Abstract
Background
Obesity is a severe public health threat worldwide. Emerging evidence suggests that gut microbiota dysbiosis is closely associated with obesity and its related metabolic complications. The aim of the present study is to investigate the effects of polysaccharide extracted from WuGuChong (PEW) on high-fat diet (HFD)-induced obesity, and the potential mechanisms involving modulation of the gut microbiota composition.
Methods
Mice were fed a normal chow diet and a high-fat diet with or without PEW (300 mg/kg/day) by oral gavage for 8 weeks. Body weight, obesity-related metabolic disorders, and gut microbiota were examined at the end of the experiment.
Results
PEW supplementation reduces body weight, adipose hypertrophy, liver steatosis, insulin resistance and systemic inflammation in HFD-fed mice, as well as maintains intestinal epithelium integrity. High-throughput 16S rRNA analysis demonstrates that PEW supplementation alters the composition of gut microbiota. The Firmicutes to Bacteroidetes ratio and the relative abundance of Proteobacteria are increased in HFD-fed mice, which are reversed by PEW supplementation to approximately the control levels.
Conclusions
Our results suggest that PEW may be used as a bioactive ingredient to prevent obesity and its related metabolic disorders by modulating the composition of gut microbiota.
Keywords: Gut microbiota, High-fat diet, Obesity, Polysaccharides, WuGuChong
Background
Obesity is a global public health issue, and the major causes of obesity are considered to be unhealthy dietary patterns and lifestyles [1, 2]. Accumulating evidence suggests that obesity, which is closely related to chronic systemic inflammation and insulin resistance, is a risk factor for cancers and some other chronic diseases such as diabetes, atherosclerosis, fatty liver diseases [3–5]. High prevalence of obesity causes great damage to public health, however, many obesity-related therapeutic approaches, such as lifestyle changes, bariatric surgery, and pharmacotherapy, lead to an increased risk of various chronic diseases [6]. Finding novel therapeutic strategies is urgently necessary and challenging for prevention of obesity.
Increasing data indicates that gut microbiota composition is associated with obesity and its related metabolic disorders [7]. The gut microbiota composition varies significantly among obese and lean people [8]. For instance, an increase in the Firmicutes/Bacteroidetes (F/B) ratio can promote the development of obesity [9, 10]. Germ-free mice with gut microbiota transplanted from high fat diet (HFD)-induced obese donors exhibited significantly increased body weight gain and metabolic syndromes [11]. In HFD-induced obese models, gut microbiota is also closely linked with intestinal permeability, which is associated with gut integrity and barrier function [12]. Meanwhile, gut microbiota plays an important role in nutrient acquisition, energy harvest and lipid metabolism, which are related to metabolic diseases [7, 13, 14]. Endotoxins, bile acids and short-chain fatty acids, produced by gut microbiota, link gut microbiota to metabolic health [15–18]. Therefore, modulating the composition of gut microbiota may be a potential new strategy for preventing obesity and its related metabolic disorders.
Recently, accumulating evidence has demonstrated that some bioactive ingredients, especially polysaccharides, can reduce obesity by modulating the composition of the gut microbiota [9, 19]. For example, supplementation with polysaccharides isolated from sea cucumber or Ganoderma lucidum, reduced body weight gain and attenuated obesity-related metabolic syndromes in association with modulation of gut microbiota in HFD-fed mice [9, 19]. These researches indicate that polysaccharides have beneficial effects on metabolism and gut microbiota.
WuGuChong, a traditional Chinese medicine with function of improving digestion disorders, has been used to treat infantile malnutrition and fulminant dysentery, as reported in traditional Chinese medical journals such as Compendium of Materia Medica and Materia Medica Companion [20]. Recently, extracts from WuGuChong have been demonstrated to have antibacterial effects, promote wound healing, and inhibit the progression of diabetes and atherosclerosis [21–23]. However, little is known about the effects of WuGuChong on gut microbiota, obesity and its related complications. In the present study, the effects of polysaccharide extracted from WuGuChong (PEW) on HFD-induced obesity and obesity-related liver steatosis, insulin resistance and systemic inflammation, as well as gut integrity were evaluated. Through the gut microbiota analysis, the results indicated that PEW had a beneficial regulation on the composition of gut microbiota, suggesting a potential mechanism by which PEW attenuated obesity and its related metabolic syndromes. Our study demonstrates that PEW may be a potential bioactive ingredient for preventing obesity.
Methods
PEW preparation
The dried bodies of WuGuChong were purchased in a traditional Chinese medicine market and identified according to the standards in “Chinese Materia Medica Standards”. The PEW was isolated according to water extraction and alcohol precipitation methods as previous reported [24]. The Sevage method and DEAE iron-exchanged chromatography method were used for deproteinization and purification of polysaccharides. The PEW was characterized by GPC (gel permeation chromatogram) and HPLC (high performance liquid chromatography). The molecular weight of PEW was determined at 32.9 kDa, and its monosaccharide composition included rhamnose, glucose, mannose, galactose, arabinose, xylose, glucuronic acid, and galacturonic acid.
Animal experiments
Eight-week old C57BL/6 male mice were obtained from the Experimental Animal Center in Dalian Medical University in China. After 1 week of acclimating to the new surroundings, the mice were randomly divided into three groups (n = 9 per group): The NCD (normal chow diet) group was conventionally fed a normal chow diet (3.656 kcal/g) containing 13.8% kcal fat (Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd. China). The HFD group was fed a high fat diet (4.73 kcal/g) containing 45% kcal fat (D12451, Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd. China). The PEW treatment group (HFD + PEW) was fed a high fat diet (D12451) and given PEW (300 mg/kg) by oral gavage once a day. The other two groups also had oral gavage with water once a day. The experimental treatment continued for 8 weeks. The mice were maintained in a light and climate controlled room at 25 ± 2 °C and 12 h light-dark cycle, with free access to water and different diets. Body weights and food intake were measured once a week. Liver and epididymal white adipose tissues (epi-WAT) were obtained and weighed immediately after treatment. The animal experimental protocols were approved by the ethics committee of Dalian Medical University (YJ-KY-SB-2019-83).
Histopathological analysis
The epi-WAT tissues were routinely fixed in 4% paraformaldehyde, processed for hematoxylin and eosin (HE) staining, and examined using a microscope (Tokyo Olympus, Japan). The liver tissues were analyzed with Oil Red O staining according to the protocol described by Chang et al. [9].
Biochemical analysis
Mice were fasted overnight after treatment, and blood samples were collected through the orbital sinus. The levels of total cholesterol (CHO), high and low density lipoprotein cholesterol (HDL-C and LDL-C), and triacylglycerol (TG) in the blood serum were measured using kits purchased from Nanjing Jiancheng Bioengineering Institute. Cytokine levels, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and lipopolysaccharide (LPS), were analyzed using ELISA kits (Shanghai Lengton Biological Technology Co., Ltd. China).
Glucose and insulin tolerance analysis
The intraperitoneal glucose and insulin tolerance tests (IPGTT and ITT) were conducted at weeks 7 and 8, respectively. In brief, mice were either fasted overnight or for 6 h, and then received an intraperitoneal injection of single-dose D-glucose (1.0 g/kg) or insulin (0.75 UI/kg; Novo Nordisk). Blood from the caudal vein was collected at 0, 15, 30, 60, and 120 min, respectively. A glucometer (Yuwell, Jiangsu, China) was used to measure blood glucose levels. Concentrations of fasting blood glucose and fasting serum insulin were measured, and the homeostasis model index of insulin resistance (HOMA-IR) was calculated according to the formula: HOMA-IR = insulin×glucose/22.5, as previously [25].
Western blot analysis
Protein samples were extracted from the ileum epithelial tissue using RIPA lysis buffer, and then quantified using a BCA protein quantification kit (KeyGEN BioTECH, China). Proteins were loaded onto an 8% SDS-PAGE gel for electrophoresis, and then transferred onto PVDF membranes (Millipore, USA). After blocking with 5% skimmed milk, membranes were incubated with primary antibodies against ZO-1 (Zonula occludin-1, 1:1000; Proteintech), occludin (1:2000; Proteintech), and GAPDH (1:2000; Proteintech) over night at 4 °C, and then with secondary antibody for 2 h. Images were taken using ChemiDoc™ MP imaging system (Bio-Rad, America).
Gene transcription analysis
Total RNA from ileum epithelial tissues was extracted using the TRIzol reagent (Invitrogen, USA) and reverse transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo, USA). Real-time qPCR was conducted using the ABI 7500 Real-time PCR System (Applied Biosystems, USA). Values were normalized against β-actin and analyzed with the 2−∆∆CT method. The real-time qPCR primers are listed in Table S1.
Intestinal microbiota analysis
Fresh stool samples obtained from the colon immediately upon sacrificing the mice were frozen in liquid nitrogen and stored at − 80 °C. A stool DNA Kit (D4015, Omega, USA) was used to isolate the fecal genomic DNA. The V3-V4 region of 16S rRNA (338F-806R, F: ACTCCTACGGGAGGCAGCAG; R: GGACTACHVGGGTWTCTAAT) was amplified for identification. The DNA template was initially denatured at 98 °C for 30 s, then amplified for 35 cycles of denaturation (98 °C for 10 s), annealing (52 °C for 30 s), and extension (72 °C for 45 s), and finally extended at 72 °C for 10 min. The PCR products were purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA) and quantified by Qubit (Invitrogen, USA). The amplicon pools were prepared for sequencing and the size and quantity of the amplicon library were assessed on Agilent 2100 Bioanalyzer (Agilent, USA) and with the Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA), respectively. The PCR products were sequenced on an Illumina MiSeq platform (LC-Bio Technology Co., Ltd., Hang Zhou, China). Paired-end reads was assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Paired-end reads were merged using FLASH [26]. Quality filtering on the raw tags were performed under specific filtering conditions to obtain the high-quality clean tags according to the fqtrim (V 0.94). Chimeric sequences were filtered using Vsearch software (v2.3.4). Sequences with≥97% similarity were assigned to the same operational taxonomic units (OTUs) by Vsearch (v2.3.4) [27]. Representative sequences were chosen for each OTU, and taxonomic data were then assigned to each representative sequence using the RDP (Ribosomal Database Project) classifier. The differences of the dominant species in different groups, multiple sequence alignment were conducted using the mafft software (V 7.310) to study phylogenetic relationship of different OTUs. OTUs abundance information were normalized using a standard of sequence number corresponding to the sample with the least sequences. Alpha diversity is applied in analyzing complexity of species diversity for a sample through 5 indices, including Chao1, Observed species, Goods coverage, Shannon, and Simpson. All the indices in the samples were calculated with QIIME (Version 1.8.0). Beta diversity analysis was used to evaluate differences of samples in species complexity. For beta-diversity analysis, principal coordinate analysis (PCoA) and unweighted pair group method with arithmetic mean (UPGMA) clustering were performed by QIIME software (Version 1.8.0) [28]. The linear discriminant analysis (LDA) effect size (LEfSe) analysis was conducted for quantitative analysis of biomarkers in different groups [29]. Briefly, Kruskal-Wallis rank-sum test and Wilcoxon rank rank-sum test were used for LEfSe analysis (P < 0.05; LDA > 3.0), to identify the most differently abundant taxa.
Statistical analysis
All data are presented as means±SD. The SPSS statistics 23.0 software (IBM, USA) was used for statistical analysis. Differences across groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison tests. Differences were considered as statistically significant if P < 0.05.
Results
PEW reduces body weight in HFD-treated mice
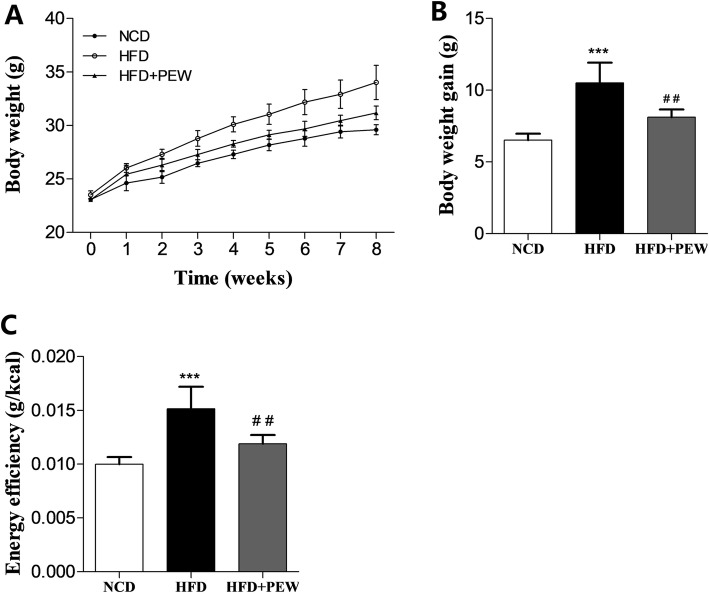
To determine the influence of PEW on body weight in HFD-treated mice, eight-week old C57BL/6 male mice were divided into three groups: NCD group, HFD group, HFD + PEW group. As expected, the HFD group exhibited increased body weight gain compared to the NCD group, which was reduced by PEW supplementation (Fig. 1a-b). Compared with the NCD group, the energy efficiency (weight gain divided by energy intake) in the HFD group was remarkably increased. Supplementation with PEW significantly decreased the energy efficiency in the HFD-fed mice (Fig. 1c), suggesting lower weight gain per kcal energy intake.
Fig. 1.
Effects of PEW on body weight (n = 5 for each group). a Body weight versus time analysis; b Body weight gain; c Energy efficiency, calculated according to the formula: body weight gain/energy intake. Data are presented as means±SD, and analyzed using the one-way ANOVA test with Tukey method. ***P < 0.001 compared with NCD; ##P < 0.05 compared with HFD
PEW inhibits liver steatosis and adipose hypertrophy in HFD-treated mice
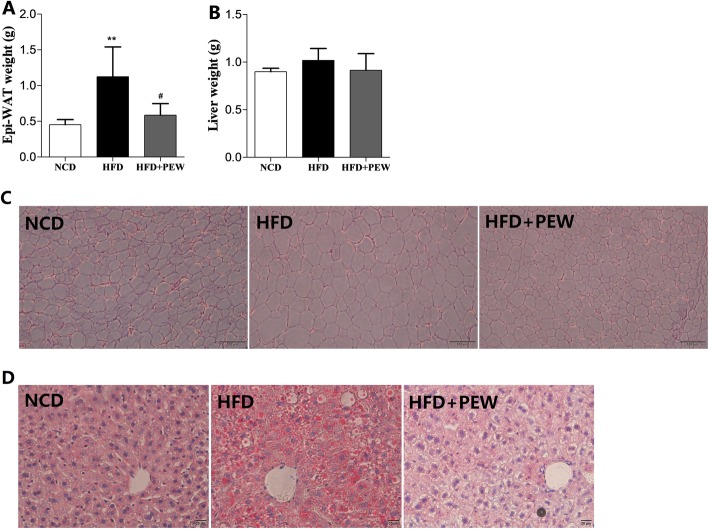
Epi-WAT and liver weights were first measured to evaluate the effect of PEW on tissue changes. The epi-WAT weight was significantly induced by the HFD and reversed by PEW supplementation, while liver weight was not affected by HFD and PEW supplementation (Fig. 2a-b). Moreover, the HFD group exhibited larger adipocyte cells compared to the NCD group and PEW supplementation group (Fig. 2c), demonstrating that PEW prevents HFD-induced adipose hypertrophy. Compared to the NCD and PEW supplementation groups, fat accumulation in the liver was increased in the HFD group as indicated by Oil Red O staining (Fig. 2d), suggesting that PEW might attenuate liver steatosis.
Fig. 2.
Effects of PEW on adipose hypertrophy and liver steatosis (n = 5 for each group). a epi-WAT weight; b Liver tissue weight. c HE staining of epi-WAT tissues (scale bar = 100 μm); d Oil red O staining of liver tissues (scale bar = 20 μm). Data are presented as means±SD, and analyzed using the one-way ANOVA test with Tukey method; **P < 0.01 compared with NCD; #P < 0.05 compared with HFD
PEW attenuates serum lipids, cholesterol levels, insulin resistance, and glucose tolerance
To assess the ability of PEW to attenuate hypercholesterolemia and hyperlipidemia, we measured serum lipid concentrations in mice. The HFD group had a significant increase in TG, CHO, HDL-C, and LDL-C concentrations (48.2, 89.2, 86.9, and 270.8%, respectively) compared to the NCD group (Fig. 3a-d). Supplementation with PEW significantly decreased the CHO, HDL-C, and LDL-C concentrations (− 22.7, − 23.0, and − 35.2%, respectively) in the HFD-fed mice. However, the TG concentration was not significantly affected by PEW supplementation. These results suggested that PEW might attenuate hypercholesterolemia and hyperlipidemia in HFD-fed mice.
Fig. 3.
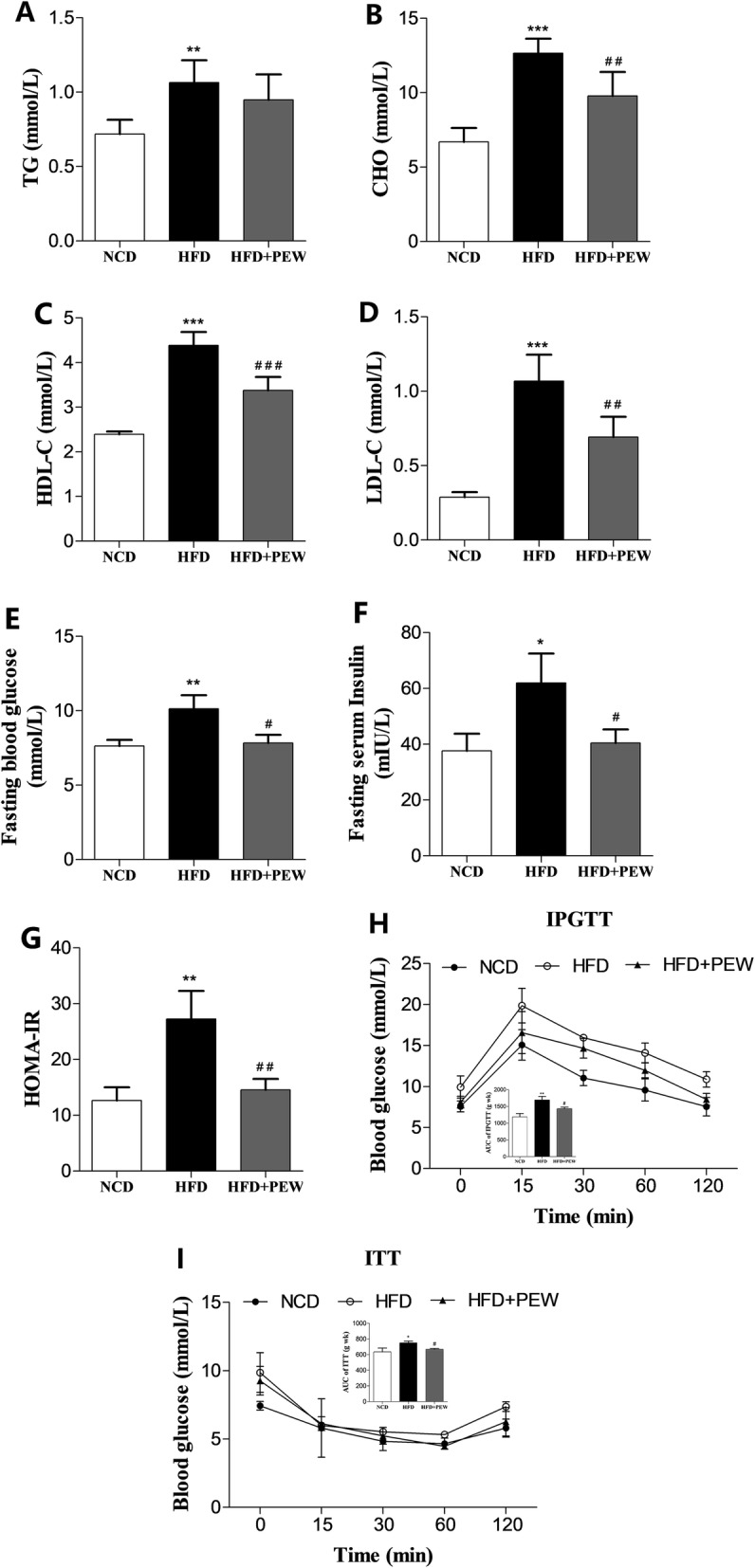
Effects of PEW on lipid concentration, glucose tolerance, and insulin resistance (n = 5 for each group). a-d TG (triacylglycerol), CHO (cholesterol), HDL-C (high density lipoprotein cholesterol), and LDL-C (low density lipoprotein cholesterol) levels in NCD, HFD, and HFD + PEW groups; e-g Fasting blood glucose, fasting serum insulin and HOMA-IR, calculated according to the formula: HOMA-IR = insulin×glucose/22.5. h-i Blood glucose versus time after intraperitoneal injection of D-glucose or insulin. The calculated AUC (inset) are also shown. Data are presented as means±SD, and analyzed using the one-way ANOVA test with Tukey method. *P < 0.05, **P < 0.01, ***P < 0.001 compared with NCD; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with HFD
As obesity is closely correlated with insulin resistance and glucose tolerance [30], fasting blood glucose and fasting serum insulin was measured, and HOMA-IR was calculated according to the established formula. In addition, IPGTT and ITT tests were also performed in the present study. Supplementation with PEW maintained insulin sensitivity in HFD-fed mice, and improved fasting blood glucose, fasting serum insulin and HOMA-IR, which were closed to the control levels (Fig. 3e-g). As shown in Fig. 3h-i, compared with the NCD or PEW supplementation groups, the HFD group exhibited higher IPGTT, ITT, and AUC (area under curve) values, further suggesting that PEW supplementation significantly decreased the induced glucose tolerance and insulin resistance in HFD-treated mice.
PEW prevents HFD-induced systemic inflammation
It was previously reported that obesity and liver steatosis are accompanied by increased levels of serum endotoxins (LPS) and inflammatory cytokines [9, 31]. The effects of PEW on LPS release and cytokines secretion were examined in this study. As expected, compared with the NCD group, the HFD group displayed a significant increase in serum LPS, TNF-α, and IL-6 levels, which was reversed by PEW supplementation (Fig. 4a-c), suggesting that PEW might prevent HFD-induced endotoxemia and systemic inflammation.
Fig. 4.
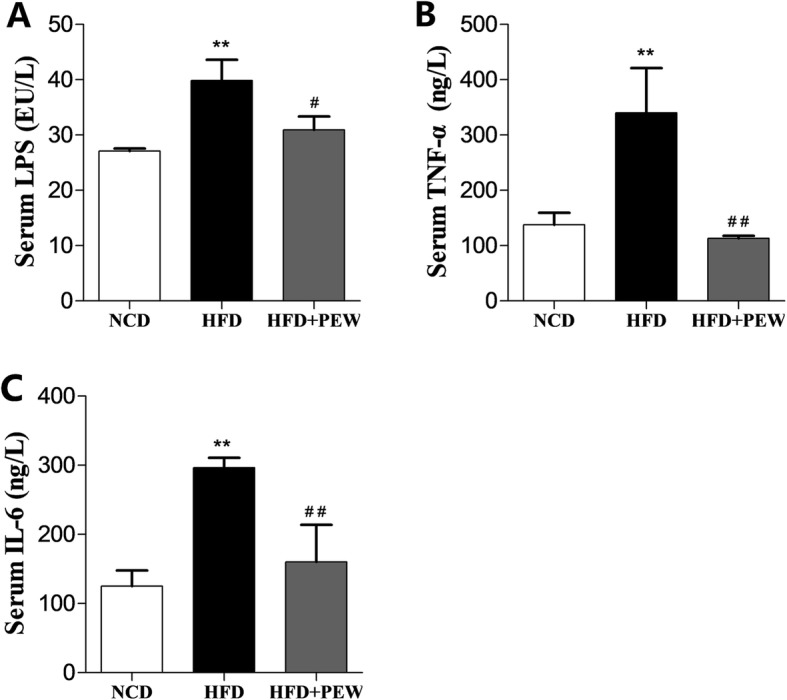
Effects of PEW on systemic inflammation (n = 5 for each group). a Serum level of endotoxins; b Serum level of TNF-α; c Serum level of IL-6. Data are presented as means±SD, and analyzed using the one-way ANOVA test with Tukey method. **P < 0.01 compared with NCD; #P < 0.05, ##P < 0.01 compared with HFD
PEW maintains intestinal epithelium integrity
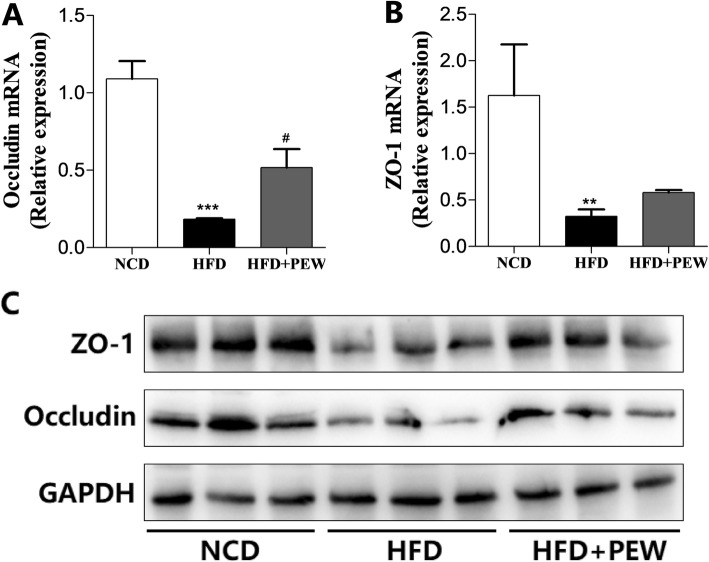
The effects of PEW on the integrity of the gut epithelium were evaluated by analyzing the expression of gut integrity biomarkers occludin and ZO-1 [32]. HFD strongly suppressed occludin and ZO-1 mRNA levels as identified by RT-PCR analysis (Fig. 5a-b). PEW supplementation significantly increased occludin levels but not ZO-1 in HFD-fed mice. However, the reduced occludin and ZO-1 protein expression in HFD-fed mice was recovered by PEW supplementation as shown by Western blot analysis (Fig. 5c). The results suggested that PEW supplementation maintained the gut integrity and barrier function.
Fig. 5.
Relative expression of occludin and ZO-1 (n = 3 for each group). a-b RT-qPCR analysis of the relative mRNA levels of occludin and ZO-1; c Western blot analysis of occludin and ZO-1. Data are presented as means±SD, and analyzed using the one-way ANOVA test with Tukey method. **P < 0.01, ***P < 0.001 compared with NCD; #P < 0.05 compared with HFD
PEW alters the intestinal microbiota composition in HFD-fed mice
The intestinal microbiota composition was determined by sequencing the 16S rRNA (V3 + V4 region) using the Illumina MiSeq platform. Based on 97% identity level, OTU averages of 2511, 3561, and 3478 were respectively clustered in the NCD, HFD, and HFD + PEW groups. As shown in Fig. S1A, the OTUs in the HFD group were higher compared to the NCD group, while PEW supplementation did not significantly decrease the OTUs. The curves of OTU rank, Chao 1, Shannon, and Simpson are presented in Fig. S1B, C, D, and E, respectively. The indexes of Chao 1 (Fig. S1F), Shannon (Fig. S1G), and Simpson (Fig. S1H) were calculated. The curves of OTU rank and Chao 1 were consistent with the number of OTUs. No significant difference was found for Shannon and Simpson indexes, suggesting that there was no difference in richness and diversity of intestinal microbiota between the three groups.
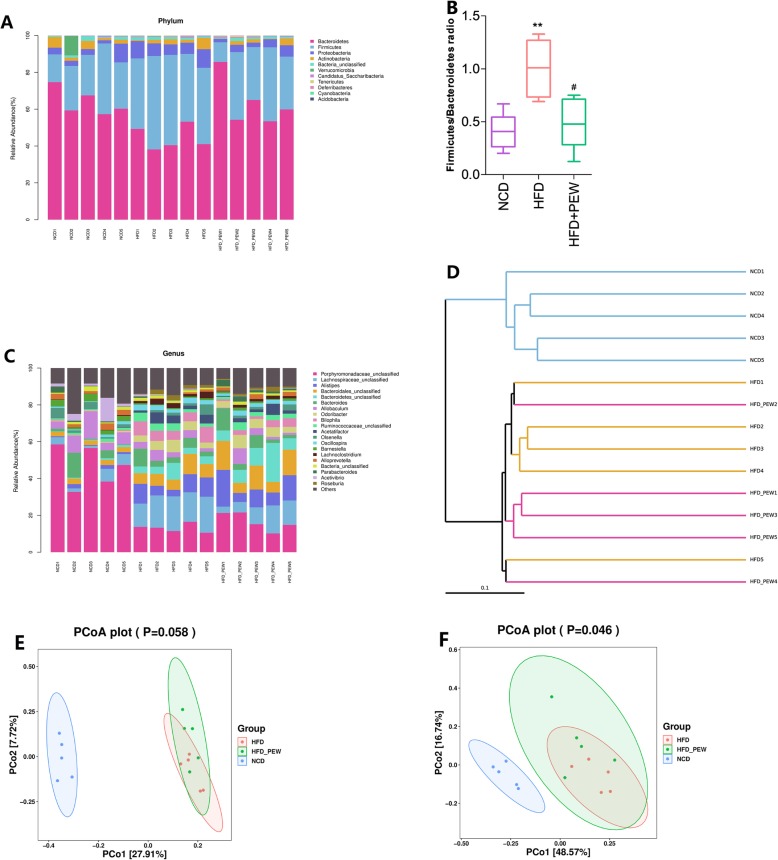
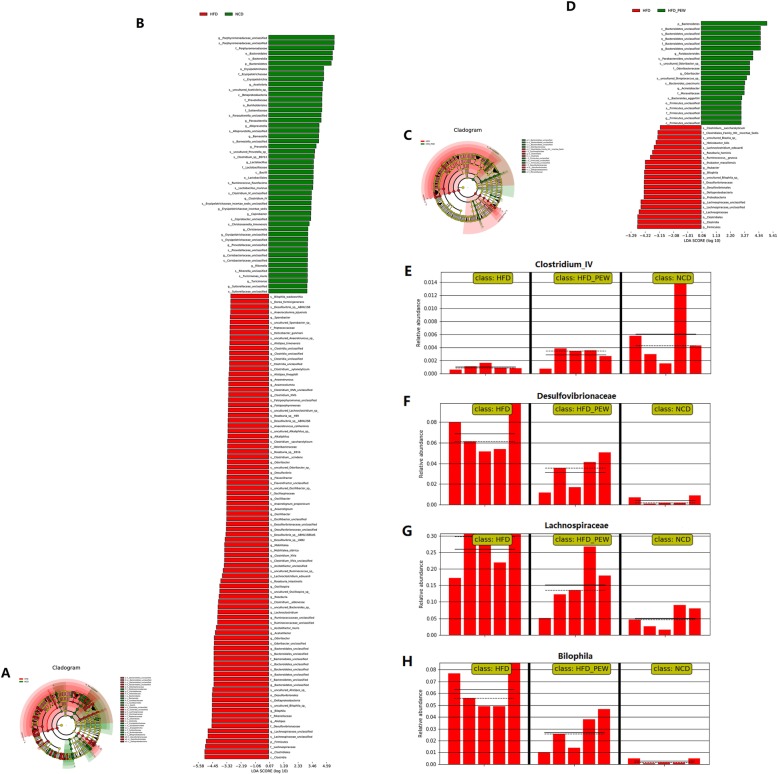
As shown in Fig. 6a, the relative decreased abundance of Bacteroidetes and increased abundance of Firmicutes and Proteobacteria were observed in the HFD group in the phylum level, and the F/B ratio was also increased in the HFD group compared to the NCD group. PEW supplementation restored the levels of Bacteroidetes, Firmicutes, and Proteobacteria, and significantly decreased the F/B ratio (Fig. 6b). The difference of genus level among each group was further investigated, and the results were consistent with the phylum level. PEW supplementation significantly increased the relative abundance of Parabacteroides belonging to the Bacteroidetes phylum, and significantly decreased the relative abundance of Ihubacter, Lachnospiraceae_unclassified, and Lachnostridium belonging to the Firmicutes phylum, as well as Bilophila belonging to the Proteobacteria phylum in HFD-treated mice (Fig. 6c). In addition, unweighted unifrac cluster based UPGMA and the unweighted and weighted unifrac distance based PCoA were performed to investigate gut microbiota structural changes. As shown in Fig. 6d-f, all three groups exhibited distinctive microbiota profiles. Furthermore, the HFD + PEW group and HFD group showed a similar microbiota structure.
Fig. 6.
The variation of gut microbiota at phylum and genus levels (n = 5 for each group). a Relative abundance of bacteria at the phylum level; b The Firmicutes to Bacteroidetes ratio in NCD, HFD, and HFD + PEW groups; c Relative abundance of bacteria at the genus level; d Unweighted UPGMA of all samples; e-f Plots of unweighted and weighted UniFrac-based PCoA. Data are presented as means±SD, and analyzed using the one-way ANOVA test with Tukey method. **P < 0.01 compared with NCD; #P < 0.05 compared with HFD
The biomarkers in the gut microbiota sequences were analyzed using the LEfSe method. Compared with the HFD group, the successive circles and bar graph showed that 50 phylotypes were higher and 90 were lower in the NCD group, and 21 phylotypes were higher and 21 were lower in the HFD + PEW group (Fig. 7a-d). The results indicate that PEW supplementation significantly increased the abundance of beneficial phylotypes Bacteroidetes, and decreased the abundance of pathogenic phylotypes Firmicutes and Proteobacteria in HFD-treated mice. As shown in Fig. 7e, PEW supplementation induced an increase in Clostridium_IV, which had a potential probiotic effect [33]. The abundance of pathogenic bacteria, including Desulfovibrionaceae, Lachnospiraceae, and Bilophila [34–36], were reduced after PEW supplementation in HFD-treated mice (Fig. 7f-h). These results indicated that PEW supplementation might prevent HFD-induced intestinal microbiota dysbiosis.
Fig. 7.
LEfSe results. a-b Cladogram showing the phylogenetic relationships of bacteria taxa and LDA scores between HFD and NCD group; c-d Cladogram showing the phylogenetic relationships of bacteria taxa and LDA scores between HFD and HFD + PEW group; e-h Relative abundance of Clostridium_IV, Desulfovibrionaceae, Lachnospiraceae, and Bilophila in gut microbiota, respectively
Discussion
Gut microbiota is closely associated with obesity and its related metabolic disorders [7]. Recent research data show that polysaccharides extracted from natural products prevent HFD-induced obesity and its related complications by modulating the composition of gut microbiota [9, 19]. However, the effects of PEW on obesity and gut microbiota has not been investigated. In the study, we investigated the effects of PEW supplementation for 8 weeks on HFD-induced obesity and gut microbiota. The results demonstrate that PEW could prevent HFD-induced obesity, and its related liver steatosis, insulin resistance and systemic inflammation by modulating the composition of gut microbiota. In addition, PEW supplementation could also maintain the intestinal epithelium integrity.
In the present study, PEW supplementation significantly prevents body weight gain, adipose hypertrophy in HFD-fed mice (Fig. 1a-b and Fig. 2a, c). As previously reported, the composition of gut microbiota in obese and lean individuals was significantly different [8]. In the gut microbiota of obese models, increased abundances of Firmicutes and decreased abundances of Bacteroidetes were observed [9, 37]. Our results show that HFD increases the abundance of Firmicutes and decreases the abundance of Bacteroidetes. Consistent with the Chang’s research [9], PEW supplementation significantly reverses the changes, and decreases the increased F/B ratio in HFD-fed mice to approximately the control levels (Fig. 6a-b), which may contribute to its anti-obesity effects. Additionally, at the phylum level, endotoxin-bearing Proteobacteria is also decreased by PEW supplementation. Obesity is often accompanied by fat accumulation in the viscera, especially in liver, and closely associated with chronic systemic inflammation, which may induce many chronic diseases [38]. As expected, PEW supplementation attenuates liver steatosis induced by HFD in the study (Fig. 2d). Previous evidence indicated that significantly increased inflammatory cytokine TNF-α and IL-6 levels were observed in obese individuals [39]. The pro-inflammatory molecules can affect the host metabolic process, as TNF-α was reported to decrease the insulin sensitivity and increase lipolysis in adipocytes, as well as IL-6 was found to contribute to hypertriglyceridemia [40, 41]. In our study, serum levels of TNF-α and IL-6, lipids, cholesterol, glucose and insulin increased significantly in HFD group (Fig. 3 and Fig. 4b-c). However, PEW supplementation significantly reduces systemic inflammation, insulin resistance, hypercholesterolemia and hyperlipidemia in HFD-fed mice. The results suggest that PEW improves obesity-related metabolic disorders by modulating systemic inflammation.
Accumulating evidence suggests that gut microbiota is closely linked to host systemic inflammation, which is a feature of obesity [39, 42]. Significantly increased inflammatory cytokine TNF-α and IL-6, as well as LPS, all markers of systemic inflammation, were observed in obese models as previous report [43], and decreased by PEW supplementation (Fig. 4a-c). Gut microbiota dysbiosis, which contributes to LPS production, is the potential mechanism for systemic inflammation induced by HFD. Endotoxins, known as LPS, which induced the release of pro-inflammatory cytokine, were predominantly derived from Gram-negative bacteria in intestinal microbiota [44, 45]. In the present study, the relative abundance of endotoxin-producing bacteria Desulfovibrionaceae is increased in HFD group, and reversed by PEW supplementation (Fig. 7f). The results are in agreement with previous research, which demonstrated that the relative abundance of Desulfovibrionaceae was decreased by dietary intervention in obese individuals [34]. The relative abundance of Bilophila has been demonstrated to be positively related to obesity and inflammation [36]. In the present study, the relative abundance of Bilophila is increased by HFD, and restored by PEW supplementation (Fig. 7h), which is consistent with the previous report. Recent research observed that the relative abundance of Lachnospiraceae was increased in high-fat/high-sucrose (HFHS)-fed mice [35]. Here, PEW supplementation significantly decreases the relative abundance of Lachnospiraceae, which is increased by HFD (Fig. 7g). In addition, Clostridium_IV, belonging to Clostridium clusters, which lacks prominent toxins and virulence factors, has potential probiotic effects. Meanwhile, Clostridium_IV was also reported to regulate host fatty acid metabolism, immune function and ameliorated colitis [33]. Our study demonstrates that PEW supplementation enhances the relative abundance of Clostridium_IV in HFD-treated mice (Fig. 7e). The results suggest that the anti-obesity and anti-inflammation effects of PEW may be due to modulation of the gut microbiota composition.
Previous study reported that HFD-induced gut microbiota dysbiosis contributed to the damage of intestinal epithelium integrity, and the release of LPS into circulation, resulting in insulin resistance, systemic inflammation and obesity [9]. Otherwise, gut microbiota could also help strengthen mucosal defense by promoting epithelial renewal and immune maturation, as well as decreasing intestinal permeability [46, 47]. In the study, PEW supplementation increases the expression of occludin and ZO-1, which are decreased by HFD (Fig. 5), resulting in maintaining intestinal epithelium integrity. This is consistent with the previous study, which reported that increased expression of occludin and ZO-1, along with modulation of the gut microbiota composition, contributed to strengthen the gut integrity and barrier function [48]. The results suggest that PEW may improve obesity-related metabolic disorders by modulating gut microbiota and maintaining intestinal epithelium integrity.
In conclusion, our results show that PEW supplementation prevents HFD-induced obesity and its related metabolic disorders, including liver steatosis, insulin resistance and systemic inflammation, by modulating gut microbiota composition and maintaining intestinal epithelium integrity. PEW supplementation decreases Firmicutes to Bacteroidetes ratio and the relative abundance of Proteobacteria in HFD-fed mice, contributing to the beneficial effects against obesity and its related disorders. Our study suggests that PEW may be used as a bioactive ingredient to prevent obesity. However, the detailed mechanisms need to be further investigated.
Supplementary information
Additional file 1: Table S1. Primers used in this study.
Additional file 2: Figure S1. The characteristics of gut microbiota. (A) OTU clusters of gut microbiota; (B-E) OTU rank curves, Chao 1 curves, Shannon curves, and Simpson curves of gut microbiota, respectively; (F-H) Chao 1, Shannon, and Simpson indexes of gut microbiota, respectively. Data are presented as means and standard deviation, and analyzed using the one-way ANOVA test. **P < 0.01 compared with NCD.
Acknowledgements
Not applicable.
Abbreviations
- CHO
Total cholesterol
- epi-WAT
Epididymal white adipose tissues
- GPC
Gel permeation chromatogram
- HE
Hematoxylin and eosin
- HFD
High-fat diet
- IPGTT
Intraperitoneal glucose test
- ITT
Insulin tolerance test
- LDA
Linear discriminant analysis
- NCD
Normal chow diet
- OTU
Operational taxonomic unit
- PEW
Polysaccharide extracted from WuGuChong
- ZO-1
Zonula occludin-1
Authors’ contributions
Junwei Zong and Shouyu Wang contributed to the study’s conception and design. Wendong Wang and Mintao Zhong contributed to drafting of the manuscript. Wendong Wang, Mintao Zhong, Tiantina Yu, Lei Chen and Lijun Shi performed experiments and data collection. Wendong Wang and Mintao Zhong performed data analysis and interpretation. The final draft was read and approved by all the authors.
Funding
This work was supported by National Natural Science Foundation of China (81573734), Liaoning Revitalization Talents Program (XLYC1802014), Liaoning Key Research and Development Planning Project (2017226015), Distinguished Professor Project of Liaoning Province, Liaoning BaiQianWan Talents Program and Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-A04–2014).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This animal study was approved by the ethics committee of Dalian Medical University (YJ-KY-SB-2019-83).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wendong Wang and Mintao Zhong contributed equally to this work.
Contributor Information
Junwei Zong, Email: aweizone@163.com.
Shouyu Wang, Email: wangshouyu666@126.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12986-020-00442-2.
References
- 1.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376:1775–1784. doi: 10.1016/S0140-6736(10)61514-0. [DOI] [PubMed] [Google Scholar]
- 2.Morgen CS, Sorensen TI. Obesity: global trends in the prevalence of overweight and obesity. Nat Rev Endocrinol. 2014;10:513–514. doi: 10.1038/nrendo.2014.124. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit R, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 5.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 6.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:1492. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 9.Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, Lai HC. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr. 2020;11:77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 15.Janssen AW, Kersten S. Potential mediators linking gut bacteria to metabolic health: a critical view. J Physiol. 2017;595:477–487. doi: 10.1113/JP272476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring fxr antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z, Zhu B, Sun Y, Ai C, Wang L, Wen C, Yang J, Song S, Liu X. Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice. Mol Nutr Food Res. 2018;62:e1800446. doi: 10.1002/mnfr.201800446. [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Li W, Niu L, Cao R, Yin W. Isolation and structural elucidation of novel antimicrobial compounds from maggots of chrysomyis megacephala fabricius. Nat Prod Res. 2015;29:239–246. doi: 10.1080/14786419.2014.948875. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Wang J, Zhang B, Liu H, Song W, He J, Lv D, Wang S, Xu X. Activity of antibacterial protein from maggots against staphylococcus aureus in vitro and in vivo. Int J Mol Med. 2013;31:1159–1165. doi: 10.3892/ijmm.2013.1291. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Wang S, Diao Y, Zhang J, Lv D. Fatty acid extracts from lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity. Lipids Health Dis. 2010;9:24. doi: 10.1186/1476-511X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei H, Xu J, He Y, Yang X, Liu W, Tian W, Zeng YU, Zhu J. Protein-rich extract of musca domestica larvae alleviated metabolic disorder in stz-induced type 2 diabetic rat model via hepatoprotective and pancreatic beta-cell protective activities. J Biosci. 2018;43:969–983. doi: 10.1007/s12038-018-9804-z. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Wen JJ, Hu JL, Nie QX, Chen HH, Xiong T, Nie SP, Xie MY. Polysaccharide from fermented momordica charantia l. with lactobacillus plantarum ncu116 ameliorates type 2 diabetes in rats. Carbohydr Polym. 2018;201:624–633. doi: 10.1016/j.carbpol.2018.08.075. [DOI] [PubMed] [Google Scholar]
- 25.Xu P, Hong F, Wang J, Cong Y, Dai S, Wang S, Wang J, Jin X, Wang F, Liu J, Zhai Y. Microbiome remodeling via the montmorillonite adsorption-excretion axis prevents obesity-related metabolic disorders. EBioMedicine. 2017;16:251–261. doi: 10.1016/j.ebiom.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magoc T, Salzberg SL. Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar RC. Search and clustering orders of magnitude faster than blast. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 28.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atamni HJ, Mott R, Soller M, Iraqi FA. High-fat-diet induced development of increased fasting glucose levels and impaired response to intraperitoneal glucose challenge in the collaborative cross mouse genetic reference population. BMC Genet. 2016;17:10. doi: 10.1186/s12863-015-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 34.Xiao S, Fei N, Pang X, Shen J, Wang L, Zhang B, Zhang M, Zhang X, Zhang C, Li M, et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol Ecol. 2014;87:357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016;6:31208. doi: 10.1038/srep31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van- Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, Shen J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 38.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y, Li Y, Du Y, Guo L, Chen M, Huang X, Yang F, Hong J, Kong X. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int J Obes. 2019;43:1631–1643. doi: 10.1038/s41366-018-0187-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular camp. Diabetes. 2002;51:2929–2935. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 41.Nonogaki K, Fuller GM, Fuentes NL, Moser AH, Staprans I, Grunfeld C, Feingold KR. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology. 1995;136:2143–2149. doi: 10.1210/endo.136.5.7720663. [DOI] [PubMed] [Google Scholar]
- 42.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 43.Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, Jia L, Zhai Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62:e12399. [DOI] [PubMed]
- 44.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639–647. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- 45.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–380. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 46.Tomas J, Reygner J, Mayeur C, Ducroc R, Bouet S, Bridonneau C, Cavin JB, Thomas M, Langella P, Cherbuy C. Early colonizing escherichia coli elicits remodeling of rat colonic epithelium shifting toward a new homeostatic state. ISME J. 2015;9:46–58. doi: 10.1038/ismej.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed. 2003;88:F52–F55. doi: 10.1136/fn.88.1.F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo B, Liu B, Wei H, Cheng KW, Chen F. Extract of the microalga nitzschia laevis prevents high-fat-diet-induced obesity in mice by modulating the composition of gut microbiota. Mol Nutr Food Res. 2019;63:e1800808. doi: 10.1002/mnfr.201800808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primers used in this study.
Additional file 2: Figure S1. The characteristics of gut microbiota. (A) OTU clusters of gut microbiota; (B-E) OTU rank curves, Chao 1 curves, Shannon curves, and Simpson curves of gut microbiota, respectively; (F-H) Chao 1, Shannon, and Simpson indexes of gut microbiota, respectively. Data are presented as means and standard deviation, and analyzed using the one-way ANOVA test. **P < 0.01 compared with NCD.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.