of the total.
Table 3.
Results from conventional, TAC and verification testing for samples with discordant results.
| No. of samples (a) | routine testing result | TAC result | verification result |
|---|---|---|---|
| 2 | negative | Adenovirus | Adenovirus |
| 11 | negative | CMV | CMV |
| 1 | negative | CMV | negative |
| 7 | negative | Coronavirus (b) | Coronavirus (b) |
| 3 | negative | hMPV | hMPV |
| 15 | negative | Influenza A | Influenza A |
| 1 | HSV1 | negative (c) | HSV1 |
| 3 | negative | Influenza A (d) | negative |
| 1 | Influenza A (e) | negative | Influenza A (e) |
| 1 | Influenza A (f) | negative | negative |
| 4 | negative | Influenza B | Influenza B |
| 1 | negative | Influenza B (g) | negative |
| 9 | negative | Rhinovirus | Rhinovirus |
| 1 | negative | Rhinovirus (h) | negative |
| 4 | negative | RSV | RSV |
(a) n = 64 (58 samples in total, 6 samples with more than one discordant result).
(b) Coronavirus OC43 (n = 2), Coronavirus 229E (n = 4), Coronavirus NL63 (n = 1).
(c) HSV1 targets not included in TAC assay.
(d) Two samples with only 1/6 and one sample with 3/6 targets for influenza A weakly positive (Ct-value > 30).
(e) Only viral culture positive, verification PCR very weakly positive (Ct-value > 36).
(f) False positive DFA.
(g) Only 1/2 targets weakly positive (Ct-value > 30).
(h) 2/2 Targets for rhinovirus weakly positive (Ct-value > 30).
Table 4.
Combinations and Ct-value of viral and non-viral pathogens identified by TAC in 17 samples.
| No. | AV | CoV | IA | IB | hMPV | PIV3 | RV | RSV A | CMV | BP | MP | LP | PJ | AS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30* | 30 | 35 | |||||||||||
| 2 | 32 (a) | 33 | 33 | |||||||||||
| 3 | 23* | 32 | ||||||||||||
| 4 | 22* | 24* | 30 | |||||||||||
| 5 | 38* | 32 | ||||||||||||
| 6 | 31* | 34 | ||||||||||||
| 7 | 30* | 33* | ||||||||||||
| 8 | 35* | 37 | ||||||||||||
| 9 | 31* | 20 | ||||||||||||
| 10 | 23* | 20 | ||||||||||||
| 11 | 31 | 27 | ||||||||||||
| 12 | 24 | 20 | ||||||||||||
| 13 | 23 | 37 | ||||||||||||
| 14 | 25* | 32 | ||||||||||||
| 15 | 29 (b) | 35 | ||||||||||||
| 16 | 22* | 27 | ||||||||||||
| 17 | 21* | 30 |
AV, adenovirus; CoV, coronavirus; IA, influenza A virus; IB, influenza B virus, hMPV, human metapneumovirus; PIV3, parainfluenza virus 3; RV, rhinovirus, RSV A, respiratory syncytial virus A; CMV, cytomegalovirus; BP, Bordetella parapertussis; MP, Mycoplasma pneumoniae; LP, Legionella pneumophila; PJ, Pneumocystis jirovecii; AS, Aspergillus species.
(a) OC43.
(b) 229E.
*Median Ct-value for different targets.
The TAC assay was significantly more likely to detect a respiratory virus compared to routine conventional testing with DFA and viral culture (McNemar P < 0.0001). When TAC assay results for viruses that could not be detected by conventional testing (coronavirus, rhinovirus, CMV in NTS samples) (n = 18) and conventional testing results for HSV (n = 1) that could not be detected by TAC testing were excluded from analysis, the difference in diagnostic performance of the TAC assay in comparison to conventional testing was still significant (P < 0.0001).
Among 58 samples on which the two techniques disagreed for viral pathogens, 55 were negative by conventional testing while positive for one or more viral pathogens by the TAC assay. Fifty of these were confirmed by verification testing. Conventional testing identified a pathogen in 3 while TAC assay was negative, of which 2 confirmed by verification PCR (Table 3). Based on these results, viral disease was considered to be present in 75 samples (52.4%) and absent in 68 samples (47.6%). The calculated sensitivity, specificity, cPPV and cNPV of the TAC assay and conventional testing to detect a respiratory viral infection are displayed in Table 5 . The cPPV of conventional testing and TAC testing was not significantly different (P = 0.25), but the cNPV of the TAC assay (96.7%) was substantially greater than that of routine conventional testing (57.8%) (P < 0.0001).
Table 5.
Calculated performance characteristics for TAC and conventional testing.
| DFA + viral culture | TAC | P value | |
|---|---|---|---|
| % cSens (95% CI) | 34.7 (24.1, 46.5) | 97.3 (90.7, 99.6) | <0.0001 |
| % cSpec (95% CI) | 98.5 (92.1, 99.8) | 91.2 (81.8, 96.7) | 0.9703 |
| % cPPV (95% CI) | 96.3 (81.0, 99.4) | 92.4 (84.2, 97.1) | 0.2485 |
| % cNPV (95% CI) | 57.8 (48.2, 66.9) | 96.9 (89.1, 99.5) | <0.0001 |
cSens, calculated sensitivity ; cSpec, calculated specificity ; cPPV, calculated positive predictive value ; cNPV, calculated negative predictive value; CI, confidence interval; DFA, direct fluorescent antibody assay; TAC, Taqman array card.
Verification testing was also performed for non-viral pathogens detected by the TAC assay. Out of 13 samples positive for P. jirovecii, 11 were confirmed. Verification testing confirmed the positive result for Aspergillus species (n = 2), M. pneumoniae (n = 1) and B. parapertussis (n = 1) but not the one for L. pneumophila.
Twenty-one patients had a NTS and BAL sample collected during the same episode of respiratory tract infection symptoms. For 9 patients, both NTS and BAL were negative. The same viral pathogen was found both in the NTS as in the BAL sample for 10 patients when applying TAC testing, compared to only 1 patient by conventional testing. For the two remaining patients with coupled NTS and BAL samples, one was positive for influenza B virus only in the BAL sample, and one for coronavirus solely in the NTS.
5. Discussion
These data demonstrate that in immunocompromised patients, the TAC assay identified significantly more viral pathogens in BAL and NTS samples than routine conventional testing, even when TAC assay results for viruses that could not be detected by conventional testing were excluded from analysis. The cPPV of conventional testing and TAC testing was not significantly different, but the cNPV of the TAC assay was substantially greater than that of routine testing. For two out of six samples classified as being false positive by TAC, more than one gene target for the specific pathogen was positive, so it is likely that the verification result was falsely negative due to a lower analytical sensitivity. For the other four results classified as false positive, the median threshold was much higher than that for the same viruses detected by the TAC assay in other samples and confirmed by validation testing. This could suggest very small amounts of virus present in these samples, leading to false-negative verification testing, although a non-specific amplification cannot be excluded.
Approximately 1 h was needed for TAC testing with card setup and run times, excluding sample extraction. While the time to result was similar for DFA assays (approximately 3 h), the results for rapid viral culture were reported after 3 days of incubation, and conventional viral culture took up to 3 weeks.
Comparing the overall positivity rate (52.4%) and the epidemiology of respiratory viruses found in this study with data from the literature is difficult. Important differences could be partially explained by the patient population, specimen type and period of sample collection from December to April (during the flu epidemic in Belgium; almost one fifth of all included samples were positive for influenza A virus), but also by the wide array of viruses included in the TAC assay and by the fact that all samples were collected from symptomatic patients. The importance of the specimen type is highlighted in several studies. In BAL specimens a diagnostic yield ranging from 3.6% to 32.0% was reported [15], [16], [17]. Soccal et al. evaluated paired nasopharyngeal and BAL fluid specimens and observed an overall viral positivity rate of 29.3% in the upper respiratory tract specimens and 17.2% in the BAL samples (P < 0.001) [18]. This study included coupled NTS and BAL samples from 21 patients, and found a similar positivity rate for both sample types (52.4% and 57.1%, respectively). When all samples were considered, the positivity rate was higher for NTS samples (63.9%) than for BAL samples (45.7%), but the difference was not statistically significant.
Dual and even triple infections are routinely reported using multiplex PCR and have accounted for between 8 and 11% of positive specimens [19], [20], [21]. The present study found 5.6% of samples with more than one viral pathogen, and 11.9% of samples showed co-infections if all included pathogens were considered. The exact clinical interpretation of these multi-pathogen screening results is not always easy. When Ct-values are moderate or higher, especially for opportunistic pathogens like CMV, P. jirovecii and Aspergillus species, benign (and asymptomatic) colonization, or contamination should be suspected. Here, carriage of these opportunistic pathogens was likely in 10 out of 17 samples for which the TAC assay found more than one pathogen. Nevertheless, low viral loads do not exclude clinical disease due to the virus [22].
The TAC format has several advantages over other multi-pathogen molecular assays. The cards are completely custom-made which offers a great flexibility. The composition of the card can be chosen in relation to the patient population of interest. To note, the card used in this study did not include herpesviruses (HSV-1/2, VZV, HHV-6) besides CMV, which could be of possible interest for an immunocompromised patient population [23]. Another advantage is the spatial separation of the 48 reaction wells which allows assays to be easily changed without the need for extensive re-optimisation and validation of a highly parallel multiplex assay. This could aid in the rapid inclusion of novel or emerging pathogens [14]. In addition, the technology uses real-time PCR which generates a semi-quantitative Ct-value, in contrast to many commercial multi-pathogen assays which only offer qualitative results. This tool is decisive for an adequate and correct interpretation of results, particularly in case of co-infections. Moreover, the workflow is simple and easy and the cost per test is relatively low in comparison to most commercial multi-pathogen molecular assays.
However, the small reaction volume of 1 μL may affect sensitivity since the equivalent of the sample in the final reaction mixture per individual well is low. PCRs in the singleplex format are theoretically one log more sensitive than the same assays in the Array Card format [10], [24]. Also, samples cannot be treated individually, so a high turnover is needed to assure an acceptable turn-around-time.
The TAC card developed by Kodani et al. [10] and modified by Weinberg et al. [24] contained duplicate wells for the detection of one same genetic target for each pathogen (except for IA virus for which 3 targets were present in double). However, due to genome variability, single target testing might lead to false-negative results [25]. The card used in this study included more than one genetic target for several pathogens, which is not only interesting to limit false-negative results, but it can also help in the interpretation of the results.
There are some limitations to our study. In contrast to conventional testing, TAC and verification testing was performed retrospectively. Therefore, the quality of the samples could have deteriorated due to the freeze-thaw cycle before TAC and verification testing. This study was also limited by the lack of verification testing on all samples.
In summary, in comparison to routine conventional testing for respiratory viruses, the TAC assay detected more viral pathogens among samples obtained from an immunocompromised population. This study provides a practical real-life assessment of the performance of the custom TAC assay in a population for whom rapid and accurate diagnosis of viral pathogens is crucial for appropriate clinical management.
Funding
None to declare.
Competing interests
None to declare.
Ethical approval
This study was approved by the ethical committee of the Erasme hospital.
Acknowledgements
We thank the technicians of our virology and molecular microbiology laboratory for their technical assistance during this study. We thank Anne Vankeerberghen of OLV Hospital Aalst, Elke Wollants and Kurt Beuselinck of University Hospital Leuven and Oriane Soetens of University Hospital Brussel and their molecular microbiology teams for part of the verification PCR work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2015.08.022.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Mahony J.B. Detection of respiratory viruses by molecular methods. Clin. Microbiol. Rev. 2008;21:716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br. J. Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englund J.A., Boeckh M., Kuypers J., Nichols W.G., Hackman R.C., Morrow R.A., Fredricks D.N., Corey L. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann. Intern. Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 4.Gutman J.A., Peck A.J., Kuypers J., Boeckh M. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant. 2007;40:809–811. doi: 10.1038/sj.bmt.1705827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ison M.G., Michaels M.G. RNA respiratory viral infections in solid organ transplant recipients. Am. J. Transplant. 2009;9(Suppl. 4):S166–S172. doi: 10.1111/j.1600-6143.2009.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamboj M., Gerbin M., Huang C.K., Brennan C., Stiles J., Balashov S., Park S., Kiehn T.E., Perlin D.S., Pamer E.G., Sepkowitz K.A. Clinical characterization of human metapneumovirus infection among patients with cancer. J. Infect. 2008;57:464–471. doi: 10.1016/j.jinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Hui A.B., Shi W., Boutros P.C., Miller N., Pintilie M., Fyles T., McCready D., Wong D., Gerster K., Waldron L., Jurisica I., Penn L.Z., Liu F.F. Robust global micro-RNA profiling with formalinfixed paraffin-embedded breast cancer tissues. Lab. Invest. 2009;89:597–606. doi: 10.1038/labinvest.2009.12. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Espiridion B., Sánchez-Aguilera A., Montalbán C., Martin C., Martinez R., González-Carrero J., Poderos C., Bellas C., Fresno M.F., Morante C., Mestre M.J., Mendez M., Mazorra F., Conde E., Castaño A., Sánchez-Godoy P., Tomas J.F., Morente M.M., Piris M.A., García J.F. Spanish Hodgkin's lymphoma study group. A TaqMan low-density array to predict outcome in advanced Hodgkin's lymphoma using paraffin-embedded samples. Clin. Cancer Res. 2009;15:1367–1375. doi: 10.1158/1078-0432.CCR-08-1119. [DOI] [PubMed] [Google Scholar]
- 9.Steg A., Wang W., Blanquicett C., Grunda J.M., Eltoum I.A., Wang K., Buchsbaum D.J., Vickers S.M., Russo S., Diasio R.B., Frost A.R., LoBuglio A.F., Grizzle W.E., Johnson M.R. Multiple gene expression analyses in paraffin-embedded tissues by TaqMan low-density array application to hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J. Mol. Diagn. 2006;8:76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodani M., Yang G., Conklin L.M., Travis T.C., Whitney C.G., Anderson L.J., Schrag S.J., Taylor T.H., Jr, Beall B.W., Breiman R.F., Feikin D.R., Njenga M.K., Mayer L.W., Oberste M.S., Tondella M.L., Winchell J.M., Lindstrom S.L., Erdman D.D., Fields B.S. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J. Clin. Microbiol. 2011;49(6):2175. doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Gratz J., Amour C., Kibiki G., Becker S., Janaki L., Verweij J.J., Taniuchi M., Sobuz S.U., Haque R., Haverstick D.M., Houpt E.R. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 2013;51(2):472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz M.H., Waller J.L., Napoliello R.A., Islam M.S., Wolff B.J., Burken D.J., Holden R.L., Srinivasan V., Arvay M., McGee L., Oberste M.S., Whitney C.G., Schrag S.J., Winchell J.M., Saha S.K. Optimization of multiple pathogen detection using the TaqMan array card: application for a population-based study of neonatal infection. PLoS One. 2013;8(6):e66183. doi: 10.1371/journal.pone.0066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodani M., Mixson-Hayden T., Drobeniuc J., Kamili S. Rapid and sensitive approach to simultaneous detection of genomes of hepatitis A, B, C, D and E viruses. J. Clin. Virol. 2014;61(2):260–264. doi: 10.1016/j.jcv.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Rachwal P.A., Rose H.L., Cox V., Lukaszewski R.A., Murch A.L., Weller S.A. The potential of TaqMan array cards for detection of multiple biological agents by real-time PCR. PLoS One. 2012;7(4):e35971. doi: 10.1371/journal.pone.0035971. e35971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren L., Gonzalez R., Wang Z., Xiang Z., Wang Y., Zhou H., Li J., Xiao Y., Yang Q., Zhang J., Chen L., Wang W., Li Y., Li T., Meng X., Zhang Y., Vernet G., Paranhos-Baccalà G., Chen J., Jin Q., Wang J. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin. Microbiol. Infect. 2009;15(12):1146–1153. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minosse C., Selleri M., Zaniratti M.S., Cappiello G., Longo R., Schifano E., Spanò A., Petrosillo N., Lauria F.N., Puro V., Capobianchi M.R. Frequency of detection of respiratory viruses in the lower respiratory tract of hospitalized adults. J. Clin. Virol. 2008;42(2):215–220. doi: 10.1016/j.jcv.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu D.L., Bridevaux P.O., Aubert J.D., Soccal P.M., Kaiser L. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am. J. Transpl. 2011;11(5):1071–1078. doi: 10.1111/j.1600-6143.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soccal P.M., Aubert J.D., Bridevaux P.O., Garbino J., Thomas Y., Rochat T., Rochat T.S., Meylan P., Tapparel C., Kaiser L. Upper and lower respiratory tract viral infections and acute graft rejection in lung transplant recipients. Clin. Infect. Dis. 2010;51(2):163–170. doi: 10.1086/653529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung J.Y., Han T.H., Kim S.W., Hwang E.S. Respiratory picornavirus infections in Korean children with lower respiratory tract infections. Scand. J. Infect. Dis. 2007;39:250–254. doi: 10.1080/00365540600999126. [DOI] [PubMed] [Google Scholar]
- 20.J.B. Mahony, S. Chong, M. Smieja, A. Petrich, S. Buracond, J. Babwah, Establishing the epidemiology of respiratory virus infections using molecular technology, abstr. C-070. Abstr. 107th Annu. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington DC. 2007.
- 21.Pierangeli A., Gentile M., Di M., arco P., Pagnotti P., Scagnolari C., Trombetti S., Russo L.L., Tromba V., Moretti C., Midulla F., Antonelli G. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J. Med. Virol. 2007;79:163––168. doi: 10.1002/jmv.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnell D., Legoff J., Mariotte E., Seguin A., Canet E., Lemiale V., Darmon M., Schlemmer B., Simon F., Azoulay E. Molecular detection of respiratory viruses in immunocompromised ICU patients: incidence and meaning. Respir. Med. 2012;106:1184–1191. doi: 10.1016/j.rmed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanghavi S.K., Bullotta A., Husain S., Rinaldo C.R. Clinical evaluation of multiplex real-time PCR panels for rapid detection of respiratory viral infections. J. Med. Virol. 2012;84:162–169. doi: 10.1002/jmv.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg G.A., Schnabel K.C., Erdman D.D., Prill M.M., Iwane M.K., Shelley L.M., Whitaker B.L., Szilagyi P.G., Hall C.B. Field evaluation of TaqMan array card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J. Clin. Virol. 2013;57(3):254–260. doi: 10.1016/j.jcv.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steensels D., Vankeerberghen A., De Beenhouwer H. Towards multi-target testing in molecular microbiology. Int. J. Microbiol. 2013:12105. doi: 10.1155/2013/121057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.