Abstract
Background
Hip fractures constitute a major health problem in elderly people and are often fall-related. Several factors can contribute to a fall episode leading to hip fracture, including fall-risk-increasing drugs (FRIDs), which are often used by elderly people.
We aimed to investigate the prevalence of medication-related falls and to assess the role of FRIDs and potentially inappropriate medications (PIMs) in a population of elderly patients hospitalized for a hip fracture.
Methods
We reviewed the patient records of 200 consecutive patients, aged ≥65 years, who were admitted for a hip fracture and evaluated whether medications were likely to have contributed to the fall episode. PIMs were identified using the Screening Tool of Older Persons’ Prescriptions version 2 (STOPP) and by evaluating indications, contra-indications and interactions of the prescribed medications for each patient.
Results
FRIDs were used by 175 patients (87.5%). Medications were considered a likely contributor to the fall in 82 patients (41%). These were most often psychotropic medications alone or in combination with antihypertensives and/or diuretics. The 82 patients with suspected medication-related falls used more medications, FRIDs and PIMs than the rest of the patients, and in 74 (90%) of the 82 patients, at least one medication considered to be a contributor to the fall was also a PIM.
Conclusions
The prevalence of suspected medication-related falls was 41%. It seems likely that a medication review could have reduced, though not eliminated, the risk of falling in this group of patients.
Keywords: Fall-risk-increasing drugs, Potentially inappropriate medication, Fall, Polypharmacy, Geriatrics
Background
A hip fracture is associated with considerable socio-economic costs [1] and constitutes a high-risk situation for an elderly patient, as the mortality for patients older than 65 years is 12–35% within the first year after the fracture [2] and remains elevated for several years [3]. Several medications have been identified as fall-risk-increasing drugs (FRIDs) [4–8]. The association between an increased risk of falling and the use of psychotropic medications, such as antidepressants, antipsychotics and benzodiazepines, seems well established, as indicated by odds ratios ranging from 1.3 to 2 in a recent metanalysis [8]. The association between falls and the use of cardiovascular medications, including antihypertensives and antiarrhythmics, does not seem quite as consistent [6]. However, in clinical practice, cardiovascular medications are often regarded as FRIDs, [9, 10] as their adverse effects can directly or indirectly cause dizziness, hypotension and orthostatic hypotension. Several reports have shown that more than 90% of elderly people experiencing a fall or a hip fracture are taking FRIDs [10, 11]. Thus, FRIDs can be regarded as a modifiable risk factor. Furthermore, a recent study showed that 85% of elderly patients with a hip fracture were prescribed potentially inappropriate medications (PIMs), which may be unnecessary or entail a high risk of adverse effects [12]. The Screening Tool of Older Persons’ Prescriptions (STOPP) [13] may guide the performance of a medication review, both by pointing out situations in which certain medications are potentially inappropriate and by identifying certain FRIDs as PIMs.
Given the high prevalence of FRIDs and PIMs among elderly patients, it may be hypothesized that medication reviews and interventions to reduce FRIDs and PIMs in this group would effectively reduce the risk of falling. However, two recent randomized studies investigating the effect of FRID-withdrawal among more than 600 elderly people experiencing a fall [9], and the effect of medication reviews [14] among 199 elderly patients with hip fractures [14] did not find an effect of these interventions on the rate of falls during a 12-month follow-up period. Possible explanations include competing risk factors for falling, such as comorbidities, and impaired cognition, balance, or vision. In addition, extrinsic factors influencing the subject [15, 16] may play a role in many falls.
Even though the prevalence of FRID users among patients with a hip fracture is high, it is not known how often FRIDs actually contribute to the fall. This knowledge is essential to understanding how much the incidence of hip fracture could potentially be reduced by stopping the use of these medications. Thus, the aims of our study were to estimate the prevalence of medication-related falls leading to a hip fracture in a population of elderly patients admitted to a joint orthopaedic and geriatric ward and to assess the role of FRIDs and PIMs.
Methods
Study design and population
This study was a retrospective cross-sectional study. Two hundred consecutive patients with hip fracture, aged 65 years or older, admitted to a Danish University Hospital during a period of 24 weeks in 2017 were identified by a search in the hospital’s database using the ICD-10 codes for fracture of the femur (DS72-DS729).
Evaluation and definition of medication-related falls, FRIDs and PIMs
A consultant in Clinical Pharmacology (CUA) reviewed all patient records, focusing on 1) the description of the fall episode, including fall-related symptoms and the conclusions regarding the causes of the fall, made by the attending geriatrician during admission; 2) comorbidities, demographic data and medication list at the time of admission; 3) blood pressure, respiratory frequency, peripheral saturation, body temperature and heart rate at the time of and during admission; 4) laboratory data including c-reactive protein, leucocyte count, electrolytes, haemoglobin, liver and renal parameters, and blood glucose; 5) the results of other diagnostic evaluations performed during admission; and 6) medication withdrawals or changes during admission.
FRIDs were defined in accordance with previous work on fall-risk-related medications [6–8, 13, 17, 18]: 1) psychotropic medications (antidepressants, antipsychotics, antiepileptic medications, medications for Parkinson’s disease, medications for dementia, first-generation antihistamines, benzodiazepines or benzodiazepine-like medications (zopiclone and zolpidem) and opioids); 2) cardiovascular medications (calcium antagonists, angiotensin converting enzyme (ACE)-inhibitors, angiotensin-II receptor (AT-II) antagonists, beta-adrenoceptor antagonists, alpha-adrenoceptor antagonists, diuretics, nitrous vasodilators, and anti-arrhythmic medications); and 3) urinary antispasmodics. PIMs were identified by review of the patients’ medication lists according to the STOPP version 2, [13] available from the Danish Geriatric Society. Furthermore, the indications, contra-indications and interactions for each prescribed medication, as listed in the Summary of Product Characteristics found on the homepage of the Danish Medicines Agency, were also considered for each patient. All data mentioned above was entered in case report forms during the initial review.
The clinical pharmacologist excluded a suspected medication-related fall if a patient had not experienced a fall, was not using any medications or FRIDs, or if a non-medication-related fall cause was described in the patient record. Otherwise, the clinical pharmacologist and a consultant in Geriatrics (POL, HU, or NA) discussed the case in order to obtain a consensus about whether one or more medications were likely contributors to the fall. Medications were generally considered likely contributors to the fall if their effects, adverse effects or interactions could have caused or aggravated symptoms or clinical findings related to the fall episode, for example, orthostatic hypotension. If we concluded that medications were likely contributors to the fall, we defined the patient as having had a suspected medication-related fall. If the fall was more likely explained by the consequences of acute or chronic disease, tripping or extrinsic factors, we did not consider medications likely contributors. Patients who were not attended by an on-site geriatrician during admission were evaluated retrospectively following the same procedure as that used for the rest of the patients.
Data handling and statistical analysis
Study data were collected and managed using REDCap (Vanderbilt, USA) electronic data capture tools hosted at Aalborg University. REDCap is a secure, web-based application designed to support data capture for research studies [19]. Data were exported for statistical analysis or graphics in STATA (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). The distribution of variables was evaluated according to histograms and Q-norm plots, and data that had a normal distribution were summarized as means ± standard deviations (SDs). Variables that were not normally distributed were summarized by medians [25th percentile, 75th percentile], and differences between two groups were tested by the Wilcoxon rank-sum test. Differences in the prevalence of diseases between patients with and without a suspected medication-related fall were analysed by two-sample z-test. Differences in age, clinical data and the number of medications and FRIDs among multiple groups were analysed by regression with a bootstrap analysis. The differences between proportions of patients with PIMs among multiple groups were compared by pairwise two-sample z-tests. Power calculation to estimate study size was not performed due to lack of data to support this. Analyses were performed without imputation of missing data. The number of patients with missing data are indicated for each variable in footnotes of tables. Percentages of patients with a given condition (e.g. sodium < 132 mmol/l) were calculated by dividing the number of confirmed cases with the total number of patients in the population (200).
Results
Prevalence of suspected medication-related falls
A fall preceded the hip fracture in 197 (98.5%) patients, and the fall was considered a low energy trauma, indicating an osteoporotic fracture, in 175 (87.5%) patients. Three patients (1.5%) (group 1) did not experience a fall, and eight (4%) (group 2) did not take any medication. In 59 patients (29.5%) (group 3), the fall episode seemed more likely to have been caused by an extrinsic factor, such as a push by a large animal or another person, or tripping. Consequences of a chronic or acute disease, such as influenza, urinary tract infection or uncontrolled atrial fibrillation, were considered the main cause of the fall in 48 patients (24%) (group 4). Finally, we considered 82 (41%) patients as having had a suspected medication-related fall (group 5) (Fig. 1). The most frequent reason for medications to be regarded as a contributor to the fall was dizziness in patients using medications with dizziness as a known adverse effect (n = 21 (10.5%)), followed by the presence of low blood pressure or symptoms of orthostatic hypotension in patients who were using medications able to decrease blood pressure (n = 17 (8.5%)). Medications were suspected to contribute to a fall by impairing the functional level in 12 patients (6%) and to contribute to accidental happenings leading to a fall by influencing cognition or balance in 11 patients (5.5%). In both instances, psychotropic drugs were the most commonly involved. Diuretics were thought to play a role by inducing hyponatremia in four patients (2%). Finally, the circumstances of the fall episode were not well described in 17 patients (8.5%), but the presence of FRIDs and no other obvious causes of the fall led us to define these as having had a suspected medication-related fall.
Fig. 1.
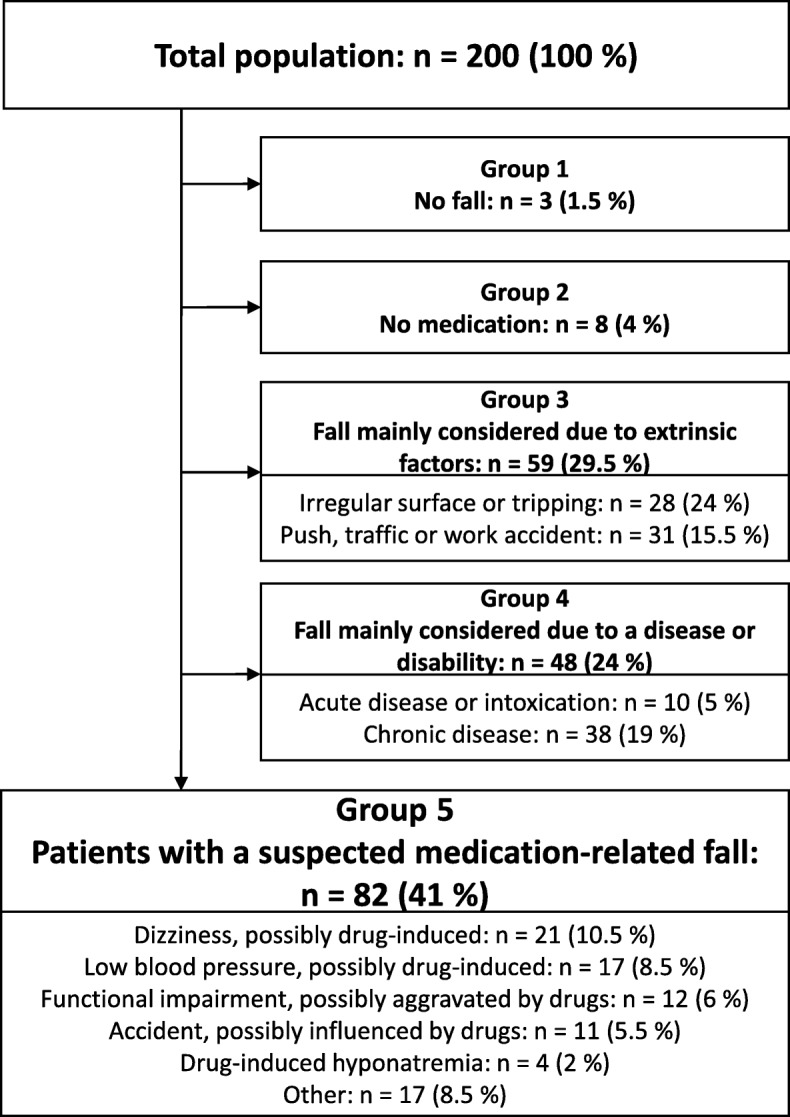
Main causes of falls. Figure 1. Flow diagram showing the selection procedure for the patients having a medication-related fall, as evaluated with the joint expertise of a clinical pharmacologist and a geriatrician
A consultant geriatrician had performed an onsite evaluation of 170 (85%) of the patients in the study population during admission.
Demographics, comorbidities and use of medication in the total population
A summary of the populations’ demographic and clinical characteristics is shown in Table 1.
Table 1.
Characteristics of the study population
| General characteristics | |
|---|---|
| Female (n (%)) | 136 (68) |
| Age (years) | 82 [76,88] |
| Age > 80 years (n (%)) | 115 (57.5) |
| Height (cm) | 166 ± 9 cm |
| Weight (kg) | 67 ± 14 kg |
| Body mass index (kg/m2) | 24 ± 4 |
| Body mass index < 18.5 (n (%)) | 17 (8.5) |
| Residence (n(%)) | |
| Private home | 158 (79) |
| Nursing home | 35 (17.5) |
| Other | 9 (4.5) |
| Type of fall and fracture (n(%)) | |
| Low-energy trauma defining an osteoporotic fracture | 175 (87.5) |
| Not low-energy trauma | 22 (11) |
| No fall | 3 (1.5) |
| Clinical findings (n(%)) | |
| First SBP at admission < 120 mmHg | 12 (6) |
| Lowest measured SBP during the admission < 120 mmHg | 135 (67.5) |
| First PS at admission < 90% | 22 (11) |
| First RF at admission > 20 /min | 23 (11.5) |
| First TP > 38.0 °C | 11 (5.5) |
| Abnormal laboratory findings at the time of admission (n(%)) | |
| Estimated glomerular filtration rate* < 60 ml/min | 74 (37) |
| Estimated glomerular filtration rate* < 30 ml/min | 13 (6.5) |
| C-reactive protein > 100 mg/l | 12 (6) |
| Haemoglobin < 6 mmol/l | 11 (5.5) |
| Sodium < 132 mmol/l | 12 (6) |
| Potassium < 3.2 mmol/l | 5 (2.5) |
| Thyroid stimulating hormone < 0.3 m IU/l or > 4.5 mIU/l | 20 (10) |
| Alanine amino transferase > 50 U/l in men or > 35 U/l in women | 12 (6) |
| Glucose < 4 mmol/l | 0 (0) |
| Glucose > 10 mmol/l | 14 (7) |
| Albumin < 34 g/l | 69 (34.5) |
Table 1. Detailed description of the characteristics of the study population. Abbreviations: CNS central nervous system SBP systolic blood pressure, PS peripheral saturation, RF respiratory frequency, TP body temperature. *: calculated by the EPI-CKD formula. Number of patients with missing data for each variable: age: 0, height: 10, weight: 17, body mass index: 24, first SBP: 2, lowest SBP: 8, PS: 4, RF: 4, TP: 8, Estimated glomerular filtration rate: 1, c-reactive protein: 9, Haemoglobin: 3, sodium: 2, potassium: 2, thyroid stimulating hormone: 37, alanine amino transferase: 8, glucose: 16, albumin: 2. Percentages of patients with a given condition (e.g. sodium < 132 mmol/l) were calculated by dividing the number of confirmed cases with the total number of patients in the population (200)
One or more comorbidities were present in 195 (97.5%) patients, and the most common comorbidities were hypertension, osteoporosis and atrial fibrillation (Table 2).
Table 2.
The prevalence of comorbidities at the time of admission occurring in 5% or more of patients
| Whole population | Patients without a medication-related fall | Patients with a medication-related fall | P value (patients with vs. without a medication-related fall) | |
|---|---|---|---|---|
| n (%) | 200 (100) | 118 (59) | 82 (41) | |
| Prevalence of comorbidity (n(%)) | ||||
| Hypertension | 90 (45) | 49 (42) | 41 (50) | 0.2 |
| Osteoporosis | 46 (23) | 28 (24) | 18 (22) | 0.8 |
| Atrial fibrillation | 44 (22) | 21 (18) | 23 (28) | 0.09 |
| Previous ischaemic stroke | 36 (18) | 21 (18) | 14 (17) | 0.9 |
| Chronic obstructive pulmonary disease | 32 (16) | 16 (13) | 16 (19) | 0.2 |
| Dementia | 30 (15) | 15 (13) | 15 (18) | 0.3 |
| Previous fracture (hip or spine) | 29 (14.5) | 12 (10) | 17 (20) | 0.04 |
| Ischaemic heart disease | 27 (13.5) | 11 (9) | 20 (24) | 0.04 |
| Type 2 diabetes | 27 (13.5) | 19 (14) | 8 (10) | 0.4 |
| Chronic renal failure | 25 (12.5) | 16 (13) | 9 (11) | 0.6 |
| Depression | 22 (11) | 9 (7) | 13 (16) | 0.07 |
| Cancer | 18 (9) | 12 (10) | 6 (7) | 0.5 |
| Chronic heart failure | 14 (7) | 10 (8) | 4 (5) | 0.3 |
| Visual or hearing impairment | 14 (7) | 13 (7) | 10 (7) | 0.9 |
| Hyperthyreosis | 12 (6) | 7 (6) | 5 (6) | 1 |
| Arthrosis | 12 (6) | 6 (5) | 5 (6) | 0.7 |
| Hypothyreosis | 10 (5) | 6 (5) | 4 (5) | 1 |
| Chronic alcoholism | 10 (5) | 5 (4) | 4 (5) | 1 |
Table 2. Comorbidities at the time of admission with a prevalence of 5 % or higher. One patient could have several diagnoses. Differences between patients with and without a medication-related fall were tested by the two-sample z-test
The median number of prescribed medications used at admission was seven, ranging from 0 to 27. At least 1 FRID was prescribed to 175 (87.5%) patients, and the median number of used FRIDs was three, ranging from zero to seven. A detailed list of medications used at the time of admission is available in Table 1S in the on-line supplementary material.
Characteristics of patients with a suspected medication-related fall
The group of patients with a suspected medication-related fall was markedly older than the group in which an extrinsic factor or tripping was considered the most likely reason (group 3). Otherwise, only the number of prescribed medications and FRIDs at the time of admission and the proportion of PIMs were significantly higher in patients with a suspected medication-related fall than in patients in the other groups (Table 3). Regarding comorbidities, patients with a suspected medication-related fall had a higher prevalence of osteoporosis and ischaemic heart disease when compared to the rest of the patients (Table 2).
Table 3.
Comparison of demographics, clinical parameters and use of medications for the groups 1 to 5 shown in Fig. 1
| Group no. | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | p-value (regression with bootstrap) | |
| n (%) | 3 (1.5) | 8 (4) | 59 (29.5) | 48 (24) | 82 (41) | |
| Description | No fall | No medication | Extrinsic factor or tripping | Disease or disability | Medication-related fall | |
| Age (years) | 78 [77–84] | 72 [70–77.5] | 79 [73–85]*# | 83 [78.5–89] | 84 [77–89] | 0.0001 |
| Body mass index (kg/m2) | 25 ± 5 | 25 ± 4 | 25 ± 4 | 24 ± 4 | 24 ± 4 | 0.7 |
| SBP at admission (mmHg) | 182 ± 27 | 150 ± 17 | 150 ± 25 | 160 ± 22 | 150 ± 27 | 0.06 |
| Lowest SBP measured during admission (mmHg) | 107 ± 33 | 116 ± 17 | 111 ± 23 | 111 ± 20 | 109 ± 20 | 0.8 |
| PS at admission (%) | 94 ± 4 | 97 ± 3 | 94 ± 5 | 94 ± 4 | 94 ± 4 | 0.1 |
| TP at admission | 37.4 ± 0.7 | 36.6 ± 0.7 | 36.8 ± 0.5# | 37.1 ± 0.8 | 36.9 ± 0.6 | 0.04 |
| Estimated glomerular filtration rate (ml/min) | 51 [6–61] | 81.5 [73–88] * | 71 [50–86] | 75 [47–86] | 66 [44–82] | 0.04 |
| Sodium (mmol/l) | 140 [137–143] | 139 [136–141] | 140 [138–141]*# | 137 [135–141] | 138 [136–141] | 0.002 |
| Number of drugs at admission | 11.6 ± 5.1 | – | 6.4 ± 4.3* | 6.4 ± 3.3* | 9.7 ± 4.0 | < 0.0001 |
| Number of FRIDS at admission | 2.3 ± 1.2 | – | 2.3 ± 1.4* | 1.75 ± 1.3* | 3.7 ± 1.2 | < 0.0001 |
| Proportion of patients with PIMs (n (%)) | 3 (100) | – | 34 (57) Ɨ | 25 (52) Ɨ | 79 (96) | – |
The table shows means ± standard deviations for parametric data and medians [interquartile range] for nonparametric data. Differences in the numeric data between groups were analysed by regression with a bootstrap analysis; *: p-value < 0.05 vs group 5. #: p-value < 0.05 vs group 4. Differences in the proportion of patients with PIMs were analysed by pairwise two-sample z-tests among groups 3, 4, and 5; Ɨ: p-value < 0.001 vs group 5. Abbreviations: SBP systolic blood pressure, PS peripheral saturation, TP temperature, FRIDS Fall-risk-increasing medications, PIMs Potentially inappropriate medications. Number of persons with missing data for each variable: age: 0, body mass index: 24 (0, 1, 6, 6, and 11 in group 1, 2, 3, 4, and 5, respectively), SBP at admission: 2 (2 in group 5), lowest blood pressure during admission: 8 (1, 2, and 5 in group 3, 4, and 5, respectively), PS: 4 (4 in group 5), TP: 8 (2 and 6 in group 3 and 5, respectively), estimated glomerular filtration rate: 1 (1 in group 4), sodium; 2 (1 in group 4 and 5), number of drugs, FRIDs and PIMs: 0
Medications playing a role in the fall episode
One medication was considered likely to play a role in the fall episode in 16 (19.5%) patients, and more than one medication was considered likely to a role in the fall episode in 66 (80.5%) of the 82 patients with a suspected medication-related fall. All medications suspected to contribute to falls were FRIDs. Psychotropic medications, including antidepressants, benzodiazepines, benzodiazepine-like medications, antipsychotics, antiepileptics and opioids, were suspected of contributing in 68 (83%) patients, whereas antihypertensive medications and diuretics likely played a role in 30 (36.5%) and 29 (35%) of the 82 patients with a suspected medication-related fall, respectively. The medication subclasses likely to have contributed to the fall episodes in more than five patients are shown in Fig. 2.
Fig. 2.
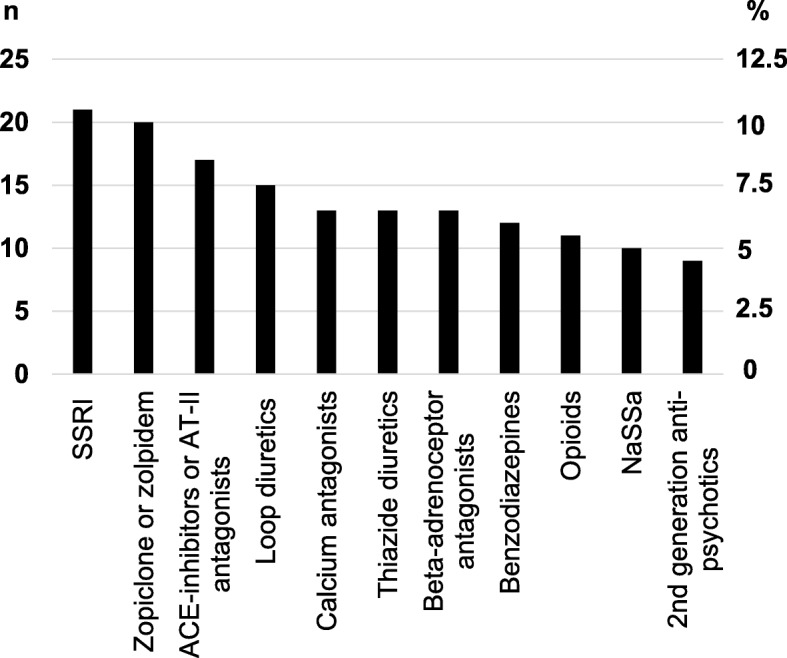
Medications most commonly suspected of contributing to falls. Figure 2. Drug classes most frequently suspected to contribute to the fall in patients with a suspected medication-related fall (group 5, Fig. 1). Left axis shows the crude number of patients in which the drug contribute. Right axis shows the percentage. Abbreviations: SSRI: selective serotonin reuptake inhibitors, ACE: angiotensin-II converting enzyme, AT-II: angiotensin-II receptor, NaSSa: Noradrenergic and specific serotonergic antidepressants
PIMs.
PIMs were identified in 141 patients (70.5%). Among patients with a suspected medication-related fall, PIMs were identified in 79 (96%) patients compared to 62 (52%) of the 118 patients without a suspected medication-related fall (p < 0.001). In 74 (90%) of the 82 patients with a suspected medication-related fall (37% of the total population), PIMs were suspected to have contributed directly to the fall leading to admission. A single medication was considered both a PIM and a contributor to the fall in 36 (44%) patients, and more than one medication was considered a PIM and contributor to the fall in 38 (46%) of the 82 patients with a suspected medication-related fall. In 42 (51%) of the patients with a suspected medication-related fall, the number of medications found likely to be involved in the fall episode was higher than the number of identified PIMs.
Discussion
We found that the prevalence of suspected medication-related falls leading to hip a fracture among elderly patients was 41%. Furthermore, we identified at least one of the medications found to contribute to the fall as potentially inappropriate in 90% of patients with a suspected medication-related fall.
The clinical data point to an influence of factors other than medication, e.g., extrinsic sources or chronic or acute illness, in the incident leading to a hip fracture in more than half of the patients. However, the estimated 41% prevalence of suspected medication-related falls suggests that medications are a major risk factor. We have not identified other studies estimating the prevalence of medication-related falls in hip fracture patients, and we were expecting a higher occurrence of suspected medication-related falls due to the frequent use of FRIDs in hip fracture patients [10, 11]. Medications can be considered modifiable risk factors, and we identified an overlap between medications found to contribute to the fall episode and medications identified as PIMs in the majority of the patients with a suspected medication-related fall. Interestingly, the number of medications suspected to be involved in the fall episode was often higher than the number identified as PIMs. Hence, a medication review may reduce the risk of medication-related falls but cannot eliminate it in all at-risk patients, suggesting that the prevalence of potentially avoidable medication-related fall-induced hip fractures might be markedly lower than 41%. This suggests that trials exploring the effect of medication reviews either requires a very large number of participants with falls or should be targeted at high risk groups. Our data may guide studies on the latter. In line with this, it has yet to be proven that withdrawal of medications reduces the risk of falls [9, 14, 20]. The randomized study by Boye et al. [9] showed that withdrawal of FRIDs did not alter the fall rate or number of FRIDs after 12 months, and they proposed a lack of compliance with the withdrawal or prescription of new medications as possible explanations [9]. Accordingly, the number of FRIDs may actually increase after a hip fracture, [21] indicating that medication reviews among elderly patients should be a primary prophylactic modality. To select patients in whom to perform a medication review, our data points towards patients prescribed a higher number of medications and FRIDs, as these were the only obvious risk factors that detected those individuals with a suspected medication-related fall. The finding of a higher prevalence of ischaemic heart disease and previous fractures may be explained by an association of these conditions with a higher usage of medications.
We found that the most common FRIDs associated with suspected medication-related falls were psychotropic medications, particularly selective serotonin reuptake inhibitors and benzodiazepines or benzodiazepine-like medications, followed by antihypertensives and diuretics. This can be explained by the known adverse effects of these drugs [6, 8] and their widespread use. Thus, it is important to focus on these medications when performing medication reviews in order to reduce the risk of medication related falls. Nevertheless, thiazides may also have a beneficial effect on bone strength by increasing renal calcium reabsorption [22]. The use of medications with an established bone-demineralizing effect, such as oral corticosteroids, aromatase inhibitors and enzyme-inducing anti-epileptics, was infrequent in our population, suggesting a limited contribution to falls by these medications among hip fracture patients. However, we could not evaluate the lifetime use of medications, and the long-term effect of prior use of such medications cannot be excluded in our study.
This study has several limitations. For one thing, the population is relatively small. The retrospective design implies that we had to rely on data obtained routinely and the possibility of focused examinations and interviews was precluded. The evaluation of clinical data and falls was not blinded to the list of medications, and the evaluations of the role of medications may have varied depending on the observer. On the other hand, the retrospective design [23] allowed us to study an unselected population of consecutive elderly patients with hip fractures, with access to clinical data, laboratory data and a detailed description of the fall episode. The validity of the description of the fall episode in the patient record is strengthened by the fact that this is a designated clinical task for the on-site consultant in geriatrics. Furthermore, the joint expertise of the clinical pharmacologist and the geriatrician in the evaluation of the individual patient records strengthens the evaluations and conclusions in our report. The female preponderance, age, high frequency of comorbidities, widespread use of medications, FRIDs, and PIMs in our patients correspond well to those from other studies of Danish and international cohorts of patients with hip fractures [11, 12, 24–26]. Thus, our population may be a representative sample of elderly patients with hip fracture. Altogether, we consider our result to be a qualified estimate of the prevalence of medication-related falls in elderly hip fracture patients.
Conclusions
The prevalence of suspected medication-related falls was 41% in elderly patients admitted with a hip fracture. It seems likely that a medication review followed by withdrawal of inappropriate medications could have reduced, though not eliminated, the risk of falling in 74 (37%) of the total population. Still, intervention studies are warranted and the present data may support planning of such studies.
Supplementary information
Additional file 1: Table S1. Medications used at the time of admission
Acknowledgements
Not applicable
Abbreviations
- FRIDS
Fall-risk-increasing drugs
- STOPP
Screening Tool of Older Persons’ Prescriptions
- PIMs
Potentially inappropriate medications
- SD
Standard deviation
- ACE
Angiotensin converting enzyme
Authors’ contributions
CUA: Conception of idea, developing design, extraction and evaluation of patient data, literature search, analysis of results, manuscript writing. POL, HQU & NA: Developing design, evaluation of patient data, literature search, and manuscript review. LPN & SA: Developing design, literature search, and manuscript review. All authors read and approved the final manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available because the Danish Patient Safety Authority has to approve transmission of the data to other researchers in each case. Data are, however, available from the authors upon reasonable request and with permission of the Danish Patient Safety Authority.
Ethics approval and consent to participate
The study was registered at the Danish Data Protection Agency. In accordance with Danish legislation (Act on Research Ethics Review of Health Research Projects § 14 stk. 2 dated 15/09/2017 and the Danish Health Act § 46 stk. 2 dated 02/11/2018), the Danish Patient Safety Authority approved the project, including transmission of the data from the patient records. Data were handled in accordance with the Danish law on Personal Data Protection.
Consent for publication
The Danish Patient Safety Authority approved the project, including publication of the results.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12877-020-01532-9.
References
- 1.Hansen L, Mathiesen AS, Vestergaard P, Ehlers LH, Petersen KD. A health economic analysis of osteoporotic fractures: who carries the burden? Arch Osteoporos. 2013;8:126. doi: 10.1007/s11657-013-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menendez-Colino R, Alarcon T, Gotor P, Queipo R, Ramirez-Martin R, Otero A, et al. Baseline and pre-operative 1-year mortality risk factors in a cohort of 509 hip fracture patients consecutively admitted to a co-managed orthogeriatric unit (FONDA cohort) Injury. 2018;49:656–661. doi: 10.1016/j.injury.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–390. doi: 10.7326/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zia A, Kamaruzzaman SB, Tan MP. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr Gerontol Int. 2017;17:463–470. doi: 10.1111/ggi.12741. [DOI] [PubMed] [Google Scholar]
- 5.Bloch F, Thibaud M, Dugue B, Breque C, Rigaud AS, Kemoun G. Psychotropic drugs and falls in the elderly people: updated literature review and meta-analysis. J Aging Health. 2011;23:329–346. doi: 10.1177/0898264310381277. [DOI] [PubMed] [Google Scholar]
- 6.De Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J Am Med Dir Assoc. 2018;19:371 e1–371 e9. doi: 10.1016/j.jamda.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Seppala LJ, van de Glind EMM, Daams JG, Ploegmakers KJ, de Vries M, Wermelink A, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372 e1–372 e8. doi: 10.1016/j.jamda.2017.12.099. [DOI] [PubMed] [Google Scholar]
- 8.Seppala LJ, Wermelink A, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19:371 e11–371 e17. doi: 10.1016/j.jamda.2017.12.098. [DOI] [PubMed] [Google Scholar]
- 9.Boye ND, van der Velde N, de Vries OJ, van Lieshout EM, Hartholt KA, Mattace-Raso FU, et al. Effectiveness of medication withdrawal in older fallers: results from the improving medication prescribing to reduce risk of FALLs (IMPROveFALL) trial. Age Ageing. 2017;46:142–146. doi: 10.1093/ageing/afw161. [DOI] [PubMed] [Google Scholar]
- 10.Beunza-Sola M, Hidalgo-Ovejero AM, Marti-Ayerdi J, Sanchez-Hernandez JG, Menendez-Garcia M, Garcia-Mata S. Study of fall risk-increasing drugs in elderly patients before and after a bone fracture. Postgrad Med J. 2018;94:76–80. doi: 10.1136/postgradmedj-2017-135129. [DOI] [PubMed] [Google Scholar]
- 11.Sjoberg C, Bladh L, Klintberg L, Mellstrom D, Ohlsson C, Wallerstedt SM. Treatment with fall-risk-increasing and fracture-preventing drugs before and after a hip fracture: an observational study. Drugs Aging. 2010;27:653–661. doi: 10.2165/11538200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Lonnbro J, Wallerstedt SM. Clinical relevance of the STOPP/START criteria in hip fracture patients. Eur J Clin Pharmacol. 2017;73:499–505. doi: 10.1007/s00228-016-2188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjoberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture-preventing and fall-risk-increasing drugs in older adults with hip fracture-a randomized controlled study. J Am Geriatr Soc. 2013;61:1464–1472. doi: 10.1111/jgs.12412. [DOI] [PubMed] [Google Scholar]
- 15.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Leavy B, Byberg L, Michaelsson K, Melhus H, Aberg AC. The fall descriptions and health characteristics of older adults with hip fracture: a mixed methods study. BMC Geriatr. 2015;15:40. doi: 10.1186/s12877-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartholt KA, Boye ND, Van der Velde N, Van Lieshout EM, Polinder S, De Vries OJ, et al. [Cost] effectiveness of withdrawal of fall-risk increasing drugs versus conservative treatment in older fallers: design of a multicenter randomized controlled trial (IMPROveFALL-study) BMC Geriatr. 2011;11:48. doi: 10.1186/1471-2318-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado-Duque ME, Castano-Montoya JP, Medina-Morales DA, Castro-Rodriguez A, Gonzalez-Montoya A, Machado-Alba JE. Drugs with anticholinergic potential and risk of falls with hip fracture in the elderly patients: a case-control study. J Geriatr Psychiatry Neurol. 2018;31:63–69. doi: 10.1177/0891988718757370. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;319:1705–1716. doi: 10.1001/jama.2017.21962. [DOI] [PubMed] [Google Scholar]
- 21.Kragh A, Elmstahl S, Atroshi I. Older adults' medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc. 2011;59:863–868. doi: 10.1111/j.1532-5415.2011.03372.x. [DOI] [PubMed] [Google Scholar]
- 22.Xiao X, Xu Y, Wu Q. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018;29:1515–1524. doi: 10.1007/s00198-018-4486-9. [DOI] [PubMed] [Google Scholar]
- 23.Clemens KK, Ouedraogo A, Speechley M, Richard L, Thain J, Shariff SZ. Hip fractures in older adults in Ontario, Canada-monthly variation, insights, and implications. Can Geriatr J. 2019;22:148–164. doi: 10.5770/cgj.22.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boddaert J, Cohen-Bittan J, Khiami F, Le Manach Y, Raux M, Beinis JY, et al. Postoperative admission to a dedicated geriatric unit decreases mortality in elderly patients with hip fracture. PLoS One. 2014;9:e83795. doi: 10.1371/journal.pone.0083795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jantzen C, Madsen CM, Abrahamsen B, Van Der Mark S, Duus BR, Howland J, et al. Pre-fracture medication use as a predictor of 30-day mortality in hip fracture patients: an analysis of 141,201 patients. Hip Int. 2019;101–106. 10.1177/1120700019832603. [DOI] [PubMed]
- 26.Frederiksen A, Abrahamsen B, Johansen PB, Sorensen HA. Danish, national cross-sectional observational study on the prevalence of prior major osteoporotic fractures in adults presenting with hip fracture-limitations and scope for fracture liaison services in prevention of hip fracture. Osteoporos Int. 2018;29:109–114. doi: 10.1007/s00198-017-4247-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Medications used at the time of admission
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available because the Danish Patient Safety Authority has to approve transmission of the data to other researchers in each case. Data are, however, available from the authors upon reasonable request and with permission of the Danish Patient Safety Authority.


