Abstract
PURPOSE
The phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin is a key pathway of survival and therapeutic resistance in breast cancer. We evaluated the pan-Akt inhibitor MK-2206 in combination with standard therapy in patients with high-risk early-stage breast cancer.
PATIENTS AND METHODS
I-SPY 2 is a multicenter, phase II, open-label, adaptively randomized neoadjuvant platform trial that screens experimental therapies and efficiently identifies potential predictive biomarker signatures. Patients are categorized by human epidermal growth factor receptor 2 (HER2), hormone receptor (HR), and MammaPrint statuses in a 2 × 2 × 2 layout. Patients within each of these 8 biomarker subtypes are adaptively randomly assigned to one of several experimental therapies, including MK-2206, or control. Therapies are evaluated for 10 biomarker signatures, each of which is a combination of these subtypes. The primary end point is pathologic complete response (pCR). A therapy graduates with one or more of these signatures if and when it has an 85% Bayesian predictive probability of success in a hypothetical phase III trial, adjusting for biomarker covariates. Patients in the current report received standard taxane- and anthracycline-based neoadjuvant therapy without (control) or with oral MK-2206 135 mg/week.
RESULTS
MK-2206 graduated with 94 patients and 57 concurrently randomly assigned controls in 3 graduation signatures: HR-negative/HER2-positive, HR-negative, and HER2-positive. Respective Bayesian mean covariate-adjusted pCR rates and percentage probability that MK-2206 is superior to control were 0.48:0.29 (97%), 0.62:0.36 (99%), and 0.46:0.26 (94%). In exploratory analyses, MK-2206 evinced a numerical improvement in event-free survival in its graduating signatures. The most significant grade 3-4 toxicity was rash (14% maculopapular, 8.6% acneiform).
CONCLUSION
The Akt inhibitor MK-2206 combined with standard neoadjuvant therapy resulted in higher estimated pCR rates in HR-negative and HER2-positive breast cancer. Although MK-2206 is not being further developed at this time, this class of agents remains of clinical interest.
INTRODUCTION
Neoadjuvant therapy for breast cancer increases rates of breast conservation while providing outcome improvements equivalent to adjuvant therapy.1 Pathologic complete response (pCR) at the time of surgery is associated with improved survival and is a US Food and Drug Administration–approved surrogate end point for accelerated approval of neoadjuvant therapies.2,3 The availability of a reliable early end point provides an opportunity to accelerate the development of effective targeted therapies and qualifying biomarkers.4 Despite increasing survival rates attributable to systemic therapies, there remain an estimated 40,000 deaths as a result of breast cancer annually in the United States,5 most from recurrence after early-stage disease. Novel agents that reduce mortality are needed along with an accelerated and discriminating model for rapid assessment of these agents.
The I-SPY 2 Trial is a phase II adaptive platform trial of neoadjuvant therapy for early-stage breast cancer at high risk for recurrence. Participants are randomly assigned to either standard-of-care control therapy or one of several experimental arms that add a novel agent or combination.6 Therapies that reach predefined thresholds of efficacy in ≥ 1 specific biomarker signatures “graduate” from the trial. Results of 2 graduated therapies have been published previously.7,8
The Akt serine/threonine kinase is a key node for growth factor receptor–initiated signaling that activates mammalian target of rapamycin (mTOR) and downstream effectors. Enhanced signaling through the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway is a key mechanism of survival and therapeutic resistance across all breast cancer receptor subtypes and contributes to trastuzumab resistance in human epidermal growth factor receptor 2 (HER2)–overexpressing breast cancer and endocrine therapy resistance in hormone receptor (HR)–positive breast cancer.9 Akt inhibitors, including the allosteric pan-Akt inhibitor MK-2206, successfully target Akt with reductions in tumor phosphorylated serine 246.10 Preclinical studies have suggested that PI3K and Akt inhibitors enhance the efficacy of antimicrotubule drugs in human breast cancer cells.11 Safety and clinical activity of MK-2206 in combination with paclitaxel, trastuzumab, or both have been described in phase I trials,12-14 providing the basis for testing MK-2206 using the I-SPY 2 platform. Here, we report the results of the investigational arm that tested MK-2206 in combination with paclitaxel with or without trastuzumab followed by anthracycline-based chemotherapy in the neoadjuvant I-SPY 2 Trial.
PATIENTS AND METHODS
Detailed descriptions of the design, eligibility, and study assessments in the I-SPY 2 Trial have been reported previously.7,8
Study Design
I-SPY 2 is an ongoing, multicenter, randomized, open-label, adaptive phase II platform trial. Using a master protocol, I-SPY 2 simultaneously tests several experimental agents or combinations against a common control arm (Data Supplement, online only).
Patients are stratified within 8 subtypes on the basis of HR, HER2, and MammaPrint score.15 Combinations of these subtypes define 10 biomarker signatures (Data Supplement). Randomization within subtype uses an adaptive algorithm that is based on Bayesian probabilities of benefit versus control that are continually updated. Patients are preferentially assigned to therapies that are more likely to improve the pCR rate for their specific biomarker subtype; 20% of patients are assigned to the control arm.7,8,16
The primary end point is pCR, which is defined as no residual invasive cancer in either breast or lymph nodes at the time of surgery. Experimental arms are continually evaluated against control for each of its possible signatures and graduate if and when they demonstrate statistical superiority in pCR rate for any of the 10 predefined signatures. Graduation is defined as ≥ 85% Bayesian predictive probability of success in a simulated phase III, 1:1 randomized, neoadjuvant trial in 300 patients with the respective signature.16,17
Eligibility and Enrollment
I-SPY 2 participants must be ≥ 18 years of age, have clinical stage II or III breast cancer, and must not have received prior surgery or systemic therapy for their breast cancer. Tumors must be ≥ 2.5 cm by imaging or physical examination and undergo expression-array profiling with the Agendia (Irvine, CA) 70-gene MammaPrint assay.15 Patients who have both HR-positive/HER2-negative and MammaPrint low-risk tumors are excluded. Patients must have an HbA1c ≤ 8 and consent to undergoing research biopsies and serial breast magnetic resonance imaging (MRI).
Treatment
Patients randomly assigned to the control arm received 12 weekly cycles of paclitaxel 80 mg/m2 followed by 4 cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 (AC) intravenously every 2 or 3 weeks. Control patients with HER2-positive tumors also received weekly trastuzumab (4 mg/kg loading dose then 2 mg/kg during weeks 2-12) in combination with paclitaxel. There was no placebo for MK-2206. Patients randomly assigned to the MK-2206 arm received oral MK-2206 135 mg/week in combination with paclitaxel (and trastuzumab if HER2 positive) that ended after 12 weeks, followed by 4 cycles of AC. In January 2014, pertuzumab became part of the standard of care in HER2-positive disease. Upon I-SPY 2 data safety and monitoring board recommendation, the control arm in HER2-positive subtypes was dropped from the trial. As a result, the last 4 patients on MK-2206 with HER2-positive disease were not randomly assigned against the control arm. They were randomly assigned against experimental arms pertuzumab plus trastuzumab plus paclitaxel and pertuzumab plus trastuzumab emtansine, both followed by AC.
Breast surgery, including management of the axilla, was performed according to National Comprehensive Cancer Network and local practice guidelines.18 Use and choice of adjuvant therapy was at the discretion of the treating physician.
Study Assessments
Patients underwent serial blood draws and bilateral breast MRI at time points before, during, and after treatment (Data Supplement). The primary end point was pCR. Surgical specimens were evaluated by pathologists trained to assess pCR using the residual cancer burden index (0 = pCR).19 Analysis was modified intention to treat. Patients who received ≥ 1 doses of study treatment were considered evaluable; patients who progressed on study therapy, changed to nonprotocol therapy, left the treating institution, withdrew consent before surgery, or did not have surgery were counted as non-pCR (13 patients in the MK-2206 arm, 8 in the control arm).
Study Oversight
The study was sponsored initially by the Foundation for the National Institutes of Health and subsequently by the nonprofit Quantum Leap Healthcare Collaborative. Merck (Kenilworth, NJ) supplied MK-2206 and some financial assistance. Neither Merck nor other pharmaceutical companies played a role in study design, data accrual, data analysis, or manuscript preparation. All participating sites received institutional review board approval. An independent data and safety monitoring board convened monthly.
Data Analysis and Statistical Considerations
Once the graduation threshold is reached (on the basis of longitudinal MRI volume and pCR data from participants who completed their surgical therapy) for ≥ 1 biomarker signature, accrual to the arm stops. Graduation probabilities are determined by this interim analysis and updated when all patients in the arm complete all chemotherapy and surgical therapy and pCR for all patients can be assessed; formal graduation status is set when accrual stops. Probability distributions of pCR rates are calculated using a Bayesian covariate-adjusted logistic model with HR, HER2, and MammaPrint statuses as covariates and are used to calculate the probability that the MK-2206 pCR rate is greater than control for each signature; similarly for the predictive probabilities of success in a future trial. Additional details of the I-SPY 2 Trial design and interpretation are published elsewhere.7,8
In an exploratory analysis, event-free survival (EFS) was evaluated for all participants in the MK-2206 (n = 78) and control (n = 202) arms (Data Supplement) who had follow-up data as of the cutoff date of February 26, 2019. Median follow-up of this exploratory EFS cohort was 4.2 years. Kaplan-Meier survival curves of the MK-2206 and control arms were prepared; within each signature, Cox proportional hazard modeling was used to estimate hazard ratios between the MK-2206 and control arms. Statistics with regard to this exploratory analysis of EFS are descriptive and not inferential. Sample sizes are small within signatures, and I-SPY 2 is not powered for EFS or other survival end points. Note that only 51 of the 202 controls included in this analysis were concurrently randomly assigned to MK-2206; no adjustments were made for potential population changes over time.
RESULTS
Patient Characteristics
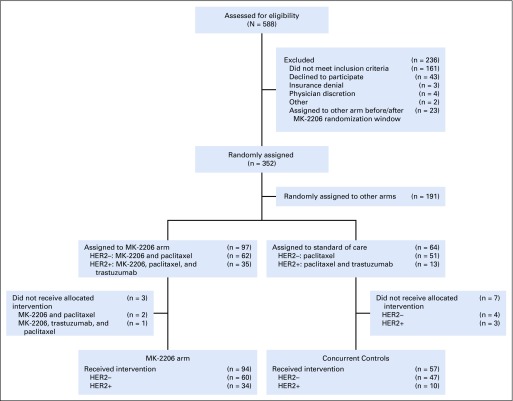
During the period MK-2206 was active (September 15, 2012, to May 14, 2014), 352 patients were randomly assigned in I-SPY 2 (Fig 1): 97 patients to the MK-2206 arm (60 HER2-negative and 34 HER2-positive patients), 191 to other arms, and 64 to the control arm. The 94 patients who received MK-2206 were included in the primary safety and efficacy analyses. Of the 64 patients concurrently randomly assigned to the control arm, 57 received standard therapy and were evaluable for pCR (47 HER2-negative and 10 HER2-positive patients).
FIG 1.
CONSORT diagram for primary efficacy analysis of MK-2206. HER2−, human epidermal growth factor receptor 2 negative; HER2+, human epidermal growth factor receptor 2 positive.
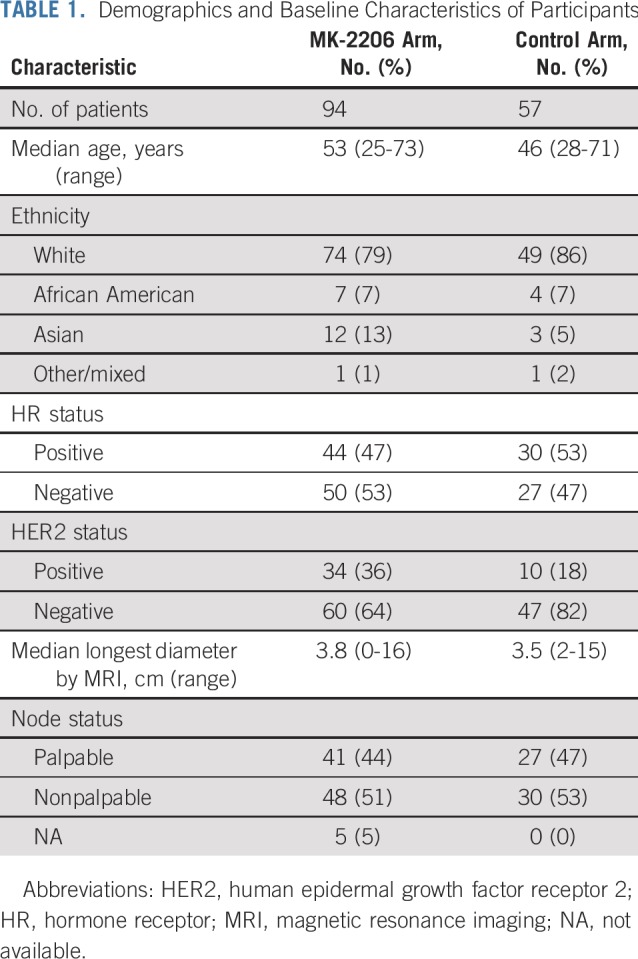
Baseline patient characteristics (Table 1) were well-balanced between the MK-2206 and control arms with respect to demographics, HR status, and pretreatment tumor size. There was an enriched population of HER2-positive patients in the MK-2206 arm compared with control (36% v 18%), an expected result of adaptive randomization.
TABLE 1.
Demographics and Baseline Characteristics of Participants
Efficacy
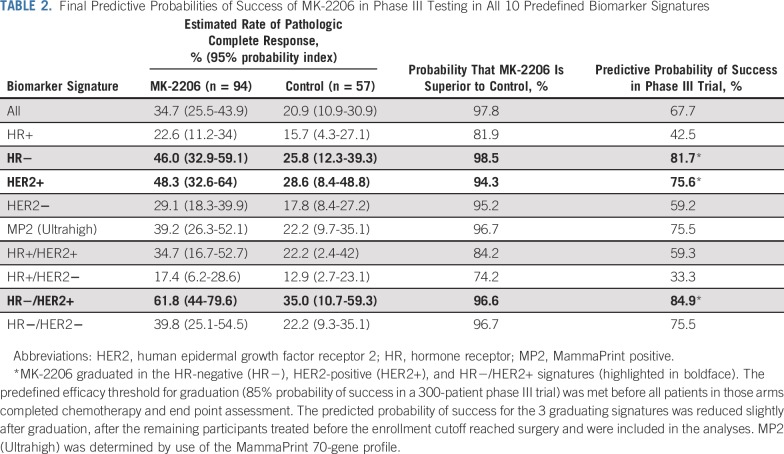
After the 97th patient assigned to MK-2206 was randomly assigned, the predefined efficacy threshold for graduation was met in 3 biomarker signatures on the basis of the patients who had surgery: HER2-positive, HR-negative/HER2-positive, and HR-negative signatures. Per protocol, enrollment to the MK-2206 arm was stopped at that time. After all patients in the arm had surgery and the final efficacy analysis was updated, probabilities in the 3 graduating signatures decreased slightly.
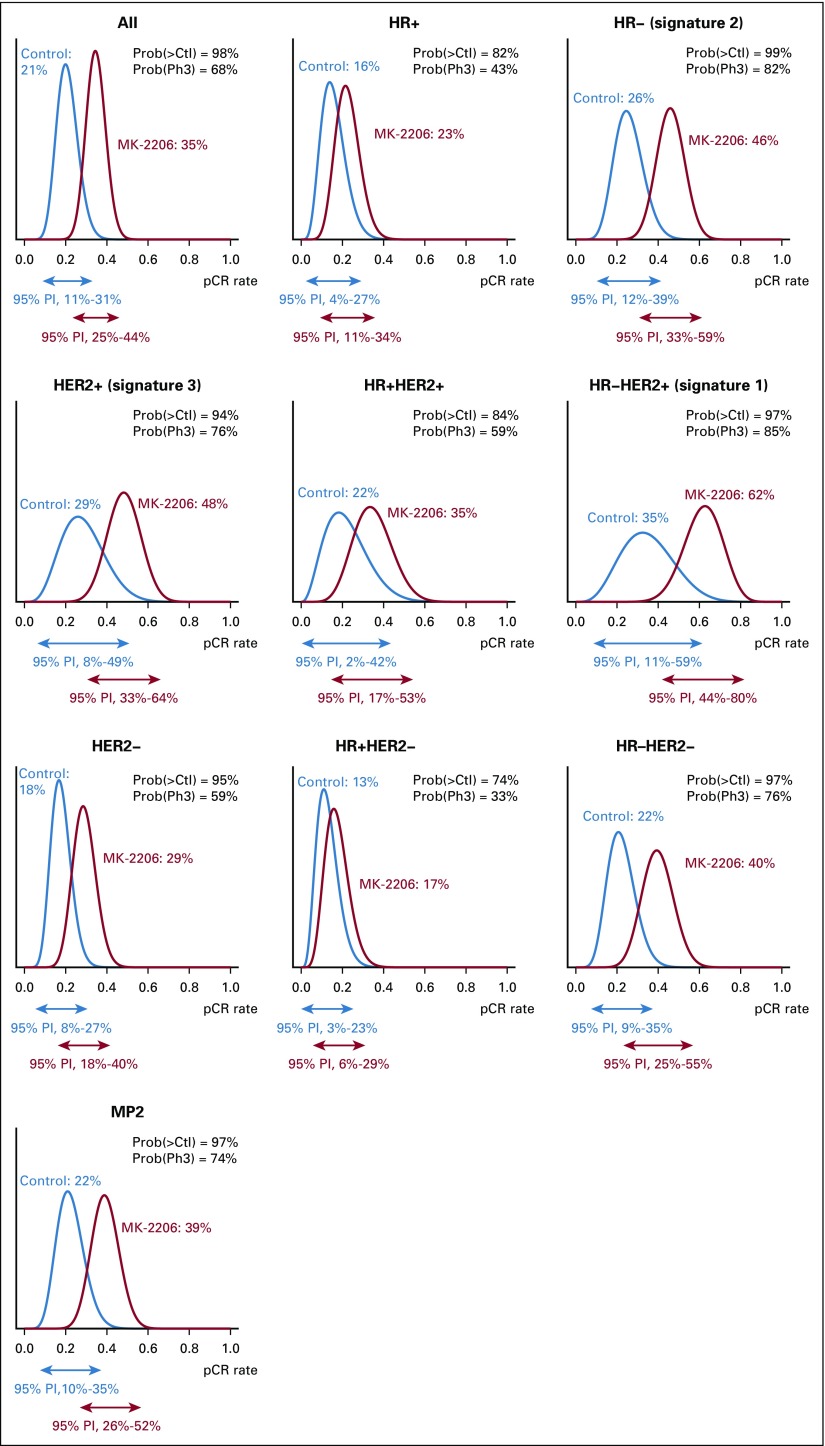
Figure 2 shows posterior probability distributions of pCR for the MK-2206 and control arms within all 10 signatures after all patients completed surgery. Table 2 lists mean pCR rates and 95% probability intervals for all 10 signatures.
FIG 2.
Probability distributions for MK-2206 and control arms within each of the predefined biomarker signatures. The corresponding 95% probability intervals (PIs) are represented by the arrows for each. The mean of each distribution is the estimated rate of pathologic complete response (pCR). HER2−, human epidermal growth factor receptor 2 negative; HER2+, human epidermal growth factor receptor 2 positive; HR–, hormone receptor negative; HR+, hormone receptor positive; MP2, MammaPrint positive; Prob(>Ctl), probability superior to control; Prob(Ph3), probability of phase III success.
TABLE 2.
Final Predictive Probabilities of Success of MK-2206 in Phase III Testing in All 10 Predefined Biomarker Signatures
The estimated pCR rate for HER2-positive patients was 48% in the MK-2206 arm compared with 29% in the control arm. The resulting probability that the MK-2206 arm was superior to the control arm for HER2-positive patients was 94%, and the probability of success for MK-2206 in a 300-patient equally randomized phase III neoadjuvant trial was 76%. The benefit seen in HER2-positive patients was concentrated in the HR-negative/HER2-positive signature, where the estimated pCR rate was 62% v 35% for the MK-2206 and control arms, respectively, with an 84.9% probability of phase III trial success. In HR-negative patients, pCR rates were 46% v 25.8% in the MK-2206 and control arms, respectively, predicting an 81.7% chance of phase III success. The benefit seen in the HR-negative subset included both HER2-negative and HER2-positive patients. Little benefit was seen in HR-positive/HER2-negative patients, with estimated pCR rates of 17% v 13% in the MK-2206 and control arms, respectively. I-SPY 2 implemented a time-adjusted model for efficacy analysis (Data Supplement) after stopping the HER2-positive control arm; the results are qualitatively similar, where the highest probability for success is observed within the 3 graduating signatures.
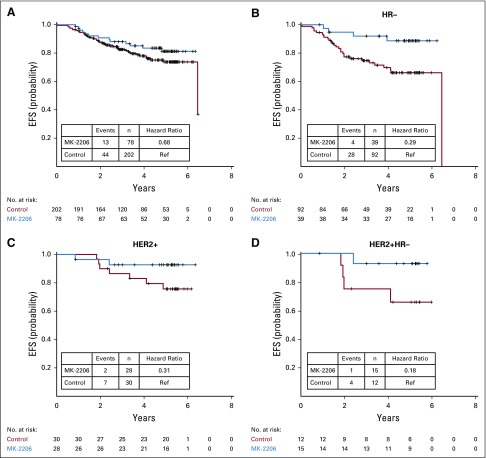
The exploratory analysis of survival outcomes suggests that patients who achieved a pCR had a better EFS than those who did not, regardless of arm (Data Supplement). In addition, patients who received MK-2206 may have had improved EFS compared with control in graduating signatures (Fig 3). However, the trial was not powered to address treatment benefits in EFS; these data are reported without significance assessment.
FIG 3.
Kaplan-Meier plots of event-free survival (EFS) in MK-2206 (blue) and control (red) arms for (A) all participants; (B) participants with hormone receptor-negative (HR−) breast cancer; (C) human epidermal growth factor receptor 2–positive (HER2+) breast cancer; and (D) HER2+/HR– breast cancer. Hazard ratios and 95% CIs are provided; however, caution is warranted in interpreting the results because of the low number of patients in each subtype and the fact that I-SPY 2 is not powered for survival end points. Ref, reference.
Adverse Events
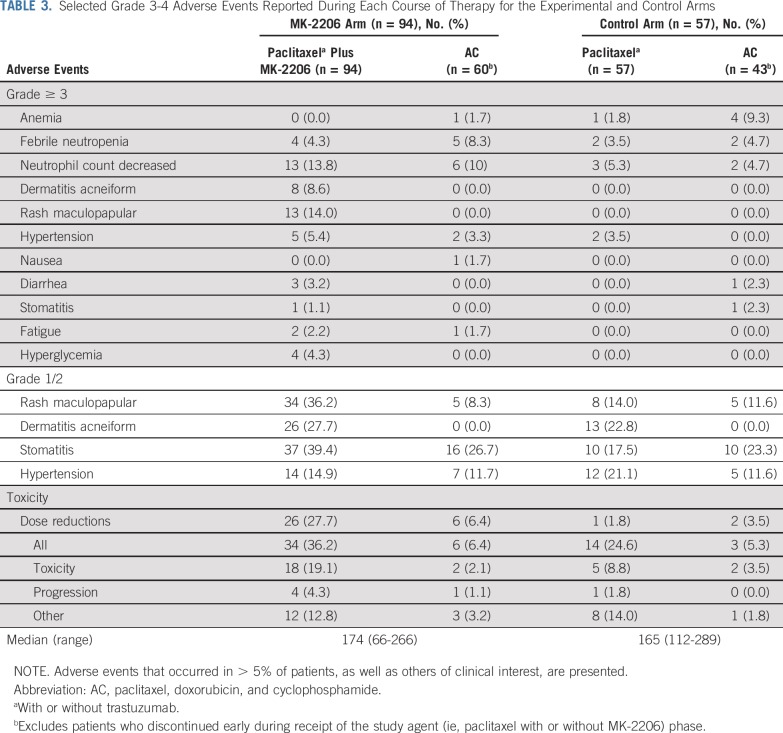
Adverse events (AEs) related to paclitaxel and MK-2206, with or without trastuzumab, were consistent with previous studies.10,14,20,21 Clinically relevant grade ≥ 3 AEs (and select grade 1/2 AEs) in the MK-2206 and control arms are listed in Table 3 (see the Data Supplement for all grade 1 and 2 AEs). AEs for HER2-positive and -negative patients were combined. The most common nonhematologic grade ≥ 3 AE associated with MK-2206 was rash. Two types of rash were reported: maculopapular and acneiform. Fifty percent of patients reported maculopapular rash in the MK-2206 arm (of which 14% were grade 3) compared with 21% in the control arm (all of which were grade 1 and 2), and 36% of patients who received MK-2206 reported acneiform rash (8.6% grade ≥ 3) compared with 23% in the control arm (all grade 1 and 2). There were 2 serious AEs as a result of grade 3 rash that were reported to be probably or definitely related to MK-2206. MK-2206–related rashes generally first presented within the first 4 weeks of therapy (median, 12 days) and resolved promptly (median, 16 days) with drug holiday, topical steroids, and/or a short course of oral steroids. Eighty-five percent of these patients were successfully rechallenged with MK-2206 after initial rash presentation.
TABLE 3.
Selected Grade 3-4 Adverse Events Reported During Each Course of Therapy for the Experimental and Control Arms
Dose reductions and early discontinuation of study therapy were more common with MK-2206 than control. In the MK-2206 arm, 26 patients (27.7%) had a dose reduction during the experimental phase (either in MK-2206 or in paclitaxel), and 18 patients (19%) discontinued therapy early because of toxicity. The most common reason for dose reduction and toxicity-related early discontinuation was rash. Paclitaxel dose reductions were similar between the MK-2206 and control arms, with low rates seen in both groups (3.2% v 1.8%, respectively). There was no significant difference in rates of dose reductions and early discontinuations during the AC phase between the MK-2206 and control arms.
DISCUSSION
I-SPY 2 establishes a new clinical trial paradigm for efficient phase II development of novel agents in patients with early-stage breast cancer at high risk of early recurrence. Through adaptive randomization that is based on prespecified biomarker signatures and shared control arms, I-SPY 2 identifies tumor subtypes that are particularly sensitive to a given experimental agent. MK-2206 was the third agent to graduate from I-SPY 2, graduating in HER2-positive, HR-negative/HER2-positive, and HR-negative disease. Our final results predict a 76%, 85%, and 82% probability, respectively, that MK-2206 will successfully demonstrate superiority to controls in a 300-person phase III neoadjuvant trial in each subtype. Consistent with other trials, data from I-SPY 2 suggest that pCR is strongly prognostic for EFS.22 An exploratory analysis suggests that MK-2206 improves EFS in patients with the sensitive biomarker signatures. However, the numbers of patients within each arm and signature are small, EFS is a secondary end point, and I-SPY 2 was not powered to evaluate EFS differences between arms.
For breast cancer, among the numerous agents that target the PI3K/Akt/mTOR pathway, only the mTOR inhibitor everolimus and the α-specific PI3K inhibitor alpelisib are approved in metastatic HR-positive/HER2-negative disease. Here, the greatest benefit was observed in HER2-positive disease, where prior studies in the metastatic setting have been disappointing. Everolimus offered no meaningful benefit in progression-free survival (PFS) when added to chemotherapy and trastuzumab in the BOLERO-1 and BOLERO-3 phase III studies of HER2-positive metastatic breast cancer in first- and later-line settings, respectively.23,24 A signal of activity was, however, observed in HR-negative/HER2-positive patients previously treated with taxane and trastuzumab, and a significant PFS benefit was seen in patients with tumors that harbored alterations in the PI3K pathway.25 PI3K pathway mutations, found in 20%-30% of all HER2-positive breast cancers, have been implicated in trastuzumab resistance; however, results have been inconsistent across multiple studies.26-30 The treatment landscape for HER2-positive early-stage breast cancer is evolving. The addition of pertuzumab, neratinib, or trastuzumab emtansine (graduates from I-SPY 2 in the HER2-positive subset8,31,32) to trastuzumab-based chemotherapy results in > 90% 4-year invasive disease-free survival in stage II-III HER2-positive disease, but at the cost of additional toxicities, and only a small subset of patients benefit.33-35 Thus, there is room for additional agents in this space, not only for the highest risk patients who need more despite dual-antibody therapy, but also for those at lower risk who may do well with targeted therapy alone and can be spared chemotherapy altogether. Our biomarker work using reverse-phase protein analysis showed that elevated phosphoproteins downstream of Akt (eg, mTOR, glycogen synthase kinase) were associated with response to MK-2206, specifically in the HER2-positive subset.36 We and others have shown that HR-positive/HER2-positive luminal tumors have lower HER2 protein expression (and HER2/CEP17 ratios) and respond poorly to HER2-targeted therapies.37 MK-2206 may be a better option for patients with this tumor profile.
MK-2206 graduated also in HR-negative disease, albeit of limited clinical utility because HR-negative/HER2-positive and triple-negative breast cancer (TNBC) are currently treated with distinct regimens. Although posterior probabilities for TNBC itself were below the I-SPY 2 graduation threshold, MK-2206 seems superior to paclitaxel alone (96.7% probability), with a predicted 75.5% chance of phase III success (per I-SPY 2 specifications). Other studies have supported a role for Akt inhibitors in TNBC because 9%-22% have PIK3CA mutations.38 A significant improvement in PFS was correlated with PI3K/Akt/PTEN alterations by the addition of the oral Akt inhibitor ipatasertib to paclitaxel in the phase II LOTUS trial of untreated metastatic TNBC.39,40 Similar findings were reported with capivasertib (AZD5363).41 However, ipatasertib added to 12 weeks of paclitaxel in the FAIRLANE study of early TNBC showed no improvement in pCR rate.42 I-SPY 2, unlike FAIRLANE, administered anthracycline chemotherapy preoperatively, which may have contributed to the higher pCR rates and different results observed in I-SPY 2. We assessed expression and protein biomarkers in the AKT pathway; and paradoxically, in the triple-negative subset, higher levels of phosphorylated Akt and its substrates were associated with reduced response to MK-2206. However, we did observe positive associations with phospho-ErbB2 (Y877) and NRG1 expression in this subtype.43 Whether MK-2206 and other Akt inhibitors have a role in TNBC remains to be seen and will depend on the performance of other promising agents in development, including pembrolizumab (an I-SPY 2 graduate that nearly tripled pCR rates compared with standard chemotherapy44).
A limitation of this study is the high rate of early discontinuation of therapy (in both the MK-2206 and the control arm). The majority of dose reductions and early discontinuations were due to rash. Because the protocol included no standard medical management guidelines for the rash, it may have affected the willingness of patients and providers to continue study therapy with a dose reduction. In general, the rash was self-limiting with holding drug and initiation of oral steroids. The majority of patients were able to tolerate the lower 90 mg/d dose. Despite a higher rate of early discontinuation in the MK-2206 arm compared with control, MK-2206 resulted in a higher pCR rate and a trend toward improved EFS.
Our data suggest activity of an Akt inhibitor combined with standard neoadjuvant treatment in HER2-positive breast cancer and possibly a signal in TNBC as well. Significant cutaneous adverse effects were seen; however, MK-2206–related rash may be manageable with increased provider and patient education as well as with clear guidelines for toxicity management. While the future development of MK-2206 remains unclear at this time, with no planned trials in breast cancer, other Akt inhibitors like ipatasertib and capivasertib are actively being tested. Akt inhibitors may very well be active agents in both early and advanced breast cancer across receptor subtypes, but strategies to prevent or mitigate the adverse effects of PI3K/Akt/mTOR pathway blockade and to predict vulnerable individuals at particular risk for toxicity will be critical for the success of this class of agents.
ACKNOWLEDGMENT
We are grateful to the pharmaceutical, biotech, and diagnostic companies who have been willing to participate in the I-SPY 2 precompetitive collaboration experiment. We thank Anna Barker for leadership in helping to launch I-SPY 2, the members of the data and safety monitoring committee (Harold Burstein, Elizabeth Frank, Steven Goodman, Clifford Hudis, Deborah Laxague, Robert Mass, Musa Meyer, Tiffany Traina, Maria Wetzel, and Janet Wittes) for meeting monthly to review the safety data, the trial coordinators, Ken Buetow and the staff of caBIG for input with the informatics design, the entire project oversight committee, and our patient advocates and investigators. We extend our gratitude to all the patients who volunteered to participate in I-SPY 2.
PRIOR PRESENTATION
Presented at the 2015 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015.
SUPPORT
Supported by Quantum Leap Healthcare Collaborative, a 501(c)3 nonprofit (2013-present); the Foundation for the National Institutes of Health (2010-2012); and National Cancer Institute Center for Biomedical Informatics and Information Technology Grant No. 28XS197. Ongoing support for the I-SPY 2 Trial is from the Safeway Foundation, the William K. Bowes Jr Foundation, and Give Breast Cancer the Boot, and initial support was from Quintiles Transnational Corporation, the San Francisco Foundation, Side Out Foundation, Harlan Family, the Avon Foundation for Women, Alexandria Real Estate Equities, and private individuals and family foundations.
Clinical trial information: NCT01042379.
AUTHOR CONTRIBUTIONS
Conception and design: A. Jo Chien, Debasish Tripathy, Kathy S. Albain, Hope S. Rugo, Michele E. Melisko, Anne M. Wallace, Andres Forero-Torres, Janice Lu, Douglas Yee, Jane Perlmutter, Angela DeMichele, Meredith B. Buxton, Julia Clennell, Donald A. Berry, Laura Esserman
Administrative support: Debasish Tripathy, Erica Stringer-Reasor, Erin D. Ellis, Rita Nanda, Douglas Yee, Meredith B. Buxton, Melissa Paoloni, Julia Clennell, Scott Berry, Katherine Steeg, Jeffrey B. Matthews, Ashish Sanil, Donald A. Berry
Provision of study material or patients: Debasish Tripathy, Hope S. Rugo, Michele E. Melisko, Richard Schwab, Erica Stringer-Reasor, Erin D. Ellis, Henry G. Kaplan, Rita Nanda, Judy C. Boughey, Anthony D. Elias, Barbara B. Haley, Claudine Isaacs, Amy S. Clark, Julie E. Lang, Janice Lu, Larissa Korde, Kristen K. Edmiston, Donald W. Northfelt, Douglas Yee, Nola M. Hylton, Angela DeMichele, Smita M. Asare
Collection and assembly of data: A. Jo Chien, Debasish Tripathy, Constantine Godellas, W. Fraser Symmans, Michele E. Melisko, Anne M. Wallace, Richard Schwab, Teresa Helsten, Andres Forero-Torres, Erica Stringer-Reasor, Henry G. Kaplan, Rita Nanda, Nora Jaskowiak, Judy C. Boughey, Barbara B. Haley, Kathleen Kemmer, Claudine Isaacs, Julie E. Lang, Janice Lu, Larissa Korde, Rebecca K. Viscusi, Douglas Yee, Nola M. Hylton, Angela DeMichele, Amy Wilson, Meredith B. Buxton, Melissa Paoloni, Julia Clennell, Jeffrey B. Matthews, Ruby Singhrao, Gillian L. Hirst, Smita M. Asare, Donald A. Berry
Data analysis and interpretation: A. Jo Chien, Debasish Tripathy, Kathy S. Albain, Hope S. Rugo, Michele E. Melisko, Anne M. Wallace, Andres Forero-Torres, Erica Stringer-Reasor, Henry G. Kaplan, Rita Nanda, Rashmi Murthy, Judy C. Boughey, Anthony D. Elias, Barbara B. Haley, Claudine Isaacs, Amy S. Clark, Janice Lu, Kristen K. Edmiston, Donald W. Northfelt, Douglas Yee, Nola M. Hylton, Laura J. van’t Veer, Angela DeMichele, Garry Peterson, Meredith B. Buxton, Melissa Paoloni, Scott Berry, Jeffrey B. Matthews, Katherine Steeg, Ashish Sanil, Christina Yau, Smita M. Asare, Donald A. Berry, Laura J. Esserman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
MK-2206 and Standard Neoadjuvant Chemotherapy Improves Response in Patients With Human Epidermal Growth Factor Receptor 2–Positive and/or Hormone Receptor–Negative Breast Cancers in the I-SPY 2 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
A. Jo Chien
Research Funding: Merck (Inst), Puma Biotechnology (Inst), Seattle Genetics (Inst)
Debasish Tripathy
Consulting or Advisory Role: Novartis, Nektar, Pfizer, Sellas Life Sciences, GlaxoSmithKline, Genomic Health, Polyphor
Research Funding: Novartis (Inst), Polyphor (Inst)
Travel, Accommodations, Expenses: Novartis
Kathy S. Albain
Consulting or Advisory Role: Novartis, Pfizer, Myriad Genetics, Genomic Health, Agendia, Genentech, Roche
Research Funding: Seattle Genetics, Seattle Genetics (Inst)
Other Relationship: Puma Biotechnology
W. Fraser Symmans
Stock and Other Ownership Interests: ISIS Pharmaceuticals, Nuvera Biosciences, Delphi Diagnostics
Consulting or Advisory Role: Merck, Almac Diagnostics
Patents, Royalties, Other Intellectual Property: Patent on Residual Cancer Burden assessment
Travel, Accommodations, Expenses: Luminex, Merck
Hope S. Rugo
Research Funding: Macrogenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), Genentech (Inst), Merck (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Pfizer, Puma Biotechnology, Mylan, Amgen, AstraZeneca, Macrogenics, Daiichi Sankyo, Merck, Novartis, OBI Pharma
Michelle E. Melisko
Stock and Other Ownership Interests: Merrimack (I)
Honoraria: Agendia, Genentech (I), Pfizer (I)
Speakers’ Bureau: Genentech (I), Agendia, Pfizer (I)
Research Funding: Genentech (Inst), Celldex (Inst), Puma Biotechnology (Inst), Eli Lilly (Inst), Nektar (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Multiple patents related to immunoliposomal drugs being studied and potentially brought to market by Merrimack (I)
Richard Schwab
Leadership: Procend
Stock and Other Ownership Interests: Samumed (I)
Patents, Royalties, Other Intellectual Property: Patent that covers sialylated glycans and antibodies that specifically bind to them for early detection and diagnosis of cancer (Inst)
Expert Testimony: Puma Biotechnology
Teresa Helsten
Research Funding: Bayer AG (Inst), Eli Lilly (Inst), Novartis (Inst), Pfizer (Inst), AbbVie (Inst), Synthon (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/627621/summary
Andres Forero-Torres
Employment: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Speakers’ Bureau: Seattle Genetics
Research Funding: Seattle Genetics (Inst), Novartis (Inst), Genentech (Inst), Roche (Inst), Daiichi Sankyo (Inst), TRACON Pharma (Inst), Gilead Sciences (Inst), Immunomedics (Inst), Oncothyreon (Inst), Pfizer (Inst), Syndax (Inst)
Erica Stringer-Reasor
Honoraria: Breast Cancer Index, Eli Lilly, Mylan
Consulting or Advisory Role: Immunomedics
Speakers’ Bureau: Eli Lilly
Research Funding: Susan G. Komen for the Cure, V Foundation
Travel, Accommodations, Expenses: Cascadian Therapeutics, Eli Lilly
Henry G. Kaplan
Consulting or Advisory Role: Daiichi-Sankyo
Rita Nanda
Consulting or Advisory Role: Merck, Genentech, Roche, Pfizer, Macrogenics, Daiichi Sankyo, Athenex, Aduro, Ionis
Research Funding: Corcept Therapeutics (Inst), Celgene (Inst), Merck (Inst), Seattle Genetics (Inst), Genentech (Inst), Roche (Inst), Odonate Therapeutics (Inst), Pfizer (Inst), AstraZeneca (Inst), AbbVie (Inst), Immunomedics (Inst), G1 Therapeutics (Inst)
Rashmi Murthy
Honoraria: Puma Biotechnology, Genentech, Daiichi Sankyo, Seattle Genetics
Consulting or Advisory Role: Puma Biotechnology, Genentech, Roche, Daiichi Sankyo, Seattle Genetics
Research Funding: Genentech (Inst), Roche (Inst), Daiichi Sankyo (Inst), Pfizer (Inst), EMD Serono (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Seattle Genetics, Puma Biotechnology, Genentech, Daiichi Sankyo
Judy C. Boughey
Research Funding: Myriad Genetics (Inst),
Patents, Royalties, Other Intellectual Property: Patent pending: Methods and materials for assessing chemotherapy responsiveness and treating cancer (Inst)
Anthony D. Elias
Stock and Other Ownership Interests: AbbVie, Merck, Gilead Sciences, Allergan, Pfizer, Abbott Laboratories, Amgen, Bristol-Myers Squibb, United Health Group, Align Oncology, Illumina, Boston Scientific, Medtronic, CVS Health, Johnson & Johnson, Exact Sciences, Thermo Fisher Scientific
Consulting or Advisory Role: HSIX, Ayala Pharmaceuticals
Research Funding: Medivation (Inst), Astellas Pharma (Inst), Genentech (Inst), Eisai (Inst), Deciphera (Inst), Xencor (Inst)
Barbara B. Haley
Research Funding: Pfizer (Inst), Eli Lilly (Inst), Daiichi Sankyo (Inst), Roche (Inst), Puma Biotechnology (Inst)
Kathleen Kemmer
Honoraria: Astellas Pharma (I)
Consulting or Advisory Role: Merck (I)
Claudine Isaacs
Honoraria: Genentech, Roche, AstraZeneca, Pfizer
Consulting or Advisory Role: Pfizer, Genentech, Roche, Novartis, AstraZeneca, Puma Biotechnology, Context Therapeutics
Speakers’ Bureau: Genentech, Pfizer, AstraZeneca
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst), Tesaro (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing, UpToDate, Wolters Kluwer, Elsevier
Amy S. Clark
Consulting or Advisory Role: Novartis
Julie E. Lang
Speakers’ Bureau: Genomic Health
Research Funding: Angle (Inst)
Janice Lu
Honoraria: Daiichi Sankyo, Pfizer, Syndex, Puma Biotechnology, Novartis
Consulting or Advisory Role: Daiichi Sankyo, Eli Lilly, Pfizer, Syndex, Puma Biotechnology, Novartis
Travel, Accommodations, Expenses: Daiichi Sankyo, Pfizer, Syndex, Novartis
Donald W. Northfelt
Research Funding: Merck (Inst), Novartis (Inst), Pfizer (Inst), Incyte (Inst), GlaxoSmithKline (Inst), Genentech (Inst), Roche (Inst)
Douglas Yee
Stock and Other Ownership Interests: Apogen Biotechnologies
Honoraria: Tempus, Puma Biotechnology, Daiichi Sankyo
Consulting or Advisory Role: Martell Diagnostic
Research Funding: Boehringer Ingelheim
Travel, Accommodations, Expenses: Boehringer Ingelheim
Open Payments Link: https://openpaymentsdata.cms.gov/physician/636443/summary
Laura J. van’t Veer
Employment: Agendia
Leadership: Agendia
Stock and Other Ownership Interests: Agendia
Angela DeMichele
Honoraria: Pfizer
Consulting or Advisory Role: Calithera Biosciences, Novartis, Context Therapeutics, Pfizer (I)
Research Funding: Pfizer (Inst), Genentech (Inst), Incyte (Inst), Millennium Pharmaceuticals (Inst), Bayer AG (Inst), Veridex (Inst), Calithera Biosciences (Inst), GlaxoSmithKline (Inst), Wyeth (Inst)
Travel, Accommodations, Expenses: Pfizer, Calithera Biosciences, Novartis,
Meredith B. Buxton
Employment: Berry Consultants
Consulting or Advisory Role: Eli Lilly (Inst), Janssen Pharmaceuticals (Inst), Amgen (Inst), Notable Labs (Inst)
Research Funding: Bayer (Inst)
Melissa Paoloni
Employment: Arcus Biosciences
Stock and Other Ownership Interests: Arcus Biosciences, Amgen, Janssen Pharmaceuticals, Eli Lilly
Julia Clennell
Employment: IQVIA
Scott Berry
Consulting or Advisory Role: Berry Consultants (Inst)
Ashish Sanil
Other Relationship: Berry Consultants
Christina Yau
Employment: NantOmics
Smita M. Asare
Research Funding: Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst), Dynavax (Inst)
Donald A. Berry
Employment: Berry Consultants
Leadership: Berry Consultants
Stock and Other Ownership Interests: Berry Consultants
Consulting or Advisory Role: Berry Consultants
Research Funding: Daiichi Sankyo
Travel, Accommodations, Expenses: Berry Consultants
Laura J. Esserman
Consulting or Advisory Role: Blue Cross Blue Shield Association
Research Funding: Merck
Travel, Accommodations, Expenses: Blue Cross Blue Shield Association
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cortazar P, Geyer CE., Jr Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015;22:1441–1446. doi: 10.1245/s10434-015-4404-8. [DOI] [PubMed] [Google Scholar]
- 2.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 3.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 4.DeMichele A, Yee D, Berry DA, et al. The neoadjuvant model is still the future for drug development in breast cancer. Clin Cancer Res. 2015;21:2911–2915. doi: 10.1158/1078-0432.CCR-14-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 6.Barker AD, Sigman CC, Kelloff GJ, et al. I-SPY 2: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 7.Rugo HS, Olopade OI, DeMichele A, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375:23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JW, Liu MC, Yee D, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375:11–22. doi: 10.1056/NEJMoa1513750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35:515–524. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 10.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 11.Morgillo F, Della Corte CM, Diana A, et al. Phosphatidylinositol 3-kinase (PI3Kα)/AKT axis blockade with taselisib or ipatasertib enhances the efficacy of anti-microtubule drugs in human breast cancer cells. Oncotarget. 2017;8:76479–76491. doi: 10.18632/oncotarget.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Angulo AM, Krop I, Akcakanat A, et al. SU2C phase Ib study of paclitaxel and MK-2206 in advanced solid tumors and metastatic breast cancer. J Natl Cancer Inst. 2015;107:dju493. doi: 10.1093/jnci/dju493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudis C, Swanton C, Janjigian YY, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. doi: 10.1186/bcr3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien AJ, Cockerill A, Fancourt C, et al. A phase 1b study of the Akt-inhibitor MK-2206 in combination with weekly paclitaxel and trastuzumab in patients with advanced HER2-amplified solid tumor malignancies. Breast Cancer Res Treat. 2016;155:521–530. doi: 10.1007/s10549-016-3701-7. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 16.Berry DA. The brave new world of clinical cancer research: Adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9:951–959. doi: 10.1016/j.molonc.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry DA. Adaptive clinical trials in oncology. Nat Rev Clin Oncol. 2011;9:199–207. doi: 10.1038/nrclinonc.2011.165. [DOI] [PubMed] [Google Scholar]
- 18.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN guidelines insights: Breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;15:433–451. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 19.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 20.Ma CX, Suman V, Goetz MP, et al. A phase II trial of neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA-mutant ER-positive and HER2-negative breast cancer. Clin Cancer Res. 2017;23:6823–6832. doi: 10.1158/1078-0432.CCR-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinsky K, Sparano JA, Zhong X, et al. Pre-surgical trial of the AKT inhibitor MK-2206 in patients with operable invasive breast cancer: A New York Cancer Consortium trial. Clin Transl Oncol. 2018;20:1474–1483. doi: 10.1007/s12094-018-1888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yee D, DeMichele A, Isaacs C, et al: Pathological complete response predicts event-free and distant disease-free survival in the I-SPY2 trial. Cancer Res 78, 2018 (suppl; abstr GS3-08) [Google Scholar]
- 23.Hurvitz SA, André F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 24.André F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 25.André F, Hurvitz S, Fasolo A, et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: Combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol. 2016;34:2115–2124. doi: 10.1200/JCO.2015.63.9161. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J, Cortés J, Im S-A, et al. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32:3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 27.Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33:1334–1339. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loibl S, Majewski I, Guarneri V, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: Pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016;27:1519–1525. doi: 10.1093/annonc/mdw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogue-Geile KL, Song N, Jeong J-H, et al. Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. J Clin Oncol. 2015;33:1340–1347. doi: 10.1200/JCO.2014.56.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gschwantler-Kaulich D, Tan YY, Fuchs E-M, et al. PTEN expression as a predictor for the response to trastuzumab-based therapy in Her-2 overexpressing metastatic breast cancer. PLoS One. 2017;12:e0172911. doi: 10.1371/journal.pone.0172911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buxton M, DeMichele AM, Chia S, et al: Efficacy of pertuzumab/trastuzumab/paclitaxel over standard trastuzumab/paclitaxel therapy for HER2+ breast cancer: Results from the neoadjuvant I-SPY 2 trial. Cancer Res 76, 2016 (suppl; abstr CT106) [Google Scholar]
- 32. DeMichele AM, Moulder S, Buxton M, et al: Efficacy of T-DM1+pertuzumab over standard therapy for HER2+ breast cancer: Results from the neoadjuvant I-SPY 2 trial. Cancer Res 76, 2016 (suppl; abstr CT042) [Google Scholar]
- 33.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 35.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 36. Wulfkuhle JD, Wolf DM, Yau C, et al: Phosphorylation of AKT kinase substrates to predict response to the AKT inhibitor MK2206 in the I-SPY 2 trial in both HER2- and HER2+ patients. J Clin Oncol 36, 2018 (suppl; abstr 12099) [Google Scholar]
- 37.Lee PRE, Zhu Z, Wolf D, et al. BluePrint luminal subtype predicts non-response to HER2-targeted therapies in HR+/HER2+ I-SPY 2 breast cancer patients. Cancer Res. 2018;78:2612. [Google Scholar]
- 38.Kriegsmann M, Endris V, Wolf T, et al. Mutational profiles in triple-negative breast cancer defined by ultradeep multigene sequencing show high rates of PI3K pathway alterations and clinically relevant entity subgroup specific differences. Oncotarget. 2014;5:9952–9965. doi: 10.18632/oncotarget.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S-B, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360–1372. doi: 10.1016/S1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dent R, Im S-A, Espié M, et al: Overall survival (OS) update of the double-blind placebo (PBO)-controlled randomized phase 2 LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for locally advanced/metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 36, 2018 (suppl; abstr 1008) [Google Scholar]
- 41. Schmid P, Abraham J, Chan S, et al: AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): A randomised, double-blind, placebo-controlled, phase II trial. J Clin Oncol 36, 2018 (suppl; abstr 1007) [Google Scholar]
- 42.Oliveira M, Saura C, Nuciforo P, et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann Oncol. 2019;30:1289–1297. doi: 10.1093/annonc/mdz177. [DOI] [PubMed] [Google Scholar]
- 43. Wolf D, Yau C, Brown-Swigart L, et al: Analysis of biomarkers for response and resistance to the AKT inhibitor MK-2206 in the neoadjuvant I-SPY 2 trial for stage II-III high-risk breast cancer. Cancer Res 78, 2018 (suppl; abstr P2-09-08) [Google Scholar]
- 44. Nanda R, Liu MC, Yau C, et al: Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol 35, 2017 (suppl; abstr 506) [Google Scholar]