Abstract
Biosimilars have the potential to broaden patient access to biologics and provide cost savings for health care systems. During the development of a biosimilar, data that directly compare the proposed biosimilar with the reference product are required. Such comparative data are generated in a stepwise hierarchical process that begins with extensive laboratory-based structural analyses and functional assays. This initial analytical phase serves as the foundation for the demonstration of biosimilarity and is followed by nonclinical in vivo testing (if required) and then clinical evaluation, including a comparative pharmacokinetics/pharmacodynamics study that is usually conducted in healthy volunteers. The development program typically culminates with a comparative clinical efficacy study. The aim of this study is to confirm clinical equivalence of the potential biosimilar and reference product on the basis of prespecified margins, using a study population and efficacy end point that are sufficiently sensitive for detecting potential product-related differences. Such studies also include detailed analyses of safety as well as evaluation of immunogenicity. As biosimilars become more widely available in oncology, especially with recent regulatory approvals of rituximab, trastuzumab, and bevacizumab biosimilars, it is critically important that clinicians understand how the comparative clinical study differs from a traditional phase III efficacy and safety study in the development of a novel biologic originator product. Here, we review the role of comparative clinical studies in biosimilar development, with a focus on trials conducted to support approved trastuzumab biosimilars. We discuss the study populations and end points used, extrapolation of indications, and the confirmatory nature of these studies within the totality of evidence supporting biosimilarity.
INTRODUCTION
Biologic products (biologics) contain an active substance from a biologic source and are manufactured by complex processes using living systems.1 They have a significant role in the clinical management of a range of medical conditions, including cancer. At a time when there is an increasing need to address the sustainability of cancer care, biosimilars have the potential to widen patient access to biologics and provide cost savings for health care systems,2-4 and detailed regulatory guidance has been created to guide their development. From a regulatory perspective, a biosimilar is a biologic that has been shown to be highly similar to an approved reference biologic product in terms of structure, biologic activity, safety, and efficacy.1,5,6 To gain regulatory approval in the United States, for example, it must be demonstrated that a proposed biosimilar is “highly similar to the reference product notwithstanding minor differences in clinically inactive components” and that “there are no clinically meaningful differences between the [biosimilar] and the reference product in terms [of] safety, purity, and potency.”6(p3) The term biosimilar reflects the fact that because of the inherent degree of natural minor variability exhibited by all biologic products, it is not possible to create a structurally identical copy of a reference product.1,6 In practice, however, biosimilars approved through a robust regulatory pathway may be considered clinically equivalent to the relevant reference product. Reflecting this, in regions such as the European Union (EU) and United States, biosimilar product labeling is aligned closely with that of the reference product.1,7 Furthermore, patient materials recently issued by the US Food and Drug Administration (FDA) describe biosimilars as having the same expected benefits and risks as their respective reference products.8
During the development of a biosimilar, an array of data that directly compare the candidate biosimilar with the reference product is required.5,6,9 This is generated in a stepwise hierarchical process, which begins with extensive characterization of the proposed biosimilar and the reference product, using a range of laboratory-based comparative structural analyses and functional assays, such as assessment of antibody-dependent cellular cytotoxicity (ADCC).5,6,10 This initial step serves as the foundation for a demonstration of biosimilarity, and the more rigorous this assessment in showing similar structure and function, the greater the justification for a selective, tailored program of nonclinical in vivo testing (if required) and clinical studies.6 The determination of biosimilarity is based on the totality of the evidence from all stages of development.5,6,9,10
With respect to the underlying scientific principles, regulatory requirements for demonstrating biosimilarity are generally consistent among stringently regulated regions, such as Australia, Canada, the EU, Japan, and the United States.11 Although biosimilar supportive care agents have been available for use in oncology for a number of years in several of these regions,12,13 it is only more recently that biosimilar monoclonal antibodies (mAbs) for the treatment of cancer, including rituximab, trastuzumab, and bevacizumab biosimilars, have received regulatory approval.14-17 Indeed, in the United States, the first bevacizumab and trastuzumab biosimilars became available for commercial sale in July 2019.18 While representing a new development in oncology, biosimilar mAbs have been used successfully for several years in the treatment of chronic inflammatory diseases,4 including conditions that were not initially studied in comparative trials as part of the biosimilarity assessment. Although oncologists may be accepting of biosimilar supportive care agents, it has been suggested that they could be less comfortable with anticancer biosimilars.19 A recent survey of US community oncologists identified educational gaps with respect to the regulatory approval framework for biosimilars, with some respondents reporting that they were uncomfortable or unfamiliar with the current process.20 A separate survey by the European Society for Medical Oncology among oncology prescribers identified gaps in knowledge related to biosimilar development, clinical trial design, and selection of end points.21 To maximize the potential of biosimilars, such knowledge gaps must be addressed.22 With the introduction of biosimilar mAbs into clinical practice, it is critically important that oncologists understand how the comparative clinical efficacy and safety study, which typically serves as the final step in the biosimilarity exercise, differs from the traditional phase III study in the development of a novel biologic originator product. In this review, we consider the role of comparative clinical studies in biosimilar development, with reference to approved trastuzumab biosimilars as an illustrative example.
COMPARATIVE CLINICAL STUDIES IN THE DEVELOPMENT OF BIOSIMILARS
The main aim of a biosimilar clinical development program is to confirm that any differences between a potential biosimilar and the reference product are not clinically meaningful.1,5,6,10 Thus, the number and scope of clinical studies performed for a potential biosimilar depend on the degree of residual uncertainty with regard to biosimilarity following the earlier analytical assessment (and nonclinical in vivo testing, if performed).6 The clinical program includes a comparative pharmacokinetics (PK) study (with a pharmacodynamics [PD] comparison where suitable biomarkers exist), which is commonly conducted in healthy volunteers.6,23,24 This is typically followed by a comparative clinical study that assesses efficacy and safety in at least one relevant indication.6,23
The aim of the comparative clinical efficacy study is not to demonstrate clinical benefit, as this has already been established independently for the reference product.23,25 Rather, the aim is to confirm clinical equivalence of the potential biosimilar and reference product on the basis of prespecified margins, using a study population and efficacy end point that are sufficiently sensitive for detecting potential product-related differences while at the same time minimizing the influence of patient- or disease-related factors.23,25 A sensitive study population would typically be one for which the treatment effect of the reference product has been shown to be robust in prior trials, which thus enhances the ability to detect small differences in efficacy.26 Factors such as prior lines of therapy and the effect of concomitant medications are also relevant to sensitivity.10 Ideally, a first-line study conducted in a homogeneous patient population (eg, in terms of disease severity) with a short-term clinical efficacy end point that measures pharmacologic activity would be recommended.1,10,25 These studies should also include a detailed analysis of safety as well as an evaluation of immunogenicity. The end point chosen may differ from that used to demonstrate the efficacy of the reference product in pivotal studies. For example, although disease-free survival (DFS), progression-free survival (PFS), or overall survival (OS) end points are often required for demonstrating clinical benefit in registration trials of novel anticancer therapeutics, short-term surrogate end points, such as overall response rate (ORR) measured at a certain time point or pathologic complete response (pCR), are considered both adequate and more appropriate for detecting potential product-related differences in a comparative clinical study of a potential anticancer biosimilar.25
To statistically test whether a biosimilar is inferior or superior to the reference product in terms of the primary efficacy end point, an equivalence study design is preferred.6,23 Equivalence is established if the CI for the selected parameter for treatment effect (eg, the difference or ratio between treatments) is completely contained within upper and lower equivalence margins; this is tantamount to performing two one-sided tests, simultaneously testing the null hypotheses of inferiority and superiority.10,27 Such margins are derived specifically for the indication and end point studied and are based on historical data that concern the efficacy of the reference product as well as on clinical judgment.1 In contrast to equivalence studies, noninferiority studies are one-sided and, hence, do not exclude the possibility that a potential biosimilar may be superior in efficacy to the reference product.10 If such superiority was considered clinically relevant, this might contradict the principle of similarity.9 Guidelines from the European Medicines Agency (EMA), FDA, and WHO state that a noninferiority design for comparative clinical studies may be appropriate and acceptable in certain circumstances,6,9,23 although a strong scientific rationale would be required.23
If biosimilarity has been successfully demonstrated on the basis of a comparative development program that includes data derived from a clinical study in one therapeutic indication, regulatory guidelines allow for the possibility of the biosimilar being approved for additional indications held by the reference product without conducting additional clinical studies (termed extrapolation).6,23,28 From scientific, cost, and ethical perspectives, biosimilar studies should not seek to replicate the efficacy and safety data of the reference product across all indications.28 However, extrapolation must be scientifically justified and considered within the context of the totality of the analytical, nonclinical, and clinical evidence supporting biosimilarity.6,23 For example, extrapolation may be challenging if the mechanism of action (MOA) of the active substance involves several receptors or binding sites, the contribution of which may vary between the tested and extrapolated indications.29
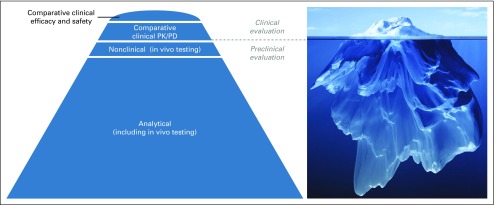
Because prescribers and clinicians are familiar with evaluating novel drugs on the basis of clinical studies, it is important that they appreciate the distinct role of comparative clinical studies in the biosimilar development paradigm.30 Although the paradigm for the development and approval of a novel biologic is that the positive benefit-risk profile is established mainly on the basis of controlled studies that demonstrate efficacy and safety in each indication approved, this is not the case for a biosimilar.1 For biosimilars, the positive benefit-risk profile is established on the basis of the totality of the evidence that demonstrates biosimilarity to the reference product, with comparative clinical efficacy trials serving a confirmatory function, and highly sensitive analytical methods providing the foundation for the data1,5,6,23 (Fig 1). Such analytical methods are generally much more sensitive than clinical studies for detecting potential differences.1,30 Furthermore, significant differences observed in quality attributes cannot be justified using clinical data.5
FIG 1.
Totality of the evidence that supports biosimilarity. Extensive analytical characterization of a proposed biosimilar and the reference product, using an array of comparative structural analyses and functional assays, provides the foundation for a demonstration of biosimilarity. Data from comparative clinical efficacy and safety studies are confirmatory and are represented as the tip of the iceberg. PD, pharmacodynamics; PK, pharmacokinetics. Iceberg image copyright © Adike/Shutterstock.com.
COMPARATIVE CLINICAL STUDIES OF TRASTUZUMAB BIOSIMILARS IN BREAST CANCER
Which Study Settings and End Points Have Been Used?
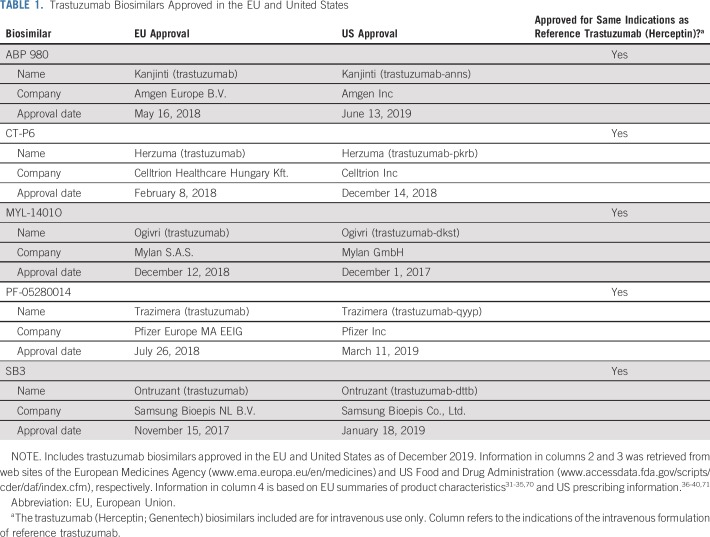
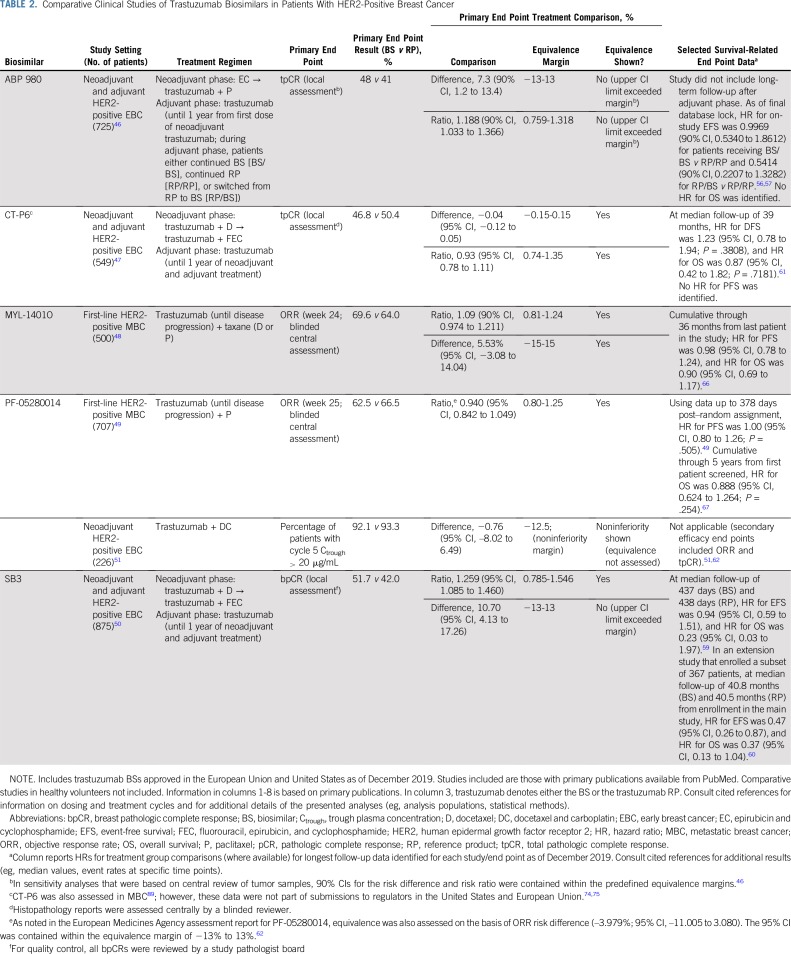
Several of the points highlighted in the previous section can be illustrated by considering the example of recently approved biosimilars in reference to trastuzumab (Herceptin; Genentech, South San Francisco, CA; Roche Registration GmbH, Grenzach-Wyhlen, Germany). As of December 2019, five trastuzumab biosimilars have been approved in the EU and United States for intravenous use31-40 (Table 1). During their respective clinical development programs, all five molecules were assessed in single-dose comparative PK similarity studies in healthy male volunteers,41-45 and in comparative clinical efficacy and safety studies in women with human epidermal growth factor receptor 2 (HER2)–positive breast cancer.46-50 There were differences in the designs of the comparative clinical efficacy studies that support biosimilarity, with the study setting (ie, patient population) representing one point of variation, although all studies used combinations with taxane-based chemotherapy46-50 (see Table 2 for an overview of the studies, including primary results). For example, the approvals of ABP 980, CT-P6, and SB3 were supported by studies that compared each biosimilar with reference trastuzumab in the neoadjuvant and adjuvant treatment of early breast cancer (EBC).46,47,50 In contrast, MYL-1401O and PF-05280014 were compared with reference trastuzumab in the first-line treatment of metastatic breast cancer (MBC).48,49 In addition, PF-05280014 was compared with reference trastuzumab in a comparative PK noninferiority study in the neoadjuvant treatment of EBC.51
TABLE 1.
Trastuzumab Biosimilars Approved in the EU and United States
TABLE 2.
Comparative Clinical Studies of Trastuzumab Biosimilars in Patients With HER2-Positive Breast Cancer
Study end points also differed across the development programs (Table 2). For example, although the EBC studies of ABP 980, CT-P6, and SB3 used a pCR primary end point, the definition of pCR varied. The studies of ABP 980 and CT-P6 assessed pCR defined as the absence of invasive tumor cells in the breast and axillary lymph nodes regardless of ductal carcinoma in situ (ie, ypT0/is ypN0, hereafter referred to as total pCR [tpCR]).46,47 In contrast, the SB3 study used the primary end point of breast pCR (bpCR) defined as the absence of invasive tumor cells in the breast regardless of ductal carcinoma in situ (ie, ypT0/is).50,52 Some experts have recommended standardizing the use of tpCR as the primary end point for evaluating neoadjuvant treatments (including biosimilars) on the grounds that tpCR is a stronger prognostic marker than bpCR.53 Indeed, both the FDA and the EMA include absence of nodal involvement in their recommended definitions of pCR as an end point in neoadjuvant studies.54,55 The investigators of the SB3 study stated that they selected bpCR to eliminate potential confounding factors related to tpCR determination that are not attributable to product-related differences, such as the extent of axillary dissection (tpCR was assessed as a secondary end point, however).50 Longer-term survival-related end points included EFS and OS in the ABP 980 and SB3 studies and DFS, PFS, and OS in the CT-P6 study; planned follow-up durations differed across the trials46,47,50,56-61 (see Table 2 for selected results available at the time of writing). The first-line MBC studies for MYL-1401O and PF-05280014 each used the primary end point of ORR on the basis of complete or partial responses achieved by week 24 and week 25, respectively.48,49 In both MBC studies, secondary efficacy end points included assessment of PFS and OS.48,49 The neoadjuvant study of PF-05280014 was a noninferiority trial that was considered supportive to the main MBC study and included a PK primary end point (the percentage of patients with trough plasma concentrations of the biosimilar or reference product > 20 μg/mL after five cycles of treatment).51 Secondary end points included tpCR and ORR.51,62
Some experts have argued that a comparative study in the neoadjuvant EBC setting using a pCR end point could offer the greatest level of homogeneity and sensitivity for detecting potential differences between a potential trastuzumab biosimilar and the reference product because patients have received the same prior treatments, have lower disease burden, and may be less likely to be immunologically impaired, for example.63,64 In addition, at the individual patient level, achieving pCR has been associated with longer EFS and OS compared with not achieving pCR.65 However, the MBC setting is also appropriate for an assessment of biosimilarity, provided that effort is made to control and minimize heterogeneity sufficiently.62 Indeed, biosimilar clinical trials conducted with MYL-1401O and PF-05280014 in the first-line MBC setting included relatively homogeneous populations. The MYL-1401O study excluded patients with prior exposure to chemotherapy or reference trastuzumab in the metastatic setting and required at least 1 year since adjuvant therapy with reference trastuzumab.48 Similarly, the PF-05280014 study excluded patients with prior systemic therapy for MBC (except endocrine therapy) along with those who had relapsed within 1 year of the last dose of adjuvant or neoadjuvant treatment (again, except endocrine therapy).49 In both studies, a low proportion of patients had prior exposure to reference trastuzumab (MYL-1401O study, 8%; PF-05280014 study, 10%).48,49 Eligibility criteria for both studies also ensured proper identification of HER2-positive patients.48,49 More generally, it is worth noting that potential heterogeneity in patient populations can be addressed by stratifying for important covariates during randomization, carefully selecting the prespecified equivalence margin, and/or increasing sample size, for example. With regard to their results, both first-line MBC studies robustly demonstrated similarity in ORR between the biosimilar and reference product48,49 (Table 2). In both the MYL-1401O and the PF-05280014 studies, no clinically meaningful differences in PFS or OS were observed compared with reference trastuzumab48,49,66,67 (selected results are listed in Table 2). An analysis of data from the MYL-1401O study also provided support for the use of ORR as a primary end point by showing a correlation between the responder/nonresponder category at week 24 and the probability of PFS (biserial correlation coefficient across all patients, 0.752).68 An additional consideration with regard to study setting is that while neoadjuvant/adjuvant therapy is given for 1 year in an EBC study, patients in a first-line MBC trial continue trastuzumab until disease progression (or unacceptable toxicity); therefore, studies in the metastatic setting offer the possibility of assessing safety and immunogenicity outcomes associated with long-term treatment.69
From a regulatory perspective, there is no requirement for potential trastuzumab biosimilars to be assessed in a comparative clinical efficacy study in the neoadjuvant setting, and with the approval of MYL-1401O and PF-05280014, the EMA and FDA clearly consider the first-line MBC setting as acceptable and sufficiently sensitive for assessing similarity. In short, both neoadjuvant EBC and first-line MBC settings provide the data needed for confirming a lack of clinically meaningful differences between a trastuzumab biosimilar and the reference product, and each has its own advantages and disadvantages.69 All five trastuzumab biosimilars discussed here have been approved for the same indications as the intravenous formulation of reference trastuzumab (ie, HER2-positive EBC, MBC, and metastatic gastric cancer in the EU and HER2-positive adjuvant breast cancer, MBC, and metastatic gastric cancer in the United States).31-40,70,71 Thus, both EBC and MBC have been considered as sufficiently sensitive settings to support extrapolation. For trastuzumab biosimilars, the scientific justification for extrapolation includes the fact that the MOA of trastuzumab is the same across indications, and the target receptor involved (HER2) is the same in each case.57,72-78 Furthermore, on the basis of data available for the reference product, there are no significant differences in expected toxicities between patient populations or indications.72,75
How Have Regulatory Authorities Interpreted Comparative Clinical Study Data Within the Context of the Totality of the Evidence?
As described earlier, biosimilarity is determined on the basis of the totality of evidence. To illustrate how regulators interpret data from comparative clinical efficacy studies within the overall assessment of biosimilarity, it is helpful to consider the evaluation of SB3 and ABP 980 by the EMA’s Committee for Medicinal Products for Human Use (CHMP) as described in European Public Assessment Reports (EPARs) and the subsequent EU approval of these biosimilars.46,50,57,73
In the SB3 study in EBC, equivalence was assessed on the basis of an analysis of the 95% CIs of both the ratio of bpCR rates and the difference in bpCR rates between arms.50 The 95% CI for the adjusted ratio of bpCR rates was contained within the predefined equivalence margin, demonstrating equivalence (Table 2). In contrast, the upper limit of the 95% CI for the adjusted difference in bpCR rates was outside the predefined equivalence margin,50 meaning that while noninferiority of SB3 was demonstrated, nonsuperiority was not. The CHMP primarily considered the difference in bpCR rates in its assessment of SB3.73 Structural and functional analyses conducted by the sponsor of numerous lots of reference trastuzumab identified that certain lots exhibited a marked downward drift in glycosylation levels, FcγRIIIa binding, and ADCC.50,79 ADCC is a known component of the trastuzumab MOA, and some of the affected lots were used in the clinical study.50,73 It was considered by the CHMP that this apparent shift in ADCC activity could have added variability to the estimation of the treatment difference, thereby contributing to the upper limit of the CI exceeding the margin.73 As noted in the EPAR for SB3, “the magnitude of the differences observed can be in part attributed to other factors and the true difference is considered likely to fall within the equivalence margins and [be] of no clinical relevance.”73(p68)
In the study of ABP 980 in EBC, equivalence was evaluated using the 90% CIs of both the risk difference and the risk ratio of locally assessed tpCR, using a sequential testing method.46 In analyses that were based on both the risk difference and the risk ratio, the upper boundaries of the 90% CIs exceeded the predefined equivalence margins (Table 2). Thus, nonsuperiority of ABP 980 was not demonstrated. In a sensitivity analysis that was based on central review of tumor samples, 90% CIs of the risk difference and risk ratio were contained within the margins, however.46 According to the EPAR for ABP 980, the CHMP seems to have considered 95% CIs (rather than 90% CIs) of the tpCR risk difference and risk ratio on the basis of local laboratory review.57 Again, the upper limits of both 95% CIs exceeded the prespecified margins.57 As with SB3, it was acknowledged in the EPAR for ABP 980 that the apparent difference between the groups was considered to be at least partly confounded by a shift in ADCC activity observed for certain lots of the trastuzumab reference product used in the study, which may have contributed to a more extreme location of the upper CI limit.57 The CHMP noted that the observed difference in efficacy results was not considered clinically relevant.57
For both SB3 and ABP 980, considering the similarity data from across all stages of the respective comparison exercises, the CHMP determined that biosimilarity to reference trastuzumab had been sufficiently shown.57,73 These examples help to illustrate that it is the totality of the evidence, with comprehensive and robust analytical data as the foundation, that is of crucial importance in a regulatory determination of biosimilarity. Data from comparative clinical studies, while clearly important, serve a confirmatory rather than a central function. As shown by the regulatory assessment of SB3 and ABP 980 in the EU, in certain circumstances, small apparent differences between a proposed biosimilar and reference product when using a sensitive clinical end point may be considered unlikely to be clinically meaningful and in view of the totality of data, may not preclude a determination of biosimilarity.
WHAT IS THE ROLE FOR COMPARATIVE CLINICAL STUDIES IN THE FUTURE DEVELOPMENT OF BIOSIMILARS?
Recently, some experts have argued that from a scientific, economic, and ethical perspective, comparative clinical efficacy studies may be unnecessary in the development of most biosimilars.80-82 A recent opinion article proposed that the current approach to biosimilar development should be replaced with a more efficient paradigm that “emphasizes analytical likeness between a biosimilar and its reference but does not generally require…in vivo nonclinical studies or clinical equivalence studies.”82(p604) The authors of the article based their proposal on the observation that
no biosimilar that has been found to be highly similar to its reference by both analytical and human pharmacokinetic studies has ever failed to be approved because it was found not to be clinically equivalent to its reference in a powered [efficacy] study.82(p604)
It should be noted that current regulatory guidelines do not mandate comparative clinical efficacy studies in all circumstances.5,6 FDA guidance, for instance, states that a comparative clinical study will be necessary “if there is residual uncertainty about whether there are clinically meaningful differences between the proposed [biosimilar] product and the reference product based on structural and functional characterization, animal testing, human PK and PD data, and clinical immunogenicity assessment.”6(p18) Factors that affect the type and extent of clinical data required include the complexity of the reference product, the magnitude of differences observed in comparative structural and functional assessment, the degree to which the MOA is understood, and the availability of a PD end point that correlates with efficacy.1,6 In the EU, regulatory requirements with regard to clinical data have evolved since the biosimilar framework was first introduced, and although comparative PK/PD studies remain essential, the strict requirement for comparative efficacy studies has been waived (or is proposed to be waived) for certain product categories, along with comparative safety/immunogenicity studies in specific circumstances.5,83,84 For granulocyte colony-stimulating factor, for example, structure, physicochemical characteristics, and biologic activity can be well characterized, and clinically relevant PD parameters are available.85 Whereas the original version of the EMA guidance concerning biosimilar granulocyte colony-stimulating factor (published in 2006)86 includes significant emphasis on comparative clinical efficacy and safety trials, a draft revision to the guideline (released in 2018 for consultation) stated that a dedicated comparative efficacy trial is “not considered necessary.”84(p7) For many biosimilar mAbs, however, the absence of robust PD efficacy measures, as well as their importance to clinical outcome, means that comparative clinical trials will likely remain necessary.87,88 In our view, the requirement for such studies is also particularly likely for oncology mAbs, where biosimilars may be used with curative intent, and prescribers will want to appraise comparative clinical data.
In summary, the paradigm for the development and approval of biosimilars differs markedly from that for novel biologics. For biosimilars, the positive benefit-risk profile is based on the totality of the evidence that demonstrates biosimilarity to the reference product rather than on efficacy and safety studies in each approved indication. In biosimilar development, the comparative clinical efficacy study aims to confirm clinical equivalence between a proposed biosimilar and its reference product on the basis of prespecified margins, along with comparable safety and immunogenicity. Such studies do not aim to establish de novo efficacy and safety. Reflecting this difference, comparative clinical studies should be performed in a sensitive population using appropriate end points to allow detection of any clinically meaningful differences between the treatments, should they exist. As is evident from experience with recently approved trastuzumab biosimilars, for certain reference products, there may be more than one appropriate design for such studies in terms of the population studied and end point used. Furthermore, there may be more than one acceptable study setting to support extrapolation. As biosimilars become more widely available in oncology, it is important that clinicians appreciate the distinct confirmatory role of comparative clinical studies in the biosimilar paradigm.
ACKNOWLEDGMENT
Medical writing support was provided by Paul Shepherd of Engage Scientific Solutions and was funded by Pfizer.
AUTHOR CONTRIBUTIONS
Conception and design: Justin Stebbing, Paul N. Mainwaring, Giuseppe Curigliano, Mark Latymer, Angel H. Bair, Hope S. Rugo
Administrative support: Mark Latymer
Collection and assembly of data: Justin Stebbing, Paul N. Mainwaring, Giuseppe Curigliano, Mark Latymer, Angel H. Bair
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Understanding the Role of Comparative Clinical Studies in the Development of Oncology Biosimilars
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Justin Stebbing
Leadership: BB Healthcare Trust, Xerion Healthcare, Springer Nature
Consulting or Advisory Role: Celltrion, TLC Biopharmaceuticals, Vor Biopharma, Lansdowne, Vitruvian, Singapore Biotech, Benevolent AI, Vaccitech
Paul N. Mainwaring
Employment: Xing Technologies
Leadership: Xing Technologies
Stock and Other Ownership Interests: Xing Technologies
Honoraria: Ipsen, Roche, Genentech, Pfizer, Janssen Oncology, Medivation, Astellas Pharma, Merck, Novartis
Consulting or Advisory Role: Pfizer, Janssen Oncology
Speakers’ Bureau: Ipsen, Roche, Genentech, Pfizer, Janssen Oncology, Medivation, Astellas Pharma, Merck, Novartis, AstraZeneca
Research Funding: Merck KGaA
Patents, Royalties, Other Intellectual Property: Four patents on nanotechnology
Travel, Accommodations, Expenses: Ipsen, Roche, Genentech, Pfizer, Janssen Oncology, Medivation, Astellas Pharma, Merck, Novartis, Xing Technologies
Giuseppe Curigliano
Honoraria: Ellipses Pharma
Consulting or Advisory Role: Roche, Genentech, Pfizer, Novartis, Eli Lilly, Foundation Medicine, Bristol-Myers Squibb, Samsung
Speakers’ Bureau: Roche, Genentech, Novartis, Pfizer, Eli Lilly, Foundation Medicine, Samsung Bioepis
Travel, Accommodations, Expenses: Roche, Genentech, Pfizer
Mark Pegram
Honoraria: Genentech, Roche
Consulting or Advisory Role: Genentech, Roche
Mark Latymer
Employment: Pfizer
Leadership: Pfizer
Stock and Other Ownership Interests: Pfizer
Travel, Accommodations, Expenses: Pfizer
Angel H. Bair
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Hope S. Rugo
Research Funding: Macrogenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), Genentech (Inst), Merck (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Pfizer, Puma Biotechnology, Mylan, Amgen, AstraZeneca, Macrogenics, Daiichi Sankyo, Merck, Novartis, OBI Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1. European Medicines Agency; European Commission: Biosimilars in the EU: Information Guide for Healthcare Professionals, 2017. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf.
- 2.Tabernero J, Vyas M, Giuliani R, et al. Biosimilars: A position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open. 2017;1:e000142. doi: 10.1136/esmoopen-2016-000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyman GH, Balaban E, Diaz M, et al. American Society of Clinical Oncology statement: Biosimilars in oncology. J Clin Oncol. 2018;36:1260–1265. doi: 10.1200/JCO.2017.77.4893. [DOI] [PubMed] [Google Scholar]
- 4.IQVIA The Impact of Biosimilar Competition in Europe. 2018 https://ec.europa.eu/docsroom/documents/31642/attachments/1/translations/en/renditions/native
- 5. European Medicines Agency: Guideline on similar biological medicinal products, 2014. https://www.ema.europa.eu/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf.
- 6. US Food and Drug Administration: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry, 2015. https://www.fda.gov/media/82647/download.
- 7. US Food and Drug Administration: Labeling for Biosimilar Products. Guidance for Industry, 2018. https://www.fda.gov/media/96894/download.
- 8. US Food and Drug Administration: Patient materials, 2019. https://www.fda.gov/drugs/biosimilars/patient-materials.
- 9. WHO: Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs), 2009. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
- 10. WHO: Guidelines on Evaluation of Monoclonal Antibodies as Similar Biotherapeutic Products (SBPs), 2017. https://www.who.int/biologicals/biotherapeutics/WHO_TRS_1004_web_Annex_2.pdf?ua=1.
- 11.Cazap E, Jacobs I, McBride A, et al. Global acceptance of biosimilars: Importance of regulatory consistency, education, and trust. Oncologist. 2018;23:1188–1198. doi: 10.1634/theoncologist.2017-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1007/s40259-018-0268-3. Aapro M, Krendyukov A, Schiestl M, et al: Epoetin biosimilars in the treatment of chemotherapy-induced anemia: 10 years’ experience gained. BioDrugs 32:129-135, 2018 [Erratum: BioDrugs 32:137-138, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio in Europe: What have we learned? Support Care Cancer. 2013;21:2925–2932. doi: 10.1007/s00520-013-1911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration: Biosimilar product information, 2019. https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm580432.htm.
- 15. European Medicines Agency: Medicines, 2019. https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar.
- 16. Genetics and Biosimilars Initiative: Biosimilars approved in Japan, 2019. http://gabionline.net/Biosimilars/General/Biosimilars-approved-in-Japan.
- 17. Genetics and Biosimilars Initiative: Biosimilars approved in Australia, 2019. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Australia.
- 18. Amgen: Amgen and Allergan’s MVASI™ (bevacizumab-awwb) and KANJINTI (trastuzumab-anns) Now Available in the United States, 2019. https://www.amgen.com/media/news-releases/2019/07/amgen-and-allergans-mvasi-bevacizumabawwb-and-kanjinti-trastuzumabanns-now-available-in-the-united-states.
- 19.Nabhan C, Valley A, Feinberg BA. Barriers to oncology biosimilars uptake in the United States. Oncologist. 2018;23:1261–1265. doi: 10.1634/theoncologist.2018-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabhan C, Jeune-Smith Y, Valley A, et al. Community oncologists’ perception and acceptance of biosimilars in oncology. J Clin Pathways. 2018;4:43–47. [Google Scholar]
- 21.Giuliani R, Tabernero J, Cardoso F, et al. Knowledge and use of biosimilars in oncology: A survey by the European Society for Medical Oncology. ESMO Open. 2019;4:e000460. doi: 10.1136/esmoopen-2018-000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard E, Wascovich M, Oskouei S, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: A systematic review. J Manag Care Spec Pharm. 2019;25:102–112. doi: 10.18553/jmcp.2019.25.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Medicines Agency: Guideline on similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues, 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf.
- 24. US Food and Drug Administration: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product: Guidance for Industry, 2016. https://www.fda.gov/media/88622/download.
- 25. European Medicines Agency: Guideline on Similar Biological Medicinal Products Containing Monoclonal Antibodies—Non-Clinical and Clinical Issues, 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf.
- 26.Krendyukov A, Schiestl M. Extrapolation concept at work with biosimilar: A decade of experience in oncology. ESMO Open. 2018;3:e000319. doi: 10.1136/esmoopen-2017-000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–196. doi: 10.1007/s11606-010-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curigliano G, O’Connor DP, Rosenberg JA, et al. Biosimilars: Extrapolation for oncology. Crit Rev Oncol Hematol. 2016;104:131–137. doi: 10.1016/j.critrevonc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Weise M, Kurki P, Wolff-Holz E, et al. Biosimilars: The science of extrapolation. Blood. 2014;124:3191–3196. doi: 10.1182/blood-2014-06-583617. [DOI] [PubMed] [Google Scholar]
- 30.Christl LA, Woodcock J, Kozlowski S. Biosimilars: The US regulatory framework. Annu Rev Med. 2017;68:243–254. doi: 10.1146/annurev-med-051215-031022. [DOI] [PubMed] [Google Scholar]
- 31. Amgen Europe: Kanjinti summary of product characteristics, 2019. https://www.ema.europa.eu/documents/product-information/kanjinti-epar-product-information_en.pdf.
- 32.Celltrion Healthcare Hungary Herzuma summary of product characteristics. 2019 https://www.ema.europa.eu/documents/product-information/herzuma-epar-product-information_en.pdf
- 33. Mylan: Ogivri summary of product characteristics, 2019. https://www.ema.europa.eu/documents/product-information/ogivri-epar-product-information_en.pdf.
- 34. Pfizer Europe: Trazimera summary of product characteristics, 2019. https://www.ema.europa.eu/en/documents/product-information/trazimera-epar-product-information_en.pdf.
- 35. Samsung Bioepis: Ontruzant summary of product characteristics, 2019. https://www.ema.europa.eu/documents/product-information/ontruzant-epar-product-information_en.pdf.
- 36.Amgen Kanjinti prescribing information. 2019 https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761073Orig1s000lbl.pdf
- 37.Celltrion Herzuma prescribing information. 2019 https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761091s001s002lbl.pdf
- 38. Mylan Pharmaceuticals: Ogivri prescribing information, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761074s004lbl.pdf.
- 39.Pfizer Trazimera prescribing information. 2019 https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761081s000lbl.pdf
- 40. Samsung Bioepis: Ontruzant prescribing information, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761100s000lbl.pdf.
- 41.Hanes V, Chow V, Zhang N, et al. A randomized, single-blind, single-dose study evaluating the pharmacokinetic equivalence of proposed biosimilar ABP 980 and trastuzumab in healthy male subjects. Cancer Chemother Pharmacol. 2017;79:881–888. doi: 10.1007/s00280-017-3286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esteva FJ, Stebbing J, Wood-Horrall RN, et al. A randomised trial comparing the pharmacokinetics and safety of the biosimilar CT-P6 with reference trastuzumab. Cancer Chemother Pharmacol. 2018;81:505–514. doi: 10.1007/s00280-017-3510-7. [DOI] [PubMed] [Google Scholar]
- 43.Waller CF, Vutikullird A, Lawrence TE, et al. A pharmacokinetics phase 1 bioequivalence study of the trastuzumab biosimilar MYL-1401O vs. EU-trastuzumab and US-trastuzumab. Br J Clin Pharmacol. 2018;84:2336–2343. doi: 10.1111/bcp.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin D, Barker KB, Li R, et al. A randomized phase 1 pharmacokinetic trial comparing the potential biosimilar PF-05280014 with trastuzumab in healthy volunteers (REFLECTIONS B327-01) Br J Clin Pharmacol. 2014;78:1281–1290. doi: 10.1111/bcp.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pivot X, Curtit E, Lee YJ, et al: A randomized phase I pharmacokinetic study comparing biosimilar candidate SB3 and trastuzumab in healthy male subjects. Clin Ther 38:1665-1673.e3, 2016. [DOI] [PubMed]
- 46.von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19:987–998. doi: 10.1016/S1470-2045(18)30241-9. [DOI] [PubMed] [Google Scholar]
- 47.Stebbing J, Baranau Y, Baryash V, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: A randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18:917–928. doi: 10.1016/S1470-2045(17)30434-5. [DOI] [PubMed] [Google Scholar]
- 48.Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: A randomized clinical trial. JAMA. 2017;317:37–47. doi: 10.1001/jama.2016.18305. [DOI] [PubMed] [Google Scholar]
- 49.Pegram MD, Bondarenko I, Zorzetto MMC, et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: A randomised, double-blind study. Br J Cancer. 2019;120:172–182. doi: 10.1038/s41416-018-0340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pivot X, Bondarenko I, Nowecki Z, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2018;36:968–974. doi: 10.1200/JCO.2017.74.0126. [DOI] [PubMed] [Google Scholar]
- 51.Lammers PE, Dank M, Masetti R, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer. 2018;119:266–273. doi: 10.1038/s41416-018-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Center for Drug Evaluation and Research: Summary review: BLA 761100 (Ontruzant), 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761100Orig1s000SumR.pdf.
- 53.Stebbing J, Baranau Y, Manikhas A, et al. Total pathological complete response versus breast pathological complete response in clinical trials of reference and biosimilar trastuzumab in the neoadjuvant treatment of breast cancer. Expert Rev Anticancer Ther. 2018;18:531–541. doi: 10.1080/14737140.2018.1457442. [DOI] [PubMed] [Google Scholar]
- 54. US Food and Drug Administration: Guidance for industry. Pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: Use as an endpoint to support accelerated approval, 2014. https://www.fda.gov/media/83507/download.
- 55.European Medicines Agency Appendix 4 to the guideline on the evaluation of anticancer medicinal products in man. 2015 https://www.ema.europa.eu/en/documents/scientific-guideline/evaluation-anticancer-medicinal-products-man-appendix-4-condition-specific-guidance-rev2_en.pdf
- 56. Amgen: ABP 980. Clinical Study Report: 20120283. Synopsis, 2017. http://filehosting.pharmacm.com/DownloadService.ashx?client=AMG&studyid=500&filename=20120283%2001.09.04.01%20Public%20Results%20Redacted%20CSR%20Synopsis%202017-01-27%20NA.pdf.
- 57. European Medicines Agency: Assessment report: Kanjinti, 2018. https://www.ema.europa.eu/en/documents/assessment-report/kanjinti-epar-public-assessment-report_en.pdf.
- 58.Pivot X, Bondarenko I, Nowecki Z, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: Final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19–27. doi: 10.1016/j.ejca.2018.01.072. [DOI] [PubMed] [Google Scholar]
- 59. Pivot X, Bondarenko IM, Zbigniew N, et al: One-year safety, immunogenicity, and survival results from a phase III study comparing SB3 (a proposed trastuzumab biosimilar) and originator trastuzumab in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment. Presented at the Eur Soc Med Oncol Congr 2017, Madrid, Spain, September 8-12, 2017. [Google Scholar]
- 60.Pivot X, Pegram M, Cortes J, et al. Three-year follow-up from a phase 3 study of SB3 (a trastuzumab biosimilar) versus reference trastuzumab in the neoadjuvant setting for human epidermal growth factor receptor 2-positive breast cancer. Eur J Cancer. 2019;120:1–9. doi: 10.1016/j.ejca.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 61. Stebbing J, Baranau Y, Baryash V, et al: 3-year follow-up of a phase III trial comparing the efficacy and safety of neoadjuvant and adjuvant trastuzumab and its biosimilar CT-P6 in HER2 positive early breast cancer (EBC). Ann Oncol 30, 2019 (suppl 5; abstr 190P)
- 62. European Medicines Agency: Assessment report: Trazimera, 2018. https://www.ema.europa.eu/documents/assessment-report/trazimera-epar-public-assessment-report_en.pdf.
- 63.Cortés J, Curigliano G, Diéras V. Expert perspectives on biosimilar monoclonal antibodies in breast cancer. Breast Cancer Res Treat. 2014;144:233–239. doi: 10.1007/s10549-014-2879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackisch C, Scappaticci FA, Heinzmann D, et al. Neoadjuvant breast cancer treatment as a sensitive setting for trastuzumab biosimilar development and extrapolation. Future Oncol. 2015;11:61–71. doi: 10.2217/fon.14.187. [DOI] [PubMed] [Google Scholar]
- 65.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 66.Waller CF, Manikhas A, Pennella EJ, et al. Biosimilar trastuzumab-dkst monotherapy versus trastuzumab monotherapy after combination therapy: Final overall survival (OS) from the phase III HERITAGE Trial.Presented at theAm Soc Clin Oncol Ann Meet Chicago, ILMay 31-June 4, 2019 [Google Scholar]
- 67.Li RK, Lipatov O, Adamchuk H, et al. Trazimera (a trastuzumab biosimilar) in HER2-positive metastatic breast cancer: Long-term safety and overall survival data.Presented at theSan Antonio Breast Cancer Symp San Antonio, TXDecember 10-14, 2019 [Google Scholar]
- 68.Rugo HS, Curigliano G, Cardoso F, et al. Settings-based efficacy comparison of trastuzumab biosimilars in breast cancer: A systematic literature review.Presented at theEur Soc Med Oncol 2018 Congr Munich, GermanyOctober 19-23, 2018 [Google Scholar]
- 69.Rugo HS, Cortes J. The new world of biosimilars in oncology: Translation of data to the clinic. Eur J Cancer. 2018;96:125–127. doi: 10.1016/j.ejca.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 70. Roche Registration: Herceptin summary of product characteristics, 2019. https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf.
- 71.Genentech Herceptin prescribing information. 2018 https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf
- 72. European Medicines Agency: Assessment report: Ogivri, 2018. https://www.ema.europa.eu/en/documents/assessment-report/ogivri-epar-public-assessment-report_en.pdf.
- 73. European Medicines Agency: Assessment report: Ontruzant, 2017. https://www.ema.europa.eu/documents/assessment-report/ontruzant-epar-public-assessment-report_en-0.pdf.
- 74. European Medicines Agency: Assessment report: Herzuma, 2017. https://www.ema.europa.eu/en/documents/assessment-report/herzuma-epar-public-assessment-report_en.pdf.
- 75. Center for Drug Evaluation and Research: Summary review: BLA 761091 (Herzuma), 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761091Orig1s000SumR.pdf.
- 76. Center for Drug Evaluation and Research: Clinical review(s): BLA 761073 (Kanjinti), 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761073Orig1s000MedR.pdf.
- 77. Center for Drug Evaluation and Research: Summary review: BLA 761074 (Ogivri), 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761074Orig1s000SumR.pdf.
- 78. Center for Drug Evaluation and Research: Summary review: BLA 761081 (Trazimera), 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761081Orig1s000SumR.pdf.
- 79.Kim S, Song J, Park S, et al. Drifts in ADCC-related quality attributes of Herceptin: Impact on development of a trastuzumab biosimilar. MAbs. 2017;9:704–714. doi: 10.1080/19420862.2017.1305530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Webster CJ, Woollett GR. Comment on “The End of Phase 3 Clinical Trials in Biosimilars Development?”. BioDrugs. 2018;32:519–521. doi: 10.1007/s40259-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frapaise FX. The end of phase 3 clinical trials in biosimilars development? BioDrugs. 2018;32:319–324. doi: 10.1007/s40259-018-0287-0. [DOI] [PubMed] [Google Scholar]
- 82.Webster CJ, Wong AC, Woollett GR. An efficient development paradigm for biosimilars. BioDrugs. 2019;33:603–611. doi: 10.1007/s40259-019-00371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolff-Holz E, Tiitso K, Vleminckx C, et al. Evolution of the EU biosimilar framework: Past and future. BioDrugs. 2019;33:621–634. doi: 10.1007/s40259-019-00377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. European Medicines Agency: Guideline on similar biological medicinal products containing recombinant granulocyte-colony stimulating factor (rG-CSF). Draft, 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-similar-biological-medicinal-products-containing-recombinant-granulocyte-colony_en.pdf.
- 85. European Medicines Agency: Concept paper on the revision of the guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant granulocyte-colony stimulating factor, 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-revision-guideline-non-clinical-clinical-development-similar-biological-medicinal_en.pdf.
- 86. European Medicines Agency: Annex to guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: Non-clinical and clinical issues: Guidance on similar medicinal products containing recombinant granulocyte-colony stimulating factor, 2006. https://www.ema.europa.eu/documents/scientific-guideline/annex-guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins_en.pdf.
- 87.Markus R, Liu J, Ramchandani M, et al. Developing the totality of evidence for biosimilars: Regulatory considerations and building confidence for the healthcare community. BioDrugs. 2017;31:175–187. doi: 10.1007/s40259-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu P, Ji P, Wang Y. Using clinical PK/PD studies to support no clinically meaningful differences between a proposed biosimilar and the reference product. AAPS J. 2018;20:89. doi: 10.1208/s12248-018-0246-1. [DOI] [PubMed] [Google Scholar]
- 89. Im YH, Odarchenko P, Grecea D, et al: Double-blind, randomized, parallel group, phase III study to demonstrate equivalent efficacy and comparable safety of CT-P6 and trastuzumab, both in combination with paclitaxel, in patients with metastatic breast cancer (MBC) as first-line treatment. J Clin Oncol 31, 2013 (suppl; abstr 629)