Abstract
Central nervous system (CNS) maintains a high level of metabolism, which leads to the generation of large amounts of free radicals, and it is also one of the most vulnerable organs to oxidative stress. Emerging evidences have shown that, as the key homeostatic cells in CNS, astrocytes are deeply involved in multiple aspects of CNS function including oxidative stress regulation. Besides, the redox level in CNS can in turn affect astrocytes in morphology and function. The complex and multiple roles of astrocytes indicate that their correct performance is crucial for the normal functioning of the CNS, and its dysfunction may result in the occurrence and progression of various neurological disorders. To date, the influence of astrocytes in CNS oxidative stress is rarely reviewed. Therefore, in this review we sum up the roles of astrocytes in redox regulation and the corresponding mechanisms under both normal and different pathological conditions.
Keywords: astrocyte, astrogliosis, central nervous system, oxidative stress, RNS, ROS
1. INTRODUCTION
Central nervous system maintains a high metabolic rate, accounting for 20% of the overall energy consumption but only 2% of body mass.123 Such high energy consumption yields large amounts of free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). Oxidative stress occurs when the production of free radicals exceeds the antioxidant capacity of CNS. Modern molecular pathophysiology studies have confirmed that oxidative stress plays an important part in various pathological changes in CNS, such as hypoxic/toxic injury, metabolic disturbance, inflammation and oncogenesis.1, 28, 60, 110, 127 Specifically, ROS and RNS could be overproduced under various CNS pathogenesis such as abnormal cell metabolism, mitochondrial damage and calcium overload, which disturbed the balance between physical oxidative reaction and antioxidative system and thus generated lipid, protein peroxidation or DNA damage on the neurons which lead to the damage of neurons.48 Previously, ROS were considered more as a detrimental substance which might cause cell damage and lead to various pathological process in CNS. Along with the in‐depth study of redox biology, ROS/RNS is also regarded as an important signal molecule which regulates various CNS activities.100
Astrocytes are the key homeostatic cells in the CNS, playing a crucial role in maintaining physiological CNS function such as providing nutrition to neurons, keeping the integrity of blood brain barrier, regulating synapse activity and processing cell metabolites.130 As the research progressed, increasing evidence has revealed the crucial role of astrocytes in regulating oxidative stress in CNS. On the one hand, a complete antioxidant response in astrocytes promotes the decomposition and clearance of free radicals produced by neurons and other cell types in the CNS thus protecting the central nervous system from oxidative stress damage. On the other hand, under certain pathological conditions, astrocytes may act as one of the main sources of detrimental ROS and RNS and these excessive free radicals can promote the activation of microglia or directly cause neural damage15, 30, 117, 118, 125 (Figure 1). As the main inherent immune cell of central nervous system, oxidative stress in CNS is well studied in microglia, while there are few reviews to summarize the role of oxidative stress in the CNS in the perspective of astrocytes. This review aims at summarizing the reported role of astrocytes in CNS oxidative stress regulation and the effects of oxidative stress on the physiological or pathological functions of astrocytes to provide a new direction for future intervention in CNS diseases.
Figure 1.
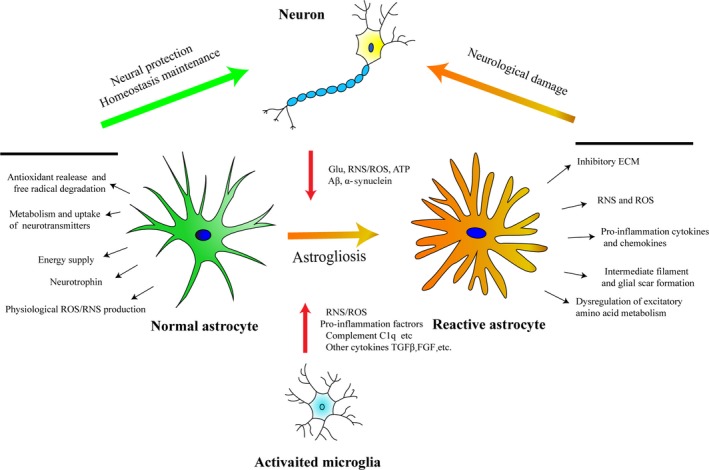
The main molecular basis of response and the interaction among astrocyte, microglia and neuron. Under physiological status, astrocytes maintain homeostasis by releasing antioxidants, degrading ROS/RNS, providing energy and neurotrophin, uptake and metabolism of neurotransmitters, etc Under pathological conditions, astrocytes could be activated via stimulation from activated microglia and degenerated neurons, causing excessive secretion of free radicals and pro‐inflammatory cytokines, glial scar formation and inhibitory ECM deposition, dysregulation of excitatory amino, etc, which lead to aggravation of neurological damage96
2. OXIDATIVE STRESS AND ANTIOXIDANT SYSTEM OF THE CENTRAL NERVOUS SYSTEM
2.1. The oxidative system of the central nervous system
In the CNS, there are two main sources of endogenous ROS: mitochondria and NADPH‐oxidized (NOX) pathway.48 Mitochondrial ROS (mROS) is mainly produced via the process of electron transfer which account for most of the total ROS.76 For another important source of ROS, seven NOXs (NOX1‐5, DUOX1, DUOX2) have been found specifically expressed on the cell membrane of different cell types, catalysing the transport process of electrons from NADPH to O2 which eventually convert to ROS.9, 90 Apart from those two main sources, ROS can also be produced by other oxidase such as cytochrome P450, xanthine oxidase, lipoxygenase and myeloperoxidase.90 RNS is mainly produced from a kind of amino acid, L‐arginine, via the metabolic process of arginine in mitochondria, which is mainly catalysed by nitric oxide synthase (NOS).24
2.2. The antioxidant system of CNS
Two major types of antioxidant response are exist in the CNS: the non‐enzymatic antioxidant system and the enzymatic antioxidant system.69, 147 The enzyme antioxidant system mainly includes several antioxidant enzymes such as glutathione peroxidase (GPxs), superoxide dismutase (SODs), catalase (CATs) and peroxidase (Prxs), and some phase II reaction enzymes, such as haeme oxygenase 1 (HO‐1), reduced coenzyme/quinone oxidoreductase 1 (NQO1) and γ‐glutamylcysteine acid ligase (GCLC).147 Nuclear factor erythroid 2‐related factor 2 (Nrf2) is an important transcription factor involved in maintenance of redox and metabolic homeostasis by regulating the expression of various antioxidant enzymes.22 Non‐enzymatic antioxidant systems include a variety of endogenous reducing substances, such as vitamin C, vitamin E, glutathione (GSH), NADPH, uric acid, bilirubin and melatonin, playing crucial role in ROS scavenging.13, 147 Besides, the thioredoxin (Trx) system is another non‐enzymatic antioxidant system in the CNS89, 100 and it acts as an antioxidant through the reversible oxidation‐reduction reaction between the active site of cysteine in Trx and NADPH.82
3. OXIDATIVE STRESS GENERATED BY ASTROCYTES
3.1. Mitochondria‐derived oxidative stress in astrocytes
Mitochondrial metabolism plays a crucial role in astrocytic redox regulation under physiological or pathological conditions.49 According to the previous view, mitochondria are only distributed in the cell body of astrocytes. However, emerging evidences have confirmed that mitochondria are also present in its thin and long processes, indicating a more complicate function of astrocytic mitochondria.115 Abnormal structure and function of astrocytes have been reported to be involved in clinical pathogenesis of Alexander's disease and amyotrophic lateral sclerosis (ALS).55, 73 In a study focused on experimental ALS, mitochondrial dysfunction is found in SOD1G93A‐expressing astrocytes which greatly contribute to motor neuron damage in the spinal cord and that damage of motor neurons could be attenuate by some mitochondrial‐specific antioxidants.16 To study the role of astrocytic electron transport chain (ETC) in neurological disorders, an astrocytic mitochondrial transcription factor A (Tfam) conditionally knockout model was established which showed increased neuronal death induced by photochemically initiated thrombosis‐induced ischaemic stroke.32 Mice with astrocyte‐specific deletion of the mitochondrial m‐AAA protease (an important protease maintaining mitochondrial homeostasis by degrading misfolded polypeptides and proteins) shows neuronal impairment and behaviour defect.87 Previous studies have suggested that the level of insulin‐like growth factor‐1 (IGF‐1) is closely associated with neuronal ageing and neurodegeneration diseases, and a recent study reveals that IGF‐1 singling could modulate the function of mitochondria and redox level in astrocytes.70 Another study confirms that mROS mediate the classical NLRP3 inflammasome activation induced by LPS in astrocytes.5 Methamphetamine (METH), a monoaminergic toxin, causes death of dopamine terminals and leads to astrogliosis in vivo by disrupting mitochondrial function and increasing ROS in astrocytes.62
3.2. NADPH‐derived oxidative stress in astrocytes
The family of NOXs contains 7 members, among which NOX2 and NOX4 are considered as the most abundant NOXs isoforms expressed in the CNS.120 Although it has been reported that NOX2 is mainly expressed in microglia40 and NOX4 is only expressed slightly in astrocytes,39 recent studies have shown that even the low expressed NOX in astrocytes also plays an important role in the regulation of oxidative stress in the central nervous system. NOX activity and superoxide level of astrocytes increase with ageing,11 and they are closely related with various diseases. In an Alzheimer's disease model, amyloid‐β could upregulate astrocytic NOX2 which induced astrogliosis.17 In the lipopolysaccharide (LPS)‐induced PD model, the expression of NADPH oxidase complex is increased and it is significantly involved in the pathogenesis of PD.114 HIV‐1 glycoprotein 120 (gp120) is a well‐known capsid protein of human immunodeficiency virus which is closely related to the AIDS‐induced neurotoxicity. Results from an in vitro study demonstrate that gp120 and methamphetamine (MA) may cause apoptotic cell death by inducing oxidative stress through NADPH oxidase (NOX) and other pathways in primary and SVGA astrocytes and such effects could be further confirmed by inhibiting NADPH‐derived ROS generation using NOX specific inhibitor or siRNA.113 In an in vitro hypoosmotic swelling model, the amount of ROS produced by NADPH oxidase is found to increase significantly in astrocytes.99 On the contrary, oxidative stress is known to cause astrocyte swelling which contributes largely to the whole brain oedema.52 To sum up, NADPH oxidase significantly affects the physiological function of astrocytes and more attention should be paid to astrocytic NADPH oxidative stress when it comes to seeking new methods to modulate NOX activity in the CNS.
3.3. RNS produced in astrocytes
Astrocytic RNS production is another important part of the astrocyte‐derived oxidative stress. There are three main NOS isoforms expressed in the CNS, namely the Ca2+/calmodulin‐dependent neuronal NOS, the endothelial NOS and the Ca2+‐independent inducible NOS (iNOS), and evidence suggests that all three NOS isoforms are expressed in astrocytes.35, 36, 85 An in vitro study confirms that LPS stimulation can increase the NO production in astrocytes.84 In a primary astrocyte‐neuronal co‐culture system, cytokine stimulation could increase astrocytic RNS production causing the dysfunction of complexes II, III and IV in neurons and this effect could be reversed when astrocytes are removed.121 Severe systemic inflammation has been reported to cause brain injury via activating astrocytic iNOS, nuclear factor kappa B (NF‐κB) and some other pathways.10 Alexander diseases have been proved to be closely associated with glial dysfunction, and a recent study confirmed that astrocytic NO is significantly involved in the astrocyte‐induced neuronal degeneration by affecting cGMP signalling in neurons.135 Additionally, emerging evidences have revealed the importance of S‐nitrosylation (S‐nitrosylate cysteine thiols in target proteins) in astrocytic NO signalling. For example, NO‐induced S‐nitrosylation of PDI (protein disulphide isomerase) is enhanced in an in vitro ischaemic/reperfusion model, causing SOD1 aggregation in astrocytes which might be involved in the pathogenesis of CNS ischaemic/reperfusion injury.18
4. THE ANTIOXIDANT RESPONSE OF ASTROCYTES
4.1. Excitatory amino acids regulated by astrocyte
Glutamate is an important excitatory neurotransmitter mainly released by excitatory neurons delivering excitatory signal in CNS. However, excessive glutamate in synaptic cleft may lead to calcium overload by overactivating NMDA receptors or non‐NMDA receptors (including AMPA receptors and kainic acid (KA) receptors) which generate large amounts of ROS and lead to neurotoxicity.107 Astrocytes are the main cells to maintain homeostasis of glutamate which indirectly affect the balance of oxidative stress (Figure 2). For example, in a study focused on the function of glutamate transporter in CNS, three glutamate transporters (GLAST, GLT‐1 and EAAC1) were, respectively, knocked down in vivo and in vitro and the results indicate that elevated extracellular glutamate and excitotoxicity mainly appeared in the astrocytic glutamate transporters' (GLAST and GLT1) knockdown group compared with EAAC1 (neuronal glutamate transporter) knockdown group, which suggested the central role of astrocytes in functional glutamate transport and prevention of glutamate neurotoxicity.105 In a transgenic rat model of SOD1 mutant‐mediated amyotrophic lateral sclerosis (ALS), researchers found significantly loss of GLT1 (EAAT2) in the ventral horn of the spinal cord and this change appeared before the motor neuron damage, suggesting a potential role for GLT1 dysfunction and oxidative glutamate toxicity in ALS pathology.46 In another study, a mouse model with a mutant caspase‐3 consensus site in the EAAT2 sequence was generated which shows delayed disease progress and longer lifespan compared with the wild‐type mice when crossed with SOD1‐G93A ALS mice.104 Apart from ALS, some neurotoxic substances such as titanium dioxide (TiO2)138 and methylmercury68, 143 or the pathological conditions such as oxygen/glucose deprivation23, 38 and could lead to abnormal glutamate transport which further result in oxidative stress and neurotoxicity.
Figure 2.
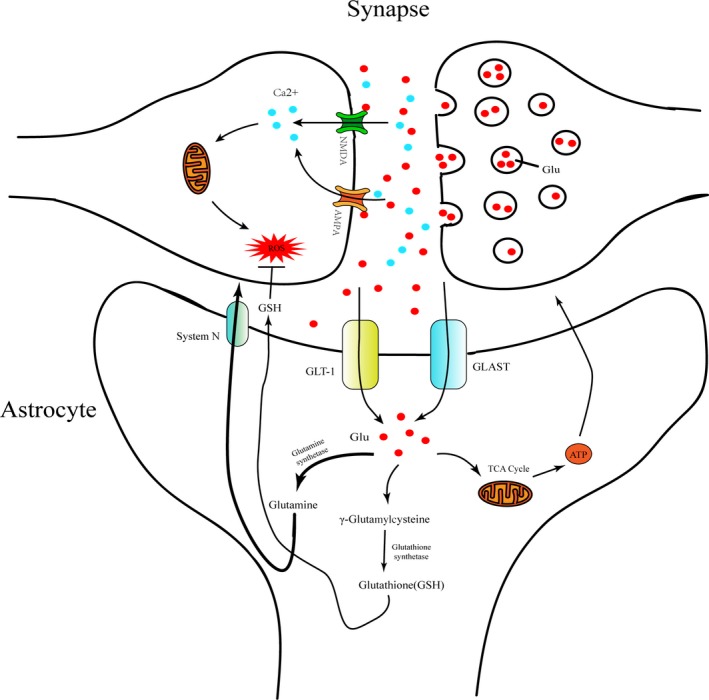
The role of astrocytes in oxidative stress regulation associated with glutamate uptake and metabolism. Under pathological conditions, excessive excitatory neurotransmitters such as glutamate were released from pre‐synaptic membrane and accumulated in the synaptic cleft which activating specific glutamate receptor NMDA and AMPA. Such activated glutamate receptor allows a large influx of Ca2 + which further leads to mitochondrial calcium overload and ROS generation. In another aspect, glutamate in synaptic cleft could be transported into astrocyte by some specific glutamate transporters like GLT1 and GLAST which are highly expressed on the cell membrane of astrocytes adjacent to the synapse. Most of glutamate accumulated in astrocytes is converted into glutamine and be delivered to neurons by the glutamine transporter (system N) maintaining excitatory neurotransmission.126 Partial glutamate in astrocytes is converted into ATP via TCA cycle in the mitochondria and GSH, respectively, catalysed by glutathione synthetase in cytoplasm. These glutamate metabolites returned into the intercellular space providing energy to neurons and inhibiting ROS/RNS130
4.2. GSH synthesis in astrocytes
Compared with neurons, astrocytes have higher capacity for the GSH production and storage, and they can protect neurons from oxidative damage by releasing GSH into the extracellular microenvironment.8, 19, 44 De novo synthesis of GSH in the brain mainly relies on Sxc− cystine/glutamate antiporter (also known as Sxc− , mainly expressed on the membrane of astrocytes and very little in neurons) which exports glutamate from the cells in exchange for cystine providing the raw material for glutathione synthesis,130 and then, the accumulated cystine in astrocytes can be converted into glutathione catalysed by γ‐glutamate‐cysteine ligase and GSH synthetase.77 A study has confirmed that enhancing Sxc‐ expression in astrocytes could increase the GSH level and providing neuroprotection effect.112 Except for the direct provision of GSH, astrocytes also supply glutathione precursor, CysGly, for neuronal glutathione generation which catalysed by astroglial ectoenzyme γ‐glutamyl transpeptidase.26 Additionally, astrocytic GSH synthesis has been reported to be regulated by some inflammation‐related signal pathways. For example, after conditional knockout of astrocyte STAT3, the GSH content of astrocytes is significantly lower than that the wild‐type cells accompanying with the lower mitochondrial membrane potential and the higher level of ROS.109 An in vitro study shows that IL‐1β can increase the production of GSH in astrocytes by activating NF‐KB.43 Some antioxidant drugs such as pramipexole, nitrogen acetylcysteine (NAC) and zonisamide have been found helpful for the treatment of Alzheimer's disease, Parkinson's disease and many other degenerative diseases of the central nervous system by increasing GSH synthesis in astrocytes.33
4.3. Nrf2‐keap1‐ARE antioxidative pathway of astrocyte
Nrf2‐keap1‐ARE pathway is an important endogenous antioxidant system in CNS. In this antioxidative system, Nrf2 is an inducible transcription factor which can be activated in response to oxidative stress146 (the specific signal pathway for Nrf2 activation is shown in Figure 3). It is reported that a specific activator of Nrf2, tBHQ, could enhance the activation the Nrf2 and the downstream antioxidative enzymes like NQO1 and GSTP1 in astrocytes while weakly in neurons.2 In another study, a ARE reporter was transiently transfected into the brain slice and primary cultures and the results indicating that high level of ARE activation and the downstream antioxidative gene expression is mainly restricted to astrocyte cell populations.86 Some researchers found that endogenous H2O2 generated in astrocytes under certain conditions could protect neurons from oxidative stress by activating Nrf2 and further activate the antioxidant stress response.41 Hyperbaric oxygen preconditioning can enhance the expression of Nrf2 in astrocytes but not neurons which increases the tolerance to ischaemic injury of spinal cord.139 In a Parkinson's model, tertiary butylhydroquinone (tBHQ) can activate astrocytes' Nrf2 to protect against neurotoxicity induced by MPP+ 4. Conditional knockout of keap1 in astrocytes could significantly alleviate the demyelinating damage in a mouse model of multiple sclerosis by activating Nrf2.25 In an in vitro study, primary Nrf2−/− astrocytes show more severe inflammatory response and cell damage effect than the wild‐type astrocytes do under a pro‐inflammatory stimulus93 All these evidences suggest that astrocytic Nrf2 is the main regulator involved in CNS oxidative homeostasis and it might be a promising target for neuroprotection.
Figure 3.
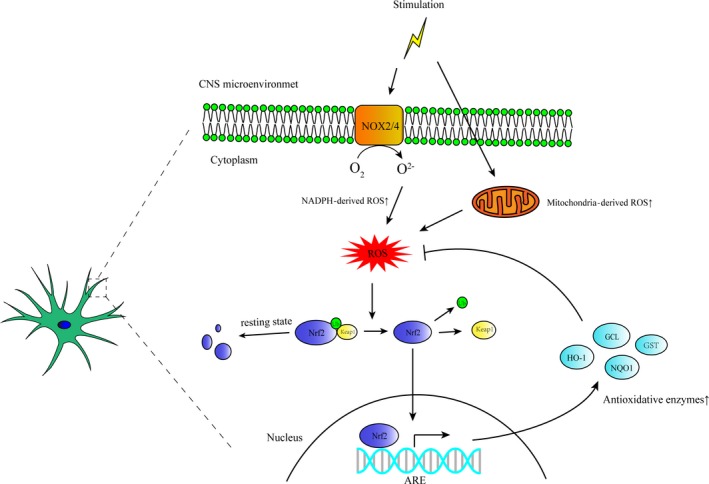
The relationship of oxidative stress and Nrf2‐regulated antioxidant response in astrocytes. Pathological stimulus in CNS microenvironment causes NOX activation or mitochondrial dysfunction both leading to excessive ROS generation in astrocytes which directly interact with keap1 causing the decrease of the activity of ubiquitin E3 ligase and thus prevents the degradation of Nrf2. The stabilized Nrf2 enters the nucleus and binds to ARE promoting the transcription of phase II antioxidative enzymes such as HO‐1, NQO‐1, GCL and GST, thus inhibiting astrocyte‐derived oxidative stress146
5. THE EFFECT OF MICROENVIRONMENTAL OXIDATIVE STRESS ON ASTROCYTES
5.1. Mediating the inflammatory response of astrocytes
Studies suggested that astrocytes are widely involved in inflammation response and innate immunity of the central nervous system. Reactive astrogliosis, also known as astrogliosis, is a general pathologic change in many CNS disorders. Concretely, overactive astrocyte persistently secretes large amounts of inflammatory factors and aggravate neuronal damage.66, 117 Oxidative stress plays an important role in astrocyte‐associated inflammation and the process of astrogliosis.102, 117 Free radicals can activate a variety of inflammatory‐related signalling pathways in astrocytes and promote the inflammatory factor release.53 NLRP3 inflammasome is a kind of intracellular ROS‐activated protein complexes that plays an important role in the innate immune response.133 Studies have shown that mitochondria‐derived ROS can activate the astrocytic NLRP3 inflammatory cascade by promoting the cleavage of pro‐caspase‐1 and the cleaved caspase‐1 could further cleave IL‐1β and IL‐18 precursors to promote the release of IL‐1β and IL‐18.122, 148 Another study confirmed that uncoupling protein 2 (UCP2), a member of mitochondrial anion carrier proteins (MACP), can inhibit astrocytic maturation of IL‐1β by reducing mitochondrial ROS in astrocyte.27 Additionally, chronic ethanol stimulation was found to activate NLRP3‐related inflammation in astrocyte by increasing mitochondria‐derived ROS.5 Similarly, NADPH oxidative‐derived ROS was also been found to be participant in the inflammatory response induced by astrocytes under the treatment of LPS or IFN‐γ.94 Except for the astrocyte‐derived oxidative stress, antioxidant response of astrocytes is also regulating the inflammatory response of CNS. For instance, astrocytes were reported to help increasing the expression of HO‐1 in microglia, decreasing the production of microglia‐derived ROS, thereby inhibiting excessive inflammation of CNS.80 Nrf2‐Keap1‐ARE, a main endogenous antioxidant stress signalling pathway, is also known to be an important anti‐inflammation pathway. In a study focused on astrocytes, researchers found that NF‐KB singling is more likely to be activated in the Nrf2 knockout cells than the wild‐type cells do and lead to more pro‐inflammatory factors released in an mechanical scratch model.93 Furthermore, a variety of other inflammatory signalling pathways have been found to be inhibited by activating Nrf2 signal pathway in astrocytes.54, 141
5.2. Reactive astrogliosis and glial scar formation
Reactive astrogliosis involved in a wide range of CNS disorders such as neurotrauma, stroke, perinatal asphyxia, brain haemorrhage, CNS infections, epilepsy or AD,96 mainly characterized by accelerated proliferation, cell hypertrophy and migration under various stimulation. On the molecular scale, such activation is associated with high expression level of certain cytoskeletal proteins such as glial fibrillary acidic protein (GFAP), vimentin (Vim) and increased secretion of extracellular matrix such as chondroitin sulphate proteoglycan (CSPG).96 In the later stage of CNS injury, astrogliosis may lead to glial scar formation which is considered as the main physical barrier that inhibits the axon regeneration of neurons. So the formation of glial scar is widely considered as one of the important factors affecting the recovery of neural function in spinal cord injury (SCI) or traumatic brain injury (TBI).34, 57, 144 A growing evidence suggests that oxidative stress and inflammation are significant factors in promoting astrogliosis and glial scar formation117 (specific factors that affect reactive astrogliosis can be seen in Figure 1). Some researchers reported that H2O2, a potent oxidant, can upregulate the expression of GFAP and ROS in astrocytes in vitro, and the intervention of molecular hydrogen could inhibit both the production of ROS and overexpression of GFAP in astrocytes.67 Rotenone is a direct mitochondrial respiratory chain blocker, and as Goswami et al37 found, it could upregulate the expression of GFAP in C6 astrocytoma cell line by increasing the mitochondrial ROS. Some inflammation inducers like LPS can stimulate astrocytes to produce NO (a main member of RNS) and increase GFAP expression.12 In an in vivo study, a cortical transplantation model was used to mimic glial activation in neurodegenerative diseases and the results suggest that sustained high levels of oxidative stress after transplantation are associated with chronic glial activation.7 MitoQ, a mitochondria‐targeted antioxidant, attenuates excessive reactive astrogliosis in the brains of Alzheimer's disease model of mice.78 Additionally, some amino acid complex, like cysteamine (CSH), promotes mitochondrial oxidative stress in astrocytes and causes astrocyte activation both in vivo and ex vivo, and the authors indicated that intervention of CSH can be used as a model to study reactive astrogliosis induced by ageing and neurodegenerative diseases.74 JAK‐STAT signal pathway is a key direct mediator of astrogliosis.91, 98, 101 Conditional knockout STAT3 in astrocytes leads to inactivation of astrocytes, and there is no glial scar formed in the injury site of astrocytic STAT3 conditional knockout mice in spinal cord injury model.137 The activation of JAK‐STAT signal was reported to be affected by oxidative stress.137 In a Parkinson's disease model, enhanced NOX activity in microglia is able to mediate microglia‐induced reactive astrogliosis by activating STAT1 or STAT3 signalling pathways.45 On the contrary, activation of endogenous antioxidant system like Nrf2‐Keap1‐ARE could inhibit the activation of excessive astrogliosis.47, 141 In conclusion, regulation of oxidative stress may be an effective way to inhibit excessive glial cell activation and glial scar formation.
5.3. Effect on glutamate transport
As reviewed in the previous section, glutamate metabolism in astrocytes plays a crucial role in maintaining the balance of oxidative stress in the CNS. Reciprocally, the oxidative stress level in CNS also significantly affects the metabolism of glutamate by astrocytes. Studies have found that in vitro peroxide intervention is able to reduce both the glutamate transporter of astrocytes and the transport capacity of astrocytes to glutamate.81 Antioxidants such as hydrogen sulphide and propofol can alleviate the inhibition effect of glutamate transporters induced by oxidative stress and thus maintain the transport of glutamate to astrocytes.72, 116 Korcok J found that LPS and IFN γ treatment significantly inhibit the astrocytic glutamate uptake and this effect could be reversed by ascorbate, an endogenic antioxidant.61 Jayakumar et al51 found that astrocytic oedema and decreased glutamate uptake of astrocyte are related to the activation of oxidative stress‐dependent MAPK pathway, while the antioxidase and antioxidants such as SOD, catalase and vitamin E can inhibit MAPK activation and significantly reduce cell oedema and enhance glutamate uptake. Another study indicated that amyloid‐β protein (Aβ) could reduce the glutamate uptake of astrocytes in vitro by increasing oxidative stress and MAPK activation.75 Reactive astrogliosis and dysfunctional transporters for l‐glutamate [excitatory amino acid transporters, (EAATs)] are the hallmarks of amyotrophic lateral sclerosis (ALS) pathology. Zagami et al145 found that oxidative stress leads to early astrogliosis and impaired EAAT activity of astrocyte, pointing to a critical role of astrocytes in response to oxidative‐induced injury in an ALS model.145 In addition to its effects on glutamate uptake, ROS also affects astrocytic glutamate secretion and this might be one of the potential reasons leading to the neurotoxicity of hippocampal neurons induced by ethanol.108 Except for the influence of ROS, RNS is proved to be associated with glutamate metabolism.97 The results of some studies confirm that the both glutamate transport and metabolism in glutamate/glutamine cycle in astrocytes can be regulated by NO and NO‐induced cysteine S‐nitrosylation.97, 142
6. ROLE OF ASTROCYTE‐RELATED OXIDATIVE STRESS IN CENTRAL NERVOUS SYSTEM DISEASES
6.1. Traumatic injury of central nervous system
Traumatic injury of CNS is always accompanied with severe inflammation and oxidative stress, which lead to the so‐called "secondary strike" effect.50, 83, 131 The drastic change of redox level after injury significantly affects the physiological function of astrocytes. For example, lower astrocytic glutamate transporter (EAATs) expression has been found in human brain biopsy tissue among traumatic brain injury patients, suggesting that the ability of astrocytic excitatory amino acid uptake might be decreased after injury.58 As reviewed in the former section, accumulation of excitatory amino acid in the microenvironment may lead to the overload of mitochondrial calcium, which aggravates the oxidative stress and neuron damage. Lu et al confirmed that the reducing substances such as H2S could increase the expression of glutamate transporter (GLT‐1) and reduce the production of ROS, thereby reducing secondary damage after mechanic injury.134 Ahmed et al3 found that traumatic tensile stress could cause mitochondrial dysfunction in astrocytes and the damaged astrocytes can further affect neuronal mitochondrial function. Additionally, astrocytic NO production significantly increased after injury and it is closely related to the secondary injury.79 Inhibition of NOS activity is reported to be an effective way to reduce neural damage.95 The antioxidant system like Nrf2 system is reported to be significantly activated, alleviating oxidative and inflammatory damage in traumatic brain injury and spinal cord injury.92, 136 Therefore, targeting astrocytes might be an effective way to reduce excessive oxidative stress and further alleviate secondary injury induced by traumatic CNS injury.
6.2. Stroke
Stroke is a clinical condition that results in CNS cell death because of the poor blood flow to the CNS. According to the aetiology, stroke can be divided into haemorrhagic stroke and ischaemic stroke. Stroke is the leading disabling and fatal diseases worldwide, and oxidative stress is the main factor leads to cell damage induced by stroke.59 Astrocytes are reported to play both neuroprotective and destructive roles in the whole pathological process of stroke by altering their cell structure and physiological function in response to the change of microenvironment induced by stroke. As the important homeostatic cells in the CNS, astrocytes are widely involved in oxidative stress modulation after stroke. On the one hand, the antioxidant response of astrocytes may save neurons from excessive oxidative stress after stroke: astrocyte‐specific overexpression of superoxide dismutase 2 (SOD2) effectively attenuates nerve damage caused by cerebral ischaemia.140 Hayakawa et al42 found that astrocytes can deliver their own functional mitochondria to neurons directly or indirectly, and blocking this process might aggravate neural damage arising from cerebral ischaemia. This phenomenon reveals a new significant mechanism for the direct regulation of oxidative stress in glial cells, and it may provide a new target for the improvement of endogenous neuroprotection after stroke. On the other hand, excessive oxidative stress induced by stroke in the central nervous system after stroke can in turn cause activation of astrocytes, which might finally form glial scars and impede neurological recovery.20, 65 In addition, activated astrocytes secrete a variety of pro‐inflammatory factors aggravating the secondary inflammatory response after stroke.106 Gouix et al found that oxygen glucose deprivation leads to dysfunction of glutamate uptake in astrocytes in vitro, which aggravate excessive oxidative stress to result in cell death. To sum up, astrocytes act as different roles in different stages of the stroke pathology and oxidative stress might be one of the important factors resulting in this difference.
6.3. Neurodegenerative diseases
Neurodegenerative disease is a series of CNS diseases arising from progressive loss of neurons and/or myelin, deteriorating over time and causing neural dysfunction, with an increasing high mobility over the world and including a wide range of CNS disease such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), Huntington's disease (HD). Astroglial asthenia often leads to the disturbance of CNS homeostasis which is reported to be highly related to these diseases.129
Alzheimer's disease is the most common neurodegenerative disorder in the world and a leading cause of dementia. Oxidative stress and astrocytes are both deeply involved in the development of the pathology of AD. The extracellular deposition of neuritic plaques and intracellular accumulation of neurofibrillary tangles which, respectively, formed by β‐amyloid and abnormal tau phosphorylation are considered as the primary neuro‐pathogenesis of AD.71 Oxidative stress has been shown in a wide range of studies to be participant in these pathogenesis of AD.14 Some in vitro studies indicated that the accumulation of Aβ could increase the production of ROS in astrocytes and exacerbate neural damage.56, 111 Regulation of astrocytic mitochondrial function by cytokines like insulin‐like growth factor‐1 (IGF‐1) is associated with learning and memory.70 Except for ROS, NO is proved to be released around the Aβ plaques by astrocytes.132 Lipoproteins from Alzheimer patients can lead to the increased production of peroxynitrite and NOS in astrocytes.88
Parkinson's disease is the second most common neurodegenerative disease over the world.102 As for its pathogenesis, α‐synuclein is considered as one of the most important pathogenesis‐related proteins and the researchers found that α‐synuclein could be transferred from neurons to astrocytes in PD model.63, 119, 128 Direct injection of human α‐synuclein into the basal ganglia of mice could lead to strong astrocytes and microglia activation.124 An in vitro study shows that alpha‐synuclein could induce generation of abundant ROS and inflammatory factors mediated by activation of TLR4 receptor of astrocyte.29 Besides, accumulation of α‐synuclein could aggravate oxidative stress in a of astrocyte‐neuron co‐culture systems, causing lipid peroxidation and death of neurons.6 Aberrant S‐nitrosylation is also reported to be associated with PD pathogenesis: S‐nitrosylated parkin, a PD associated protein, can be found in the brain of PD patient and mouse models of PD21; however, the potential role of astrocytes in this pathology remains unclear.102
As for ALS study, approximately 90% of familial ALS patients have mutations in SOD1 which encode an important antioxidant enzyme.64 An experimental ALS study shows that astrocytes expressing mutant superoxide dismutase 1 are toxic to normal motoneurons.31 In addition, SOD1 mutant astrocytes could secrete more TGFβ, expressing higher level of inflammasomes like NLRP3, and activate NF‐KB signalling pathway to aggravate the inflammatory response in an ALS model.64 Compared to the control group, astrocytes expressing mutant SOD1 and TDP43 result in worse nitroxidative stress which cause more death of motoneurons.103 In conclusion, astrocytes are one of the potential targets for oxidative stress regulation to improve the outcome of neurodegenerative diseases.
7. SUMMARY
In summary, the health state of CNS is closely associated with the balance between oxidative and antioxidative factors. Astrocytes as the main supportive cells in CNS are significantly involved in the redox homeostasis maintaining under physiological or pathological conditions. Accumulating evidence shows that astrocytes play a dual role in ROS/RNS regulation: on the one hand, astrocytes could protect the central nervous system from oxidative injury by producing various antioxidant, removing the excitatory amino acids and activating some endogenic antioxidative systems like Nrf2 as the neuroprotective role. On the other hand, under certain circumstances, astrocytes could be an important source of excessive ROS and RNS because of mitochondrial dysfunction, impaired excitatory amino acid metabolism and antioxidant generation, which plays a detrimental role. The pathogenesis of numerous neurological disorders, such as stroke, trauma, infection and neurodegenerative diseases, is reported to be highly associated with astrocytic redox homeostasis. Reciprocally, excessive free radicals in the microenvironment of CNS may lead to reactive astrogliosis aggravating inflammation and glial scar formation, both of which burden the CNS. Increasing evidence indicates that astrocyte might be a promising target for oxidative stress modulation in CNS and this may provide us with future therapies on those related diseases. Additionally, personalized intervention needs to be considered because of the different effects of astrocytes under different conditions. Understanding the mechanisms of CNS redox biology and the relative role of specific cell types will pave the way for effective therapeutics targeting for oxidative stress in CNS disorders.
CONFLICT OF INTEREST
The authors have no competing interests to disclose.
AUTHOR CONTRIBUTIONS
LZ, AT, JX and GG contributed to conception and design of the study; YC and CQ wrote the first draft of the manuscript; JH and XT wrote sections of the manuscript; and CL and KH searched the literature. All authors revised the manuscript and approved the submitted version.
ACKNOWLEDGEMENTS
This work was supported by 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18007); the National Natural Science Foundation of China (31471286 and 81772693); and the National Major Scientific and Technological Special Project for Significant New Drugs Development (2019ZX09301‐147).
Chen Y, Qin C, Huang J, et al. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020;53:e12781 10.1111/cpr.12781
Contributor Information
Aiping Tong, Email: aipingtong@scu.edu.cn.
Liangxue Zhou, Email: liangxue_zhou@126.com.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Abdul‐Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015;51:966‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahlgren‐Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H:quinone oxidoreductase and glutathione‐S‐transferases in primary cultures of rat neurons and glia: role of the antioxidant/electrophile responsive element. Glia. 1999;25:131‐142. [PubMed] [Google Scholar]
- 3. Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch‐induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74:1951‐1960. [PubMed] [Google Scholar]
- 4. Alarcón‐Aguilar A, Luna‐López A, Ventura‐Gallegos JL, et al. Primary cultured astrocytes from old rats are capable to activate the Nrf2 response against MPP+ toxicity after tBHQ pretreatment. Neurobiol Aging. 2014;35:1901‐1912. [DOI] [PubMed] [Google Scholar]
- 5. Alfonso‐Loeches S, Urena‐Peralta JR, Morillo‐Bargues MJ, Oliver‐De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol‐induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angelova PR, Horrocks MH, Klenerman D, Gandhi S, Abramov AY, Shchepinov MS. Lipid peroxidation is essential for alpha‐synuclein‐induced cell death. J Neurochem. 2015;133:582‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bates KA, Martins RN, Harvey AR. Oxidative stress in a rat model of chronic gliosis. Neurobiol Aging. 2007;28:995‐1008. [DOI] [PubMed] [Google Scholar]
- 8. Baxter PS, Hardingham GE. Adaptive regulation of the brain's antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016;100:147‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bedard K, Krause KH. The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245‐313. [DOI] [PubMed] [Google Scholar]
- 10. Bellaver B, dos Santos JP, Leffa DT, et al. Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol Neurobiol. 2018;55:2685‐2695. [DOI] [PubMed] [Google Scholar]
- 11. Bellaver B, Souza DG, Souza DO, Quincozes‐Santos A. Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol. 2017;54:2969‐2985. [DOI] [PubMed] [Google Scholar]
- 12. Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930‐4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braunersreuther V, Jaquet V. Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr Pharm Biotechnol. 2012;13:97‐114. [DOI] [PubMed] [Google Scholar]
- 14. Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabezas R, El‐Bacha RS, Gonzalez J, Barreto GE. Mitochondrial functions in astrocytes: neuroprotective implications from oxidative damage by rotenone. Neurosci Res. 2012;74:80‐90. [DOI] [PubMed] [Google Scholar]
- 16. Cassina P, Cassina A, Pehar M, et al. Mitochondrial dysfunction in SOD1G93A‐bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial‐targeted antioxidants. J Neurosci. 2008;28:4115‐4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chay KO, Nam Koong KY, Hwang S, Kim JK, Bae CS. NADPH oxidase mediates beta‐amyloid peptide‐induced neuronal death in mouse cortical cultures. Chonnam Med J. 2017;53:196‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Guan T, Li C, et al. SOD1 aggregation in astrocytes following ischemia/reperfusion injury: a role of NO‐mediated S‐nitrosylation of protein disulfide isomerase (PDI). J Neuroinflammation. 2012;9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione‐dependent mechanism. J Neurochem. 2001;77:1601‐1610. [DOI] [PubMed] [Google Scholar]
- 20. Choudhury GR, Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis. 2016;85:234‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung KK, Thomas B, Li X, et al. S‐nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328‐1331. [DOI] [PubMed] [Google Scholar]
- 22. Cuadrado A, Rojo AI, Wells G, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295‐317. [DOI] [PubMed] [Google Scholar]
- 23. Dal‐Cim T, Poluceno GG, Lanznaster D, de Oliveira KA, Nedel CB, Tasca CI. Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: involvement of A1 and A2A adenosine receptors and PI3K, MEK, and PKC pathways. Purinergic Signal. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Draheim T, Liessem A, Scheld M, et al. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia. 2016;64:2219‐2230. [DOI] [PubMed] [Google Scholar]
- 26. Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du R‐H, Wu F‐F, Lu M, et al. Uncoupling protein 2 modulation of the NLRP3 inflammasome in astrocytes and its implications in depression. Redox Biol. 2016;9:178‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duan X, Wen Z, Shen H, Shen M, Chen G. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev. 2016;2016:1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fellner L, Irschick R, Schanda K, et al. Toll‐like receptor 4 is required for alpha‐synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandez‐Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012;443:3‐11. [DOI] [PubMed] [Google Scholar]
- 31. Ferraiuolo L, Higginbottom A, Heath PR, et al. Dysregulation of astrocyte‐motoneuron cross‐talk in mutant superoxide dismutase 1‐related amyotrophic lateral sclerosis. Brain. 2011;134:2627‐2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fiebig C, Keiner S, Ebert B, et al. Mitochondrial dysfunction in astrocytes impairs the generation of reactive astrocytes and enhances neuronal cell death in the cortex upon photothrombotic lesion. Front Mol Neurosci. 2019;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Finsterwald C, Magistretti PJ, Lengacher S. Astrocytes: new targets for the treatment of neurodegenerative diseases. Curr Pharm Des. 2015;21:3570‐3581. [DOI] [PubMed] [Google Scholar]
- 34. Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gabbott PL, Bacon SJ. Localisation of NADPH diaphorase activity and NOS immunoreactivity in astroglia in normal adult rat brain. Brain Res. 1996;714:135‐144. [DOI] [PubMed] [Google Scholar]
- 36. Galea E, Feinstein DL, Reis DJ. Induction of calcium‐independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992;89:10945‐10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goswami P, Gupta S, Joshi N, Sharma S, Singh S. Astrocyte activation and neurotoxicity: a study in different rat brain regions and in rat C6 astroglial cells. Environ Toxicol Pharmacol. 2015;40:122‐139. [DOI] [PubMed] [Google Scholar]
- 38. Gouix E, Buisson A, Nieoullon A, et al. Oxygen glucose deprivation‐induced astrocyte dysfunction provokes neuronal death through oxidative stress. Pharmacol Res. 2014;87:8‐17. [DOI] [PubMed] [Google Scholar]
- 39. Gray SP, Jandeleit‐Dahm KA. The role of NADPH oxidase in vascular disease–hypertension, atherosclerosis & stroke. Curr Pharm Des. 2015;21:5933‐5944. [DOI] [PubMed] [Google Scholar]
- 40. Greaves DR, Gordon S. Macrophage‐specific gene expression: current paradigms and future challenges. Int J Hematol. 2002;76:6‐15. [DOI] [PubMed] [Google Scholar]
- 41. Haskew‐Layton RE, Payappilly JB, Smirnova NA, et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2‐independent pathway. Proc Natl Acad Sci U S A. 2010;107:17385‐17390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He Y, Jackman NA, Thorn TL, Vought VE, Hewett SJ. Interleukin‐1beta protects astrocytes against oxidant‐induced injury via an NF‐kappaB‐dependent upregulation of glutathione synthesis. Glia. 2015;63:1568‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hirrlinger J, Schulz JB, Dringen R. Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res. 2002;69:318‐326. [DOI] [PubMed] [Google Scholar]
- 45. Hou L, Zhou X, Zhang C, et al. NADPH oxidase‐derived H2O2 mediates the regulatory effects of microglia on astrogliosis in experimental models of Parkinson's disease. Redox Biol. 2017;12:162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howland DS, Liu J, She Y, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant‐mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci U S A. 2002;99:1604‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hubbs AF, Benkovic SA, Miller DB, et al. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170:2068‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Islam MT. Oxidative stress and mitochondrial dysfunction‐linked neurodegenerative disorders. Neurol Res. 2017;39:73‐82. [DOI] [PubMed] [Google Scholar]
- 49. Jackson JG, Robinson MB. Regulation of mitochondrial dynamics in astrocytes: mechanisms, consequences, and unknowns. Glia. 2018;66:1213‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jantzie L, El Demerdash N, Newville JC, Robinson S. Time to reconsider extended erythropoietin treatment for infantile traumatic brain injury? Exp Neurol. 2019;318:205‐215. [DOI] [PubMed] [Google Scholar]
- 51. Jayakumar AR, Panickar KS, Murthy CHR, Norenberg MD. Oxidative stress and mitogen‐activated protein kinase phosphorylation mediate ammonia‐induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774‐4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jayakumar AR, Rama Rao KV, Tong XY, Norenberg MD. Calcium in the mechanism of ammonia‐induced astrocyte swelling. J Neurochem. 2009;109:252‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol. 2013;8:824‐839. [DOI] [PubMed] [Google Scholar]
- 54. Jeong YH, Park JS, Kim DH, Kim HS. Lonchocarpine Increases Nrf2/ARE‐mediated antioxidant enzyme expression by modulating AMPK and MAPK signaling in brain astrocytes. Biomol Ther (Seoul). 2016;24:581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson AB, Brenner M. Alexander's disease: clinical, pathologic, and genetic features. J Child Neurol. 2003;18:625‐632. [DOI] [PubMed] [Google Scholar]
- 56. Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta‐amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182‐193. [DOI] [PubMed] [Google Scholar]
- 57. Karimi‐Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury‐beneficial and detrimental effects. Mol Neurobiol. 2012;46:251‐264. [DOI] [PubMed] [Google Scholar]
- 58. Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016;173:692‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167‐1186. [DOI] [PubMed] [Google Scholar]
- 60. Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92:1419‐1424. [DOI] [PubMed] [Google Scholar]
- 61. Korcok J, Wu F, Tyml K, Hammond RR, Wilson JX. Sepsis inhibits reduction of dehydroascorbic acid and accumulation of ascorbate in astroglial cultures: intracellular ascorbate depletion increases nitric oxide synthase induction and glutamate uptake inhibition. J Neurochem. 2002;81:185‐193. [DOI] [PubMed] [Google Scholar]
- 62. Lau JW, Senok S, Stadlin A. Methamphetamine‐induced oxidative stress in cultured mouse astrocytes. Ann N Y Acad Sci. 2000;914:146‐156. [DOI] [PubMed] [Google Scholar]
- 63. Lee HJ, Suk JE, Patrick C, et al. Direct transfer of alpha‐synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262‐9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee J, Hyeon SJ, Im H, Ryu H, Kim Y, Ryu H. Astrocytes and microglia as non‐cell autonomous players in the pathogenesis of ALS. Exp Neurobiol. 2016;25:233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li L, Stary CM. Targeting glial mitochondrial function for protection from cerebral ischemia: relevance, mechanisms, and the role of MicroRNAs. Oxid Med Cell Longev. 2016;2016:6032306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu F‐T, Xu S‐M, Xiang Z‐H, et al. Molecular hydrogen suppresses reactive astrogliosis related to oxidative injury during spinal cord injury in rats. CNS Neurosci Ther. 2014;20:778‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu W, Xu Z, Yang T, Deng Y, Xu B, Feng S. Tea polyphenols protect against methylmercury‐induced cell injury in rat primary cultured astrocytes, involvement of oxidative stress and glutamate uptake/metabolism disorders. Mol Neurobiol. 2016;53:2995‐3009. [DOI] [PubMed] [Google Scholar]
- 69. Lizama‐Manibusan B, McLaughlin B. Redox modification of proteins as essential mediators of CNS autophagy and mitophagy. FEBS Lett. 2013;587:2291‐2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Logan S, Pharaoh GA, Marlin MC, et al. Insulin‐like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid‐beta uptake in astrocytes. Mol Metab. 2018;9:141‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu M, Hu LF, Hu G, Bian JS. Hydrogen sulfide protects astrocytes against H(2)O(2)‐induced neural injury via enhancing glutamate uptake. Free Radic Biol Med. 2008;45:1705‐1713. [DOI] [PubMed] [Google Scholar]
- 73. Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77‐87. [DOI] [PubMed] [Google Scholar]
- 74. Manganaro F, Chopra VS, Mydlarski MB, Bernatchez G, Schipper HM. Redox perturbations in cysteamine‐stressed astroglia: implications for inclusion formation and gliosis in the aging brain. Free Radic Biol Med. 1995;19:823‐835. [DOI] [PubMed] [Google Scholar]
- 75. Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid‐beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen‐activated protein kinase cascades. Neuroscience. 2008;156:898‐910. [DOI] [PubMed] [Google Scholar]
- 76. Mazat JP, Devin A, Ransac S. Modelling mitochondrial ROS production by the respiratory chain. Cell Mol Life Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McBean GJ. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42:199‐205. [DOI] [PubMed] [Google Scholar]
- 78. McManus MJ, Murphy MP, Franklin JL. The mitochondria‐targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703‐15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Menzel L, Kleber L, Friedrich C, et al. Progranulin protects against exaggerated axonal injury and astrogliosis following traumatic brain injury. Glia. 2017;65:278‐292. [DOI] [PubMed] [Google Scholar]
- 80. Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase‐1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26:1880‐1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miralles VJ, Martínez‐López I, Zaragozá R, et al. Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res. 2001;922:21‐29. [DOI] [PubMed] [Google Scholar]
- 82. Mohammadi F, Soltani A, Ghahremanloo A, Javid H, Hashemy SI. The thioredoxin system and cancer therapy: a review. Cancer Chemother Pharmacol. 2019;84:925‐935. [DOI] [PubMed] [Google Scholar]
- 83. Morganti‐Kossmann MC, Semple BD, Hellewell SC, Bye N, Ziebell JM. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol. 2019;137:731‐755. [DOI] [PubMed] [Google Scholar]
- 84. Moriyama M, Fujitsuka S, Kawabe K, Takano K, Nakamura Y. Zinc potentiates lipopolysaccharide‐induced nitric oxide production in cultured primary rat astrocytes. Neurochem Res. 2018;43:363‐374. [DOI] [PubMed] [Google Scholar]
- 85. Murphy S, Minor RL Jr, Welk G, Harrison DG. Evidence for an astrocyte‐derived vasorelaxing factor with properties similar to nitric oxide. J Neurochem. 1990;55:349‐351. [DOI] [PubMed] [Google Scholar]
- 86. Murphy TH, Yu J, Ng R, et al. Preferential expression of antioxidant response element mediated gene expression in astrocytes. J Neurochem. 2001;76:1670‐1678. [DOI] [PubMed] [Google Scholar]
- 87. Murru S, Hess S, Barth E, et al. Astrocyte‐specific deletion of the mitochondrial m‐AAA protease reveals glial contribution to neurodegeneration. Glia. 2019;67:1526‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nanetti L, Vignini A, Moroni C, et al. Peroxynitrite production and NOS expression in astrocytes U373MG incubated with lipoproteins from Alzheimer patients. Brain Res. 2005;1054:38‐44. [DOI] [PubMed] [Google Scholar]
- 89. Nasoohi S, Ismael S, Ishrat T. Thioredoxin‐Interacting Protein (TXNIP) in cerebrovascular and neurodegenerative diseases: regulation and implication. Mol Neurobiol. 2018;55:7900‐7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nayernia Z, Jaquet V, Krause KH. New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal. 2014;20:2815‐2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oliva AA Jr, Kang Y, Sanchez‐Molano J, Furones C, Atkins CM. STAT3 signaling after traumatic brain injury. J Neurochem. 2012;120:710‐720. [DOI] [PubMed] [Google Scholar]
- 92. Pan H, Wang H, Wang X, Zhu L, Mao L. The absence of Nrf2 enhances NF‐kappaB‐dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm. 2012;2012:217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pan H, Wang H, Zhu L, Mao L, Qiao L, Su X. Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem Res. 2011;36:2434‐2441. [DOI] [PubMed] [Google Scholar]
- 94. Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540‐551. [DOI] [PubMed] [Google Scholar]
- 95. Pearse DD, Chatzipanteli K, Marcillo AE, Bunge MB, Dietrich WD. Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J Neuropathol Exp Neurol. 2003;62:1096‐1107. [DOI] [PubMed] [Google Scholar]
- 96. Pekny M, Pekna M, Messing A, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323‐345. [DOI] [PubMed] [Google Scholar]
- 97. Raju K, Doulias P‐T, Evans P, et al. Regulation of brain glutamate metabolism by nitric oxide and S‐nitrosylation. Sci Signal. 2015;8:ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reichenbach N, Delekate A, Plescher M, et al. Inhibition of Stat3‐mediated astrogliosis ameliorates pathology in an Alzheimer's disease model. EMBO Mol Med. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Reinehr R, Görg B, Becker S, et al. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55:758‐771. [DOI] [PubMed] [Google Scholar]
- 100. Ren X, Zou L, Zhang XU, et al. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid Redox Signal. 2017;27:989‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Renault‐Mihara F, Okano H. STAT3‐regulated RhoA drives reactive astrocyte dynamics. Cell Cycle. 2017;16:1995‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rizor A, Pajarillo E, Johnson J, Aschner M, Lee E. Astrocytic oxidative/nitrosative stress contributes to parkinson's disease pathogenesis: the dual role of reactive astrocytes. Antioxidants (Basel). 2019;8:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rojas F, Cortes N, Abarzua S, Dyrda A, van Zundert B. Astrocytes expressing mutant SOD1 and TDP43 trigger motoneuron death that is mediated via sodium channels and nitroxidative stress. Front Cell Neurosci. 2014;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rosenblum LT, Shamamandri‐Markandaiah S, Ghosh B, et al. Mutation of the caspase‐3 cleavage site in the astroglial glutamate transporter EAAT2 delays disease progression and extends lifespan in the SOD1‐G93A mouse model of ALS. Exp Neurol. 2017;292:145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rothstein JD, Dykes‐Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675‐686. [DOI] [PubMed] [Google Scholar]
- 106. Roy Choudhury G, Ryou M‐G, Poteet E, et al. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke. Brain Res. 2014;1551:45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rueda CB, Llorente‐Folch I, Traba J, et al. Glutamate excitotoxicity and Ca2+‐regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim Biophys Acta. 2016;1857:1158‐1166. [DOI] [PubMed] [Google Scholar]
- 108. Salazar M, Pariente JA, Salido GM, Gonzalez A. Ethanol induces glutamate secretion by Ca2+ mobilization and ROS generation in rat hippocampal astrocytes. Neurochem Int. 2008;52:1061‐1067. [DOI] [PubMed] [Google Scholar]
- 109. Sarafian TA, Montes C, Imura T, et al. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS ONE. 2010;5:e9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schachtele SJ, Hu S, Little MR, Lokensgard JR. Herpes simplex virus induces neural oxidative damage via microglial cell Toll‐like receptor‐2. J Neuroinflammation. 2010;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schubert D, Soucek T, Blouw B. The induction of HIF‐1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci. 2009;29:1323‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Seib TM, Patel SA, Bridges RJ. Regulation of the system x(C)‐ cystine/glutamate exchanger by intracellular glutathione levels in rat astrocyte primary cultures. Glia. 2011;59:1387‐1401. [DOI] [PubMed] [Google Scholar]
- 113. Shah A, Kumar S, Simon SD, Singh DP, Kumar A. HIV gp120‐ and methamphetamine‐mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013;4:e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sharma N, Nehru B. Curcumin affords neuroprotection and inhibits alpha‐synuclein aggregation in lipopolysaccharide‐induced Parkinson's disease model. Inflammopharmacology. 2018;26:349‐360. [DOI] [PubMed] [Google Scholar]
- 115. Shih EK, Robinson MB. Role of astrocytic mitochondria in limiting ischemic brain injury? Physiology (Bethesda). 2018;33:99‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sitar SM, Hanifi‐Moghaddam P, Gelb A, Cechetto DF, Siushansian R, Wilson JX. Propofol prevents peroxide‐induced inhibition of glutamate transport in cultured astrocytes. Anesthesiology. 1999;90:1446‐1453. [DOI] [PubMed] [Google Scholar]
- 117. Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Song YJC, Halliday GM, Holton JL, et al. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol. 2009;68:1073‐1083. [DOI] [PubMed] [Google Scholar]
- 120. Sorce S, Stocker R, Seredenina T, et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: what is the evidence? Free Radic Biol Med. 2017;112:387‐396. [DOI] [PubMed] [Google Scholar]
- 121. Stewart VC, Sharpe MA, Clark JB, Heales SJ. Astrocyte‐derived nitric oxide causes both reversible and irreversible damage to the neuronal mitochondrial respiratory chain. J Neurochem. 2000;75:694‐700. [DOI] [PubMed] [Google Scholar]
- 122. Strowig T, Henao‐Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278‐286. [DOI] [PubMed] [Google Scholar]
- 123. Sure VN, Sakamuri SSVP, Sperling JA, et al. A novel high‐throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience. 2018;40:365‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sznejder‐Pacholek A, Joniec‐Maciejak I, Wawer A, Ciesielska A, Mirowska‐Guzel D. The effect of alpha‐synuclein on gliosis and IL‐1alpha, TNFalpha, IFNgamma, TGFbeta expression in murine brain. Pharmacol Rep. 2017;69:242‐251. [DOI] [PubMed] [Google Scholar]
- 125. Tacconi MT. Neuronal death: is there a role for astrocytes? Neurochem Res. 1998;23:759‐765. [DOI] [PubMed] [Google Scholar]
- 126. Todd AC, Marx MC, Hulme SR, Broer S, Billups B. SNAT3‐mediated glutamine transport in perisynaptic astrocytes in situ is regulated by intracellular sodium. Glia. 2017;65:900‐916. [DOI] [PubMed] [Google Scholar]
- 127. Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Valdinocci D, Radford RA, Siow SM, Chung RS, Pountney DL. Potential Modes of Intercellular alpha‐Synuclein Transmission. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Verkhratsky A, Marutle A, Rodriguez‐Arellano JJ, Nordberg A. Glial asthenia and functional paralysis: a new perspective on neurodegeneration and alzheimer's disease. Neuroscientist. 2015;21:552‐568. [DOI] [PubMed] [Google Scholar]
- 130. Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98:239‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. von Leden RE, Parker KN, Bates AA, Noble‐Haeusslein LJ, Donovan MH. The emerging role of neutrophils as modifiers of recovery after traumatic injury to the developing brain. Exp Neurol. 2019;317:144‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta‐amyloid plaques. Exp Neurol. 1997;144:266‐272. [DOI] [PubMed] [Google Scholar]
- 133. Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84‐97. [DOI] [PubMed] [Google Scholar]
- 134. Wang JF, Li Y, Song JN, Pang HG. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int. 2014;64:37‐47. [DOI] [PubMed] [Google Scholar]
- 135. Wang L, Hagemann TL, Kalwa H, Michel T, Messing A, Feany MB. Nitric oxide mediates glial‐induced neurodegeneration in Alexander disease. Nat Commun. 2015;6:8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang X, de Rivero Vaccari JP, Wang H, et al. Activation of the nuclear factor E2‐related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J Neurotrauma. 2012;29:936‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wanner IB, Anderson MA, Song B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3‐dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870‐12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wilson CL, Natarajan V, Hayward SL, Khalimonchuk O, Kidambi S. Mitochondrial dysfunction and loss of glutamate uptake in primary astrocytes exposed to titanium dioxide nanoparticles. Nanoscale. 2015;7:18477‐18488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xu J, Huang G, Zhang K, et al. Nrf2 activation in astrocytes contributes to spinal cord ischemic tolerance induced by hyperbaric oxygen preconditioning. J Neurotrauma. 2014;31:1343‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu MX, Zhu YF, Chang HF, Liang Y. Nanoceria restrains PM2.5‐induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF‐kappaB pathway in Nrf2 deficient mice. Free Radic Biol Med. 2016;99:259‐272. [DOI] [PubMed] [Google Scholar]
- 142. Yamada T, Kawahara K, Kosugi T, Tanaka M. Nitric oxide produced during sublethal ischemia is crucial for the preconditioning‐induced down‐regulation of glutamate transporter GLT‐1 in neuron/astrocyte co‐cultures. Neurochem Res. 2006;31:49‐56. [DOI] [PubMed] [Google Scholar]
- 143. Yin Z, Milatovic D, Aschner JL, et al. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Young W. Spinal cord regeneration. Cell Transplant. 2014;23:573‐611. [DOI] [PubMed] [Google Scholar]
- 145. Zagami CJ, Beart PM, Wallis N, Nagley P, O'Shea RD. Oxidative and excitotoxic insults exert differential effects on spinal motoneurons and astrocytic glutamate transporters: Implications for the role of astrogliosis in amyotrophic lateral sclerosis. Glia. 2009;57:119‐135. [DOI] [PubMed] [Google Scholar]
- 146. Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurogibol. 2013;100:30‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J. Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal. 2011;15:2867‐2908. [DOI] [PubMed] [Google Scholar]
- 148. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221‐225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


