Abstract
Objective:
Brain imaging studies of structural abnormalities in OCD have yielded inconsistent results, partly due to limited statistical power, clinical heterogeneity, and methodological differences. Here, we perform meta- and mega-analyses comprising the largest study of cortical morphometry in OCD ever undertaken.
Methods:
T1-weighted MRI scans of 1905 OCD patients and 1760 healthy controls from 27 sites worldwide were processed locally using FreeSurfer to assess cortical thickness and surface area. Effect sizes for differences between patients and controls, and associations with clinical characteristics, were calculated using linear regression models controlling for age, sex, site, and intracranial volume.
Results:
In adult OCD patients versus controls, we found significantly lower surface area of the transverse temporal cortex and a thinner inferior parietal cortex. Medicated adult OCD patients also showed thinner cortices throughout the brain. In pediatric OCD patients versus controls, we found significantly thinner inferior and superior parietal cortices, but none of the regions analyzed showed significant differences in surface area. However, medicated pediatric OCD patients had lower surface area in frontal regions. Cohen’s d effect sizes varied between −0.10 and −0.33.
Conclusion
The parietal cortex was consistently implicated both in adults and children with OCD. More widespread cortical thickness abnormalities were found in medicated adult OCD patients, and more pronounced surface area deficits (mainly in frontal regions) were found in medicated pediatric OCD patients. These cortical measures represent distinct morphological features and may be differentially affected during different stages of development and illness, and possibly moderated by disease profile and medication.
Keywords: OCD, MRI, FreeSurfer, Cortical Thickness, Surface Area
Introduction
Disease models of obsessive-compulsive disorder (OCD) propose that abnormalities in the cortico-striato-thalamo-cortical (CSTC) circuits are key to the pathophysiology of OCD. More recent findings also implicate the involvement of fronto-limbic and fronto-parietal regions in pediatric and adult OCD (1–3). An important limitation of brain imaging research is the typically small samples that limit sensitivity and presumably contributing to the lack of reproducibility and reliability (4). This issue may be partially addressed by the use of meta- and mega-analysis of multiple study samples. We therefore initiated the OCD working group within the Enhancing Neuro-Imaging Genetics through Meta-Analysis (ENIGMA) consortium (5) in which researchers around the world collaborate to boost statistical power, with the aim of elucidating brain abnormalities in OCD.
Recently, we performed meta- and mega-analyses on data from 3589 individuals and reported subcortical volume differences between OCD patients and healthy controls that were related to clinical characteristics. Distinct subcortical volume abnormalities were detected in adults and children with OCD. Adult OCD patients had significantly smaller hippocampal and larger pallidal volumes. The smaller hippocampal volume seemed to be driven by comorbid depression and an adult illness onset. The larger pallidal volume was more pronounced in adult OCD patients with a childhood illness onset. Children with OCD had larger thalamic volumes compared to control children (6).
With regard to the cortex, prior magnetic resonance imaging (MRI) studies consistently show abnormalities in dorsomedial prefrontal and anterior cingulate cortices (ACC) (7–10), findings that are supported by mega-analyses from the OCD Brain Imaging Consortium (11; 12). Also abnormalities in fronto-parietal and temporo-parietal regions have been reported (10; 12–14). Findings regarding the orbitofrontal cortex (OFC) (8; 11; 15) and operculum have been rather inconsistent (8; 10; 11; 13; 16). These inconsistencies may be partially explained by differences in processing protocols, limited statistical power, and clinical heterogeneity related to variation in disease profile and developmental stage.
Most of these studies were predominantly based on volumetric measures using voxel-based morphometry (VBM). Volumetric measures, however, depend on a combination of changes in gray matter thickness and surface area (17). Fewer studies have used surface-based methods to generate detailed maps of cortical thickness and surface area. These measures represent distinct features of cortical morphometry that are somewhat genetically independent and are driven by different neurobiological processes (18). Studying these properties independently will make it easier to interpret the cortical abnormalities reported in OCD in the context of the postulated neurodevelopmental basis for OCD (19) (Supplementary Information SI1).
Here we performed the largest coordinated worldwide study of cortical measures in patients with OCD compared to healthy controls. We extracted cortical thickness and surface area estimates of 1905 OCD patients and 1760 healthy controls, using harmonized data processing and analysis strategies across 27 sites. We also aimed to establish the potential modulating effects of demographic and clinical characteristics. Based on the prior literature, we expected lower cortical thickness in ACC, OFC, dorsomedial prefrontal cortex (dmPFC) and parietal regions, in OCD patients compared to healthy controls. In addition, we explored the cortical surface area profile in OCD.
Method
Samples
The ENIGMA-OCD working group includes 38 data sets from 27 international research institutes, with neuroimaging and clinical data from OCD patients and typically developing healthy control subjects (i.e., free of psychopathology), including both children and adults (participating sites are mapped in Supplementary Figure S1). Six (i.e. the entire OBIC sample) out of these 38 data sets were identical to those included in the OBIC mega-analyses using VBM (11) and vertex-based FreeSurfer (12). We defined adults as individuals aged ≥18 years and children as individuals aged <18 years. The split at the age of 18 followed from a natural selection of the age ranges used in these samples, as most samples used the age of 18 years as a cut-off for inclusion. Each sample’s demographic and clinical characteristics are detailed in Supplementary Tables S1 and S2. In total, we analyzed data from 3665 subjects including 1905 OCD patients (407 children and 1498 adults) and 1760 control subjects (324 children and 1436 adults). All local institutional review boards permitted the use of measures extracted from the anonymized data for mega-analyses.
Image Acquisition and Processing
Structural T1-weighted MRI brain scans were acquired and processed locally. Image acquisition parameters for each site are given in Supplementary Table S3. All cortical parcellations were performed with the fully automated segmentation software FreeSurfer, version 5.3 (20), following standardized ENIGMA protocols to harmonize analyses and quality control procedures across multiple sites (see http://enigma.usc.edu/protocols/imaging-protocols/). Segmentation of 68 (34 left and 34 right) cortical gray matter regions based on the Desikan-Killiany atlas (21) and two whole-hemisphere measures were visually inspected and statistically evaluated for outliers. Details on image exclusion criteria and quality control are presented in Supplementary Information SI1.
Statistical framework
We performed both a meta-analysis (i.e., using group statistics from the independent studies) and mega-analysis (i.e., pooling extracted measures from individual subjects across sites, while adjusting for site effects) to be consistent with our prior paper. In this manuscript we will focus on the mega-analysis. See Supplementary Information SI3 for methods, results and discussion of the meta-analysis.
We examined differences between OCD patients and controls within a mega-analytical framework by pooling the extracted cortical thickness and surface area measures from each site. Each of the 70 cortical regions of interest (68 regions and two whole-hemisphere average thickness or total surface area measures) served as the outcome measure and a binary indicator of diagnosis as the predictor of interest in multiple linear regression models. All cortical thickness models were adjusted for age and sex; cortical surface area models were corrected for intracranial volume (ICV, see Supplementary Information SI1), age, age2, sex, age-by-sex, and age2-by-sex, to account for any higher-order effects on cortical surface area of age and sex as well as head size, which do not appear to be detectable for cortical thickness measures (22). Additionally, all models were also adjusted for site, coded by using dummy variables. Effect size estimates were calculated using the Cohen’s d metric computed from the t-statistic of the diagnosis indicator variable from the regression models. Similarly, for models testing interactions (i.e., sex-by-diagnosis and age-by-diagnosis), a multiplicative predictor was the predictor of interest with the main effect of each predictor included in the model. The effect size was calculated using the same procedure.
To detect potentially different effects of disease with age, we performed all analyses separately for pediatric and adult participants. We performed stratified analyses comparing the medicated group and unmedicated group of OCD patients separately to controls and to each other. Likewise, stratified analyses were performed to investigate effect of comorbid major depressive disorder, comorbid anxiety disorders, and OCD symptom dimensions (using the Yale-Brown Obsessive Compulsive Scale [Y-BOCS] and the Children’s Yale-Brown Obsessive Compulsive Scale [CY-BOCS] symptom checklist; see Supplementary Information SI2). To study the neurodevelopmental aspects of illness within the adult samples, we performed separate stratified analyses comparing childhood-onset OCD patients (onset <18 years) and adult-onset OCD patients (onset ≥18 years). Furthermore, we examined associations with age at onset, illness duration and illness severity (using the total severity score from the (C)Y-BOCS (23; 24)) as continuous variables. In these analyses, effect sizes were expressed as partial-correlation Pearson’s r after removing nuisance variables (age, sex, site and ICV). Throughout the manuscript, we report P-values corrected for multiple comparisons using the Benjamini-Hochberg procedure to ensure a false-discovery rate (FDR) limited at 5% for 70 cortical measures.
Results
An overview of the demographic and clinical characteristics of the pooled samples is provided in Table 1.
Table 1:
Mega-analytical demographics and clinical characteristics.
| Characteristic | Adult OCD Patients (N=1498) | Adult Healthy Controls (N=1436) | Pediatric OCD Patients (N=407) | Pediatric Healthy Controls (N=324) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 32.1* | 9.7 | 30.5* | 9.7 | 13.8 | 2.5 | 13.6 | 2.6 |
| OCD illness severity scorea | 24.4 | 6.9 | 21.4 | 7.3 | ||||
| Age at onset of clinical symptoms (years) | 19.8 | 9.1 | 10.6 | 3.1 | ||||
| N | % | N | % | N | % | N | % | |
| Male | 756 | 50.5 | 713 | 49.7 | 220 | 54.1 | 164 | 50.6 |
| Medication use at time of scan | 646 | 43,1 | 183 | 45.0 | ||||
| Current comorbid anxiety disorder | 224 | 15.0 | 132 | 32.4 | ||||
| Current comorbid major depression diagnosis | 167 | 11.1 | 29 | 7.1 | ||||
| Current comorbid Tourette’s Disorder** | 24 | 1.6 | 30 | 7.4 | ||||
| Current comorbid ADHD** | 13 | 0.9 | 42 | 10.3 | ||||
| Current comorbid ASD** | 0 | 0 | 5 | 1.2 | ||||
| OCD symptom dimensionsb | ||||||||
| Aggressive/checking | 927 | 61.9 | 195 | 47.9 | ||||
| Contamination/cleaning | 791 | 52.8 | 172 | 42.3 | ||||
| Symmetry/ordering | 640 | 42.7 | 181 | 44.5 | ||||
| Sexual/religious | 487 | 32.5 | 92 | 22.6 | ||||
| Hoarding | 379 | 25.3 | 92 | 22.6 | ||||
As measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) total score
As measured with the YBOCS symptom checklist
Statistically significant difference (t(2932)=−4.222, p<0.001)
not assessed in each sample
abbreviations: ADHD attention deficit hyperactivity disorder; ADS autism spectrum disorder
Mega-analysis
Cortical thickness and surface area differences between OCD patients and controls
Adults:
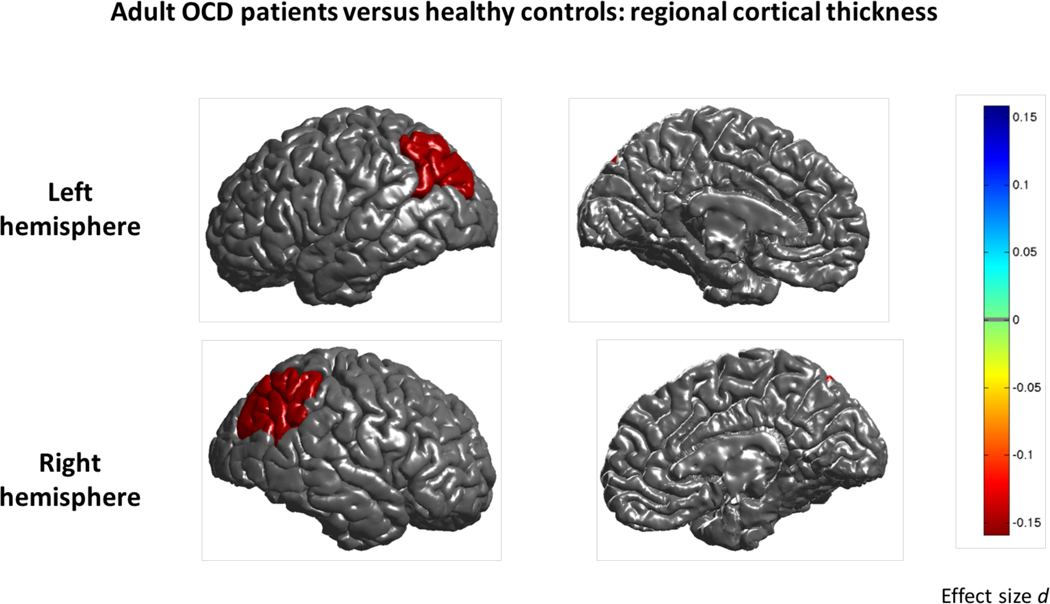
Lower cortical thickness was observed in adult OCD patients (N=1498) compared to controls (N=1436) in the bilateral inferior parietal cortex (Cohen’s d effect size −0.14; Figure 1 and Supplementary Table S4). In all tables, regions are listed in order of effect size (strongest to weakest). A lower surface area was observed in the left transverse temporal cortex (Cohen’s d −0.16; Supplementary Table S5 and Figure S2). None of the regions showed significant sex-by-diagnosis or age-by-diagnosis interaction effects.
Figure 1:
Mega-analysis effect sizes for regions that showed a significant (q<0.05) difference in cortical thickness between adult OCD patients and healthy controls. Negative effect sizes d (red) indicate cortical thinning in OCD compared to controls
Children:
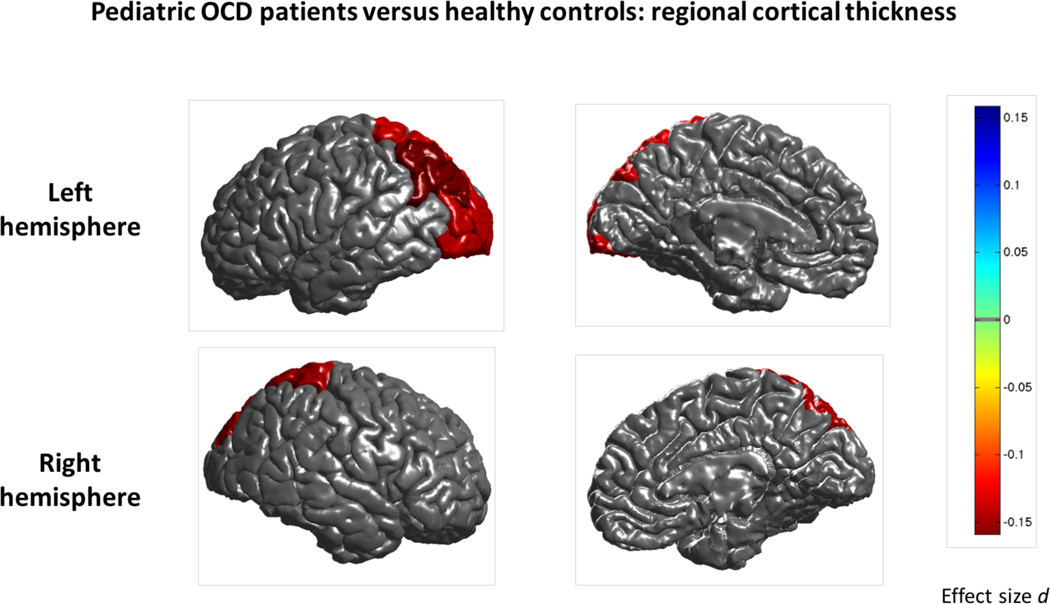
We found significantly thinner cortices in pediatric OCD patients (N=407) compared to controls (N=324) in bilateral superior parietal and left inferior parietal cortices (Figure 2) and left lateral occipital cortex (Cohen’s d between −0.24 and −0.31; Supplementary Table S6). None of the regions analyzed showed significant differences in cortical surface area or evidence of sex-by-diagnosis or age-by-diagnosis interaction effects (Supplementary Table S7).
Figure 2:
Mega-analysis effect sizes for regions that showed a significant (q<0.05) difference in cortical thickness between pediatric OCD patients and healthy controls. Negative effect sizes d (red) indicate cortical thinning in OCD compared to controls
Influence of medication on cortical thickness and surface area
Adults:
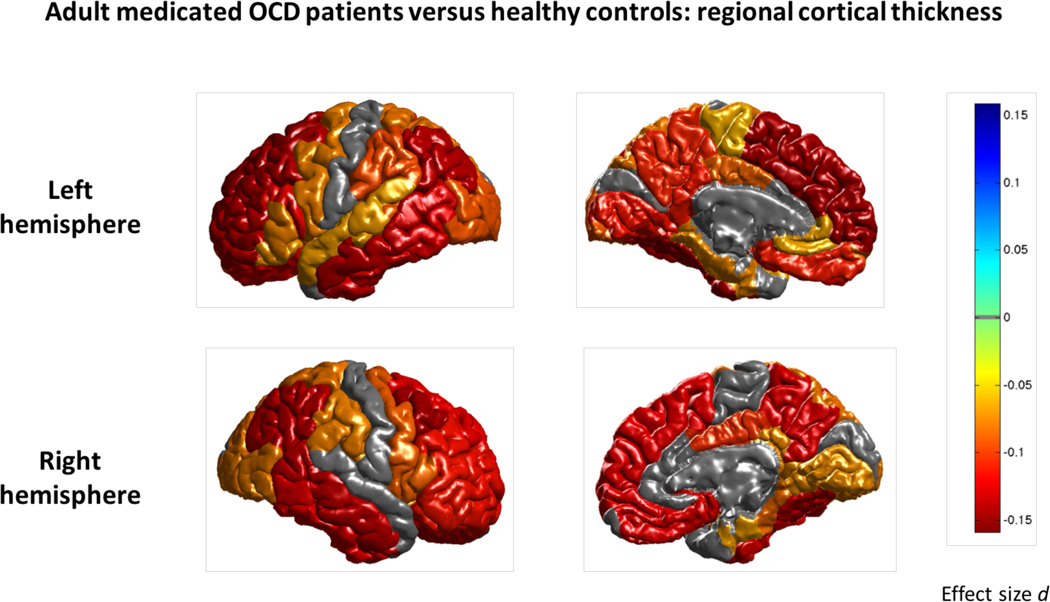
Left and right hemisphere cortical thickness was lower in medicated OCD patients (N=646) compared to controls (N=1436). Regionally, we found significantly thinner cortices in frontal, temporal, parietal and occipital regions of adult medicated OCD patients (Cohen’s d between −0.10 and −0.26; Figure 3 and Supplementary Table S8a). We did not detect significant differences in cortical thickness in unmedicated OCD patients (N=831) compared to controls (Supplementary Table S8b). Medicated OCD patients compared to unmedicated patients showed lower cortical thickness in frontal, temporal and parietal regions (Cohen’s d between −0.13 and −0.21; Supplementary Table S8c and Figure S3). Similar to the main group comparison, we found lower surface area of the left transverse temporal cortex in medicated OCD patients versus controls (Cohen’s d −0.20; Supplementary Table S9a and Figure S4). We did not detect differences in surface area in unmedicated OCD patients compared to controls and when comparing medicated and unmedicated patients directly (Supplementary Tables S9b–c).
Figure 3:
Mega-analysis effect sizes for regions that showed a significant (q<0.05) difference in cortical thickness between adult medicated OCD patients and healthy controls. Negative effect sizes d (red) indicate cortical thinning in OCD compared to controls.
Children:
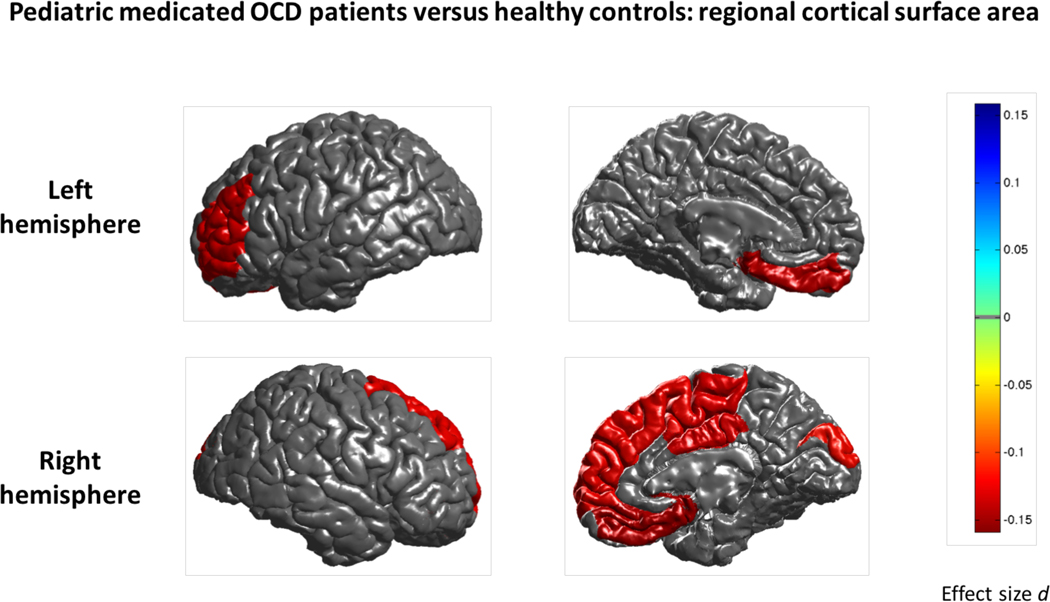
Compared to controls (N=324), medicated children with OCD (N=183) showed lower cortical thickness of the bilateral inferior parietal and superior parietal cortices and left lateral occipital cortex (Cohen’s d ~−0.31; Supplementary Table S10a and Figure S5). We did not detect significant differences in cortical thickness in unmedicated pediatric OCD patients (N=222) compared to controls and when comparing medicated with unmedicated patients (Supplementary Tables S10b–c). More widespread surface area differences were detected when comparing medicated pediatric OCD patients to controls mainly in several frontal regions (Cohen’s d between −0.27 and −0.33; Supplementary Table S11a and Figure 4). No differences in surface area were observed when comparing unmedicated patients to controls (Supplementary Table S11b). We did observe lower surface area of the right lingual (Cohen’s d −0.34) and pericalcarine (Cohen’s d −0.40) cortices in medicated compared to unmedicated pediatric OCD patients (Supplementary Table S11c and Figure S6).
Figure 4:
Mega-analysis effect sizes for regions that showed a significant (q<0.05) difference in cortical surface area between pediatric medicated OCD patients and healthy controls. Negative effect sizes d (red) indicate reduced cortical surface area in OCD compared to controls.
Influence of comorbidities on cortical thickness and surface area
We did not detect any associations between cortical thickness or surface area and current comorbid depression or anxiety disorder in adults (respectively N=167 and N=224) or in children (respectively N=29 and N=132). These numbers, however, are too small because of the lack of systematic assessment of comorbidities in some samples and reflect an underestimation of comorbidity due to exclusion of comorbid cases in other samples. See Supplementary Information SI4 for full details.
Influence of symptom dimensions on cortical thickness and surface area
Adults:
Regression analyses within OCD patients on symptom dimensions (N=1214) showed no associations between the presence of a particular symptom dimension and cortical thickness or surface area with any of the regions.
Children:
Pediatric OCD patients with ordering and symmetry symptoms (N=181) showed a higher surface area of the left cuneus (Cohen’s d 0.49; Supplementary Table S20 and Figure S14). None of the regions analyzed showed significant differences in cortical thickness or evidence of associations with the other symptom dimensions.
Influence of age at onset and illness duration on cortical thickness and surface area
Adult OCD patients with an adult illness onset (N=775) compared to controls (N=1436) showed thinner cortices in left and right hemisphere overall. Regionally, we observed thinner cortices in frontal and temporal regions of adult-onset patients (Cohen’s d between −0.11 and −0.16; Supplementary Table S21a and Figure S15). We also found lower surface area of the left transverse temporal cortex (Cohen’s d −0.17) and the left pars opercularis (Cohen’s d −0.14) in adult OCD patients with an adult illness onset (Supplementary Table S22a and Figure S16). We did not detect significant differences in cortical thickness or surface area in adult OCD patients with a childhood illness onset (N=646) compared to controls or when comparing adult-onset and childhood-onset patients directly (Supplementary Tables S21b–c and Supplementary Tables S22b–c).
Furthermore, we did not observe any significant linear (Supplementary Tables S23–S26) or quadratic (Supplementary Tables S37–S40) associations between age at onset or illness duration as continuous variables and cortical thickness or surface area changes in the adult (N=1419) or pediatric (N=708) OCD groups.
Association between illness severity and cortical thickness and surface area
We did not detect any significant linear (Supplementary Tables S27–S28) or quadratic (Supplementary Tables S41–S42) associations in either the adult (N=1453) or the pediatric (N=404) OCD patients between illness severity ([C]Y-BOCS) and cortical thickness or surface area.
Meta-analysis
Decreased cortical thickness of the inferior parietal cortex was present in adult patients with OCD compared to healthy controls, but at a less stringent significance threshold (Cohen’s d≈ −0.14; p <0.01, uncorrected). The meta-analysis did show significant widespread effects of medication on cortical thickness and a lower surface area of the transverse temporal cortex in adult OCD patients. The pediatric meta-analysis, also on a less stringent significance threshold (Cohen’s d≈ −0.31; p <0.05, uncorrected), showed decreased cortical thickness of the inferior and superior parietal cortex in children with OCD. In addition, field strength did not significantly explain the effect size estimates of cortical thickness or surface area differences in adult and pediatric OCD patients compared to controls (Supplementary Information SI3)
Discussion
Cortical thickness
This is the largest neuroimaging study conducted on cortical measures in OCD to date. We found that the parietal cortex was consistently implicated both in adult and pediatric OCD, which is consistent with prior VBM and FreeSurfer studies (12; 25). Lower cortical thickness of the inferior parietal cortex in adult OCD patients compared to controls is in accordance with results reported by Kuhn et al. (10) and the OBIC consortium (12). Lower cortical thickness of the inferior and superior parietal cortex in children with OCD is a novel finding. The only other study of cortical thickness in pediatric OCD found lower cortical thickness of another parietal region, the supramarginal gyrus (29). Other imaging studies have reported lower gray matter volume in the parietal lobe, especially in the angular gyrus of the inferior parietal lobe in children and adults with OCD (25; 30).
In contrast to previous mega-analyses from the OBIC consortium, we did not find cortical thickness abnormalities in the OFC, ACC, or dmPFC. Six (i.e. the entire OBIC sample) out of our 38 data sets were identical to those included in the OBIC mega-analysis. Apart from a much larger sample size including samples from more different countries, no discrepancies between demographic and clinical characteristics could be found between this sample and the OBIC sample. Thus these inconsistencies are likely to reflect differences in analysis methods and the overall sample size. While FreeSurfer measures thickness and surface area separately, it segments whole structures based on probabilistic information from a predefined atlas (20), compared to VBM’s voxel-wise registration (26). Mainly global or regional differences in structure can be inferred from these atlas-based FreeSurfer analyses, as opposed to local morphology as with VBM. Moreover, the FreeSurfer mega-analysis of the OBIC consortium (12) was conducted using vertex-based analyses rather than the atlas-based approach we used in the current study. It is thus possible that certain abnormalities on vertex level are not detectable when averaging data across whole regions (27). Notably, the OBIC sample included only 1.5T scans and was processed using an older version of FreeSurfer V4.5. Future research using higher resolution parcellation (e.g., (28)) is necessary to validate our results.
In the present study we had sufficient statistical power to detect subtle (Cohen’s d, −0.15 to −0.31) cortical abnormalities in OCD (Supplemental Information SI5). Large-scale studies such as ours are well powered to distinguish consistent, generalizable findings from false positives. Structural MRI provides a crude and indirect measure of putative alterations at the molecular level, but these subtle abnormalities in the parietal cortex may still be relevant from a pathophysiological perspective (31). These results provide insight into what systems are affected, and promote further research to evaluate specific pathways implicated in the pathophysiology of OCD.
Neuroimaging studies of normal brain maturation demonstrate a continuous increase in parietal thickness reaching peak values around age 12, followed by a steady decrease over subsequent decades (32). In terms of neurodevelopmental abnormalities, our results may be cautiously interpreted as evidence for a relationship between the expression of OCD and disturbances in factors influencing radial cortical expansion, which influences gray matter thickness rather than factors influencing the tangential expansion that determines the overall surface area (33). In this context, our results could indicate an altered cortical maturation in OCD resulting in a thinner parietal cortex in early childhood, persisting into adulthood, although further confirmatory work using longitudinal samples is needed.
Cognitive studies in OCD suggest that the parietal cortex plays a significant role in accounting for the cognitive deficits seen in OCD patients. Parietal lobe activation may be related to attention, set shifting, planning and response inhibition, which are also reported to be impaired in OCD patients (34) and reflect a lack of cognitive flexibility that may be related to the repetitive nature of OCD symptoms and behaviors. The inferior parietal cortex is an important node in both the fronto-parietal network and the default mode network. Several studies reported altered connectivity within these networks in patients with OCD (35–37). The phenomenology of the disorder is consistent with the idea of a disrupted relationship between ongoing internal thought and external information, in that patients often excessively focus on internally generated fears that are inconsistent with evidence present in the external environment (38).
We reported lower cortical thickness of numerous regions throughout the brain of medicated adult OCD patients. These medication effects partially overlap with prior research (12). Although these findings need to be interpreted with caution, it has been suggested that antidepressants might modulate plasticity in the brain (39). Additionally, post-hoc analyses suggest that these medication effects are strongest in those patients taking antidepressants with adjuvant antipsychotics (Supplementary Tables S36a–c and Supplementary Info SI4). Alternatively, those patients taking medication could represent a more clinically severe cohort that manifests these morphometric abnormalities. Results may have been confounded by a higher illness severity and a higher percentage of comorbid depression of the medicated adult OCD group (Supplementary Table S35). However, results of post-hoc analyses comparing the most severe (YBOCS>30) unmedicated OCD patients to controls did not resemble the same pattern of medication effects. Nevertheless, the cortical abnormalities in currently medicated OCD patients could reflect persistent abnormalities related to increased OCD severity before treatment. In addition, medication effects persisted after adding a covariate correcting for illness severity (data not shown). The lack of association between severity according to the (C)Y-BOCS and cortical measures could be due to medication reducing the symptom severity. Additionally, current symptom severity might not be optimal to capture the long-term disease severity.
With regard to retrospectively ascertained age at onset in adult patients, the lack of inferior parietal abnormalities in the adult sample with childhood onset might be explained by insufficient power. When looking at the effect sizes, decreased cortical thickness of the inferior parietal cortex was present in adult patients with a childhood disease onset compared to healthy controls, but at a less stringent significance threshold. The effect size was even slightly larger than the effect size of the main group comparison suggesting a power issue, rather than a lack of inferior parietal abnormalities. In contrast, adult illness onset was associated with widespread thinner cortices. The adult-onset group is older than the childhood-onset group, but also has a higher percentage of medicated patients. Post hoc analyses showed that these effects mostly disappear when correcting for medication status suggesting that these findings are mainly driven by medication.
Cortical structural deficits were not associated with comorbid depression or anxiety. The effect sizes of these small sub-groups with comorbid anxiety or depression indicate insufficient statistical power to address this issue with certainty. From a clinical point of view comorbid Tourette’s syndrome and attention-hyperactivity-deficit disorder are more relevant to study in children with OCD. Because of the lack of systematic clinical investigation of comorbidities we were unable to investigate this. Common comorbidities may be more aptly termed interacting variables, as they interact in complex ways. Therefore, excluding comorbid conditions will ignore complex interactions that are often integral to the disorder.
Surface area
The transverse temporal cortex surface area deficit was consistent across analyses in adult OCD. This region belongs to the primary auditory cortex and has not been implicated in OCD pathophysiology before. Lower cortical thickness and lower volume of this region have been associated with auditory hallucinations in schizophrenia (40). Prior approaches to detect structural alteration in this region may have been hampered by small samples or the modest sensitivity of conventional volumetric approaches. The advantage of high statistical power allows us to examine abnormalities throughout the brain without the need to pre-specify regions of interest and thus identify new regions putatively associated with the disorder. Further research is necessary understand the involvement of the transverse temporal cortex in OCD.
Medicated children with OCD had smaller left and right hemisphere total surface area, reflecting a diffuse pattern of frontal surface area deficits. These findings cannot be explained by differences in illness severity, comorbidity or age at onset (Supplementary Table S35). This may indicate delayed cortical maturation, although longitudinal studies are needed to prove that. The surface area of these frontal regions matures over a more prolonged time course during adolescence (41) and may be especially prone to a maturational delay in pediatric OCD, possibly affected by medication status. Such delayed maturation may alter functional connections with other regions through decreases in growth and branching of dendritic trees and the number of synapses associated with gray matter volume (42), which may persist into adult OCD even if surface area measures normalize when transitioning into adulthood. The absence of cortical surface area abnormalities in the adult OCD patients with a childhood-onset could indicate such normalization.
Limitations
When combining existing data across samples worldwide, data collection protocols were not prospectively harmonized. Imaging acquisition protocols and clinical assessments therefore differed across studies, which limits analysis of sources of heterogeneity.As another limitation we note that the T1-weighted scans were not collected with direct measures of head motion, which might have introduced potential motion-induced bias in cortical measures (43).
In addition, FreeSurfer measurements may benefit from manual edits if they are made consistently across all scans. Although we had an extensive standardized protocol for quality checking, the individual sites did not perform manual editing, as this could have resulted in increased variation in the data across sites due to high number of sites involved.
We reported widespread medication effects both in adults and children with OCD. However, the current study did not allow a reliable investigation of medication effects because of its cross-sectional design and lack of detailed information on history, duration, type, and dosage of psychotropic treatment. We therefore must interpret our results with caution and cannot make any premature conclusions about the effect of anti-OCD medication. Further efforts, e.g. intervention studies with comparisons before and after medication, are required to draw valid conclusions on the impact of medication use on cortical structure.
Several studies using a symptom dimensional approach suggest that symptom dimensions may be mediated by partially distinct neural systems (44; 45). Except for the association between a higher surface area of the left cuneus and ordering/symmetry dimension in children, we did not detect thickness or surface area effects of the other symptom dimensions in children and adults. An explanation may be that symptom subtype differences are more focal and remain undetected in this atlas-based analysis. On the other hand, variance in use of instruments across the participating sites might have led to suboptimal harmonization of the symptom dimension scores and therefore might explain the absence of associations with the symptom dimensions in our study. During harmonization, we defined symptom dimensions in a binary manner as absent or present for each participant based on the (C)Y-BOCS symptom checklist, whereas prior studies have correlated the dimension scores with cortical measures.
As a minor limitation, we note that followed from a natural selection of study samples we split the data in adults (≥ 18 years) and children (< 18 years), which might not be the optimal cut-off related to the onset and evolution of OCD (46; 47) In addition, the pediatric sample represents a wide age range, including puberty. We did not have enough data on pubertal stage to take pubertal development into account. Given the role of hormonal influence on cortical structures this will be useful to pursue in future research.
Conclusion
The parietal cortex was implicated in both adult and pediatric OCD. These results support the hypothesis that the pathophysiology of OCD cannot be solely explained by alterations of the classical CSTC regions and emphasize the importance of parietal regions. Widespread cortical thickness abnormalities were found in medicated adult OCD patients, while more pronounced surface area deficits were found in medicated pediatric OCD patients. Cortical thickness and surface area represent distinct features of the cortex and may be differentially affected by OCD and possibly moderated by medication status. Further work, using longitudinal designs, and incorporating genetic and environmental variables, will be useful in understanding the precise mechanisms underlying the structural abnormalities preceding the onset of the illness and occurring during the course of the illness.
Supplementary Material
Disclosures and acknowledgements:
All authors have no conflicts of interest related to this study. Dan J. Stein has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun in the past 3 years.
The ENIGMA-Obsessive Compulsive Disorder Working-Group gratefully acknowledges support from the NIH BD2K award U54 EB020403–02 (PI: Dr. Thompson) and Neuroscience Amsterdam, IPB-grant to Dr. Schmaal & Dr. van den Heuvel. Supported by the Hartmann Muller Foundation (No. 1460 to Dr. Brem); the International Obsessive-Compulsive Disorder Foundation (IOCDF) Research Award to Dr. Gruner; the Dutch Organization for Scientific Research (NWO) (grants 912–02-050, 907–00-012, 940–37-018, and 916.86.038); the Netherlands Society for Scientific Research (NWO-ZonMw VENI grant 916.86.036 to Dr. van den Heuvel; NWO-ZonMw AGIKO stipend 920–03-542 to Dr. de Vries), a NARSAD Young Investigators Award to Dr. van den Heuvel, and the Netherlands Brain Foundation (2010(1)-50 to Dr. van den Heuvel); Oxfordshire Health Services Research Committee (OHSRC) (Dr. Anthony James); the Deutsche Forschungsgemeinschaft (DFG) (KO 3744/2–1 to Dr. Koch); the Marató TV3 Foundation grants 01/2010 and 091710 to Dr. Lazaro; the Wellcome Trust and a pump priming grant from the South London and Maudsley Trust, London, UK (Project grant no. 064846) to Dr. Mataix-Cols; the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT KAKENHI No. 26461753 to Dr. Nakamae); International OCD Foundation Research Award 20153694 and an UCLA Clinical and Translational Science Institute Award (to Dr. Nurmi); National Institutes of Mental Health grant R01MH081864 (to Drs. O’Neill and Piacentini) and grant R01MH085900 (to Drs. O’Neill and Feusner); the Government of India grants to Prof. Y.C. Janardhan Reddy (SR/S0/HS/0016/2011) and Dr. Janardhanan C. Narayanaswamy (DST INSPIRE faculty grant -IFA12-LSBM-26) of the Department of Science and Technology; the Government of India grants to Prof. Y.C. Janardhan Reddy (No.BT/PR13334/Med/30/259/2009) and Dr. Janardhanan C. Narayanaswamy (BT/06/IYBA/2012) of the Department of Biotechnology; the Wellcome-DBT India Alliance grant to Dr. Ganesan Venkatasubramanian (500236/Z/11/Z); the Carlos III Health Institute (CP10/00604, PI13/00918, PI13/01958, PI14/00413/PI040829); FEDER funds/European Regional Development Fund (ERDF), AGAUR (2014 SGR 1672 and 2014 SGR 489); a “Miguel Servet” contract (CP10/00604) from the Carlos III Health Institute to Dr. Soriano-Mas; the Italian Ministry of Health (RC10–11-12–13-14–15A to Dr. Spalletta); the Swiss National Science Foundation (No. 320030_130237 to Dr. Walitza); and the Netherlands Organization for Scientific Research (NWO VIDI 917–15-318 to Dr. van Wingen).
Further we wish to acknowledge Nerisa Banaj, Ph.D., Silvio Conte, Sergio Hernandez B.A., Yu Jin Ressal and Alice Quinton.
Footnotes
See Full Consortium List excel file for the complete list of ENIGMA-OCD Working-Group members
References
- 1.van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, Denys D, Goudriaan AE, Veltman DJ: Brain circuitry of compulsivity. Eur. Neuropsychopharmacol. 2016; 26:810–827 [DOI] [PubMed] [Google Scholar]
- 2.Milad MR, Rauch SL: Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012; 16:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piras F, Piras F, Chiapponi C, Girardi P, Caltagirone C, Spalletta G: Widespread structural brain changes in OCD: A systematic review of voxel-based morphometry studies. Cortex 2015; 62:89–108 [DOI] [PubMed] [Google Scholar]
- 4.Button KS, Ioannidis JP a, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR: Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013; 14:365–76 [DOI] [PubMed] [Google Scholar]
- 5.Thompson PM, Andreassen OA, Arias-Vasquez A, Bearden CE, Boedhoe PS, Brouwer RM, Buckner RL, Buitelaar JK, Bulaeva KB, Cannon DM, Cohen RA, Conrod PJ, Dale AM, Deary IJ, Dennis EL, de Reus M a., Desrivieres S, Dima D, Donohoe G, Fisher SE, Fouche J-P, Francks C, Frangou S, Franke B, Ganjgahi H, Garavan H, Glahn DC, Grabe HJ, Guadalupe T, Gutman B a., Hashimoto R, Hibar DP, Holland D, Hoogman M, Pol HEH, Hosten N, Jahanshad N, Kelly S, Kochunov P, Kremen WS, Lee PH, Mackey S, Martin NG, Mazoyer B, McDonald C, Medland SE, Morey RA, Nichols TE, Paus T, Pausova Z, Schmaal L, Schumann G, Shen L, Sisodiya SM, Smit DJ a., Smoller JW, Stein DJ, Stein JL, Toro R, Turner J a., van den Heuvel M, van den Heuvel O a., van Erp TGM, van Rooij D, Veltman DJ, Walter H, Wang Y, Wardlaw JM, Whelan CD, Wright MJ, Ye J: ENIGMA and the Individual: Predicting Factors that Affect the Brain in 35 Countries Worldwide. Neuroimage 2017; 145;389–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boedhoe PSW, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, Benedetti F, Beucke JC, Bollettini I, Bose A, Brem S, Calvo A, Cheng Y, Cho KIK, Dallaspezia S, Denys D, Fitzgerald KD, Fouche J-P, Giménez M, Gruner P, Hanna GL, Hibar DP, Hoexter MQ, Hu H, Huyser C, Ikari K, Jahanshad N, Kathmann N, Kaufmann C, Koch K, Kwon JS, Lazaro L, Liu Y, Lochner C, Marsh R, Martínez-Zalacaín I, Mataix-Cols D, Menchón JM, Minuzzii L, Nakamae T, Nakao T, Narayanaswamy JC, Piras F, Piras F, Pittenger C, Reddy YCJ, Sato JR, Simpson HB, Soreni N, Soriano-Mas C, Spalletta G, Stevens MC, Szeszko PR, Tolin DF, Venkatasubramanian G, Walitza S, Wang Z, van Wingen GA, Xu J, Xu X, Yun J-Y, Zhao Q, Thompson PM, Stein DJ, van den Heuvel OA, Heuvel OA van den: Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis. Am. J. Psychiatry 2017; 174:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radua J, Mataix-Cols D: Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 2009; 195:393–402 [DOI] [PubMed] [Google Scholar]
- 8.Rotge J-Y, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, Aouizerate B, Burbaud P: Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation metaanalysis. Neuropsychopharmacology 2010; 35:686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatasubramanian G, Zutshi A, Jindal S, Srikanth SG, Kovoor JME, Kumar JK, Janardhan Reddy YC: Comprehensive evaluation of cortical structure abnormalities in drug-naive, adult patients with obsessive-compulsive disorder: A surface-based morphometry study. J. Psychiatr. Res. 2012; 46:1161–1168 [DOI] [PubMed] [Google Scholar]
- 10.Kuhn S, Kaufmann C, Simon D, Endrass T, Gallinat J, Kathmann N: Reduced thickness of anterior cingulate cortex in obsessive-compulsive disorder. Cortex 2013; 49:2178–2185 [DOI] [PubMed] [Google Scholar]
- 11.de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, Stein DJ, Fouche JP, Soriano-Mas C, Sato JR, Hoexter MQ, Denys D, Nakamae T, Nishida S, Kwon JS, Jang JH, Busatto GF, Cardoner N, Cath DC, Fukui K, Jung WH, Kim SN, Miguel EC, Narumoto J, Phillips ML, Pujol J, Remijnse PL, Sakai Y, Shin NY, Yamada K, Veltman DJ, Van Den Heuvel OA.: Multicenter voxelbased morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatry 2014; 171:340–349 [DOI] [PubMed] [Google Scholar]
- 12.Fouche J-P, du Plessis S, Hattingh C, Roos A, Lochner C, Soriano-Mas C, Sato JR, Nakamae T, Nishida S, Kwon JS, Jung WH, Mataix-Cols D, Hoexter MQ, Alonso P, OCD Brain Imaging Consortium, de Wit SJ, Veltman DJ, Stein DJ, van den Heuvel OA: Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br. J. Psychiatry 2017; 210:67–74 [DOI] [PubMed] [Google Scholar]
- 13.Nakamae T, Narumoto J, Sakai Y, Nishida S, Yamada K, Kubota M, Miyata J, Fukui K: Reduced cortical thickness in non-medicated patients with obsessive-compulsive disorder. Prog. NeuroPsychopharmacology Biol. Psychiatry 2012; 37:90–95 [DOI] [PubMed] [Google Scholar]
- 14.Fan Q, Palaniyappan L, Tan L, Wang J, Wang X, Li C, Zhang T, Jiang K, Xiao Z, Liddle PF: Surface anatomical profile of the cerebral cortex in obsessive–compulsive disorder: a study of cortical thickness, folding and surface area. Psychol. Med. 2013; 43:1081–1091 [DOI] [PubMed] [Google Scholar]
- 15.Radua J, van den Heuvel O a, Surguladze S, Mataix-Cols D: Meta-analytical comparison of voxelbased morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch. Gen. Psychiatry 2010; 67:701–711 [DOI] [PubMed] [Google Scholar]
- 16.Narayan VM, Narr KL, Philips OR, Thompson PM, Toga AW, Szeszko PR: Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport 2008; 19:1551–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutton C, Draganski B, Ashburner J, Weiskopf N: A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 2009; 48:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler AM, Kochunov P, Fox PT, Duggirala R, Almasy L, Blangero J, Glahn DC: Heritability of volume, surface area and thickness for anatomically defined cortical brain regions estimated in a large extended pedigree. Neuroimage 2009; 47:S162 [Google Scholar]
- 19.Rosenberg DR, Keshavan MS: Toward a neurodevelopmental model of obsessive-compulsive disorder. Biol. Psychiatry 1998; 43:623–640 [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM: Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355 [DOI] [PubMed] [Google Scholar]
- 21.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ: An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–980 [DOI] [PubMed] [Google Scholar]
- 22.Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM: Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage 2010; 52:172–185 [DOI] [PubMed] [Google Scholar]
- 23.Scahill L, Riddle M a., McSWIGGIN-HARDIN M, Ort SI, King R a., Goodman WK, Cicchetti D, Leckman JF: Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and Validity. J. Am. Acad. Child Adolesc. Psychiatry 1997; 36:844–852 [DOI] [PubMed] [Google Scholar]
- 24.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale. Arch 1989; 46:1006–1011 [DOI] [PubMed] [Google Scholar]
- 25.Lázaro L, Bargalló N, Castro-Fornieles J, Falcón C, Andrés S, Calvo R, Junqué C: Brain changes in children and adolescents with obsessive-compulsive disorder before and after treatment: A voxelbased morphometric MRI study. Psychiatry Res. - Neuroimaging 2009; 172:140–146 [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ: Voxel-based morphometry--the methods. Neuroimage 2000; 11:805–821 [DOI] [PubMed] [Google Scholar]
- 27.Clarkson MJ, Cardoso MJ, Ridgway GR, Modat M, Leung KK, Rohrer JD, Fox NC, Ourselin S: A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage 2011; 57:856–865 [DOI] [PubMed] [Google Scholar]
- 28.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC: A multi-modal parcellation of human cerebral cortex. Nature 2016; 536:171–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fallucca E, MacMaster FP, Haddad J, Easter P, Dick R, May G, Stanley JA, Rix C, Rosenberg DR: Distinguishing Between Major Depressive Disorder and Obsessive-Compulsive Disorder in Children by Measuring Regional Cortical Thickness. 2011; 68:527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopřivová J, Horáček J, Tintěra J, Praško J, Raszka M, Ibrahim I, Höschl C: Medial frontal and dorsal cortical morphometric abnormalities are related to obsessive-compulsive disorder. Neurosci. Lett. 2009; 464:62–66 [DOI] [PubMed] [Google Scholar]
- 31.Boedhoe PSW, Schmaal L, Mataix-cols D, ENIGMA-OCD working group, Thompson PM, Stein D, van den Heuvel OA: Association and Causation in Brain Imaging : The Case of OCD - Response to McKay et al. Am. J. Psychiatry 2017; 174(6), 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis a C, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM: Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004; 101:8174–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakic P: Specification of Cerebral Cortical Areas. Science. 1988; 241:170–176 [DOI] [PubMed] [Google Scholar]
- 34.Graybiel AM, Rauch SL: Toward a neurobiology of obsessive-compulsive disorder. Neuron 2000; 28:343–347 [DOI] [PubMed] [Google Scholar]
- 35.Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF: Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One 2012; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, Blair Simpson H: Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2017; 38:678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, Yue Q, Tang H, Yan C, Lui S, Huang X, Chan RCK, Zang Y, He Y, Gong Q: Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J. Psychiatry Neurosci. 2011; 36:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor K, Aardema F: Fusion or confusion in obsessive compulsive disorder. Psychol. Rep. 2003; 93:227–232 [DOI] [PubMed] [Google Scholar]
- 39.Hoexter MQ, Duran DS, Alcante CCD, Dougherty DD, Shavitt RG, Lopes AC, Diniz JB, Deckersbach T, Batistuzzo MC, Bressan RA, Miguel EC, Busatto GF: Gray Matter Volumes in Obsessive-Compulsive Disorder Before and After Fluoxetine or Cognitive-Behavior Therapy : A Randomized Clinical Trial. Neuropsychopharmacology 2012; 37:734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Liang S, Pu W, Song Y, Mwansisya TE, Yang Q, Liu H, Liu Z, Shan B, Xue Z: Reduced cortical thickness in right Heschl’s gyrus associated with auditory verbal hallucinations severity in first-episode schizophrenia. BMC Psychiatry 2015; 15:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wierenga LM, Langen M, Oranje B, Durston S: Unique developmental trajectories of cortical thickness and surface area. Neuroimage 2014; 87, 120–126. [DOI] [PubMed] [Google Scholar]
- 42.Anderson BJ: Plasticity of gray matter volume: The cellular and synaptic plasticity that underlies volumetric change. Dev. Psychobiol. 2011; 53(5), 456–465. [DOI] [PubMed] [Google Scholar]
- 43.Savalia NK, Agres PF, Chan MY, Feczko EJ, Kennedy KM, Wig GS: Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Hum. Brain Mapp. 2017; 38:472–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HBM, Van Balkom AJLM, Veltman DJ: The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain 2009; 132:853–868 [DOI] [PubMed] [Google Scholar]
- 45.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML: Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2004; 61:564–576 [DOI] [PubMed] [Google Scholar]
- 46.Leckman JF, Denys D, Simpson HB, Mataix-Cols D, Hollander E, Saxena S, Miguel EC, Rauch SL, Goodman WK, Phillips KA, Stein DJ: Obsessive-compulsive disorder: A review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress. Anxiety 2010; 27:507–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anholt GE, Aderka IM, van Balkom AJLM, Smit JH, Schruers K, van der Wee NJA, Eikelenboom M, De Luca V, van Oppen P: Age of onset in obsessive-compulsive disorder: admixture analysis with a large sample. Psychol. Med. 2014; 44:185–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.