Abstract
Background
Arsenic toxicity induces a range of metabolic responses in plants, including DNA methylation. The focus of this paper was on the relationship between As-induced stress and plant senescence in the hyperaccumulator Pteris cretica var. Albo-lineata (Pc-Al). We assume difference in physiological parameters and level of DNA methylation in young and old fronds as symptoms of As toxicity.
Results
The As accumulation of Pc-Al fronds, grown in pots of haplic chernozem contaminated with 100 mg As kg− 1 for 122 days, decreased with age. Content of As was higher in young than old fronds for variants with 100 mg As kg− 1 (2800 and 2000 mg As kg− 1 dry matter, respectively). The highest As content was determined in old fronds of Pc-Al grown in pots with 250 mg As kg− 1. The increase with age was confirmed for determined nutrients – Cu, Mg, Mn, S and Zn. A significant elevation of all analysed nutrients was showed in old fronds. Arsenic accumulation affected DNA methylation status in fronds, but content of 5-methylcytosine (5mC) decreased only in old fronds of Pc-Al (from 25 to 12%). Determined photosynthetic processes showed a decrease of fluorescence, photosynthetic rate and chlorophylls of As treatments in young and old fronds. Water potential was decreased by As in both fronds. Thinning of the sclerenchymatous inner cortex and a reduction in average tracheid metaxylem in the vascular cylinder was showed in roots of As treatment. Irrespective to fronds age, physiological parameters positively correlated with a 5mC while negatively with direct As toxicity. Opposite results were found for contents of Cu, Mg, Mn, S and Zn.
Conclusions
The results of this paper point to changes in the metabolism of the hyperaccumulator plant Pc-Al, upon low and high exposure to As contamination. The significant impact of As on DNA methylation was found in old fronds. Irrespective to fronds age, significant correlations were confirmed for 5mC and As toxicity. Our analysis of the very low water potential values and lignification of cell walls in roots showed that transports of assimilated metabolites and water between roots and fronds were reduced. As was showed by our results, epigenetic changes could affect studied parameters of the As hyperaccumulator plant Pc-Al, especially in old fronds.
Keywords: Pteridaceae, Long-term stress, Toxic element, Epigenetic change, DNA demethylation
Background
Environmental pollution with arsenic (As) poses a risk to plant, animal and human health. Uptake and accumulation of this element by plants vary according to plant species. For most plants, significantly reduced growth and fitness is evident at soil arsenic concentrations of 25.0–85.0 mg kg− 1 total As [1]. In contrast, some fern species of the Pteridaceae family can tolerate As and accumulate it in their above-ground tissues to > 1000 mg As kg− 1 dry weight [2, 3]. The cultivars of Pteris cretica (var. Albo-lineata, Wimsetti and Alexandrae) were identified as an arsenic hyperaccumulators by Zhao et al. [3], who reported that this species accumulates As to the levels found in P. vittata, the first As-hyperaccumulating species identified. Arsenate (AsV) taken up by roots of P. vittata from the soil was reduced to arsenite (AsIII), which was rapidly transported to the vacuoles of the upper and lower epidermal cells and trichomes of the fronds [4]. Tu and Ma [5] found that As in the fronds of P. vittata was primarily contained as inorganic arsenite (average of 94%). According to these authors arsenite re-oxidation to arsenate occurred more often with senescence of fronds. Koller et al. [6] confirmed the effect of the development stage of fronds of Pteris umbrosa on As content. The senesced fronds had a significantly lower As content in contrast to green fronds while expanding fronds had the highest As content. The different result - increase in As concentration from young to mature and old fronds was shown in P. vittata [5].
Arsenic stress can provoke numerous toxic effects in plants. As is widely reported to inhibit the rate of photosynthesis in plants and to reduce chlorophyll concentration [7]. Agnihotri and Seth [8] showed a decline in photosynthetic pigments and gaseous exchange parameters in plants exposed to As, indicating the onset of senescence. Foyer and Noctor [9] also confirmed that oxidative stress activates senescence associated with the degradation of photosynthetic pigments and remobilises the basic nutrients C, N, P, and S. Modified stress metabolism due to extended reversible senescence releases nutrients from catabolic processes and transports them from old leaves into young leaves more efficiently.
Studies have also revealed that stress-inducing abiotic factors, including As, can trigger epigenetic changes (in particular DNA methylation/demethylation), which may contribute to the regulation of gene expression in chronic stress conditions [10]. Phenotypic manifestations of epigenetic changes include reduced plant growth (dwarfism) and development (in particular seed germination [11]), flowering period [12, 13] and male fertility/sterility of anthers and/or pollens [14].
The primary detoxification of As in the cells of terrestrial plants relies on rapid reduction of AsV to AsIII and the formation of AsIII-glutathione or AsIII-phytochelatin complexes, which are eventually transported to the vacuole. Disturbances in cellular processes, caused by a toxic excess of As, induce oxidative stress responses [15–17], methylation of both As forms in P. cretica [18], and epigenetic changes in DNA [19]. In this study, we aimed to gain insight into the context of As hyperaccumulation and plant senescence in Pteris cretica var. Albo-lineata. In addition, we examined related changes in selected physiological parameters and DNA methylation status as potentially indicative of epigenetic modification. The degree of senescence was evaluated with respect to different changes found in young and old fronds of P. cretica var. Albo-lineata.
Results
Growth and elemental content of As-exposed P. cretica var. Albo-lineata
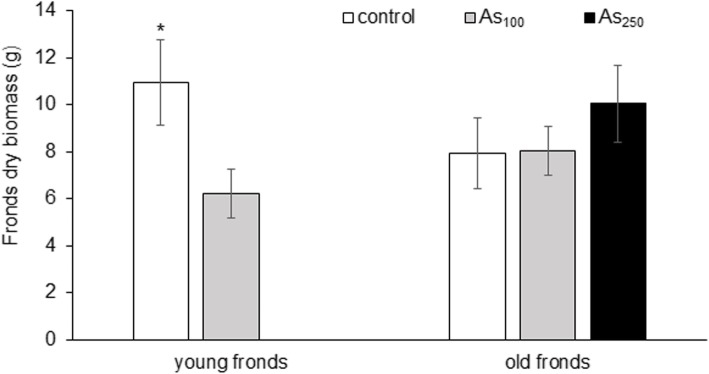
The effect of As100 soil was observed only on young fronds. The dry biomass of Pc-Al young fronds was decreased by 43% (Fig. 1). The effect of As250 soil was not observed. Differences between young and old fronds of Pc-Al were not statistically significant. Symptoms of As toxicity were not observed.
Fig. 1.
Dry biomass of young and old fronds of P. cretica var. Albo-lineata. Values represent mean ± SD. The Kruskal-Wallis test was used to compare the significance (p < 0.05) between: i) treatments of young fronds (control and As100) and old fronds (control, As100 and As250) and ii) young and old fronds of control and As100 treatment. Treatments significantly different from each other are marked with asterisks. Differences between young and old frond was not significant
The analysis of the concentration of elements in dried fronds of Pc-Al revealed that the highest As concentration was determined in As250 old fronds (Table 1). In the control and As100 soil condition, young fronds had approximately 1.5 times higher concentrations of As than old ones (Table 1). Compared with controls, ferns grown in the As100 soils increased As concentrations 150- and 170-fold in young and old fronds, respectively. Ferns grown in the As250 soil increased As concentration 421-fold compared to control. Irrespective of the presence of added As in the soils, old fronds tended to accumulate higher concentrations of Cu, Mg, Mn, S and Zn than young fronds (Table 1, Fig. 2). The effect of high soil As – As100 and As250 on the Cu accumulation showed an increase in young fronds (by 17%) and old fronds (by 100%), respectively. The increases in S (59%) and Zn (86%) concentration were only observed in old fronds. When grown in As100 and As250 soils, the concentrations of Mg and Mn increased in old fronds of Pc-Al (Mg by 60% and Mn by 66%) but decreased in young fronds (Mg by 6% and Mn by 18%).
Table 1.
Content of elements in young and old fronds of P. cretica var. Albo-lineata
| Parameters | Young fronds (mg kg− 1 dry weight) | Old fronds (mg kg− 1 dry weight) | |||
|---|---|---|---|---|---|
| control | As100 | control | As100 | As250 | |
| x̄ ± SD | x̄ ± SD | x̄ ± SD | x̄ ± SD | x̄ ± SD | |
| As | 19 ± 0.6aB | 2847 ± 63bB | 12 ± 0.1aA | 2034 ± 47abA | 5056 ± 143b |
| Cu | 5.1 ± 0.1aA | 6.0 ± 0.1bA | 5.5 ± 0.1aB | 7.0 ± 0.2abB | 11 ± 0.2b |
| Mg | 2277 ± 61bA | 2139 ± 43aA | 2509 ± 40aB | 3342 ± 35abB | 4034 ± 106b |
| Mn | 28 ± 0.5bA | 23 ± 0.9aA | 35 ± 2aB | 52 ± 1.3abB | 58 ± 4b |
| S | 1326 ± 27aA | 1372 ± 43aA | 1521 ± 51aB | 2195 ± 17abB | 2420 ± 179b |
| Zn | 17 ± 0.2aA | 18 ± 0.7aA | 21 ± 0.4aB | 27 ± 0.3abB | 39 ± 1.2b |
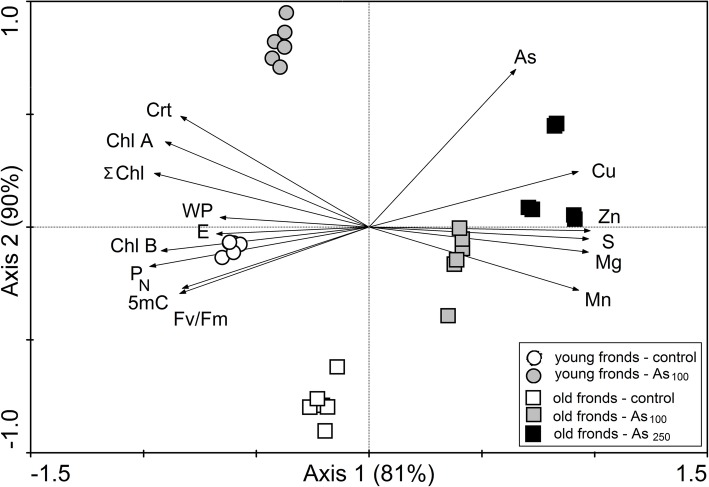
Fig. 2.
Ordination diagram showing the results of PCA analysis with selected parameters in fronds of P. cretica var. Albo-lineata. Treatment abbreviations: control, treated with 0 mg As kg− 1 soil; As100, treated with 100 mg As kg− 1 soil; As250, treated with 250 mg As kg− 1 soil. Parameter abbreviations: Crt, carotenoids; Chl A, chlorophyll a; Chl B, chlorophyll b; Σ Chl, total chlorophyll; WP, water potential; PN, net photosynthetic rate; E, transpiration rate; Fv/Fm, fluorescence; 5mC, 5-methylcytosine; As, Cu, Mg, Mn, S and Zn; total content of elements
Values with the same letter were not statistically significant at the 0.01 level by the Kruskal-Wallis test. Different letters indicate significantly different values (p < 0.01): a, b comparison between the treatments of young fronds (control and As100) and old fronds (control, As100 and As250); A, B comparison between young and old fronds for control and As100 treatment. Results for comparison between control and As100 of young and old fronds are in Additional File 1. Coefficients of variation (CV, %) are in Additional File 2.
DNA methylation status of As-exposed P. cretica var. Albo-lineata
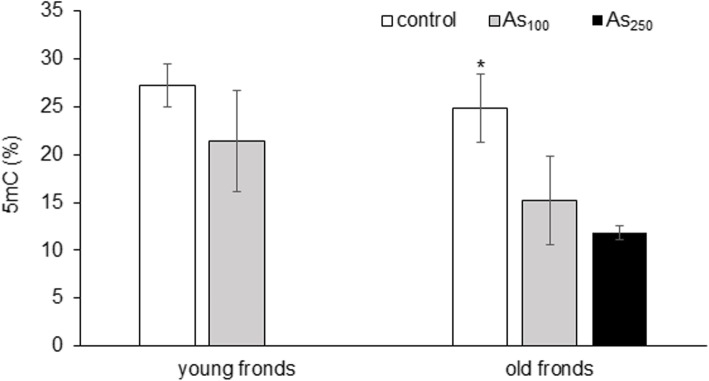
Since As and senescence might affect the methylation of DNA at cytosines in plants, the 5-methylcytosine content (5mC, %) of Pc-Al DNA was analysed (Figs. 2 and 3). Compared with controls, the overall DNA methylation status in fronds of ferns grown in As100 soils was fluctuated from 27 to 21% in young and from 25 to 15% in old fronds. The 5mC content in old fronds of ferns grown in As250 soil was 12%. The decrease was only proved in old fronds. The effect of frond senescence on 5mC content was not statistically significant, but the trend, in terms of average 5mC content, was lower in old fronds.
Fig. 3.
Content of 5-methylcytosine (5mC) in young and old fronds of P. cretica var. Albo-lineata. Values represent mean ± SD. The Kruskal-Wallis test was used to compare the significance (p < 0.05) between: i) treatments of young fronds (control and As100) and old fronds (control, As100 and As250) and ii) young and old fronds of control and As100 treatment. Treatments significantly different from each other are marked with asterisks. Differences between young and old frond was not significant. Coefficients of variation (CV, %) are in Additional File 2
Pigment content, fluorescence, WP and GEP of As-exposed P. cretica var. Albo-lineata
Growth in As100 and As250 soil resulted in a decrease of chlorophyll contents (Chl A, Chl B and Σ Chl) of Pc-Al (Table 2). The content of carotenoids (Crt) was reduced by As, but not significantly. Irrespective of the presence of added As in the soil, contents of all analysed pigments were higher in young fronds than in old fronds. Compared with controls, pigment content of ferns grown in the As100 soils was higher in young fronds, especially Chl A and Crt contents (5-fold and 6-fold higher than those in old fronds, respectively). While the average value of Chl A and Chl B ratio remained unaffected by As in old fronds, it increased in young fronds of ferns grown in As100 soils (Table 2).
Table 2.
Physiological parameters in young and old fronds of P. cretica var. Albo-lineata
| Parameters | Young fronds | Old fronds | |||
|---|---|---|---|---|---|
| control | As100 | control | As100 | As250 | |
| x̄ ± SD | x̄ ± SD | x̄ ± SD | x̄ ± SD | x̄ ± SD | |
| Chl A (nmol ml−1) | 11 ± 0.2bB | 10 ± 0.5aB | 2.6 ± 0.6bA | 1.5 ± 0.4abA | 1.0 ± 0.5a |
| Chl B (nmol ml− 1) | 4.8 ± 1.0bB | 2.8 ± 0.2aB | 2.6 ± 0.7bA | 1.9 ± 0.4abA | 0.6 ± 0.2a |
| Chl A/Chl B (−) | 2.4 ± 0.4aB | 3.6 ± 0.03bB | 1.1 ± 0.7aA | 0.9 ± 0.5aA | 1.4 ± 0.4a |
| Σ Chl (nmol ml− 1) | 16 ± 1.0bB | 13 ± 0.7aB | 5.2 ± 0.1bA | 3.4 ± 0.3abA | 1.6 ± 0.7a |
| Crt (nmol ml− 1) | 2.4 ± 0.1aB | 2.3 ± 0.3aB | 0.5 ± 0.2aA | 0.3 ± 0.1aA | 0.3 ± 0.04a |
| Fv/Fm (µmol m-2 s-1) | 0.8 ± 0.02bB | 0.7 ± 0.03aB | 0.7 ± 0.02bA | 0.6 ± 0.07aA | 0.5 ± 0.1a |
| WP (MPa) | −1.4 ± 0.09aB | −1.9 ± 0.03bB | −1.7 ± 0.04aA | −3.7 ± 0.03bA | −2.5 ± 0.4ab |
| E(mmol H2O m−2 s−1) | 0.7 ± 0.2aA | 0.9 ± 0.2bA | 0.8 ± 0.1bA | 0.7 ± 0.1aA | 0.3 ± 0.04a |
| PN (μmol CO2 m− 2 s− 1) | 8.1 ± 0.1bB | 7.7 ± 0.04aB | 7.7 ± 0.04bA | 7.2 ± 0.03abA | 6.7 ± 0.1a |
| WUE (−) | 12 ± 2.7bA | 8.6 ± 1.3aA | 9.7 ± 1.2aA | 9.4 ± 0.6aA | 24.4 ± 1.6b |
Chlorophyll fluorescence (Fv/Fm), as an indicator of plant photosynthetic activity, was lower in old fronds than in young fronds. The value of Fv/Fm for young fronds from control plants (0.82 μmol m− 2 s− 1) responded to the quantum yield of photosystem II. During As100 stress Fv/Fm of fronds decreased compared with controls (by 12.5% in young fronds and 14% in old fronds). Also As250 stress decreased Fv/Fm of old fronds – by 29% (Table 2). The lowest value of Fv/Fm (71% of the value observed with the controls) was measured in As250 conditions in old fronds. Observed declines in pigment content and Fv/Fm in old leaves of fern in As100 and As250 soils indicated a faster progression of senescence.
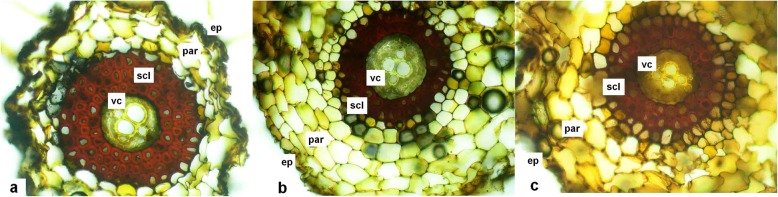
Water potential (WP) was decreased in young and old fronds of the plants grown in As100 soil (by 36 and 118%, respectively). Higher values of WP were observed in young fronds, irrespective of added As. To explore potential changes in roots, cross-section analysis through adventitious roots was performed. The roots of As treated plants showed thinning of the sclerenchymatous inner cortex and a reduction in average tracheid metaxylem in the vascular cylinder, compared to controls (Fig. 4).
Fig. 4.
Cross-section through an adventitious root of P. cretica var. Albo-lineata. Treatment: control (4a; 0 mg As kg− 1 soil); As100 (4b; 100 mg As kg− 1 soil); As250 (4c; 250 mg As kg− 1 soil). Abbreviations: vc, vascular cylinder; scl, sclerenchymatous inner cortex; par, parenchymatous outer cortex; ep, epidermis
From these data, the water-use efficiency was estimated (WUE = PN/E). Values with the same letter were not statistically significant at the 0.01 level by the Kruskal-Wallis test. Different letters indicate significantly different values (p < 0.01): a, b comparison between the treatments of young fronds (control and As100) and old fronds (control, As100 and As250); A, B comparison between young and old fronds for control and As100 treatment. Results for comparison between control and As100 of young and old fronds are in Additional File 1. Coefficients of variation (CV, %) are in Additional File 2.
The PN and the rate of transpiration (E) were determined to gain further insight into the photosynthetic performance in Pc-Al fronds (Table 2). The PN and E data indicated a higher photosynthetic activity in the young fronds of the control ferns (Table 2, Fig. 2), and a decrease in the photosynthesis rate under As conditions (by 5% in young fronds and 13% in old fronds). 28.5% increase of transpiration was observed in young fronds after As application. Compared with controls, added As decreased WUE only in young fronds (by 29%).
Principal component analysis of physiological parameters
The first axis of the PCA analysis explained 81% of the variability of all analysed data, the first two axes explained 90% of the variability, and the first four axes together explained 99% of the variability. Diagramming PCA analysis was used for visualisation of all relationships between Pc-Al parameters (Fig. 2; data only for As100 are in Additional File 3). In the PCA diagram, the first ordination axis divided the young fronds group on the left side from old fronds on the right side. This division indicated a large effect of frond senescence on all studied parameters. For young and old fronds, marks for treatments (control, As100) were located in the different parts of the diagram, which indicated a high effect of the treatments on all the recorded data. As observed with primary data, PCA confirmed that the accumulation of Cu, S, Zn, Mg and Mn was more pronounced in old fronds of Pc-Al grown in As100 and As250 soils and that Chl B, PN, WP, E, Fv/Fm, as well as 5mC were higher in young fronds of control plants. Arsenic content was negatively correlated with relative 5mC content of DNA as the angle between the vectors for As and 5mC was > 90°. Relationships visualised in the PCA diagram were confirmed by linear correlations (Table 3). The results in Table 3 showed an effect of As and 5mC of all treatments on other measured parameters. Correlations of As effect and 5mC on other parameters were calculated in different old fronds, where senescence was evaluated as a difference of tested parameters between young and old fronds (Table 3). The content of As in Pc-Al fronds significantly correlated with 5mC and other physiological parameters, except Crt. Negative relationships of As were confirmed for pigments, Fv/Fm, WP, E and PN. By comparison, these parameters were positively correlated with 5mC. Negative relationships were found between 5mC and Cu, Mg, Mn, S and Zn. By comparison, these parameters were positively correlated with As. The effect of As and 5mC of low As treatment (As100) is showed in Additional File 4.
Table 3.
Linear correlation of As and 5mC with selected parameters of P. cretica var. Albo-lineata
| As | r | 5mC | r |
|---|---|---|---|
| 5mC | −0.74*** | As | −0.74*** |
| Mg | 0.71*** | Mg | −0.77*** |
| Cu | 0.89*** | Cu | −0.76*** |
| Zn | 0.79*** | Zn | −0.76*** |
| Mn | 0.59** | Mn | −0.74*** |
| S | 0.69*** | S | −0.78*** |
| Chl A | −0.38* | Chl A | 0.59** |
| Chl B | −0.75*** | Chl B | 0.69*** |
| Crt | −0.31n.s. | Crt | 0.56** |
| Fv/Fm | −0.77*** | Fv/Fm | 0.74*** |
| WP | −0.41* | WP | 0.64*** |
| E | −0.56*** | E | 0.44* |
| PN | −0.87*** | PN | 0.81*** |
*p < 0.05, **p < 0.01, ***p < 0.001; n.s., not statistically significant
Discussion
Our results show that when grown in chernozem soils spiked with 100 and 250 mg As kg−1, As could accumulate in the fronds of P. cretica var. Albo-lineata to > 2000 mg As kg− 1 dry mass. These data confirmed the As hyperaccumulation status of Pc-Al and were consistent with results reported by Zhao et al. [3] for this fern and by Tu and Ma [5] for P. vittata. The As content in young fronds of control and As100 variants was higher than in old fronds. Effect of the development stage of fronds on As content was determined in Pteris umbrosa by Koller et al. [6]. During maturation of young fronds and senescence, As concentration in P. umbrosa exposed to 100 and 600 mg As L− 1 declined. This finding is consistent with our results for control and As100 variants.
Arsenic stress induces epigenetic changes in organisms, resulting in a decrease or increase in DNA methylation [19]. Analysis of 5mC in Pc-Al showed that As reduced the extent of DNA methylation. Similar results for heavy metals were published by Aina et al. [20]. The first paper focused on the effect of As on DNA methylation in plants was published by Erturk et al. [21]. Their results showed DNA hypermethylation of some genes in germinating maize seeds exposed to low As levels.
An increase of DNA methylation increases plant growth and transcriptionally represses genes involved in flavonoid biosynthesis [22]. A decrease of DNA methylation reduces plant growth and stimulates flowering, formation and growth of buds [13, 23, 24]. This finding was confirmed by our results. Dry biomass of Pc-Al young fronds was decreased by 43% (Fig. 1). As revealed by PCA analysis (Fig. 2), physiological parameters of the plant are affected more strongly by the methylation status of Pc-Al DNA than by direct As toxicity.
Some publications suggest that parts of DNA are sensitive to epigenetic changes [25]. However, in plants, the conservative parts of DNA without changes in DNA methylation were observed. Little information about the epigenetic activation of transcription of silenced plant genes of primary and secondary metabolites is known. Cazzonelli [26] described epigenetic changes linked to the regulation of metabolic pathways leading to carotenoid biosynthesis in relation to abscisic acid (control of carotenogenesis). According to Zhang et al. [27] epigenetic changes are linked with the biosynthesis of chlorophylls and tocopherols whose precursor is phytyl diphosphate. Lushchak and Semchuk [28] were also interested in these epigenetic changes. According to these authors, plants can increase photosynthesis by chlorophylls biosynthesis or by synthesizing the antioxidant metabolites tocopherols. We showed the continuity of changes in methylation/demethylation of cytosine DNA in relation to the photosynthetic pigments carotenoids and chlorophylls (primary relationship) and also to gas-exchange parameters (GEP) or to Fv/Fm, which are indicators of plant photosynthetic activity.
It has been well documented that stress-related senescence processes involve the degradation of photosynthetic pigments [9], accompanied by a reduction in photosynthetic efficiency. Our results indicated that excess As reduced the level of chlorophylls and affected photosynthetic processes in Pc-Al fronds (Fig. 2, Tables 2 and 3). Farooq et al. [29] reported that As decreased GEP and pigment content in Brassica napus and, according to Wang et al. [30] this toxic element significantly affected Fv/Fm in P. vittata within 60 days of exposure. An association between epigenetic changes in old fronds, resulting from As stress, and a reduced chlorophyll content might be indicated by the correlation between 5mC and Chl A and Chl B levels (Table 3). The decline in the levels of carotenoids and chlorophylls in young fronds of As100 treated plants was less than that seen in older fronds. A significant increase of the Chl A/Chl B ratio was confirmed for young fronds of As100 plants. These changes, together with the values of PN, E and Fv/Fm, pointed to a senescence in As100 plants. During senescence, plant metabolites are remobilised from old leaves to young leaves after expression of genes typical for senescence [31]. We found that As toxicity slightly increased leaf senescence (Fig. 2, Tables 1, 2 and 3). The opposite correlations results for 5mC, in contrast to direct As toxicity (Table 3), showed that decreases of chlorophylls, fluorescence and PN could be affected by epigenetic changes. Ay et al. [32] published similar conclusions for the effects of epigenetic changes on physiological processes in plants.
A decrease of 5mC content in As plants led to a significant negative correlation with increased amounts of Cu, Zn and Mn, cofactors of superoxide dismutases, and with S, a key element in the biosynthesis of cysteine and methionine. Increased accumulation of tested elements could be affected by epigenetic changes as showed by the significant correlations for 5mC (Table 3). While there was an elevation in the concentrations of Cu, Mn, and Zn, in P. vittata exposed to As contamination [5], in Pc-Al it was more pronounced in old fronds as compared to young ones. These authors observed a same trend as we did – higher Mg content in old fronds compared to young ones in P. vittata. It was reasonable to assume that these elements were significant as cofactors of antioxidative metalloenzymes [33–35] as a part of chlorophylls (Mg), and as non-enzymatic antioxidants, protecting against As-induced stress. Increased concentrations of S can be linked to the accumulation of glutathione and phytochelatins involved in detoxification of cellular As in P. cretica var. Mayii [36]. Based on this, the observation that the concentration of S and Zn remained unaffected and the concentrations of Mn and Mg were reduced in young fronds of Pc-Al grown in As100 soil was surprising. We hypothesised that changes in Mn and Mg content were linked with N metabolism.
Water consumption by ferns is directly proportional to As contamination. One of the causes of changes in water content in fronds is the lignification of the conducting tissues in the roots. We found very low WP values for As100 and As250 plants as a result of stress attributable to As contamination (Table 2). Induced stress in cell walls leads primarily to the lack of water in fronds and secondarily to osmotic stress, which is a limiting factor for the growth and development of these plants. Leaf senescence together with the effect of As resulted in lignification of conducting tissue (Fig. 4). Similar reports of WP reduction as a result of the lignification of conducting tissue in plants exposed to stress conditions were published by Hare and Cress [37] and Yamaguchi et al. [38]. If the photosynthetic membrane system is protected by flavonoids, ascorbate and tocopherols [28, 39–41], then cell walls are protected by lignin [38]. Reduced DNA methylation in Pc-Al increases biosynthesis of sterols, tocopherols, flavonoids, isoflavonoids and lignins because these plant metabolites are epigenetically regulated by silencing genes [40, 42, 43]. Cross-sections through adventitious roots of As100 and As250 plants showed the deformation of root cell walls as a result of lignification (Fig. 4). Zanella et al. [44] observed morphological changes in tobacco roots growing in As and Cd contaminated solutions. They found increased cell wall thickness due to lignin over-deposition in the rhizodermal and external cortical parenchyma cells of the primary structure zone, which led to premature exodermis formation. Similar changes in root, lignification upon exposure to metals were confirmed by Piršelová et al. [45]. According to cited works, the lignification-induced cellular changes result in a reduction in water uptake by plants. This finding is in line with our results: changes in WP values and morphological changes in the roots. Reduction of water content and metabolites subsequently limits the ability of the plant to overcome As toxicity.
Conclusions
The results of this paper point to changes in the metabolism of the As-hyperaccumulator plant Pteris cretica var. Albo-lineata, on exposure to 100 and 250 mg As kg− 1 contamination. Compared with controls, ferns grown in the As100 soils increased As concentrations in young and old fronds, 150- and 170-fold, respectively. Ferns grown in the As250 soil increased As concentration 421-fold compared to control. Higher As content was found in the young fronds in comparison to old fronds of control and As100 treatments. Analysis of 5mC content showed that accumulation of As was associated with affected DNA methylation. The decrease of 5mC was confirmed in old fronds (from 25 to 15% and 12% in contrast to control). As revealed by PCA and correlations, physiological parameters of the Pteris cretica var. Albo-lineata are strongly affected by the methylation status of DNA and by direct As toxicity. Increased accumulation of tested nutrients (Cu, Mn, Zn, Mg and S) or decreased chlorophylls, Fv/Fm, PN and WP could be affected by epigenetic changes as was showed by our results.
Accumulation of As in plants affected photosynthetic processes and the content of pigments in fronds. There was a decrease of chlorophylls (ΣChl by 69%) and Fv/Fm (by 29%) in old fronds of As treatment, indicating a faster progression of senescence. These changes together with values of PN and E, indicated reversible senescence in As plants. Based on our determination of very low values for WP (from − 1.4 to − 3.7) and morphological changes in the roots (lignification of cell walls) we proposed that transport of assimilated metabolites between roots and fronds might be reduced.
Methods
Plant material and experimental design
Plants of Pteris cretica (L.) var. Albo-lineata (Pc-Al) were obtained from the garden centre Tulipa Praha (Czech Republic). Ferns at the 10–15 fronds stage were planted in 5 L pots (1 fern for pot) under greenhouse conditions (natural photoperiod; temperature 22–24 °C; relative humidity approximately 60%) for 122 days. Each pot contained 5 kg of haplic chernozem mixed with 0.5 g N, 0.16 g P and 0.4 g K per 1 kg of soil (supplemented as NH4NO3 and K2HPO4). The soil used in this experiment (Table 4) was collected from a non-polluted area in Prague-Suchdol, Czech Republic (50°8ˊ8˝ N, 14°22ˊ43˝ E). Ferns were grown in this soil without As supplement (control) and with two As dose - 100 mg As per kg soil (As100) and 250 mg As per kg soil (As250). Arsenic was added as a solution of Na2HAsO4 and was thoroughly mixed with the soil; maturation period of spiked soil was ten days. Each treatment was replicated three times. Above-ground biomass of control and As100 variants were separated to young and old fronds. The young and old fronds were separated according to their location in plant habit and their size. The young fronds were located lower ground of fern and their area did not exceed to 5 × 10 cm. The larger fronds from full habit were indicated as old fronds. Growth of new fronds were not found at As250 variants and senescent fronds were indicate as old fronds. After being harvested, the fronds were treated as described below. Cross-sections through an adventitious root were inspected using a Nikon E 200 microscope equipped with DS camera head and the NIS-Elements application (Nikon Instruments, Inc., Melville, NY, USA).
Table 4.
Basic properties of soil
| pHKCl | Corg | CEC | Total As | Water extractable As | Sand | Silt | Clay | Bulk density |
|---|---|---|---|---|---|---|---|---|
| (−) | (%) | (mmol+ kg−1) | (mg kg− 1) | (mg kg− 1) | (%) | (%) | (%) | (g cm− 3) |
| 7.1 | 1.83 | 258 | 16 ± 1.7 | 0.10 ± 0.01 | 26 | 72 | 2 | 2.57 |
Corg, organic carbon; CEC, cation exchange capacity.
Determination of arsenic and other elements
Fronds were oven-dried for three days at 40 °C. Homogenised material (0.5 ± 0.05 g) was digested with a mixture of HNO3 and H2O2 (4:1, v/v) in an Ethos 1 device (MLS GmbH, Leutkirch im Allgäu, Germany). Contents of As, Cu, Mg, Mn, and Zn were determined using inductively coupled plasma-optical emission spectrometry (ICP-OES; Agilent 720, Agilent Technologies Inc., Santa Clara, CA, USA). Certified reference material (CRM NIST 1573a Tomato leaves, Analytika®, Czech Republic) was mineralized under the same conditions for quality assurance.
Isolation of DNA and determination of relative DNA methylation status based on % 5-methylcytosine
The fronds were weighed, frozen in liquid nitrogen and stored at − 80 °C prior to DNA methylation analysis. To isolate total DNA, the fronds (1 g fresh weight) were ground to a fine powder in liquid nitrogen by mortar and pestle. DNA was extracted from 100 mg of powdered tissue using a NucleoSpin Plant II molecular kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany), as instructed in the user manual. The global DNA methylation status of DNA was determined using 100 ng of isolated DNA and a MethylFlash Methylated DNA Quantification Kit (Fluorometric; Epigentek Group Inc., Farmingdale, NY, USA) according to the manufacturer’s instructions. A SpectraMax MiniMax 300 Imaging Cytometer (Molecular Devices LLC, San Jose, CA, USA) with excitation at 530 nm was used to measure the fluorescence at 590 nm.
Determination of pigments
Pigment content in the leaves was measured photometrically with an Evolution 2000 UV-Vis (Thermo Fisher Scientific Inc., Waltham, MA, USA). A vessel-free leaf segment (0.5 cm2) excised from a freshly separated frond was incubated in the dark in 1 ml dimethylformamide with shaking for 24 h. The absorbance of the extract was measured at wavelengths 480, 646.8, and 663.8 nm. Absorbance values at 710 nm were subtracted from these measurements. Data pigment contents were calculated from these data:
Chlorophyll A (Chl A; nmol ml− 1): Chl A = 12.0 × A663.8–3.11 × A646.8.
Chlorophyll B: (Chl B; nmol ml− 1): Chl B = 20.78 × A646.8–4.88 × A663.8.
Total chlorophyll (Σ Chl; nmol ml− 1): Chl A + Chl B = 7.12 × A663.8 + 17.67 × A646.8.
Carotenoids (Crt; nmol ml− 1): Crtx + c = (1000 × A480–1.12 Chl A – 34.07 Chl B) / 245.
Determination of fluorescence
The chlorophyll fluorescence [variable fluorescence (Fv)/maximal fluorescence (Fm); μmol m− 2 s− 1] was measured using a modulated chlorophyll fluorometer OS1-FL (Opti-Sciences, ADC, BioScientific, Ltd., Hoddesdon, UK). The fresh leaf was obscured by clipping after 20 min to set up a dark-adapted state. Chlorophyll fluorescence was excited by a 660 nm solid-state light source, with filters blocking radiation longer than 690 nm. Saturation of the photosystem being measured was achieved by using a filtered 35 W halogen lamp (350–690 nm) with a pulse of 15,000 μmol m− 2 s− 1 during 0.8 s.
Determination of water potential
Water potential (WP; MPa), a measure of the energy status of the water in a system, was measured using a dew point PotentiaMeter (Decagon Devices, Inc., Pullman, WA, USA). The leaves of the plants were placed in a disposable syringe, the air was drawn off from the syringe, and the syringe was tightly closed with parafilm. The specimen was frozen at − 18 °C, then thawed, and the sap flow was pushed out into the measuring chamber of the PotentiaMeter.
Determination of selected photosynthesis parameters with gas-exchange parameters (GEP)
The net photosynthetic rate (PN; μmol CO2 m−2 s−1) and the rate of transpiration (E; mmol H2O m−2 s−1) were determined with the portable gas exchange system LCpro+ (ADC BioScientific, Ltd., Hoddesdon, UK). The water-use efficiency parameter (WUE) was calculated from these determined values (WUE = PN/E). Values of PN and E were determined between 8:00 and 11:30 Central European Time (CET) and conditions of measurement chamber were described previously [46, 47].
Statistical analysis
All data were checked for homogeneity of variance and normality by Levene and Shapiro-Wilk tests. Collected data did not meet the conditions for the use of analysis of variance (ANOVA) and were thus evaluated by non-parametric Kruskal-Wallis test in the Statistica 12.0 program (StatSoft, Inc., Tulsa, OK, USA). Significant differences were assessed as effect of i) treatment on physiological parameters and ii) age of fronds on physiological parameters. A principal component analysis (PCA), in the CANOCO 4.5 program, was applied to all collected data as a single set. We used standardisation of species because data of different characters were analysed together. PCA was used to draw correlations from the complex data set. The results were visualised in the form of bi-plot ordination diagrams using the CanoDraw program [48]. Correlations were confirmed using a linear correlation (r, p < 0.05, p < 0.01, p < 0.001) by Statistica 12.0.
Supplementary information
Additional file 1. Table S1. Content of elements and physiological parameters in young and old fronds of P. cretica var. Albo-lineata growing on low As dose – As100.
Additional file 2. Table S2. Coefficients of variation (CV, %) for the content of elements, DNA methylation and physiological parameters in P. cretica var. Albo-lineata.
Additional file 3. Figure S1. Ordination diagram showing the results of PCA analysis with selected parameters in fronds of P. cretica var. Albo-lineata growing on low As dose – As100. Treatment abbreviations: control, treated with 0 mg As kg− 1 soil; As100, treated with 100 mg As kg− 1 soil. Parameter abbreviations: Crt, carotenoids; Chl A, chlorophyll a; Chl B, chlorophyll b; Σ Chl, total chlorophyll; WP, water potential; PN, net photosynthetic rate; E, transpiration rate; Fv/Fm, fluorescence; 5mC, 5-methylcytosine; As, Cu, Mg, Mn, S and Zn; total content of elements. The first axis of the PCA analysis explained 74% of the variability of all analysed data, the first two axes explained 94% of the variability, and the first four axes together explained 99% of the variability.
Additional file 4. Table S3. Linear correlation of As and 5mC with selected parameters of P. cretica var. Albo-lineata growing on low As dose – As100.
Acknowledgements
We thank Ms. Hana Zámečníková, from the Czech University of Life Sciences Prague, for analyses of arsenic and other elements.
Abbreviations
- 5mC
5-methylcytosine content
- As100
Treatment with 100 mg As per kg soil
- As250
Treatment with 250 mg As per kg soil
- Chl A
Chlorophyll A
- Chl B
Chlorophyll B
- Crt
Carotenoids
- E
Rate of transpiration
- Fv/Fm
Chlorophyll fluorescence
- GEP
Gas-exchange parameters
- PCA
Principal Component Analysis
- Pc-Al
Pteris cretica var. Albo-lineata
- PN
Net photosynthetic rate
- WP
Water potential
- WUE
Water-use efficiency
Authors’ contributions
MP and DP conceived and designed the experiments. MP and VZ calculated the relative DNA methylation status. FH calculated selected photosynthesis parameters. JČ performed cross-sections through the roots. VZ, DP, PK, MP analysed the data and wrote the paper. All authors have read and approved the final version of the manuscript.
Funding
This research was supported by the Czech Science Foundation, Grant No. 17-10591S and by the Ministry of Education, Youth and Sports from European Regional Development Fund-Project “Centre for the investigation of synthesis and transformation of nutritional substances in the food chain in interaction with potentially harmful substances of anthropogenic origin: comprehensive assessment of soil contamination risks for the quality of agricultural production” [grant number CZ.02.1.01/0.0/0.0/16_019/0000845]. The funding bodies provided the financial support to the research projects, but did not involve in study design, data collection, analysis, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The plant material was bought from the Tulipa Praha garden centre (Czech Republic). No other permissions were necessary to buy and to cultivate these plants.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12870-020-2325-6.
References
- 1.Eisler R. Eisler’s encyclopedia of environmentally hazardous priority chemicals. Amsterdam: Elsevier; 2007. [Google Scholar]
- 2.Ye WL, Wood BA, Stroud JL, Andralojc PJ, Raab A, McGrath SP, et al. Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol. 2010;154(3):1505–1513. doi: 10.1104/pp.110.163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao FJ, Dunham SJ, McGrath SP. Arsenic hyperaccumulation by different fern species. New Phytol. 2002;156(1):27–31. doi: 10.1046/j.1469-8137.2002.00493.x. [DOI] [Google Scholar]
- 4.Su YH, McGrath SP, Zhu YG, Zhao FJ. Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol. 2008;180(2):434–441. doi: 10.1111/j.1469-8137.2008.02584.x. [DOI] [PubMed] [Google Scholar]
- 5.Tu C, Ma LQ. Effects of arsenic on concentration and distribution of nutrients in the fronds of the arsenic hyperaccumulator Pteris vittata L. Environ Pollut. 2005;135(2):333–340. doi: 10.1016/j.envpol.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Koller CE, Patrick JW, Rose RJ, Offler CE, MacFarlane GR. Pteris umbrosa R. Br. As an arsenic hyperaccumulator: accumulation, partitioning and comparison with the established as hyperaccumulator Pteris vittata. Chemosphere. 2007;66(7):1256–1263. doi: 10.1016/j.chemosphere.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, et al. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health. 2018;15(1):59. doi: 10.3390/ijerph15010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agnihotri A, Seth CS. Exogenously applied nitrate improves the photosynthetic performance and nitrogen metabolism in tomato (Solanum lycopersicum L. cv Pusa Rohini) under arsenic (V) toxicity. Physiol Mol Biol Plants. 2016;22(3):341–349. doi: 10.1007/s12298-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 2009;11(4):861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 10.Yaish MW. Editorial: epigenetic modifications associated with abiotic and biotic stresses in plants: an implication for understanding plant evolution. Front Plant Sci. 2017;8:1983. doi: 10.3389/fpls.2017.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechat MM, Brun G, Montiel G, Véronési C, Simier P, Thoiron S, et al. Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parasitic plant, Phelipanche ramosa L. Pomel J Exp Bot. 2015;66(11):3129–3140. doi: 10.1093/jxb/erv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossdorf O, Arcuri D, Richards CL, Pigliucci M. Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol Ecol. 2010;24(3):541–553. doi: 10.1007/s10682-010-9372-7. [DOI] [Google Scholar]
- 13.Iwase Y, Shiraya T, Takeno K. Flowering and dwarfism induced by DNA demethylation in Pharbitis nil. Physiol Plant. 2010;139(1):118–127. doi: 10.1111/j.1399-3054.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 14.Ba Q, Zhang G, Wang J, Niu N, Ma S, Wang J. Gene expression and DNA methylation alterations in chemically induced male sterility anthers in wheat (Triticum aestivum L.) Acta Physiol Plant. 2014;36(2):503–512. doi: 10.1007/s11738-013-1431-6. [DOI] [Google Scholar]
- 15.Bona E, Cattaneo C, Cesaro P, Marsano F, Lingua G, Cavaletto M, et al. Proteomic analysis of Pteris vittata fronds: two arbuscular mycorrhizal fungi differentially modulate protein expression under arsenic contamination. Proteomics. 2010;10(21):3811–3834. doi: 10.1002/pmic.200900436. [DOI] [PubMed] [Google Scholar]
- 16.Campos NV, Araújo TO. Arcanjo-Silva S, Freitas-Silva L, Azevedo AA, Nunes-Nesi A. Arsenic hyperaccumulation induces metabolic reprogramming in Pityrogramma calomelanos to reduce oxidative stress. Physiol Plant. 2016;157(2):135–146. doi: 10.1111/ppl.12426. [DOI] [PubMed] [Google Scholar]
- 17.He S, Hu Y, Wang H, Wang H, Li Q. Effects of indole-3-acetic acid on arsenic uptake ad antioxidative enzymes in Pteris cretica var. nervosa and Pteris ensiformis. Int J Phytoremediat. 2017;19(3):231–238. doi: 10.1080/15226514.2016.1207609. [DOI] [PubMed] [Google Scholar]
- 18.Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010;8(3):199–216. doi: 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- 19.Ushijima T, Okochi-Takada E. Aberrant methylations in cancer cells: where do they come from? Cancer Sci. 2005;96(4):206–211. doi: 10.1111/j.1349-7006.2005.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aina R, Sgorbati S, Santagostino A, Labra M, Ghiani A, Citterio S. Specific hypomethylation of DNA is induced by heavy metals in white clover and industrial hemp. Physiol Plant. 2004;121(3):472–480. doi: 10.1111/j.1399-3054.2004.00343.x. [DOI] [Google Scholar]
- 21.Erturk FA, Aydin M, Sigmaz B, Taspinar MS, Arslan E, Agar G, et al. Effects of As2O3 on DNA methylation, genomic instability, and LTR retrotransposon polymorphism in Zea mays. Environ Sci Pollut Res. 2015;22(23):18601–18606. doi: 10.1007/s11356-015-5426-2. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, He H, Li J, Chen W, Wang X, Guo L, et al. Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell. 2012;24(3):875–892. doi: 10.1105/tpc.111.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burn JE, Bagnall DJ, Metzger JD, Dennis ES, Peacock WJ. DNA methylation, vernalization, and the initiation of flowering. Proc Natl Acad Sci U S A. 1993;90(1):287–291. doi: 10.1073/pnas.90.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albrechtová JTP, Ullmann J, Krekule J, Seidlová F. Effect of 5-azacytidine on growth pattern in Chenopodium rubrum. Plant Sci. 1994;96(1–2):211–215. doi: 10.1016/0168-9452(94)90238-0. [DOI] [Google Scholar]
- 25.Arase S, Kasai M, Kanazawa A. In planta assays involving epigenetically silenced genes reveal inhibition of cytosine methylation by genistein. Plant Methods. 2012;8:10. doi: 10.1186/1746-4811-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cazzonelli CI. Carotenoids in nature: insights from plants and beyond. Funct Plant Biol. 2011;38(11):833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Zhang W, Ren G, Li D, Cahoon RE, Chen M, et al. Chlorophyll synthase under epigenetic surveillance is critical for vitamin E synthesis, and altered expression affects tocopherol levels in Arabidopsis. Plant Physiol. 2015;168(4):1503–1511. doi: 10.1104/pp.15.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lushchak VI, Semchuk NM. Tocopherol biosynthesis: chemistry, regulation and effects of environmental factors. Acta Physiol Plant. 2012;34(5):1607–1628. doi: 10.1007/s11738-012-0988-9. [DOI] [Google Scholar]
- 29.Farooq MA, Gill RA, Ali B, Wang J, Islam F, Ali S, et al. Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology. 2016;25(2):350–366. doi: 10.1007/s10646-015-1594-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang HB, Xie F, Yao YZ, Zhao B, Xiao QQ, Pan YH, et al. The effects of arsenic and induced-phytoextraction methods on photosynthesis in Pteris species with different arsenic-accumulating abilities. Environ Exp Bot. 2012;75:298–306. doi: 10.1016/j.envexpbot.2011.08.002. [DOI] [Google Scholar]
- 31.Gaufichon L, Reisdorf-Cren M, Rothstein SJ, Chardon F, Suzuki A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010;179(3):141–153. doi: 10.1016/j.plantsci.2010.04.010. [DOI] [Google Scholar]
- 32.Ay N, Janack B, Humbeck K. Epigenetic control of plant senescence and linked processes. J Exp Bot. 2014;65(14):3875–3887. doi: 10.1093/jxb/eru132. [DOI] [PubMed] [Google Scholar]
- 33.Cakmak I. Tansley review no. 111. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146(2):185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Ocaña A, Chaki M, Luque F, Gómez-Rodríguez MV, Carreras A, Valderrama R, et al. Functional analysis of superoxide dismutases (SODs) in sunflower under biotic and abiotic stress conditions. Identification of two new genes of mitochondrial Mn-SOD. J Plant Physiol. 2011;168(11):1303–1308. doi: 10.1016/j.jplph.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Zemanová V, Pavlík M, Pavlíková D. Cadmium toxicity induced contrasting patterns of concentrations of free sarcosine, specific amino acids and selected microelements in two Noccaea species. PLoS One. 2017;12(5):e0177963. doi: 10.1371/journal.pone.0177963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raab A, Feldmann J, Meharg AA. The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol. 2004;134(3):1113–1122. doi: 10.1104/pp.103.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hare PD, Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997;21(2):79–102. doi: 10.1023/A:1005703923347. [DOI] [Google Scholar]
- 38.Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, Rogers EE, et al. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ. 2010;33(2):223–243. doi: 10.1111/j.1365-3040.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- 39.Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78(6):661–669. doi: 10.1006/anbo.1996.0175. [DOI] [Google Scholar]
- 40.Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007;143(4):1720–1738. doi: 10.1104/pp.106.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang YL, Ren WW, Zhang L, Tang KX. Molecular cloning and characterization of gene coding for γ-tocopherol methyltransferase from lettuce (Lactuca sativa) Genet Mol Res. 2011;10(4):3204–3212. doi: 10.4238/2011.December.21.2. [DOI] [PubMed] [Google Scholar]
- 42.Anterola AM, Lewis NG. Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry. 2002;61(3):221–294. doi: 10.1016/S0031-9422(02)00211-X. [DOI] [PubMed] [Google Scholar]
- 43.Gas-Pascual E, Simonovik B, Schaller H, Bach TJ. Inhibition of cycloartenol synthase (CAS) function in tobacco BY-2 cells. Lipids. 2015;50(8):761–772. doi: 10.1007/s11745-015-4036-6. [DOI] [PubMed] [Google Scholar]
- 44.Zanella L, Fattorini L, Brunetti P, Roccotiello E, Cornara L, D'Angeli S, et al. Overexpression of AtPCS1 in tobacco increases arsenic and arsenic plus cadmium accumulation and detoxification. Planta. 2016;243(3):605–622. doi: 10.1007/s00425-015-2428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piršelová B, Kuna R, Libantová J, Moravčíková J, Matušíková I. Biochemical and physiological comparison of heavy metal-triggered defense responses in the monocot maize and dicot soybean roots. Mol Biol Rep. 2011;38(5):3437–3446. doi: 10.1007/s11033-010-0453-z. [DOI] [PubMed] [Google Scholar]
- 46.Zemanová V, Pavlík M, Pavlíková D, Hnilička F, Vondráčková S. Responses to cd stress in two Noccaea species (Noccaea praecox and Noccaea caerulescens) originating from two contaminated sites in Mežica, Slovenia and Redlschlag. Austria Arch Environ Contam Toxicol. 2016;70(3):464–474. doi: 10.1007/s00244-015-0198-8. [DOI] [PubMed] [Google Scholar]
- 47.Holá D, Benešová M, Fischer L, Haisel D, Hnilička F, Hniličková H, et al. The disadvantages of being a hybrid during drought: a combined analysis of plant morphology, physiology and leaf proteome in maize. PLoS One. 2017;12(4):e0176121. doi: 10.1371/journal.pone.0176121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ter Braak CJF, Šmilauer P. CANOCO reference manual and CanoDraw for windows user’s guide: software for canonical community ordination (version 4.5) Ithaca: Microcomputer Power; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Content of elements and physiological parameters in young and old fronds of P. cretica var. Albo-lineata growing on low As dose – As100.
Additional file 2. Table S2. Coefficients of variation (CV, %) for the content of elements, DNA methylation and physiological parameters in P. cretica var. Albo-lineata.
Additional file 3. Figure S1. Ordination diagram showing the results of PCA analysis with selected parameters in fronds of P. cretica var. Albo-lineata growing on low As dose – As100. Treatment abbreviations: control, treated with 0 mg As kg− 1 soil; As100, treated with 100 mg As kg− 1 soil. Parameter abbreviations: Crt, carotenoids; Chl A, chlorophyll a; Chl B, chlorophyll b; Σ Chl, total chlorophyll; WP, water potential; PN, net photosynthetic rate; E, transpiration rate; Fv/Fm, fluorescence; 5mC, 5-methylcytosine; As, Cu, Mg, Mn, S and Zn; total content of elements. The first axis of the PCA analysis explained 74% of the variability of all analysed data, the first two axes explained 94% of the variability, and the first four axes together explained 99% of the variability.
Additional file 4. Table S3. Linear correlation of As and 5mC with selected parameters of P. cretica var. Albo-lineata growing on low As dose – As100.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The plant material was bought from the Tulipa Praha garden centre (Czech Republic). No other permissions were necessary to buy and to cultivate these plants.