Abstract
New treatments for multiple sclerosis (MS) focused on B cells have created an atmosphere of excitement in the MS community. B cells are now known to play a major role in disease, demonstrated by the highly impactful effect of a B cell-depleting antibody on controlling MS. The idea that a virus may play a role in the development of MS has a long history and is supported mostly by studies demonstrating a link between B cell-tropic Epstein–Barr virus (EBV) and disease onset. Efforts to develop antiviral strategies for treating MS are underway. Although gaps remain in our understanding of the etiology of MS, the role, if any, of viruses in propagating pathogenic immune responses deserves attention.
Keywords: multiple sclerosis, Epstein–Barr virus, ocrelizumab, vaccination, cell-based therapies, immune evasion
Multiple Sclerosis and EBV: Evolution of the Theory
MS is a chronic immune-mediated disease with a complex etiology involving a dysregulated immune system with bouts of peripherally mediated inflammation, as well as ongoing central nervous system (CNS)-compartmentalized inflammation leading to loss of neural tissue and worsening disability [1,2]. Intermittent waves of aberrant regulation and/or activation of immune cell subsets result in their trafficking and perivascular infiltration across the blood–brain barrier (BBB) into the CNS where immune cells become reactivated and impact on the underlying tissue resulting in disease relapses. The biological underpinnings of nonrelapsing progressive MS are not well understood but are thought, at least in part, to be driven by CNS-compartmentalized inflammation involving persistence of immune cells and their activation both around perivascular lesions and in the meninges. With respect to the etiology of MS, both genetic susceptibility and environmental exposures are thought to be involved [3, 4, 5]. The lifetime incidence of MS in the general population ranges from 2.0 to 9.6 per 100 000 patient-years. The concordance rate of MS in genetically identical twins, that ranges from ∼30% in northern populations to 15% or even 6% in countries such as Italy and France, highlights not only the contribution of genetic risk but also the importance of the environment acting on a genetically predisposed host. T cell and, more recently, B cell interactions have been shown to play a crucial role in driving new relapses [6, 7, 8, 9, 10]. Highly effective disease control observed using the B cell-depleting antibody ocrelizumab reinforces interest in developing additional B cell-mediated treatments. Building on the success of these studies could include the design and execution of an approach targeting the implicit role of a B cell-tropic virus in MS.
Specific environmental exposures are relevant to both triggering MS and modulating disease course. Virus infection is one crucial environmental factor. Of all viruses considered in MS pathogenesis, EBV, a highly B cell-tropic virus, is the best-studied (Table 1 ). Defective control of EBV is associated with infectious mononucleosis (IM) in addition to Hodgkin's lymphoma, Burkitt's lymphoma, gastric cancer, nasopharyngeal carcinoma, and conditions associated with HIV, such as hairy cell leukemia and other lymphomas [11,12]. There is also evidence that infection with EBV and associations with transcription factors, implicating EBV nuclear antigen 2 (EBNA2), operating across different disease loci are linked to a higher risk of autoimmune diseases other than MS, including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren's syndrome [13, 14, 15, 16].
Table 1.
Viruses Implicated in MS: Virus, Disease Involvement, and Association with MS
| Virus | Disease involvement | Association with MS |
|---|---|---|
| EBV, HHV-4, lymphocryptovirus Double-stranded (ds) DNA virus, neurotropic Tropism: B cells, epithelial cells Cell latency: memory B cells |
Infectious mononucleosis, Hodgkin and non-Hodgkin’s lymphoma, Burkett’s lymphoma, gastric and nasopharyngeal cancer, hairy cell leukemia, MS | Mononucleosis predisposes to MS, reduced activity of EBV-specific cytotoxic T cells (e.g., exhausted) in MS patients, EBV seropositive-epidemiological studies, virus present in MS brain, EBV prolongs the lifespan of B cells |
| HHV-6, roseolovirus dsDNA, neurotropic Tropism: broad; hematopoietic and epithelial cells Cell latency: lymphocytes and monocytes |
Exanthema subitum (roseola infantum) and pneumonitis, MS | Present in MS plaques, reactivation during relapses, high levels found in oligodendrocytes and areas of demyelination, elevated levels are found early in MS and during relapses/exacerbations, anti-HPV IgG and IgM titers are reported to predict relapses |
| CMV, betaherpesvirinae dsDNA, neurotropic Tropism: broad; hematopoietic cells, smooth muscle, monocytes, epithelial and endothelial cells, fibroblasts, connective tissue Cell latency: cells of the myeloid lineage |
Retinitis, hepatitis, colitis, pneumonia, encephalitis, MS | Both detrimental and beneficial properties reported, large meta-analysis MS versus controls did not yield a conclusive link between CMV and MS |
| Varicella zoster virus (VZV), HHV-3 dsDNA, neurotropic Tropism: mononuclear cells Cell latency: sensory ganglia |
Chickenpox, shingles, MS | Virus is present during relapses, recent studies failed to show an increased risk of MS associated with varicella or zoster infections |
| HERV-W Tropism: cells of the nervous system, syncytiotrophoblast layer of the placenta Cell latency: multiple |
MS, diabetes, autoimmune arthritis, and schizophrenia. In most cases the observed expression profiles of specific HERV-W sequences have not led to a definitive association with human disease pathology | Present in infiltrating macrophages and activated MS lesions, MSRV Env protein is detected in blood of active MS patients, drives the expression of proinflammatory cytokines, reduces myelin protein, expression and kills oligodendrocyte precursors |
EBV appears to be involved across the clinical spectrum of MS, including early pediatric-onset MS, established relapsing-remitting (RRMS), and progressive forms (PMS), as well as in patients with both mild and severe disease course (Box 1 ). Viral-induced animal models of neuroinflammation, demyelination, and neurodegeneration provide additional proof of principle that viruses play a role in autoimmune disease (Box 2 ). Over the past 20 years, convergent studies from multiple investigators point to a link between EBV infection and the development (i.e., etiology) of MS [17, 18, 19, 20, 21]. MS does not develop in the absence of exposure to EBV, and EBV is a 'required', but insufficient on its own, contributor in early disease pathophysiology. Independent support comes from an observed higher rate of EBV activation in MS [22,23], a role for EBV in triggering new relapses consistent with a reduced ability of EBV-specific CD8+ T cells (e.g., T cell exhaustion, see Glossary) to limit EBV reactivation in MS patients [24,25], and a strong correlation between the presence of anti-EBV antibodies in blood and disease onset [26, 27, 28, 29]. Evidence of CNS involvement comes from reports of elevated anti-EBNA1 IgG serology associated with the appearance of new gadolinium (Gd)-enhancing brain lesions [30] and the presence, in patients, of EBNA1-specific T cells recognizing myelin [31]. In addition to EBV-infected B cells in the periphery contributing to relapsing disease, EBV may play a crucial role in propagating CNS-compartmentalized inflammation and injury, potentially contributing to progressive (nonrelapsing) aspects of MS. B cells may traffic not only into the CNS but also out of the CNS into deep cervical lymph nodes [32,33]. These B cells include those infected with EBV, which could then activate aberrant T cell responses in the periphery. One can speculate that EBV from CNS pools may contribute not only to CNS-compartmentalized inflammation but also to further disease relapses. In support of this contention is evidence of antibodies in cerebrospinal fluid (CSF) of MS patients that recognize EBV antigens [34], and evidence that EBV-infected B cells and plasma cells accumulate in MS brain in meningeal immune-cell collections (Box 3 ). Although the presence of such EBV-infected B cells in MS brain has not been observed in all studies [35], the potential for such cells to participate in germinal center (GC)-like reactions in the meninges could support ongoing immune cell activation and the maintenance of a pool of pathogenic EBV-infected cells in the CNS.
Box 1. EBV Involvement across the MS Spectrum.
Evidence supporting an essential role for EBV in MS is derived from studies including pediatric and adult-onset patients with RRMS, patients with SPMS and PMS, as well as aggressive forms of MS. It is therefore worth reviewing the association of EBV across the broad spectrum of MS. In a cohort of 1047 clinically isolated syndrome (CIS) cases only one was seronegative for EBNA-1 [89]. This study represents the largest EBV-CIS population evaluated to date. In a different study, including CIS, RRMS, and PMS, all had evidence of EBV serology and confirmed diagnosis of MS. Further support comes from a recent study by Gieß et al. [90] reporting association of EBV infection with early-onset MS. EBV serology correlated with early diagnostic conversion from CIS to MS. However, neither EBNA-1 nor viral capsid antigen (VCA) IgG antibodies in serum, nor EBV DNA load in saliva, were associated with radiological or clinical disease activity. EBV infection is strongly associated with pediatric MS [91, 92, 93, 94]. Herpes simples virus (HSV)-1 seropositivity was associated with pediatric MS cases negative for HLA-DRB1*15:01, highlighting the complex nature of viral exposure and genetic factors. Multivariate analysis in the same study revealed a reduction in the risk of developing MS associated with CMV infection and no influence on MS status associated with HSV-1 infection [91]. Taken together, a role for EBV in early MS is supported by convergent pediatric MS studies. As in adult MS, these studies are consistent with a role for EBV as ‘required but insufficient’, likely playing one or more key contributing roles across the MS spectrum, intersecting with genetic susceptibility and additional environmental factors.
Box 2. Virus-Induced Animal Models of Inflammation, Demyelination, and Degeneration.
Animal models can be used to explore virus-specific mechanisms contributing to autoimmune and demyelinating diseases including MS [95, 96, 97]. EBV itself does not infect mice, which has contributed to the challenge of studying the role of EBV in models of CNS inflammation including experimental autoimmune encephalomyelitis (EAE). Nevertheless, the EBV-like virus, murine gammaherpesvirus-68 (gHV-68), exacerbates EAE [98, 99, 100] and leads to a type I IFN-dependent increase in heparan sulfate and responsiveness to proliferation-inducing ligands, and inhibition of viral reactivation [101]. The Theiler’s murine encephalomyelitis virus (TMEV) model [95] correlates infection with late-stage demyelination and entry of TMEV into the CNS [102,103]. In contrast to MS, B cell depletion in the TMEV model caused worsening of disease, hinting that prolonged B cell depletion might worsen viral infection and progression of disability [102]. The mouse hepatitis (corona) virus (MHV) model causes a chronic inflammatory demyelinating disease resembling MS [104]. In marmoset EAE, infection with endogenous viruses such as EBV or CMV alters immune responses and recruits intensely pathogenic T cells from the anti-effector memory cell population [97]. EBV-infected B cells mediate disease progression through MHC class Ib (Caja-E)-restricted cytotoxic T cells activated by gammaherpesvirus, causing demyelination of cortical grey matter [105]. Anti-CD20 antibody causes depletion of EBV-like CalHV3 from lymphoid organs, supporting a key role for CD20+ B cells in MS. The marmoset EAE model of MS suggests that EBV infection leads to increased citrullination of peptides in conjunction with autophagy during antigen presentation, allowing B cells to cross-present autoantigens to CD8+CD56+ T cells and leading to disease progression [97,106]. EBV also upregulated the antigen-presenting machinery of infected B cells and facilitated cross-presentation of immunogenic MOG peptides to CD8+ T cells [107]. In a variety of animal models, EBV-like viruses and EBV itself lead to the development of autoimmune, neurodegenerative, and MS-like disease pathologies.
Box 3. EBV in MS Brain.
Several studies report detection of EBV-infected B cells and plasma cells in the brain of MS patients [30,35,46, 47, 48,108, 109, 110, 111]. In earlier studies, meningeal B cells within specific structures, referred to as tertiary lymphoid follicles with a GC-like architecture, were described as major sites of EBV persistence in MS brain [46,47]. More recently, the presence of EBV in both MS and healthy brains has been reported [108, 109, 110]. Veroni [109] identified widespread EBV infection in meninges of MS patients, and EBV-related gene expression profiles (associated with latent EBV infection) in both meningeal and white matter tissue. Of further interest was the reported detection of gene expression in EBV-infected cells associated with IFN-γ signaling, type I immunity effector functions, B cell differentiation, proliferation, lipid-antigen presentation, and T cell and myeloid cell recruitment. In another study, brain EBV was detected by PCR or EBV encoding region (EBER) in situ hybridization (ISH) in 90% of all MS cases compared with only 24% of non-MS samples [108]. EBNA1 was detected by immunohistochemistry (IHC) in MS brain sections as was, to a lesser extent, the intermediate-early EBV transactivator gene, BZLF-1. Of note, this study also reported the detection of EBV in astrocytes and microglia. Viruses other than EBV (e.g., HSV-1, CMV, HHV-6) were not detected by PCR. A further study analyzed the expression of EBV latent proteins as well as proteins associated with lytic infection in archived brain samples [110]. EBV-encoded protein and mRNA were detected by IHC and in situ hybridization in both MS and control brains. The EBV early lytic protein, BZLF1, was observed in 46.1% of MS and 44.4% of non-MS samples. Latent virus was described to be more prevalent in MS brains, while lytic virus was only found in chronic MS plaques, consistent with a role for EBV in disease pathogenesis. In recent studies, EBV was identified across all stages of MS, distributed throughout the brain, in various cells types, and was shown to be present in white matter and within meningeal structures. Despite these studies, the presence of EBV in MS brain remains an active area of debate. As noted by Lassmann et al. [35], contrasting results from various groups may be due in part to differences in the methods employed across studies, including tissue preparation, antibodies used for detection, and the ability to preserve and detect meningeal structures containing ectopic B cell follicles [35,112,113].
Mechanistic Hypotheses
The prevalence of EBV worldwide reflects the overall tolerance and efficient immune control of this virus in the human population [36]. EBV commonly establishes a lifelong latent infection in human B cells with little or no adverse effects. EBV, also known as human herpesvirus 4 (HHV-4) and a member of a family comprising eight known human herpesviruses, is one of the most common DNA viruses found in humans and infects ∼95% of the world adult population.
Various intrinsic properties of EBV enable it to evade the immune system by establishing latent infection in B memory cells [37]. EBV achieves this by utilizing a series of distinct latency transcription programs that exploit normal B cell differentiation pathways, and drives these infected B cells to transition from activated B cell blasts to latently (latency I and II) infected resting memory B cells [38].
Several hypotheses have been proposed to explain how EBV infection may play a role in MS pathophysiology (Table 2 ). EBV may be involved in both peripheral immune responses that contribute to relapses, as well as within the CNS as part of CNS-compartmentalized inflammation, that is likely to be crucial in progressive aspects of the disease. In the periphery, EBV may contribute to aberrant activation and trafficking of CNS-reactive immune cells, resulting in disease relapses. The molecular mimicry theory [22,31] describes how T cells primed by exposure to EBV antigens crossreact to recognize and attack CNS antigens. Another theory suggests how αB-crystallin may be recognized mistakenly as a self-protein [39]. This hypothesis describes how EBV infection of peripheral B cells may induce expression of αB-crystallin in lymphoid cells, which then triggers a CD4+ T cell response to αB-crystallin that is also expressed in oligodendrocytes. Another theory describes the potential for EBV to preferentially drive proinflammatory B cell cytokine responses [e.g., tumor necrosis factor (TNF), lymphotoxin (LT), IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) expression] [40,41] and interfere with the downregulatory function of IL-10. Another theory based on the ability of EBV to induce expression of EBV-induced G protein-coupled receptor 2 (EBI2/GPR183) [42, 43, 44, 45] supports, in part, migration of autoreactive T cells and EBV-infected B cells into the CNS.
Table 2.
EBV Hypotheses: Mechanisms, Evidence For, and Evidence Against
| Hypothesis | Mechanism | Evidence | Evidence against | Refs |
|---|---|---|---|---|
| EBV-infected autoreactive B cells (Pender hypothesis) | EBV exposure is essential for MS onset in genetically susceptible individuals. EBV-infected autoreactive B cells accumulate in the CNS where they produce pathogenic antibodies and provide co-stimulatory survival signals to autoreactive T cells that would otherwise die in the CNS by apoptosis. Loss of EBV control due to a defective EBV-specific CD8+ T cell response. Sunlight/vitamin D may protect against MS by increasing EBV-specific cytotoxic T cells. | EBV-infected B cells and plasma cells are present in MS brain. Presence of EBV-infected autoreactive plasma cells in the synovium in rheumatoid arthritis and in the salivary glands in Sjögren’s syndrome. Defective T cell control of EBV infection in MS. Beneficial effect of B cell depletion in MS. Beneficial effect of EBV-specific T cell therapy in MS. Higher frequency of EBV seropositivity in MS patients compared with controls. No MS in the absence of EBV serology. Blood samples collected from US military personnel before the onset of MS showed that high titers of serum IgG antibodies to EBNA1 increase the risk of developing MS. History of IM predisposes to MS. | CMV, VZV, HHV6, and HERV are also implicated in MS. Evidence for the presence of EBV in MS brain has been challenged. No virus has been unequivocally associated with lesion formation in MS. |
[8,24,25,50,114,86,87] |
| EBV bystander damage | Inflammation in the CNS in MS primarily directed against EBV results in bystander damage. | Bystander T cells contribute to EAE pathogenesis. Virus infections can lead to significant activation of APCs such as dendritic cells which could activate autoreactive T cells, thus initiating autoimmune disease. | The mechanism of bystander killing of uninfected neighboring cells in MS brain remains unclear and requires further study. | [47] |
| αB-Crystallin 'mistaken self' |
Exposure to infectious agents induces the expression of αB-crystallin, a small heat-shock protein, in lymphoid cells. The immune system mistakes self αB-crystallin for a microbial antigen and generates a CD4+ T cell response, attacking αB-crystallin in oligodendrocytes, causing inflammatory demyelination. | αB-Crystallin is reported to be an immunodominant antigen in the CNS. αB-Crystallin is the dominant myelin-associated activator of human T cells and accumulates in oligodendrocytes. EBV induces the expression of αB-crystallin in B cells, which present the protein to CD4+ T cells in an HLA-DR-restricted manner. |
A connection between initial development and persistent CNS inflammation related to αB-crystallin reactivity is not clearly accounted for by this hypothesis. | [39] |
| Molecular mimicry, EBV crossreactivity |
T cells primed by exposure to EBV antigens crossreact with and attack CNS antigens. | 3–4% of EBNA1-specific CD4+ T cells in healthy subjects and MS patients react with peptides derived from myelin proteins. IgGs recognizing peptides from EBV and MBP 85–98 are elevated in MS patients. | Detailed mechanisms of molecular mimicry are limited by prolonged periods of disease latency, lack of statistical power in epidemiological studies, the potential role of genetics, and limited understanding of T cell repertoire and B cell responses. | [115, 116, 117] |
| HHV6A/EBV; potential astrocyte involvement | EBV infection of astrocytes activates HERV-W/MSRV/syncytin-1. HHV-6A actives latent EBV in B cells in MS lesions. HHV-6A is a neurotropic virus that infects astrocytes. HHV-6A and EBV may both be fundamental to the pathogenetic processes in MS. Infection with neurotropic HHV-6A leads to transformation of latent EBV-infected B cells in the CNS. |
Induces human endogenous retrovirus HERV-K18-encoded superantigen. MS subjects that fail to suppress HHV-6 during IFN-β treatment show a poor clinical response. | The prevalence of viral coinfection and their combined effects in MS are unknown. | [27,49] |
| EBI2; EBV-induced G protein-coupled receptor 2 (GPR183) | EBI2 is a mediator of CNS autoimmunity and contributes to the migration of lymphocytes. Not a B cell versus T cell hypothesis. |
Highly expressed in MS lesions. Promotes early CNS migration of encephalitogenic CD4 T cells and B cell migration within secondary lymphoid organs. EBI2 receptor regulates myelin development and inhibits lysophosphatidylcholine (LPC, lysolecithin)-induced demyelination. EBI2 mediates the oxysterol–EBI2 pathway that is involved in immune regulation, and differential expression of this receptor mediates B and T-dependent antibody responses. | Knowledge of the role of EBI2 in EBV infection is incomplete. Studies are limited to animal models. |
[42, 43, 44, 45] |
| EBV-induced B cell cytokine response | EBV infection of B cells induces the expression of proinflammatory cytokines. Success of B cell therapies may lie in restoring and maintaining a favorable balance between pro- and anti-inflammatory B cell activities in patients. | Proinflammatory cytokines play a key role in MS pathology. EBV infection may interfere with the downregulatory function of innate IL-10, potentially through the production of vIL-10. Cytokine-secreting B cells in the periphery may influence new disease activity and play a role disease activity in the CNS. | Functional heterogeneity in the B cell pool is poorly understood, as are the activities of B cells in the CNS. | [40,41,118] |
Within the CNS, EBV may contribute to propagating target organ inflammation and injury. This is supported by the idea that EBV could elicit ‘bystander damage’ [46, 47, 48] by inducing an antiviral immune response against infected cells in the CNS. Another hypothesis focuses on how HHV-6A and EBV may play a cooperative pathogenic role [27,49]. According to this theory, EBV infection of astrocytes may activate human endogenous retrovirus-W (HERV-W)/MS-associated retrovirus (MSRV)/syncytin-1. An additional hypothesis (autoreactive B cell) provides some ‘unifying’ principles [18,50], and describes how EBV infection is essential for developing MS and emerges, in part, through reduced control of EBV reinfection by exhaustion of EBV-specific cytotoxic CD8+ T cells. Deficient CD8+ killing of EBV (T cell exhaustion) results in the accumulation of EBV-infected autoreactive B cells in the MS brain that drive inflammation through interaction with autoreactive T cells.
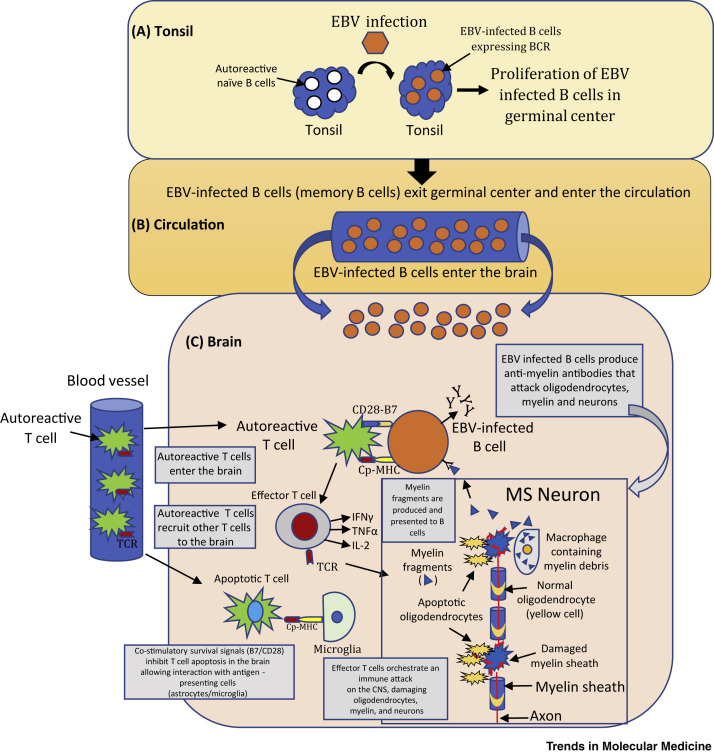
According to the autoreactive B cell hypothesis, defective elimination of EBV-infected B cells by cytotoxic CD8+ T cells results in the accumulation of EBV-infected autoreactive B cells in lymphoid structures and target organs implicated in MS (Figure 1 ) [13,25,50]. EBV infection and reactivation are normally controlled by functional EBV-specific cytotoxic CD8+ T cells. The autoreactive B cell hypothesis proposes that, in susceptible hosts, EBV infection confers abnormal survival and proliferation advantages to EBV-infected autoreactive B cells. Latently infected cells accumulate in lymphoid tissues in the MS brain, resulting in prolonged exposure to local antigens (such as myelin). Reports of EBV-infected autoreactive plasma cells in the synovium of rheumatoid arthritis patients and in salivary glands of patients with Sjögren’s syndrome provide general support for this hypothesis across other autoimmune diseases.
Figure 1.
The Autoreactive B Cell Hypothesis
(A) Tonsil to germinal center (GC): naïve B cells (white) are infected by Epstein–Barr Virus (EBV, orange) and proliferate in GCs. B cell receptor (BCR) and EBV proteins are expressed on latently infected autoreactive memory B cells. (B) Circulation: EBV-infected memory B cells exit the tonsil into the circulation (orange). EBV-specific cytotoxic CD8+ T cells normally control the number of EBV-infected B cells but, in the case of multiple sclerosis (MS), there is a defect in this mechanism. (C) EBV-infected B cells enter the MS brain residing for long periods of time where they produce oligoclonal IgG bands and pathogenic autoantibodies which attack and damage neurons. EBV-infected B cells provide co-stimulatory survival signals (B7) to CD28 receptors expressed on autoreactive T cells (green) that normally undergo apoptosis. Autoreactive T cells entering the brain are reactivated by EBV-infected B cells presenting CNS antigens (Cp) bound to MHC molecules. Autoreactive T cells produce cytokines [e.g., IL2, interferon (IFN)-γ, tumor necrosis factor (TNF)-β] and recruit other inflammatory cells (effector and cytotoxic T cells) that damage oligodendrocytes (yellow), myelin, and neurons. Adapted, with permission, from [18]. Abbreviations: B7, co-stimulatory molecule; CD28, T cell surface receptor; CNS, central nervous system; Cp–MHC, CNS peptides bound to MHC molecules; TCR, T cell receptor.
EBV can impact on MS-relevant immune responses of both memory B cells and memory T cells, whose interactions are now considered to play a key role in disease pathophysiology [51, 52, 53]. In theory, CNS-autoreactive T cells may be activated in lymphoid tissue, potentially through interaction with EBV-infected B cells, and migrate into the CNS where they receive both co-stimulatory and survival signals from EBV-infected B cells. Enhanced B cell-mediated antigen presentation to autoreactive T cells and inhibition of their apoptosis may contribute to persistence of local inflammation, including recruitment of other inflammatory cells, which together result in both antigen-directed injury as well as bystander injury to CNS tissue. Recent studies suggest that, in MS, memory B cells can drive autoproliferation of type 1 T helper (Th1) cell brain-homing CD4+ T cells that recognize autoantigens in both B cells and in MS lesions [51]. These cells reportedly migrate to the brain and induce inflammation through interaction with HLA-DR and the RAS guanyl-releasing protein 2 (RASGPR2) [54]. Jelcic and colleagues [51] report that natalizumab, known to be an effective treatment for MS [55], can block this migration. Interestingly, RASGRP1 impairs T cell expansion, leading to susceptibility to EBV infection, and this provides a potential link between anti-EBV immunity and expansion of activated T cells [54].
A common underlying theme that emerges from these theories is that EBV-infected memory B cells contribute to the recognition of self-antigens in MS brain, leading to propagation of aberrant immune responses both in the periphery and the CNS.
Immune Evasion by EBV Plays a Key Role in Viral Persistence and Prolongation of B Cell Lifespan
As a result of evolutionary, symbiotic relationships, viruses and host cells have developed the capacity to coexist [51,56]. This coevolution has led to the development of mechanisms that allow viruses to escape immune surveillance, resulting in prolonged virus survival, with the potential for reactivation and infection [18,57].
When GCs form, EBV-infected cells enter and reside undetected in GCs as memory B cells. EBV-induced proteins EBNA2, latent member protein 1 (LMP1), and LMP2A play a key role in this process [57]. Of interest is that persistence of EBV-infected memory B cells has a relatively minor effect on GC processes, resulting in low levels of self-reactivity and poly-reactivity. This provides EBV with a distinct advantage by allowing an increase in the number of memory B cells, thus providing an ever-present population of infectable cells.
Another important protein to consider is the viral IL-10 homolog (BCRF1/vIL-10) which is present in the serum and plasma of IM patients [12]. IL-10 is normally expressed not only by myeloid cells but also by T cells and B cells. IL-10 can limit autoimmunity by inhibiting T cell growth, T cell expression of interferon (IFN)-γ, and cytokine production by lipopolysaccharide (LPS)-stimulated monocytes [58,59]. IL-10 is also known to inhibit apoptotic cell death in IM. vIL-10 and native IL-10 have significant differences in activity. vIL-10 supports the persistence and immune evasion of EBV in human cells, impairs natural killer (NK) cell-mediated killing of infected B cells, interferes with CD4+ T cell activity, and modulates cytokine responses [60]. vIL-10 can also reduce antigen presentation and recognition of newly infected cells by EBV-specific CD8+ T cells, and appears to diminish the immunogenicity of EBV during the initial, pre-latent phase of infection, thereby strengthening the establishment of latent EBV infection. Immune evasion provides EBV with an unusually long lifespan in human cells. This is of major survival benefit to EBV but can be catastrophic to humans by leading to development of cancer, autoimmune disease, and other serious illnesses.
Therapeutic Implications of the EBV Theory for Disease Control
Effective control of EBV infection has been proposed as a means to prevent or cure autoimmune diseases. In MS, controlling EBV infection could be accomplished by B cell depletion, antiviral drugs, boosting immunity, or improving immune surveillance.
Antiviral Compounds
Several antiviral compounds have been evaluated as treatments for MS, including but not limited to famciclovir, stavudine, zidovudine, abacavir, and raltegravir. The use of antiviral compounds was encouraged, in part, by their effectiveness in treating AIDS/HIV, the association of HERVs with MS [61], and anti-EBV effects including inhibition of EBV DNA replication. Famciclovir and acyclovir are effective in treating herpes zoster, shingles, chickenpox, and genital herpes, but have not been evaluated as a treatment for MS or failed in a placebo-controlled study in RRMS [62]. Antiherpesviral nucleoside analogs (acyclovir, penciclovir, and ganciclovir) have also received attention. Unfortunately, studies in MS have been discouraging or failed to show any effect on disease activity [63,64].
The reason for a lack of efficacy may be due to the low impact of a given virus on disease or to challenges in study design, including the mechanism of action (MOA) of a specific agent, inactivation by a viral kinase, limited duration of studies, single agent versus a combination of antiviral drugs (e.g., effective in HIV), and small sample sizes. Continued reports highlighting evidence that HHVs and HERVs are involved in MS, and case reports of remission, warrant further study particularly because larger studies will be necessary because existing studies were not designed to show clinical efficacy.
The reader should be reminded that a potent antiviral protein, human IFN-β, remains one of the top five treatment options for MS [65, 66, 67]. The MOA of IFN-β in MS is not clear but is thought to involve anti-inflammatory mechanisms. Nevertheless, IFN-β is known to inhibit the infectivity of EBV, cytomegalovirus (CMV), and other viruses, influence proliferative T cell responses to EBNA1 [68], and decrease the memory B cell compartment that is considered to be a pathogenic cell subset and key trigger in MS [69]. It is tempting to suggest that the MOA of IFN-β and other disease-modifying therapies (DMTs) [70] includes overlapping antiviral and anti-inflammatory mechanisms that support future simultaneous testing of MS drugs with divergent MOAs.
Vaccination
History is full of examples of the eradication of human disease by vaccination [71] or other means of controlling viral propagation [72]. Presently, there is no available vaccine to protect against EBV infection. One possible approach to developing such a vaccine would be to target gp350 or other viral proteins [73]. A small trial evaluating a vaccine against EBV showed limited results in MS [74]. Recent advances in vaccination with a focus on genomic vaccines, that are able to deliver multiple protein-coding sequences, could help to advance vaccination studies in MS [75]. Lessons can be learned from monitoring clinical studies that are underway using genomic vaccines to test their safety and immunogenicity for Ebola virus, hepatitis C virus, and breast, brain, and other cancers.
The prospect of developing a prophylactic vaccine to block or prevent acute EBV infection as a strategy to prevent the development of MS is nevertheless appealing, although it is also fraught with major challenges. A challenge in designing an EBV vaccine is that providing sterile immunity against any herpesvirus is almost an improbable endpoint. Ideally a vaccine which can prevent acute IM may be sufficient to reduce the risk of developing MS [71,76,77]. Recent studies on other herpesvirus vaccines have provided promising results supporting the concept that designing a vaccine which can prevent disease rather than infection may be possible [78]. Challenges exist in development of an EBV vaccine, but efforts have begun and may yield surprising results [79].
Anti-EBV Antibodies and Targeting Viral Pathways
Antibodies recognizing EBV proteins expressed during latency, including EBNA1, LMP1, LMP2a, would increase immunity against EBV. To date no studies focused on the control of EBV infection using antibodies directed against EBV-specific proteins have been reported.
Other viral targets could be obtained from a series of genome-wide association studies (GWAS) which have identified ∼250 variants that contribute to MS disease susceptibility [3, 4, 5]. In addition, the growing wealth of human genetic data grouped as viral interactomes or transcriptomes of B cells and EBV-infected B cells may be useful when selecting new targets, especially because EBV genetic variants are associated with MS [80,81]. Targeting B cell pathways (e.g., BAFF, BHRF1) or employing siRNAs targeting EBV genes (e.g., LMP1, LMP2a and EBNA1) to downregulate expression and induce apoptosis in EBV-infected cells might also be effective in reducing EBV reactivation.
Cell-Based Immunotherapies
Cell-based immunotherapies, especially those targeting EBV-infected transformed cells, have shown efficacy [82, 83, 84]. A similar benefit may result from treating autoimmune disease(s) such as MS with cell-based immunotherapies aimed at reducing EBV reactivation, especially immunotherapies targeting cell types known to be implicated in disease pathology. It is encouraging to note that such studies are now underway. Cell-based therapies include depletion of the immune system via immunoablation, followed by mesenchymal and related stem cell transplantation, autologous hematopoietic stem cell transplantation, transplantation of oligodendrocyte progenitor cells, and introduction of endogenous stem cells followed by enhancement of their reparative capabilities [85]. Although these are potentially breakthrough treatment approaches, there are methodological and ethical challenges in designing such studies, and our understanding of potential benefits and safety concerns is limited, thereby relegating these approaches to aggressive or hard-to-treat MS for which treatments are desperately needed but few are available.
The recent application of an autologous or allogeneic T cell therapy targeting EBV-infected B cells using an EBV-specific cytotoxic CD8+ T cell therapy [18,86] provides an alternative cell-based approach. An example has been demonstrated in a patient with secondary progressive MS (SPMS), where infusion of EBV-specific CD8+ cytotoxic T cells had no adverse effects and the individual showed clinical improvement with reduced disease activity on magnetic resonance imaging (MRI) and decreased intrathecal immunoglobulin production, highlighting the likelihood of treatment effects occurring in the CNS [87]. This was accompanied by an increase in the percent of circulating LMP and B-lymphoblastic cell line (LCL)-reactive effector CD8+ memory cell populations. Success observed in this study led to a Phase I clinical trial of autologous EBV-specific T cell therapy in progressive MS [86]. The authors noted no serious adverse effects, including no disease exacerbation. They also reported that seven of the 10 treated patients showed clinical improvement. A Phase I study to evaluate the safety of an allogeneic EBV-specific cytotoxic T cell therapy in subjects with RRMS and progressive MS is underway (NCT03283826 i). The advantages of a cell-based approach targeting EBV-infected cells include limited off-target effects and the potential to target infected B cells in the periphery and CNS, perhaps providing benefit in both relapsing and nonrelapsing (progressive) MS.
Another registered study is underway to assess the safety and feasibility of adoptive cell therapy with autologous EBV-specific cytotoxic T lymphocytes (CTLs) in patients with a first clinical episode highly suggestive of MS (NCT02912897 ii). The purpose of this study is to develop an immune intervention capable of restoring the host–EBV balance. The study will assess the feasibility and safety of autologous transfer of several concentrations of CD8+ T cells directed against autologous EBV-transformed B cell lines to restore efficient control of EBV reactivation in MS patients.
Cell-based therapies represent an innovative and exciting tactic for controlling MS. The outcome of ongoing studies is much anticipated and may open the door to a vastly different treatment approach. It should be kept in mind that any cell-based treatment for MS will need to have an excellent safety profile, duration of effect, and a distinct advantage over currently available treatments of which there are many.
Concluding Remarks
Most approved disease-modulating therapies in MS are now understood to have a direct and/or indirect impact on both memory B cells and memory T cells, whose interactions are thought to play key roles in disease pathophysiology [2,10,52]. Most recently, the importance of B cell involvement in MS is strongly supported by clinical studies (NCT00676715 iii; NCT01412333 iv; NCT01194570 v; NCT02545868 vi) using a B cell-depleting antibody, ocrelizumab, which substantially reduces annualized relapse rate and dramatically limits the appearance of new Gd-enhanced or new T2 lesions, as well as delaying progression of disability [6,88]. Ocrelizumab represents a breakthrough treatment for MS, and its impact on limiting disease activity and CNS injury while selectively targeting B cells would be consistent with pathophysiologic role(s) for EBV-infected B cells in MS. It is unclear whether ocrelizumab modulates the regulation, activation, or trafficking of memory B cells, or broadly depletes them, but it is interesting to speculate to what extent the observed benefits reflect an impact of these therapies on EBV-mediated disease mechanisms. Cell-based therapies selectively targeting subsets of B cells, such as those infected with EBV, could provide an innovative approach for achieving this, with potential implications for treating both relapsing and progressive forms of MS, and perhaps even preventing the development of MS.
None of the environmental factors or genetic susceptibility variants identified are sufficient, in isolation, to cause disease. Instead, disease onset appears to involve a combination of factors constituting 'a perfect storm', resulting in dysregulated autoimmunity and inflammation in the CNS. Harmful inflammation occurring both in the periphery and in the CNS are central to MS pathophysiology and contribute to relapsing disease and nonrelapsing progressive disease. With respect to understanding B cell involvement in MS, efforts until the 1980s focused on the nature of the aberrant intrathecal antibody response, with analysis of oligoclonal bands and the net synthesis of immunoglobulin within the CNS. In the 1980s, largely driven by the increasing use of experimental autoimmune encephalomyelitis (EAE) as an animal model of MS, there was a more T cell-centric view of MS pathophysiology. Currently MS disease pathology is thought to invoke key interactions between T and B cells, particularly between their memory subsets, all supported by convergence of clinical trial results and laboratory-based studies [6, 7, 8, 9, 10,18,25,52,86]. The high tropism that EBV has for B cells, and its mechanisms of immune avoidance, serve to promote long-term survival and persistence (of both the virus and memory B cells), which fundamentally alter B cell biology, resulting in persistence and accumulation of disease-relevant EBV-infected autoreactive B cells.
EBV may contribute both to episodic peripherally mediated inflammation that underlies disease relapses and associated perivascular CNS injury, as well as to persistent CNS-compartmentalized inflammation such as in the meninges, thus contributing to more diffuse, nonrelapsing progressive injury. Ultimately, proof will come from interventional studies that selectively eliminate and/or modulate EBV-infected memory B cells. It is far from clear whether antiviral therapies targeting EBV or other viruses will provide benefit in MS (Clinician’s Corner). Nevertheless, the involvement of viruses in autoimmune disease remains understudied despite a long history of reports implicating viruses in processes leading to inflammation. Continued research is warranted not only on the role of EBV in MS but also on viruses in general and their impact on autoimmune disease (see Outstanding Questions).
Outstanding Questions.
How should clinical studies be designed to best capture a reliable measurement of progression of disability in studies on the potential benefits of B cell-directed therapies and those aimed at controlling EBV immunity?
More research should be focused on understanding the role, if any, of meningeal immune-cell aggregates in the MS brain, including the possible role of EBV-infected B cells in forming these aggregates and their relationship to disease pathology.
What is the mechanistic contribution of EBV to the development of MS, and what is its impact on established disease? For instance, ‘no EBV seropositivity, no MS’ is well established, therefore previous infection with EBV is necessary but insufficient on its own for the development of MS. In this regard, what is the best way to design clinical studies to prove that targeting EBV or viral exposure in general would be an effective MS treatment or cure?
Clinician’s Corner.
Several effective treatments for MS are available. A recently introduced therapy aimed at depleting B cells (ocrelizumab) provides excellent control of disease and represents an important advance in the treatment of MS.
B cell depletion efficiently suppresses acute inflammatory disease activity in RRMS and, unlike many other established treatments, may also slow down the progression of disability in progressive MS.
Additional approaches are being evaluated that focus on depleting a pathogenic subset of B cells, either by blockade of B cell proliferation or viral reactivation pathways (e.g., B cell-activating factor antagonists, siRNAs) or by targeting B cell-tropic viruses using cell-based methods.
Slowing and ultimately halting progressive injury associated with MS remains a major challenge and concern for both patients and the treating neurologist. Available treatments improve quality of life measures in part by reducing the frequency of clinical exacerbations and limiting the development of focal inflammatory lesions, but have relatively little effect on delaying disability.
Disclaimer Statement
A.B-R. has served on the scientific advisory boards of Receptos-Celgene, Sanofi/Genzyme, Roche/Genentech, Novartis, GSK, Atara Therapeutics, Guthy-Jackson Greater Good Foundation, and Immune Tolerance Network; received travel funding and/or speaker honoraria from Receptos-Celgene, Roche/Genentech, Novartis, Sanofi-Genzyme, and GSK; consulted for Receptos-Celgene, Roche/Genentech Novartis, Sanofi-Genzyme, GSK, and Atara Therapeutics; and received research support from Novartis, Genzyme-Sanofi, and Biogen. M.P. received consulting fees and research funding from Atara Biotherapeutics and is a member of the Neurology Clinical Advisory Panel of Atara Biotherapeutics. K.R. is a consultant and member of the scientific advisory board of Atara Biotherapeutics and has received a license fee payment and research funding from Atara Biotherapeutics. L.S. served on the scientific advisory board of Novartis, Receptos, Atreca, Tolerion, Teva, and AbbVie; received travel funding and/or speaker honoraria from Celgene and AbbVie; has a patent pending on cytokines and type 2 interferons; has multiple patents on antigen specific tolerance; received research support from Atara Biotherapeutics, Celgene, and Biogen; holds stock options and board membership in Tolerion; and is a member of the board of directors of BioAtla. H.P.H. has received fees for consulting, speaking, and serving on steering committees from Bayer Healthcare, Biogen, Geneuro, MedImmune, Merck, Novartis, Opexa, Receptos Celgene, Roche, Sanofi, Genzyme, and Teva. T.M. was previously employed by Atara Biotherapeutics. E.C. is a consultant for Atara Biotherapeutics. B.T.A. is an employee of, and owns equity shares in, Atara Biotherapeutics. G.G. has received fees for advisory board participation and speaker fees for Merck, and speaker fees from Atara Biotherapeutics. M.J.J. is an employee of, and owns equity shares in, Atara Biotherapeutics.
Acknowledgments
We acknowledge Dr Brian Wilburn for help in assembling and publishing the manuscript.
Glossary
- Allogeneic T cells
alloreactive T cells stimulated by donor antigen-presenting cells (APCs) which express both allogeneic MHC and co-stimulatory activity.
- Autologous T cells
autoreactive T cells from the same individual stimulated by self-APCs expressing specific antigens.
- Autoreactive cells
T cells acting against host cells or tissues; may function to enhance B cell responses.
- BHRF1
the EBV homolog of Bcl-2; protects human B cells from programmed cell death.
- αB-Crystallin
a member of the heat-shock protein family; functions as a molecular chaperone that binds to misfolded proteins to prevent protein aggregation; inhibits apoptosis and contributes to intracellular architecture. Elevated expression is found in many neurological diseases.
- Ectopic B cell follicles
primary or secondary lymphoid follicles in lymphoid organs containing B cells involved in antibody responses.
- Experimental autoimmune encephalomyelitis (EAE)
a T cell-mediated animal model resulting in brain inflammation and demyelination in the CNS; resembles acute disseminated encephalomyelitis (ADEM) but is also used as an animal model of MS; however, EAE is unable to reproduce some pathological patterns observed in human MS.
- Genomic vaccines
includes multiple DNA or RNA sequences that eliminate the need to vaccinate with protein by recruiting host cells to express a selected protein that enhances a given immune response to that protein.
- Germinal centers (GCs)
sites within secondary lymphoid organs where B cells proliferate, differentiate, and, through somatic hypermutation, can switch antibody class and increase affinity.
- Gp350
a highly conserved EBV envelope protein that enables EBV attachment to susceptible host cells.
- Immunoablation
the destruction of immune resistance for a medical purpose.
- Latent infection
a hidden, inactive, or dormant infection that allows a virus to lie dormant or silent, thus escaping immune detection, a type of persistent viral infection.
- Lytic infection
a reproductive cycle that leads to destruction of the infected cell and membrane; released viruses are replicated in newly infected cells using host DNA replication machinery.
- Meningeal structures
cranial structures consisting of three protective layers, the dura, arachnoid, and pia mater, that are continuous between the brain and spinal cord. Follicle-like aggregates in meninges contain mostly B cells infected with EBV; inflammation in the meninges is associated with grey matter (cortical) demyelination.
- Memory cells
long-lived lymphocytes that can respond to a specific reintroduced antigen long after initial, prior exposure.
- Oligoclonal IgG bands
immunoglobulins seen on electrophoresis of cerebrospinal fluid that provide evidence of immunoglobulin synthesis within the CNS compartment. A characteristic of MS.
- Prophylactic vaccine
a method for stimulating the immune response of an individual to prevent or attenuate the effects of future infection by any natural or 'wild-type' pathogen.
- T cell exhaustion
a condition where T cells display poor effector function, thus preventing efficient control of infection and tumor growth.
Resources
- i
- ii
- iii
- iv
- v
- vi
References
- 1.Hauser S.L., et al. Multiple sclerosis: prospects and promise. Ann. Neurol. 2013;74:317–327. doi: 10.1002/ana.24009. [DOI] [PubMed] [Google Scholar]
- 2.Weiner H.L. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch. Neurol. 2004;61:1613–1635. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 3.Baranzini S.E., Oksenberg J.R. The genetics of multiple sclerosis: from 0 to 200 in 50 years. Trends Genet. 2017;33:960–970. doi: 10.1016/j.tig.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikram M.A., et al. Genetic susceptibility to multiple sclerosis: brain structure and cognitive function in the general population. Mult. Scler. 2017;23:1697–1706. doi: 10.1177/1352458516682104. [DOI] [PubMed] [Google Scholar]
- 5.Jokubaitis V.G., Butzkueven H. A genetic basis for multiple sclerosis severity: red herring or real? Mol. Cell. Probes. 2016;30:357–365. doi: 10.1016/j.mcp.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Mulero P., et al. Ocrelizumab: a new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 2018;11 doi: 10.1177/1756286418773025. 1756286418773025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan Y., et al. The role of Epstein–Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen. Res. 2019;14:373–386. doi: 10.4103/1673-5374.245462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser S.L., et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 9.Bittner S., et al. Targeting B cells in relapsing-remitting multiple sclerosis: from pathophysiology to optimal clinical management. Ther. Adv. Neurol. Disord. 2017;10:51–66. doi: 10.1177/1756285616666741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose T. Role of immunological memory cells as a therapeutic target in multiple sclerosis. Brain Sci. 2017;7:E148. doi: 10.3390/brainsci7110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheik-Ali S. Infectious mononucleosis and multiple sclerosis – updated review on associated risk. Mult. Scler. Relat. Disord. 2017;14:56–59. doi: 10.1016/j.msard.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Endriz J., et al. Time correlation between mononucleosis and initial symptoms of MS. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e308. doi: 10.1212/NXI.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pender M.P. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003;24:584–588. doi: 10.1016/j.it.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Pender M.P. CD8+ T-cell deficiency, Epstein–Barr virus infection, vitamin D deficiency and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis. 2012;2012:189096. doi: 10.1155/2012/189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tracy S.I., et al. Persistence of Epstein–Barr virus in self-reactive memory B cells. J. Virol. 2012;86:12330–12340. doi: 10.1128/JVI.01699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harley J.B., et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat. Genet. 2018;50:699–707. doi: 10.1038/s41588-018-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Menéndez S., et al. Epstein–Barr virus and multiple sclerosis. From evidence to therapeutic strategies. J. Neurol. Sci. 2016;361:213–219. doi: 10.1016/j.jns.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Pender M.P., Burrows S.R. Epstein–Barr virus and multiple sclerosis: potential opportunities for immunotherapy. Clin. Transl. Immunol. 2014;3:e27. doi: 10.1038/cti.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pender M.P. The essential role of Epstein–Barr virus in the pathogenesis of multiple sclerosis. Neuroscientist. 2011;17:351–367. doi: 10.1177/1073858410381531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascherio A., Munger K.L. Epstein–Barr virus infection and multiple sclerosis: a review. J. Neuroimmune Pharmacol. 2010;5:271–277. doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 21.Ascherio A., Munger K.L. Environmental risk factors for multiple sclerosis. Part I. The role of infection. Ann. Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 22.Angelini D.F., et al. Increased CD8+ T cell response to Epstein–Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013;9:e1003220. doi: 10.1371/journal.ppat.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mechelli R., et al. Epstein–Barr virus nuclear antigen-1 B-cell epitopes in multiple sclerosis twins. Mult. Scler. 2011;17:1290–1294. doi: 10.1177/1352458511410515. [DOI] [PubMed] [Google Scholar]
- 24.Cencioni M.T., et al. Programmed death 1 is highly expressed on CD8+ CD57+ T cells in patients with stable multiple sclerosis and inhibits their cytotoxic response to Epstein–Barr virus. Immunology. 2017;152:660–676. doi: 10.1111/imm.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pender M.P., et al. Defective T-cell control of Epstein–Barr virus infection in multiple sclerosis. Clin. Transl. Immunol. 2017;6:e126. doi: 10.1038/cti.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeba E., et al. Evaluation of Epstein–Barr virus-specific antibodies in Cypriot multiple sclerosis patients. Mol. Immunol. 2019;105:270–275. doi: 10.1016/j.molimm.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Mameli G., et al. Expression and activation by Epstein–Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS One. 2012;7:e44991. doi: 10.1371/journal.pone.0044991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lünemann J.D., et al. Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 2010;67:159–169. doi: 10.1002/ana.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ascherio A., et al. Epstein–Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA. 2001;286:3083–3088. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 30.Tzartos J.S., et al. Association of innate immune activation with latent Epstein–Barr virus in active MS lesions. Neurology. 2011;78:15–23. doi: 10.1212/WNL.0b013e31823ed057. [DOI] [PubMed] [Google Scholar]
- 31.Lünemann J.D., et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J. Exp. Med. 2008;205:1763–1773. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern J.N., et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci. Transl. Med. 2014;6:248ra107. doi: 10.1126/scitranslmed.3008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Büdingen H.C., et al. B cell exchange across the blood–brain barrier in multiple sclerosis. J. Clin. Invest. 2012;122:4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nociti V., et al. Epstein–Barr virus antibodies in serum and cerebrospinal fluid from multiple sclerosis, chronic inflammatory demyelinating polyradiculoneuropathy and amyotrophic lateral sclerosis. J. Neuroimmunol. 2010;225:149–152. doi: 10.1016/j.jneuroim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Lassmann H., et al. Epstein–Barr virus in the multiple sclerosis brain: a controversial issue report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain. 2011;134:2772–2786. doi: 10.1093/brain/awr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Gorman C., et al. Environmental risk factors for multiple sclerosis: a review with a focus on molecular mechanisms. Int. J. Mol. Sci. 2012;13:11718–11752. doi: 10.3390/ijms130911718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ressing M.E., et al. Immune evasion by Epstein–Barr virus. Curr. Top. Microbiol. Immunol. 2015;391:355–381. doi: 10.1007/978-3-319-22834-1_12. [DOI] [PubMed] [Google Scholar]
- 38.Thorley-Lawson D.A., Gross A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 39.van Noort J.M., et al. Mistaken self, a novel model that links microbial infections with myelin-directed autoimmunity in multiple sclerosis. J. Neuroimmunol. 2000;105:46–57. doi: 10.1016/s0165-5728(00)00181-8. [DOI] [PubMed] [Google Scholar]
- 40.Li R., et al. Cytokine-defined B cell responses as therapeutic targets in multiple sclerosis. Front. Immunol. 2015;6:626. doi: 10.3389/fimmu.2015.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Addario M., et al. Epstein–Barr virus and its glycoprotein-350 upregulate IL-6 in human B-lymphocytes via CD21, involving activation of NF-κB and different signaling pathways. J. Mol. Biol. 2001;308:501–514. doi: 10.1006/jmbi.2001.4589. [DOI] [PubMed] [Google Scholar]
- 42.Wanke F., et al. EBI2 is highly expressed in multiple sclerosis lesions and promotes early CNS migration of encephalitogenic CD4 T cells. Cell Rep. 2017;18:1270–1284. doi: 10.1016/j.celrep.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Rutkowska A., et al. EBI2 regulates pro-inflammatory signaling and cytokine release in astrocytes. Neuropharmacology. 2018;133:121–128. doi: 10.1016/j.neuropharm.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Clottu A.S., et al. EBI2 expression and function: robust in memory lymphocytes and increased by natalizumab in multiple sclerosis. Cell Rep. 2017;18:213–224. doi: 10.1016/j.celrep.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Daugvilaite V., et al. Oxysterol–EBI2 signaling in immune regulation and viral infection. Eur. J. Immunol. 2014;44:1904–1912. doi: 10.1002/eji.201444493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magliozzi R., et al. B-cell enrichment and Epstein–Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 2013;72:29–41. doi: 10.1097/NEN.0b013e31827bfc62. [DOI] [PubMed] [Google Scholar]
- 47.Serafini B., et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serafini B., et al. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fierz W. Multiple sclerosis: an example of pathogenic viral interaction? Virol. J. 2017;14:42–48. doi: 10.1186/s12985-017-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pender M.P., et al. Decreased T cell reactivity to Epstein–Barr virus infected lymphoblastoid cell lines in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2009;80:498–505. doi: 10.1136/jnnp.2008.161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jelcic I., et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell. 2018;175:85–100. doi: 10.1016/j.cell.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li R., et al. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018;19:696–707. doi: 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 53.Bose T. Role of immunological memory cells as a therapeutic target in multiple sclerosis. Brain Sci. 2017;7:E148. doi: 10.3390/brainsci7110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winter S., et al. Loss of RASGRP1 in humans impairs T-cell expansion leading to Epstein–Barr virus susceptibility. EMBO Mol. Med. 2018;10:188–199. doi: 10.15252/emmm.201708292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinman L. The discovery of natalizumab, a potent therapeutic for multiple sclerosis. J. Cell Biol. 2012;199:413–416. doi: 10.1083/jcb.201207175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durzyńska J., Goździcka-Józefiak A. Viruses and cells intertwined since the dawn of evolution. Virol. J. 2015;12:169. doi: 10.1186/s12985-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tracy S.I., et al. Persistence of Epstein–Barr virus in self-reactive memory B cells. J. Virol. 2012;86:12330–12340. doi: 10.1128/JVI.01699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng C.T., Oldstone M.B., et al. IL-10: achieving balance during persistent viral infection. Curr. Top. Microbiol. Immunol. 2014;380:129–144. doi: 10.1007/978-3-662-43492-5_6. [DOI] [PubMed] [Google Scholar]
- 59.Blackburn S.D., Wherry E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Jochum S., et al. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8:e1002704. doi: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Küry P. Human endogenous retroviruses in neurological diseases. Trends Mol. Med. 2018;24:379–394. doi: 10.1016/j.molmed.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lycke J. Trials of antivirals in the treatment of multiple sclerosis. Acta Neurol. Scand. 2017;201:45–48. doi: 10.1111/ane.12839. [DOI] [PubMed] [Google Scholar]
- 63.Drosu N.C., et al. Could antiretrovirals be treating EBV in MS? A case report. Mult. Scler. Relat. Disord. 2018;22:19–21. doi: 10.1016/j.msard.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gold J., et al. A phase II baseline versus treatment study to determine the efficacy of raltegravir (Isentress) in preventing progression of relapsing remitting multiple sclerosis as determined by gadolinium-enhanced MRI: the INSPIRE study. Mult. Scler. Relat. Disord. 2018;24:123–128. doi: 10.1016/j.msard.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Croze E. Differential gene expression and translational approaches to identify biomarkers of interferon beta activity in multiple sclerosis. J. Int. Cyt. Res. 2010;30:743–749. doi: 10.1089/jir.2010.0022. [DOI] [PubMed] [Google Scholar]
- 66.Kieseier B.C. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Jakimovski D., et al. Interferon β for multiple sclerosis. Cold Spring Harb. Perspect. Med. 2018;8:a032003. doi: 10.1101/cshperspect.a032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Comabella M., et al. EBV-specific immune responses in patients with multiple sclerosis responding to IFNβ therapy. Mult. Scler. 2012;18:605–609. doi: 10.1177/1352458511426816. [DOI] [PubMed] [Google Scholar]
- 69.Rizzo F., et al. Interferon-β therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS-mediated apoptosis. Immunol. Cell Biol. 2016;94:886–894. doi: 10.1038/icb.2016.55. [DOI] [PubMed] [Google Scholar]
- 70.Zivadinov R., et al. Teriflunomide's effect on humoral response to Epstein–Barr virus and development of cortical gray matter pathology in multiple sclerosis. Mult. Scler. Relat. Disord. 2019;36:101388. doi: 10.1016/j.msard.2019.101388. [DOI] [PubMed] [Google Scholar]
- 71.Cohen J.I. Vaccine development for Epstein–Barr virus. Adv. Exp. Med. Biol. 2018;1045:477–493. doi: 10.1007/978-981-10-7230-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutton M.Y., et al. A review of the Centers for Disease Control and Prevention's response to the HIV/AIDS crisis among Blacks in the United States, 1981–2009. Am. J. Public Health. 2009;99(Suppl. 2):S351–S359. doi: 10.2105/AJPH.2008.157958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogembo, et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J. Transl. Med. 2015;13:50–62. doi: 10.1186/s12967-015-0415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith C., Khanna R. The development of prophylactic and therapeutic EBV vaccines. Curr. Top. Microbiol. Immunol. 2015;391:455–473. doi: 10.1007/978-3-319-22834-1_16. [DOI] [PubMed] [Google Scholar]
- 75.Williams J.A. Improving DNA vaccine performance through vector design. Curr. Gene Ther. 2014;14:170–189. doi: 10.2174/156652321403140819122538. [DOI] [PubMed] [Google Scholar]
- 76.Steinman L. Immune therapy for autoimmune diseases. Science. 2004;305:212–216. doi: 10.1126/science.1099896. [DOI] [PubMed] [Google Scholar]
- 77.Endriz J., et al. Time correlation between mononucleosis and initial symptoms of MS. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e308. doi: 10.1212/NXI.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belshe R.B., et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balfour H., et al. The promise of a prophylactic Epstein–Barr virus vaccine. Pediatr. Res. 2019 doi: 10.1038/s41390-019-0591-5. Published online October 3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mechelli R., et al. Epstein–Barr virus genetic variants are associated with multiple sclerosis. Neurology. 2015;84:1362–1368. doi: 10.1212/WNL.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Afrasiabi A., et al. Evidence from genome wide association studies implicates reduced control of Epstein–Barr virus infection in multiple sclerosis susceptibility. Genome Med. 2019;11:26–38. doi: 10.1186/s13073-019-0640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu J.L., Glaser S.L. Epstein–Barr virus-associated malignancies: epidemiologic patterns and etiologic implications. Crit. Rev. Oncol. Hematol. 2000;34:27–53. doi: 10.1016/s1040-8428(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 83.Kanakry J.A., Ambinder R.F. EBV-related lymphomas: new approaches to treatment. Curr. Treat Options Oncol. 2013;14:224–236. doi: 10.1007/s11864-013-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLaughlin L.P., et al. EBV-directed T cell therapeutics for EBV-associated lymphomas. Methods Mol. Biol. 2017;1532:255–265. doi: 10.1007/978-1-4939-6655-4_19. [DOI] [PubMed] [Google Scholar]
- 85.Scolding N.J., et al. Cell-based therapeutic strategies for multiple sclerosis. Brain. 2017;140:2776–2796. doi: 10.1093/brain/awx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pender M.P., et al. Epstein–Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight. 2018;3:e124714. doi: 10.1172/jci.insight.124714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pender M.P., et al. Epstein–Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Mult. Scler. 2014;20:1541–1544. doi: 10.1177/1352458514521888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Syed Y. Ocrelizumab: a review in multiple sclerosis. CNS Drugs. 2018;32:883–890. doi: 10.1007/s40263-018-0568-7. [DOI] [PubMed] [Google Scholar]
- 89.Dobson R., et al. Epstein–Barr-negative MS: a true phenomenon? Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e318. doi: 10.1212/NXI.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gieß R.M., et al. Epstein–Barr virus antibodies in serum and DNA load in saliva are not associated with radiological or clinical disease activity in patients with early multiple sclerosis. PLoS One. 2017;12:e0175279. doi: 10.1371/journal.pone.0175279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nourbakhsh B., et al. Heterogeneity in association of remote herpesvirus infections and pediatric MS. Ann. Clin. Transl. Neurol. 2018;5:1222–1228. doi: 10.1002/acn3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waubant E., et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology. 2011;76:1989–1995. doi: 10.1212/WNL.0b013e31821e552a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banwell B., et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol. 2007;6:773–781. doi: 10.1016/S1474-4422(07)70196-5. [DOI] [PubMed] [Google Scholar]
- 94.Pohl D., et al. High seroprevalence of Epstein–Barr virus in children with multiple sclerosis. Neurology. 2006;67:2063–2065. doi: 10.1212/01.wnl.0000247665.94088.8d. [DOI] [PubMed] [Google Scholar]
- 95.Lassmann H., Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133:223–244. doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palumbo S., Pellegrini S. In: Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Zagon I.S., Mclaughlin P.J., editors. Codon Publications; 2017. Experimental in vivo models of multiple sclerosis: state of the art. [PubMed] [Google Scholar]
- 97.'tHart B.A., et al. EBV Infection and multiple sclerosis: lessons from a marmoset model. Trends Mol. Med. 2016;22:1012–1024. doi: 10.1016/j.molmed.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Márquez A.C., Horwitz M.S. The role of latently infected B cells in CNS autoimmunity. Front. Immunol. 2015;6:544–562. doi: 10.3389/fimmu.2015.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casiraghi C., et al. Gammaherpesvirus latency accentuates EAE pathogenesis: relevance to Epstein–Barr virus and multiple sclerosis. PLoS Pathog. 2012;8:e1002715. doi: 10.1371/journal.ppat.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peacock J.W., et al. Exacerbation of experimental autoimmune encephalomyelitis in rodents infected with murine gammaherpesvirus-68. Eur. J. Immunol. 2003;33:1849–1858. doi: 10.1002/eji.200323148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jarousse N., et al. Virally-induced upregulation of heparan sulfate on B cells via the action of type I IFN. J. Immunol. 2011;187:5540–5547. doi: 10.4049/jimmunol.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilli F., et al. The effect of B-cell depletion in the Theiler’s model of multiple sclerosis. J. Neurol. Sci. 2015;359:40–47. doi: 10.1016/j.jns.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 103.Martinez N.E., et al. Protective and detrimental roles for regulatory T cells in a viral model for multiple sclerosis. Brain Pathol. 2014;24:436–451. doi: 10.1111/bpa.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bender S.J., Weiss S.R. Pathogenesis of murine coronavirus in the central nervous system. J. Neuroimmune Pharmacol. 2010;5:336–354. doi: 10.1007/s11481-010-9202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.'tHart B.A., et al. The primate autoimmune encephalomyelitis model; a bridge between mouse and man. Ann. Clin. Transl. Neurol. 2015;2:581–593. doi: 10.1002/acn3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morandi E., et al. EBV infection empowers human B Cells for autoimmunity: role of autophagy and relevance to multiple sclerosis. J. Immunol. 2017;199:435–448. doi: 10.4049/jimmunol.1700178. [DOI] [PubMed] [Google Scholar]
- 107.Dunham J., et al. Analysis of the crosstalk of Epstein–Barr virus-infected B cells with T cells in the marmoset. Clin. Transl. Immunology. 2017;6:e127. doi: 10.1038/cti.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassani A., et al. Epstein–Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS ONE. 2018;13:e0192109. doi: 10.1371/journal.pone.0192109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Veroni C., et al. Transcriptional profile and Epstein–Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J. Neuroinflammation. 2018;15:18. doi: 10.1186/s12974-017-1049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moreno M.A., et al. Signature of Epstein–Barr virus infection in multiple sclerosis brain lesions. Neurol. Neuroimmunol. Neuroinflamm. 2018;5:e466. doi: 10.1212/NXI.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angelini D.F., et al. Increased CD8+ T cell response to Epstein–Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013;9:e1003220. doi: 10.1371/journal.ppat.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peferoen L., et al. Epstein–Barr virus is not a characteristic feature in the central nervous system in established multiple sclerosis. Brain. 2009;133:e137. doi: 10.1093/brain/awp296. [DOI] [PubMed] [Google Scholar]
- 113.Opsahl M.L., Kennedy P.G. An attempt to investigate the presence of Epstein Barr virus in multiple sclerosis and normal control brain tissue. J. Neurol. 2007;254:425–430. doi: 10.1007/s00415-006-0316-7. [DOI] [PubMed] [Google Scholar]
- 114.Jilek S., et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008;131:1712–1721. doi: 10.1093/brain/awn108. [DOI] [PubMed] [Google Scholar]
- 115.Lünemann J.D., et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J. Exp. Med. 2008;205:1763–1773. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Libbey J.E., et al. Molecular mimicry in multiple sclerosis. Int. Rev. Neurobiol. 2007;79:127–147. doi: 10.1016/S0074-7742(07)79006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Michel L., et al. B cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS compartmentalized inflammation. Front. Immunol. 2015;6:636. doi: 10.3389/fimmu.2015.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]