Abstract
Background:
Among patients prescribed long-term opioid therapy (LTOT) for chronic pain, no study has yet examined how clinicians respond to evidence of illicit drug use and whether the decision to discontinue opioids is influenced by a patient’s race.
Methods:
Among outpatients of black and white race initiating LTOT through the VA between 2000 and 2010, we reviewed electronic medical records to determine whether opioids were discontinued within 60 days of a positive urine drug test. Logistic regression was used to examine differences by race.
Results:
Among 15,366 patients of black (48.1%) or white (51.9%) race initiating LTOT from 2000–2010, 20.5% (25.5% of blacks vs. 15.8% of whites, P <. 001) received a urine drug test within the first 6 months of treatment; 13.8% tested positive for cannabis and 17.4% for cocaine. LTOT was discontinued in 11.4% of patients who tested positive for cannabis and in 13.1% of those who tested positive for cocaine. Among patients testing positive for cannabis, blacks were 2.1 times more likely than whites to have LTOT discontinued (adjusted odds ratio [AOR] 2.06, 95% confidence interval [CI] 1.04–4.08). Among patients testing positive for cocaine, blacks were 3.3 times more likely than whites to have LTOT discontinued (AOR 3.30, CI 1.28–8.53).
Conclusions:
Among patients testing positive for illicit drug use while receiving LTOT, clinicians are substantially more likely to discontinue opioids when the patient is black. A more universal approach to administering and responding to urine drug testing is urgently needed.
Keywords: Opioid Analgesics, Practice Guideline, Quality of Health Care, Racial Disparities, Urine Drug Testing
1. Introduction
In 2015, there were more than 33,000 overdose deaths in the U.S. attributed to prescription and illicit opioids (Rudd et al., 2016b). Although heroin and illicitly manufactured fentanyl are increasingly driving this evolving epidemic, more than 40% of all opioid overdose deaths in the U.S. are due to prescription opioids (Braden et al., 2008; Centers for Disease Control and Prevention, 2017; Manchikanti et al., 2011; Rudd et al., 2016b; Von Korff et al., 2008).
In recent years, efforts to prevent poisonings from prescription opioids have largely focused on limiting patients’ exposure to these medications by urging clinicians to be more judicious in how opioids are prescribed (Califf et al., 2016; Dart et al., 2015; Edelman et al., 2013; Korff et al., 2008; Volkow et al., 2011). Much less attention has been paid to how well clinicians monitor patients for signs of adverse events, including opioid use disorder, other substance abuse, and overdose, once opioids are initiated (Becker et al., 2013; Dowell et al., 2016; Gaither et al., 2016a; b; Gaither et al., 2014; Starrels et al., 2010).
Urine drug testing has long been considered one of the best tools clinicians have for monitoring patients for opioid misuse (Chou et al., 2009; US Department of Veterans Affairs/Department of Defense, 2003). Opioid prescribing guidelines recommend that clinicians test for illicit drug use in all patients receiving long-term opioid therapy (LTOT) (Chou et al., 2009; Dowell et al., 2016; US Department of Veterans Affairs/Department of Defense, 2003; 2010). Illicit drug use concurrent with LTOT is considered a moderate predictor of opioid misuse and a behavior suggestive of addiction (US Department of Veterans Affairs/Department of Defense, 2010). Experts recommend that clinicians increase the frequency of monitoring in patients testing positive for illicit drug use, and if the behavior continues, clinicians are advised to taper the patient off opioids and to initiate a referral to an addiction specialist if appropriate (Chou et al., 2009). Immediate discontinuation of opioids is recommended for patients who refuse addiction treatment (US Department of Veterans Affairs/Department of Defense, 2010). Clinician compliance with these recommendations has yet to be examined.
Prior research has revealed racial differences with respect to LTOT and accompanied safety monitoring. Blacks with chronic pain are less likely than whites to be prescribed opioids (Burgess et al., 2014). Blacks are also more likely than whites to undergo urine drug testing (Becker et al., 2014; Becker et al., 2011), even though whites—particularly white males—are more likely to misuse opioids and are at higher risk for overdose and death (Rudd et al., 2016a). When faced with evidence of illicit drug use, whether clinicians are more likely to discontinue opioids when a patient is black is unknown.
The purpose of this study, therefore, was to more closely examine urine drug testing in a sample of approximately equal numbers of black and white patients receiving care through the Department of Veterans Affairs (VA). Military veterans have been hit particularly hard by the opioid crisis due to the high prevalence of chronic pain and mental health and substance use disorders within this population (Seal et al., 2012). Use of a VA sample, among whom access to care is guaranteed by charter, also allowed us to control for differences in healthcare coverage, an important issue that often confounds disparities research (Asch et al., 2006).
Our primary outcome was discontinuation of LTOT in patients who tested positive for cannabis or cocaine and to determine whether this response differed based on the race of the patient. We also examined rates of urine drug testing and use of illicit substances in patients overall and by race.
2. Materials and methods
2.1. Study overview
In a large sample of outpatients initiating LTOT through the VA between 2000 and 2010, we examined discontinuation of LTOT following a positive urine drug test in the sample overall and by race. The methods for identifying patients and measuring outcomes have been previously described; we summarize this information here (Gaither et al., 2016a; b; Gaither et al., 2014).
2.2. Data source
From the electronic medical record, we abstracted administrative, clinical, laboratory, and pharmacy data on patients participating in the Veterans Aging Cohort Study (VACS), a prospective cohort of HIV+ patients matched (1:2) by age, sex, race (via self-report), and VA site-of-care to HIV− controls. The VACS is HIPAA compliant and has been approved by the institutional review boards for the VA Connecticut Healthcare System and the Yale School of Medicine. The requirement for informed consent was waived for this study.
2.3. Study population
From the VA Pharmacy Benefits Management (PBM) database, we captured outpatient prescriptions for oral and transdermal opioids filled between 2000 and 2010. LTOT was defined as receipt of at least a 3-month supply of opioids prescribed for chronic pain but not for an opioid use disorder. To focus on incident versus prevalent cases, we excluded from this cohort patients filling opioid prescriptions within the prior 3 months. We also excluded patients with less than 3 months of follow-up and those who had a baseline ICD-9-CM (International Classification of Disease Clinical Modification) code for palliative or end-of-life care. Further details regarding the specific diagnoses we used to define chronic pain and the specific opioids we examined are available elsewhere (Edelman et al., 2013; Gaither et al., 2016a).
There were 17,044 patients initiating LTOT between 2000 and 2010. We limited the analytic sample to those of black and white race (n=15,366); prior research suggests that it is between these two groups that disparities in patient care are most pronounced (Abdus et al., 2015; Asch et al., 2006; Becker et al., 2014; Becker et al., 2011; Burgess et al., 2014).
2.4. Outcome variables
We examined the first urine drug test received by patients during the initial 6 months of LTOT. Our primary outcome was discontinuation of LTOT following a positive test for cannabis or cocaine. To assess for discontinuation, we determined whether a patient received an opioid prescription within 60 days of a positive drug test; we considered non-receipt within this time period to be discontinuation.
In accordance with recommendations from opioid prescribing guidelines, we also assessed whether patients were administered a urine drug test within 1, 3, and 6 month(s) of starting LTOT (Chou et al., 2009; US Department of Veterans Affairs/Department of Defense, 2003; 2010).
Finally, we examined by race the proportion of patients testing positive for cannabis or cocaine, the two substances in the standard urine drug test that are by definition illicit, during the first 6 months of starting LTOT. While taking unprescribed controlled substances is also of concern, we did not examine amphetamine, benzodiazepine, barbiturate, or opioid use because in our administrative data there was no valid way of ascertaining whether these medications were being legitimately prescribed outside the VA. Similarly, we did not examine absence of prescribed opioids in urine drug test results, a potential indicator of diversion, because an accurate assessment of absence requires data that are not typically obtained in clinical settings (e.g., quantification of a qualitative enzyme-mediated immunosorbent assay result or performance of higher sensitivity gas chromatography/mass spectrometry test). Finally, because heroin rapidly metabolizes to other opiates that appear in a screening test as “opiates,” there is no way to accurately identify heroin use in administrative data.
2.5. Covariates
To characterize the sample at baseline, we extracted demographic data from the VA National Patient Care Database. Clinical characteristics were based on ICD-9-CM codes and laboratory data. The VACS Index was used to reflect overall severity of illness—higher scores are indicative of greater all-cause mortality risk in both HIV+ and HIV− patients; extensive details on the development and use of the VACS Index have been published previously (Justice et al., 2010; Justice et al., 2013; Tate et al., 2013). Variables related to opioid dose, duration, and DEA (Drug Enforcement Administration) controlled substance schedule were obtained from the PBM database.
The diagnosis of a substance use disorder (SUD) was based on ICD-9-CM codes for an alcohol or drug use disorder, receipt of inpatient or outpatient SUD treatment, or scores ≥ 4 on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption). Patients were considered to have a SUD history if they met any of the above criteria at baseline (i.e., LTOT initiation) or any time prior (i.e., lifetime prevalence).
2.6. Statistical analyses
For demographic and clinical characteristics, we used proportions and means to characterize the sample at baseline. Differences according to race were assessed with χ2-tests for categorical variables and, as appropriate, t, ANOVA, or Wilcoxon rank sum tests for continuous variables.
To assess for receipt of urine drug testing and discontinuation of LTOT by race, we used unadjusted and multivariable logistic regression. In the multivariable models, we controlled for patient characteristics listed in Table 1 that were associated with both race and the outcome of interest at P=.20 (Skelly et al., 2012). Specifically, the full model included the following: age, HIV status, hepatitis C status, diabetes, serious mental illness, substance use disorder, VACS Index, and benzodiazepine co-prescriptions. We then used backward elimination to develop the parsimonious models shown in Tables 1 and 2. Regardless of statistical significance, however, we retained in the models variables we determined a priori to be of clinical significance including SUD history, serious mental illness, and HIV status. HIV status is relevant because opioid prescribing and monitoring practices may differ between HIV-specialty providers (who treat the majority of VA patients with HIV) and general-medical practitioners (Starrels et al., 2016).
Table 1.
Patient characteristics by race at initiation of long-term opioid therapy
| Characteristic | White (n=7,976) | Black (n=7,390) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 50.0 (9.8) | 50.3 (8.7) | .03* |
| Male Sex, n (%) | 7828 (98.1) | 7151 (97.8) | < .001* |
| HIV, n (%) | 2388 (29.9) | 2369 (32.1) | .005 |
| Hepatitis C, n (%) | 1835 (23.0) | 2536 (34.3) | < .001 |
| Diabetes, n (%) | 2194 (27.5) | 2371 (32.1) | < .001 |
| BMI, mean (SD) | 28.6 (6.5) | 28.1 (6.4) | < .001* |
| Smoking Status, n (%) | < .001 | ||
| Never | 1506 (19.9) | 1537 (21.5) | |
| Current | 4823 (63.6) | 4619 (64.7) | |
| Former | 1257 (16.6) | 980 (13.7) | |
| Serious Mental Illness, n (%) | 1639 (20.6) | 1513 (20.5) | .91 |
| Substance Use Disorder History, n (%) | 2301 (28.9) | 3209 (43.4) | < .001 |
| VACS Index, median (IQR) | 17 .0 (10.0–30.0) | 22 .0 (12.0–39.0) | < .001 |
| Average Daily Opioid Dose, median (IQR), mg MEQa | 16.5 (9.7–34.4) | 15.4 (9.4–28.6) | .006 |
| Short-Acting Schedule II Opioids, n (%) | 2706 (33.9) | 2492 (33.7) | .79 |
| Concurrent Benzodiazepine Receipt, n (%) | 2188 (27.4) | 992 (13.4) | < .001 |
| CD4 Count, median (IQR), cells/μLb | 384.0 (212.0–583.0) | 332.0 (161.0–545.0) | < .001 |
| HIV-1 RNA, Log10 Viral Load, < 500 copies/ml, n (%)b | 1052 (63) | 913 (53) | < .001 |
Abbreviations: BMI, body mass index; IQR, interquartile range; PTSD, post-traumatic stress disorder; MEQ, morphine equivalent; VACS, Veterans Aging Cohort Study.
Note: Although statistically significant, the actual clinical differences are very small.
Average daily opioid dose calculated by dividing the total morphine equivalents received in the year since starting long-term opioid by total days supply.
Among HIV-infected patients.
Table 2.
Final multivariable-adjusted logistic regression model examining discontinuation of long-term opioid therapy by race among patients testing positive for cannabis
| Characteristic | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Race | ||
| White (Reference) | — | — |
| Black | 1.98 (1.01–3.86) | 2.06 (1.04–4.08) |
| Age, y | — | 0.94 (0.91–0.98) |
| HIV | — | 1.56 (0.81–3.00) |
| Substance Use Disorder History | — | 0.69 (0.36–1.33) |
| Serious Mental Illness | — | 0.94 (0.44–1.99) |
Age, BMI, VACS Index, average daily opioid dose, and CD4 were continuous variables. Smoking status and HIV-1 RNA viral load were coded as categorical variables. All other variables, including discontinuation of long-term opioid therapy, were coded as binary variables.
All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC). We applied a 2-sided statistical significance level of 0.05 to all analyses.
3. Results
3.1. Characteristics of the study cohort
We identified 15,366 patients of white or black race initiating LTOT between 2000 and 2010. The sample was predominately male (97.5%) with a mean (SD) age of 50.1 (9.3) years. Thirty-one percent were infected with HIV and 28.5% with hepatitis C. More than half (64.1%) were current smokers. The mean (SD) BMI was 28.4 (6.4). The proportion of patients with a serious mental illness or history of SUD was 20.5% and 35.9%, respectively.
As shown in Table 1, blacks were more likely than whites to have a diagnosis of HIV, hepatitis C, diabetes, serious mental illness, or an SUD. Blacks were also more likely to be engaged with mental health services or SUD treatment.
Whites were more likely than blacks to receive a higher average daily dose of opioids and a greater days’ supply. Whites were also more than three times more likely to receive benzodiazepines concurrent with opioids. We found no difference in receipt of short-acting Schedule II opioids according to race.
3.2. Discontinuation of opioid therapy by race
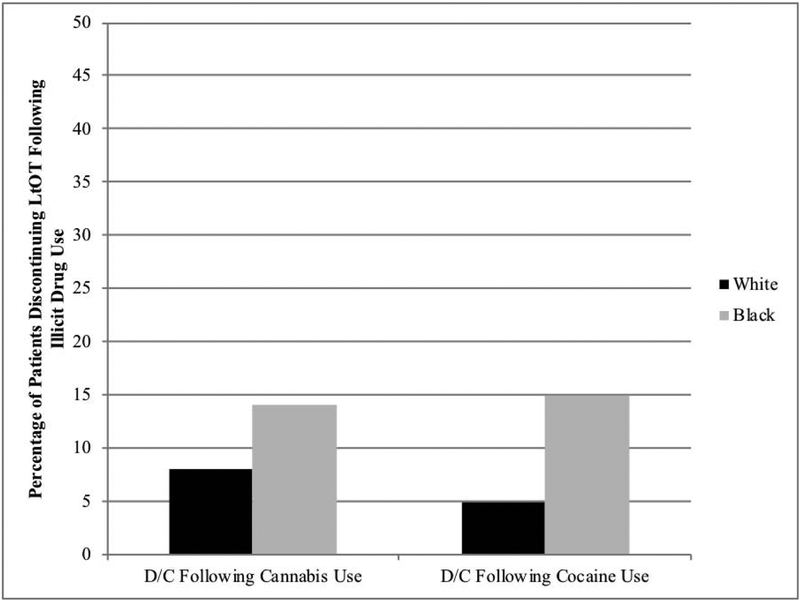
Among patients who tested positive for cannabis, 11.3% had opioids discontinued within 60 days (Fig. 1). Blacks were 2.0 times more likely than whites to have opioids discontinued (OR 1.98, 95% CI 1.01–3.86). As shown in Table 2, these associations persisted in the adjusted model.
Figure 1.
Percentage of patients discontinuing long-term opioid therapy by race following illicit drug use.
Abbreviations: D/C, discontinuation; LTOT, long-term opioid therapy.
Among patients who tested positive for cocaine, 13.1% had opioids discontinued within 60 days (Fig. 1). Blacks were 3.0 times more likely than whites to have opioids discontinued (OR 3.05, 95% CI 1.19–7.81). As shown in Table 3, these associations persisted in the adjusted model.
Table 3.
Final multivariable-adjusted logistic regression model examining discontinuation of long-term opioid therapy by race among patients testing positive for cocaine
| Characteristic | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Race | ||
| White (Reference) | — | — |
| Black | 3.05 (1.19–7.81) | 3.30 (1.28–8.53) |
| Age, y | — | 0.97 (0.93–1.01) |
| HIV | — | 0.72 (0.42–1.23) |
| Substance Use Disorder History | — | 0.74 (0.41–1.34) |
| Serious Mental Illness | — | 1.09 (0.62–1.01) |
To assess whether these results could be attributed to higher discontinuation rates among blacks regardless of urine drug testing results, we also examined by race discontinuation among those testing negative for illicit drug use. These findings were not significant: among those testing negative for cannabis, we found no association between race and opioid discontinuation (P =.34); among those testing negative for cocaine, we found no association between race and opioid discontinuation (P =.83).
To address the potential for changes over time in rates of discontinuation, we included a variable for year in the adjusted models. The results remained virtually unchanged with this inclusion (cannabis: AOR 2.04, 95% CI 1.02–4.05; cocaine: 2.96 (1.15–7.60)).
3.3. Receipt of urine drug testing by race
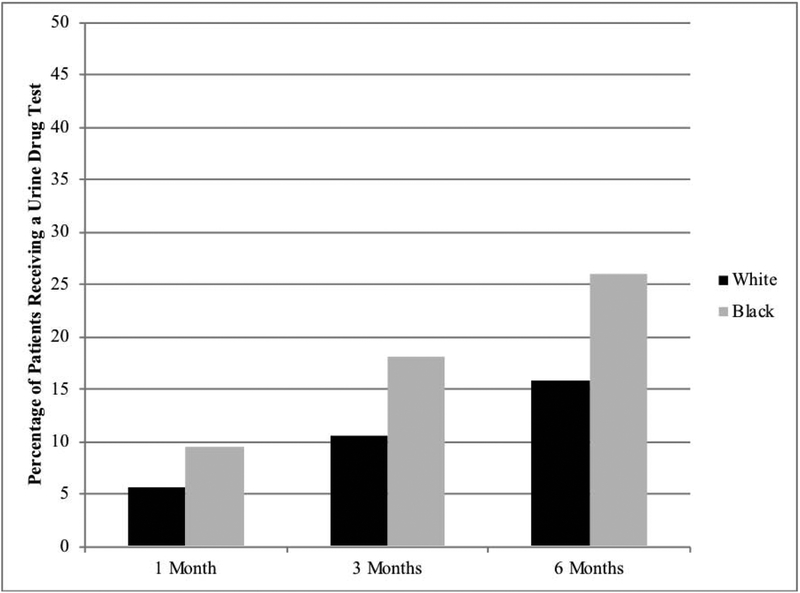
Twenty-one percent of patients received a urine drug test within the first 6 months of treatment. As shown in Fig. 2, blacks were more likely to receive a urine drug test within 1 month, 3 months, and 6 months of initiating opioid therapy (all P < .001).
Figure 2.
Percentage of patients receiving a urine drug test by race
In the adjusted models, blacks were 1.6 times more likely to receive a urine drug test (adjusted odds ratio [AOR] 1.57, 95% confidence interval [CI], 1.44–1.71, P < .001) in the first 6 months of starting LTOT (final model adjusted for age, HIV, hepatitis C, diabetes, SUD history, and receipt of benzodiazepine co-prescriptions).
To account for changes over time in receipt of urine drug testing by race, we included a variable for time in the adjusted models, which did not change the overall results (AOR 1.52, 95% CI, 1.39–1.66).
3.4. Aberrant results by race
Whites were more likely than blacks (15.5% vs. 12.6%, P < .001) to test positive for cannabis, whereas blacks were more likely to test positive for cocaine (23.7% vs. 7.9%, P < .001).
Thirty-four percent of patients who tested positive for cannabis or cocaine were receiving concurrent SUD treatment. Among these patients, blacks were more likely than whites to be engaged in SUD treatment (36.7% vs. 27.8%, P=.01).
4. Discussion
In this sample of 15,366 patients of black or white race initiating LTOT between 2000 and 2010, nearly 90% of patients who tested positive for cannabis or cocaine refilled an opioid prescription within the following 60 days. Blacks were twice as likely to have opioids discontinued after testing positive for cannabis and three times more likely after testing positive for cocaine.
Moreover, only 20% of patients received a urine drug test within the first 6 months of starting opioids, a time when patients are at high risk for overdose and death (Chou et al., 2009).
Urine drug testing rates were lowest for non-Hispanic white males—a group at high risk for opioid misuse and abuse (Rudd et al., 2016b). Blacks were nearly twice as likely to receive a urine drug test at all time points: 1 month, 3 months, and 6 months following initiation. Among those receiving a urine drug test, 1 in 4 tested positive for illicit drug use. Whites were 1.3 times more likely than blacks to test positive for cannabis, whereas blacks were more than 3.6 times more likely to test positive for cocaine.
To our knowledge, this is the first study to examine opioid discontinuation among those who test positive for illicit drug use overall and by race. By examining administration of urine drug testing in the VA, we are also able to show that blacks are vulnerable to racial bias even in settings where access to care is more equitable. Finally, with this research we add to the literature a more nuanced view of how illicit drug use differs according to race.
Our findings are in keeping with previous studies showing that clinicians have been slow to integrate urine drug testing into patient care, even for patients at high risk for opioid misuse and abuse (Gaither et al., 2016a; b; Gaither et al., 2014; Starrels et al., 2010). For more than a decade, physicians have been encouraged to monitor all patients prescribed opioid therapy for illicit drug use and diversion through baseline and periodic urine drug testing (US Department of Veterans Affairs/Department of Defense, 2003), yet rates remain low in the VA and the general medical population (Gaither et al., 2014).
Why some clinicians have failed to adopt this universal approach to urine drug testing and how patients are selected for testing remains unclear. In an earlier study, we found that only 50% of patients with a substance use disorder at the time of initiating opioid therapy received a drug test within the first 6 months (Gaither et al., 2014). These patients were no more likely than those without alcohol and drug use disorders to be tested, which is consistent with prior studies (Starrels et al., 2011). This study also examined the association between receipt of urine drug testing and all-cause mortality but found no association. The findings from this current study may in part explain why: this study provides evidence to support reports that many clinicians lack an understanding of which patients to test, how to interpret the results, and what steps to take when a patient tests positive for illicit drug use (Starrels et al., 2012).
Balsa and McGuire argue that clinical uncertainty of this type often contributes to disparities in healthcare among racial minorities; without clear guidelines on how patients should be treated, physicians fall back on ingrained belief systems such as racial stereotyping (Balsa and McGuire, 2003).
The primary limitations of this study are those common to observational research involving large clinical datasets. First, we examined only discontinuation of opioids. Further research is needed to examine other responses to aberrant urine toxicology results, specifically: tapering of opioid dose, referral to addiction treatment, switching to alternative pain medications, and initiation of more intensive monitoring. Second, according to the leading clinical practice guidelines (Chou et al., 2009; Dowell et al., 2016; US Department of Veterans Affairs/Department of Defense, 2010), in addition to the misuse of illicit drugs, the decision to discontinue opioids should take into account, at minimum, the following: 1) effectiveness of opioids on pain control and overall functionality, 2) existence of side effects or adverse events, 3) issues related to patient adherence to the treatment plan, and 4) importantly, patient’s desire to discontinue the medications. However, because this study relies on administrative, pharmacy, and electronic medical record data, we are unable to provide a full clinical picture of the circumstances that led to opioid discontinuation, including how often discontinuation was initiated by the patient and how differences in patient and provider characteristics may have influenced this decision, whether made on the part of the patient or provider.
To address these limitations, we attempted to rigorously control for the presence of serious mental illness, substance use disorders, and other comorbidities that may have factored into the decision to discontinue opioids (see methods). While our multivariable models should control for the higher rates of serious mental illness and substance use disorders found among blacks in our sample, there is always the risk for unmeasured confounding in research that relies on observational data.
Additionally, the VA setting and the focus on patients with HIV limit the generalizability of our findings. Moreover, the pattern of drug use appears to differ for non-VA patients: according to recent data from the National Survey on Drug Use and Health, black adults are more likely than white adults to use both cannabis and cocaine (refers to past-year [2015–2016] drug use and not lifetime prevalence) (Substance Abuse and Mental Health Services Administration, 2018).
Despite these limitations, we believe our findings should be considered in the context of a substantial body of literature that shows that blacks often face disparities in care (Abdus et al., 2015; Asch et al., 2006; Balsa and McGuire, 2003; Guerrero et al., 2013; Mennis and Stahler, 2016; Sahker et al., 2015), including in relation to opioid prescribing and treatment (Becker et al., 2014; Becker et al., 2011; Burgess et al., 2014).
5. Conclusions
There is a general consensus among experts in the field of pain management that urine drug testing is one of the best tools clinicians have for identifying opioid misuse, illicit drug use, and the concomitant use of sedatives or other substances that may increase the risk of overdose (Dowell et al., 2016). The results of our study demonstrate that a more universal approach to urine drug testing is urgently needed both within and outside of the VA.
While our data do not fully capture all the nuances clinicians consider in determining whether or not to discontinue opioids following illicit drug use, the magnitude of the discrepancy we found in discontinuation rates for blacks compared to whites suggests that, in the absence of clearer urine drug testing guidelines, extraneous factors unrelated to the risks and benefits of LTOT—including racial stereotypes—may enter into the decision-making. In light of this potential, we believe that the safest and most judicious way forward is for clinicians to adhere to the latest guidelines from the CDC, which recommend that urine drug testing be administered to all patients prior to opioid initiation and then at least annually thereafter (Dowell et al., 2016). In turn, clinicians need clearer guidance on how to respond to aberrant toxicology results in a manner that is both less biased and more effective in identifying patients at risk for overdose.
Highlights.
Blacks are more likely to be tested for drug use but are at less risk of overdose.
Blacks are more likely to have long-term opioid therapy (LTOT) discontinued following illicit drug use.
A more universal approach to urine drug testing is needed.
Role of Funding Source
Research reported in this paper was supported by grants from the National Institute on Drug Abuse (F31DA035567; K12DA033312), National Center for Advancing Translational Sciences (KL2TR001862), National Institute on Alcohol Abuse and Alcoholism (U10AA013566; U01AA020790; U24AA020794), and the National Institute of Mental Health (P30MH062294). These organizations had no role in the design, conduct, or reporting of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest to report.
References
- Abdus S, Mistry KB, Selden TM, 2015. Racial and ethnic disparities in services and the Patient Protection and Affordable Care Act. Am. J. Public Health 105 Suppl. 5, S668–S675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch SM, Kerr EA, Keesey J, Adams JL, Setodji CM, Malik S, McGlynn EA, 2006. Who is at greatest risk for receiving poor-quality health care? N. Engl. J. Med 354, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Balsa AI, McGuire TG, 2003. Prejudice, clinical uncertainty and stereotyping as sources of health disparities. J. Health Econ 22, 89–116. [DOI] [PubMed] [Google Scholar]
- Becker WC, Fraenkel L, Edelman EJ, Holt SR, Glover J, Kerns RD, Fiellin DA, 2013. Instruments to assess patient-reported safety, efficacy, or misuse of current opioid therapy for chronic pain: A systematic review. Pain 154, 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Meghani S, Tetrault JM, Fiellin DA, 2014. Racial/ethnic differences in report of drug testing practices at the workplace level in the U.S. Am. J. Addict 23, 357–362. [DOI] [PubMed] [Google Scholar]
- Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ, 2011. Racial differences in primary care opioid risk reduction strategies. Ann. Fam. Med 9, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden JB, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD, 2008. Trends in use of opioids by noncancer pain type 2000–2005 among Arkansas Medicaid and HealthCore enrollees: Results from the TROUP study. J. Pain 9, 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DJ, Nelson DB, Gravely AA, Bair MJ, Kerns RD, Higgins DM, van Ryn M, Farmer M, Partin MR, 2014. Racial differences in prescription of opioid analgesics for chronic noncancer pain in a national sample of veterans. J. Pain 15, 447–455. [DOI] [PubMed] [Google Scholar]
- Califf RM, Woodcock J, Ostroff S, 2016. A proactive response to prescription opioid abuse. N. Engl. J. Med 374, 1480–1485. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2017. Prescription opioid-related overdose deaths. Centers for Disease Control and Prevention, Atlanta, GA: [WWW].https://www.cdc.gov/drugoverdose/data/overdose.html. [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C, American Pain Society—American Academy of Pain Medicine Opioids Guidelines, 2009. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain 10, 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL, 2015. Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med 372, 241–248. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm. Rep 65, 1–49. [DOI] [PubMed] [Google Scholar]
- Edelman EJ, Gordon K, Becker WC, Goulet JL, Skanderson M, Gaither JR, Brennan Braden J, Gordon AJ, Kerns RD, Justice AC, Fiellin DA, 2013. Receipt of opioid analgesics by HIV-infected and uninfected patients. J. Gen. Intern. Med 28, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither JR, Goulet JL, Becker WC, Crystal S, Edelman EJ, Gordon K, Kerns RD, Rimland D, Skanderson M, Justice AC, Fiellin DA, 2016a. The association between receipt of guideline-concordant long-term opioid therapy and all-cause mortality. J. Gen. Intern. Med 31, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither JR, Goulet JL, Becker WC, Crystal S, Edelman EJ, Gordon K, Kerns RD, Rimland D, Skanderson M, Justice AC, Fiellin DA, 2016b. The effect of substance use disorders on the association between guideline-concordant long-term opioid therapy and all-cause mortality. J. Addict. Med 10, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither JR, Goulet JL, Becker WC, Crystal S, Edelman EJ, Gordon K, Kerns RD, Rimland D, Skanderson M, Weisberg DF, Justice AC, Fiellin DA, 2014. Guideline-concordant management of opioid therapy among Human Immunodeficiency Virus (HIV)-infected and uninfected veterans. J. Pain 15, 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero EG, Marsh JC, Duan L, Oh C, Perron B, Lee B, 2013. Disparities in completion of substance abuse treatment between and within racial and ethnic groups. Health Serv. Res 48, 1450–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, Rimland D, Rodriguez-Barradas MC, Oursler KK, Brown ST, Braithwaite RS, May M, Covinsky KE, Roberts MS, Fultz SL, Bryant KJ, Team VP, 2010. Towards a combined prognostic index for survival in HIV infection: The role of ‘non-HIV’ biomarkers. HIV Med. 11, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, Kitahata MM, Horberg MA, Brooks JT, Buchacz K, Rourke SB, Rachlis A, Napravnik S, Eron J, Willig JH, Moore R, Kirk GD, Bosch R, Rodriguez B, Hogg RS, Thorne J, Goedert JJ, Klein M, Gill J, Deeks S, Sterling TR, Anastos K, Gange SJ, NA-ACCORD and VACS Project Teams, 2013. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: A North American cross cohort analysis. J. Acquir. Immune Defic. Syndr 62, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff MV, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C, 2008. De facto long-term opioid therapy for noncancer pain. Clin. J. Pain 24, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, Sehgal N, Shah R, Benyamin R, Vallejo R, Fellows B, Christo PJ, 2011. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician 14, 91–121. [PubMed] [Google Scholar]
- Mennis J, Stahler GJ, 2016. Racial and ethnic disparities in outpatient substance use disorder treatment episode completion for different substances. J. Subst. Abuse Treat 63, 25–33. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM, 2016a. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb. Mortal. Wkly. Rep 64, 1378–1382. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016b. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Sahker E, Toussaint MN, Ramirez M, Ali SR, Arndt S, 2015. Evaluating racial disparity in referral source and successful completion of substance abuse treatment. Addict. Behav 48, 25–29. [DOI] [PubMed] [Google Scholar]
- Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC, 2012. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 307, 940–947. [DOI] [PubMed] [Google Scholar]
- Skelly AC, Dettori JR, Brodt ED, 2012. Assessing bias: The importance of considering confounding. Evid. Based Spine Care J 3, 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ, 2010. Systematic review: Treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann. Intern. Med 152, 712–720. [DOI] [PubMed] [Google Scholar]
- Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ, 2011. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J. Gen. Intern. Med 26, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrels JL, Fox AD, Kunins HV, Cunningham CO, 2012. They don’t know what they don’t know: Internal medicine residents’ knowledge and confidence in urine drug test interpretation for patients with chronic pain. J. Gen. Intern. Med 27, 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrels JL, Peyser D, Haughton L, Fox A, Merlin JS, Arnsten JH, Cunningham CO, 2016. When human immunodeficiency virus (HIV) treatment goals conflict with guideline-based opioid prescribing: A qualitative study of HIV treatment providers. Subst. Abuse 37, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2018. Reports and detailed tables from the 2016 National Survey on Drug Use and Health (NSDUH). Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Nattermann J, Lampe FC, Bucher HC, Sterling TR, Crane HM, Kitahata MM, May M, Sterne JA, 2013. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 27, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Veterans Affairs, 2003. Clinical practice guideline for management of opioid therapy for chronic pain. US Department of Veterans Affairs, Washington, D.C. [Google Scholar]
- US Department of Veterans Affairs, 2010. Clinical practice guideline for management of opioid therapy for chronic pain. US Department of Veterans Affairs, Washington, D.C. [Google Scholar]
- Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR, 2011. Characteristics of opioid prescriptions in 2009. JAMA 305, 1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C, 2008. De facto long-term opioid therapy for noncancer pain. Clin. J. Pain 24, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]