Abstract
PURPOSE
Radiation dose to the neuroregenerative zone of the hippocampus has been found to be associated with cognitive toxicity. Hippocampal avoidance (HA) using intensity-modulated radiotherapy during whole-brain radiotherapy (WBRT) is hypothesized to preserve cognition.
METHODS
This phase III trial enrolled adult patients with brain metastases to HA-WBRT plus memantine or WBRT plus memantine. The primary end point was time to cognitive function failure, defined as decline using the reliable change index on at least one of the cognitive tests. Secondary end points included overall survival (OS), intracranial progression-free survival (PFS), toxicity, and patient-reported symptom burden.
RESULTS
Between July 2015 and March 2018, 518 patients were randomly assigned. Median follow-up for alive patients was 7.9 months. Risk of cognitive failure was significantly lower after HA-WBRT plus memantine versus WBRT plus memantine (adjusted hazard ratio, 0.74; 95% CI, 0.58 to 0.95; P = .02). This difference was attributable to less deterioration in executive function at 4 months (23.3% v 40.4%; P = .01) and learning and memory at 6 months (11.5% v 24.7% [P = .049] and 16.4% v 33.3% [P = .02], respectively). Treatment arms did not differ significantly in OS, intracranial PFS, or toxicity. At 6 months, using all data, patients who received HA-WBRT plus memantine reported less fatigue (P = .04), less difficulty with remembering things (P = .01), and less difficulty with speaking (P = .049) and using imputed data, less interference of neurologic symptoms in daily activities (P = .008) and fewer cognitive symptoms (P = .01).
CONCLUSION
HA-WBRT plus memantine better preserves cognitive function and patient-reported symptoms, with no difference in intracranial PFS and OS, and should be considered a standard of care for patients with good performance status who plan to receive WBRT for brain metastases with no metastases in the HA region.
INTRODUCTION
Brain metastases have a substantial impact on patients and the health care system because up to 30% of patients with cancer will develop brain metastases.1 Whole-brain radiotherapy (WBRT) remains an important treatment modality in the majority of patients with brain metastases because it palliates symptoms, significantly improves intracranial control, and diminishes the chance of death as a result of neurologic causes.2-4 However, the majority of patients experience cognitive deterioration after WBRT, which raises concerns about the toxicity of WBRT.5
Investigations into the cognitive toxicity of brain irradiation have revealed mechanisms and opportunities to prevent these adverse effects of WBRT. Memantine is an N-methyl-d-aspartate (NMDA) receptor antagonist that blocks pathologic excessive stimulation of NMDA receptors and has been shown to be beneficial in dementia and neuroprotective in preclinical models of brain irradiation.6,7 In a placebo-controlled phase III trial, prophylactic memantine during and after WBRT demonstrated better preservation of cognitive function with the secondary end point time to cognitive decline and was well tolerated with adverse events rates similar to placebo, establishing memantine as a standard of care for patients with better prognosis receiving WBRT.8
Preclinical and clinical studies have suggested that relatively low doses of radiation received by the neural stem cells within the subgranular zone of the hippocampal dentate gyrus may contribute to radiotherapy (RT)–induced cognitive toxicity.9,10 Our research group developed techniques using intensity-modulated RT (IMRT) to deliver therapeutic doses of WBRT while limiting radiation dose to the bilateral hippocampal dentate gyri (hippocampal avoidance [HA]; Fig 1) and hypothesized that HA would prevent WBRT-associated cognitive toxicity.11,12 In a multi-institution phase II trial, HA-WBRT for patients with brain metastases was associated with highly promising preservation of memory and quality of life (QOL) compared with historical controls.13 To validate the hypothesis that conformal avoidance of the hippocampal neural stem cells using IMRT improves cognitive outcomes, NRG CC001 (ClinicalTrials.gov identifier: NCT02360215), a prospective multi-institutional randomized phase III trial, investigated the role of WBRT plus memantine with or without HA in patients with brain metastases.
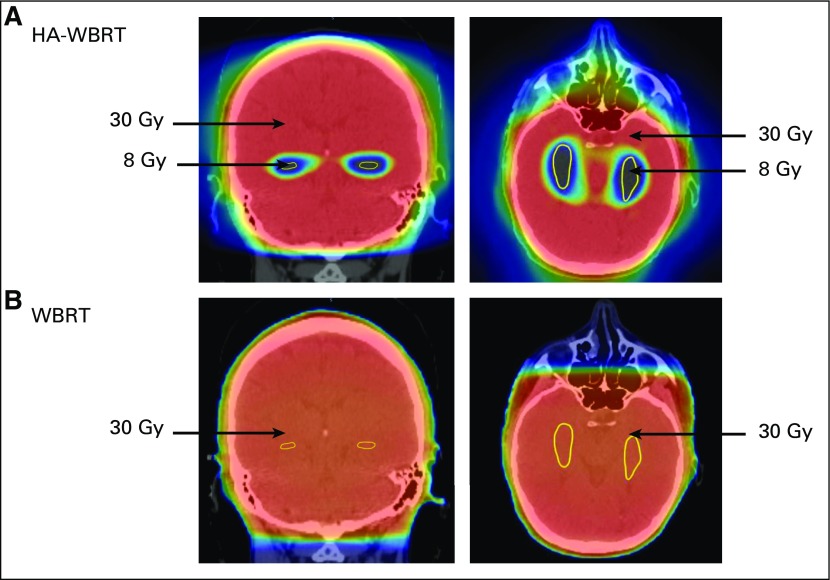
FIG 1.
Several-fold reduction in radiation dose to hippocampi (yellow) using (A) hippocampal avoidant whole-brain radiotherapy (HA-WBRT) v (B) conventional WBRT.
METHODS
Trial Patients
Adult patients (≥ 18 years of age) with brain metastases outside a 5-mm margin around either hippocampus were eligible. Eligibility criteria included Karnofsky performance score ≥ 70 and pathologically proven diagnosis of solid tumor malignancy. Prior resection of brain metastases or radiosurgery was allowed. Exclusion criteria included radiographic evidence of hydrocephalus or other architectural distortion of the ventricular system, leptomeningeal metastases, planned cytotoxic chemotherapy during WBRT, prior WBRT, allergy to memantine, or current use of other NMDA antagonists. The complete eligibility criteria are provided in the trial protocol (Data Supplement, online only). Institutional review board approval was required. All patients were required to provide informed consent.
Trial Design and Treatment
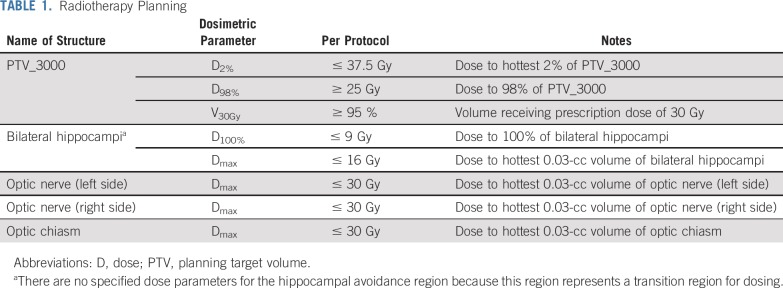
Patients were stratified according to recursive partitioning analysis class (1 v 2) and prior therapy (none v radiosurgery or surgical resection) and randomly assigned, using a permuted block procedure, to WBRT plus memantine or HA-WBRT plus memantine.14,15 In both study arms, memantine for twice-daily dosing was prescribed as follows: 5-mg morning dose week 1, 5 mg twice a day week 2, morning dose 10 mg and evening dose 5 mg week 3, and 10 mg twice a day weeks 4-24.8 Extended-release formulation was prescribed as follows: 7-mg daily dose week 1, 14-mg daily dose week 2, 21-mg daily dose week 3, and 28-mg daily dose weeks 4-24. For both study arms, the prescribed WBRT dose was 30 Gy in 10 fractions. Hippocampal contouring and HA-WBRT planning directives have been previously described,11,13 with bilateral hippocampal contours manually generated on the fused thin-slice magnetic resonance imaging (MRI)-computed tomography image set and expanded by 5 mm to generate the HA region. The planning target volume (PTV) was defined as the whole-brain parenchyma, excluding the HA region; no setup margin was added to the PTV. IMRT was used to deliver the conformal RT plan for patients treated with HA-WBRT (Table 1; for protocol details, see the Data Supplement). Before enrolling patients in this trial, all participating sites completed a credentialing exercise in which they would generate an HA-WBRT treatment plan on a sample case that would be reviewed centrally. For the first patient planned for HA-WBRT by a radiation oncologist, rapid central review of hippocampal contours and HA-WBRT planning was conducted in real time before initiation of treatment. If the initial plan was deemed acceptable, subsequent treatment plans were reviewed post-treatment in a timely manner to provide close monitoring, assessment of plan quality, and ongoing feedback (Data Supplement).
TABLE 1.
Radiotherapy Planning
Assessments
Before random assignment, each patient underwent a baseline evaluation that consisted of history and physical examination, neurologic examination, performance status, and thin-slice MRI and completed cognitive testing and measures of patient-reported QOL and symptom burden. All baseline evaluations along with an assessment of adverse events were repeated at month 2, 4, 6, and 12. The cognitive testing was administered by a trained, certified member of the site study team (Data Supplement). The same validated battery of cognitive tests used in the prior memantine trial was administered in this trial to assess learning and memory (Hopkins Verbal Learning Test-Revised [HVLT-R]), verbal fluency (Controlled Oral Word Association), processing speed (Trail Making Test [TMT] Part A), and executive function (TMT Part B [TMT-B]).8,16,17 QOL and symptom burden were assessed by the EQ-5D-5L and the MD Anderson Symptom Inventory-Brain Tumor (MDASI-BT) module, respectively.18,19 For the index and visual analog scale scores from the EQ-5D-5L, higher scores indicate better QOL, while for the MDASI-BT factors symptom severity and symptom interference, higher scores indicate increased symptoms. All treatment-related toxicities and adverse events were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
End Points
The primary end point was time to cognitive failure, defined as cognitive decline determined by the reliable change index on at least one of the cognitive tests.8 Patients and clinicians were not blinded to treatment assignment, although the neurocognitive chair who scored the cognitive tests was blinded to treatment assignment. Secondary end points included intracranial progression-free survival (PFS), overall survival (OS), toxicity, patient-reported symptoms (focusing on symptom severity, symptom interference, neurologic factor, and cognitive factor subscale scores as well as fatigue, neurologic factor items, and cognitive factor items), QOL, and cognitive function as measured separately on the individual cognitive tests and the Clinical Trial Battery composite score.8
Statistical Analysis
Because of the competing risk of death, the method described by Pintilie20 was used to estimate sample size for the primary end point using data from NRG Oncology’s RTOG 0614. The memantine arm showed a cognitive failure rate of 53.8% at 6 months (corresponding to a monthly hazard of 0.198 by Pintilie’s method) and a death rate (as a competing risk) of 30.7% (corresponding to a monthly hazard of 0.113). It was assumed that there was an 11% absolute reduction in cognitive failure (monthly hazard rate of 0.129) and a similar death rate using HA-WBRT, resulting in a hazard ratio (HR) of 0.65. Using a two-sided α = .05, 230 events were required to achieve 90% statistical power using Pintilie’s method. The probability of cognitive failure, provided that some failures may not be observed because of death, was calculated over a 63-month study (57 months for accrual and 6 months of additional follow-up) as 60.1%, resulting in a total sample size of 382 patients. The target accrual was inflated by 25% to account for noncompliant patients.
A key secondary end point of interest, symptom severity (a subscale of MDASI-BT), had 80% statistical power to detect a moderate effect size of 0.5 with a two-sided type I error of 0.05. The analysis was conducted on all randomly assigned patients on an intention-to-treat basis. The cumulative incidence approach was used to estimate the median time to cognitive failure to account for the competing risk of death. Gray’s test was used to test for a statistically significant difference in the distribution of cognitive failure times.21 OS and intracranial PFS were estimated using the Kaplan-Meier method, and differences between treatment arms were tested using the log-rank test.22,23 OS and intracranial PFS were measured from the date of random assignment to the date of intracranial progression for intracranial PFS only, death, or the last follow-up date on which the patient was reported alive. Adjusted and unadjusted Cox proportional hazards models were used to obtain HRs and 95% CIs for OS and intracranial PFS, while cause-specific Cox models were used for cognitive failure.24 Between-arm comparisons of categorical variables, such as cognitive deterioration and adverse events, were tested using χ2 tests. Change from baseline scores for QOL and symptom items were compared between arms using a t test. Trends across time on the MDASI-BT were modeled with mixed-effects models using maximum likelihood estimation. Covariates consisting of baseline score, age, stratification factors, and interaction terms were considered for each model, with differences at 6 months tested using a t test. Individual MDASI-BT items were compared between arms for cognitive and neurologic factors, if the factor score was significantly different between arms, and for fatigue. As a sensitivity analysis because of missing data, models were run using imputed data for alive patients with consent who were missing symptom data. Multiple imputation used a Markov chain Monte Carlo method with 20 iterations. The 6-month tests were two-sided using an overall significance level of .05 as Hochberg’s procedure was used to adjust for multiplicity.25
RESULTS
Study Patients
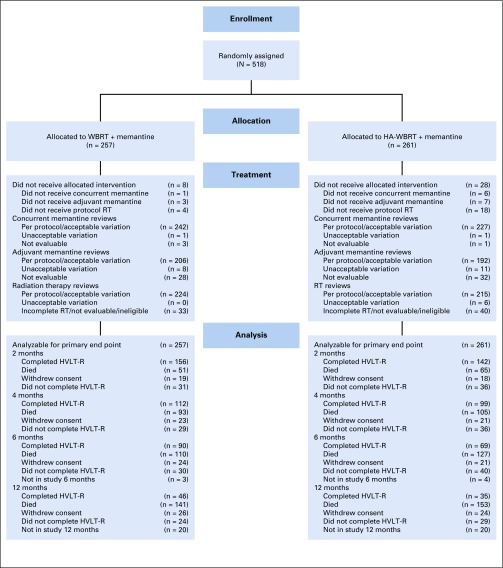
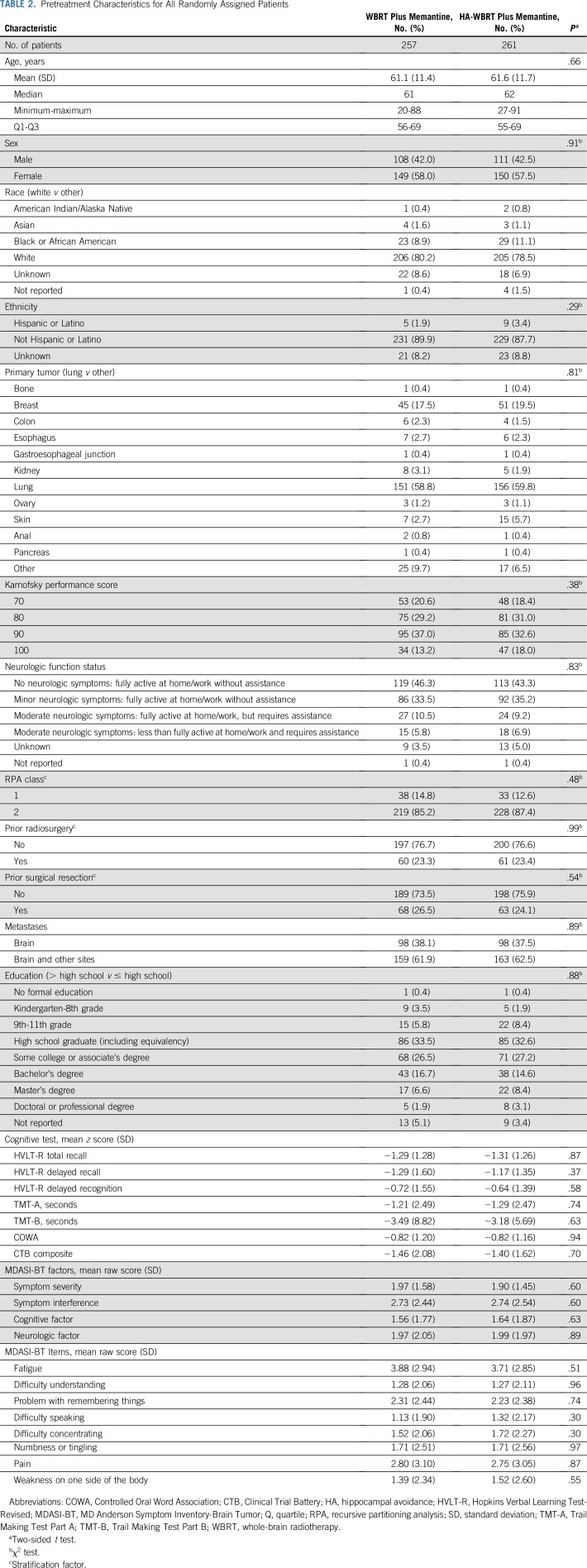
Between July 13, 2015, and March 12, 2018, 518 patients were randomly assigned to WBRT plus memantine or HA-WBRT plus memantine at 112 participating institutions in the United States and Canada (Fig 2). Twenty-seven patients were found to be ineligible (11 in the WBRT plus memantine arm and 16 in the HA-WBRT plus memantine arm) most commonly because of incomplete prerandomization cognitive assessment. Baseline characteristics, including baseline cognitive function, were well balanced between the study arms (Table 2). Median age was 61.5 years (range, 20-91 years), and the majority of patients had primary lung cancer (57.7%). The median follow-up for alive patients was 7.9 months (range, 0-15.6 months). Compliance rates for cognitive testing and patient-reported outcomes are provided in the Data Supplement.
FIG 2.
CONSORT diagram. HA, hippocampal avoidance; HVLT-R, Hopkins Verbal Learning Test-Revised; RT, radiotherapy; WBRT, whole-brain radiotherapy.
TABLE 2.
Pretreatment Characteristics for All Randomly Assigned Patients
Treatment Outcomes
Primary analysis and cognitive outcomes.
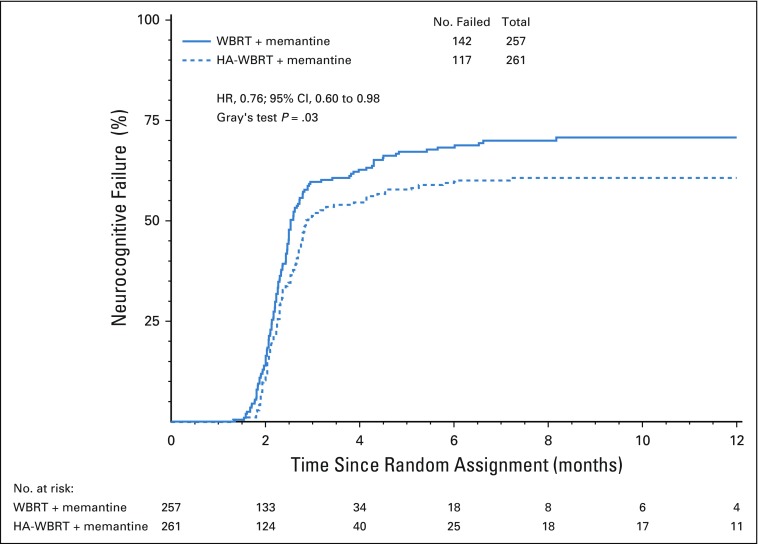
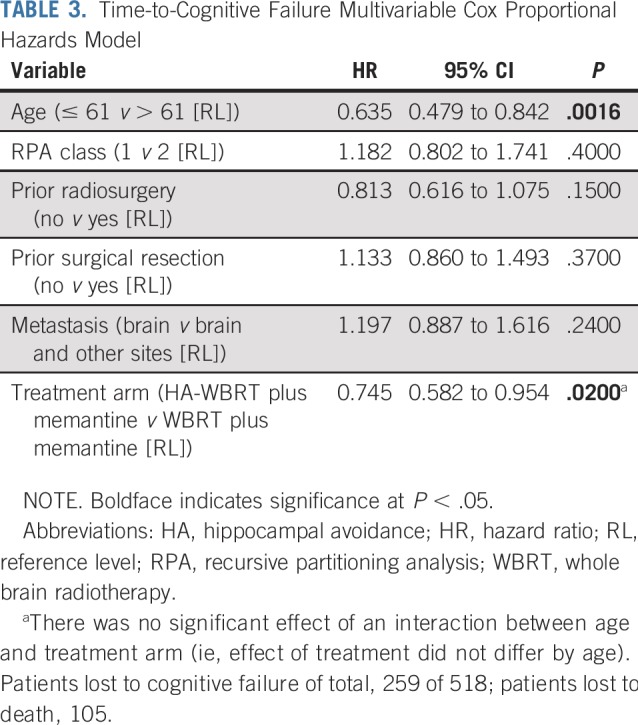
Cognitive failure risk was significantly lower in the HA-WBRT plus memantine arm compared with the WBRT plus memantine arm (HR, 0.76; 95% CI, 0.60 to 0.98; P = .03; Fig 3; Data Supplement). The unadjusted cause-specific treatment effect was significant (HR, 0.76; 95% CI, 0.60 to 0.98; P = .03; Data Supplement). In the adjusted cause-specific analysis, the treatment effect was even more pronounced in favor of HA-WBRT plus memantine (HR, 0.74; 95% CI, 0.58 to 0.95; P = .02; Table 3).
FIG 3.
Kaplan-Meier graph showing time to cognitive failure. HA, hippocampal avoidance; WBRT, whole-brain radiotherapy.
TABLE 3.
Time-to-Cognitive Failure Multivariable Cox Proportional Hazards Model
Analysis of each cognitive test separately revealed no difference in cognitive deterioration rates between arms at 2 months (Data Supplement), while the HA-WBRT plus memantine arm was less likely to have deterioration in TMT-B at 4 months (23.3% v 40.4%, respectively; P = .01; Data Supplement) and HVLT-R total recall and delayed recognition at 6 months (11.5% v 24.7% [P = .049] and 16.4% v 33.3% [P = .02], respectively; Data Supplement). Although no significant treatment effects were seen, analysis of cognitive test standardized scores demonstrated better cognitive outcomes over time in all cognitive domains in the HA-WBRT plus memantine arm (Data Supplement).
Symptom burden and QOL.
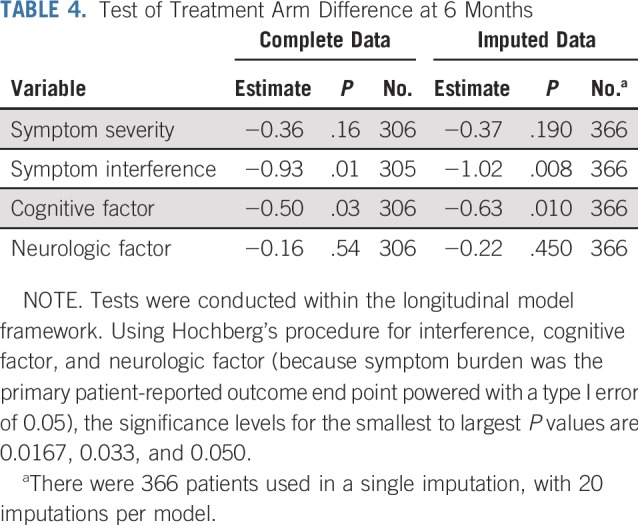
Mixed-effects modeling for symptom severity showed significant interaction between treatment arm and time, with the between-arm difference favoring HA-WBRT plus memantine with longer follow-up (estimates, −0.10; P = .03; Data Supplement). Symptom interference, cognitive factor, and neurologic factor did not show any significant treatment effects (Data Supplement). After imputation, the symptom severity treatment arm-by-time interaction effect remained (estimate, −0.11; P = .04), and HA-WBRT plus memantine was significantly associated with fewer cognitive symptoms (estimate, −0.33; P = .022).
After applying Hochberg’s multiplicity adjustment on imputed data, both symptom interference and cognitive factor showed significant between-arm differences at 6 months (Table 4). Specifically, patients in the HA-WBRT plus memantine arm experienced less symptom interference and fewer cognitive symptoms at 6 months (estimate, −1.02 [P = .008] and −0.63 [P = .01], respectively). Symptom severity and neurologic factor did not show a significant treatment effect at 6 months (estimate, −0.37 [P = .19] and −0.22 [P = .45], respectively; Table 4).
TABLE 4.
Test of Treatment Arm Difference at 6 Months
Cognitive factor differences at 6 months were driven primarily by two items: problems with remembering things and difficulty speaking. At 6 months, patients in the HA-WBRT plus memantine arm reported less difficulty with remembering things (mean, 0.16 v 1.29; P = .01) and less difficulty speaking (mean, −0.20 v 0.45; P = .049) compared with the WBRT plus memantine arm. Greater improvement in fatigue at 6 months was reported in the HA-WBRT plus memantine arm compared with the WBRT plus memantine arm (mean, 0.93 v −0.16; P = .04). No differences were seen between study arms at baseline or over time for the EQ-5D-5L (Data Supplement).
Survival, intracranial progression, and toxicity.
There was no difference between the HA-WBRT plus memantine and WBRT plus memantine arms in terms of OS (median, 6.3 v 7.6 months, respectively; HR, 1.13; 95% CI, 0.90 to 1.41; P = 0.31; Data Supplement), intracranial PFS (median, 5.0 v 5.3 months, respectively; HR, 1.14; 95% CI, 0.93 to 1.41; P = .21; Data Supplement), or percentage of deceased patients at each cognitive testing time point (Data Supplement). Relapses in the HA region were 11 in the HA-WBRT plus memantine arm v 16 in the WBRT plus memantine arm. There was no difference in grade ≥ 3 toxicity between the WBRT plus memantine and HA-WBRT plus memantine arms with regard to attribution (61.7% v 58.8%, respectively; P = .53) or related to treatment (20.3% v 19.4%, respectively; P = .83; Data Supplement).
DISCUSSION
This phase III trial provides confirmation that conformal avoidance of the hippocampal neuroregenerative stem-cell niche using IMRT during WBRT better preserves cognitive function and patient-reported symptoms, while observing no difference between treatment arms in toxicity, intracranial PFS, or OS, in patients with brain metastases. With these findings, HA-WBRT plus memantine should be considered a standard of care for patients with good performance status with brain metastases, but no metastases in the HA region, who plan to receive WBRT. These findings affect the estimated 200,000 patients per year who receive WBRT in the United States alone and potentially the design of RT for primary brain tumors, although additional clinical studies in these patient populations are needed.10,26
To our knowledge, this trial provides the first definitive clinical evidence that the hippocampal neuroregenerative niche is important to pathophysiology of RT-induced cognitive decline and builds on extensive preclinical work and prior clinical studies.9,10,12,13,27,28 This trial’s observation of better preservation of cognitive domains after HA supports the hypothesis of hippocampal stem-cell radiosensitivity because HA uses IMRT to generate a several-fold reduction in radiation dose delivered to the mitotically active neural stem cells located in the hippocampal dentate gyrus and thereby permits better conservation of hippocampal neurogenesis (Fig 1). Of note, there were fewer HA regional relapses in the HA-WBRT plus memantine arm. Because the HA region accounts for only 2% of the whole-brain parenchyma, development of metastases in the HA region is an uncommon event, and evaluation of differences between study arms for such a rare event would require a substantially larger trial.11-13
In the current trial, the benefit of HA-WBRT emerges robustly with ≥ 4 months follow-up. For patients with life spans much shorter than 4 months, prior trials have shown that the predominant impact on cognition is likely deterioration as a result of disease progression or other factors that contribute to shorter survival.5,13 Therefore, it seems reasonable to forego HA during WBRT in patients with survival expected to be < 4 months.18 To aid in decision making, prognostic systems have been developed to provide survival time estimates for patients with brain metastases.29
Given the potential for symptoms and impaired function from uncontrolled brain metastases, one of the intents of brain metastases management is palliation through the prevention and/or improvement of neurologic signs and symptoms. Within this context, the current trial’s demonstration of better preservation of patient-reported cognition and symptom interference and specific symptoms, including difficulty speaking, difficulty with remembering, and fatigue, emphasizes the importance of HA-WBRT in achieving the palliative objectives of brain metastasis management. These findings specific to patient-reported overall cognitive symptom burden and preservation of memory and speech meaningfully complement the cognitive preservation findings on objective cognitive tests and the cognition-specific rationale for HA.
The results of this trial also contribute to the evolving debate over whether stereotactic radiosurgery (SRS) and/or WBRT are the optimal approaches to the management of brain metastases. Prior randomized trials that demonstrated more-favorable cognitive outcomes with SRS in lieu of WBRT for 1-3 brain metastases did not include modern cognitive preservation strategies of prophylactic memantine or HA.5,30 This paradigm remains the subject of ongoing (ClinicalTrials.gov identifier: NCT03550391) and planned phase III trials of SRS compared with HA-WBRT plus memantine and will continue to evolve as newer systemic and immunotherapy agents improve survival and demonstrate activity in brain metastases.
Consistent with the majority of clinical trials that have evaluated different forms of RT, in the current trial, participants were not blinded to treatment. Because delivery of HA-WBRT and standard WBRT are significantly different, it would be logistically difficult to keep the patient blinded to treatment. In addition, because only the trial participant is kept in ignorance in the process of masking treatment assignment, it raises ethical issues because the treatment team needs to engage in active deception.31 Finally, although lack of blinding could potentially have an impact on patient-reported outcomes, it is not expected to have any meaningful impact on the objective cognitive assessment battery results.32
In conclusion, use of HA during WBRT with memantine effectively spares the hippocampal neuroregenerative niche to better preserve cognitive function and patient-reported symptoms. No differences were observed in toxicity, intracranial PFS, or OS compared with standard WBRT and memantine.
ACKNOWLEDGMENT
We acknowledge the efforts of George Ballinger RT (R)(T); Roseann Bonanni, CTR, CCRP; Denise Manfredi, BS, RT (T); Kathryn Okrent, MA; and Tiffani Simpson-Small, RN, MPH, at the NRG Oncology Statistics and Data Management Center for their efforts in developing and managing the clinical trial and Rebecca Stull with NRG Oncology Operations for her editorial assistance.
PRIOR PRESENTATION
Presented at the 2018 Annual Meeting of the American Society for Radiation Oncology, San Antonio, TX, October 21-24, 2018; 2018 Annual Meeting of Society for Neuro-Oncology, New Orleans, LA, November 15-18, 2018; and 2019 Annual Meeting of the American Academy of Neurology, Philadelphia, PA, May 4-11, 2019.
SUPPORT
Supported by National Cancer Institute grants UG1CA189867 (National Cancer Institute Community Oncology Research Program) and U10CA180868 (NRG Oncology Operations).
See accompanying Editorial on page 1003
AUTHOR CONTRIBUTIONS
Conception and design: Paul D. Brown, Vinai Gondi, Stephanie Pugh, Wolfgang A. Tome, Jeffrey S. Wefel, Terri S. Armstrong, Joseph A. Bovi, Andre Konski, Deepak Khuntia, Tammie L. S. Benzinger, Deborah Bruner, Mark R. Gilbert, Minesh P. Mehta, Lisa A. Kachnic
Financial support: Deborah Bruner
Administrative support: Tammie L. S. Benzinger, Minesh P. Mehta
Provision of study material or patients: Cliff Robinson, Andre Konski, David Roberge, Vijayananda Kundapur, Kiran Devisetty, Kenneth Usuki, Nadia N. Laack, Wenyin Shi, Minesh P. Mehta
Collection and assembly of data: Vinai Gondi, Stephanie Pugh, Wolfgang A. Tome, Jeffrey S. Wefel, Cliff Robinson, David Grosshans, Tammie L. S. Benzinger, David Roberge, Vijayananda Kundapur, Sunjay Shah, Kenneth Usuki, Baldassarre Stea, Harold Yoon, Jing Li, Tim J. Kruser, Steven J. Chmura, Wenyin Shi
Data analysis and interpretation: Paul D. Brown, Vinai Gondi, Stephanie Pugh, Jeffrey S. Wefel, Terri S. Armstrong, Joseph A. Bovi, Cliff Robinson, Andre Konski, Deepak Khuntia, David Grosshans, Deborah Bruner, Mark R. Gilbert, David Roberge, Kiran Devisetty, Kenneth Usuki, Bethany Marie Anderson, Baldassarre Stea, Nadia N. Laack, Tim J. Kruser, Steven J. Chmura, Wenyin Shi, Snehal Deshmukh, Minesh P. Mehta, Lisa A. Kachnic
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paul D. Brown
Honoraria: UpToDate
Stephanie Pugh
Research Funding: Pfizer (Inst), Astellas (Inst), Millennium Pharmaceuticals (Inst)
Wolfgang A. Tome
Stock and Other Ownership Interests: Johnson & Johnson
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Archeus
Research Funding: Varian Medical Systems (Inst), Accuray (Inst), Chrysalis Biotherapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Patents held by the Wisconsin Research Foundation
Jeffrey S. Wefel
Consulting or Advisory Role: Angiochem (Inst), Bayer AG, Genentech, Roche, Juno Therapeutics (Inst), Novocure (Inst), Vanquish Oncology
Joseph A. Bovi
Honoraria: Elekta
Consulting or Advisory Role: Elekta
Cliff Robinson
Leadership: Radialogica
Stock and Other Ownership Interests: Radialogica
Consulting or Advisory Role: Varian Medical Systems, AstraZeneca, EMD Serono
Research Funding: Varian Medical Systems (Inst), Elekta (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Noninvasive imaging and treatment system for cardiac arrhythmias WO 2017078757 A1, US Provisional Application No. 62/598,162: System and method for determining segments for ablation
Travel, Accommodations, Expenses: Siemens Healthineers
Andre Konski
Employment: University of Pennsylvania
Stock and Other Ownership Interests: ImmunoCellular Therapeutics, Trevena
Deepak Khuntia
Employment: Varian Medical Systems
Leadership: Varian Medical Systems
Stock and Other Ownership Interests: Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Patent on FLASH radiation technology where Varian Medical Systems owns all rights to that patent (Inst)
Tammie L. S. Benzinger
Research Funding: Avid Radiopharmceuticals (Inst), Eli Lilly (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending for advanced diffusion MRI using diffusion basis spectrum imaging for neuroinflammation
David Roberge
Stock and Other Ownership Interests: Croton Healthcare
Honoraria: Pfizer, EMD Serono, Brainlab, Siemens Healthineers
Research Funding: Varian Medical Systems (Inst), Siemens Healthineers (Inst), Elekta (Inst), Bristol-Myers Squibb (Inst)
Kiran Devisetty
Leadership: Radiation & Retina Research, Radiation & Retina Research (I)
Stock and Other Ownership Interests: Abbott Laboratories (I), AbbVie (I)
Consulting or Advisory Role: Novocure
Travel, Accommodations, Expenses: Augmenix
Kenneth Usuki
Honoraria: Brainlab
Travel, Accommodations, Expenses: Brainlab
Jing Li
Research Funding: Bristol-Myers Squibb (Inst)
Nadia N. Laack
Research Funding: Bristol-Myers Squibb (Inst)
Tim J. Kruser
Consulting or Advisory Role: AstraZeneca
Speakers’ Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Steven J. Chmura
Employment: Astellas Pharma (I)
Honoraria: Reflexion Medical
Research Funding: Merck, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: UptoDate
Wenyin Shi
Consulting or Advisory Role: Novocure, Varian Medical Systems, Brainlab
Research Funding: Novocure (Inst), Brainlab (Inst), Regeneron Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems, Novocure, Brainlab
Minesh P. Mehta
Leadership: Oncoceutics
Stock and Other Ownership Interests: Oncoceutics
Consulting or Advisory Role: AstraZeneca, Celgene, Tocagen, AbbVie, Blue Earth Diagnostics
Research Funding: Novocure (Inst)
Patents, Royalties, Other Intellectual Property: Wisconsin Alumni Research Foundation patent 14/934,27: Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy
No other potential conflicts of interest were reported.
REFERENCES
- 1.Brown PD, Ahluwalia MS, Khan OH, et al. Whole-brain radiotherapy for brain metastases: Evolution or revolution? J Clin Oncol. 2018;36:483–491. doi: 10.1200/JCO.2017.75.9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: Secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015;1:457–464. doi: 10.1001/jamaoncol.2015.1145. [DOI] [PubMed] [Google Scholar]
- 4.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisberg B, Doody R, Stöffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 7.Duman JG, Dinh J, Zhou W, et al. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro-oncol. 2018;20:655–665. doi: 10.1093/neuonc/nox203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 10.Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85:348–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 16.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 17.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: Results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 18.Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. doi: 10.1007/s11060-006-9135-z. Armstrong TS, Mendoza T, Gning I, et al: Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol 80:27-35, 2006 [Erratum: J Neurooncol 80:37, 2006] [DOI] [PubMed] [Google Scholar]
- 20.Pintilie M. Dealing with competing risks: Testing covariates and calculating sample size. Stat Med. 2002;21:3317–3324. doi: 10.1002/sim.1271. [DOI] [PubMed] [Google Scholar]
- 21.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 24.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 25.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 26.Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: A phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33:1653–1659. doi: 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizumatsu S, Monje ML, Morhardt DR, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 28.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 29.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 31.Miller FG, Kaptchuk TJ. Sham procedures and the ethics of clinical trials. J R Soc Med. 2004;97:576–578. doi: 10.1258/jrsm.97.12.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler A, Hermann C. Placebo- and nocebo-effects in cognitive neuroenhancement: When expectation shapes perception. Front Psychiatry. 2019;10:498. doi: 10.3389/fpsyt.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]