Abstract
Background
Porcine reproductive and respiratory syndrome (PRRS) caused by PRRS virus (PRRSV) results in economic losses in the swine industry globally. Several studies have investigated the use of plant extracts in the prevention and control of PRRS outbreaks. Thai medicinal plants may be useful for treating PRRSV infection in pigs. Therefore, we investigated the in vitro anti-PRRSV and antioxidant properties of seven Thai medicinal plants: Caesalpinia sappan Linn., Garcinia mangostana Linn., Houttuynia cordata, Perilla frutescens, Clinacanthus nutans, Phyllanthus emblica, and Tiliacora triandra.
Results
Using antiviral screening, we observed that T. triandra extract strongly inhibited PRRSV infectivity in MARC-145 cells [virus titer 3.5 median tissue culture infective dose (TCID50)/ml (log10)] at 24 h post-infection, whereas C. sappan extract strongly inhibited PRRSV replication [virus titer 2.5 TCID50/ml (log10)] at 72 h post-infection. C. sappan extract had the highest total phenolic content [220.52 mM gallic acid equivalent/g] and lowest half-maximal inhibitory concentration [1.17 mg/ml in 2,2-diphenyl-1-picrylhydrazyl and 2.58 mg/ml in 2,2-azino-bis (3-ethylbenzothiazo-line-6-sulfonic acid) diammonium salt].
Conclusion
T. triandra extract could inhibit PRRSV infectivity, whereas C. sappan extract was the most effective in inhibiting PRRSV replication in MARC-145 cells. This study elucidates the antiviral activities of Thai medicinal plant extracts in vivo. The results promise that Thai medicinal plant extracts, particularly T. triandra and C. sappan extracts, can be developed into pharmaceutical drugs for the prevention of PRRS in pigs.
Keywords: Porcine reproductive and respiratory syndrome, Porcine reproductive and respiratory syndrome virus, Antiviral activity, Thai medicinal plants
Background
Porcine reproductive and respiratory syndrome virus (PRRSV) is endemic in most pig-producing countries, and it results in enormous economic losses to the swine industry globally [1]. This enveloped, positive-sense, single-stranded RNA virus belongs to the Arteriviridae family (order Nidovirales), which also includes the equine arteritis virus, mouse lactate dehydrogenase-elevating virus, and simian hemorrhagic fever virus [2]. In general, PRRSV infection causes a disease that is characterized by reproductive failure in sows and respiratory infections in growing pigs [3], and this disease predisposes pigs to infection by bacteria and other viral pathogens [4, 5]. This disease is known as porcine reproductive and respiratory syndrome (PRRS) and has become endemic in many countries throughout the world following an epidemic phase [6, 7]. Its incidence was first reported in Thailand in 1989, and since then, several outbreaks have been reported [8]. It has become a major infectious disease that causes high mortality in swine and production losses in the swine industry in this country.
Preventative measures such as gilt acclimatization, vigilant biosecurity, and vaccination have been shown to be useful in controlling PRRS outbreaks, and supportive treatments are available for alleviating its severity; however, no specific treatment for PRRS is available [9, 10]. Antiviral therapeutics are a critical tool for combating viral infections, particularly in cases wherein no vaccines are available against the circulating virus. Thus, pharmacological intervention may represent an alternative approach in controlling PRRSV. A number of natural compounds and compositions have been shown to possess antiviral activities against PRRSV. Gao et al. [11] showed that Cryptoporus volvatus extract exhibited antiviral activity against PRRSV infection and replication. Pringproa et al. [12] reported that crude Cynodon dactylon extract significantly inhibited PRRSV replication as early as 24 h post-infection (hpi). Therefore, the antiviral activities of other Thai medicinal plants against PRRSV should also be investigated. Thai medicinal plants such as Caesalpinia sappan Linn., Garcinia mangostana Linn., Houttuynia cordata, Perilla frutescens, Clinacanthus nutans, Phyllanthus emblica, and Tiliacora triandra are known to have antioxidant and antiviral activities. These plants have already been promoted for use in primary health care and have been classified according to their pharmacological actions [13–18]. Therefore, the aim of this study was to determine the antiviral activities of Thai medicinal plant extracts against PRRSV infection in vitro and to measure their phytochemical contents to develop an alternative anti-PRRSV therapy for use in veterinary medicine.
Results
Cytotoxic activities of plant extracts
Prior to determining antiviral activity, we evaluated the cytotoxicity of the seven Thai medicinal plant extracts on the viability of MARC-145 cells, and viability is expressed as 50% cytotoxic concentration (CC50). The results showed that the CC50 of the seven plant extracts ranged from 78 to 2500 μg/ml, and the effect of Thai medicinal plant extract concentration on the tested cells increased in a dose-dependent manner (Fig. 1). P. emblica extract had the lowest CC50 of 78 μg/ml. The CC50 of G. mangostana extract was the second lowest (312.5 μg/ml) and that of C. sappan extract was 625 μg/ml. Further, T. triandra and H. cordata extracts had CC50 of 1250 μg/ml, whereas C. nutans and P. frutescens extracts had the highest CC50 (2500 μg/ml).
Fig. 1.
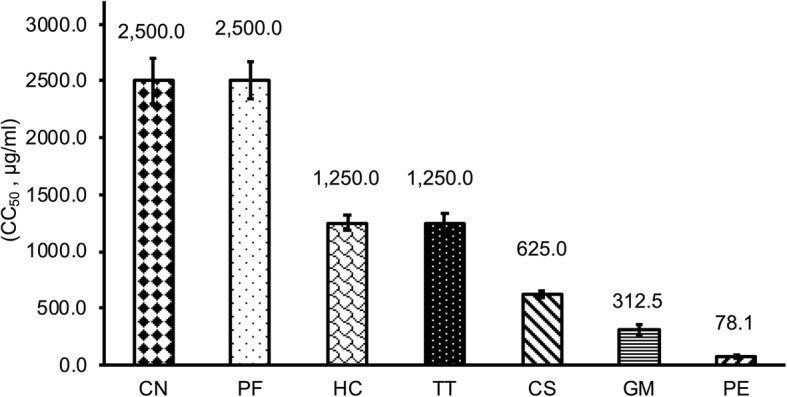
Cytotoxity of the seven Thai medicinal plant extracts on MARC-145 cells determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. MARC-145 cells were incubated with various concentrations of these plant extracts or control without plant extract for 72 h prior to the MTT assay. Values are expressed as mean ± standard error. CN, Clinacanthus nutans; PF, Perilla frutescens; HC, Houttuynia cordata; TT, Tiliacora triandra; CS, Caesalpinia sappan Linn.; GM, Garcinia mangostana Linn.; PE, Phyllanthus emblica; CC50, 50% cytotoxic concentration
Inhibition of PRRSSV infection by Thai medicinal plant extracts
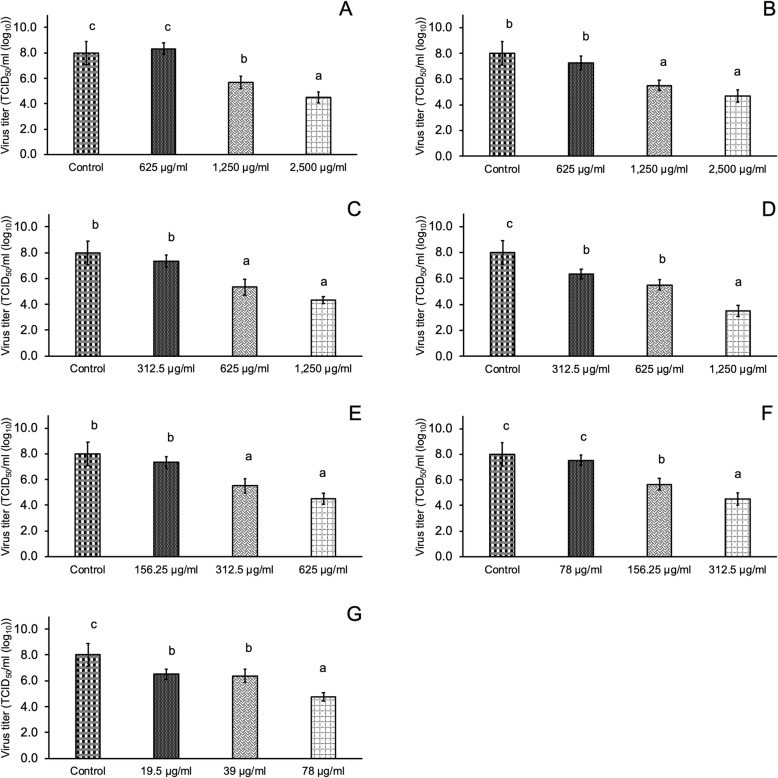
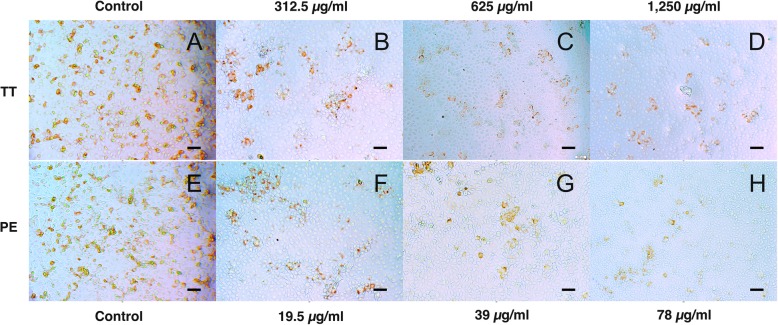
We treated PRRSV with different concentrations of Thai medicinal plant extracts that were determined based on their CC50 values so that these plant extracts did not affect the proliferative activity of MARC-145 cells. The screening results of the inhibition of PRRSV infectivity showed the potential of Thai medicinal plant extracts to inhibit PRRSV infectivity (Fig. 2). T. triandra extract significantly inhibited PRRSV infectivity in MARC-145 cells at 24 hpi when supplied at a concentration of 1250 μg/ml (P < 0.05), and the observed virus titer at this concentration was 3.5 TCID50/ml (log10). Interestingly, P. emblica extract at a low concentration of 78 μg/ml could inhibit PRRSV infectivity [virus titer = 4.5 TCID50/ml (log10)]. As shown in Fig. 3, immunoperoxidase monolayer assay (IPMA) indicated that T. triandra and P. emblica extracts blocked PRRSV infectivity in MARC-145 cells, as shown by slight brown staining of cells.
Fig. 2.
Virus titer for the inhibition of PRRSV infectivity of seven Thai medicinal plant extracts at 24 h post-infection (hpi). AClinacanthus nutans; BPerilla frutescens; CHouttuynia cordata; DTiliacora triandra; ECaesalpinia sappan Linn.; FGarcinia mangostana Linn. and GPhyllanthus emblica. a, b, and c, P-value of < 0.05 compared with different concentrations of the plant extracts
Fig. 3.
Immunoperoxidase monolayer assay (IPMA) showing the inhibition of PRRSV infection in MARC-145 cells by Tiliacora triandra (TT) extract at concentrations of 312.5, 625, and 1250 μg/ml (a–d) and P. emblica (PE) extract at concentrations of 19.5, 39, and 78 μg/ml (e–h). Scale bar in the figure: 200 μm
Thai medicinal plant extracts inhibit PRRSV replication
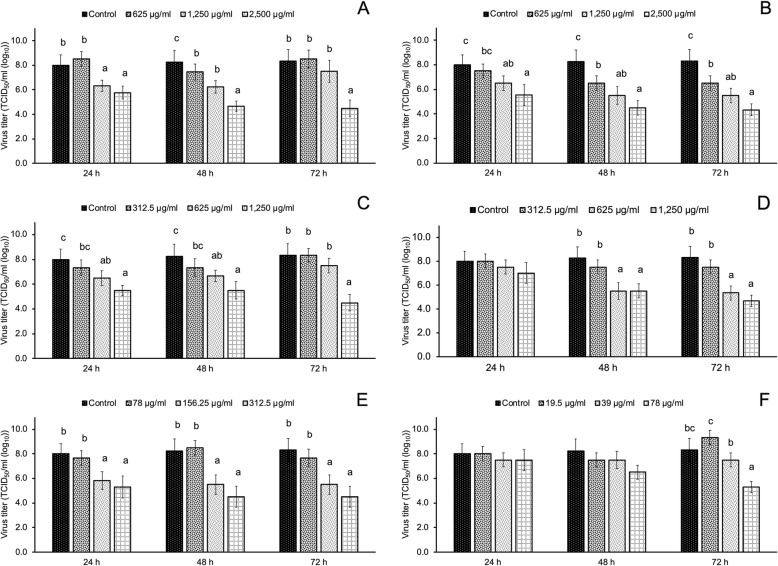
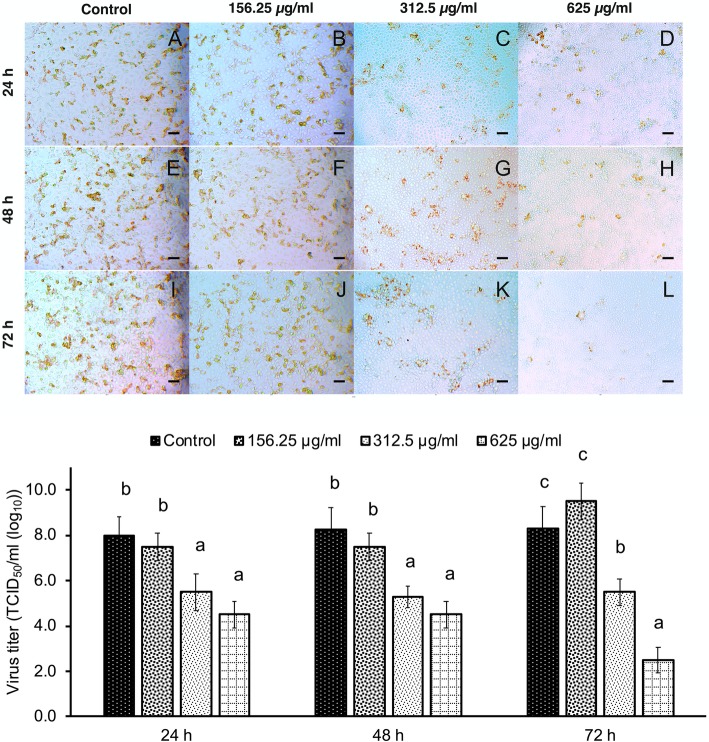
Different Thai medicinal plant extracts were tested in an in vitro inhibitor screening assay to determine inhibition of PRRSV replication at three time intervals (24, 48, and 72 hpi). At various time points after the infection, PRRSV in supernatants was quantified for determining virus titer by IPMA. Results of screening were the same as those of the inhibition test of PRRSV infectivity, i.e., PRRSV replication was inhibited in a dose-dependent manner (Fig. 4). Interestingly, as shown in Fig. 5, we found that C. sappan extract had significant potential to inhibit PRRSV replication in vitro. As shown in Fig. 5L, few cells that were stained brown showed the efficiency of C. sappan extract at a concentration of 625 μg/ml, and the inhibition of PRRSV replication by C. sappan extract was significantly stronger than that by other plant extracts at 72 hpi [2.7 TCID50/ml (log10)].
Fig. 4.
Virus titer for the inhibition of PRRSV replication of seven Thai medicinal plant extracts at 24, 48, and 72 h post-infection (hpi). AClinacanthus nutans; BPerilla frutescens; CHouttuynia cordata; DTiliacora triandra; EGarcinia mangostana Linn. and FPhyllanthus emblica. a, b, and c; P-value of < 0.05 compared with different concentrations of the plant extracts
Fig. 5.
IPMA of Caesalpinia sappan Linn. inhibiting PRRSV replication in MARC-145 cells at 24 (A–D), 48 (E and F), and 72 h post-infection (hpi) (I–L). a, b, and c: P-value of < 0.05 compared with different concentrations of C. sappan. Scale bar in the figure: 200 μm
Phytochemical contents of Thai medicinal plant extracts
The total phenolic contents of the seven Thai medicinal plant extracts were determined using the Folin–Ciocalteu assay by constructing a standard curve of gallic acid. Total phenolic content was the highest in C. sappan extract [mean ± standard error: 220.52 ± 4.47 mM gallic acid equivalent (GAE)/g sample], followed by G. mangostana extract (91.16 ± 4.62 mM GAE/g sample), with the lowest total phenolic content was observed in H. cordata extract (8.51 ± 0.04 mM GAE/g sample) (Table 1).
Table 1.
Total phenolic contents and antioxidant activities of seven Thai medicinal plant extracts
| Total phenolic (mM GAE/g) |
DPPH (IC50, mg/ml) |
ABTS (IC50, mg/ml) |
FRAP (mM Fe2+/g) |
|
|---|---|---|---|---|
| Caesalpinia sappan | 220.52 ± 4.47 | 1.17 ± 0.06 | 2.57 ± 0.16 | 334.78 ± 13.15 |
| Garcinia mangostana | 91.16 ± 4.62 | 4.82 ± 0.58 | 4.98 ± 0.10 | 46.12 ± 1.27 |
| Houttuynia cordata | 14.25 ± 0.20 | 97.79 ± 4.14 | 72.02 ± 4.01 | 8.55 ± 0.18 |
| Perilla frutescens | 29.86 ± 0.41 | 11.68 ± 0.51 | 21.37 ± 1.28 | 43.32 ± 0.92 |
| Clinacanthus nutans | 25.52 ± 0.22 | 50.34 ± 5.60 | 37.82 ± 1.25 | 18.39 ± 0.54 |
| Phyllanthus emblica | 44.35 ± 0.24 | 3.49 ± 0.17 | 4.95 ± 0.11 | 94.17 ± 0.62 |
| Tiliacora triandra | 30.45 ± 1.51 | 17.77 ± 0.22 | 21.16 ± 1.06 | 30.58 ± 1.13 |
DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2-azino-bis (3-ethylbenzothiazo-line-6-sulfonic acid) diammonium salt, FRAP ferric reducing antioxidant power, GAE gallic acid equivalents, IC50 half maximal inhibitory concentration
Antioxidant activity
C. sappan extract had the highest antioxidant activity, with IC50 values of 1.17 ± 0.06 mg/ml in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2.57 ± 0.16 mg/ml n 2,2-azino-bis(3-ethylbenzothiazo-line-6-sulfonic acid) diammonium salt (ABTS) and a reducing power of 334.78 ± 13.15 mM Fe2+/g in the ferric-reducing antioxidant power (FRAP) assay (Table 1). P. emblica extract had the second strongest antioxidant activity against free radicals, with IC50 values of 3.49 ± 0.17 mg/ml in DPPH and 4.95 ± 0.11 mg/ml in ABTS and a reducing power of 94.17 ± 0.62 mM Fe2+/g sample in the FRAP assay.
Discussion
PRRSV outbreak causes significant economic loss in the swine industry worldwide. The current commercial PRRSV vaccines are inadequate to protect pigs from PRRSV infections [19]. Medicinal plants have progressively been explored as suitable alternative sources of antiviral agents [20]. Thai medicinal plants have widely been used as a source of herbal medicines because of their high bioactive compound contents that are effective against various diseases. In this study, seven Thai medicinal plant extracts were screened for their antiviral activity against PRRSV.
Before determining the antiviral properties of a compound, it is essential that a cytotoxicity assay is performed to determine the concentrations that can be used to avoid cell damage and ensure PRRSV selectivity in vitro. In this study, we reported cytotoxicity as CC50, which indicates the concentration of a substance that can inhibit virus activity by 50%. We found that P. emblica extract showed the highest cell toxicity (78.1 μg/ml). In this study, high-potential plant extracts were found to be C. sappan and T. triandra extracts, with CC50 of 625 and 1250 μg/ml, respectively. Antiviral compounds should be highly effective while showing minimal toxicity to normal cells and tissues [21].
In this study, we investigated the antiviral activity of seven Thai medicinal plant extracts against PRRSV by assessing the inhibition of PRRSV infection and replication in MARC-145 cells. The range of plant extract concentrations was determined based on their CC50 values. P. emblica extract inhibited PRRSV infection in MARC-145 cells and in vitro. P. emblica extract at a concentration of 78 μg/ml inhibited PRRSV infectivity at a virus titer of 4.5 TCID50/ml (log10). In this study, P. emblica extract showed the highest cytotoxicity to MARC-145 cells with CC50 of < 100 μg/ml. Therefore, the antiviral activity of other plant extracts were investigated in this study. We found that T. triandra extract at a concentration of 1250 μg/ml significantly inhibited PRRSV infectivity at a virus titer of 3.5 TCID50 (log10). While T. triandra extract has been used as anti-inflammatory [22], anticancer [23], and antimicrobial agents against Mycobacterium tuberculosis [24], its antiviral activity, particularly against PRRSV, has not been investigated previously. Therefore, this is the first report to indicate that T. triandra extract could significantly prevent the entry of PRRSV into MARC-145 cells. However, T. triandra extract was not found to be effective in inhibiting PRRSV replication. All studied plant extracts could inhibit PRRSV replication when applied at high concentrations, as shown by the linear regression model from 24 to 72 hpi after incubation with PRRSV. C. sappan extract at a concentration of 625 μg/ml could inhibit PRRSV replication as 72 hpi [virus titer 2.7 TCID50 (log10)]. Although the antiviral activity of C. sappan extract against the influenza virus [13] and the antimicrobial properties of C. sappan [25] have previously been investigated, this is the first study to reveal the inhibitory activity of C. sappan extract on PRRSV replication in MARC-145 cells.
Regarding phytochemical content, C. sappan extract had the highest total phenolic content (220.52 ± 4.47 mM GAE/g sample). The total phenolic content of a plant is considered an indicator of its antioxidant capacity because the redox properties of phenolic compounds allow them to act as reducing agents, hydrogen donors, and radical scavengers [22]. Previously, Lee et al. [26] reported that ethanolic C. sappan extract had a total phenolic content of 723.67 μg GAE/mg. The values of total phenolic content in this study were slightly different from those reported previously. This may be because of the different durations, geographical variations, or extraction methods, which may have altered the phenolic content. Ethanolic plant extracts can be used for the investigation of antiviral activity in a cell line. Abu-Jafar and Huleihel [27] reported that ethanolic Eucalyptus camaldulensis leave extracts had strong antiviral activity against different members of the herpes virus family (HSV-1, HSV-2, and VZV). Ramalingam et al. [28] reported that the ethanolic extracts of Andrographis paniculata have the highest antiviral inhibitory effects against dengue virus in Vero cells.
The screening of plants as possible sources of antiviral agents has led to the discovery of potent inhibitors of in vitro viral replication, thereby increasing the probability of identifying new bioactive plant compounds [29]. These findings suggest the appropriate species and concentration of plant extract that could effectively inhibit PRRSV replication, with both T. triandra and C. sappan extracts being highly effective in inhibiting PRRSV infection in vitro by interfering with viral attachment and inhibiting viral replication and/or virus release, respectively. The modes of action of T. triandra and C. sappan extracts against PPRSV require further investigation but are likely to be related to the natural compounds they contain. Therefore, it was speculated that both T. triandra and C. sappan extracts are potential candidates for preventing PRRSV infection in pigs. However, the plant extracts used for testing antiviral activity was crude extracts. In future, we plan to purify the most effective Thai medicinal plant extracts (T. triandra and C. sappan extracts) for screening the active compound that is highly effective against PRRSV.
Conclusion
Thai medicinal plant extracts exhibit antiviral activity against PRRSV. T. triandra extract effectively inhibited PRRSV infection. and C. sappan extract had the strongest antiviral activity against PRRSV replication. These activities can be presumably attributed to the total phenolic contents and antioxidant activities of these plant extracts. Although several previous studies have shown the antiviral activity of plant extracts against PRRSV, there are no reports on the antiviral activities of T. triandra and C. sappan extracts against PRRSV. To the best of our knowledge, this study is the first to report the inhibitory activity of T. triandra and C. sappan extracts against PRRSV activity in vitro. Further studies are required to elucidate the mechanisms of action of these plant extracts on PRRSV.
Methods
Chemicals
All chemicals used in this study were of analytical grade or higher. Ethanol and methanol were obtained from Merck (Darmstadt, Germany). ABTS, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), DPPH, Folin–Ciocalteu phenol reagent, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), sodium carbonate, and 2,4,6-tri-pyridyl-s-triazine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ferric chloride hexahydrate and potassium persulfate were procured from LOBA CHEMIE PVT (Mumbai, India). Gallic acid was procured from Fluka Chemical Co. (Buchs, Switzerland). Dulbecco’s modified Eagle’s medium (DMEM) was procured from Gibco (Massachusetts, USA).
Plant extracts, cells, and viruses
Ethanolic C. sappan, G. mangostana, H. cordata, P. frutescens, C. nutans, P. emblica, and T. triandra extracts were purchased from Specialty Natural Product Co. Ltd. (Thailand).
MARC-145 tissue culture cells were grown in DMEM containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin and incubated at 37 °C in a 5% CO2 atmosphere. To produce inoculated cells, PRRSV (VR2332 North American genotype) was propagated in MARC-145 cells, and virus titer was quantified using IPMA.
Cytotoxicity assay
The cytotoxicity of the seven Thai medicinal plant extracts was determined using the MTT assay. Briefly, MARC-145 cells were plated at a density of 5000 cells/well in 96-well plates and incubated in a 5% CO2 atmosphere at 37 °C for 24 h. When cells had at least 90% confluence, the medium was removed and replaced with medium containing two-fold serial dilutions of the plant extracts. In addition, medium without plant extract was used as a positive control. Incubation was then continued in a 5% CO2 atmosphere at 37 °C for 72 h. After this, the medium was removed, 20 μl of freshly prepared MTT solution (5 mg/ml) was added to each well, and the plates were incubated at 37 °C for 4 h. Then, the medium was replaced with 150 μl DMSO to dissolve the crystals, and the plates were incubated at 37 °C for 5 min to dissolve any air bubbles before measuring the MTT signal at an absorbance of 550 nm. Results are reported as CC50.
Inhibition of virus infection assay
The inhibition of virus infection assay was performed as previously described [12]. Briefly, the plant extracts at the concentration that was determined in the cytotoxicity test outlined above and at two lower concentrations in two-fold dilution were mixed with PRRSV at 108 TCID50/ml at a ratio of 1:1 and incubated at 37 °C for 1 h. DMSO (1%) containing medium mixed with PRRSV served as the control. Thereafter, the mixture of PRRSV and plant extracts as well as controls were inoculated in MARC-145 cells at a density of 5000 cells/well in a 96-well plate and incubated at 37 °C for 1 h. Subsequently, the medium was removed and replaced with a fresh medium containing 10% FBS. The plates with MARC-145 cells were cultured under standard conditions for 24 h hpi, and supernatants were collected to quantify virus titer.
Inhibition of viral replication assay
The inhibition of viral replication assay was performed as previously described [12]. Briefly, MARC-145 cells were plated at a density of 5000 cells/well in 96-well plates and infected with PRRSV at a multiplicity of infection of 1 at 37 °C for 1 h. Then, PRRSV was removed from each well and replaced with the diluted plant extracts at the concentration that was determined in the cytotoxicity test and at two lower concentrations ins two-fold dilution. Further, 1% DMSO was mixed to medium as the control. The plates were cultured under standard conditions; supernatants were collected at 24, 48, and 72 hpi; and virus titer was quantified.
Virus titer
Virus titer was further assessed by IPMA as previously described [30]. Briefly, cells were fixed with 100 μl of 4% cold formalin for 15 min at room temperature (RT), washed once with 100 μl of phosphate-buffered saline (PBS) and twice with 100 μl of 0.5% PBS Tween-20 (PBST), and blocked with 100 μl of 1% BSA in 0.5% PBST for 30 min at RT. After blocking, the cells were stained with 70 μl of anti-PRRSV NC protein monoclonal antibody (Median Diagnostics, Gangwon-do, Korea) diluted at a ratio of 1:400 at RT for 60 min, washed, and incubated with peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (H + L) (Jackson ImmunoResearch, Pennsylvania, USA) diluted at a ratio of 1:1200 for 60 min at RT. After washing thrice with PBS, the cells were counter stained with 1,5-diaminopentane substrate and examined under a microscope. Virus titer is expressed as TCID50 and was determined using the Reed–Muench method.
Phytochemical analysis
The total phenolic contents of the plant extracts were determined using the Folin–Ciocalteu method [31], and their free radical-scavenging activities were determined using the DPPH-scavenging and ABTS-scavenging assays, as previously reported [32, 33]. Antioxidant activities were determined using the FRAP assay, according to the Benzie and Strain method [34].
Statistical analysis
Differences in antiviral activities among the different concentrations of each plant extract were tested using one-way analysis of variance with Tukey’s post hoc test for a comparison of means. CC50 was calculated using regression analysis of dose–response curves for the MTT assay. All statistical analyses were performed using the SPSS 23.0 software (SPSS Inc., Chicago, IL, USA) with a significance level of P-value of ≤0.05.
Acknowledgments
The authors thank Dr. Wolfram Spreer of the University of Hohenheim for his critical comments on this article and thank Enago (https://www.enago.com) for the English language review.
Abbreviations
- PRRS
Porcine reproductive and respiratory syndrome
- PRRSV
Porcine reproductive and respiratory syndrome virus
- TCID50
Median tissue culture infective dose
- RNA
Ribonucleic acid
- hpi
Hour post-infection
- CC50
50% cytotoxic concentration
- IPMA
Immunoperoxidase monolayer assay
- GAE
Gallic acid equivalent
- DPPH
2, 2-diphenyl-1-picrylhydrazyl
- ABTS
2, 2-azino-bis(3-ethylbenzothiazo-line-6-sulfonic acid) diammonium salt
- FRAP
Ferric-reducing antioxidant power
- IC50
Half-maximal inhibitory concentration
- DMEM
Dulbecco’s Modified Eagle Medium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- CO2
Carbon dioxide
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- PBS
Phosphate-buffered saline
- RT
Room temperature
Authors’ contributions
KP, SH, and KS contributed to the study design. CA performed the experiments, carried out the statistical analysis, and drafted the manuscript. KP, SH and KS contributed to the statistical analysis and critically reviewed the manuscript. KP, MS, SM, WR, and KS conceived the study, coordinated the work described, and contributed to the manuscript preparation. All authors read and approved the final manuscript.
Funding
CA was supported financially by a Ph.D. scholarship of Research and Researcher for Industries Projects (RRi), Thailand Science Research and Innovation, under contract no. PHD61I0042. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. Also, this project was partially supported by Chiang Mai University.
Availability of data and materials
The datasets supporting the results of this article are available in the figshere (https://figshare.com/s/97bfdb8d693a8c95ffaf).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Q, Yoo D. PRRS virus receptors and their role for pathogenesis. Vet Microbiol. 2015;177:229–241. doi: 10.1016/j.vetmic.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Lunney JK, Fang Y, Ladinig A, Chen N, Li Y, Rowland B, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci. 2016;4:129–154. doi: 10.1146/annurev-animal-022114-111025. [DOI] [PubMed] [Google Scholar]
- 3.Nilubol D, Tripipat T, Hoonsuwan T, Kortheerakul K. Porcine reproductive and respiratory syndrome virus, Thailand, 2010–2011. Emerg Infect Dis. 2012;18:2039–2043. doi: 10.3201/eid1811.111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, et al. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J Vet Diagnostic Investig. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- 5.Pu X, Liang J, Shang R, Wang X, Wang Z, Hua L, et al. Influence of Hypericum perforatum extract on piglet infected with porcine respiratory and reproductive syndrome virus. Agric Sci China. 2009;8:730–739. doi: 10.1016/S1671-2927(08)60272-2. [DOI] [Google Scholar]
- 6.Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997;55:309–316. doi: 10.1016/S0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- 7.Thanapongtharm W, Linard C, Pamaranon N, Kawkalong S, Noimoh T, Chanachai K, et al. Spatial epidemiology of porcine reproductive and respiratory syndrome in Thailand. BMC Vet Res. 2014;10:174. doi: 10.1186/s12917-014-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damrongwatanapok S, Arsayuth K, Kongkrong C, Parchariyanon S, Pinyochon WTU. Serological studies and isolation of porcine reproductive and respiratory syndrome (PRRS) virus in Thailand. J Thai Vet Med Assoc. 1996;47:19–30. [Google Scholar]
- 9.Labarque G, Van Gucht S, Van Reeth K, Nauwynck H, Pensaert M. Respiratory tract protection upon challenge of pigs vaccinated with attenuated porcine reproductive and respiratory syndrome virus vaccines. Vet Microbiol. 2003;95:187–197. doi: 10.1016/S0378-1135(03)00157-3. [DOI] [PubMed] [Google Scholar]
- 10.Anantikulchai P, Emprom P, Pringproa K, Yamsakul P. In vitro Cytotoxicity Test and Antiviral Activity of Curcuminoids from Turmeric Extract Against PRRS Virus. Vet Integr Sci. 2017;15:199–205. doi: 10.14456/cmvj.2017.X. [DOI] [Google Scholar]
- 11.Gao L, Zhang W, Sun Y, Yang Q, Ren J, Liu J, et al. Cryptoporus volvatus extract inhibits porcine reproductive and respiratory syndrome virus (PRRSV) in vitro and in vivo. PLoS One. 2013;8:e63767. doi: 10.1371/journal.pone.0063767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pringproa K, Khonghiran O, Kunanoppadol S. In Vitro Virucidal and Virustatic Properties of the Crude Extract of Cynodon dactylon against Porcine Reproductive and Respiratory Syndrome Virus In Vitro Virucidal and Virustatic Properties of the Crude Extract of Cynodon dactylon against Porcine Reprodu. Vet Med Int. 2014;2014:947589. doi: 10.1155/2014/947589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu A, Shu S, Qin H, Ming S, Lee Y, Wang Y, et al. In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Med. 2009;75:337–339. doi: 10.1055/s-0028-1112208. [DOI] [PubMed] [Google Scholar]
- 14.Chen S-X. Min wan B-NL. Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med. 1996;62:381–382. doi: 10.1055/s-2006-957916. [DOI] [PubMed] [Google Scholar]
- 15.Chiow KH, Phoon MC, Putti T, Tan BKH, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. Extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med. 2016;9:1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahata T, Otake T, Mori H, Kojima Y, Oishi I, Oka S, et al. A novel substance purified from Perilla Frutescens Britton inhibits an early stage of HIV-1 replication without blocking viral adsorption. Antivir Chem Chemother. 2002;13:283–288. doi: 10.1177/095632020201300503. [DOI] [PubMed] [Google Scholar]
- 17.Haetrakul T, Dunbar SG, Chansue N. Antiviral activities of Clinacanthus nutans (Burm.F.) Lindau extract against cyprinid herpesvirus 3 in koi (Cyprinus carpio koi) J Fish Dis. 2018;41:581–587. doi: 10.1111/jfd.12757. [DOI] [PubMed] [Google Scholar]
- 18.Xiang Y, Pei Y, Qu C, Lai Z, Ren Z, Yang K, et al. In vitro anti-herpes simplex virus activity of 1,2,4,6-tetra-O-galloyl-β-d-glucose from Phyllanthus emblica L. (Euphorbiaceae) Phyther Res. 2011;25:975–982. doi: 10.1002/ptr.3368. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, Bai X, Cui T, Zhou H, Chen Y, Xie J, et al. In vitro antiviral activity of Germacrone against porcine reproductive and respiratory syndrome virus. Curr Microbiol. 2016;73:317–323. doi: 10.1007/s00284-016-1042-8. [DOI] [PubMed] [Google Scholar]
- 20.Mehrbod P, Abdalla MA, Njoya EM, Ahmed AS, Fotouhi F, Farahmand B, et al. South African medicinal plant extracts active against influenza a virus. BMC Complement Altern Med. 2018;18:112. doi: 10.1186/s12906-018-2184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adnan A, Allaudin ZN, Hani H, Loh H-S, Khoo T-J, Ting KN, et al. Virucidal activity of Garcinia parvifolia leaf extracts in animal cell culture. BMC Complement Altern Med. 2019;19:169. doi: 10.1186/s12906-019-2586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weerawatanakorn M, Rojsuntornkitti K, Pan M-H, Wongwaiwech D. Some Phytochemicals and Anti-inflammation Effect of Juice from Tiliacora triandra Leaves. J Food Nutr Res. 2018;6:32–38. doi: 10.12691/jfnr-6-1-6. [DOI] [Google Scholar]
- 23.Rattana S, Cushnie B, Taepongsorat L, Phadungkit M. Chemical constituents and in vitro anticancer activity of Tiliacora triandra leaves. Pharmacogn J. 2016;8. 10.5530/pj.2016.1.1.
- 24.Sureram S, Senadeera SPD, Hongmanee P, Mahidol C, Ruchirawat S, Kittakoop P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of mycobacterium tuberculosis. Bioorg Med Chem Lett. 2012;22:2902–2905. doi: 10.1016/j.bmcl.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan R, Selvam GG, Karthik S, Mathivanan K, Baskaran R, Karthikeyan M, et al. in vitro antimicrobial activity of Caesalpinia sappan L. Asian Pac J Trop Biomed 2012;2:S136–S139. doi:10.1016/S2221-1691(12)60144-0.
- 26.Lee M-J, Lee H-S, Kim H, Yi H-S, Park S-D, Moon H-I, et al. RETRACTED: antioxidant properties of benzylchroman derivatives from Caesalpinia sappan L. against oxidative stress evaluated in vitro. J Enzyme Inhib Med Chem. 2010;25:608–614. doi: 10.3109/14756360903373376. [DOI] [PubMed] [Google Scholar]
- 27.Abu-jafar A, Huleihel M. Antiviral activity of Eucalyptus camaldulensis leaves ethanolic extract on herpes viruses infection. Int J Clin Virol. 2017;1:001–009. doi: 10.29328/journal.ijcv.1001001. [DOI] [Google Scholar]
- 28.Ramalingam S, Karupannan S, Padmanaban P, Vijayan S, Sheriff K, Palani G, et al. Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. Ayu. 2018;39:87–91. doi: 10.4103/ayu.AYU_144_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn LK, Foglio MA, Rodrigues RA. Sousa IM de O, martini MC, Padilla MA, Lima Neto DF de AC. In-vitro antiviral activities of extracts of plants of the Brazilian Cerrado against the avian Metapneumovirus (aMPV) Brazilian J Poult Sci. 2015;17:275–280. doi: 10.1590/1516-635X1703275-280. [DOI] [Google Scholar]
- 30.Zhang J, Liu W, Chen W, Li C, Xie M, Bu Z. Development of an Immunoperoxidase Monolayer Assay for the Detection of Antibodies against Peste des Petits Ruminants Virus Based on BHK-21 Cell Line Stably Expressing the Goat Signaling Lymphocyte Activation Molecule. 2016. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slinkard K, Singleton VL. Total phenol analysis: automation ans comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 32.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 33.Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- 34.Benzie IFF, Strain JJBT-M in E. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Oxidants and Antioxidants Part A: Academic; 1999. p. 15–27. 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the results of this article are available in the figshere (https://figshare.com/s/97bfdb8d693a8c95ffaf).