Abstract
PURPOSE
To provide guidelines for the accurate pathologic diagnosis of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL), the preoperative evaluation of the patient with suspected BIA-ALCL, and the pathologic evaluation of the capsulectomy specimen.
METHODS
To better inform patients and healthcare providers about BIA-ALCL, we convened to review diagnostic procedures used in the evaluation of patients with suspected BIA-ALCL. We focused on the processing of the seroma fluid/effusion surrounding the implant, the handling of capsulectomy specimens following removal of implant(s), and the preoperative evaluation of the patient with suspected BIA-ALCL. Recommendations were based on the published literature and our experience to optimize procedures to obtain an accurate diagnosis and assess for tumor invasion and the extent of the disease.
RECOMMENDATIONS
Early diagnosis of BIA-ALCL is important as the disease can progress and deaths have been reported. Because the most common presentation of BIA-ALCL is swelling of the breast with fluid collection, an accurate diagnosis requires cytologic evaluation of the effusion fluid surrounding the affected implant. The first priority is cytocentrifugation and filtration of fresh, unfixed effusion fluid to produce air-dried smears that are stained with Wright-Giemsa or other Romanowsky-type stains. Preparation of a cell block is desirable to allow for hematoxylin and eosin staining and immunohistochemical analysis of formalin-fixed, paraffin-embedded histologic sections. Cell block sections can be used for polymerase chain reaction–based investigation of T-cell receptor gene rearrangement to detect clonality. Fixation and mapping of the capsulectomy specimen to select multiple representative sections are advised to assess for microscopic tumor involvement and capsular invasion. It is appropriate to assess lymph node involvement by excisional biopsy material rather than fine needle aspiration, due to propensity for focal involvement.
INTRODUCTION
Breast implant–associated anaplastic (BIA) large-cell lymphoma (ALCL) is a rare form of ALCL, formally recognized as a specific entity by WHO in 2016.1 BIA-ALCL typically develops in the fluid and the capsule adjacent to the breast implant, with the most common clinical presentation being that of a seroma or swelling around the breast implant.2 The first patient with BIA-ALCL was reported in 1997,3 and additional patients have been reported worldwide.4,5 In 2011, the US Food and Drug Administration (FDA) reported a possible association between women with breast implants and ALCL, based on its analysis of 34 published reports of ALCL.6 This report was accompanied by a safety communication on FDA’s Web site, which has been regularly updated to provide physicians and patients with additional information (https://www.fda.gov/medical-devices/implants-and-prosthetics/breast-implants). To gather additional information to better characterize BIA-ALCL, the FDA collaborated with the American Society of Plastic Surgeons and Plastic Surgery Foundation to develop a registry of women with breast implants and ALCL, named the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE) Registry.2 Other countries also have compiled data concerning incidence and risk for BIA-ALCL in implant recipients.7-9 In March 2019, FDA held a public advisory committee meeting on the safety of breast implants, including BIA-ALCL. FDA encourages reporting of all confirmed patients with BIA-ALCL to MedWatch, the FDA’s global-safety Information and Adverse Event Reporting Program, as well as to the PROFILE Registry, which collects information regarding patients within the United States.
As of July 2019, FDA had received 573 unique BIA-ALCL medical device reports (MDRs), in which patient death was observed among 33 patients, representing a notable increased number of MDR reports of patients with BIA-ALCL and BIA-ALCL–related MDR deaths reported to FDA.10 When detailed information was available, the most commonly reported clinical presentation was seroma, although other symptoms may be present without seroma, such as mass or hardening adjacent to the implant. A preponderance of patients had textured-surface implants at the time of diagnosis, which occurred a median of 8 years after initial implant placement. In particular, the risk of BIA-ALCL was significantly higher with Allergan’s (Dublin, Ireland) BIOCELL textured breast implant than with other textured breast implants. This resulted in Allergan’s voluntary recall of these products not only from the US market but worldwide. Although the risk of BIA-ALCL appears to be higher with textured-surface breast implants, the exact etiology remains unknown.
Professional organizations and the National Comprehensive Cancer Network have published information to help physicians understand the disease and provide diagnosis and treatment. Most reported patients with BIA-ALCL have demonstrated a relatively indolent clinical course with therapy when disease is limited to the effusion fluid and invasion of the capsule has not occurred. However, there have been reports of deaths due to the progression of the disease.2,11,12 Therefore, prompt diagnosis and treatment are important.
Based on the discussions from the March 2019 public advisory committee meeting, patients and health care providers may not be adequately informed about BIA-ALCL, including its presentation and diagnosis. As such, the FDA invited one of the Advisory Committee members (E.S.J.) to prepare a “best practices guideline” for the diagnosis of BIA-ALCL, soliciting contributions from experts in the field and incorporating a comprehensive review of the literature. The objective of this article is to provide additional guidance to health care providers, (pathologists, surgeons, radiologists, and oncologists) regarding the recognition and diagnosis of BIA-ALCL using a standardized pathologic protocol for clinical evaluation of patients. This protocol includes evaluation of the periprosthetic fluid when present and processing of capsulectomy specimens. With increased education for both the public and health care providers, the hope is to identify and diagnose BIA-ALCL early when intervention can be curative.
CLINICAL EVALUATION OF THE PATIENT WITH BREAST IMPLANTS AND SYMPTOMS (EFFUSION/SEROMA)
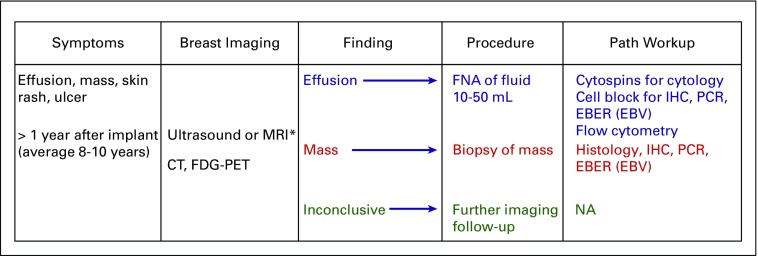
The most common clinical presentation of patients with BIA-ALCL is the onset of periprosthetic fluid collection surrounding an implant, typically occurring greater than 1 year after initial implantation. There have been case reports of BIA-ALCL diagnoses as early as 4 and 10 months,2 although the median time to diagnosis was 8 to 10 years.13,14 This may manifest as breast swelling, asymmetry, or a sensation of fullness, occasionally accompanied by pain. Effusions around implants have been mistaken for implant rupture or capsular contracture, which is a buildup of scar tissue around the implant. Patients present with symptoms a median of 8 to 10 years after initial implantation, and it is not uncommon for patients to have had multiple devices over time, although there is a higher risk with textured breast implants.7,15,16 When information was available, indications for implant placement were nearly evenly split between cosmetic augmentation and reconstruction.17 Most patients with BIA-ALCL have an effusion around the implant that is confined by a fibrous capsule. The effusion is confirmed optimally by ultrasound.18 Less commonly, patients present with a grossly detected mass that is assessed by imaging, such as ultrasound, computerized tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography (PET) scan. Documentation of a mass should be followed by incisional, excisional, or core biopsy.19
Because most patients present with effusion (seroma), ultrasound-guided aspiration (fine-needle aspiration [FNA]) of the effusion is essential to obtaining an accurate diagnosis of BIA-ALCL. Percutaneous aspiration alone is not therapeutic and is only considered to be a diagnostic option. Note that a small incidental volume of periprosthetic fluid may be normal and common around breast implants. A larger volume of fluid yields a more accurate diagnosis, but a minimum of 10 to 50 mL is appropriate to provide enough material for preparation of cytopathology smears, cell block with immunohistochemistry for CD30 and other lineage-associated markers, and, when possible, flow cytometry and molecular genetic studies.20 FNA evaluation after previous serial drainages may artificially lower tumor burden, rendering diagnosis difficult; a larger volume sample or concentration of the cellular contents by centrifugation may be important in such instances.
EVALUATION OF EFFUSION FLUID FOR DIAGNOSIS
Sample Preparation and Processing
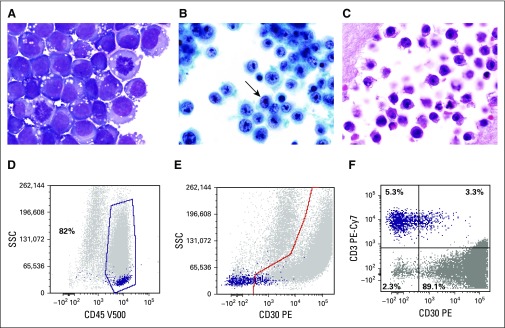
Periprosthetic fluid aspirated from the effusion is typically the initial sample submitted for pathologic evaluation. In some instances, this fluid may contain more diagnostic cellular material than capsulectomy specimens.21 The simplest preparation method involves cytocentrifugation and filtration of fresh, unfixed seroma fluid followed by air-drying and staining with Giemsa, Wright-Giemsa, or other Romanowsky-type stain within 48 hours. This technique allows for enrichment of cells with minimal distortion and good preservation of morphologic detail, and is well suited for the evaluation of hematolymphoid cells (Fig 1A).22 Additional slides can be alcohol fixed and stained with Papanicolaou, hematoxylin and eosin (H&E), or both. If available, automated processing methods for liquid-based cytology may be used to generate thin-layer Papanicolaou-stained slides (Fig 1B).23 Additional air-dried cytocentrifuged slides may be reserved for immunocytochemistry. However, because many of the antibodies important for diagnosis of BIA-ALCL and exclusion of other entities in the differential diagnosis may not be validated on air-dried cytocentrifuged slides, preparation of a cell block is desirable to allow for H&E staining and immunohistochemical analysis of formalin-fixed, paraffin-embedded cell block sections (Table 1; Fig 1C).24 Two or more preparation methods (ie, one cytologic staining method and cell block preparation) are appropriate to improve diagnostic yield and to allow for special stains.23,24 Leftover fresh fluid may be used for ancillary testing by flow cytometry or molecular genetic analysis.
FIG 1.
Cytologic features of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL). (A) The neoplastic cells are large and pleomorphic with irregular nuclei, large prominent nucleoli, conspicuous mitotic activity, and moderate cytoplasm, which is basophilic and contains prominent vacuoles on air-dried preparations stained with a Romanowsky-type method (Giemsa, ×1,000). (B) The vesicular chromatin and nucleolar prominence and shape are better appreciated on the alcohol-fixed thin-layer preparation, which also contains some kidney-shaped hallmark cells (arrow; Papanicolaou, ×1,000). (C) Similar features are seen on the cell block preparation (hematoxylin and eosin, ×1,000). (D) Flow cytometric analysis of a BIA-ALCL effusion; in a histogram plotting CD45 versus side scatter, ALCL cells usually distribute in the lymphocyte (purple dots) and monocyte (gray dots) regions, that in this particular patient, represent 82% of cells in the specimen. (E) In a histogram plotting CD30 versus side scatter, CD30+ cells are distributed below the highlighted area (gray dots), whereas most lymphocytes are small and negative for CD30 (purple dots). (F) Histogram shows the distribution of selected (gated) cells in the histogram at top. It shows that 89.1% of gated cells express CD30, whereas 5.3% of cells are CD3+ lymphocytes but CD30−. It can be concluded that the aberrant cells (gray dots) are CD45+, CD30+, and CD3−, whereas the normal small T lymphocytes (purple dots) are CD45+, CD30−, and CD3+. PE-Cy7, phycoerythrin cyanine 7; SSC, side scatter; V500-A, Becton-Dickinson Horizon V500 (San Jose, CA) for flow cytometry.
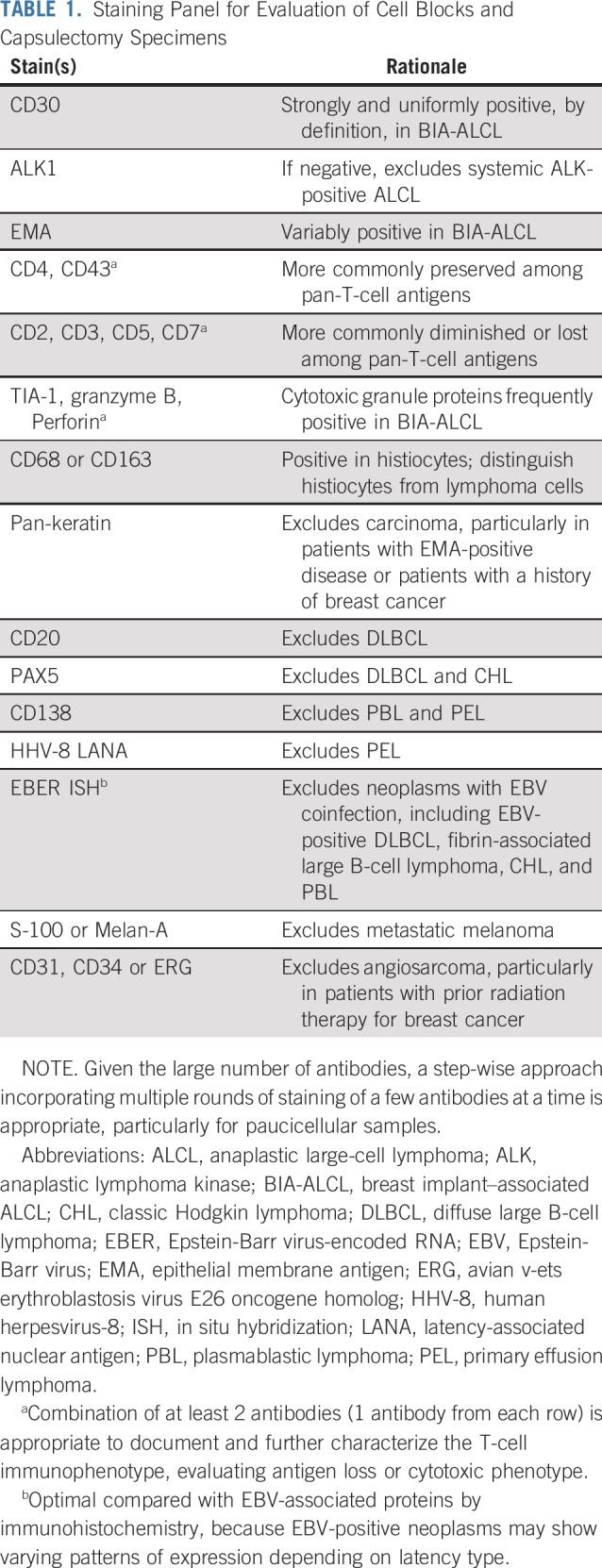
TABLE 1.
Staining Panel for Evaluation of Cell Blocks and Capsulectomy Specimens
Morphologic Features
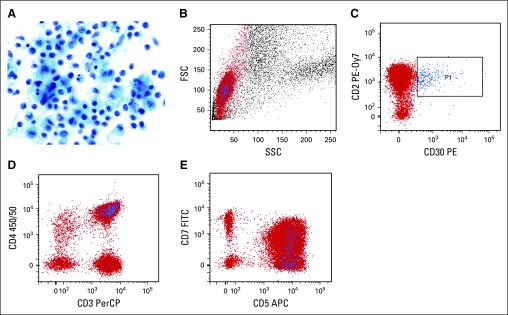
Cytologic preparations demonstrate discohesive large pleomorphic cells that are several times larger than a normal small lymphocyte. The atypical cells have irregular nuclei with dispersed or vesicular chromatin, prominent nucleoli, and moderately abundant cytoplasm, which may also display small vacuoles.21,25 Cell size and nuclear shape are best assessed on air-dried, Romanowsky-stained preparations, while nuclear and chromatin detail is more clearly visible on Papanicolaou- or H&E-stained slides. A subpopulation of cells with horseshoe- or kidney-shaped nuclei, so-called “hallmark cells,” seen in other forms of ALCL is typically present.26 Atypical mitotic figures may be seen. Because the main entity in the differential diagnosis is a peri-implant inflammatory reaction, recognition of the composition of benign seroma aspirates is essential; these typically contain small lymphocytes and histiocytes (Fig 2A). The latter may be medium sized, with abundant cytoplasmic microvacuoles, oval to irregular nuclei, and small nucleoli; however, cell size is about half that of ALCL cells or smaller.21
FIG 2.
Benign seroma aspirate containing nonspecific peri-implant inflammatory reaction. (A) There is a predominance of histiocytes (macrophages) and small lymphocytes. Some of the histiocytes show reactive features of curved, irregular nuclei, small nucleoli, and moderately abundant granular cytoplasm. However, their cell size is less than half that of ALCL cells, and the chromatin features are more delicate, with smaller nucleoli (Papanicolaou, ×1,000). (B-E) Flow cytometry was performed for T-cell markers and CD30. (B) On light scatter analysis, gating is performed on lymphocytes, highlighted in red, based on their low forward scatter (FSC) and side scatter (SSC) properties. (C) CD30-positive cells, highlighted in blue (P1), are small based on light scatter properties (B) and have a normal T-cell phenotype with preserved expression of CD2(C), CD3 (D) and CD5 (E), normal variable expression of CD7 (E), and predominant staining for CD4 (D), consistent with a reactive T-cell population. APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PE-0y7, phycoerythrin-cyanine 7; PerCP, peridinin chlorophyll protein.
Differential Diagnosis
In series of unselected patients, approximately 90% of primary breast lymphomas are B-cell lymphomas, most often diffuse large B-cell lymphoma (DLBCL; 50.1%) and marginal zone lymphoma (18.3%),7 whereas approximately 12% are T-cell lymphomas, with the most common subtype being anaplastic lymphoma kinase (ALK)-negative ALCL.7,27 However, among patients with breast implants, ALK-negative ALCL is far more common. A novel lesion occurring rarely in this setting is fibrin-associated large B-cell lymphoma, an Epstein-Barr virus (EBV)–related lesion with some morphologic similarities to DLBCL associated with chronic inflammation but distinct clinicopathologic features.28 Other histologic types associated with EBV infection, including extranodal natural killer/T-cell lymphoma, nasal type,29 and plasmablastic lymphoma,30 have been reported.
Immunohistochemical Studies
A panel of immunohistochemical stains is important to confirm the diagnosis of BIA-ALCL and exclude other entities in the differential diagnosis.24 Like other forms of ALK-negative ALCL, BIA-ALCL cells are strongly and uniformly positive for CD30 and negative for ALK, with frequent expression of cytotoxic molecules, incomplete expression of pan-T-cell antigens, and variable positivity for epithelial membrane antigen.5,24,31 For a comprehensive consideration of other entities (see Differential Diagnosis), stains for B-cell–associated antigens (CD20, PAX5, and CD138), human herpesvirus-8, and in situ hybridization for EBV-encoded RNA are advised.28 A pan-keratin stain is appropriate to exclude poorly differentiated carcinoma, particularly in patients with a prior history of breast cancer.32 Other poorly differentiated neoplasms to exclude include metastatic melanoma and angiosarcoma. If cytomorphology is not well preserved, immunohistochemistry for CD68 or CD163 is helpful in identifying reactive histiocytes and distinguishing them from lymphoma cells.
Laboratories with flow cytometry capability and a clinically validated CD30 antibody may opt to analyze its expression on lymphocytes in conjunction with a screening panel for T- and B-cell antigens.24 However, this analysis is regarded as second line. If enough material remains for only 1 additional evaluation method after preparation of cytology slides, then it is appropriate to prioritize the creation of a cell block over flow cytometry because many stains important in excluding entities in the differential diagnosis of BIA-ALCL are not routinely available by flow cytometry. In addition, caution should be exerted in interpreting CD30 expression on T cells, because this activation antigen may be expressed by reactive T-cell subsets, requiring correlation with light scatter properties and concurrent cytology (Fig 2B-2E).
Molecular Genetic Testing
The mainstay of molecular testing in the evaluation of possible BIA-ALCL is polymerase chain reaction (PCR) for T-cell receptor (TCR) gene rearrangements, particularly using BIOMED-2 primers.33 Clonal TCR gene rearrangements have been identified in nearly all BIA-ALCLs reported.2,5 If sufficient cellular material can be obtained, PCR studies are a valuable diagnostic tool. In addition to establishing clonality, which supports the presence of a neoplastic T-cell process, the specific sizes of the peaks identified by capillary electrophoresis of the PCR products can be used to compare the clone with other synchronous or metachronous samples from the same patient. It is essential to interpret the results of TCR PCR in the context of other clinical and pathologic features and not as the sole finding leading to a diagnosis of BIA-ALCL, because both false-positive and false-negative results may be obtained. Clonal or oligoclonal peaks can be seen in non-neoplastic conditions, especially when the overall number of T cells is low. False-negative results also may occur, particularly if the overall number of neoplastic cells is low, and abundant reactive T cells are present. PCR for immunoglobulin gene rearrangements may occasionally be indicated in breast implant–associated specimens if the differential diagnosis includes a B-cell neoplasm. Clonal TCR gene rearrangements also have been detected in BIA-ALCL using next-generation sequencing (NGS),34 although this is not currently required in the routine clinical setting.
Activating mutations of STAT3 have been reported in up to 64% of BIA-ALCL; other mutated genes include JAK1, JAK3, DNMT3A, and TP53.2,35-37 Germline TP53 mutations have been detected in some patients.34 NGS or other mutational testing approaches might be indicated in the setting of specific clinical research protocols but do not currently have a documented role in the routine evaluation of BIA-ALCL. Fluorescence in situ hybridization (FISH) for recurrent chromosomal rearrangements likewise is not routinely required. BIA-ALCLs reported to date have been ALK negative,5,36 and FISH for ALK rearrangements is not necessary if ALK immunohistochemistry can be adequately interpreted. Rearrangements of DUSP22 and/or TP63 that have been reported in subsets of ALK-negative ALCL and primary cutaneous ALCL38 have been uniformly absent in BIA-ALCLs reported to date.5,36,39 FISH testing might be helpful if the primary site of the tumor is in question, but it is not required in routine evaluation of patients with localized disease.
PROCESSING OF CAPSULECTOMY SPECIMENS
BIA-ALCL in the effusion fluid is treated with implant removal and complete capsulectomy.48 The College of American Pathologists has not as yet issued guidelines for the processing of such specimens. However, in their recent review of 50 patients with BIA-ALCL in which complete capsulectomy was performed, Lyapichev et al40 analyzed the gross and microscopic features to assess both the distribution of residual disease and presence of invasion They distinguished patients with grossly identifiable tumor from those in which no gross lesion was evident in the capsulectomy specimen. No tumor was found in 12% of the specimens, in which lymphoma cells were only identified in the effusion. Based on these data, the following approach is proposed for the examination of capsulectomy specimens with known or suspected BIA-ALCL.
Preparation and Sectioning
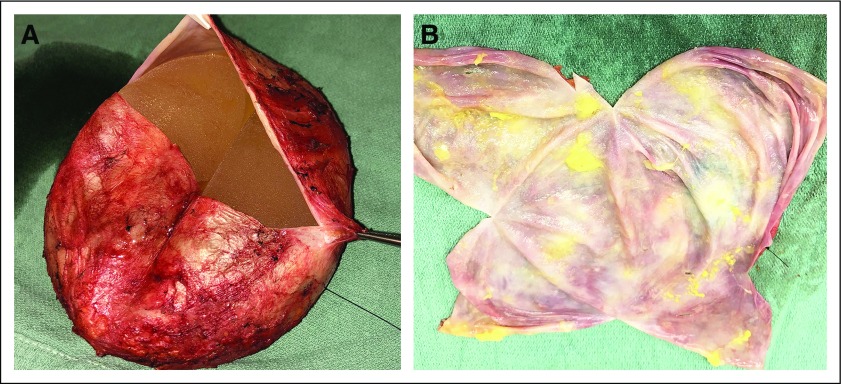
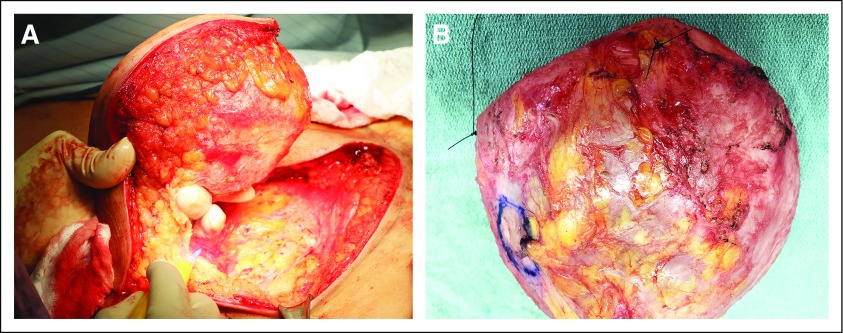
Ideally, the specimen is an en bloc resection with the intact capsule containing the implant, surrounding effusion, and any associated masses (Appendix Fig A1, online only). The surgeon provides orientation of the specimen. A needle is inserted to aspirate any remaining effusion, saving it for cytologic examination. The specimen is opened transversely on the posterior aspect of the capsule (Fig 3A) and then opened longitudinally through the superior and inferior aspects of the capsule, until the capsule lies open and flat (Fig 3B). The implant, which is usually loosely attached to the capsule, is removed carefully, and any floating material or material attached to the implant or capsule is submitted for histologic evaluation. The capsule is pinned flat on a paraffin board and submerged overnight in buffered formalin (Appendix Fig A2, online only).
FIG 3.
(A) The pathologist acknowledges the specimen is intact and contains landmark sutures denoting lateral (long-stitch) and superior (short-stitch) margins. After aspiration of any fluid/effusion, open the specimen through a transverse incision of the posterior aspect of the specimen. (B) After the transverse incision, a superior-to-inferior longitudinal incision allows exposure of the luminal side of the capsule, as well as assessment of the integrity of the implant.
Histologic Evaluation of Capsulectomy
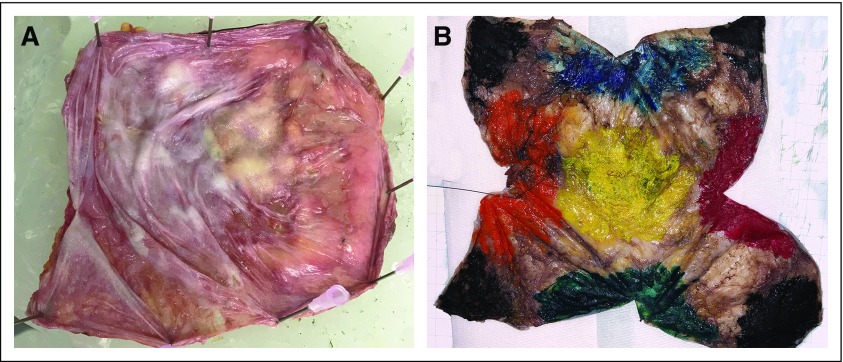
After overnight fixation, the external surface of the capsule is inked using 6 different colors for each of the sides or aspects of the specimen, representing essentially 6 sides of a cube (Appendix Fig A2). We propose submitting 2 sections “on edge” of each of the 6 aspects, for a total of 12 sections, a figure based on a mathematic model that calculated the number of sections required to reliably detect tumor in a specimen with no gross lesions.40
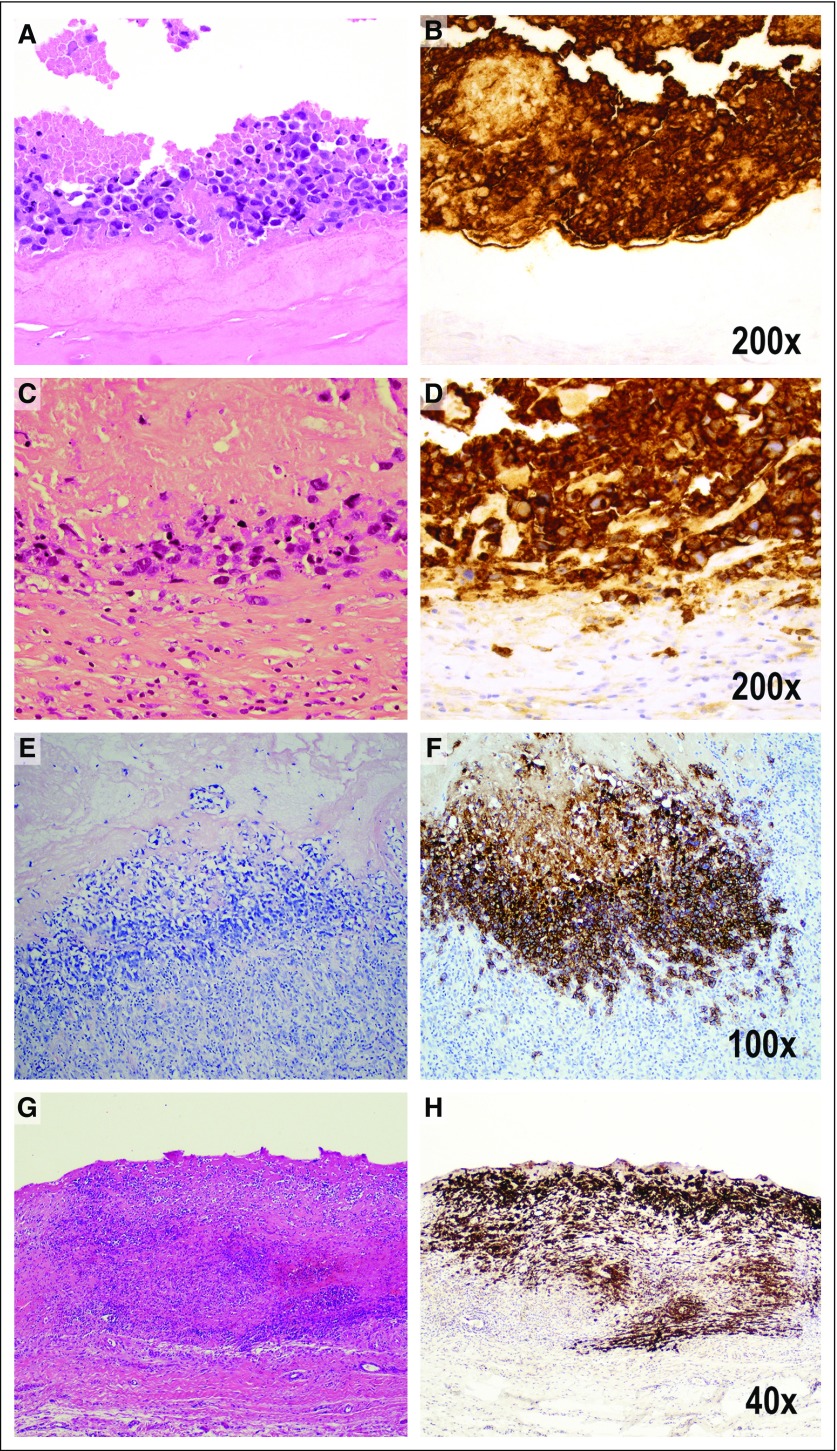
In addition to routine H&E-stained sections, CD30 immunohistochemistry is important to detect microscopic evidence of involvement by BIA-ALCL. Occasional or rare small lymphocytes or plasma cells in the stroma may react with CD30, but they lack the characteristic cytologic features of ALCL. A common finding is the presence of abundant necrosis, ghost lymphoma cells, and karyorrhexis with a minor component of viable tumor cells (Fig 4). The necrotic material may retain immunoreactivity for CD30, aiding in diagnosis.
FIG 4.
Pathologic staging that reflects tumor progression from the luminal surface to the outer boundaries of the capsule. Pathologic stage T0 is not illustrated because it indicates the presence of tumor cells in the effusion, but not detected in the capsule. Pathologic stage T1 indicates (A) the lymphoma cells lie on the luminal side of the capsule, and CD30 highlights both viable and necrotic tumor cells, many of which are (B) ghosts in a background of granular material indicating cellular remnants. The tumor cells are insulated from the rest of the capsule by a dense acellular collagenous layer. Therefore, CD30 is much more extensive than the amount of identifiable viable tumor cells (A, hematoxylin and eosin [H&E]; B, CD30 immunohistochemistry, ×200). Pathologic stage T2 indicates few inflammatory cells admixed with deeper lymphoma cells (C, H&E; D, CD30 immunohistochemistry, ×200). Stage T3 indicates that numerous inflammatory cells surround lymphoma cells (E, H&E; F, CD30 immunohistochemistry, ×100). Stage 4 indicates that the tumor cells are beyond the boundaries of the fibrous capsule (G, H&E; H, CD30 immunohistochemistry, ×40).
STAGING AND PREOPERATIVE EVALUATION
The presumed natural history of BIA-ALCL based on published reports influences the approach to diagnosis, staging, and therapeutic management (Fig 5). The earliest evidence of disease consists of ALCL cells floating in the effusion. At this stage, the capsule may not disclose evidence of involvement, either on the capsular surface or with invasion of the luminal surface.17,41 Accumulation of tumor cells on the luminal side of the capsule is followed by capsular invasion and, ultimately, invasion into the breast or surrounding soft tissue.31 These latter pathologic stages may be palpated by the patient or detected through imaging studies. In addition, axillary lymph node involvement may occur.
FIG 5.
Flowchart for the diagnosis of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL). Color coding indicates the preferred diagnostic approach based on the results of imaging studies, disclosing effusion, mass, or inconclusive findings. CT, computed tomography; FDG-PET, [18F]fluorodeoxyglucose–positron emission tomography; EBER, in situ hybridization for EBV; EBV, Epstein-Barr virus; FNA, fine-needle aspiration; IHC, immunohistochemistry; MRI, magnetic resonance imaging; NA, not applicable; PCR, polymerase chain reaction for T-cell receptor gene rearrangement, or immunoglobulin rearrangement, as indicated. (*) Preferred initial imaging approach. Figure modified from the 2018 National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology.19
A previously proposed TNM staging system evaluated the extent of tumor from T1 to T4.17,40 The rationale was based on a series of 60 patients, in which patients with a mass had decreased overall survival compared with patients without a mass.5,42 T1 indicates tumor cells confined to the effusion or the luminal side of the capsule. T2 indicates early capsular invasion, whereas T3 indicates tumor cell aggregates or sheets within the capsule. T4 indicates tumor beyond the capsule, with or without lymph node involvement. The clinical significance of minimal invasion remains to be determined.17
Nodal involvement is often primarily sinusoidal, limiting assessment by FNA. As such, excision of clinically suspicious lymph nodes is advised at the time of surgery. Core biopsies offer another alternative, but if negative, cannot reliably exclude involvement by ALCL. Lymph node enlargement also can occur in the absence of involvement by lymphoma; in those patients, reactive lymphoid and histiocytic hyperplasia may be seen as a reaction to silicone or polyurethane, sometimes in association with implant rupture.43,44
Ultrasound is the preferred initial imaging modality and can be used to identify associated periprosthetic masses (sensitivity, 46%; specificity, 100%) and enlarged regional lymph nodes.18 MRI can be used if ultrasound is indeterminate. It is appropriate for capsular masses to undergo core needle biopsy to establish a tissue diagnosis. In patients with a confirmed diagnosis of BIA-ALCL, PET-CT may be useful to establish the extent of disease and identify pathologic lymph nodes for biopsy.19
FUTURE AREAS OF INVESTIGATION
There is still an incomplete understanding of the natural history of the disease, particularly related to precursor lesions, which have not been fully defined. The advanced end of the disease spectrum, when lymphoma extends to regional lymph nodes, chest wall, or distant sites, will also benefit from better definition.45 The relationship of BIA-ALCL to other forms of ALK-negative ALCL is not fully resolved, and instances of disease have been reported in patients with other types of surgical implants that might provoke a chronic inflammatory response.46
Appendix
FIG A1.
(A) En bloc resection of the intact capsule containing the implant, with or without effusion or tumor mass, to assess margins and the extent of disease. (B) En bloc resected specimen handled for pathologic examination. It is essential to orient the specimen; in this photo, the lateral margin is marked with a long stitch and the superior margin with a short stich.
FIG A2.
(A) Once the capsule lies flat, the capsule is pinned flat on a paraffin board and submerged in neutral formalin overnight. (B) After overnight fixation, the specimen is oriented, and the outer surface of the capsule is inked with 6 different colors denoting 6 different aspects of the specimen. For each aspect/side, different colors are used. Illustrated are anterior (yellow), superior (blue), inferior (green), medial (red), lateral (orange), and posterior (black). The color pattern allows mapping any lesion that is not grossly identifiable and is discovered on microscopic examination. The ink also serves to indicate the distance between the deepest site of involvement and the margin.
Footnotes
This article reflects the views of the authors and should not be construed to represent the Food and Drug Administration’s views or policies.
AUTHOR CONTRIBUTIONS
Conception and design: Elaine S. Jaffe, Binita S. Ashar, Sung W. Yoon
Administrative support: Elaine S. Jaffe, Binita S. Ashar
Collection and assembly of data: Elaine S. Jaffe, Mark W. Clemens, Andrew L. Feldman, Philippe Gaulard, Roberto N. Miranda, Aliyah R. Sohani, Timothy Stenzel, Sung W. Yoon
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Best Practices Guideline for the Pathologic Diagnosis of Breast Implant–Associated Anaplastic Large-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elaine S. Jaffe
Stock and Other Ownership Interests: Gilead Sciences, Merck, Teva
Binita S. Ashar
Employment: INOVA Health System
Andrew L. Feldman
Research Funding: Seattle Genetics (Inst)
Patents, Royalties, Other Intellectual Property: A.L.F. is an inventor of technology for which Mayo Clinic holds an unlicensed patent or has submitted a patent application (Inst)
Roberto N. Miranda
Honoraria: Allergan
Timothy Stenzel
Employment: Invivoscribe
Leadership: Invivoscribe
Stock and Other Ownership Interests: Invivoscribe
Travel, Accommodations, Expenses: Invivoscribe
No other potential conflicts of interest were reported.
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1097/PRS.0000000000005571. McCarthy CM, Loyo-Berrios N, Qureshi AA, et al: Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE): Initial report of findings, 2012-2018. Plast Reconstr Surg 143:65s-73s, 2019 (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 4.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1093/annonc/mdv575. Laurent C, Delas A, Gaulard P, et al: Breast implant-associated anaplastic large cell lymphoma: Two distinct clinicopathological variants with different outcomes. Ann Oncol 27:306-314, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Center for Devices and Radiological Health, US Food and Drug Administration: FDA update on the safety of silicone gel-filled breast implants. https://www.fda.gov/media/80685/download.
- 7.Laurent C, Haioun C, Brousset P, et al. New insights into breast implant-associated anaplastic large cell lymphoma. Curr Opin Oncol. 2018;30:292–300. doi: 10.1097/CCO.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 8.de Boer M, van Leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018;4:335–341. doi: 10.1001/jamaoncol.2017.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becherer BE, de Boer M, Spronk PER, et al. The Dutch Breast Implant Registry: Registration of breast implant-associated anaplastic large cell lymphoma-a proof of concept. Plast Reconstr Surg. 2019;143:1298–1306. doi: 10.1097/PRS.0000000000005501. [DOI] [PubMed] [Google Scholar]
- 10. FDA: Medical device reports of breast implant-associated anaplastic large cell lymphoma. https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma.
- 11. doi: 10.1097/PRS.0000000000005568. Collins MS, Miranda RN, Medeiros LJ, et al: Characteristics and treatment of advanced breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 143:41S-50S, 2019 (suppl) [DOI] [PubMed] [Google Scholar]
- 12.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg. 2011;128:112e–118e. doi: 10.1097/PRS.0b013e318221db96. [DOI] [PubMed] [Google Scholar]
- 13.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: Analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. doi: 10.1097/PRS.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 14. Food and Drug Administration: Medical device reports of breast implant-associated anaplastic large cell lymphoma. https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma.
- 15.Doren EL, Miranda RN, Selber JC, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139:1042–1050. doi: 10.1097/PRS.0000000000003282. [DOI] [PubMed] [Google Scholar]
- 16.Le Bras F, Laurent C, Bosc R, et al. Breast implant associated-anaplastic large cell lymphoma (BIA-ALCL): The French Lymphoma Study Association (LYSA) registry data Blood 132:16412018 (suppl 1). 29866817 [Google Scholar]
- 17. doi: 10.1200/JCO.2015.63.3412. Clemens MW, Medeiros LJ, Butler CE, et al: Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol 34:160-168, 2016 [Erratum: J Clin Oncol 34:888, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adrada BE, Miranda RN, Rauch GM, et al. Breast implant-associated anaplastic large cell lymphoma: Sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat. 2014;147:1–14. doi: 10.1007/s10549-014-3034-3. [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.6004/jnccn.2022.0015. National Comprehensive Cancer Network: NCCN Clinical practice guidelines in oncology (NCCN guidelines) T cell lymphomas. Version 2. December 17, 2018 https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf. [DOI] [PubMed]
- 20.Burke JS, Sheibani K, Rappaport H. Dermatopathic lymphadenopathy. An immunophenotypic comparison of cases associated and unassociated with mycosis fungoides. Am J Pathol. 1986;123:256–263. [PMC free article] [PubMed] [Google Scholar]
- 21.Granados R, Lumbreras EM, Delgado M, et al. Cytological diagnosis of bilateral breast implant-associated lymphoma of the ALK-negative anaplastic large-cell type. Diagn Cytopathol. 2016;44:623–627. doi: 10.1002/dc.23485. [DOI] [PubMed] [Google Scholar]
- 22. Galagan KA, Blomberg D, Corenbleet PJ, et al: Color Atlas of Body Fluids: An Illustrated Field Guide Based on Proficiency Testing. Northfield, IL, CAP Press, 2006. [Google Scholar]
- 23. Cibas ES, Ducatman BS. Cytology: Diagnostic Principles and Clinical Correlates. Philadelphia, PA, Elsevier, 2014. [Google Scholar]
- 24. doi: 10.1111/cyt.12678. Barbe E, de Boer M, de Jong D: A practical cytological approach to the diagnosis of breast-implant associated anaplastic large cell lymphoma. Cytopathology 30:363-369, 2019. [DOI] [PubMed] [Google Scholar]
- 25. doi: 10.1111/cyt.12541. Ronchi A, Montella M, Argenzio V, et al: Diagnosis of anaplastic large cell lymphoma on late peri-implant breast seroma: Management of cytological sample by an integrated approach. Cytopathology 29:294-299, 2018. [DOI] [PubMed] [Google Scholar]
- 26. Swerdlow SH, Campo E, Harris NL, et al (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (rev ed 4). Lyon, France, International Agency for Research on Cancer, 2017. [Google Scholar]
- 27.Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: A study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 28.Boyer DF, McKelvie PA, de Leval L, et al. Fibrin-associated EBV-positive large B-cell lymphoma: An lindolent neoplasm with features distinct from diffuse large B-cell lymphoma associated with chronic inflammation. Am J Surg Pathol. 2017;41:299–312. doi: 10.1097/PAS.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 29.Aladily TN, Nathwani BN, Miranda RN, et al. Extranodal NK/T-cell lymphoma, nasal type, arising in association with saline breast implant: Expanding the spectrum of breast implant-associated lymphomas. Am J Surg Pathol. 2012;36:1729–1734. doi: 10.1097/PAS.0b013e31826a006f. [DOI] [PubMed] [Google Scholar]
- 30.Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28:736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 31.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large cell lymphoma associated with breast implants: A report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Filie A, Arthur D, et al. Breast implant-associated anaplastic large cell lymphoma in a patient with Li-Fraumeni syndrome. Histopathology. 2015;67:925–927. doi: 10.1111/his.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 34.Blombery P, Thompson E, Ryland GL, et al. Frequent activating STAT3 mutations and novel recurrent genomic abnormalities detected in breast implant-associated anaplastic large cell lymphoma. Oncotarget. 2018;9:36126–36136. doi: 10.18632/oncotarget.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blombery P, Thompson ER, Jones K, et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica. 2016;101:e387–e390. doi: 10.3324/haematol.2016.146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oishi N, Brody GS, Ketterling RP, et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132:544–547. doi: 10.1182/blood-2017-12-821868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letourneau A, Maerevoet M, Milowich D, et al. Dual JAK1 and STAT3 mutations in a breast implant-associated anaplastic large cell lymphoma. Virchows Arch. 2018;473:505–511. doi: 10.1007/s00428-018-2352-y. [DOI] [PubMed] [Google Scholar]
- 38.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124:1473–1480. doi: 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerbe A, Alame M, Dereure O, et al. Systemic, primary cutaneous, and breast implant-associated ALK-negative anaplastic large-cell lymphomas present similar biologic features despite distinct clinical behavior. Virchows Arch. 2019;475:163–174. doi: 10.1007/s00428-019-02570-4. [DOI] [PubMed] [Google Scholar]
- 40. doi: 10.1038/s41379-019-0337-2. Lyapichev KA, Pina-Oviedo S, Medeiros LJ, et al: A proposal for pathologic processing of breast implant capsules in patients with suspected breast implant anaplastic large cell lymphoma. Mod Path . [epub ahead of print on August 5, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quesada AE, Medeiros LJ, Clemens MW, et al. Breast implant-associated anaplastic large cell lymphoma: A review. Mod Path . 2019;32:166–188. doi: 10.1038/s41379-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 42.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: Long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katzin WE, Centeno JA, Feng LJ, et al. Pathology of lymph nodes from patients with breast implants: A histologic and spectroscopic evaluation. Am J Surg Pathol. 2005;29:506–511. doi: 10.1097/01.pas.0000155145.60670.e4. [DOI] [PubMed] [Google Scholar]
- 44.Egan C, Jaffe ES. Non-neoplastic histiocytic and dendritic cell disorders in lymph nodes. Semin Diagn Pathol. 2018;35:20–33. doi: 10.1053/j.semdp.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. doi: 10.1093/asj/sjy097. Loghavi S, Medeiros LJ, Javadi S, et al: Breast implant-associated anaplastic large cell lymphoma with bone marrow involvement. Aesthet Surg J 38:NP92-NP96, 2018. [DOI] [PubMed] [Google Scholar]
- 46.Mendes J, Jr, Mendes Maykeh VA, Frascino LF, et al. Gluteal implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;144:610–613. doi: 10.1097/PRS.0000000000005910. [DOI] [PubMed] [Google Scholar]